Abstract
Ganirelix is a gonadotropin-releasing hormone (GnRH) antagonist with high antagonistic activity that blocks the GnRH receptor by competitive binding. A daily dose of 0.25 mg of ganirelix was sel5ected after a phase II study because it was the minimal, effective daily dose to prevent premature luteinizing hormone surges and this dose yielded the highest ongoing pregnancy rate per started cycle. After subcutaneous administration, ganirelix is rapidly absorbed, reaching peak levels within 1–2 hours (tmax), and has a high absolute bioavailability (>90%). Prospective, comparative studies have demonstrated the advantages of GnRH antagonists over long GnRH agonist treatment in assisted reproduction, including the immediate reversibility of drug effects, a requirement for less follicle-stimulating hormone, a shortened duration of stimulation, a reduced incidence of ovarian hyperstimulation syndrome, and reduced patient burden. Combined analyses concluded that in the general in vitro fertilization population, there is a trend for a slightly lower ongoing pregnancy rate and a lower risk of ovarian hyperstimulation syndrome that is largely eliminated when considering triggering with GnRH agonist instead of human chorionic gonadotropin. Regardless of all the research, it is still not fully elucidated why the long GnRH agonist protocol has a trend for higher pregnancy rates after fresh transfer of the same number of good-quality embryos.
Key Words: GnRH antagonist, pregnancy rates, OHSS, prevention of premature LH surges, ganirelix
Structure and mechanism of action
Ganirelix (United States Adopted Name: Ganirelix acetate) is a third-generation gonadotropin-releasing hormone (GnRH) antagonist with high antagonistic activity and minimal histamine-releasing properties. It blocks the GnRH receptor by competitive binding but does not activate the receptor in the pituitary gland (1).
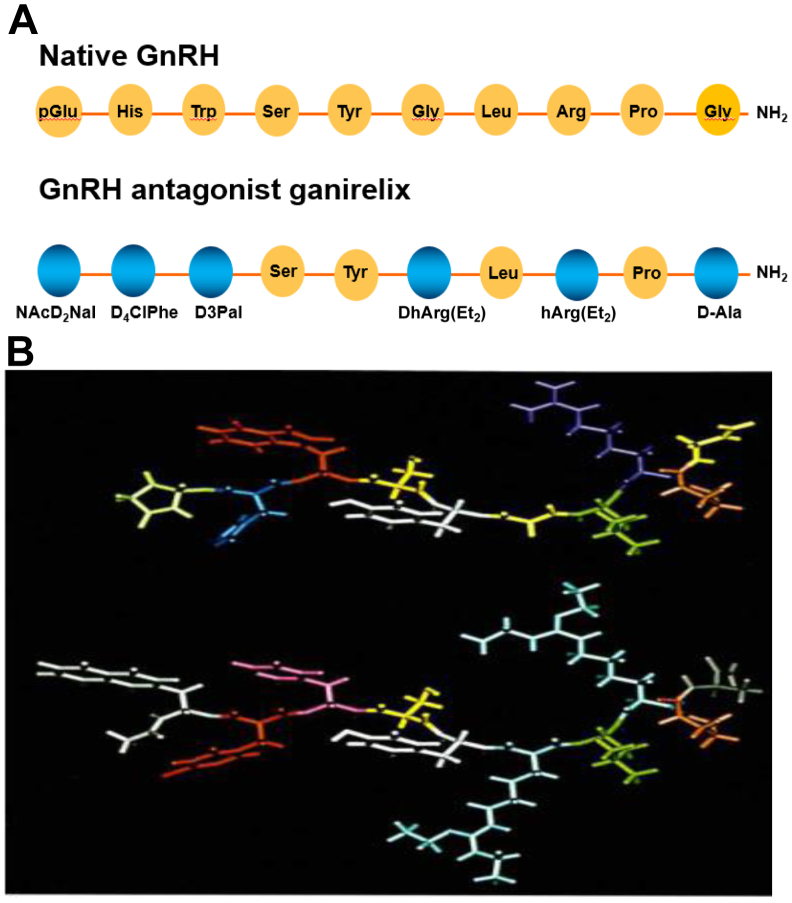
As a result, a rapid, profound, and reversible suppression of the release of pituitary gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and consequently of gonadal function occurs. The decapeptide contains several amino acids that are unnatural in stereochemistry and/or in structure (Fig. 1).
Figure 1.
(A) Schematic presentation of the amino acid sequence of native gonadotropin-releasing hormone (GnRH) and ganirelix. (B) A 3-dimensional model of native GnRH and ganirelix.
Its molecular weight is 1570.4, and substitutions may be found at positions 1, 2, 3, 6, 8, and 10. The latter determine its physical and chemical properties, which include high aqueous solubility, high stability, and high receptor-binding affinity. As a result, ganirelix has a ninefold higher GnRH receptor–binding affinity (Dissociation constant [KD] = 0.4 nM) than GnRH (KD = 3.6 nM). Additional experiments investigated the specificity of binding of ganirelix to other receptors of the G-protein–coupled receptor family and other types of receptors. Subsequently, specificity and efficacy of action were demonstrated in in vivo animal pharmacology with respect to both general and reproductive pharmacology. Special attention has been paid to the reversibility of the effects of ganirelix on female reproductive function.
Nonclinical pharmacokinetic studies show that the in vitro and in vivo stability of ganirelix is considerably greater than that of native GnRH owing to the use of D-amino acids for substitution. There are many conditions for which suppression of endogenous hormones would be helpful for treatment; for example, suppression of estradiol concentrations in endometriosis could lead to reduced menstrual cycle–related pain, and such treatment could diminish the growth of sex hormone–dependent malignant neoplasms, such as prostatic and breast cancers. Nonclinical research on ganirelix has been performed mainly by Syntex Research (Palo Alto, CA; RS-26306-298) for the long-term treatment of various hormone-dependent disorders, such as prostate cancer.
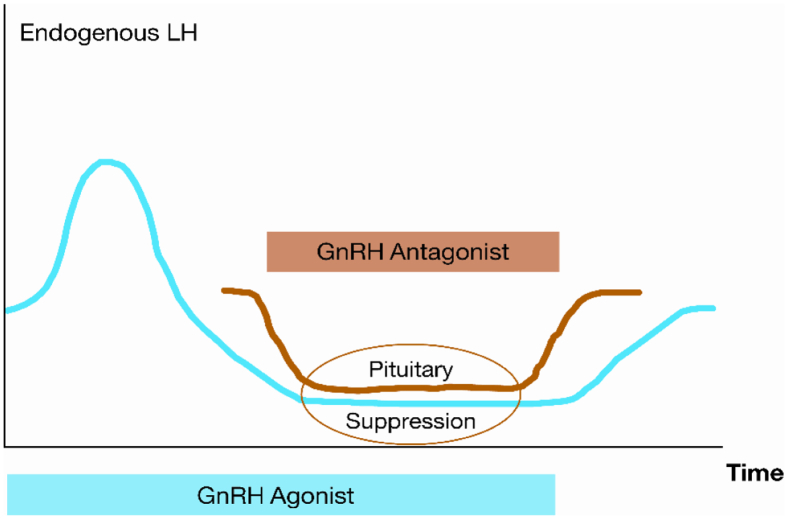
In the framework of nonclinical safety and efficacy testing, ganirelix was compared with second-generation GnRH antagonists, such as detirelix, in several studies. The compound was developed by NV Organon (Oss, The Netherlands) for the prevention of premature LH surges in women undergoing ovarian stimulation before assisted reproductive technology. In contrast to a GnRH agonist, a GnRH antagonist causes immediate and reversible blockage of the GnRH receptor at the pituitary gland and, therefore, reduces the secretion of FSH and LH within several hours after injection (Fig. 2). In 1999, ganirelix was the first approved GnRH antagonist in the United States after a first-priority review by the Food and Drug Administration.
Figure 2.
Mechanism of action of a gonadotropin-releasing hormone (GnRH) agonist and a GnRH antagonist, the latter causing immediate and reversible blockage of the GnRH receptor. LH = luteinizing hormone.
Global clinical development
Clinical development of ganirelix for the prevention of premature LH surges started in 1996 with a dose-finding trial in patients undergoing in vitro fertilization (IVF). The application of ganirelix during ovarian stimulation required the selection of a relatively low dose of ganirelix that would prevent premature LH surges but would not result in a too profound LH suppression that could compromise the clinical outcome. Therefore, a double-blinded, randomized, dose-finding study including 6 different dosages of ganirelix between 0.0625 mg and 2 mg was performed (2).
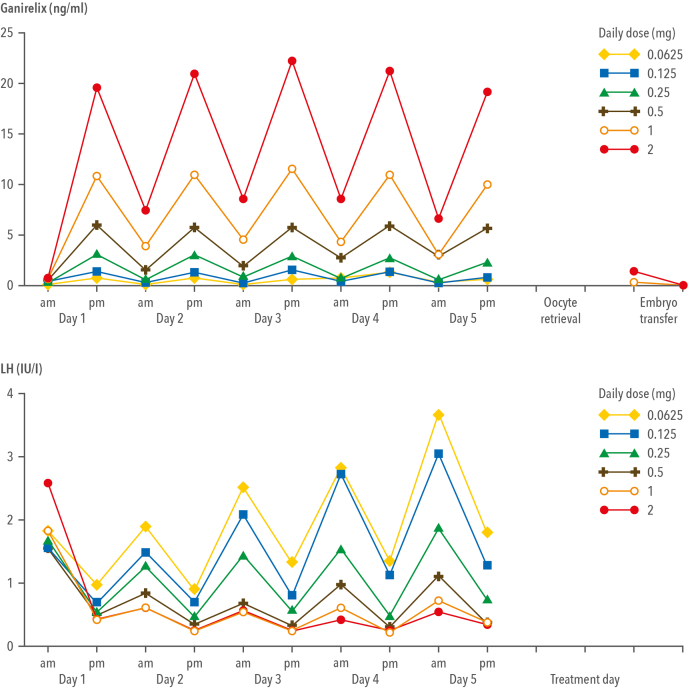
The ganirelix dose-finding study is a typical example of a classic, double-blinded, dose-finding study that included 333 patients and 6 dosages of ganirelix to establish the optimal dose for further development. The pharmacokinetics and pharmacodynamics of ganirelix as established in these patients are presented in Figure 3. Circulating ganirelix levels increased in a linear, dose-proportional manner, and a steady state was reached within 2 days. The effect of increasing ganirelix levels on serum LH levels is immediate and reversible; thus, drug compliance to prevent premature LH surges is essential.
Figure 3.
Serum ganirelix levels (upper panel) and serum luteinizing hormone (LH) levels (lower panel) measured just before ganirelix injection in the morning and approximately 8 hours later in the afternoon for patients who received at least 5 days of ganirelix treatment. (Adapted from the ganirelix dose-finding study group [2], 1998.).
During the trial, the lowest and highest doses were terminated prematurely because of too low and too high pituitary suppression, respectively. The data justifying the termination of these 2 dose groups during the trial are shown in Table 1. Treatment with daily 0.0625-mg ganirelix and the 0.125-mg dose resulted in premature LH rises during ovarian stimulation; thus, ganirelix levels were too low to completely block the GnRH receptors, resulting in endogenous LH release from the pituitary gland under the influence of native, pulsatile GnRH. The highest dose group treated with daily 2-mg ganirelix was discontinued because no ongoing pregnancies were established in the first cohort of 30 patients, most probably because endogenous LH was too suppressed. Follow-up data on cryopreserved embryos obtained during the dose-finding study indicated that the high antagonist dosages had not adversely affected the potential of embryos to establish pregnancy in freeze-thaw cycles (3). Because no pregnancies were established in the 2-mg group after fresh embryo transfer, the effect of daily treatment with 2-mg ganirelix on endometrial development was studied in oocyte donors; however, in comparison to 0.25-mg ganirelix, no relevant alteration was observed in the endometrial development in the early and midluteal phases (4).
TABLE 1.
Main clinical findings in the phase II dose-finding study of ganirelix.
| Daily dose of ganirelix (mg) |
||||||
|---|---|---|---|---|---|---|
| 0.0625 (n = 31) | 0.125 (n = 65) | 0.25 (n = 69) | 0.5 (n = 69) | 1.0 (n = 65) | 2.0 (n = 30) | |
| Serum LH rise of ≥10 IU/L during ganirelix treatment | 5 | 6 | 1 | - | - | - |
| Serum LH (IU/L) on the day of hCG treatment | 3.6 | 2.5 | 1.7 | 1.0 | 0.6 | 0.4 |
| Serum estradiol (pg/mL) on the day of hCG treatment | 1475 | 1130 | 1160 | 823 | 703 | 430 |
| Oocytes | 9.0 | 9.5 | 10.0 | 8.8 | 9.3 | 8.6 |
| Good-quality embryos (day 3) | 3.8 | 3.3 | 3.3 | 2.5 | 3.3 | 3.5 |
| Implantation rate (%) | 14.2 | 16.6 | 21.9 | 9.0 | 8.8 | 1.5 |
| Ongoing pregnancy rate (%) per started stimulation | 23.3 | 23.1 | 33.8 | 10.1 | 14.1 | 0 |
| Ongoing pregnancy rate (%) per transfer | 25.9 | 25.0 | 37.1 | 13.0 | 15.3 | 0 |
Note: hCG = human chorionic gonadotropin; LH = luteinizing hormone.
The ganirelix dose-finding study resulted in the selection of a daily dose of 0.25-mg of ganirelix for further clinical development because it was the minimal, effective daily dose to prevent premature LH surges, and this dose also yielded the highest implantation rate and ongoing pregnancy rate per started cycle. After the phase II study, the pharmacokinetic and pharmacodynamic characteristics of the selected 0.25-mg dose of ganirelix were further studied in healthy female volunteers of reproductive age. Ganirelix was rapidly absorbed, reaching peak levels within 1–2 hours (tmax) after a single injection and had a relatively short half-life of approximately 13 hours (5). Moreover, ganirelix injected subcutaneously had a high absolute bioavailability (>90%) in comparison to intravenous injection. A subsequent multiple-dose study indicated that exposure increased in a dose-proportional manner, with an elimination half-life of 16 hours and a steady state being acheived within 2–3 days after daily 0.25-mg ganirelix administration (6).
The first comparative phase III efficacy and safety study of ganirelix (7) was designed as an open-label, noninferiority trial to show that the clinical outcome of the ganirelix regimen was “no worse” than the comparator, that is, a long GnRH agonist protocol. This first and largest comparative phase III trial included approximately 700 patients who were treated in a ratio of 2:1 with ganirelix or a long protocol of intranasal buserelin. The primary end point was the number of cumulus-oocyte complexes recovered, and the lower limit of the treatment difference was set at −3 oocytes. In addition, for the ongoing pregnancy rate per started cycle, a predefined treatment difference of −5% was considered to be acceptable, anticipating an ongoing pregnancy rate of approximately 22%. After the first large study, 2 smaller randomized controlled studies, each including approximately 300 patients, were performed using a long protocol of leuprolide acetate and triptorelin as a reference (8, 9).
Gonadotropin-releasing hormone (GnRH) antagonist vs. long GnRH agonist protocols, what are the differences?
Studies comparing the GnRH antagonist with long protocols of GnRH agonist have consistently indicated that the cohort of recruited follicles is smaller after GnRH antagonist treatment. The difference in the largest ganirelix trial (7) was −1 oocyte, and the lower limit of the 95% confidence interval (CI) was within the predefined noninferiority margin of −3 oocytes. The treatment difference for the ongoing pregnancy rate was −5.4%, which was not statistically significant; however, the point estimate passed the predefined treatment difference (delta) of 5%. After this first randomized controlled trial, many other prospective controlled studies and (systematic) reviews have demonstrated the advantages of GnRH antagonist treatment over GnRH agonists in assisted reproduction, including the immediate reversibility of drug effects, a requirement for less FSH, a shortened duration of stimulation, a reduced incidence of ovarian hyperstimulation syndrome (OHSS), and reduced patient burden (10). After several Cochrane analyses, the last analysis (11) concluded that there is moderate-quality evidence that the use of GnRH antagonist compared with long GnRH agonist protocols is associated with a substantial reduction in OHSS without reducing the likelihood of achieving a live birth. Additional combined analyses indicated that the incidences of severe OHSS [5.1% (27 of 528) vs. 8.9% (44 of 495)] and moderate OHSS [10.2% (54 of 528) vs.15.6% (77 of 495)] were significantly lower with the GnRH antagonist protocol than with the long GnRH agonist protocol (12). The review by Lambalk et al. (13), including 50 studies, concluded that the ongoing pregnancy rate was significantly lower in the GnRH antagonist group than in the agonist group (relative risk (RR), 0.89; 95% CI, 0.82–0.96) in the general IVF population, whereas in women with polycystic ovary syndrome and in women with poor ovarian response, there was no evidence of a difference in ongoing pregnancy between the antagonist (RR, 0.97; 95% CI, 0.84–1.11) and agonist (RR, 0.87; 95% CI, 0.65–1.17) groups. With respect to OHSS, they concluded that antagonists resulted in significantly lower OHSS rates both in general IVF patients (RR, 0.63; 95% CI, 0.50–0.81) and in women with polycystic ovary syndrome (RR, 0.53; 95% CI, 0.30–0.95). An evaluation of all combined analyses provided clear evidence that the GnRH antagonist protocol is a safer treatment protocol, certainly when considering the treatment option of GnRH agonist triggering (14, 15). Regardless of all the research, it is still unclear why the long GnRH agonist protocol has a trend for higher pregnancy or live birth rates after fresh embryo transfer. This may be related to a higher chance of selecting a top-quality embryo for transfer because the ovarian response is higher, or it may be related to a direct and diverse effect of these GnRH analogues on embryo quality and implantation (16).
Ganirelix in current treatment regimens
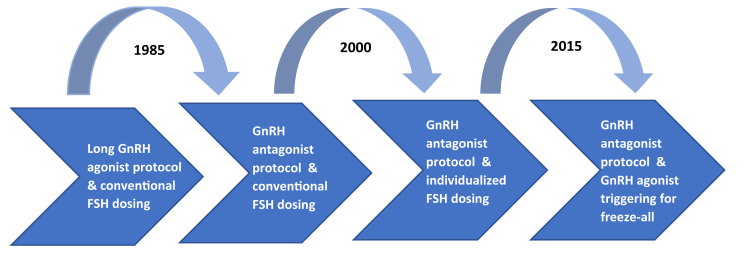
The development of GnRH antagonist for the prevention of premature LH surges is considered a major step forward in infertility treatment being more convenient and safer for patients (Fig. 4). Moreover, the GnRH antagonist protocol allows the triggering of final oocyte maturation with a GnRH agonist in combination with freezing of all embryos (freeze-all strategy). Initially, this segmentation of IVF treatment was thought to eliminate OHSS (17). First tested in 2000 by Itskovitz et al. (18), it became clear that GnRH agonist triggering induces an initial flare-up of endogenous LH that lasts for 24–36 hours (19), completes oocyte maturation, and reduces the risk of OHSS due to rapid luteolysis (20). Today, the GnRH antagonist protocol with GnRH agonist triggering and freezing of all embryos is the preferred approach for high responders, oocyte donors, and patients requiring preimplantation genetic testing. However, GnRH agonists are not indicated for triggering final oocyte maturation, and thus, ganirelix was developed in a protocol using only a single bolus of human chorionic gonadotropin for triggering, followed by fresh embryo transfer.
Figure 4.
The development of gonadotropin-releasing hormone (GnRH) analogue protocols for in vitro fertilization treatment over time. FSH = follicle-stimulating hormone.
In phase III registration trials, ganirelix was started at stimulation day 6, with conventional FSH starting doses of 150 or 225 IU. In current practice, ganirelix is most frequently started at day 5 or 6 to prevent early LH rises, although these have a limited impact on the chance of pregnancy (21). During the last decades, the GnRH antagonist protocol has moved away from conventional FSH dosing before fresh embryo transfer and has been replaced by individualized FSH dosing that reduces the risk of overdosing and underdosing as well as dose adjustments during stimulation (22). For frozen cycles, the GnRH antagonist protocol with GnRH agonist triggering has become the preferred treatment option, which allows more robust stimulation and the recovery of more oocytes without compromising safety. Overall, thanks to the development of ganirelix, patients are treated more safely and more successfully than they were 20 years ago, when only GnRH agonists were available for the prevention of LH surges.
Footnotes
B.M. is currently an employee of Organon and a former employee of Ferring Pharmaceuticals.
References
- 1.Mannaerts B., Gordon K. Embryo implantation and GnRH antagonists: GnRH antagonists do not activate the GnRH receptor. Hum Reprod. 2000;15:1882–1883. doi: 10.1093/humrep/15.9.1882. [DOI] [PubMed] [Google Scholar]
- 2.A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod. 1998;13:3023–3031. [PubMed] [Google Scholar]
- 3.Kol S., Lightman A., Hillensjö T., Devroey P., Fauser B., Tarlatzis B., et al. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod. 1999;14:2242–2244. doi: 10.1093/humrep/14.9.2242. [DOI] [PubMed] [Google Scholar]
- 4.Simon C., Oberyé J., Bellver J., Vidal C., Bosch E., Horcajadas J.A., et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–3327. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 5.Oberyé J.J.L., Mannaerts B.M.J.L., Kleijn H.J., Timmer C.J. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999;72:1001–1005. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- 6.Oberyé J.J.L., Mannaerts B.M.J.L., Huisman J.A.M., Timmer C.J. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 7.The European Orgalutran Study Group. Borm G., Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. The European Orgalutran Study Group. Hum Reprod. 2000;15:1490–1498. doi: 10.1093/humrep/15.7.1490. [DOI] [PubMed] [Google Scholar]
- 8.Fluker M., Grifo J., Leader A., Levy M., Meldrum D., Muasher S.J., et al. North American Ganirelix Study Group Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertil Steril. 2001;75:38–45. doi: 10.1016/s0015-0282(00)01638-1. [DOI] [PubMed] [Google Scholar]
- 9.European. Middle East Orgalutran Study Group Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod. 2001;16:644–651. doi: 10.1093/humrep/16.4.644. [DOI] [PubMed] [Google Scholar]
- 10.Tarlatzis B.C., Fauser B.C., Kolibianakis E.M., Diedrich K., Rombauts L., Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12:333–340. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 11.Al-Inany H.G., Youssef M.A., Ayeleke R.O., Brown J., Lam W.S., Broekmans F.J. Gonadotrophin–releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toftager M., Bogstad J., Bryndorf T., Løssl K., Roskær J., Holland T., et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31:1253–1264. doi: 10.1093/humrep/dew051. [DOI] [PubMed] [Google Scholar]
- 13.Lambalk C.B., Banga F.R., Huirne J.A., Toftager M., Pinborg A., Homburg R., et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23:560–579. doi: 10.1093/humupd/dmx017. [DOI] [PubMed] [Google Scholar]
- 14.Humaidan P., Alsbjerg B. GnRHa trigger for final oocyte maturation: is HCG trigger history? Reprod Biomed Online. 2014;29:274–280. doi: 10.1016/j.rbmo.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Engmann L., Benadiva C., Humaidan P. GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: a SWOT analysis. Reprod Biomed Online. 2016;32:274–285. doi: 10.1016/j.rbmo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Raga F., Casañ E.M., Kruessel J., Wen Y., Bonilla-Musoles F., Polan M.L. The role of gonadotropin-releasing hormone in murine preimplantation embryonic development. Endocrinology. 1999;140:3705–3712. doi: 10.1210/endo.140.8.6899. [DOI] [PubMed] [Google Scholar]
- 17.Devroey P., Polyzos N.P., Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 18.Itskovitz-Eldor J., Kol S., Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication. Hum Reprod. 2000;15:1965–1968. doi: 10.1093/humrep/15.9.1965. [DOI] [PubMed] [Google Scholar]
- 19.Fauser B.C., de Jong D., Olivennes F., Wramsby H., Tay C., Itskovitz-Eldor J., et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 20.Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril. 2004;81:1–5. doi: 10.1016/j.fertnstert.2003.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Frattarelli J.L., Hillensjö T., Broekmans F.J., Witjes H., Elbers J., Gordon K., et al. Clinical impact of LH rises prior to and during ganirelix treatment started on day 5 or on day 6 of ovarian stimulation. Reprod Biol Endocrinol. 2013;11:90. doi: 10.1186/1477-7827-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen A.N., Nelson S.M., Fauser B.C.J.M., García-Velasco J.A., Klein B.M., Arce J.C., ESTHER-1 Study Group Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107:387–396.e4. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]