Abstract
This book chapter presents the most important clinical aspects concerning the gonadotropin-releasing hormone antagonist cetrorelix and its importance in reproductive medicine. After an overview of the historical milestones in the development and establishment of cetrorelix in the context of ovarian stimulation treatment, its dosage, effects, and side effects are evaluated. The chapter terminates with a conclusion emphasizing the ease of use and the increase in patient safety because of a significantly reduced risk of ovarian hyperstimulation syndrome with cetrorelix compared with the agonist protocol
Introduction
Cetrorelix is a decapeptide and belongs to the group of gonadotropin-releasing hormone (GnRH) antagonists. Its main function is the inhibition of the luteinizing hormone (LH) secretion from the anterior pituitary gland, thereby preventing ovulation and inhibiting the production of sex steroids. After its initial applications in oncology, cetrorelix has gained importance in reproductive medicine as part of the short antagonist protocol for controlled ovarian stimulation. The great merit of GnRH antagonists is the almost 100% prevention of ovarian hyperstimulation syndrome. Overall, the GnRH antagonist cetrorelix, injected subcutaneously once or twice a day by the patient, is characterized by good efficacy and tolerability. However, an allergic local skin reaction with redness, swelling, and pain can be observed occasionally in patients using the drug. In conclusion, GnRH antagonists, such as cetrorelix, are currently the standard for in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatments.
Historical Development
The basis for developing GnRH antagonists was laid in 1971 with the structural elucidation and synthesis of GnRH by Schally and Guillemin (1). The GnRH is produced in a pulsatile manner by hypothalamic neurons in the area of the arcuate nucleus and controls pituitary-ovarian function. The main problem with gonadotropin stimulation is premature LH surge because of the positive feedback signal from estradiol to the pituitary gland. Consequences are premature ovulation, a reduction in oocyte and embryo quality, and, thus, a reduced pregnancy rate. Therefore, there was a need for further pharmacological research to develop a drug inhibiting a premature rise in LH (2). In 1981, the research continued with the synthesis of GnRH agonists, and in 1988, cetrorelix, the first GnRH antagonist, was discovered by Schally (3). In 1999, cetrorelix was the first “third generation" GnRH antagonist available on the European Union market for controlled ovarian stimulation in IVF and ICSI. One year later, ganirelix was approved in the EU as the second “third generation" GnRH antagonist, which was approved by the Food and Drug Administration in 1999. Important milestones for the clinical introduction of cetrorelix were the scientific works of Reissmann et al. (4) who published study results about the clinical efficacy and effectiveness of cetrorelix. Diedrich et al. (5) who published a dose-finding study for cetrorelix, provided further data for the clinical implementation of cetrorelix. Furthermore, Albano et al. (6) published a prospective, randomized open-label study, establishing a multidose administration protocol for cetrorelix and demonstrating the noninferiority of cetrorelix in preventing an endogenous LH surge compared with the GnRH agonist buserelin. In the following years, cetrorelix was quickly established as a useful tool in reproductive medicine.
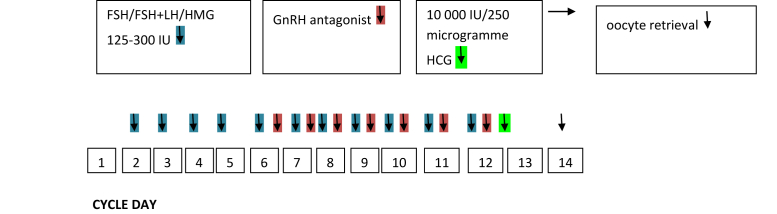
The GnRH antagonist cetrorelix was originally used to treat prostate carcinoma and benign prostatic hyperplasia. Cetrorelix is now 1 of the 2 injectable GnRH antagonists for controlled ovarian stimulation with assisted reproduction techniques. With this relatively young drug, the imminent complication of gonadotrophin stimulation ovarian hyperstimulation syndrome (OHSS) was reduced to almost zero in the short antagonist protocol because OHSS is triggered by human chorionic gonadotropin (HCG). In the short antagonist protocol, there is the possibility of triggering ovulation with a GnRH agonist, thus preventing HCG application (7). For this approach, 2 injections of the GnRH agonist triptorelin are performed usually about 36 hours before oocyte retrieval (8). Although the first Cochrane analysis by Al-Inany et al. (9) in 2006 showed a reduced pregnancy rate for the short antagonist protocol compared with the long agonist protocol, Griesinger et al. (10) in a new meta-analysis published in Human Reproduction Update in the same year, could show a comparable life birth rate between the 2 stimulation protocols. The European Society of Human Reproduction and Embryology recommended the short antagonist protocol as the standard stimulation protocol. This is primarily because of the greater safety of the patient. In addition, the stimulation duration and gonadotrophin consumption are reduced in the antagonist protocol. Whereas the number of oocytes retrieved is slightly reduced, there is equi-effectiveness in terms of oocyte quality and pregnancy rate (11). A scheme showing the course of the medication depending on the cycle day in ovarian stimulation with the short antagonist protocol is illustrated in Figure 1. Table 1 compares GnRH antagonists and GnRH agonists.
FIGURE 1.
Short antagonist protocol applied in IVF and ICSI cycles.
ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization.
TABLE 1.
Comparison of the GnRH antagonist cetrorelix with GnRH analogs.
| Criteria | GnRH antagonist cetrorelix | GnRH agonists |
|---|---|---|
| Onset of action | Immediate | Delayed (flare-up effect) |
| Efficacy | High | High |
| Route of administration | Subcutaneous injection | Subcutaneous injection |
| Daily dose | 1–2 injections | One injection per day or depot preparation |
| Indications | Controlled ovarian stimulation, ovarian cancer, prostate cancer, benign prostate hyperplasia, fertility preservation | Controlled ovarian stimulation, endometriosis, fibroids, prostate cancer, fertility preservation |
| Risk profile | favorable (minimal risk for OHSS) | unfavorable (risk for OHSS) |
| Risk for local allergic reaction | higher | lower |
| Mode of action | competitive binding to the GnRH receptors to suppress the GnRH effects | down-regulation of the GnRH receptors and desensitization of the gonadotrophic cells |
| Reversibility | immediately | after 6 weeks |
| Clinical benefits | simple stimulation no increase of gonadotrophin dose at GnRH antagonist initiation avoidance of OHSS and ovarian cysts shorter stimulation lower gonadotrophin consumption |
more oocytes retrieved potential suppression of endometriosis |
| Price | about 56 € per injection | about 17 € per injection |
GnRH = gonadotropin-releasing hormone; OHSS = ovarian hyperstimulation syndrome.
Dosage Form
Cetrorelix exists in 2 preparations for clinical use. One formulation is a single-dose preparation with 3 mg cetrorelix, and the second is a multiple-dose preparation with 0.25 mg cetrorelix. Meanwhile, the multidose administration starting at a follicle size > 12 mm has developed to be the golden standard, as single and multiple-dose protocols are absolutely comparable with each other concerning pregnancy rates, embryo transfer, oocyte pick-up, and HCG administration (12). For this cause, multiple-dose preparation has prevailed in everyday clinical practice. Each vial contains 0.25 mg of cetrorelix as acetate in the form of a white powder. An ampoule with a colorless solvent is also supplied. After mixing the powder and solvent, each ampoule contains 0.25 mg cetrorelix per ml. The patient administers the mixed ampoule into the abdomen's subcutaneous fat tissue. Most patients do not need assistance for the injection. The absolute bioavailability of cetrorelix is 85%. Anecdotal evidence suggests that an allergic skin reaction could be prevented by cooling the ampoule beforehand.
Areas of Application and Dosage
Cetrorelix is used to prevent premature ovulation as part of controlled ovarian stimulation treatment. The content of 1 vial (0.25 mg) is administered once a day every 24 hours. In individual cases, it can also be given twice a day at intervals of 12 hours. On average, the first cetrorelix application is performed on day 6 of the menstrual cycle, depending on the sonographic follicle size and serum estradiol level. Individual deviations are possible, especially in patients with accelerated follicle growth with an initiation of the GnRH antagonist on the 4th or 5th day of stimulation. Most experts recommend commencing the GnRH antagonist at a follicle size between 12 mm and 14 mm. This is usually the case on the 5th or 6th day of stimulation. The last application takes place on the day of ovulation induction. The administration of cetrorelix is contraindicated in the event of an allergy to the active ingredient, during pregnancy and breastfeeding, and in the case of severe kidney function impairment.
Effect
The half-life after a single injection of cetrorelix is approximately 30 hours, which is twice the half-life of GnRH agonists. After multiple injections, the half-life is up to 80 hours. The maximum plasma concentration is reached 1 to 2 hours after the injection. Albumin binding is around 85%. Cetrorelix's mechanism of action can be regarded as competitive binding to GnRH receptors, preventing GnRH circulating in the blood from interacting with its specific receptor. This results in the suppression of plasma LH and follicle stimulating hormone levels within 8 hours after injection. The effects of cetrorelix were already shown to be reversible in the following cycle, as spontaneous ovulations were observed in the following cycle (13, 14).
Interactions
No interaction studies have been performed with cetrorelix. However, interactions in the cytochrome P450 enzyme system of the liver could largely be ruled out. In clinical use, allergic skin reactions with painful swelling and redness are regularly observed, but they are mild and quickly reversible (15).
Conclusion
Over the years, the GnRH antagonist cetrorelix has developed into a standard drug in reproductive medicine, being an integral part of controlled stimulation protocols (the so-called short antagonist protocols). The main advantages of using GnRH antagonist protocols can be regarded in the simple and patient-friendly approach, lower duration of stimulation, decreased consumption of gonadotrophins with lower drug costs, much lower risk for OHSS, and less hormonal disturbances like ovarian cyst formation or hormonal withdrawal while achieving an equal pregnancy rate compared with the stimulation protocols using GnRH agonists.
Footnotes
S.F. has nothing to disclose. K.D. has nothing to disclose.
References
- 1.Guillemin R. Purification, isolation, and primary structure of the hypothalamic luteinizing hormone-releasing factor of ovine origin. A historical account. Am J Obstet Gynecol. 1977;129:214–218. doi: 10.1016/0002-9378(77)90749-9. [DOI] [PubMed] [Google Scholar]
- 2.Reissmann T., Felberbaum R., Diedrich K., Engel J., Comaru-Schally A.M., Schally A.V. Development and applications of luteinizing hormone-releasing hormone antagonists in the treatment of infertility: an overview. Hum Reprod. 1995;10:1974–1981. doi: 10.1093/oxfordjournals.humrep.a136219. [DOI] [PubMed] [Google Scholar]
- 3.Schally A.V. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 4.Reissmann T., Schally A.V., Bouchard P., Riethmiiller H., Engel J. The LHRH antagonist cetrorelix: a review. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 5.Diedrich K., Diedrich C., Santos E., Zoll C., al-Hasani S., Reissmann T., et al. Suppression of the endogenous luteinizing hormone surge by the gonadotrophin-releasing hormone antagonist Cetrorelix during ovarian stimulation. Hum Reprod. 1994;9:788–791. doi: 10.1093/oxfordjournals.humrep.a138597. [DOI] [PubMed] [Google Scholar]
- 6.Albano C., Felberbaum R.E., Smitz J., Riethmüller-Winzen H., Engel J., Diedrich K., et al. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. European Cetrorelix Study Group. Hum Reprod. 2000;15:526–531. doi: 10.1093/humrep/15.3.526. [DOI] [PubMed] [Google Scholar]
- 7.Devroey P., Polyzos N.P., Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 8.Devroey P., Adriaensen P. OHSS Free Clinic. Facts Views Vis Obgyn. 2011;3:43–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Inany H.G., Abou-Setta A.M., Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;19:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Griesinger G., Diedrich K., Devroey P., Kolibianakis E.M. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 11.Bosch E., Broer S., Griesinger G., Grynberg M., Humaidan P., Kolibianakis E., et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;2020 doi: 10.1093/hropen/hoaa009. hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howles C.M. The place of gonadotrophin-releasing hormone antagonists in reproductive medicine. Reprod Biomed Online. 2002;4:64–71. doi: 10.1016/s1472-6483(12)60120-5. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig M., Albano C., Olivennes F., Felberbaum R.E., Smitz J., Ortmann O., et al. Plasma and follicular fluid concentrations of LHRH antagonist cetrorelix (Cetrotide) in controlled ovarian stimulation for IVF. Arch Gynecol Obstet. 2002;266:12–17. doi: 10.1007/pl00007490. [DOI] [PubMed] [Google Scholar]
- 14.Leroy I., d'Acremont M., Brailly-Tabard S., Frydman R., de Mouzon J., Bouchard P. A single injection of a gonadotropin-releasing hormone (GnRH) antagonist (Cetrorelix) postpones the luteinizing hormone (LH) surge: further evidence for the role of GnRH during the LH surge. Fertil Steril. 1994;62:461–467. doi: 10.1016/s0015-0282(16)56932-5. [DOI] [PubMed] [Google Scholar]
- 15.Engel J.B., Schultze-Mosgau A., Diedrich K. Five years' clinical use of GnRH antagonists: evaluation of safety and allergic potential. Reprod Biomed Online. 2005;10:61–65. doi: 10.1016/s1472-6483(11)60392-1. [DOI] [PubMed] [Google Scholar]