Abstract
People with cystic fibrosis (pwCF) were considered to be clinically vulnerable to COVID-19 and were therefore given priority in the vaccination campaign. Vaccines induced a humoral response in these patients that was comparable to the response observed among the general population. However, the role of the cell-mediated immune response in providing long-term protection against SARS-CoV-2 in pwCF has not yet been defined. In this study, humoral (antibody titre) and cell-mediated immune responses (interferon-γ release) to the BNT162b2 vaccine were measured at different time points, from around 6–8 months after the 2nd dose and up to 8 months after the 3rd dose, in 118 CF patients and 26 non-CF subjects. Subjects were sampled between November 2021 and September 2022 and followed-up for breakthrough infection through October 2022. pwCF mounted a cell-mediated response that was similar to that observed in non-CF subjects. Low antibody titres (<1st quartile) were associated with a higher risk of breakthrough infection (HR: 2.39, 95 % CI: 1.17–4.88), while there was no significant association with low INF-γ levels (<0.3 IU/mL) (HR: 1.38, 95 % CI: 0.64–2.99). Further studies are needed in subgroup of pwCF receiving immunosuppressive therapy, such as organ transplant recipients. This data is important for tailoring vaccination strategies for this clinically vulnerable population.
Keywords: Cystic fibrosis, COVID-19, SARS-CoV-2, Vaccine, Immunogenicity, Immune response
1. Introduction
Cystic fibrosis (CF) is a genetic and multisystemic disease that affects approximately 100,000 people worldwide. It is caused by mutations of the CF Transmembrane Regulator (CFTR) gene, an ion channel whose defect causes abnormal secretions in many organs. The main clinical manifestations of CF are malabsorption, which is treated with enzyme replacement therapy, and recurrent lung infections that lead to progressive lung disease. Despite the remarkable advances in the management of CF, lung disease still represents the main cause of death for these patients [1].
Viral infections in people with CF (pwCF) superimpose with bacterial infections and trigger pulmonary exacerbations [2]. This is an important factor in the progression of lung damage and deterioration of lung function. Since the beginning of the COVID-19 pandemic, pwCF have been considered a clinical vulnerable population who deserved special attention. However, the clinical impact of COVID-19 on this population was less severe than expected, and only a minority of them - those with impaired lung function and transplant recipients - are at high risk of severe COVID-19 [3], [4], [5]. Recent data show that a dysfunctional CFTR channel reduces viral entry and replication, thus protecting pwCF from severe SARS-CoV-2 infection [6].
A coordinated immune response, involving both innate immunity and T and B-cell-based immunity, is required to effectively control SARS-CoV-2 infection [7], [8], [9]. T-cell mediated immunity is particularly important against variants of the virus [10] and in clinically vulnerable populations [11], [12].
The immune response to SARS-CoV-2 infection and to vaccination has been studied to lower extent in pwCF. We have recently demonstrated that pwCF have antibody titres after two doses of the BNT162b2 vaccine against SARS-CoV-2 comparable to that observed among the general population [13] and this was confirmed in a different cohort of patients [14]. However, the role of the cell-mediated immune response, which may be more relevant in long-term protection against SARS-CoV-2, has not yet investigated in pwCF. Therefore, this study aims to measure humoral and cell-mediated immune responses elicited by a mRNA-based vaccine against SARS-CoV-2 in pwCF and to evaluate their relation with the subsequent risk of infection.
2. Methods
2.1. Study population
This study is based on data collected in a project aiming at evaluating the safety and effectiveness of mRNA-based vaccines against SARS-CoV-2 in pwCF. In this work, we reported the results on the humoral and cell-mediated responses elicited by the BNT162b2 vaccine. Patients who agreed to be sampled between November 2021 and September 2022 were enrolled. Samples were collected for each subject in different times over a period around 6–8 months after the 2nd dose and up to 8 months after the 3rd dose. The number of collected samples by time periods are reported in the Appendix A. Supplementary data.
A control group of individuals without CF was also enrolled among the health care workers of the CF centre and their families to evaluate whether cell-mediated response to vaccines were comparable to that observed in the population without CF.
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the IRCCS, Istituto Nazionale per le Malattie Infettive, Lazzaro Spallanzani, Rome, Italy (protocol number: 354 2020/2021).
2.2. Laboratory tests
Humoral response was quantified by measuring the total antibody titre against the S1 receptor binding domain (S1-RBD) of SARS-CoV-2, while cell-mediated response was quantified by measuring the plasma concentration of spike-induced interferon-gamma (INF-γ) release. All analyses were performed centrally at the Clinical Laboratory of the IRCCS Ca' Granda Ospedale Maggiore Policlinico Foundation, Milan, Italy.
2.3. Anti-SARS-CoV2 IgG measurement
Quantification of antibodies to the SARS-CoV-2 S1-RBD was determined by Elecsys® anti-SARS-CoV-2 enzyme immunoassay (Roche Diagnostics, Monza, Italy) (positivity cut off = 0.8 U/mL, lower limit of quantification, LLOQ = 0.4 U/mL, upper limit of quantification, ULOQ = 12,500 U/mL, sensitivity 98.8 % and specificity 100 %). Values below the LLOQ were set to LLOQ/2 and values above the ULOQ were set to ULOQ before analysis. This serological assay is an in vitro quantitative determination of high-affinity antibodies against SARS-CoV-2, using a recombinant protein with the S1-RBD in a one-step double antigen sandwich assay format.
2.4. Quantification of cell-mediated immune response according to INF-γ release
Cell-mediated immune response was measured with the STANDARDTM F CoviFERON FIA (IFN-γ) system, a new rapid IFN-γ Release Assay (IGRA) by which plasma from stimulated samples in Covi-FERON tubes can be used for detection of IFN-γ using Standard F2400 analyzer (SD Biosensor, Inc.Korea), with positivity cut off = 0.30 IU/mL.
Blood samples were collected from patients and ejected respectively 1 mL into each Covi-FERON Tube (Nil tube, Original SP Antigen tube, Variant SP Antigen tube, NP antigen tube and Mitogen tube). As soon as the tube was filled with blood, we mixed it 10 times gently to allow the entire surface of the tube to be immersed in blood in order to mix blood cells with the antigen on the tube wall. All the tubes were incubated at 37 °C for 16 to 24 h. After incubation, we collected plasma by centrifuging tubes for 15 min at relative centrifugal force of 2200 g. All the samples were prepared as follows: we took 100 µL of Sample Dilution Buffer into a conjugate tablet vial and 100 µL of plasma sample, we mixed the sample and the dissolved conjugate tablet by pipetting. We applied 100 µL of the specimen to the test device and the analyzer gave us automatically the test result within 15 min.
2.5. Statistical analysis
Humoral and cell-mediated responses were evaluated across the following periods: 180–250 days after the 2nd dose, and 60–119, 120–179 and 180–250 days after the 3rd dose of the vaccine. The statistical significance of the period effect on anti-SARS-CoV-2 antibody titre and INF-γ release was verified through a likelihood ratio test comparing two linear quantile mixed models with random intercept which included or not “period” as predictor [15]. These models allow the analysis of skewed data with repeated measures on the same subject, and they were also used to evaluate the relationship between antibody titre and INF-γ release. To this purpose we used the models to estimate the expected change (β) in the median value (quantile = 0.5) of INF-γ for an increment of 1000 U/mL of antibody titre and its corresponding 95 % confidence interval CI. CI was estimated using bootstrap replications (n = 1000).
Patients were followed-up for SARS-CoV-2 infection through October 31, 2022. SARS-CoV-2 infections were confirmed by a RT-PCR or antigen test on nasopharyngeal swab. Cumulative incidence of first positive test was computed according to the degree of humoral response to the vaccine (<1st quintile of antibody titre vs ≥ 1st quintile of antibody titre) and according to cell-mediated immune response (INF-γ < 0.3 IU/ml vs INF-γ ≥ 0.3 IU/ml) using the extended Kaplan-Meier estimator [16]. In the extended Kaplan-Meier curves, antibody titre and INF-γ release were considered as time-varying covariates; thus, patients contributed to different risk sets during the follow-up according to the values of antibody titre and INF-γ at the beginning of the interval. Since all patients entered the risk set at their first determination of antibody titre or INF-γ; the two analyses were based on a different number of patients at risk and on a different number of SARS-CoV-2 infections at each time point. The hazard ratio, estimated through a Cox regression model, and the corresponding 95 % CI, were used to evaluate whether low responses were associated with increased risk of infection. Days elapsed from blood withdrawal was used as time scale.
3. Results
The study included 118 paediatric and adult patients (age range: 13–38 years) and 26 non-CF controls whose characteristics are described in Table 1 . Controls were more likely males and older than pwCF. Two-thirds of the CF patients carried the F508del CFTRvariant and 59 % of them presented pancreatic insufficiency. Most patients had a slight/moderate impairment of lung function and none had a percent predicted forced expiratory volume in one second (ppFEV1) < 40. Less than 30 % of the patients were receiving CFTR modulators, half were taking inhaled corticosteroids and only two patients were being treated with systemic steroids.
Table 1.
Participants’ characteristics.
| Total number of patients | pwCF (N: 118) n ( %) |
Non-CF subjects (N: 26) n ( %) |
|---|---|---|
| Male sex | 59 (50.0) | 19 (73.1) |
| Age (years), median (range) | 21 (13–38) | 43 (30–76) |
| F508del homozygous | 31 (26.3) | |
| F508del heterozygous | 48 (40.7) | |
| Other variants | 39 (33.1) | |
| Pancreatic insufficiency | 70 (59.3) | |
| Respiratory infection by P. aeruginosa | 54 (45.8) | |
| ppFEV, median (IQR) | 96 (47–129) | |
| ppFEV < 40 | 0 | |
| Oxygen therapy | 0 | |
| Lung transplantation | 0 | |
| Diabetes | 10 (8.5) | |
| Cirrhosis | 4 (3.4) | |
| Inhaled steroids | 59 (50.0) | |
| Systemic steroids | 2 (1.7) | |
| CFTR modulators | 33 (28.0) |
Data are numbers ( %) unless otherwise specified.
CFTR: cystic fibrosis transmembrane conductance regulator. IQR: interquartile range. ppFEV1: percent predicted forced expiratory volume in one second. pwCF: people with cystic fibrosis.
Over the study period, 287 samples were collected to evaluate the humoral response (122 collected before receiving the 3rd dose and 165 after) to the COVID-19 vaccine and 237 samples to evaluate the cell-mediated immune response (64 collected before receiving the 3rd dose and 173 after).
Median plasma antibody titre was 1086 U/mL after 180–250 days from the 2nd dose and exceeded 10,000 U/mL after the 3rd dose. (p-value for the period effect <0.001) (Fig. 1 a). All samples collected during the different periods were above the threshold for a positive humoral response.
Fig. 1.
Humoral (anti-SARS-CoV-2 S1-RBD antibody titre) and T cell-mediated immune response (spike-induced T-cell interferon-γ release, INF-γ) following the 2nd and the 3rd dose of the BNT162b2 COVID-19 vaccine in cystic fibrosis. Panel a) shows the distribution of the antibody titre at different periods after the 2nd and 3rd dose. Panel b) shows the distribution of interferon-γ concentrations at different periods after the 2nd and 3rd dose. Panel c) shows the proportion of patients who mounted a humoral response during the different periods considered. Circles are individual values, empty circles indicate patients who had a SARS-CoV-2 infection before sample collection.
Median INF-γ released by blood cells stimulated with SARS-CoV-2 antigens significantly increased from 0.27 IU/mL after 180–250 days from the 2nd dose to 1.69, 1.29 and 1.56 IU/mL after 60–119, 120–179 and 180–250 days from the 3rd dose, respectively (p-value for the period effect < 0.001) (Fig. 1 b). Similarly, the percentages of positive responses increased from 50 % after 180–250 days from the 2nd dose to 82.1, 84.2 and 91.7 % after 60–119, 120–179 and 180–250 days from the 3rd dose, respectively (Fig. 1 c).
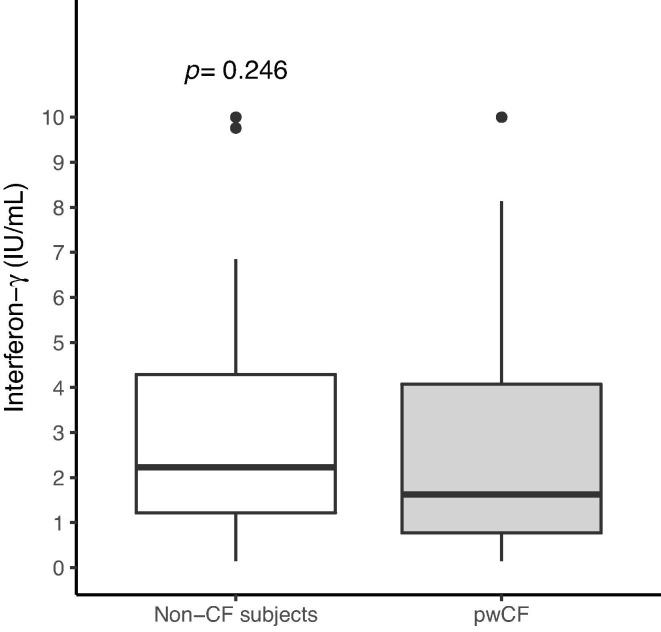
INF-γ release evaluated 6–8 months after the 3rd dose was not significantly different between pwCF and the control group (median INF-γ: 1.62, IQR: 0.77; 4.07 vs 2.23, IQR: 1.21; 4.29, p-value = 0.246) (Fig. 2 ). The absence of a significant difference remained when we adjusted for sex and age (estimated difference in median INF-γ release = -0.62, 95 % CI: −1.91; 0.60) and these two confounders were not associated neither with antibody titre nor with INF-γ release (Appendix A. Supplementary data).
Fig. 2.
Spike-induced T-cell interferon-γ release measured 6–8 months after the 3rd dose of the BNT162b2 COVID-19 vaccine among people with cystic fibrosis (pwCF) and among subjects without cystic fibrosis. Th p-value was obtained from a likelihood ratio test between two quantile regression models (at quantile = 0.5) including or not the group indicator (non-CF subjects vs pwCF) and adjusted for sex and age. CF: cystic fibrosis.
Levels of antibody titre were associated with higher cell-mediated immune response (Fig. 3 ). The estimated increase in the median of INF-γ concentration for an increment of 1000 U/mL in antibody titre was 0.12 IU/mL (95 % CI: 0.06–0.17).
Fig. 3.
Relationship between anti-S1-RBD-SARS-CoV-2 antibody titre and spike-induced T-cell interferon-γ release measured after receiving the BNT162b2 COVID-19 vaccine in patients with cystic fibrosis. The line indicates the predicted median values of interferon-γ release (y-axis) for a given value of antibody titre (x-axis). The shadowed area indicates the 95 % confidence band of the predicted values. β is the slope of the line estimated from a linear quantile mixed-effects regression model at quantile = 0.5.
Fig. 4 shows the cumulative incidences of first SARS-CoV-2 infection according to the degree of humoral and cell-mediated response to the vaccine. In the analysis by humoral responses, we registered 36 cases (14 cases occurred after low responses and 22 after high responses), while in the analysis by cell-mediated response we registered 29 cases (10 occurred after low responses and 19 after high responses). Low antibody titres were associated with higher infection rate (HR: 2.39, 95 % CI: 1.17–4.88), whereas there was no significant association with INF-γ release by blood cells (HR for low vs high responses: 1.38, 95 % CI: 0.64–2.99). None of the patients required hospitalization from COVID-19 or died during the study period.
Fig. 4.
Cumulative probability of SARS-CoV-2 infection according to degree of humoral response [anti-S1-RBD-SARS-CoV-2 antibody titre < 801 U/mL (1st quintile) vs ≥ 801 U/mL] to the BNT162b2 COVID-19 vaccine (Panel a) and according to cell-mediated immune response (spike-induced T-cell interferon-γ release, INF-γ < 0.3 vs INF-γ ≥ 0.3 IU/mL) during the follow-up time (Panel b). Quintiles of antibody titre were calculated from the whole set of values. Curves were drawn using the extended Kaplan–Meier methods, where patients contribute to different risk sets during follow-up times.
4. Discussion
In this relatively large cohort of pwCF we characterized the humoral and cell-mediated immune responses to BNT162b2 vaccine against SARS-CoV-2. We found that both virus-specific antibody and INF-γ productions remarkably increased after receiving the booster dose (3rd dose), reaching levels comparable to those observed in non-CF subjects. We also found that although the two responses were related, only low antibody levels were significantly associated with a higher risk of breakthrough infection. On the other hand, the extent of cellular response did not seem to affect the risk of infection.
Similar to what has been observed in non-CF populations, we reported a relevant interindividual variability in both humoral and cell-mediated immune response to COVID-19 vaccines. The reasons for this variability are likely to be found in the genetic background of the individuals who underwent vaccination. In this regard, several authors showed that many genetic risk factors may account for the variable outcome of SARS-CoV-2 infection and to the extent of cellular immune response characterized by excessive production of pro-inflammatory cytokines, which is a major determinant of COVID-19 severity [17], [18].
Several studies suggest that the different components of adaptive immunity response against viral infections can contribute by multiple mechanisms to protective immunity against COVID-19. Both humoral (antibody-mediated) and cellular (T-cell-mediated) immune responses concur to the development of resistance to pathogens [19]. The existence of a relationship between antibody titre and protection against infection in the first months post-vaccination and the physiological decrease in the circulating antibody titre within 4–6 months of vaccination are now well established [20], [21], [22]. Moreover, several lines of evidence also suggest important contributions of T and B cell memory responses in protective immunity against infection [7].
Since immunoglobulins G plays a key role in the development of humoral immunity [23] and most studies measured specific immunoglobulins directed against the spike protein epitopes, primarily against the S1-RBD of the SARS-CoV-2 spike protein [24]. However, the published studies show important differences in the method used and in the detection times which make it difficult to compare the results obtained. In addition, most studies have focused on the type of humoral response to SARS-CoV-2 as it is easier to determine and less expensive than the cellular one.
The cellular response to SARS-CoV-2 infection has been less investigated than the humoral response but, there is evidence that it can be equally effective and long-lasting. Even in patients who fail to mount a significant antibody response [25], cell-mediated immunity appears to play a key role in promoting rapid virus clearance [26]. Furthermore, according to some studies, up to 30 % of patients with asymptomatic infection or with mild symptoms may not present a protective humoral response: in these cases, cell-mediated immunity might be more important to fight the infection [27]. In addition, cell-mediated immunity can play a key role against emerging variants that escape humoral immunity [10], [28], [29], [30]. However, in our study INF-γ release after S1-RBD stimulation was not significantly associated with the risk of breakthrough SARS-CoV-2 infection.
Traditionally, cellular response is assessed by determining the presence of specific lymphocytes (such as Th1 CD4+ T and CD8+ T cells) using ELISpot or flow cytometry [31], [32]. However, these methods cannot be used routinely in SARS-CoV-2 diagnostics both for technical problems and for high costs. Alternatively, T-cell response can be assessed by measuring the concentration of IFN-γ in whole blood platform test. IFN-γ is produced by lymphocytes T in contact with specific pathogenic antigens and is essential for the development of innate and adaptive immunity. IGRA, which has long been used in the diagnosis of tuberculosis, has been suitably modified and applied successfully to the detection of SARS-CoV-2 (SARS-CoV-2 IGRA version) [33]. This tool is fast, relative expensive and has a high sensitivity and specificity [34].
To our knowledge, this is the first study measuring humoral and cell-mediated responses to COVID-19 vaccine in pwCF, a population particularly vulnerable to viral respiratory infections. We also evaluated for the first time in this population how these responses are related to subsequent SARS-CoV-2 infection.
Our study has some limitations. First, CF cases and controls were not comparable in terms of sex and age: controls were more likely male and older than pwCF. However, we can reasonably exclude that this has significantly biased the comparison, since 80 % of pwCF mounted a cell-mediated response and in our sample sex and age were not important determinants of cell-mediated immune response. Second, the Covi-FERON stimulatory peptide set was created before the appearance of the Delta and Omicron variants, therefore it did not contain specific antigen against those variants. However, the peptide used to stimulate pertain to a highly preserved epitope on the virus's spike protein receptor binding domain which is common to all SARS-CoV-2 variants. Third, we did not consider an important measure of the effectiveness of immune response, i.e. virus-neutralizing antibodies. Neutralizing antibodies seem to play a major role in prevention of infection, while cellular immunity might be a key factor in disease severity and resolution of infection [35]. Finally, the clinical relevance of our study is partly limited by the fact that we did not include transplanted subjects. These patients receive chronic immunosuppressive treatment, which negatively affect both humoral and cell-mediated immune response to vaccines [28], [36], [37], [38].
5. Conclusions
The BNT162b2 vaccine against SARS-CoV-2 elicits humoral and cell-mediated immune response in pwCF that are similar to those observed in the general population. However, future studies are needed to determine the persistence of the observed immune responses over time. It will be important to extend the follow-up to assess how the immune response will change after repetitive COVID-19 vaccine boosters and to evaluate subgroups of pwCF with suboptimal immunity, such as organ transplant recipients [14]. This point is important in defining the risk of reinfection and in tailoring the vaccination booster schedule for fragile populations such as pwCF [39], [40].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.05.041.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Shteinberg M., Haq I.J., Polineni D., Davies J.C. Cystic fibrosis. Lancet. 2021;397:2195–2211. doi: 10.1016/S0140-6736(20)32542-3. [DOI] [PubMed] [Google Scholar]
- 2.Wark P.A.B., Tooze M., Cheese L., Whitehead B., Gibson P.G., Wark K.F., et al. Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur Respir J. 2012;40:510–512. doi: 10.1183/09031936.00202311. [DOI] [PubMed] [Google Scholar]
- 3.Colombo C., Cipolli M., Daccò V., Medino P., Alghisi F., Ambroni M., et al. Clinical course and risk factors for severe COVID-19 among Italian patients with cystic fibrosis: a study within the Italian Cystic Fibrosis Society. Infection. 2022;50(3):671–679. doi: 10.1007/s15010-021-01737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corvol H., de Miranda S., Dehillotte C., Lemonnier L., Chiron R., Danner-Boucher I., et al. Cumulative incidence and risk factors for severe coronavirus disease 2019 in french people with cystic fibrosis. Clin Infect Dis. 2022;75(12):2135–2144. doi: 10.1093/cid/ciac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr S.B., McClenaghan E., Elbert A., Faro A., Cosgriff R., Abdrakhmanov O., et al. Factors associated with clinical progression to severe COVID-19 in people with cystic fibrosis: A global observational study. J Cyst Fibros. 2022;21(4):e221–e231. doi: 10.1016/j.jcf.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezzerri V., Gentili V., Api M., Finotti A., Papi C., Tamanini A., et al. SARS-CoV-2 viral entry and replication is impaired in Cystic Fibrosis airways due to ACE2 downregulation. Nat Commun. 2023;14(1) doi: 10.1038/s41467-023-35862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraccioli G., Gremese E., Golett D., Petrone L., Cantini F., Ugel S., et al. Immune-guided therapy of COVID-19. Cancer Immunol Res. 2022;10(4):384–402. doi: 10.1158/2326-6066.CIR-21-0675. [DOI] [PubMed] [Google Scholar]
- 9.Goletti D., Petrone L., Manissero D., Bertoletti A., Rao S., Ndunda N., et al. The potential clinical utility of measuring severe acute respiratory syndrome coronavirus 2-specific T-cell responses. Clin Microbiol Infect. 2021;27(12):1784–1789. doi: 10.1016/j.cmi.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrone L., Tortorella C., Aiello A., Farroni C., Ruggieri S., Castilletti C., et al. Humoral and cellular response to spike of delta SARS-CoV-2 variant in vaccinated patients with multiple sclerosis. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.881988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrone L., Picchianti-Diamanti A., Sebastiani G.D., Aiello A., Laganà B., Cuzzi G., et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis. 2022;121:24–30. doi: 10.1016/j.ijid.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alicandro G., Daccò V., Cariani L., Rosazza C., Sciarrabba C.S., Ferraro F., et al. Immunogenicity of BNT162b2 mRNA-based vaccine against SARS-CoV-2 in people with cystic fibrosis according to disease characteristics and maintenance therapies. Biomedicines. 2022;10(8) doi: 10.3390/biomedicines10081998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucca F., Bezzerri V., Danese E., Olioso D., Peserico D., Boni C., et al. Immunogenicity and safety of the BNT162b2 COVID-19 vaccine in patients with cystic fibrosis with or without lung transplantation. Int J Mol Sci. 2023;24(2):908. doi: 10.3390/ijms24020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraci M. Linear quantile mixed models: The lqmm package for laplace quantile regression. J Stat Softw. 2014;57:1–29. doi: 10.18637/jss.v057.i13. [DOI] [Google Scholar]
- 16.Snapinn S.M., Jiang Q., Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat. 2005;59:301–307. doi: 10.1198/000313005X70371. [DOI] [Google Scholar]
- 17.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., et al. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi T., Pigazzini S., Degenhardt F., Cordioli M., Butler-Laporte G., Maya-Miles D., et al. Age-dependent impact of the major common genetic risk factor for COVID-19 on severity and mortality. J Clin Invest. 2021;131(23) doi: 10.1172/JCI152386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiello A., Coppola A., Vanini V., Petrone L., Cuzzi G., Salmi A., et al. Accuracy of QuantiFERON SARS-CoV-2 research use only assay and characterization of the CD4+ and CD8+ T cell-SARS-CoV-2 response: comparison with a homemade interferon-γ release assay. Int J Infect Dis. 2022;122:841–849. doi: 10.1016/j.ijid.2022.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldridge R.W., Yavlinsky A., Nguyen V., Eyre M.T., Shrotri M., Navaratnam A.M.D., et al. SARS-CoV-2 antibodies and breakthrough infections in the Virus Watch cohort. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-32265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science (80-) 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury J., Najjar-Debbiny R., Hanna A., Jabbour A., Abu Ahmad Y., Saffuri A., et al. COVID-19 vaccine – Long term immune decline and breakthrough infections. Vaccine. 2021;39(48):6984–6989. doi: 10.1016/j.vaccine.2021.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J., Deng Y., Zhao Z., Mao B., Lu M., Lin Y., et al. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol. 2022;19(2):150–157. doi: 10.1038/s41423-021-00774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tretyn A., Szczepanek J., Skorupa M., Jarkiewicz-Tretyn J., Sandomierz D., Dejewska J., et al. Differences in the concentration of anti-sars-cov-2 igg antibodies post-covid-19 recovery or post-vaccination. Cells. 2021;10(8) doi: 10.3390/cells10081952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagannathan P., Wang T.T. Immunity after SARS-CoV-2 infections. Nat Immunol. 2021;22:539–540. doi: 10.1038/s41590-021-00923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6) doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seraceni S., Zocca E., Cervone T.E., Tomassetti F., Polidori I., Valisi M., et al. T-cell assay after COVID-19 vaccination could be a useful tool? a pilot study on interferon-gamma release assay in healthcare workers. Diseases. 2022;10(3):49. doi: 10.3390/diseases10030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farroni C., Picchianti-Diamanti A., Aiello A., Nicastri E., Laganà B., Agrati C., et al. Kinetics of the B- and T-cell immune responses after 6 months from SARS-CoV-2 mRNA vaccination in patients with rheumatoid arthritis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.846753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccines. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Iketani S., Guo Y., Chan J.-W., Wang M., Liu L., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 31.Woldemeskel B.A., Kwaa A.K., Garliss C.C., Laeyendecker O., Ray S.C., Blankson J.N. Healthy donor T cell responses to common cold coronaviruses and SARS-CoV-2. J Clin Invest. 2020;130:6631–6638. doi: 10.1172/JCI143120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalewska M., Fus W., Konka A., Wystyrk K., Bochenek A., Botor H., et al. An immune response to heterologous ChAdOx1/BNT162b2 vaccination against COVID-19: evaluation of the anti-RBD specific IgG antibodies titers and interferon gamma release assay (IGRA) test results. Vaccines. 2022;10(9) doi: 10.3390/vaccines10091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EUROIMMUN Launches SARS-CoV-2 Test System to Detect T-Cell Response | Technology Networks n.d. https://www.technologynetworks.com/immunology/product-news/euroimmun-launches-sars-cov-2-test-system-to-detect-t-cell-response-344589 (accessed March 9, 2023).
- 34.Brand I., Gilberg L., Bruger J., Garí M., Wieser A., Eser T.M., et al. Broad T cell targeting of structural proteins after SARS-CoV-2 infection: high throughput assessment of T cell reactivity using an automated interferon gamma release assay. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.688436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedzierska K., Thomas P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Reports Med. 2022;3 doi: 10.1016/j.xcrm.2022.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Offizi G., Agrati C., Visco‐Comandini U., Castilletti C., Puro V., Piccolo P., et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42(1):180–186. doi: 10.1111/liv.15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picchianti Diamanti A., Luchetti M.M., Nicastri E., Rosado M.M., Editorial L.B. Chronic autoimmune arthritis, infections and vaccines. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1058152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tortorella C, Aiello A, Gasperini C, Agrati C, Castilletti C, Ruggieri S, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022;98:E541–54. https://doi.org/10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed]
- 39.Agrati C., Castilletti C., Goletti D., Sacchi A., Bordoni V., Mariotti D., et al. Persistent Spike-specific T cell immunity despite antibody reduction after 3 months from SARS-CoV-2 BNT162b2-mRNA vaccine. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-07741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desmecht S., Tashkeev A., El Moussaoui M., Marechal N., Perée H., Tokunaga Y., et al. Kinetics and persistence of the cellular and humoral immune responses to BNT162b2 mRNA vaccine in SARS-CoV-2-naive and -experienced subjects: impact of booster dose and breakthrough infections. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.863554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.