Abstract
Background
People with severe mental illness are twice as likely to develop type 2 diabetes as those without severe mental illness. Treatment guidelines for type 2 diabetes recommend that structured education should be integrated into routine care and should be offered to all. However, for people with severe mental illness, physical health may be a low priority, and motivation to change may be limited. These additional challenges mean that the findings reported in previous systematic reviews of diabetes self management interventions may not be generalised to those with severe mental illness, and that tailored approaches to effective diabetes education may be required for this population.
Objectives
To assess the effects of diabetes self management interventions specifically tailored for people with type 2 diabetes and severe mental illness.
Search methods
We searched the Cochrane Library, MEDLINE, EMBASE, PsycINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the International Clinical Trials Registry Platform (ICTRP) Search Portal, ClinicalTrials.gov and grey literature. The date of the last search of all databases was 07 March 2016.
Selection criteria
Randomised controlled trials of diabetes self management interventions for people with type 2 diabetes and severe mental illness.
Data collection and analysis
Two review authors independently screened abstracts and full‐text articles, extracted data and conducted the risk of bias assessment. We used a taxonomy of behaviour change techniques and the framework for behaviour change theory to describe the theoretical basis of the interventions and active ingredients. We used the GRADE method (Grades of Recommendation, Assessment, Development and Evaluation Working Group) to assess trials for overall quality of evidence.
Main results
We included one randomised controlled trial involving 64 participants with schizophrenia or schizoaffective disorder. The average age of participants was 54 years; participants had been living with type 2 diabetes for on average nine years, and with their psychiatric diagnosis since they were on average 28 years of age. Investigators evaluated the 24‐week Diabetes Awareness and Rehabilitation Training (DART) programme in comparison with usual care plus information (UCI). Follow‐up after trial completion was six months. Risk of bias was mostly unclear but was high for selective reporting. Trial authors did not report on diabetes‐related complications, all‐cause mortality, adverse events, health‐related quality of life nor socioeconomic effects. Twelve months of data on self care behaviours as measured by total energy expenditure showed a mean of 2148 kcal for DART and 1496 kcal for UCI (52 participants; very low‐quality evidence), indicating no substantial improvement. The intervention did not have a substantial effect on glycosylated haemoglobin A1c (HbA1c) at 6 or 12 months of follow‐up (12‐month HbA1c data 7.9% for DART vs 6.9% for UCI; 52 participants; very low‐quality evidence). Researchers noted small improvements in body mass index immediately after the intervention was provided and at six months, along with improved weight post intervention. Diabetes knowledge and self efficacy improved immediately following receipt of the intervention, and knowledge also at six months. The intervention did not improve blood pressure.
Authors' conclusions
Evidence is insufficient to show whether type 2 diabetes self management interventions for people with severe mental illness are effective in improving outcomes. Researchers must conduct additional trials to establish efficacy, and to identify the active ingredients in these interventions and the people most likely to benefit from them.
Plain language summary
Self management interventions for type 2 diabetes in adults with severe mental illness
Review question
What are the effects of diabetes self management interventions specifically tailored for adults with type 2 diabetes and severe mental illness?
Background
Diabetes is one of the most common long‐term conditions, affecting around 415 million people worldwide. People with severe mental illness are twice as likely to develop diabetes as those without mental health problems because of many factors, including antipsychotic medication side effects and inadequate 'lifestyle' such as poor diet and low levels of physical activity. Once diagnosed, type 2 diabetes is managed through a combination of medication and behavioural changes. When diabetes is poorly managed, people can develop severe and life‐threatening complications. Healthcare providers have developed patient education programmes to help people to self manage their diabetes, and to reduce the likelihood of these complications. Although many programmes for type 2 diabetes have been found to be effective, little is known about programmes that have been specifically tailored to meet the needs of people with severe mental illness.
Study characteristics
We identified one study, which recruited 64 adults with type 2 diabetes and schizophrenia or schizoaffective disorder. Researchers compared usual care plus information leaflets with a 24‐week education programme delivered once a week for 90 minutes (Diabetes Awareness and Rehabilitation Training). This programme provided basic diabetes education and information about nutrition and exercise. The average age of participants was 54 years; participants had been living with type 2 diabetes for on average nine years and with their psychiatric diagnosis since they were on average 28 years old. People in the included study were monitored for six months after the programme ended.
This evidence is up to date as of 07 March 2016.
Key results
In summary, few studies have evaluated the effects of diabetes self management programmes for adults with severe mental illness. Study authors of the single included study did not report diabetes‐related complications, all‐cause mortality, adverse events, health‐related quality of life nor socioeconomic effects. They described small improvements in body mass index and body weight, as well as in diabetes knowledge and self efficacy. Current evidence is insufficient to show that these types of programmes can help people with type 2 diabetes and severe mental illness to better manage their diabetes and its consequences.
Quality of the evidence
We rated the overall quality of the evidence as very low, mainly because of the small numbers of included studies and participants, and because reported study results showed inconsistency.
Summary of findings
for the main comparison.
| Self management interventions for type 2 diabetes in adult people with severe mental illness | ||||||
|
Population: adults with type 2 diabetes and severe mental illness Setting: community Intervention: diabetes self management Comparison: usual care + information | ||||||
| Outcomes | Usual care + information | Diabetes self management | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | Comments |
| Diabetes‐related complications | See comment | See comment | See comment | See comment | See comment | Not reported |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Not reported |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Not reported |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Not reported |
|
Self care behaviours: physical activity (measured by total energy expenditure in kcal) Follow‐up: 6 months (6 months after the end of the intervention) |
Mean energy expenditure was 2148 kcal | Mean energy expenditure was 652 kcal higher | ‐ | 52 (1) | ⊕⊝⊝⊝ Very lowa | Trial authors stated that this difference reflected no improvement |
|
HbA1c [%] Follow‐up: 6 months (6 months after the end of the intervention) |
Mean HbA1c was 7.9% | Mean HbA1c was 1% lower | ‐ | 52 (1) | ⊕⊝⊝⊝ Very lowa | Trial authors stated that this difference reflected no improvement |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Not reported |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; kcal: kilocalories | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded by three levels because of selective reporting bias, indirectness and imprecision
Background
Description of the condition
Diabetes is a common and serious global health problem, currently affecting an estimated 9% of adults ‐ 415 million people worldwide ‐ and taking up 12% of international health expenditures (International Diabetes Federation 2015). In high‐income countries, approximately 87% to 91% of all people with diabetes are estimated to have type 2 diabetes (International Diabetes Federation 2015). The condition typically develops in adulthood, usually in people over the age of 40 years, but younger onset is becoming more common. Diabetes is characterised by poorly regulated blood glucose levels, which may arise from defects in insulin secretion (insulin deficiency), in its action (insulin resistance) or both. The aim of treatment is to manage blood glucose levels to alleviate short‐term symptoms while preventing or delaying the development of long‐term complications. Individuals can initially control elevated glucose in the blood, known as hyperglycaemia, through lifestyle management, such as changes to diet and exercise, but given the progressive nature of type 2 diabetes, it is likely that most individuals will ultimately require pharmacological intervention as well. This may initially consist of oral hypoglycaemic drugs and, if the disease remains uncontrolled, insulin therapy.
The primary symptoms of type 2 diabetes are increased thirst and urination; however, not all individuals will experience these symptoms. Therefore, many people remain undiagnosed for a sustained period of time. Undetected hyperglycaemia can have implications for the outcome of diabetes, including greater risk of macrovascular and microvascular complications. Microvascular complications that primarily affect people with type 2 diabetes involve the eyes, kidneys and nervous system, and include coronary heart disease and major stroke (The Emerging Risk Factors Collaboration 2010).
The prevalence of type 2 diabetes is increasing rapidly worldwide and is predicted to more than double in the years between 2000 and 2030 (Wild 2004). Although no single causal factor has been attributed to development of the condition, increasing urbanisation and ageing populations are strongly linked to global changes in the incidence and prevalence of diabetes. One important risk factor is a diagnosis of severe mental illness such as schizophrenia, bipolar disorder or other psychoses, with research suggesting an almost two‐fold increase in the risk of diabetes among people with severe mental illness (Osborn 2008). This increased risk has been linked to a combination of factors including patient behaviour, in particular physical inactivity and poor diet (De Hert 2011) and higher rates of smoking (Lawrence 2009). Alongside lifestyle and behavioural factors, medications commonly prescribed for severe mental illness are strongly associated with development of metabolic abnormalities and weight gain, which significantly increase the risk of type 2 diabetes (De Hert 2011).
The World Health Organization (WHO) recognises mental disorder as an important contributing factor to the global burden of non‐communicable diseases, such as diabetes, and emphasises that equitable access to effective programmes and healthcare interventions is needed (WHO 2013a). As such, the WHO Comprehensive Mental Health Action Plan for 2013 to 2020 states that developing good‐quality mental health services requires the use of evidence‐based protocols and practices. This plan suggests that health workers must not limit interventions to those that improve mental health but must also attend to the physical health needs of people with a mental disorder (WHO 2013b). In the United Kingdom, the Schizophrenia Commission (The Schizophrenia Commission 2012) and the Royal College of Psychiatrists (Royal College of Psychiatrists 2009) recognise that the poorer physical health of people with severe mental illness must be urgently addressed, and they include amongst their advice the need for tailored health promotion programmes that can help people to manage better their physical health, including chronic illnesses.
Given the importance of lifestyle changes in the management of type 2 diabetes, it is essential that people possess the skills needed to manage their condition. Patient education and self management are an integral part of diabetes care. People with type 2 diabetes have the right to receive education about their condition and treatment options, as well as information and training on how they can best manage their illness. National Institute for Health and Care Excellence (NICE) guidelines for type 2 diabetes (NICE 2015) recommend that structured education must be integrated into routine care and should be offered to all. In addition, the National Health Service (NHS) report on commissioning of mental health and diabetes services in the UK (NHS Diabetes 2011) states that people with severe mental illness who develop diabetes should have access to appropriate diabetes care. However, despite evidence suggesting that diabetes self management programmes have a positive impact on clinical, lifestyle and psychosocial outcomes (Deakin 2005; Duke 2009; Pal 2013; Steed 2003; Steinsbekk 2012; Thorpe 2013), it remains unclear whether a diagnosis of severe mental illness has an impact on the effectiveness of such interventions, as people with severe mental illness are not likely to receive standard diabetes education (Goldberg 2007b).
For people with severe mental illness, physical health may not be a priority (Buhagiar 2011) and motivation to change may be limited, presenting additional challenges for successful self management. Therefore, it cannot be assumed that the findings reported in existing systematic reviews of diabetes self management interventions can be generalised to those with severe mental illness.
Description of the intervention
Diabetes self management interventions are complex, as they consist of several interacting components (Craig 2008). Self management refers to an individual's ability to manage the clinical and psychosocial consequences, along with the lifestyle changes, inherent in living with a chronic condition (Barlow 2002). On the basis of this broad definition, the content and complexity of diabetes self management interventions vary significantly, not only in terms of their aims and the behaviour/s they target (e.g. self monitoring of blood glucose, insulin titration, diet, exercise), but also in terms of their intensity, duration, place of delivery (i.e. primary or secondary care), mode of delivery (i.e. group, individual, online), type and training of the facilitator (i.e. diabetes and/or mental healthcare professional/s or lay person), active ingredients within the intervention and theoretical background.
Adverse effects of the intervention
Little evidence suggests that diabetes self management interventions are associated with adverse effects. However, adverse effects could occur if:
the content of the diabetes self management intervention is not evidence‐based, potentially resulting in incorrect information and training for people with type 2 diabetes;
participants misunderstand the information given or are unable to perform the required behaviours;
participants became anxious as a result of being more engaged, for example, if self monitored blood glucose readings are high and participants are unable to understand why (Peel 2004);
being more engaged leads to inappropriate use of healthcare services;
exercise leads to injury or increased pain and fatigue; or
participants make decisions that are detrimental to their health and well‐being, such as insulin titration that leads to hypoglycaemia.
How the intervention might work
Development of self management interventions has been influenced by several theories of health behaviour change, including social cognitive theory (Bandura 1986), the theory of reasoned action and planned behaviour (Ajzen 1991), self regulation theory (Leventhal 1984) and the transtheoretical model (Prochaska 1997). All of these theories identify concepts that predict health behaviour, with primary focus on beliefs, attitudes and expectations. Resulting self management interventions differ in their theoretical underpinnings and hence in the techniques they adopt to change behaviour. For example, a diabetes self management intervention based on social cognitive theory (Bandura 1986) may seek to reduce carbohydrate intake by increasing diet‐related self efficacy. Bandura proposed several ways in which self efficacy can be enhanced, including skills mastery wherein a person gains confidence by successfully achieving a goal, observation of someone performing the behaviour and verbal persuasion. These behaviour change techniques are proposed to be the 'active ingredients' that explain how a self management intervention might work.
In addition to the active ingredients, behaviour change interventions involve other key features, including the behaviour or behaviours they aim to change (i.e. diet, exercise, self monitoring) and their duration, intensity, setting and mode of delivery and type and training of the facilitator, all of which can influence engagement and the efficacy and replicability of an intervention (Hoffman 2014). Figure 1 presents a simplified schematic representation of the conceptual framework for diabetes self management interventions, which acknowledges their complex nature, along with the best‐established self management behaviour change techniques included in these types of interventions.
1.
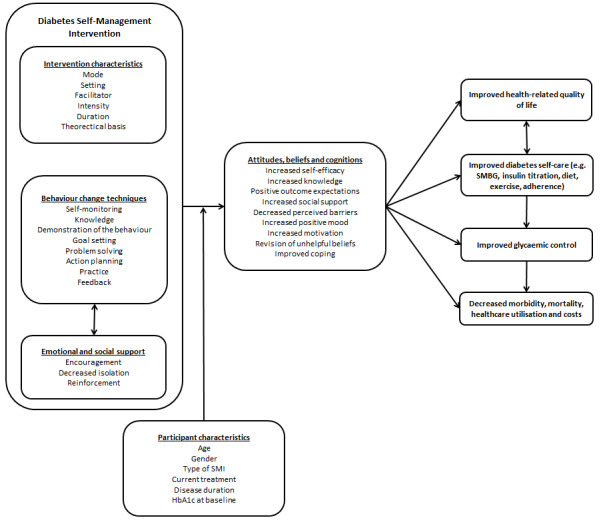
Schematic representation of diabetes self management.
Why it is important to do this review
Although some evidence indicates statistically and clinically significant benefits derived from diabetes self management interventions in the general population (Deakin 2005; Duke 2009; Pal 2013; Steed 2003; Steinsbekk 2012; Thorpe 2013), little evidence suggests that these interventions are effective in changing outcomes for people with severe mental illness and type 2 diabetes. A systematic review of diabetes self management specifically for those with schizophrenia or schizoaffective disorder found that approaches delivered in both inpatient and outpatient settings can be effective in managing type 2 diabetes, particularly those that address diet and exercise behaviour, but concluded that intervention packages need to be tailored to the unique challenges associated with decreased cognition and motivation, limited resources and the loss of energy and weight gain associated with use of antipsychotics (Cimo 2012). This review aims to broaden the inclusion criteria of this previous systematic review (Cimo 2012) to severe mental illnesses other than schizophrenia and schizoaffective disorder and other outcomes, including patient‐reported and socioeconomic outcomes.
This review will evaluate the effects of diabetes self management interventions for people with severe mental illness and type 2 diabetes, and it will provide us with the opportunity to describe, using established reporting systems, the active components of these interventions and the theoretical frameworks within which they were developed to establish how they work. Medical Research Council (MRC) guidelines for developing complex interventions (Craig 2008) and the Consolidated Standards of Reporting Trials (CONSORT) statement for randomised controlled trials (RCTs) of non‐pharmacological interventions (Boutron 2008) acknowledge the need for improved methods of specifying and reporting intervention content. In response, the Behaviour Change Technique Taxonomy (BCTTv1) (Michie 2013) was developed. This taxonomy provides standardised descriptions of different techniques, so that a shared language is used in the field of behaviour change, and links these techniques to published theories of behaviour. This systematic review will use the BCTTv1 (Michie 2013) to classify intervention content. Applying this method will help to provide a cumulative understanding, across the field of behaviour change, of how diabetes self management interventions change behaviour and improve outcomes. In addition, we will apply a coding system to assess the way in which these interventions have applied theory (Michie 2010). This theoretical coding system will enable an assessment of how, and to what extent, theory has been used to develop the intervention. Use of these coding systems will also prove helpful in systematically identifying and documenting the content of diabetes self management interventions for people with severe mental illness and type 2 diabetes, and will establish which components and theories are most effective. By undertaking subgroup analysis, review authors will attempt to identify whether intervention effects vary not only by intervention characteristics, but also by participant characteristics, to establish which type of self management intervention works best, for whom and under what conditions.
Objectives
To assess the effects of diabetes self management interventions specifically tailored for adults with type 2 diabetes and severe mental illness.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled clinical trials (RCTs).
Types of participants
Adults with severe mental illness and type 2 diabetes. We defined adult participants as those 18 years of age and older. Diagnosis of type 2 diabetes should have been consistent with the standard classification criteria valid at the time of the trial (e.g. ADA 1999; ADA 2008; WHO 1998). We defined severe mental illness as psychosis, schizophrenia, schizoaffective disorder, bipolar disorder, personality disorder or depression with psychotic features, however diagnosed.
Types of interventions
Intervention
Interventions were targeted to improve self management of type 2 diabetes mellitus; these could include interventions that targeted, for example, self monitoring of blood glucose, diet or exercise behaviour. Interventions may or may not have included self management of severe mental illness, but we excluded interventions that focused solely on the self management of mental health. The intervention could be of any duration.
Comparator
The comparison group provided another active intervention or usual/standard care.
Exclusions
Any intervention that:
included only participants with type 1 diabetes;
included participants without severe mental illness;
involved participants younger than 18 years of age, including trials that included both adults and children;
was targeted at healthcare professionals; or
focused exclusively on self management of mental health.
We included trials that recruited participants with both type 1 and 2 diabetes only if we could extract results for participants with type 2 diabetes. We included trials that recruited participants with and without severe mental illness only if we could extract results for participants with severe mental illness.
Types of outcome measures
Primary outcomes
Self care behaviours.
Diabetes‐related complications.
Adverse events.
Secondary outcomes
All‐cause mortality.
Health‐related quality of life.
Diabetes knowledge.
Self efficacy.
Progression of severe mental illness.
Glycosylated haemoglobin A1c (HbA1c).
Body mass index (BMI).
Weight.
Blood pressure.
Change in medication or in intensity of drug treatment.
Socioeconomic effects.
Methods of outcome measurement
Self care behaviours: evaluated with a validated instrument such as the Summary of Diabetes Self care Activities measure (Toobert 2000).
Diabetes‐related complications: defined as vascular complications (angina pectoris, myocardial infarction, stroke or peripheral vascular disease), neuropathy, nephropathy, retinopathy, diabetic foot and lower limb amputation and heart failure.
Adverse events of the intervention: defined as, for example, hypoglycaemia, pain, fatigue and anxiety.
All‐cause mortality: defined as death from any cause.
HbA1c: measured as glycosylated haemoglobin A1c.
Health‐related quality of life: evaluated with a validated generic or disease‐specific instrument, such as Short Form (SF)‐36 (McHorney 1993; Ware 1992) or the Diabetes Health Profile (Meadows 2000).
Diabetes knowledge: evaluated with a validated instrument such as the Brief Diabetes Knowledge Test (Fitzgerald 1998).
Self efficacy (general or diabetes‐specific): evaluated with a validated instrument such as the Diabetes Empowerment Scale (Anderson 2000).
Progression of severe mental illness: assessed by a disease‐specific measure, such as the Positive and Negative Syndrome Scale (Kay 1987), or by generic measures such as the Clinical Global Impressions Scale (Busner 2007) or the Health of the Nation Outcome Scale (Wing 1998).
BMI: measured as body weight in kilograms per meter squared (kg/m²).
Weight: in kilograms or pounds.
Blood pressure: systolic and diastolic blood pressure in millimetres of mercury (mmHg).
Change in medication or intensity of drug treatment: intensity of type 2 diabetes treatment defined as an increase in medication dose or the introduction of an additional drug; intensity of severe mental illness treatment defined as an increase in medication dose or the introduction of an additional drug.
Socioeconomic effects: direct costs defined as admission/re‐admission rates, average length of stay, visits to general practitioner, accident/emergency visits; indirect costs defined as resources lost as the result of illness of participants or family members.
Timing of outcome measurement
We classified the timing of outcome measurements as short, medium and long term. Short‐term follow‐up was defined as measurement taken within one month of the end of the intervention period, therefore capturing immediate effects of the intervention; medium‐term follow‐up was defined as between one and six months post intervention, and long‐term follow‐up as six months and longer.
Summary of findings
We present a 'Summary of findings table' to report the following outcomes, listed according to priority.
Diabetes‐related complications.
All‐cause mortality.
Adverse events.
Health‐related quality of life.
Self care behaviours.
HbA1c.
Socioeconomic effects.
Search methods for identification of studies
We planned to search the Allied and Complementary Medicine Database (AMED) (McBain 2014); however, on the recommendation of the Cochrane Metabolic and Endocrine Disorders Group (CMED), we deemed AMED redundant, as it was unlikely to reveal any relevant trials above and beyond the included databases.
Electronic searches
We searched the following sources from inception of each database to the specified date, and we placed no restrictions on the language of publication.
Cochrane Library (7 March 2016).
MEDLINE <1946 to Present> (7 March 2016).
EMBASE <1974 to 2016 Week 10> (7 March 2016).
PsycINFO <1806 to March Week 1 2016> (7 March 2016).
CINAHL (7 March 2016).
ClinicalTrials.gov (7 March 2016).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/) (7 March 2016).
We continuously applied a MEDLINE (via Ovid SP) email alert service to identify newly published trials using the same search strategy as described for MEDLINE (for details on search strategies, see Appendix 1). After supplying the final review draft for editorial approval, CMED performed a complete update search on all databases available at the editorial office and sent the results of this search to the review authors.
Searching other resources
We planned to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved articles, including trials, (systematic) reviews, meta‐analyses and health technology assessment reports. We searched unpublished literature by using the following databases.
BASE: Bielefeld Academic Research Engine (http://www.base‐search.net/).
Open Grey (http://www.opengrey.eu/).
NHS Evidence (http://www.evidence.nhs.uk/).
UK Clinical Research Network Study Portfolio (http://public.ukcrn.org.uk/search/).
Data collection and analysis
Selection of studies
Two review authors (HM, MH) independently scanned the abstract, title or both of every record retrieved. We rejected articles at this stage if they did not meet the inclusion criteria. If it was not possible to reject at this point, we retrieved full‐text copies of the article. Two review authors (HM, JJ) then independently scanned the full text of all remaining articles. We resolved differences between review authors by discussing them with the review team and by contacting trial authors for clarification. We included an adapted PRISM (Preferred Reporting Items for Systematic Reviews and Meta‐analyses) diagram of trial selection (Liberati 2009). We present a PRISMA flowchart showing the process of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfilled the inclusion criteria, two review authors (HM, KM) independently extracted key participant and intervention characteristics and reported data on efficacy outcomes and adverse events by using standard data extraction templates, with disagreements resolved by discussion (see Characteristics of included studies; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9).
We presented Information, including trial identifier, about potentially relevant ongoing studies in the Characteristics of ongoing studies table. We planned to find the protocol of each included trial and to report primary, secondary and other outcomes in comparison with data derived from publications in a joint appendix titled "Matrix of trial endpoints (publications and trial documents)" (Appendix 6).
We emailed the authors of all included trials to enquire whether they would be willing to answer questions regarding their trials. Appendix 10 shows the results of this survey. We sought relevant missing information on the trial from the primary author of the article, when required.
We coded both intervention and comparator groups for their use of theory and behaviour change techniques.
Use of theory
A theory coding scheme has been developed that assesses how and to what extent theory has been used to develop an intervention (Michie 2010). This coding scheme consists of 19 items, each requiring a 'yes', 'no' or 'do not know' response. The scheme classifies these 19 questions into six categories: (1) Is theory mentioned? (2) Are the relevant theoretical constructs targeted? (3) Is theory used to select recipients or to tailor an intervention? (4) Are the relevant theoretical constructs measured? (5) Is theory tested? and (6) Has theory been refined? For the purposes of any analysis, if the theoretical basis for the intervention group was the same as for the control group, we coded the intervention as not having a theoretical basis (except for descriptive purposes) because theory was unable to explain the difference in effect size between the two groups.
Use of behaviour change techniques
We used the Behaviour Change Technique Taxonomy (BCTTv1) (Michie 2013) to code both intervention and control groups. We provided appropriate training for those extracting and coding behaviour change techniques. If the same behaviour change technique (BCT) was employed within both intervention and control groups, we coded the intervention as not containing the BCT (except for descriptive purposes) because the BCT would not explain differences in effect size between the two conditions.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised yield of information by collating all available data and using the most complete data set aggregated across all known publications. In case of doubt, we planned to assign priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (HM, KM) independently assessed risk of bias for each included trial and resolved disagreements by consensus. We assessed risk of bias by using the tool of The Cochrane Collaboration for assessment of risk of bias (Higgins 2011a; Higgins 2011b) based on the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We rated risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a 'Risk of bias summary' figure and assessed the impact of individual bias domains on trial results at endpoint and trial levels. In case of high risk of selection bias, we marked all endpoints investigated in the associated trial as 'high risk'.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessors), we evaluated risk of bias separately for each outcome (Hróbjartsson 2013). We noted whether outcomes were self reported, investigator assessed or adjudicated outcome measures, for example, whether hypoglycaemia was reported by participants or by trial personnel.
We considered the implications of missing outcome data from individual participants, such as high drop‐out rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
We assessed outcome reporting bias by integrating the results of the appendix 'Examination of outcome reporting bias' (Appendix 7), the appendix 'Matrix of trial endpoints (publications and trial documents)' (Appendix 6) and the section 'Outcomes (outcomes reported in abstract of publication)' of the Characteristics of included studies tables. This analysis formed the basis of our judgement of selective reporting (reporting bias).
We defined the following endpoints as self reported outcomes.
Health‐related quality of life.
Self care behaviours.
Diabetes knowledge.
Self efficacy.
Adverse events, depending on measurement.
Body mass index (BMI), depending on measurement.
Weight, depending on measurement.
Change in medication or intensity of drug treatment, depending on measurement.
We defined the following outcomes as investigator‐assessed outcomes.
HbA1c.
All‐cause mortality.
Diabetes‐related complications.
BMI, depending on measurement.
Weight, depending on measurement.
Blood pressure.
Change in medication or intensity of drug treatment, depending on measurement.
Socioeconomic effects.
Measures of treatment effect
We planned to express dichotomous outcomes as risk ratios (RRs), along with 95% confidence intervals (95% CIs). For continuous outcomes when the same measurement scale was used (e.g. HbA1c), we measured treatment effects as the difference in mean changes from baseline. For continuous outcomes with different measurement scales, such as quality of life, we measured treatment effects as standardised mean differences (SMDs). The definition of SMD used in Cochrane reviews is the effect size known in social science as Hedges’ g (adjusted) (Hedges 1985). If Hedges’ g was not reported, we calculated it as the difference between the two means (intervention and control) divided by the pooled standard deviation. If this was not possible, we planned to describe the results of each trial in a narrative synthesis. We planned to express time‐to‐event data as hazard ratios (HRs) with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. We planned to extract data from cross‐over trials for intervention and control groups at baseline and at the time point immediately preceding cross‐over. In case of a unit of analysis error in cluster‐RCTs, we planned to adjust for the design effect by reducing the size of the trial to its "effective sample size" (Rao 1992). We would have calculated this by dividing the original sample size by the 'design effect'. The design effect is 1 + (M ‐ 1) * ICC, where M is the average cluster size, and ICC is the intra‐cluster correlation coefficient. For dichotomous data, we planned to divide the number of participants and the number experiencing the event by the design effect. For continuous data, we planned to reduce only sample sizes, leaving means and standard deviations unchanged (Higgins 2011a).
Dealing with missing data
We attempted to obtain missing data from trial authors and carefully evaluated important numerical data such as screened, randomised participants, as well as intention‐to‐treat, as‐treated and per‐protocol populations. We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals) and we critically appraised issues of missing data and use of imputation methods (e.g. last observation carried forward, mean imputation, imputing based on predicted values from a regression analysis).
When standard deviations for outcomes were not reported and we did not receive the information from trial authors, we planned to impute these values by assuming the standard deviation of the missing outcome to be the average of standard deviations from those trials for which this information was reported. We planned to investigate the impact of imputation on meta‐analyses by performing sensitivity analysis.
When trial authors failed to respond within one month of the first contact, we made a second attempt. If we received no response after two months, we recorded data as missing.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we would not report trial results as the pooled effect estimate in a meta‐analysis. We planned to identify heterogeneity (inconsistency) by visually inspecting forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also planned to consider the I² statistic, which quantifies inconsistency across trials, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); an I² statistic of 75% or more indicates a considerable level of heterogeneity (Higgins 2011a). We expected type of diabetes treatment (i.e. insulin‐dependent vs non‐insulin‐dependent type 2 diabetes) and a diagnosis of severe mental illness to introduce clinical heterogeneity.
Assessment of reporting biases
If we had included 10 or more trials that had investigated a particular outcome, we planned to use funnel plots to assess small‐study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore planned to interpret results carefully (Sterne 2011).
Data synthesis
Unless good evidence suggested homogeneous effects across trials, we planned to summarise primarily 'low risk of bias' data by using a random‐effects model (Wood 2008). We planned to interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects and to present a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). We planned to perform statistical analyses according to the statistical guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We presented overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. Two review authors (HM, KM) independently rated the quality of evidence for each outcome. We present a summary of the evidence in Table 1, which provides key information about the best estimate of the magnitude of effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and the rating of overall confidence in effect estimates for each outcome. We created Table 1 on the basis of methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results on outcomes in the Types of outcome measures section. Meta‐analysis was not possible; therefore, we presented results in a narrative Table 1.
In addition, we established an appendix titled 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) (Appendix 11) to help with standardisation of Table 1.
Subgroup analysis and investigation of heterogeneity
Clearly the efficacy of diabetes self management for people with severe mental illness is important, but it is also important to identify optimal content and delivery methods, as well as participant characteristics, that lead to the most improved outcomes. We planned to perform subgroup analyses to establish whether intervention effects varied with different participant populations or intervention characteristics. We used these comparisons only to generate hypotheses.
We expected the following characteristics to introduce clinical heterogeneity, and we planned to carry out subgroup analyses to investigate interactions.
Age.
Gender.
Disease duration of both type 2 diabetes and severe mental illness at baseline.
Insulin‐treated versus non‐insulin‐treated type 2 diabetes.
Severe mental illness treatment (i.e. antipsychotic medication vs no antipsychotic medication, typical (first‐generation) vs atypical (second‐generation) antipsychotic medication, olanzapine or clozapine treatment vs other antipsychotic treatment).
Diagnosis of severe mental illness (i.e. psychosis, schizophrenia, schizoaffective disorder, bipolar disorder, personality disorder or depression with psychotic features).
Targeted behaviour (e.g. self monitoring, self titration of drug/insulin, exercise, diet).
HbA1c at baseline.
Behaviour change techniques used.
Use of a theory to inform the intervention.
Intensity of the intervention provided.
Intervention setting (i.e. primary or secondary care or community).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting analysis to the following.
Published trials.
Taking into account risk of bias, as specified in the Assessment of risk of bias in included studies section.
Very long or large trials to establish the extent to which they dominate the results.
Trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry vs other) or country.
We also planned to test the robustness of our results by repeating the analysis using different measures of effect size (RR, odds ratio (OR), etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see Table 2, Characteristics of included studies', 'Characteristics of excluded studies, and 'Characteristics of ongoing studies' sections.
1. Overview of trial populations.
| Intervention and comparator | Sample sizea | Screened/eligible [N] | Randomised [N] | Analysed [N] | Finishing trial [N] | Randomised finishing trial [%] | Follow‐upb | |
| McKibbin 2010 | I: Diabetes Awareness and Rehabilitation Training (DART) | ‐ | 77 | 32 | 26 | 26 | 81.3 | 24 weeks (6 months post intervention) |
| C: usual care plus information (UCI) | 32 | 26 | 26 | 81.3 | ||||
| Total: | 64 | 52 | 52 | 81.3 | ||||
aAccording to power calculation in trial publication or report
bDuration of intervention and/or follow‐up under randomised conditions until end of trial
"‐" denotes not reported
C: comparator; I: intervention
Results of the search
After removal of duplicates, the search of 11 electronic bibliographic databases yielded a total of 3080 citations. HM and MH performed independent screening of the abstracts of these articles, and CF resolved disagreements. We retrieved full papers for all abstracts that the reviewers could not confidently exclude. HM and JJ assessed 60 full‐text articles for eligibility. One trial (three reports) and nine ongoing trials fulfilled the inclusion criteria. We summarised our search results in Figure 2.
2.
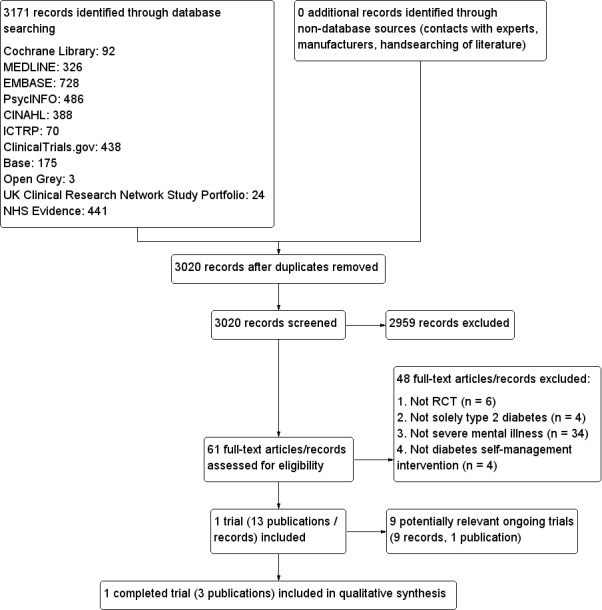
Study flow diagram.
Included studies
We included one trial (three trial reports) with 64 participants. We presented a detailed description of the characteristics of this trial elsewhere (see Characteristics of included studies). Nine additional trials were ongoing and provided no published data; we presented details of these trials in the Characteristics of ongoing studies table.
Source of data
We obtained the data presented in this review from three published articles and through correspondence with the trial author.
Comparisons
The trial was a randomised controlled trial comparing Diabetes Awareness and Rehabilitation Training (DART) with usual care plus information (UCI).
Overview of trialpopulations
Investigators approached a total of 77 patients to participate in the trial; 11 declined to take part and two were already participating in other psychoeducational or medication trials. A total of 64 participants provided consent to participate in the trial ‐ 32 in each arm. Two did not complete the trial because of inpatient hospitalisation, one was unable to complete the follow‐up assessment, one relocated, one died before receiving the intervention, one had psychiatric decompensation and one lost interest. Researchers reported results for 57 participants (29 in the control arm and 28 in the intervention arm) immediately post intervention (i.e. six months from the time of entry into the trial; known as 'short‐term follow‐up') and for 52 participants (26 in each arm) at six months post intervention (i.e. 12 months from entry into the trial; known as 'long‐term follow‐up'). Five other participants were lost to long‐term follow‐up, as they had moved out of the area.
Trial design
Investigators conducted the RCT at a single site. They did not report the time frame in which the trial was completed, nor whether blinding of participants or personnel to group allocation was undertaken. The trial did not include a run‐in period, nor was it terminated early. A trained interviewer, masked to group allocation, conducted a 90‐minute interview to collect trial outcomes. However, measures taken during this interview remain unclear.
Settings
Investigators conducted the trial in the San Diego healthcare system and did not report the site of recruitment.
Participants
Participants were primarily women (65%). The RCT included only adults over 40 years of age, with a mean age of 54 years. Most individuals in the sample were white (61%) and were living in board‐of‐care facilities (83%). Average length of education was 12 years. The sample consisted of 46 participants with schizophrenia and nine with schizoaffective disorder. The mean age of participants at onset of psychiatric illness was 28 years. The mean duration of diabetes was nine years. Trial authors did not report the presence of co‐morbidities. Most participants were receiving oral treatment (68%) for their diabetes; 12% controlled their diabetes through dietary changes only, 7% with insulin and 9% with a combination of an oral agent and insulin. Medical treatment for their psychiatric illness consisted predominantly of risperidone or quetiapine (47%); remaining participants received aripiprazole or ziprasidone (23%), clozapine or olanzapine (30%).
Scores of psychiatric symptom severity, measured on the Positive and Negative Syndrome Scale (PANSS), indicated a mean positive symptom score of 14, a negative symptom score of 5 and a general symptoms score of 4. The mean baseline score on the Hamilton Depression Scale was 14 and on the Mattis Dementia Rating Scale 128.
Mean glycosylated haemoglobin A1c (HbA1c) of participants at baseline was 7%, body mass index (BMI) was 33 kg/m²and on average, participants weighed 217 lbs; their mean systolic blood pressure was 133 mmHg and mean diastolic blood pressure 84 mmHg.
Diagnosis
Although providers confirmed the diagnosis, they did not report the clinical diagnostic criteria used to identify type 2 diabetes or severe mental illness. .
Intervention
The DART intervention was a group‐based, face‐to‐face, 24‐week self management programme. The intervention took place weekly, and each session lasted for 90 minutes. DART comprised three modules: (1) basic diabetes education (sessions one to four, repeated at sessions 13 to 16); (2) nutrition (sessions five to eight, repeated at sessions 17 to 20); and (3) lifestyle exercise (sessions 9 to 12, repeated at sessions 21 to 24). Each module contained four 90‐minute manualised sessions. Basic diabetes education included an explanation of motivation and a review of blood sugar and symptoms of low and high blood sugar levels, diabetes complications, how to use a glucose meter, how to talk with your doctor and types of medication available for treatment. Nutrition education included a review of food groups, portion sizes, healthy meals and food labels, along with ways to replace sugar with fat and fibre. Lifestyle and exercise sessions presented different types of exercise, as well as their impact on blood sugar levels, use of a pedometer to track exercise and care of the foot during exercise.
Personnel adapted educational materials for people of middle age and older with schizophrenia or schizoaffective disorder by introducing one or two topics per session, providing an overview and summary of the materials, implementing a teach and query training method and using mnemonic aids and print materials with larger font and limited text. They provided participants with simple guidelines about how they might lead a healthier lifestyle, such as switching from regular soda or fruit punch to diet soda or water.
One diabetes‐trained mental health professional delivered the intervention. Thus facilitators did not make contact with participants' healthcare provider during the intervention but encouraged participants to speak to their physician about their diabetes and provided guidance on how to record laboratory results and examination findings.
Trial reports state that the intervention was based on social cognitive theory but provide no other details on how and to what extent theory was used to develop the intervention. As a result, the trial scored only one point on a scale of 0 to 8, on the basis of the theory coding scheme (Michie 2010). Trial authors stated that they employed the following behavioural change strategies within the intervention: self monitoring (e.g. pedometers, weekly weigh‐ins), modelling, practice (i.e. healthy food sampling), goal setting and reinforcement for attendance and behavioural change (i.e. raffle tickets for small health‐related prizes). Through independent coding of intervention descriptions, HM and KM used the Behaviour Change Technique Taxonomy (BCTTv1) (Michie 2013) to identify 14 behaviour change techniques in the intervention arm: self monitoring outcome(s) of the behaviour; social support (unspecified); material reward (behaviour); behaviour substitution; graded tasks; instruction on how to perform the behaviour; credible source; feedback on outcome(s) of the behaviour; objects added to the environment; self monitoring of behaviour; body changes; behavioural practice/rehearsal; demonstration of the behaviour; and goal setting (outcome).
Comparator
The comparator ‐ usual care plus Information (UCI) ‐ consisted of usual care provided by participants' providers and three brochures provided by the American Diabetes Association that were relevant to diabetes management (i.e. basic diabetes education, nutrition, exercise). Researchers did not specify the theoretical underpinnings of the control arm, hence a score of zero on the theory coding scheme (Michie 2010) and independent coding identified only one reported BCT: social support (unspecified).
Outcomes
Trial authors did not specify a primary outcome; they measured a range of outcomes as part of the trial and reported different outcomes at each follow‐up. They provided short‐term follow‐up immediately post intervention (i.e. six months from baseline) and long‐term follow‐up six months after completion of the intervention (i.e. 12 months from baseline). See Appendix 8 and Appendix 9.
Investigators assessed the short‐term efficacy of the intervention in accordance with self care behaviours (total energy expenditure, total activity, total kilocalories consumed and total minutes of activity), weight, BMI, waist circumference, blood pressure, changes to diabetes and antipsychotic treatment, fasting blood glucose, HbA1c, cholesterol, lipoprotein, triglycerides, diabetes knowledge and self efficacy. A total of 57 participants contributed to the analysis of these outcome measures. At long‐term follow‐up, researchers explored differences between groups across 52 participants, for BMI, changes to diabetes and antipsychotic medication, weight, waist circumference, HbA1c, diabetes knowledge and energy expenditure.
To measure dietary intake, investigators asked participants to rank how often they consumed 70 different foods over the past month on the Block Brief 2000 Revision of the Health and Habits and History Questionnaire (Block 1990). They measured physical activity by using the Yale Physical Activity Scale (YPAS; Dipietro 1993), which provides two indices: total energy expenditure (TEE) and total activity summary index (TASI). Researchers calculated the TEE by using an activities checklist to assess time spent in various activities during a typical week in the past month. They calculated the TASI by summing the hours spent in different types of activities weighted by their intensity. They derived the total number of minutes of moderate and vigorous activity from each day of monitoring (i.e. at least three days of data, 10 hours per day) by using an accelerometer and averaged these values across the three days.
Trial authors measured diabetes knowledge on the 23‐item Diabetes Knowledge Test (Fitzgerald 1998) and self efficacy on the 28‐item Diabetes Empowerment Scale (Anderson 2000), which consists of three subscales: managing psychosocial aspects of diabetes (MPAD), dissatisfaction and readiness for change (DRFC) and setting and achieving diabetes goals (SADG).
Investigators measured positive and negative symptoms by using the Positive and Negative Syndrome Scale (PANSS) (Kay 1987), depressive symptom severity by using the Hamilton Depression Rating Scale (HAM‐D) (Hamilton 1960) and cognitive functioning by using the Dementia Rating Scale (DRS) (Mattis 1973). They assessed these measures only at baseline to describe the sample and used the PANSS immediately following the intervention to explore its effect as a moderator of intervention effectiveness (McKibbin 2010).
Excluded studies
After evaluation of full texts, we excluded 48 articles from the review. Of these, six were not RCTs; in 34 papers, included participants did not meet our definition of severe mental illness (psychosis, schizophrenia, schizoaffective disorder, bipolar disorder, personality disorder or depression with psychotic features); in four papers, participants were not solely those diagnosed with type 2 diabetes and data could not be extracted for type 2 participants only; and in the final four papers, researchers did not evaluate a diabetes self management intervention.
Risk of bias in included studies
For details on risk of bias of included trials, see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual trials, see Figure 3. Overall, risk of bias was unclear for most aspects, as articles provided insufficient details for review authors to make an assessment.
3.
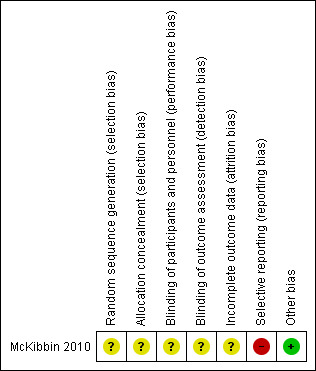
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Researchers reported no information on allocation concealment or method of randomisation; therefore, risk of selection bias was unclear.
Blinding
Blinding of participants and intervention facilitators would not have been possible, and trial authors did not report blinding of other trial personal to group allocation; hence, we classified this trial as having unclear risk of performance and detection bias. A blinded trained interviewer undertook a 90‐minute interview with each participant to collect data, but trial authors failed to specify which outcomes were measured by this interview.
Incomplete outcome data
Trial authors did not perform intention‐to‐treat (ITT) analyses, and they reported no information on how missing data were treated. From baseline to immediately post intervention, 11% of the overall sample, and from baseline to six months post intervention 19%, failed to complete both baseline and follow‐up assessments. Researchers did not report reasons for drop‐out by trial arm.
Selective reporting
We judged risk of reporting bias as high. We were unable to find a published protocol for the trial. The article reporting long‐term outcomes failed to present results for several of the outcomes measured at short‐term follow‐up, including blood pressure, fasting blood glucose, cholesterol, lipoprotein, triglycerides, self efficacy, total activity, total kilocalories consumed and total minutes of activity.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
See Table 1 for the main patient‐relevant outcomes.
Baseline characteristics
For details of baseline characteristics, see Appendix 3, Appendix 4 and Appendix 5.
Diabetes Awareness and Rehabilitation Training (DART) programme versus usual care plus information (UCI)
Primary outcomes
Self care behaviours
Trial investigators measured physical activity by using the Yale Physical Activity Scale (Dipietro 1993). The TEE subscale did not improve with the DART programme in comparison with UCI at short‐term or long‐term follow‐up. The TASI improved immediately following the DART programme in comparison with UCI. Researchers observed no substantial difference in the total number of minutes of daily activity performed by participants between DART and UCI at short‐term follow‐up. The mean energy expenditure six months after completion of the intervention was 2148 kcal for the DART group and 2800 kcal for the UCI group. Trial authors reported that the difference of 652 kcal did not reflect an improvement. For measurement of dietary intake, participants completed the Brief 2000 Revision of the Health and Habits and History Questionnaire (Block 1990), which estimates the total calories consumed in kilocalories. Participation in the DART programme did not result in improvement in the number of calories consumed at short‐term follow‐up compared with UCI. Trial authors did not report effects at long‐term follow‐up for the TASI, minutes of daily activity or dietary intake.
This trial did not measure or report outcomes in relation to diabetes‐related complications and adverse events.
Secondary outcomes
This trial did not measure or report outcomes in relation to all‐cause mortality, health‐related quality of life nor socioeconomic effects. Although investigators measured positive and negative affect and depression at baseline, they did not use these scales to measure progression of mental health across the trial period.
Diabetes knowledge
Diabetes knowledge, as measured by the Diabetes Knowledge Test (Fitzgerald 1998), improved following completion of the DART programme compared with UCI at both short‐term and long‐term follow‐up.
Self efficacy
Trial authors assessed self efficacy by using the Diabetes Empowerment Scale (Anderson 2000). Scores on all three subscales improved immediately after completion of the DART programme in comparison with UCI. Trial authors did not report results at long‐term follow‐up.
Glycaemic control
Glycaemic control, as measured by HbA1c, showed no statistically significant effect of the DART programme in comparison with UCI at short‐term (mean difference (MD) 0.6%) or long‐term follow‐up (end of trial values 7.9% for DART vs 6.9% for UCI). Also, fasting blood glucose levels showed no marked differences between intervention and comparator groups, and this outcome was reported only at short‐term follow‐up.
Body mass index (BMI)
Researchers observed improvement in favour of DART in BMI at short‐term (MD 1.7 units) and long‐term follow‐up (MD 2.4 units).
Weight
Weight improved immediately following completion of the intervention compared with UCI. Although trial authors reported that participants in the DART group experienced weight loss at long‐term follow‐up and UCI participants gained weight, they did not provide pre‐post data.
Blood pressure
Both systolic blood pressure and diastolic blood pressure failed to improve at short‐term follow‐up in the DART programme compared with UCI.
Change in medication or intensity of drug treatment
Trial authors reported few changes in antipsychotic and diabetes treatment type in the short term or over the long term. Groups were also similar in terms of antipsychotic and diabetes treatment type at both follow‐up intervals. Investigators reported no data for either of these outcomes.
Other outcomes
We did not specify several other secondary outcomes in our protocol, but trial authors included them in the trial and reported that they showed an effect for the intervention. Waist circumference in inches improved as a result of the DART programme compared with UCI, both at short‐term and long‐term follow‐up. Researchers presented short‐term effects for triglycerides but no substantial short‐term effects on levels of cholesterol in the DART programme in comparison with UCI, or for high‐density or low‐density lipoproteins.
Subgroup analyses
Trial authors explored the moderating effects of schizophrenia symptoms following the intervention, as measured by the PANSS (Kay 1987), on changes in diabetes knowledge and self efficacy from baseline to short‐term follow‐up. These results indicated that differences in changes in diabetes knowledge between the DART programme and UCI were dependent on the prevalence and severity of schizophrenia symptoms. When the total psychiatric symptom severity score was low at baseline, change in diabetes knowledge was greater in the DART group than in the UCI group at short‐term follow‐up. However, when the total psychiatric symptom severity score was high at baseline, investigators reported no difference in the change in diabetes knowledge between the two groups at short‐term follow‐up. They observed interaction effects for both negative and general symptom scores on the PANSS (Kay 1987). When negative or general symptom scores were low at baseline, the DART group performed better in relation to their diabetes knowledge than the UCI group. However, when negative or general scores were high, trial authors reported no differences between the two arms. Positive symptom severity did not interact with trial arm on any of the three self efficacy subscales.
Sensitivity analyses
We performed no sensitivity analyses because of the limited number of trials included in the review (n = 1).
Assessment of reporting bias
We did not draw funnel plots because the number of included trials was limited (n = 1).
Ongoing studies
We found nine ongoing RCTs, seven in progress in the USA, one in Germany and another in Canada. In seven trials, inclusion criteria included type 2 diabetes and at least one of the included severe mental illnesses. Hence, these trials would be included in subsequent updates of this review only if suitable subgroup analyses were performed.
Discussion
Summary of main results
Effects of the intervention on clinical outcomes
We included one trial involving 64 participants with type 2 diabetes and either schizophrenia or schizoaffective disorder. This randomised controlled trial (RCT) compared the 24‐week Diabetes Awareness and Rehabilitation Training (DART) programme ‐ a group‐based face‐to‐face self management intervention covering general diabetes education, nutrition and exercise ‐ with usual care plus information (UCI). Most individuals in the sample were women (65%), and the mean age of participants was 54 years. The mean age of onset of psychiatric illness was 28 years, and the mean duration of diabetes nine years. Investigators recorded outcome measures immediately following the intervention (i.e. short‐term follow‐up) and six months post intervention (i.e. long‐term follow‐up).
Trial authors observed no substantial effects on glycaemic control, blood pressure, cholesterol, high and low lipoprotein or total number of minutes of activity per day. They reported observable improvements in body mass index (BMI) and waist circumference at short‐term and long‐term follow‐up in the DART programme compared with UCI, and in triglycerides and weight immediately post intervention only.
Effects of the intervention on patient‐reported outcomes
Diabetes knowledge, self efficacy and total activity levels of participants improved immediately following the DART programme in comparison with UCI. Participants maintained improvements in diabetes knowledge at long‐term follow‐up. Total calories consumed by participants and their total energy expenditure failed to improve as a consequence of the programme in comparison with usual care.
Behaviour change techniques used in the intervention and mechanisms of action
Trial authors did not specify how and to what extent theory had been used to develop the content for the intervention or control group. Coding of DART revealed 13 behaviour change techniques unique to the DART programme.
Overall completeness and applicability of evidence
The primary limitation of this review is the overall lack of trials. We identified only one RCT with 64 participants that met the inclusion criteria. This RCT targeted only older adults (40+ years) with schizophrenia or schizoaffective disorder; we found no suitable trials that recruited younger participants or those with other severe mental illnesses. Another significant limitation was lack of measurement and reporting of outcome measures specified in the protocol. The included RCT did not measure or report findings on adverse events, diabetes‐related complications, mortality, health‐related quality of life, progression of mental health nor socioeconomic effects. Although the intervention was reported to be grounded in social cognitive theory, trial authors presented no information on how and to what extent social cognitive theory had been used to develop the DART programme. Subgroup analysis to explore the effects on intervention effectiveness of participant and intervention characteristics, such as active ingredients, was not possible.
Quality of the evidence
We rated the quality of the only trial included in this review as very low. Researchers did not measure outcomes related to diabetes‐related complications, all‐cause mortality, adverse events, health‐related quality of life and socioeconomic effects. Trial authors did not provide details about the randomisation process. The nature of the intervention precluded participant blinding, and it was unclear whether personnel or outcome assessors were blinded to group allocation. Investigators defined self care behaviour in terms of physical activity and food consumption. Whilst some of these measures were objective, such as total minutes of physical activity measured by an accelerometer, the remainder involved subjective reports.
We noted selective reporting bias in relation to weight, blood pressure, fasting blood glucose, cholesterol, high‐density and low‐density lipoproteins, triglycerides, self efficacy and several self care behaviours. Although researchers reported the effects of the intervention at short‐term follow‐up for these outcomes, they did not report long‐term effects, possibly indicating that these analyses were not statistically significant and hence were not reported. In addition, investigators did not explore the moderating effects of symptoms in relation to self care behaviours nor glycosylated haemoglobin (HbA1c). The small sample size and the number of included trials significantly reduced the precision of this review.
Potential biases in the review process
This Cochrane review addresses a specific and well‐defined research question. The search of the literature was extensive and sensitive, but publication bias remains a possibility. The final review includes only English language articles, although we did not limit our search criteria to publications in English.
Although the inclusion criteria were clearly defined, we noted continued ambiguity in the wider literature on the definition of diabetes self management. We deliberately kept this definition broad, so as not to exclude potentially important interventions, as long as the primary focus of the intervention was to enable participants to better manage their type 2 diabetes; however, as a result of often brief descriptions, we based judgements about inclusion on limited data.
Selection of trials followed the protocol and different review authors were responsible for selecting trials at each stage of the review, which may have introduced bias into the selection process. However, we ensured that one review author was involved at all stages to maintain some consistency .
We excluded trials in which the sample combined individuals with type 1 and type 2 diabetes, or those who had been diagnosed with a severe mental illness not listed in our inclusion criteria if subgroup analyses had not been performed; hence important and relevant data may be missing from this review.
We made the decision to include all three articles reporting one RCT, to maximise the quantity of data available for this review. We did not treat these three articles as three individual trials because each article described different aims. We have emphasised this fact throughout the review, and awareness of this is important when the findings and conclusions of this review are considered.
Agreements and disagreements with other studies or reviews
A review of effective lifestyle interventions for improving type 2 diabetes self management in people with schizophrenia or schizoaffective disorder by Cimo 2012 reported reductions in weight and BMI, but limited evidence for improved glycaemic control. Our review supports these findings. Cimo 2012 concluded that lifestyle interventions can be effective in management of type 2 diabetes, particularly when the intervention incorporates diet and exercise components. However, the review includes only four papers ‐ two were short‐term and long‐term follow‐up articles reported in this systematic review (McKibbin 2006; McKibbin 2010), and two were quasi‐experimental trials. Hence these conclusions may be overestimated. Consistent with this review, Cimo 2012 recommended that future research should focus on the long‐term sustainability of diabetes self management interventions for people with severe mental illness, and on addressing the needs of a younger population.
Authors' conclusions
Implications for practice.
Evidence is insufficient to show whether type 2 diabetes self management interventions for people with severe mental illness are effective in improving clinical, psychosocial, behavioural or economic outcomes.
Implications for research.
The small number of published trials reveals a significant gap in the literature for theory‐ and evidence‐based interventions that enable service users with severe mental illness to manage their type 2 diabetes. Several ongoing trials may meet the inclusion criteria in future updates of this review. However, the inclusion criteria for most of these ongoing trials include but are not exclusive to type 2 diabetes and severe mental illness, and therefore will contribute to the objectives of this review only if subgroup analyses are performed for this subset of participants.
We therefore recommend that theory‐ and evidence‐based interventions should be developed that address the specific challenges experienced by people with severe mental illness when they attempt to manage their diabetes, and that these interventions should be evaluated in robust randomised controlled trials. Future publications should ensure that the theoretical basis, active ingredients (behaviour change techniques) and doses of these ingredients (frequency of behaviour change techniques) are clearly described in published protocols and final reports. This will lead to a better understanding of which elements of an intervention are the most effective components for changing diabetes‐related behaviours and outcomes.
Finally, we affirmed a clear need to establish whether these interventions have effects on all‐cause mortality, health‐related quality of life and socioeconomic aspects, or whether they lead to adverse events, such as hypoglycaemic events or diabetes‐related complications.
Notes
Portions of the background and methods sections, the appendices, additional tables and Figures 1 to 3 of this review are based on a standard template established by the Cochrane Metabolic and Enocrine Disorders Group. We have based parts of the background and methods sections, the appendices, additional tables and Figures 1 to 3 of this review on a standard template established by the Cochrane Metabolic and Enocrine Disorders Group.
Acknowledgements
We thank Karla Bergerhoff and Maria‐Inti Metzendorf, former and current Trials Search Co‐ordinators for the Cochrane Metabolic and Endocrine Disorders Group, for designing and implementing the search strategy. We also would like to thank the Cochrane Metabolic and Endocrine Disorders Group for help and guidance provided during the review process.
Appendices
Appendix 1. Search strategies
| Cochrane Library |
| 1. [mh "Diabetes Mellitus, Type 2"] 2. ("MODY" or "NIDDM" or T2D*):ti,ab 3. (("non insulin*" next depend*) or (noninsulin* next depend*) or noninsulindepend* or "non" next "insulindepend*"):ti,ab 4. ((typ* next (2 or II)) near/4 diabet*):ti,ab 5. ((("late" or adult* or matur* or "slow" or stabl*) near/4 "onset") and diabet*):ti,ab 6. {or #1‐#5} 7. [mh "Diabetes Insipidus"] 8. (diabet* next "insipidus"):ti,ab 9. #7 or #8 10. #6 not #9 11. [mh ^"Mental Disorders"] 12. [mh "Affective Disorders, Psychotic"] 13. [mh "Personality disorders"] 14. [mh "Schizophrenia and Disorders with Psychotic Features"] 15. ("mental" near/4 (disorder* or "illness")):ti,ab 16. (schizo* or psychos?s or "psychotic"):ti,ab 17. (("bipolar" or "affective" or "personality") next disorder*):ti,ab 18. [mh ^"Depressive Disorder, Major"] 19. (("major" or "unipolar" or "clinical" or "recurrent") next depress*):ti,ab 20. {or #11‐#19} 21. #10 and #20 |
| MEDLINE (Ovid SP) |
| 1. exp Diabetes Mellitus, Type 2/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. exp Diabetes Insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. Mental Disorders/ 12. exp Affective Disorders, Psychotic 13. exp Personality disorders/ 14. exp "Schizophrenia and Disorders with Psychotic Features"/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. Depressive Disorder, Major/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. Patient Education as Topic/ 23. Patient Compliance/ 24. exp Self Care/ 25. exp Health Promotion/ 26. exp Behavior Therapy/ 27. exp Health Behavior/ 28. Program Evaluation/ 29. Life style/ 30. Weight Loss/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42‐52: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version] 42. randomised controlled trial.pt. 43. controlled clinical trial.pt. 44. randomi?ed.ab. 45. placebo.ab. 46. drug therapy.fs. 47. randomly.ab. 48. trial.ab. 49. groups.ab. 50. or/42‐49 51. exp animals/ not humans/ 52. 50 not 51 53. 41 and 52 |
| EMBASE (Ovid SP) |
| 1. non insulin dependent diabetes mellitus/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. exp diabetes insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. mental disease/ 12. major affective disorder/ 13. exp personality disorder/ 14. exp psychosis/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. major depression/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. exp health education/ 23. exp patient attitude/ 24. exp self care/ 25. behavior therapy/ 26. exp health behavior/ 27. exp program evaluation/ 28. lifestyle/ 29. weight reduction/ 30. weight control/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42:Wong 2006a"sound treatment studies" filter – BS version] 42. random*.tw. or clinical trial*.mp. or exp health care quality/ 43. 41 and 42 44. limit 43 to embase |
| PsycINFO (Ovid SP) |
| 1. Diabetes Mellitus/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. Diabetes Insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. Mental Disorders/ 12. exp Affective Disorders/ 13. exp Personality Disorders/ 14. exp Psychosis/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. exp Major Depression/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. Health Education/ or Health Literacy/ or Client Education/ 23. Disease Management/ or Coping Behavior/ or Self Care Skills/ 24. Health Behavior/ or Treatment Compliance/ 25. Health Promotion/ or Health Attitudes/ 26. "Physical Illness (Attitudes Toward)"/ or Illness Behavior/ 27. exp Program Evaluation/ 28. exp Behavior Therapy/ 29. exp Lifestyle/ 30. Weight Loss/ or Weight Control/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42:Eady 2008"PsycInfo Search Strategies" filter ‐ BS version] 42. control*.tw. OR random*.tw. OR exp Treatment/ 43. 41 and 42 |
| CINAHL (via EBSCO) |
| S1 MH "Diabetes Mellitus, Type 2+" S2 TX (MODY OR NIDDM OR T2D*) S3 TX ("non insulin* depend*" OR "noninsulin* depend*" OR noninsulin#depend* OR "non insulin#depend*") S4 TX (("typ* 2" OR "typ* II" OR typ#2 OR typ#II) N3 diabet*) S5 TX (((late OR adult* OR matur* OR slow OR stabl*) N3 onset) AND diabet*) S6 S1 OR S2 OR S3 OR S4 OR S5 S7 MH "Mental Disorders" OR MH "Mental Disorders, Chronic" OR MH "Psychotic Disorders+" OR MH "Personality Disorders+" OR (MH "Depression+") S8 TX (mental N3 (disORder* OR disease* OR illness)) S9 TX (schizo* OR psychos#s OR psychotic) S10 TX ((bipolar OR affective OR personality) N1 disorder) S11 TX ((major OR unipolar OR clinical OR recurrent) N1 depress*) S12 S7 OR S8 OR S9 OR S10 OR S11 S13 S6 AND S12 S14 MH "Health Education+" OR MH "Health Behavior+" OR MH "Coping" OR MH "Self Care+" OR MH "Health Promotion" S15 MH "Behavior Therapy+" OR MH "Program Evaluation" S16 MH "Life Style+" OR MH "Weight Loss" OR MH "Weight Control" S17 TX (self OR monitor* OR manage* OR educat* OR knowledge OR behav* OR psychoth* OR psychosocial OR aware* OR adjust* OR adher* OR compliance) S18 TX (intervention# OR program# OR programme# OR lifestyle OR "life style") S19 TX (weight N3 (management OR los* OR reduct*)) S20 S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 S13 AND S20 [S22:Wong 2006b"therapy studies" filter ‐ BS version] S22 MH "prognosis+" OR MH "study design+" OR random* S23 S21 AND S22 |
| ICTRP Search Portal (Standard search) |
| diabet* AND mental illness* OR diabet* AND mental disorder* OR diabet* AND mental disease* OR diabet* AND schizo* OR diabet* AND psychosis OR diabet* AND psychoses OR diabet* AND psychotic OR diabet* AND bipolar OR diabet* AND affective disorder* OR diabet* AND personality disorder* OR diabet* AND major depress* OR diabet* AND unipolar depress* OR diabet* AND clinical depress* OR diabet* AND recurrent depress* OR diabet* AND severe depress* |
| ClinicalTrials.gov (Advanced search) |
|
Search Terms: (diabetes OR diabetic) AND (mental OR schizophrenia OR psychosis OR psychoses OR psychotic OR bipolar OR affective OR personality OR major depression OR major depressive OR clinical depression OR unipolar depression OR recurrent depression) Study Type: Interventional Studies Age Group: Adult, Senior |
Appendix 2. Description of interventions
| Intervention | Comparator | |
| McKibbin 2010 | The Diabetes Awareness and Rehabilitation Training (DART) intervention was a group, face‐to‐face, 24‐week self management programme. DART comprised 3 modules: (1) basic diabetes education (sessions 1 to 4, repeated at sessions 13 to 16); (2) nutrition (sessions 5 to 8, repeated at sessions 17 to 20); and (3) lifestyle exercise (sessions 9 to 12, repeated at sessions 21 to 24). Each module contained four 90‐minute manualised sessions. Basic education included an explanation of motivation and a review of blood sugar in symptoms of low and high blood sugar, diabetes complications, how to use a glucose meter, doctor visits and how to talk with your doctor and medication. Nutrition education included a review of food groups, portion sizes, healthy meals and food labels, and replacing sugar with fat and fibre. Lifestyle and exercise education reviewed different types of exercise, how exercise impacts blood sugar, tracking exercise using a pedometer and foot care during exercise Personnel adapted educational materials for people of middle age and older with schizophrenia by introducing 1 or 2 topics per session, providing an overview and summary of the materials, implementing a teach and query training method, using mnemonic aids and print materials with larger font and limiting text. Participants were given simple guidelines about how they might lead a healthier lifestyle, such as switching from regular soda or fruit punch to diet soda or water One diabetes‐trained mental health professional delivered the intervention. These facilitators did not make contact with participants' healthcare providers, and they encouraged participants to speak with their physician about their diabetes and provided guidance on how to record laboratory results and examination findings |
Usual care plus information (UCI) consisted of usual care delivered by participants' providers and 3 brochures provided by the American Diabetes Association relevant to diabetes management (i.e. basic diabetes education, nutrition, exercise) |
Appendix 3. Baseline characteristics (I)
| Intervention and comparator | Duration of intervention (duration of follow‐up) | Description of participants | Trial period (year to year) | Country | Setting | |
| McKibbin 2010 | I: DART | 24 weeks (6 months post intervention) | Participants with type 2 diabetes and schizophrenia/schizoaffective disorder | ‐ | USA | Community |
| C: UCI | ||||||
| "‐" denotes not reported C: comparator; DART: Diabetes Awareness and Rehabilitation Training; UCI: usual care plus information; I: intervention; SD: standard deviation | ||||||
Appendix 4. Baseline characteristics (II)
| Intervention and comparator | Sex [female %] | Age [mean years (SD)] | Ethnicity [%] | Duration of diabetes [mean years (SD)] | Type of severe mental illness [%] | Age of onset of severe mental illness [mean years (SD)] | |
| McKibbin 2010 | I: DART | 38 | 52 (10.1) | White: 45 African American: 31 Hispanic: 17 Asian: 7 Native American: 0 | 8.9 (5.8) | Schizophrenia: 79 Schizoaffective: 21 |
25.7 (12.3) |
| C: UCI | 38 | 54 (8.4) | White: 72 African American: 10 Hispanic: 7 Asian: 3 Native American: 7 | 8.6 (6.5) | Schizophrenia: 90 Schizoaffective: 10 |
29.3 (11.8) | |
| "‐" denotes not reported C: comparator; DART: Diabetes Awareness and Rehabilitation Training; I: intervention; SD: standard deviation; UCI: usual care plus information | |||||||
Appendix 5. Baseline characteristics (III)
| Intervention and comparator | HbA1c [mean % (SD)] | BMI [mean kg/m² (SD)] | Diastolic blood pressure [mean mmHg (SD)] | Systolic blood pressure [mean mmHg (SD)] | Glucose control agents [%] | Antipsychotic medication [%] | Comorbidities [%] | |
| McKibbin 2010 | I: DART | 7.4 (2.9) | 33.6 (6.8) | 83 (10) | 134 (17) | Diet only: 15 Oral agent only: 69 Insulin only: 12 Oral agent and insulin: 4 | Apripiprazole or ziprasidone: 25 Risperidone or quetiapine: 46 Clozapine or olanzapine: 29 | ‐ |
| C: UCI | 6.7 (2.1) | 32.9 (6.2) | 85 (13) | 132 (15) | Diet only: 10 Oral agent only: 72 Insulin only: 3 Oral agent and insulin: 14 | Apripiprazole or ziprasidone: 21 Risperidone or quetiapine: 48 Clozapine or olanzapine: 313 | ‐ | |
| "‐" denotes not reported BMI: body mass index; C: comparator; DART: Diabetes Awareness and Rehabilitation Training; HbA1c: glycosylated haemoglobin A1c; I: intervention; SD: standard deviation; UCI: usual care plus information | ||||||||
Appendix 6. Matrix of trial endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a |
Trial results posted
in trial register [Yes/No] |
Publications specified
in trial register [No/Citation] |
Endpoints quoted in publicationb | |
| McKibbin 2010 | N/T | No | No | Diabetes knowledge, self efficacy (Leutwyler 2010 (see McKibbin 2006) Weight, body mass index, waist circumference, blood pressure, fasting blood glucose, HbA1c, cholesterol, high‐density lipoprotein, low‐density lipoprotein, triglycerides, diabetes knowledge, self efficacy, energy expenditure, activity levels, total kilocalories consumed, total minutes of activity (McKibbin 2006) BMI, weight, waist circumference, HbA1c, diabetes knowledge, energy expenditure (McKibbin 2010) |
|
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers)
bPublication refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary trial) EMA: European Medicines Agency; FDA: Food and Drug Administration (US); N/T: no trial document available | ||||
Appendix 7. Examination of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| McKibbin 2010 | Self care behaviours | N/A | N/A | Total activity, total calories consumed and total minutes of activity were measured immediately following the intervention, but not at 6‐month follow‐up, and were not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored. Total energy expenditure was measured both immediately following the intervention and at 6‐month follow‐up but was not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A |
| Diabetes‐related complications | N/I | N/I | N/I | N/I | |
| Adverse events | N/I | N/I | N/I | N/I | |
| All‐cause mortality | N/I | N/I | N/I | N/I | |
| Self efficacy | N/A | N/A | Self efficacy was measured and analysed immediately following the intervention and was analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored. Self efficacy at 6 months post intervention was not reported | ||
| Progression of severe mental illness | N/A | N/A | Symptoms were measured at baseline and following the intervention by the PANSS and the Hamilton Depression Scale, as indicated in Leutwyler 2010 (see McKibbin 2006), but these results are not reported in McKibbin 2006 nor McKibbin 2010 | N/A | |
| HbA1c | N/A | N/A | HbA1c was measured immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Body mass index | N/A | N/A | BMI was measured and analysed immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Weight | N/A | N/A | Weight was measured and analysed immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Blood pressure | N/A | N/A | Blood pressure was measured and analysed immediately following the intervention, but results are not reported at 6‐month follow‐up and were not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Change in antipsychotic treatment type | The article reports no significant changes in antipsychotic treatment type from baseline to 6‐month follow‐up; however, no data were provided | N/A | N/A | N/A | |
| Change in diabetes treatment type | The article reports no significant changes in antipsychotic treatment type between trial arms over time; however, no data were provided | N/A | N/A | N/A | |
| Socioeconomic effects | N/I | N/I | N/I | N/I | |
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant
(Classification 'A', table 2, Kirkham 2010)
bClear that outcome was measured and analysed; trial report states that outcome was analysed but reports no results
( Classification 'D', table 2, Kirkham 2010)
cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results
(Classification 'E', table 2, Kirkham 2010)
dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results
(Classification 'G', table 2, Kirkham 2010) N/A: not applicable N/I: not investigated | |||||
Appendix 8. Definition of endpoint measurement (I)
| Self care behaviours [IO, SO]a | Diabetes‐related complications | Adverse events | All‐cause mortality | Health‐related quality of life | Diabetes knowledge [SO]a | Self efficacy [SO]a | Progression of severe mental illness [SO]a | |
| McKibbin 2010 | For measure of dietary intake, participants were asked to rank how often they consumed 70 different foods in the past month on the Block Brief 2000 Revision of the Health and Habits and History Questionnaire. Outcome is total calories consumed, lower is positive (SO) For measure of physical activity, participants completed the Yale Physical Activity Scale (YPAS). The YPAS provides 2 indices: total energy expenditure (TEE) and total activity summary index (TASI). Higher scores are positive (SO) Physical activity was also measured by an accelerometer (AM7164) (Computer Science and Applications (CSA), a small, lightweight device that is worn on a belt around the waist. The number of minutes of moderate and vigorous activity (MVA) was derived for each valid day of monitoring (i.e. ≥ 3 days of data, 10 hours per day) and averaged across those days. Higher scores positive (IO) |
N/I | N/I | N/I | N/I | 23‐Item diabetes knowledge test. Higher scores reflect greater knowledge (SO) | 28‐Item Diabetes Empowerment Scale. Higher scores reflect higher confidence (SO) | Depressive symptom severity was measured using the 28‐item Hamilton Depression Rating Scale (HAM‐D). Unable to tell whether higher is positive (SO) Positive and negative mood was measured using the Positive and Negative Syndrome Scale (PANSS). Unable to tell whether higher is positive (SO) |
|
aMethod of endpoint evaluation.
AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self reported outcome measurement N/I: not investigated | ||||||||
Appendix 9. Definition of endpoint measurement (II)
| HbA1c [AO]a | Body mass index [SO]a | Weight [SO]a | Blood pressure [IO]a | Change in medication or intensity of drug treatment | Socioeconomic effects | |
| McKibbin 2010 | A 10‐mL blood sample was collected after a 12‐hour fast and was assayed by the UCSD Clinical Research Center using established protocols. Lower scores are positive (IO) | Calculated from height and weight as kg/m2 measured at awakening in light clothing. Lower scores are positive (IO) | Weight in kg measured at awakening in light clothing. Lower scores are positive (IO) | A single‐seated blood pressure reading was obtained after a 5‐minute rest with a validated automated oscillometric sphygmomanometric device (Omron model HEM‐705‐CP, Omron Healthcare Inc., Vernon Hills, IL, USA). Biceps circumference was measured to select the appropriate size cuff, and participants were seated with the forearm resting on the table. Lower scores are positive (IO) | N/I | N/I |
|
aMethod of endpoint evaluation. AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self reported outcome measurement HbA1c: glycosylated haemoglobin A1c; N/I: not investigated | ||||||
Appendix 10. Survey of trial investigators providing information on included trials
| Date trial author contacted | Date trial author replied | Date trial author was asked for additional information [short summary] | Date trial author provided data [short summary] | |
| McKibbin 2010 | 08/07/15 12/10/15 |
08/07/15 No reply |
|
For Table 1 in 2010 paper, the column on the right should reflect the DART programme participant data. Twenty‐six participants were included in each arm for 6‐month follow‐up |
| DART: Diabetes Awareness and Rehabilitation Training | ||||
Appendix 11. Checklist to aid consistency and reproducibility of GRADE assessments
| Diabetes‐related complications | All‐cause mortality | Adverse events | Health‐related quality of life | Self care behaviours | HbA1c | Socioeconomic effects | ||
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | N/A | N/A | N/A | N/A | Unclear | Unclear | N/A |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | ||||||
| Were participants and personnel blinded (i.e. no potential for performance bias)? | Unclear | Unclear | ||||||
| Was outcome assessment blinded (i.e. no potential for detection bias)? | Unclear | Unclear | ||||||
| Was an objective outcome used? | No (↓) | Yes | ||||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | No (↓) | No (↓) | ||||||
| No other biases reported (i.e. no potential for other bias)? | Yes | Yes | ||||||
| Did trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyb | Point estimates did not vary widely? | Yes | Yes | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 included studies point estimate; some: confidence intervals but not all overlap at least 1 point estimate; no: at least 1 outlier: where the confidence intervals of some studies do not overlap with those of most included studies)? | N/A | N/A | ||||||
| Was the direction of effect consistent? | N/A | N/A | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² = 40% to 60%), high I² > 60%)? | N/A | N/A | ||||||
| Was the test for heterogeneity statistically significant (P value < 0.1)? | N/A | N/A | ||||||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | |||||
| Were the interventions in included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | No (↓) | No (↓) | ||||||
| Was the outcome time frame sufficient? | Sufficient | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | ||||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | N/A | ||||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| Was no industry influence noted in studies included in the review? | Yes | Yes | ||||||
| Was no evidence of funnel plot asymmetry found? | N/A | N/A | ||||||
| Was no discrepancy in findings noted between published and unpublished trials? | Unclear | Unclear | ||||||
|
aQuestions on risk of bias are answered in relation to most of the aggregated evidence in the meta‐analysis rather than to individual trials
bQuestions on inconsistency are based primarily on visual assessment of forest plots and statistical quantification of heterogeneity based on I² cWhen judging the width of the confidence interval, it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials eDepends on the context of the systematic review area (↓): key item for possible downgrading of the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
McKibbin 2010.
| Methods |
Parallel randomised controlled clinical trial Superiority design |
|
| Participants |
Inclusion criteria
Exclusion criteria
Diagnostic criteria: ‐ |
|
| Interventions |
Number of study centres: ‐ Treatment before study: ‐ Intervention: Diabetes Awareness and Rehabilitation Training (DART), a 24‐week group‐based intervention, consisting of weekly 90‐minute sessions. Covers basic education, nutrition and exercise Control: Usual care plus information (UCI) condition consisted of usual care provided by participants' physicians and three brochures provided by the American Diabetes Association relevant to diabetes management (i.e. basic diabetes education, nutrition and exercise) Provider: 1 diabetes‐trained mental health professional |
|
| Outcomes |
Outcomes reported in abstract of publication
Outcomes reported in abstract of publication (McKibbin 2006)
Outcomes reported in abstract of publication (McKibbin 2010)
|
|
| Study details |
Run‐in period: ‐ Trial terminated before regular end: no |
|
| Publication details |
Language of publication: English Funding: Betty Irene Moore Foundation and National Institute of Nursing Research; National Insititute of Mental Health grants and Department of Veterans Affairs (McKibbin 2006); National Institute for Mental Health and National Center for Research Resourses (McKibbin 2010) Publication status: peer‐reviewed journal |
|
| Stated aim for study |
Quote from publication: "To explore the relationship between the symptoms of schizophrenia experienced by older persons diagnosed with schizophrenia and type 2 diabetes mellitus and their response to a health promoting intervention" Quote from publication (McKibbin 2006): "To test the efficacy of a novel, manualised 24‐week lifestyle intervention to reduce obesity in middle‐aged and older persons with schizophrenia and type‐2 DM" Quote from publication (McKibbin 2010): "To test the sustained impact of a 6‐month diabetes management intervention in middle‐aged and older adults with schizophrenia and type 2 diabetes mellitus" |
|
| Notes | Long‐term follow‐up of McKibbin 2006 and Leutwyler 2010 (see McKibbin 2006) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The total sample was composed of 64 subjects from board and care, day treatment programs, and community clubhouses that were randomly assigned to treatment (DART) and control groups (UCI)" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient evidence to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient evidence to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient evidence to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient evidence to permit judgement |
| Selective reporting (reporting bias) | High risk |
Comment: the paper does not report on outcomes related to progression of severe mental illness, change in medications, blood pressure, fasting blood glucose, cholesterol, lipoprotein, triglycerides, self efficacy, total activity, total kilocalories or total minutes of activity, despite evidence indicating that these outcomes were measured Comment Leutwyler 2010 (see McKibbin 2006): this paper reports only on outcomes related to knowledge and self efficacy; several other outcomes were measured CommentMcKibbin 2006: this paper does not report on outcomes related to progression of severe mental illness or change in medications, despite evidence indicating that these outcomes were measured |
| Other bias | Low risk | Comment: nothing detected |
"‐" denotes not reported
BMI: body mass index; DART: Diabetes Awareness and Rehabilitation Training; HbA1c: glycosylated haemoglobin A1c; UCI: usual care plus information
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12614000138684 | Not a randomised controlled trial (RCT) |
| Bogner 2010 | Not a severe mental illness |
| Bogner 2012 | Not a severe mental illness |
| Ell 2009 | Not a severe mental illness |
| Gois 2009 | Not a severe mental illness |
| Green 2015 | Includes type 1 and type 2 diabetes |
| Hjorth 2014 | Includes type 1 and type 2 diabetes |
| Huang 2002 | Not a severe mental illness |
| Huang 2004 | Not a severe mental illness |
| ISRCTN13762819 | Not a diabetes self management intervention |
| Katon 2004 | Not a severe mental illness |
| Katon 2006 | Not a severe mental illness |
| Katon 2008 | Not a severe mental illness |
| Katon 2012 | Not a severe mental illness |
| Lamers 2011 | Not a severe mental illness |
| Lustman 1998a | Not a severe mental illness |
| Lustman 1998b | Not a severe mental illness |
| NCT00253240 | Not a randomised controlled trial (RCT) |
| NCT00468676 | Not a severe mental illness |
| NCT00564070 | Not a severe mental illness |
| NCT00627029 | Not a severe mental illness and not a diabetes self management intervention |
| NCT01098253 | Not a severe mental illness |
| NCT01106885 | Not a severe mental illness |
| NCT01228032 | Not a diabetes self management intervention |
| NCT01890226 | Not a diabetes self management intervention |
| NCT02027259 | Not a severe mental illness |
| NCT02029989 | Not a diabetes self management intervention |
| NCT02053714 | Not a randomised controlled trial (RCT) |
| NCT02160639 | Not a severe mental illness |
| Nelson 2014 | Not a severe mental illness |
| Petrak 2013 | Not a severe mental illness |
| Pibernick‐Okanovic 2009 | Not a severe mental illness |
| Piette 2011a | Not a severe mental illness |
| Piette 2011b | Not a severe mental illness |
| Robinson 2010 | Not a randomised controlled trial (RCT) |
| Safren 2014 | Not a severe mental illness |
| Sajatovic 2011 | Not a randomised controlled trial (RCT) |
| Salisbury 2014 | Not a diabetes self management intervention |
| Schneider 2011 | Not a severe mental illness |
| Simon 2007 | Not a severe mental illness |
| Spencer 2013 | Not a severe mental illness |
| Stiefel 2008 | Not a severe mental illness |
| Taveira 2011 | Includes type 1 and type 2 diabetes |
| van Bastelaar 2009 | Not a severe mental illness |
| van Bastelaar 2011a | Not a severe mental illness |
| van Bastelaar 2011b | Not a severe mental illness |
| van Bastelaar 2012 | Not a severe mental illness |
| van Dijk 2013 | Not a severe mental illness |
Characteristics of ongoing studies [ordered by study ID]
Dwinger 2013.
| Trial name or title | Acronym: Intervention Trial to Decrease Cardiovascular Risk in Persons With Serious Mental Illness (IDEAL) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: unblinded Primary purpose: interventional |
| Participants |
Condition: those with one or more diagnoses of the following: diabetes, coronary artery disease, asthma, hypertension, heart failure, chronic obstructive pulmonary disease (COPD), chronic depression or schizophrenia Enrolment: 1670 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): telephone‐based health coaching Comparator(s): no coaching (treatment as usual) |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Other outcome(s)
|
| Starting date |
Trial start date: 2011 Trial completion date: unknown |
| Contact information | Responsible party/principal investigator: Prof. Martin Härter; m.haerter@uke.de |
| Study identifier | German Clinical Trials Register (Deutsches Register Klinischer Studien, DRKS):DRKS00000584 |
| Official title | Telephone‐Based Health Coaching for Chronically Ill Patients: Study Protocol for a Randomised Controlled Trial |
| Stated purpose of study | Quote: "Aim of this study is to evaluate telephone‐based health coaching for chronically ill patients in Germany" |
| Notes | ‐ |
NCT00525304.
| Trial name or title | A Self‐Management Program for Adults With Both Schizophrenia and a Co‐occurring Medical Condition |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: supportive care |
| Participants |
Condition: schizophrenia Enrollment: 100 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): behavioural: self management programme for chronic illness. Self management programme for chronic illness will include between 10 and 16 psychoeducational and supportive group sessions Comparator(s): not reported |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
|
| Starting date |
Trial start date: September 2007 Trial completion date: May 2015 |
| Contact information | Responsible party/principal investigator: Richard W. Goldberg, PhD; 410‐706‐8473; rgoldber@psych.umaryland.edu |
| Study identifier | NCT number: NCT00525304 |
| Official title | Optimizing Chronic Illness Self‐Management for Individuals With Schizophrenia |
| Stated purpose of study | Quote: "This study will develop and evaluate the effectiveness of a self‐management program for adults living with both schizophrenia and a co‐occurring medical condition" |
| Notes | Acccording to ClinicalTrials.gov the information of this record has not been verified recently. Last accessed: 14.04.2016. |
NCT01410357.
| Trial name or title | Improving Outcomes for Individuals With Serious Mental Illness and Diabetes (TTIM) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: supportive care |
| Participants |
Conditions
Enrolment: 212 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): targeted training in illness management (TTIM): This intervention blends psychoeducation, problem identification/goal setting, behavioural modelling and reinforcement via use of peer educators and health care linkage; it has been adapted to the primary care setting and targeted for SMI‐DM participants. Generalisability is enhanced by relatively brief in‐person participation requirements, and by inclusion of professional staff typically found in primary care. TTIM will stress information sharing that is accessible to participants and, through a collaborative process, will foster motivation for severe mental illness diabetes self management Comparator(s): treatment as usual (TAU): Participants in this arm will continue to receive treatment as usual from their usual medical and mental health care providers. They will not receive any intervention |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Other Outcome Measure(s)
|
| Starting date |
Trial start date: July 2011 Trial completion date: July 2015 |
| Contact information | Responsible party/principal investigator: Martha Sajatovic, MD; Case Western Reserve University |
| Study identifier | NCT number: NCT01410357 |
| Official title | Improving Outcomes for Individuals With Serious Mental Illness and Diabetes |
| Stated purpose of study | Quote: "This project tests a model for improving illness self‐management among persons who have both serious mental illness and diabetes and will be performed within a primary care setting at a safety net hospital system" |
| Notes | Study completed. No study results nor publications available. Last accessed: 14.04.2016. |
NCT01725815.
| Trial name or title | Acronym: Health Access and Recovery Peer Program (HARP) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Conditions
Enrolment: 400 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): behavioural: HARP intervention The HARP intervention is a 6‐week, 6‐session, group format intervention designed to improve self management of chronic medical diseases. Each group lasts 90 minutes and includes 8 to 12 attendees. Between groups, participants work with partners from the group to troubleshoot problems and accomplish action plans identified during the session. At the end of the programme, monthly alumni groups meet for 6 months to reinforce lessons from the intervention, to monitor progress and to maintain peer support Comparator(s): no intervention control |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
|
| Starting date |
Trial start date: June 2011 Trial completion date: April 2016 |
| Contact information | Responsible party/principal investigator: Benjamin Druss, MD, MPH; Emory University |
| Study identifier | NCT number: NCT01725815 |
| Official title | A Peer‐Led, Medical Disease Self‐Management Program for Mental Health Consumers |
| Stated purpose of study | Quote: "establish the first fully peer‐led, evidence‐based intervention for improving physical self‐management in this vulnerable population" |
| Notes | ‐ |
NCT01828931.
| Trial name or title | Lifestyle Intervention for Diabetes and Weight Management in Psychosis (Healthy LIFE) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Conditions
Enrolment: 120 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): lifestyle intervention ‐ a lifestyle intervention based on the Look AHEAD trial intervention, involving counselling related to dietary and physical activity habits Comparator(s): usual care ‐ standard care provided via participants' family physicians, diabetes nurses and psychiatrists |
| Outcomes |
Primary outcome(s)
|
| Starting date |
Trial start date: December 2012 Trial completion date: December 2015 |
| Contact information | Responsible party/principal investigator: Margaret K Hahn, MD; Centre for Addiction and Mental Health |
| Study identifier | NCT number: NCT01828931 |
| Official title | Effectiveness of Intensive Lifestyle Interventions in the Management of Diabetes in Individuals With Psychosis |
| Stated purpose of study | Quote: "We propose a 3‐year randomised controlled trial examining the effectiveness of a lifestyle intervention (LI) aimed at reducing caloric intake and increasing physical activity in overweight or obese individuals (N=150) suffering from both a psychotic illness and T2DM" |
| Notes | ‐ |
NCT02011529.
| Trial name or title | Acronym: TEAMcare for Diabetes in Mental Health Centers |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: 40 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): TEAMcare is an evidence‐based collaborative care approach to the treatment of diabetes and psychiatric illness. It involves structured visits with a trial nurse for monitoring of psychiatric symptoms, control of medical disease and performance of self care activities. Nurses use motivational coaching to help participants solve problems and set goals for improved self care and medication adherence. Medications for diabetes, hypertension and hyperlipidaemia are monitored and therapy intensified on the basis of treat‐to‐target guidelines. All of these processes and outcome measures are tracked in a registry designed for the trial, and nurses receive weekly supervision by a psychiatrist, an endocrinologist and a psychologist to review new cases and to track progress. Once a participant achieves targeted levels for relevant measures, the participant and the nurse develop a maintenance plan Comparator(s): treatment as usual: Participants randomised to treatment as usual will receive their usual mental health treatment and primary care treatment |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Other outcome(s)
|
| Starting date |
Trial start date: November 2013 Trial completion date: September 2015 |
| Contact information | Responsible party/principal investigator: Lydia Chwastiak, Associate Professor, University of Washington |
| Study identifier | NCT number: NCT02011529 |
| Official title | A Team Approach to Improve the Quality of Diabetes Care for Patients With Schizophrenia |
| Stated purpose of study | Quote: "To demonstrate the feasibility and acceptability of adapting TEAMcare for patients with schizophrenia. The aim of this innovative mental health center‐based team intervention is to improve diabetes, cardiovascular and psychiatric outcomes among patients with poorly controlled type 2 diabetes" |
| Notes | Study completed. No study results nor publications available. Last accessed: 14.04.2016. |
NCT02127671.
| Trial name or title | Acronym: Intervention Trial to Decrease Cardiovascular Risk in Persons With Serious Mental Illness (IDEAL) |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: prevention |
| Participants |
Condition: 1 of the following CVD risk factors: hypertension, diabetes mellitus or dyslipidaemia Enrollment: 250 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): individual cardiovascular risk reduction counselling, co‐ordination with primary care providers to ensure appropriate management of risk factors, collaboration with mental health staff and social supports. All participants will be offered group exercise classes, and programmes will be provided with instruction to provide more healthy meals Comparator(s): control ‐ All participants will be offered group exercise classes, and programmes will be provided with instruction to provide more healthy meals |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Other outcome(s)
|
| Starting date |
Trial start date: December 2013 Trial completion date: January 2018 |
| Contact information | Responsible party/principal investigator: Gail L. Daumit, MD, MHS; Johns Hopkins University |
| Study identifier | NCT number: NCT02127671 |
| Official title | Comprehensive CVD Risk Reduction Trial in Persons With Serious Mental Illness |
| Stated purpose of study | Quote: "This study will determine whether a program where a health coach works with participants on heart healthy behaviours and treatment of risk factors is coordinated with primary care can reduce overall heart disease risk in people with serious mental illness" |
| Notes | ‐ |
NCT02188732.
| Trial name or title | Self‐Management Training and Automated Telehealth to Improve SMI Health Outcomes |
| Methods |
Type of trial: interventional Allocation: randomised Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: supportive care |
| Participants |
Conditions
Enrollment: 300 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s)
Comparator(s): Community‐Based Health Home (CBHH): Each team has a staff‐to‐participant ratio of approximately 1:12, and each team serves approximately 120 participants with severe mental illness by using person‐centred planning and recovery‐oriented, flexible service models. Each team provides mobile outreach and includes a team leader; a peer counsellor; a psychiatric nurse co‐ordinator; a clinical care co‐ordinator; specialists in substance abuse (dual diagnosis), community integration, rehabilitation, employment and housing; and a medical nurse practitioner (MNP) and a health outreach worker (HOW) |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
Other outcome(s)
|
| Starting date |
Trial start date: September 2014 Trial completion date: August 2019 |
| Contact information | Responsible party/principal investigator: Stephen J. Bartels, MD, MS; sbartels@dartmouth,edu; or Maghan Santos, MSW; maghan.m.santos@dartmouth.edu |
| Study identifier | NCT number: NCT02188732 |
| Official title | Self‐Management Training and Automated Telehealth to Improve SMI Health Outcomes |
| Stated purpose of study | Quote: "To evaluate outcomes for n=100 in a Community Based Health Home alone (CBHH), compared to n=100 also receiving Self‐Management Training (CBHH+SMT), and n=100 also receiving Automated Telehealth (CBHH+AT)" |
| Notes | ‐ |
NCT02318797.
| Trial name or title | Optimizing Behavioral Health Homes for Adults With Serious Mental Illness (PCORI OH) |
| Methods |
Type of trial: interventional Allocation:randomised Intervention model: parallel assignment Masking: single‐blind (investigator) Primary purpose: Health Services Research |
| Participants |
Conditions
Enrollment: 1229 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): patient self directed care, patient self management toolkits, web portal with information on health conditions, personal health care use data, health tracking tools, wellness programmes Comparator(s): provider‐supported integrated care registered nurse on staff at community mental health centres with access to patient‐level physical health information. to work with participants on co‐ordinating their care, to enhance communication between providers and payer and to provide patient wellness support and education |
| Outcomes |
Primary outcome(s)
Secondary outcome(s)
|
| Starting date |
Trial start date: October 2013 Trial completion date: January 2017 |
| Contact information | Responsible party/principal investigator: Charles F. Reynolds, MD; University of Pittsburgh; UPMC Center for High‐Value Health Care |
| Study identifier | NCT number: NCT02318797 |
| Official title | Optimizing Behavioral Health Homes by Focusing on Outcomes That Matter Most for Adults With Serious Mental Illness |
| Stated purpose of study | Quote: "test two promising ways for promoting the health, wellness, and recovery of adults with SMI" |
| Notes | ‐ |
"‐" denotes not reported
Differences between protocol and review
The protocol specified that review authors would search the Allied and Complementary Medicine Database (AMED) for articles; however, on advice from the Trials Search Co‐ordinator in the Cochrane Metabolic and Endocrine Disorders Group, we removed this database from the search strategy.
Contributions of authors
Hayley McBain (HM): protocol draft, search strategy development, acquisition of trial reports, trial selection, data extraction, data analysis, data interpretation, review of drafts and future review updates.
Kathleen Mulligan (KM): protocol draft, search strategy development, data extraction, data analysis, data interpretation, review of draft and future review updates.
Mark Haddad (MH): protocol draft, trial selection, data analysis, data interpretation, review of draft and future review updates.
Chris Flood (CF): protocol draft, trial selection, data analysis, data interpretation, review of draft and future review updates.
Julia Jones (JJ): protocol draft, data analysis, data interpretation, review of draft and future review updates.
Alan Simpson (AS): protocol draft, data extraction, data analysis, data interpretation, review of draft and future review updates
Declarations of interest
HM: none known.
KM: none known.
MH: none known.
CF: none known.
JJ: none known.
AS: none known.
New
References
References to studies included in this review
McKibbin 2010 {published and unpublished data}
- Leutwyler HC, Wallhagen M, McKibbin C. The impact of symptomatology on response to a health promoting intervention among older adults with schizophrenia. The Diabetes Educator 2010;36(6):945‐55. [DOI] [PubMed] [Google Scholar]
- McKibbin CL, Golshan S, Griver K, Kitchen K, Wykes TL. A healthy lifestyle intervention for middle‐aged and older schizophrenia patients with diabetes mellitus: a 6 month follow‐up analysis. Schizophrenia Research 2010;121:203‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, Mudaliar S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophrenia Research 2006;86:36‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12614000138684 {published data only}
- ACTRN12614000138684. Comparison of the health outcomes of patients with severe mental illness and at least one co‐morbid chronic physical health problem who attend a physical health clinic at Fremantle Mental Health Services with a similar group of patients who are managed by a general practitioner in the community. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365704 (accessed 14 April 2016).
Bogner 2010 {published data only}
- Bogner HR, Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African‐Americans: a randomized controlled pilot trial. The Diabetes Educator 2010;36(2):284‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bogner 2012 {published data only}
- Bogner HR, Morales KH, Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Annals of Family Medicine 2012;10:15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ell 2009 {published data only}
- Ell K, Katon W, Cabassa LJ, Xie B, Lee PJ, Kapetanovic S, et al. Depression and diabetes among low‐income Hispanics: design elements of a socioculturally adapted collaborative care model randomized controlled trial. International Journal of Psychiatry in Medicine 2009;39(2):113‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gois 2009 {published data only}
- Gois C. IPT in physical diseases. The diabetes mellitus case. European Psychiatry 2009;24(1):S278. [Google Scholar]
Green 2015 {published data only}
- Green CA, Yarborough BJH, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. American Journal of Psychiatry 2015;172(1):71‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjorth 2014 {published data only}
- Hjorth P, Davidsen AS, Kilian R, Pilgaard Erikson S, Jensen AOW, Sørensen HØ, et al. Improving the physical health of long‐term psychiatric inpatients. Australian & New Zealand Journal of Psychiatry 2014;48(9):861‐70. [DOI] [PubMed] [Google Scholar]
Huang 2002 {published data only}
- Huan X, Lei S, Li T, Li J, Li N, Wu S. Effect of health education and psychosocial intervention on depression in patients with type 2 diabetes. Chinese Mental Health Journal 2002;16(3):149‐51. [Google Scholar]
Huang 2004 {published data only}
- Huang FL, Ye JH, Huang F. Effect of mental intervention on type 2 diabetes: randomized controlled trial. Chinese Journal of Clinical Rehabilitation 2004;8(27):5795‐8. [Google Scholar]
ISRCTN13762819 {published data only}
- ISRCTN13762819. Managing cardiovascular risk for people with severe mental illnesses. A clinical trial in primary care (PRIMROSE). http://www.isrctn.com/ISRCTN13762819 (accessed 14 April 2016).
Katon 2004 {published data only}
- Katon WJ, Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Archives of General Psychiatry 2004;61(10):1042‐9. [DOI] [PubMed] [Google Scholar]
Katon 2006 {published data only}
- Katon, W, Unũtzer J, Fan MY, Willians JW Jr, Schoenbaum M, Lin EH, et al. Cost‐effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care 2006;29(2):265‐70. [DOI] [PubMed] [Google Scholar]
Katon 2008 {published data only}
- Katon WJ, Russo JE, Korff M, Lin EH, Ludman E, Ciechanowski PS. Long‐term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care 2008;31(6):1155‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katon 2012 {published data only}
- Katon W, Russo J, Lin EHB, Schmittdiel J, Ciechanowski P, Ludman E, et al. Cost‐effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Archives of General Psychiatry 2012;69(5):506‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamers 2011 {published data only}
- Lamers F, Jonkers CCM, Bosma H, Knottnerus JA, Eijk JTM. Treating depression in diabetes patients: does a nurse‐administered minimal psychological intervention affect diabetes‐specific quality of life and glycaemic control? A randomized controlled trial. Journal of Advanced Nursing 2011;67(4):788‐99. [DOI] [PubMed] [Google Scholar]
Lustman 1998a {published data only}
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Predicting response to cognitive behavior therapy of depression in type 2 diabetes. General Hospital Psychiatry 1998;20:302‐6. [DOI] [PubMed] [Google Scholar]
Lustman 1998b {published data only}
- Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RA. Cognitive behavior therapy for depression in type 2 diabetes mellitus: a randomized, controlled trial. Annals of Internal Medicine 1998;129:613‐21. [DOI] [PubMed] [Google Scholar]
NCT00253240 {published data only}
- NCT00253240. Diabetes screening, risk management and disease management in a high‐risk mental health population. https://clinicaltrials.gov/ct2/show/NCT00253240 (accessed 14 April 2016).
NCT00468676 {published data only}
- NCT00468676. Nurse‐led case management for diabetes and cardiovascular disease patients with depression. https://clinicaltrials.gov/ct2/show/NCT00468676 (accessed 14 April 2016).
NCT00564070 {published data only}
- NCT00564070. CBT for adherence and depression in diabetes. https://clinicaltrials.gov/ct2/show/NCT00564070 (accessed 14 April 2016).
NCT00627029 {published data only}
- NCT00627029. Evaluation of programs of coordinated care and disease management. https://clinicaltrials.gov/ct2/show/NCT00627029 (accessed 14 April 2016).
NCT01098253 {published data only}
- NCT01098253. Integrating depression services into type 2 diabetes mellitus management. https://clinicaltrials.gov/ct2/show/NCT01098253 (accessed 14 April 2016).
NCT01106885 {published data only}
- NCT01106885. Effective care management of depressed diabetes patients (the Positive Steps study). https://clinicaltrials.gov/ct2/show/NCT01106885 (accessed 14 April 2016).
NCT01228032 {published data only}
- NCT01228032. The Health Outcomes Management and Evaluation (HOME) study. https://clinicaltrials.gov/ct2/show/NCT01228032 (accessed 14 April 2016).
NCT01890226 {published data only}
- NCT01890226. A mobile personal health record for behavioral health homes (mPHR). https://clinicaltrials.gov/ct2/show/NCT01890226 (accessed 14 April 2016).
NCT02027259 {published data only}
- NCT02027259. Behavioral activation therapy for both depression and diabetes vs. diabetes alone delivered via group visits (BA‐MEDIC). https://www.clinicaltrials.gov/ct2/show/NCT02027259 (accessed 14 April 2016).
NCT02029989 {published data only}
- NCT02029989. Point‐of‐care testing (POCT) detection and management of metabolic syndrome in patients with mental illness. https://clinicaltrials.gov/ct2/show/NCT02029989 (accessed 14 April 2016).
NCT02053714 {published data only}
- NCT02053714. Improving diabetes outcomes for persons with severe mental illness. https://clinicaltrials.gov/ct2/show/study/NCT02053714 (accessed 14 April 2016).
NCT02160639 {published data only}
- NCT02160639. Evaluating the impact of year long, augmented diabetes self management support. https://clinicaltrials.gov/ct2/show/NCT02160639 (accessed 14 April 2016). [DOI] [PMC free article] [PubMed]
Nelson 2014 {published data only}
- Nelson K, Drain N, Robinson J, Kapp J, Hebert P, Taylor L, et al. Peer Support for Achieving Independence in Diabetes (Peer‐AID): design, methods and baseline characteristics of a randomized controlled trial of community health worker assisted diabetes self‐management support. Contempory Clinical Trials 2014;38:361‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrak 2013 {published data only}
- Petrak F, Herpertz S, Albus C, Hermanns N, Hiemke C, Hiller W, et al. Study protocol of the Diabetes and Depression Study (DAD): a multi‐center randomized controlled trial to compare the efficacy of a diabetes‐specific cognitive behavioral group therapy versus sertraline in patients with major depression and poorly controlled diabetes mellitus. BMC Psychiatry 2013;13:206‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pibernick‐Okanovic 2009 {published data only}
- Pibernik‐Okanovic M, Begic D, Ajdukovic D, Andrijasevic N, Meteljo Z. Psychoeducation versus treatment as usual in diabetic patients with subthreshold depression: preliminary results of a randomized controlled trial. Trials 2009;10:78‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piette 2011a {published data only}
- Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M, et al. A randomized trial of telephone counseling plus walking for depressed diabetes patients. Medical Care 2011;49(7):641‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piette 2011b {published data only}
- Piette JD, Valenstein M, Himle J, Duffy S, Torres T, Vogel M, et al. Clinical complexity and the effectiveness of an intervention for depressed diabetes patients. Chronic Ilness 2011;7(4):267‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2010 {published data only}
- Robinson SJ, Conrad G, Gibson M, Morris C, DesLauriers C, Charlebois H, et al. Multimodal healthy lifestyle intervention for prevention and or mitigation of weight gain, diabetes and dyslipidemia in a first episode psychosis population. Early Intevention in Psychosis 2010;4(Suppl 1):73. [Google Scholar]
Safren 2014 {published data only}
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBTAD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014;37:625‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sajatovic 2011 {published data only}
- Sajatovic M, Dawson NV, Perzynski AT, Blixen CE, Bialko CS, McKibbin CL, et al. Best practices: optimizing care for people with serious mental illness and comorbid diabetes. Psychiatric Services 2011;62(9):1001‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salisbury 2014 {published data only}
- Salisbury C. The 3D study: improving care for multimorbidity patients. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=16067 (accessed 14 April 2016).
Schneider 2011 {published data only}
- Schneider KL, Pagoto SJ, Handschin B, Panza E, Bakke S, Liu Q, et al. Design and methods for a pilot randomized clinical trial involving exercise and behavioral activation to treat comorbid type 2 diabetes and major depressive disorder. Mental Health and Physical Activity 2011;4(14):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2007 {published data only}
- Simon GE, Katon WJ, Lin EH, Rutter C, Manning WG, Korff M, et al. Cost‐effectiveness of systematic depression treatment among people with diabetes mellitus. Archives of General Psychiatry 2007;64(1):65‐72. [DOI] [PubMed] [Google Scholar]
Spencer 2013 {published data only}
- Spencer MS, Hawkins J, Espitia NR, Sinco B, Jennings T, Lewis C, et al. Influence of a community health worker intervention on mental health outcomes among low‐income Latino and African American adults with type 2 diabetes. Race and Social Problems 2013;5:137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiefel 2008 {published data only}
- Stiefel F, Zdrojewski C, Bel Hadj F, Boffa D, Dorogi Y, So A, et al. Effects of a multifaceted psychiatric intervention targeted for the complex medically ill: a randomized controlled trial. Psychotherapy and Psychosomatics 2008;77:247‐56. [DOI] [PubMed] [Google Scholar]
Taveira 2011 {published data only}
- Taveira TH, Dooley AG, Cohen LB, Khatana SAM, Wu WC. Pharmacist‐led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. The Annals of Pharmacotherapy 2011;45:1346‐55. [DOI] [PubMed] [Google Scholar]
van Bastelaar 2009 {published data only}
- Bastelaar KMP, Pouwer F, Cuijpers P, Snoek FJ. Web‐based cognitive behavioural therapy for diabetes patients with comorbid depression: first findings. Diabetologia 2009;52(Suppl 1):S393. [Google Scholar]
van Bastelaar 2011a {published data only}
- Bastelaar K, Cuijpers P, Pouwer F, Riper H, Snoek FJ. Development and reach of a web‐based cognitive behavioural therapy programme to reduce symptoms of depression and diabetes‐specific distress. Patient Education and Counselling 2011;84:49‐55. [DOI] [PubMed] [Google Scholar]
van Bastelaar 2011b {published data only}
- Bastelaar KMP, Pouwer F, Cuijpers P, Riper H, Snoek FJ. Web‐based depression treatment for type 1 and type 2 diabetic patients. Diabetes Care 2011;34(2):320‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Bastelaar 2012 {published data only}
- Bastelaar KMP, Pouwer F, Cuijpers P, Riper H, Twisk JWR, Snoek FJ. Is a severe clinical profile an effect modifier in a web‐based depression treatment for adults with type 1 or type 2 diabetes? Secondary analyses from a randomized controlled trial. Journal of Medical Internet Research 2012;14(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dijk 2013 {published data only}
- Dijk SEM, Pols AD, Adriaanse MC, Bosmans JE, Elders PJM, Marwijk HWJ, et al. Cost‐effectiveness of a stepped‐care intervention to prevent major depression in patients with type 2 diabetes mellitus and/or coronary heart disease and subthreshold depression: design of a cluster‐randomized controlled trial. BMC Psychiatry 2013;13:128‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Dwinger 2013 {published data only}
- DRKS00000584. Evaluation of a telephonebased health coaching in chronic diseases. http://www.drks.de/DRKS00000584 (accessed 14 April 2016).
- Dwinger S, Dirmaier J, Herbarth L, König H, Eckardt M, Kriston L, et al. Telephone‐based health coaching for chronically ill patients: study protocol for a randomized controlled trial. Trials 2013;14:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00525304 {published data only}
- NCT00525304. A self‐management program for adults with both schizophrenia and a co‐occurring medical condition. https://www.clinicaltrials.gov/ct2/show/NCT00525304 (accessed 14 April 2016).
NCT01410357 {published data only}
- NCT01410357. Improving outcomes for individuals with serious mental illness and diabetes (TTIM). https://clinicaltrials.gov/ct2/show/NCT01410357 (accessed 14 April 2016).
NCT01725815 {published data only}
- NCT01725815. The Health Access and Recovery Peer program (HARP). https://clinicaltrials.gov/ct2/show/NCT01725815 (accessed 14 April 2016).
NCT01828931 {published data only}
- NCT01828931. Lifestyle intervention for diabetes and weight management in psychosis (Healthy LIFE). https://clinicaltrials.gov/ct2/show/NCT01828931 (accessed 14 April 2016).
NCT02011529 {published data only}
- NCT02011529. TEAMcare for diabetes in mental health centers. https://clinicaltrials.gov/ct2/show/NCT02011529 (accessed 14 April 2016).
NCT02127671 {published data only}
- NCT02127671. Intervention trial to decrease cardiovascular risk in persons with serious mental illness (IDEAL). https://clinicaltrials.gov/ct2/show/NCT02127671 (accessed 14 April 2016).
NCT02188732 {published data only}
- NCT02188732. Self‐management training and automated telehealth to improve SMI health outcomes. https://clinicaltrials.gov/ct2/show/NCT02188732 (accessed 14 April 2016).
NCT02318797 {published data only}
- NCT02318797. Optimizing behavioral health homes for adults with serious mental illness (PCORI OH). https://clinicaltrials.gov/ct2/show/NCT02318797 (accessed 14 April 2016).
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22:S5‐19. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31:S12‐54. [DOI] [PubMed] [Google Scholar]
Ajzen 1991
- Ajzen I. The theory of planned behavior. Organisational Behavior and Human Decision Processes 1991;50:179‐211. [Google Scholar]
Anderson 2000
- Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self‐efficacy. Diabetes Care 2000;23:739‐43. [DOI] [PubMed] [Google Scholar]
Bandura 1986
- Bandura A. Social. Social Foundations of Thought and Action: A Social Cognitive Theory. Upper Saddle River, NJ: Prentice‐Hall, 1986. [Google Scholar]
Barlow 2002
- Barlow JH. How to use education as an intervention in osteoarthritis. Balliere's Best Practice & Research. Clinical Rheumatology 2001;15(4):545‐8. [DOI] [PubMed] [Google Scholar]
Block 1990
- Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1:58‐64. [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, for the CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148:295‐309. [DOI] [PubMed] [Google Scholar]
Buhagiar 2011
- Buhagiar K, Parsonage L, Osborn DP. Physical health behaviours and health locus of control in people with schizophrenia‐spectrum disorder and bipolar disorder: a cross‐sectional comparative study with people with non‐psychotic mental illness. BMC Psychiatry 2011;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Busner 2007
- Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007;4(7):28‐37. [PMC free article] [PubMed] [Google Scholar]
Cimo 2012
- Cimo A, Stergiopoulos E, Cheung C, Bonato S, Dewa CS. Effective lifestyle interventions to improve type II diabetes self‐management for those with schizophrenia or schizo‐affective disorder. BMC Psychiatry 2012;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintrye S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Hert 2011
- Hert M, Correll CU, Bobes J, Cetkovich‐Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10(1):52‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deakin 2005
- Deakin TA, McShane CE, Cade JE, Williams R. Group based training for self‐management strategies in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003417.pub2] [DOI] [PubMed] [Google Scholar]
Dipietro 1993
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Medicine and Science in Sport and Exercise 1993;25(5):628‐42. [PubMed] [Google Scholar]
Duke 2009
- Duke SAS, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005268.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eady 2008
- Eady AM, Wilczynski NL, Haynes RB. PsycINFO search strategies identified methodologically sound therapy studies and review articles for use by clinicians and researchers. Journal of clinical epidemiology 2008;61(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 1998
- Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care 1998;21:706‐10. [DOI] [PubMed] [Google Scholar]
Goldberg 2007b
- Goldberg RW, Kreyenbuhl LA, Medoff DR, Dickerson FB, Wohlheiter K, Fang LJ, et al. Quality of diabetes care among adults with serious mental illness. Psychiatric Services 2007;58(4):536‐43. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical Methods for Meta‐analysis. New York: Academic Press, 1985. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172:137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2014
- Hoffman TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185:E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
International Diabetes Federation 2015
- International Diabetes Federation. Diabetes: A global emergency. IDF Diabetes Atlas, 7th edition. Brussels, Belgium: International Diabetes Federation, 2015. [Google Scholar]
Kay 1987
- Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Lawrence 2009
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health 2009;9:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leventhal 1984
- Leventhal H, Nerenz DR, Steele DF. Illness representations and coping with health threats. In: Baum A, Singer J editor(s). A Handbook of Psychology and Health. Hillsdales, NJ: Erlbaum, 1984:219‐52. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattis 1973
- Mattis S. Dementia Rating Scale. Psychological Assessment Resources, Inc., Odessa, FL, 1973.
McBain 2014
- McBain H, Mullihan K, Haddad M, Flood C, Jones J, Simpson A. Self‐management interventions for type 2 diabetes in adult people with severe mental illness (Protocol). Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011361] [DOI] [PMC free article] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE, Raczek AE. The MOS 36‐item short‐form health survey: II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31:247‐63. [DOI] [PubMed] [Google Scholar]
McKibbin 2006
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophrenia Research 2006;86:36‐44. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meadows 2000
- Meadows KA, Abrams C, Sandbaek A. Adaptation of the Diabetes HealthProfile (DHP‐1) for use with patients with type 2 diabetes mellitus: psychometric evaluation and cross‐cultural comparison. Diabetic Medicine 2000;17:572‐80. [DOI] [PubMed] [Google Scholar]
Michie 2010
- Michie S, Prestwich A. Are interventions theory based? Development of a theory coding scheme. Health Psychology 2010;29:1‐8. [DOI] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Johnston M, Abraham C, Francis J, Eccles MP. The behaviour change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behaviour change interventions. Annals of Behavioural Medicine 2013;46:81‐95. [DOI] [PubMed] [Google Scholar]
NHS Diabetes 2011
- NHS Diabetes. Commissioning Mental Health and Diabetes Services. London: NHS Diabetes, 2011. [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Type 2 Diabetes in Adults: Management. London: NICE, 2015. [PubMed] [Google Scholar]
Osborn 2008
- Osborn D, Wright C, Levy G, King M, Deo R, Nazareth I. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and meta analysis. BMC Psychiatry 2008;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2013
- Pal K, Eastwood SV, Michie S, Farmer AJ, Barnard ML, Peacock R, et al. Computer‐based diabetes self‐management interventions for adults with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD008776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peel 2004
- Peel E, Parry O, Douglas M, Lawton J. Blood glucose self‐monitoring in non‐insulin‐treated type 2 diabetes: a qualitative study of patients' perspectives. British Journal of General Practice 2004;54:183‐8. [PMC free article] [PubMed] [Google Scholar]
Prochaska 1997
- Prochaska JO, Velicer WF. The transtheoretical model of health behaviour change. American Journal of Health Promotion 1997;12:38‐48. [DOI] [PubMed] [Google Scholar]
Rao 1992
- Rao JNK, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48:577‐85. [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Royal College of Psychiatrists 2009
- Royal College of Psychiatrists. OP67 Physical Health in Mental Health: Final Report of a Scoping Group. London: Royal College of Psychiatrists, 2009. [Google Scholar]
Steed 2003
- Steed L, Cooke D, Newman S. A systematic review of psychosocial outcomes following education, self‐management and psychological interventions in diabetes mellitus. Patient Education and Counseling 2003;51:5‐15. [DOI] [PubMed] [Google Scholar]
Steinsbekk 2012
- Steinsbekk A, Rygg L, Lisulo M, Rise MB, Fretheim A. Group based diabetes self‐management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review and meta‐analysis. BMC Health Services Research 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
The Emerging Risk Factors Collaboration 2010
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010;375:2215‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Schizophrenia Commission 2012
- The Schizophrenia Commission. The Abandoned Illness: a Report by the Schizophrenia Commission. London: Rethink Mental Illness, 2012. [Google Scholar]
Thorpe 2013
- Thorpe CT, Fahey LE, Johnson H, Deshpande M, Thorpe JM, Fisher EB. Facilitating healthy coping in patients with diabetes: a systematic review. Diabetes Educator 2013;39:33‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toobert 2000
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self‐care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943‐50. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHO 2013a
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013‐2020. Geneva, Switzerland: WHO Press, 2013.
WHO 2013b
- World Health Organization. Mental Health Action Plan 2013‐2020. Geneva, Switzerland: WHO Press, 2013.
Wild 2004
- Wild S, Roglie G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047‐53. [DOI] [PubMed] [Google Scholar]
Wing 1998
- Wing KJ, Beevor AS, Curtis RH, Park SB, Hadden S, Burns A. Health of the Nation Outcome Scale (HoNOS). Research and development. British Journal of Psychiatry 1998;172:11‐8. [DOI] [PubMed] [Google Scholar]
Wong 2006a
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wong 2006b
- Wong SSL, Wilczynski N, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. Journal of Nursing Scholarship 2006;38(2):194‐9. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


