Abstract
Background
Infections by SARS-CoV-2 in liver transplant recipients (LT) patients are of particular concern, notably due to perceived added risks related to immunosuppression and comorbidity burden. Current literature on this topic often relies on small, non-standardized, and geographically limited studies. This manuscript describes COVID-19 presentations and causes for elevated mortality in a large cohort of LT recipients.
Methods
This study was designed as a multicentric historical cohort, including LT recipient patients with COVID-19 in 25 study centers, with the primary endpoint being COVID-related death. We also collected demographic, clinical, and laboratory data regarding presentation and disease progression.
Results
Two hundred and thirty-four cases were included. The study population was predominantly male and White and had a median age of 60 years. The median time from transplantation was 2.6 years (IQR 1-6). Most patients had at least one comorbidity (189, 80.8%). Patient age (P = .04), dyspnea (P < .001), intensive care unit admission (P < .001), and mechanical ventilation (P < .001) were associated with increased mortality. Modifications of immunosuppressive therapy (P < .001), specifically the suspension of tacrolimus, maintained significance in multivariable analysis.
Conclusions
Attention to risk factors and the individualization of patient care, especially regarding immunosuppression management, is crucial for delivering more precise interventions to these individuals.
The COVID-19 pandemic has drastically shifted the routine of transplantation centers worldwide. Current literature on the disease characteristics in this population comprises cohort studies from Europe and North America [1], [2], [3], [4], [5], [6]. However, these studies often report low numbers of patients with varying methodologies.
Descriptions regarding the role of immunosuppression on disease outcomes have yielded conflicting results. Belli et al reported a seemingly protective role of tacrolimus against mortality, whereas Miarons et al argued that CYP3A4 inhibition in SARS-CoV-2 infection could lead to tacrolimus toxicity [2,7].
This lack of clear evidence is of particular concern because the population of liver transplant (LT) recipients is perceived as susceptible to higher rates of severe disease due to immunosuppression and an increased number of comorbidities [8]. This study seeks to report the impact of COVID-19, standardizing disease characteristics, progression, and outcomes in a large sample of LT recipients.
Materials and Methods
We designed this study as a multicentric retrospective historical cohort by analyzing patient records submitted using an online standardized questionnaire in all enrolled institutions.
Informed consent was obtained from all participants, following institutional and international standards in all participant centers. Institutional Board Review approval numbers for each institution are outlined in the supplementary material section. All data is derived from transplants involving organs obtained from willing and consented donations.
The study included adult LT recipient patients with previously established post-transplant follow-up at any of the participating institutions who sought health care services between December 2019 and October 2021 and had a confirmatory reverse transcription polymerase chain reaction or rapid antigen test. Follow-up was maintained until either death or the date of the study conclusion. The primary endpoint of the study was COVID-19–related death.
Data on previous medical conditions, immunosuppressive regimens, and symptoms at admission were collected for all patients. Imaging tests were performed if medically indicated and considered abnormal if presented with interstitial alterations, consolidations, or ground glass opacities, unilaterally or bilaterally. Data on specific covid-treatments was collected, yet, due to the period in which the study was conducted, most of the current covid-specific treatments were either unknown or not yet in use. As such, the study mostly focused on collecting data on the use of anticoagulation or corticosteroid therapy.
Due to the study beginning before any vaccines were available in the country, vaccination status was not collected, and patients were presumed to be unvaccinated because, until the last months of the study, <10% of the country's population was fully vaccinated [9].
We collected patient data using a standardized online formulary filled out by participating institutions. Statistical analyses were performed using SPSS version 23 (IBM SPSS, Inc, Armonk, NY, United States) and R (version 4.0; R Foundation for Statistical Computing, Vienna, Austria). We performed descriptive analysis and parametric or nonparametric comparative tests. Mann-Whitney Q test was used for non-normal continuous variables, the Student t test and analysis of variance were used for normal variables, and the Wilcoxon rank sum test and Fisher's exact test for non-normal variables. We performed a χ2 test for normal and non-normal discrete binary variables. Select variables were submitted to multivariable analysis by binary logistic regression with Wald tests for independent variable significance and Pseudo-R2 estimation. Kaplan Meier with log-rank tests was also performed and plotted in survival probabilities to showcase some specific variables' effects on mortality.
Results
Demographics and General Characteristics of the Study Population
Among the 25 participating centers, 234 patients were included in this study. Diagnosis of COVID-19 was predominantly performed by reverse transcription polymerase chain reaction (98%), with a small percentage of rapid antigen tests (2.1%). Detailed descriptions of demographic data and outcomes are displayed in Table 1 .
Table 1.
Demographic and Outcome Data
| Characteristic | N | Overall N = 234* |
Survived N = 186* |
Dead N = 48* |
P Value† |
|---|---|---|---|---|---|
| Sex | 234 | .063 | |||
| Female | 64 (27%) | 56 (30%) | 8 (17%) | ||
| Male | 170 (73%) | 130 (70%) | 40 (83%) | ||
| Age | 234 | 60 (54, 66) | 59 (53, 66) | 63 (58, 68) | .033 |
| Ethnicity | 234 | .9 | |||
| Not disclosed | 6 (2.6%) | 5 (2.7%) | 1 (2.1%) | ||
| Asian | 3 (1.3%) | 3 (1.6%) | 0 (0%) | ||
| Black | 13 (5.6%) | 12 (6.5%) | 1 (2.1%) | ||
| Mixed race | 66 (28%) | 52 (28%) | 14 (29%) | ||
| White | 146 (62%) | 114 (61%) | 32 (67%) | ||
| Years since LTX | 232 | 2.6 (1.0, 6.6) | 3.1 (1.1, 6.6) | 1.6 (0.9, 5.9) | .11 |
| Diabetes | 234 | 110 (47%) | 89 (48%) | 21 (44%) | .6 |
| Dyslipidemia | 234 | 7 (3.0%) | 5 (2.7%) | 2 (4.2%) | .6 |
| Chronic Kidney Disease | 234 | 29 (12%) | 21 (11%) | 8 (17%) | .3 |
| Hypertension | 234 | 111 (47%) | 88 (47%) | 23 (48%) | > .9 |
| Cardiovascular disease | 234 | 22 (9.4%) | 19 (10%) | 3 (6.2%) | .6 |
| Obesity | 211 | 55 (26%) | 37 (22%) | 18 (44%) | .004 |
| Fever | 234 | 121 (52%) | 94 (51%) | 27 (56%) | .5 |
| Nausea | 234 | 13 (5.6%) | 9 (4.8%) | 4 (8.3%) | .3 |
| Odynophagia | 234 | 6 (2.6%) | 5 (2.7%) | 1 (2.1%) | > .9 |
| Cough | 234 | 115 (49%) | 93 (50%) | 22 (46%) | .6 |
| Choryza | 234 | 36 (15%) | 32 (17%) | 4 (8.3%) | .13 |
| Dyspnea | 234 | 77 (33%) | 48 (26%) | 29 (60%) | < .001 |
| Abdominal pain | 234 | 5 (2.1%) | 5 (2.7%) | 0 (0%) | .6 |
| Diarrhea | 234 | 43 (18%) | 35 (19%) | 8 (17%) | .7 |
| Lost sense of smell | 234 | 26 (11%) | 25 (13%) | 1 (2.1%) | .026 |
| Assymptomatic | 234 | 12 (5.1%) | 11 (5.9%) | 1 (2.1%) | .5 |
| Disease wave | 229 | .12 | |||
| First | 111 (48%) | 84 (46%) | 27 (59%) | ||
| Second | 118 (52%) | 99 (54%) | 19 (41%) | ||
| Hospitalized | 234 | 146 (62%) | 98 (53%) | 48 (100%) | < .001 |
| ICU | 234 | 64 (27%) | 23 (12%) | 41 (85%) | < .001 |
| Mechanical Ventilation | 234 | 44 (19%) | 5 (2.7%) | 39 (81%) | < .001 |
| Mechanical ventilation Length | 45 | 12 (5, 20) | 8 (5, 12) | 13 (4, 20) | .3 |
| Need for pronation | 45 | 19 (40%) | 3 (43%) | 16 (40%) | > .9 |
| Need for renal replacement therapy | 234 | 36 (15%) | 9 (4.8%) | 27 (56%) | < .001 |
| Follow up time (d) | 221 | 106 (40, 273) | 162 (73, 297) | 22 (10, 35) | < .001 |
| CXR alterations | 33 | 23 (70%) | 11 (58%) | 12 (86%) | .13 |
| Chest CT alterations | 143 | 128 (90%) | 88 (85%) | 40 (100%) | .012 |
Descriptive variables displayed as “count (percentage)” and continuous variables displayed as “median (IQR).”
n (%); Median (IQR).
CT = computed tomography; CXR = Chest X-Ray; ICU = INtensive Care Unit; LTX = Liver transplantation.
Pearson's χ2 test; Wilcoxon rank sum test; Fisher's exact test.
Disease onset was symptomatic in 95% of cases, with fever (52%) and cough (49%) being the most common at admission. Imaging findings were present in 90% of the chest computed tomography scans, with an association with increased mortality (P = .012).
Regarding COVID-specific treatments, 48 patients (21%) were treated with anticoagulants (unfractionated heparin, low-molecular-weight heparin), and 82 (35%) received corticosteroid therapy. Only 1 patient received remdesivir, 1 received basiliximab, and 1 received convalescent serum. Corticosteroid and anticoagulation (unfractionated or low molecular weight heparin) therapy were both associated with increased mortality in univariate tests (P < .001 for both) but did not show significance in multivariable analysis.
Outcomes and Complications
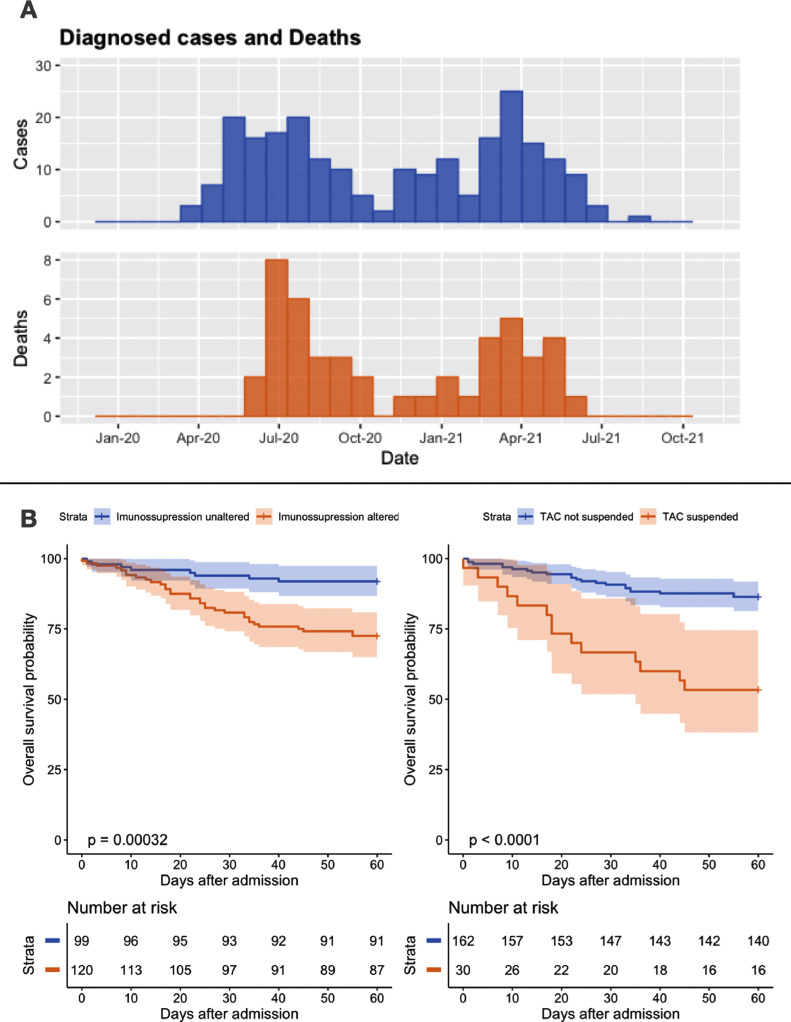
There were 48 deaths in our sample. The lethality and case incidence followed a bimodal trend (Fig 1 A) similar to that observed nationwide during the same period. There were no significant differences in disease incidence or death between COVID waves.
Fig 1.
(A) Histograms detailing temporal variations in the number of diagnosed cases of COVID-related deaths in our study. Both case incidence and deaths display a bimodal pattern in liver transplant recipients, akin to those described in the general population. (B) Kaplan Meier with log-rank test plots describing the overall 60-day survival probability of patients with and without changes to their immunosuppressive regimens overall (left) and, specifically, suspension of tacrolimus (right). The graph displays a higher likelihood of death in patients with changes to their immunosuppressive regimen, which is exacerbated when tacrolimus suspension is specified. TAC, tacrolimus.
Most patients required hospitalization (62%), with 63 (27%) requiring intensive care. Forty-four patients (19%) required mechanical ventilation with a median ventilator length of 12 days. Nineteen (8.1%) required pronation, and 36 (15%) required renal replacement therapy. Overall, COVID-related lethality was 20.5%, with an intensive care unit (ICU) mortality rate of 75%.
Patient age (P = .033) and obesity (P = .004) were associated with increased mortality. Dyspnea at presentation, mechanical ventilation, and renal replacement therapy were also strongly correlated with increased mortality (P < .001 for all).
Immunosuppression management and correlation with mortality
There were many combinations of immunosuppressants, the most common being tacrolimus + mycophenolate (118 cases; 50%), followed by tacrolimus + everolimus (33 cases; 14%), and stand-alone tacrolimus (28 cases; 12%).
After admission, 121 patients (55%) underwent modifications to their immunosuppressive regimen (partial or total suspension of any medication). Most modifications occurred in hospitalized patients (102 cases; 84%). There was a strong correlation between modifications of immunosuppressive therapy and increased mortality (P < .001).
In a detailed analysis, alterations to each drug in such regimens were described and categorized in either dose reduction or complete suspension. There was a significant association between the suspension of tacrolimus (15% of regimens with the drug) and COVID-related death (P < .001). The same was true for prednisone suspension (P = .025). The use of mycophenolate at admission also was associated with mortality (P = .009). Dose reduction of any of the drugs was not associated with death. Detailed information on managing individual immunosuppressive drugs is displayed in Table 2 . Figure 1B displays the accumulated survival probability in patients with immunosuppressive modifications and tacrolimus suspension.
Table 2.
Alterations to Immunosuppressive Agents, Segmented by Total Drug Suspension or Dose Reduction
| Characteristic | N | Overall N = 234* |
Survived N = 186* |
Dead N = 48* |
P Value† |
|---|---|---|---|---|---|
| TAC Reduction | 194 | 31 (16%) | 22 (14%) | 9 (22%) | .2 |
| TAC Suspension | 194 | 30 (15%) | 14 (9.1%) | 16 (40%) | < .001 |
| MFS Reduction | 138 | 6 (4.3%) | 4 (3.9%) | 2 (5.7%) | .6 |
| MFS Suspension | 138 | 70 (51%) | 48 (47%) | 22 (63%) | .10 |
| EVR Reduction | 42 | 0 (0%) | 0 (0%) | 0 (0%) | - |
| EVR Suspension | 42 | 8 (19%) | 6 (18%) | 2 (25%) | .6 |
| PRED Reduction | 18 | 0 (0%) | 0 (0%) | 0 (0%) | - |
| PRED Suspension | 18 | 3 (17%) | 0 (0%) | 3 (50%) | .025 |
| AZA Reduction | 5 | 0 (0%) | 0 (0%) | 0 (0%) | - |
| AZA Suspension | 5 | 3 (60%) | 1 (33%) | 2 (100%) | .4 |
| CsA Reduction | 10 | 0 (0%) | 0 (0%) | 0 (NA%) | - |
| CsA Suspension | 10 | 4 (40%) | 4 (40%) | 0 (NA%) | > .9 |
| Any partial or total supension to Imunossupression | 222 | 121 (55%) | 84 (48%) | 37 (80%) | < .001 |
Values are displayed as “count (percent).”Bolded = significance (p > 0.05).
AZA = Azathioprine; CsA = Cyclosporine A; EVR = Everolimus; MFS = Mycophenolate Sodium; PRED = Prednisone; Tac = Tacrolimus.
n (%).
Pearson's χ2 test test; Fisher's exact test.
We performed multivariable analysis to reduce the confounding effect of covid severity in relation to immunosuppression and mortality. In binary logistic regression, the correlation of tacrolimus suspension and mortality-maintained significance after accounting for dyspnea and hospitalization (P = .013) or ICU admission (P = .046) and during follow-up, as surrogates for COVID severity. These models explained 38.2 % (Nagelkerke R2) of the variance in patient mortality and correctly classified 79.9 % of cases in the model considering dyspnea and hospitalization. When accounting for dyspnea and ICU admission, 55.9% of the variance was explained by the model, with 87.1% of cases correctly classified.
Conversely, overall immunosuppression modifications did not correlate to increased mortality when accounting for these factors (P = .162 and P = .172, respectively).
Discussion
Our study sheds light on the presentation and disease course of COVID-19 in LT recipients. To our knowledge, this is the most extensive study to date on this topic. Our results revealed several crucial factors correlated with increased mortality, notably the important role of immunosuppression management in COVID, denoting a very high mortality rate in LT recipients.
Regarding vaccination status in our population, the study began before any immunizations were widely available in Brazil. Therefore, all patients included in the study were considered non-vaccinated, as national data shows that until the last months of the study, <10% of the country's population were unvaccinated [9].
Although these characteristics set the sample apart from most vaccinated LT recipients, such differences may be attenuated by evidence of the lower effectiveness of vaccines in this population [10,11]. Moreover, the results may still represent the fraction of patients with incomplete or absent vaccination, knowingly those in which more deaths and severe cases currently occur (and those in which more concerns are raised regarding modifications to immunosuppression) [12], [13], [14].
The data collected in this study showed a paradoxical association of anticoagulation or corticosteroid treatment with increased mortality. This may be explained by a biased indication of treatment (selection bias), which was administered mostly in hospitalized (68%) and ICU patients (89%).
A crucial finding of this study was the grossly elevated mortality rate. Our study included 48 deaths, accounting for an overall lethality of 20.5% and an ICU lethality of 75%. These numbers are much higher than the 2.2% national lethality observed during the same period [15]. Other studies have reported similarly high lethality in LT recipients [16].
Although our study did not collect COVID variants within the studied population, the distribution of deaths in 2 distinct waves correlated to nationwide case surges of the Alpha and Gamma COVID variants, respectively [17].
Our sample had a very high hospital admission rate, with 146 patients (62%) being treated in an inpatient setting. This finding is also displayed in previous studies [4,6,18]. However, the subjective nature of medical decisions and the availability bias in large transplant centers may partially explain such high admission rates.
Previous studies have suggested factors that may be associated with patient deaths and be responsible for such high lethality. Older age, ICU admission, and the need for mechanical ventilation [2,3,[5], [6], [7]] have been observed as risk factors, and our study concurs with such findings.
Our study also found novel correlations with mortality in patients with increased BMI (P = .02) and the presence of dyspnea at presentation (P < .001). These factors may explain the increased lethality in this population and show that these patients were frailer at admission than previously thought.
Be that as it may, immunosuppression regimen modifications and tacrolimus suspension were strongly correlated with mortality in our study in single variable analysis (P < .001), which have been reported by several studies [2,7,18]. The detailed analysis performed on this finding indicates the crucial effect of immunosuppression management on patient outcomes in these cases.
The strong association of tacrolimus suspension to death is in line with the findings of Belli et al [2], which suggested a protective effect of tacrolimus in LT recipients. Yet, our findings further such hypotheses by sustaining such association in multivariable analysis, including hospitalization and symptomatology at admission.
Our study showed a seemingly important detrimental effect of tacrolimus suspension, irrespective of hospitalization, ICU admission, or dyspnea at presentation, surrogates for COVID severity.
The findings of our study are strengthened by the fact that it had a robust, multicentric, and geographically well-distributed population. Be that as it may, it has limitations, notably the retrospective design and lack of complete data collection in some cases, such as the lack of the predominant covid variants during the study.
In light of the myriad findings in this study, we can conclude that managing LT recipients with COVID-19 is a complex, multifaceted effort that often involves patients with a previously high disease burden. Attention to risk factors and individualization of patient care, especially in immunosuppression management, is crucial for delivering better and more precise interventions to these individuals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
The authors would like to thank all participants of the COVID-19 Brazilian Transplantation Study Group:
Tercio Genzini, Hospital das Clinicas–HCA/FUNDHACRE; Regina Gomes Santos, Hospital Leforte; Lucio Figueira Pacheco Moreira, IDOR–Instituto D´Or de Ensino e Pesquisa; Laura Cristina Machado Pinto, IDOR–Instituto D´Or de Ensino e Pesquisa; Jose Huygens Parente Garcia, Hospital Universitario Walter Cantidio/UFC; Ilka FSF Boin, Hospital de Clinicas–Unicamp; Raquel SB Stucchi, Hospital de Clinicas–Unicamp; Elaine Cristina Ataide, Hospital de Clinicas–Unicamp; Eduardo Riccetto, Hospital de Clinicas–Unicamp; Simone Reges Perales, Hospital de Clinicas–Unicamp; Leticia Zanaga, Hospital de Clinicas–Unicamp; Renato Fereira da Silva, FAMERP/SP Faculdade de Medicina São Jose do Rio Preto; Rita CM Fereira da Silva, FAMERP/SP Faculdade de Medicina São Jose do Rio Preto; Luciana Haddad, Hospital das Clínicas–FMUSP; Luiz AC D´Albuquerque, Hospital das Clínicas–FMUSP; Marcio Dias de Almeida, Hospital Israelita Alberto Einstein/SP; Andre Watanabe, ICDF–Instituto de Cardiologia do Distrito Federal; Gustavo S Peixoto, Hospital Meridional/ES; Claudio Moura Lacerda de Melo, IMIP/PE Instituto de Medicina Integral Professor Fernando Figueira; Renata Ferreira Bezerra, IMIP/PE Instituto de Medicina Integral Professor Fernando Figueira; Nertan Luiz Tefilli, Hospital São Vicente Curitiba; Marcia Halpern, Hospital Universitário Clementino Fraga Filho–UFRJ; Maira Silva Godoy, Hospital Santa Isabel de Blumenau; Marcelo Nogara, Hospital Santa Isabel de Blumenau; Jorge Marcelo Padilla Mancero, Hospital Beneficência Portuguesa; Huda Maria Noujaim, Hospital Beneficência Portuguesa; Erika Bevilaqua Rangel, Hospital São Paulo/UNIFESP; Agnaldo Soares Lima, SCMBH–Santa Casa Misericórdia Belo Horizonte; Ivelise Regina Canito Brasil, HGF/SUS–Hospital Geral de Fortaleza; Renato Hidalgo, Hospital Miguel Soeiro/Sorocaba; Christian Evangelista Garcia, Hospital Municipal São Jose; Ajith Kumar Sankarankutty, Hospital das Clinicas/FMRPUSP; Adriano Mizziara Gonzalez, Hospital São Paulo/UNIFESP; Andre Ibhraim David, Hospital Samaritano SP; Mauricio Barros, Pro-Figado; Jose Ben-Hur de Escobar Ferraz Neto, Hospital Alemão Oswaldo Cruz/SP; Claudemiro Quireze Junior, Hospital Estadual Geral de Goiania Dr Alberto Rossi; Eduardo Fonseca, Hospital Sirio Libanes/SP.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.transproceed.2023.05.007.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Becchetti C, Gschwend SG, Dufour J-F, Banz V. COVID-19 in liver transplant recipients: a systematic review. J Clin Med. 2021;10:4015. doi: 10.3390/jcm10174015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151–1163. doi: 10.1053/j.gastro.2020.11.045. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumortier J, Duvoux C, Roux O, Altieri M, Barraud H, Besch C, et al. Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45 doi: 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2021;73:e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miarons M, Larrosa-García M, García-García S, Los-Arcos I, Moreso F, Berastegui C, et al. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation. 2021;105:138–150. doi: 10.1097/TP.0000000000003460. [DOI] [PubMed] [Google Scholar]
- 8.Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987–2999. doi: 10.3748/wjg.v26.i22.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus Pandemic (COVID-19). <https://ourworldindata.org/coronavirus>; 2023 [accessed 08.08.2022].
- 10.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toniutto P, Falleti E, Cmet S, Cussigh A, Veneto L, Bitetto D, et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. 2022;77:152–162. doi: 10.1016/j.jhep.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardgrave H, Wells A, Nigh J, Klutts G, Krinock D, Osborn T, et al. COVID-19 mortality in vaccinated vs. unvaccinated liver & kidney transplant recipients: a single-center United States propensity score matching study on historical data. Vaccines (Basel) 2022;10:1921. doi: 10.3390/vaccines10111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma E, Ai J, Zhang Y, Zheng J, Gao X, Xu J, et al. Omicron infections profile and vaccination status among 1881 liver transplant recipients: a multi-centre retrospective cohort. Emerg Microbes Infect. 2022;11:2636–2644. doi: 10.1080/22221751.2022.2136535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamm SR, Rezahosseini O, Møller DL, Loft JA, Poulsen JR, Knudsen JD, et al. Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: real-life data from Denmark. Am J Transplant. 2022;22:2637–2650. doi: 10.1111/ajt.17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saúde CN de S de. PAINEL NACIONAL: COVID-19. PAINEL NACIONAL: COVID-19. <https://www.conass.org.br/painelconasscovid19/>; 2023 [accessed 20.12.2021].
- 16.Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, et al. Impact of COVID-19 on liver transplant recipients-a systematic review and meta-analysis. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moura EC, Cortez-Escalante J, Cavalcante FV, Barreto IC de HC, Sanchez MN, Santos LMP. Covid-19: temporal evolution and immunization in the three epidemiological waves, Brazil, 2020–2022. Rev Saúde Pública. 2022;56:105. doi: 10.11606/s1518-8787.2022056004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll E, Fernández-Ruiz M, Padilla M, Moreso F, Hernández-Vicente A, Yañez Í, Molina M, et al. COVID-19 in Solid Organ Transplant Recipients in Spain Throughout 2020: catching the Wave? Transplantation. 2021;105:2146–2155. doi: 10.1097/TP.0000000000003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.