Abstract
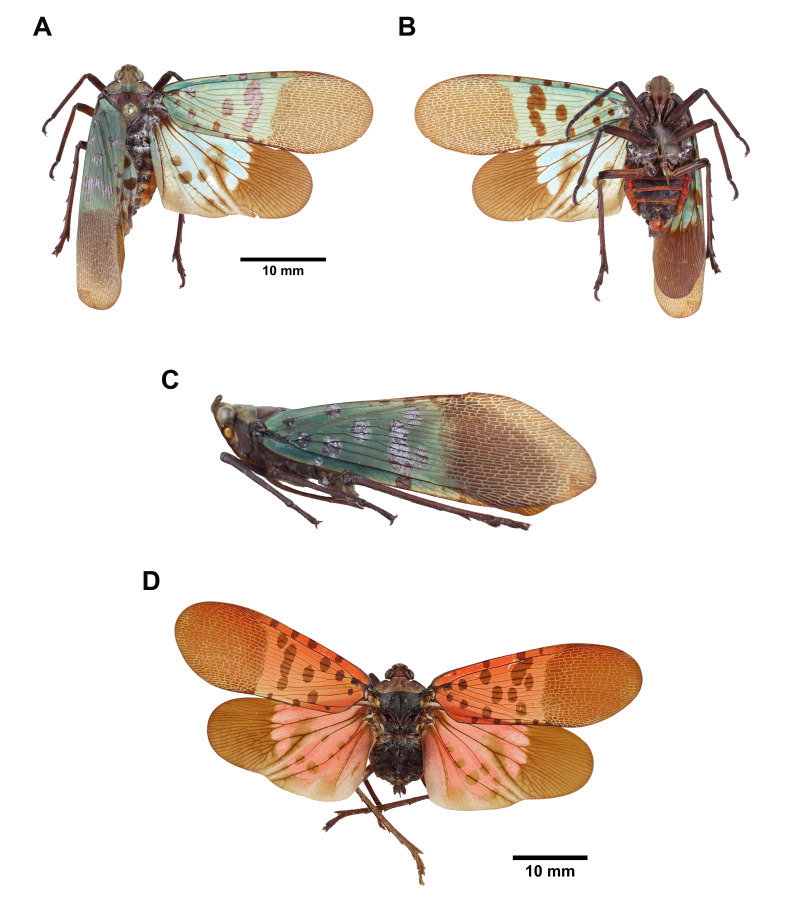
The family Fulgoridae belongs to the order Hemiptera, superfamily Fulgoridea, with approximately 770 described species worldwide. Their extraordinary appearance attracts the attention of both entomologists and the public. In addition to the evolutionary issue of their special appearance, certain species are also considered notorious pests (e.g., Lycorma delicatula). Several issues widely exist in previous taxonomic studies of lanternflies: (1) application of uncertain morphological characters leads to synonymy or misidentification; (2) descriptions of male genitalia are incomplete; (3) information of nymphal morphology is insufficient. Therefore, this study aims to provide a comprehensive taxonomic study of Fulgoridae from Taiwan. In this study, eight species in six genera from Taiwan were reported, of which Limois westwoodii was recorded for the first time from Taiwan. Lycorma olivacea was proposed as a new junior synonym of L. meliae. The fifth-instar nymph of Saiva formosana was described for the first time. Detailed descriptions of these lanternflies and an identification key to adults of Fulgoridae from Taiwan were also included.
Keywords: Fulgorid, Synonym, Male genitalia, New record, Nymph
BACKGROUND
Fulgoridae is the family with the largest body size within the superfamily Fulgoroidea, containing about 770 described species to date (Bourgoin 2022). Most lanternflies are distributed in tropical regions and feed on the sap of woody plants (Bartlett et al. 2014). The spectacular body coloration and exaggerated cephalic process of representative species make this family recognizable not only to entomologists but also to wildlife photographers and specimen collectors. Since little is known about lanternfly biology and phylogeny, it is difficult to understand the adaptive value and evolutionary process of their cephalic processes (Urban and Cryan 2009). In addition, certain species have been reported to be serious pests, such as Lycorma delicatula (White), which causes great economic losses to the grape industry (Du et al. 2021).
The fauna of Taiwanese Fulgoridae were mainly surveyed during the Japanese ruled period. So far, 11 species have been recorded from Taiwan and surrounding islands. However, previous taxonomic studies of lanternflies commonly suffer from the following issues: (1) general application of coloration in taxonomy of lanternflies may lead to synonymy or misidentification due to frequent variation within species; (2) descriptions of male genitalia for many species remains absent or incomplete; (3) morphology of nymphal stages is poorly described for majority of species.
In the present study, a comprehensive survey of Taiwanese Fulgoridae fauna was conducted, and eight species in six genera from Taiwan were recognized and redescribed, including one newly recorded species, Limois westwoodii (Hope). The records of Lycorma delicatula, Pyrops spinolae (Westwood) and Zanna chinensis (Distant) in Taiwan were considered questionable. In addition, Lycorma olivacea Kato was proposed as a new junior synonym of Lycorma meliae Kato based on the morphology of male genitalia. The fifth-instar nymph of Saiva formosana Kato was described for the first time. An identification key to the species of adults of Fulgoridae from Taiwan is provided.
MATERIALS AND METHODS
Dry pinned specimens were used for descriptions and photography. Photographs of the specimens were taken with a Canon EOS 800D camera with Contax Carl Zeiss S-Planar 60 mm F2.8 AEG lens. Measurements were taken from photographs with ImageJ software (Abramoff et al. 2004). The methods for dissection of male genitalia were modified from Constant and Pham (2017): male genitalia were cut from the abdomen by dissecting scissors and then heated for about one hour in a 10% solution of potassium hydroxide (KOH). The pieces were examined in ethanol and then placed in glycerol for preservation. Photographs of male genitalia were taken with a Leica DVM6 digital microscope. All photographs were optimized with Adobe Photoshop CS6 software (Adobe Systems Inc) and stacked with Helicon Focus 6.7.1 software (Kozub et al. 2000). The morphological terminology used in this study follows O'Brien (1988) for external morphology and Yang and Chang (2000) for male genitalia.
Specimens examined were deposited at the following institutions: Hokkaido University Insect Collection (HUIC, Sapporo, Japan), National Chung Hsing University (NCHU, Taichung, Taiwan), National Museum of Natural Sciences (NMNS, Taichung, Taiwan), National Taiwan University (NTU, Taipei, Taiwan), Oxford University Museum of Natural History (OUMNH, Oxford, UK), Senckenberg Deutsche Entomologische Institut (SDEI, Müncheberg, Germany), Taiwan Agricultural Research Institute (TARI, Taichung, Taiwan), Taiwan Forestry Research Institute (TFRI, Taipei, Taiwan), and University Museum, University of Tokyo (UMUT, Tokyo, Japan).
RESULTS
TAXONOMY
Order Hemiptera Linnaeus, 1758
Superfamily Fulgoroidea Latreille, 1807
Family Fulgoridae Latreille, 1807
Identification key to the species of Fulgoridae from Taiwan
1. Anal area of hindwings hyaline, without cross-veins (Fig. 1A); genital styles with a hook on each lateral margin over 1/2 from base in ventral view (Fig. 2C) .................................................................................................. Dichoptera similis Schumacher, 1915
-Anal area of hindwings colored, with cross-veins (Figs. 7A, 9A, D, 14A, 16A, 19A, 22A, D); genital styles with a hook on each lateral margin at 1/2 or below from base in ventral view (Figs. 5C, 8C, 10C, 11C, 15C, 17C, 20C, 23C) ................................... 2
2. Head without cephalic process (Fig. 14C); 10th abdominal segment with apical margin nearly as broad as basal margin in dorsal view (Fig. 15B) ............................................................................................ Penthicodes pulchella (Guérin-Méneville, 1838)
-Head with cephalic process (Figs. 4C, 7C, 9C, 16C, 19C, 22C); 10th abdominal segment with apical margin broader than basal margin in dorsal view (Figs. 5B, 8B, 10B, 11B, 17B, 20B, 23B) ..................................................................................................... 3
3. Cephalic process long, protruding forward (Figs. 16C, 19C, 22C); 9th abdominal segment with anterior margin flat in lateral view (Figs. 17A, 20A, 23A) ....................................................... 4
-Cephalic process short, not protruding forward (Figs. 4C, 7C, 9C); 9th abdominal segment with anterior margin concave or convex in lateral view (Figs. 5A, 8A, 11A) ............................... 6
4. Cephalic process narrowing suddenly beyond eyes (Fig. 22A, D); ventral pair of lateral phallobasal lobes slightly sclerotized (Fig. 23D) ................................................ Saiva formosana Kato, 1929
-Cephalic process narrowing gradually beyond eyes (Figs. 16A, 19A, D); ventral pair of lateral phallobasal lobes membranous (Figs. 17D, 20D) ......................................................................... 5
5. Cephalic process with apex narrowing and compressed laterally (Fig. 16A, B); tegmina with 1 transverse band near base (Fig. 16A); phallobasal conjunctival processes exposed (Fig. 17D, E) ............................................. Pyrops candelaria (Linnaeus, 1758)
-Cephalic process with apex strongly inflated (Fig. 19A, B, D); tegmina without transverse band near base (Fig. 19A, D);
phallobasal conjunctival processes not exposed (Fig. 20D, E) ............................................... Pyrops watanabei (Matsumura, 1913)
6. Tegmina not hyaline (Fig. 9A, D); labium not reaching apex of abdomen (Fig. 9B); phallobasal conjunctival processes with apexes folding backward and downward in ventral view (Figs. 10D, 11D) ......................................... Lycorma meliae Kato, 1929
-Tegmina partially hyaline (Figs. 4A, 7A); labium reaching apex of abdomen (Figs. 4B, 7B); phallobasal conjunctival processes with apexes not folding backward and downward in ventral view (Figs. 5D, 8D) ............................................................................. 7
7. Frons with 3 longitudinal carinae extending from ventral side of cephalic process, median carina sometimes indistinct terminally (Fig. 4D); 9th abdominal segment with dorso-posterior margin angulate in lateral view (Figs. 5A, 6A); 10th abdominal segment with apical margin medially concave as “U” shape dorsally and ventrally in dorsal view (Figs. 5B, 6C); genital styles suboval in ventral view (Figs. 5C, 6E) ............... Limois kikuchii Kato, 1932
-Frons with 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 7D); 9th abdominal segment with dorso-posterior margin rounded in lateral view (Figs. 6B, 8A); 10th abdominal segment with apical margin medially concave as “V” shape dorsally but slightly concave as “U” shape ventrally in dorsal view (Figs. 6D, 8B); genital styles subtriangular in ventral view (Figs. 6F, 8C) ................... Limois westwoodii (Hope, 1843)
Note: Lycorma delicatula (White, 1845), Pyrops spinolae (Westwood, 1842) and Zanna chinensis (Distant, 1893) were excluded from the identification key since their records in Taiwan are questionable.
Genus Dichoptera Spinola, 1839
Dichoptera similis Schumacher, 1915
(Figs. 1, 2, 3)
Dichoptera similis Schumacher, 1915: 130; Kato, 1933: pl. 3, fig. 6.
Type locality: Kosempo [= Jiasian, Kaohsiung, Taiwan] (Schumacher 1915).
Description: Measurements: body length, male (n = 8) 27.1 mm (24.4–29.8 mm), female (n = 5) 30.2 mm (25.9–33 mm); tegmen length, male (n = 8) 22.5 mm (19.8–24.3 mm), female (n = 5) 25.1 mm (22.6–27.6 mm).
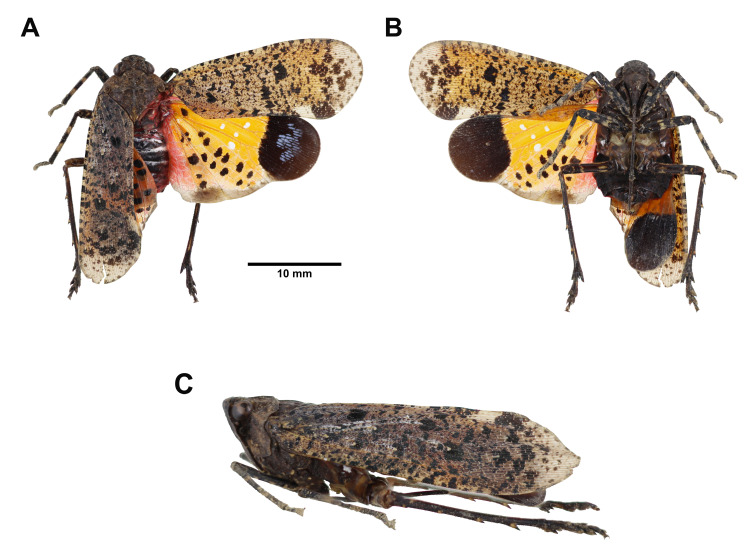
Head: general color yellowish brown to pale green (Fig. 1A, B, C); cephalic process short, protruding upward, with 1 black longitudinal band on dorsal side and each lateral side (Fig. 1A, C); vertex as long as broad, with 1 black hollow-arrowheaded marking on disc, disconnected at each lateral side, lateral margins strongly carinate (Fig. 1A, C); frons longer than broad, with 3 longitudinal carinae extending from ventral side of cephalic process, stongly protruding (Fig. 1B); genae with 1 black transverse band passing through eye, connected with lateral band extending from cephalic process (Fig. 1C); occiput with 2 black spots along each postocular flange (Fig. 1A); antennae brown (Fig. 1C); clypeus longer than frons (Fig. 1B); labium elongate, not reaching apex of abdomen, terminal of each segment dark brown (Fig. 1B).
Thorax: (Fig. 1A, C) general color yellowish brown to pale green; pronotum with 1 black marking like reverse “V” on anterior angle, 1 dark brown marking like reverse parentheses on disc, and 1 dark brown reticulated marking on each lateral area; mesonotum with 1 pair of black semicircular patch between 1 pair of black reverse-subtriangular patch along anterior margin, 1 black marking like Greek letter “Ω” between lateral carinae, surrounding 1 pair of brown semioval patch connected with 1 pair of brown obtuse-triangular patch posteriorly, and 1 black marking like arrowhead bending outward on each lateral area outside lateral carinae.
Tegmina: (Fig. 1A) general color hyaline, divided into basal area and apical area by a distinct serrate nodal line with venation of apical area reticulate; basal area with several brown patches along anterior margin, 1 weak brown spot in front of disc, 1 dark brown spot connected with 1 weak brown spot anteriorly behind disc; apical area with 1 yellowish brown to pale green pterostigma, 1 gradient brown band along nodal line, 1 big dark brown hollow-subtriangular marking along end of anterior margin, and several weak brown spot along apical margin.
Hindwings: (Fig. 1A) general color hyaline, apical angle dark brown, anal area without cross-veins.
Legs: (Fig. 1A, B) general color yellowish brown to pale green; femora with several brown irregular patches; fore-and mesotibiae with 1 dark brown ring on apex and middle; metatibiae with 7 lateral spines; 3rd fore-and mesotarsomeres dark brown.
Abdomen: general color reddish brown to yellowish brown (Fig. 1A, B); 1st, 7th and 8th tergites black, 2nd–6th tergites with 1 pair of big black suboval patch on disc of each tergite (Fig. 1A); laterosternites sometimes with black spots (Fig. 1B); 5th and 6th sternites with 1 black transverse band along both ends of anterior margin, 7th sternite black anteriorly, 8th sternite black (Fig. 1B).
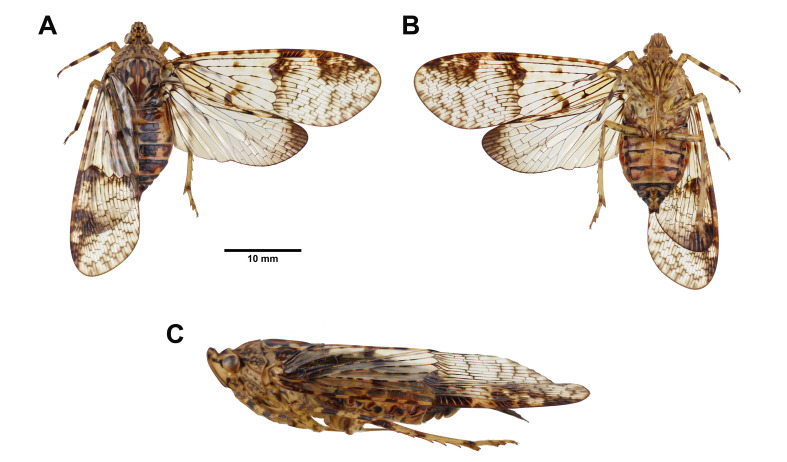
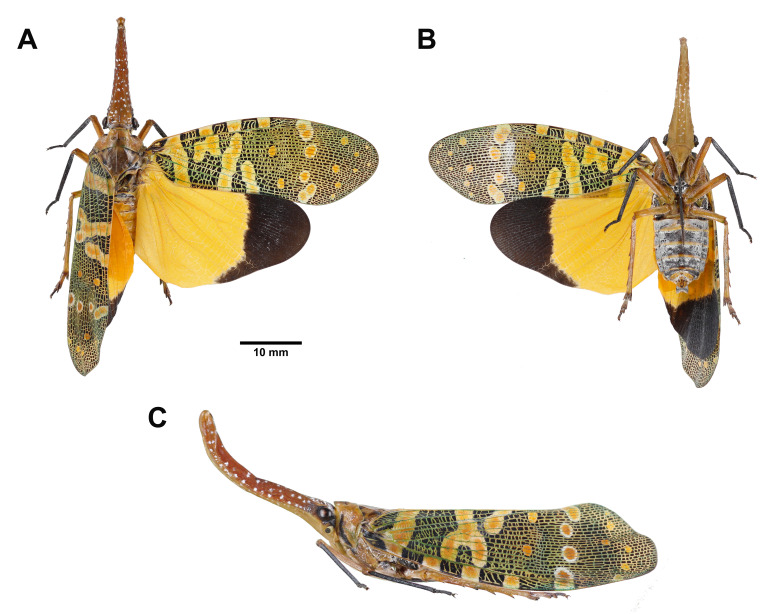
Fig. 1.
Dichoptera similis Schumacher, 1915, adult female. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale).
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin concave, dorso-anterior margin acute and strongly protrudent, dorso-posterior margin angulate, ventro-posterior margin slightly protrudent (Fig. 2A); 10th abdominal segment with ventral margin strongly concave at 4/5 from apex in lateral view (Fig. 2A), apical margin nearly as broad as basal margin, strongly concave as “U” shape dorsally but weakly concave ventrally in dorsal view (Fig. 2B); 11th abdominal sternite about 3 times longer than 11th abdominal tergite (Fig. 2B); genital styles shorter than 10th abdominal segment in lateral view (Fig. 2A), subtrapezoid with a hook on each lateral margin at 2/3 from base in ventral view (Fig. 2C); ventral pair of lateral phallobasal lobes sclerotized, dorsal pair of lateral phallobasal lobes membranous (Fig. 2D, E); phallobasal conjunctival processes exposed, with middle sinuate, apexes and basal 2/3 sclerotized, about 6 times longer than sheath, apexes acute (Fig. 2D, E).
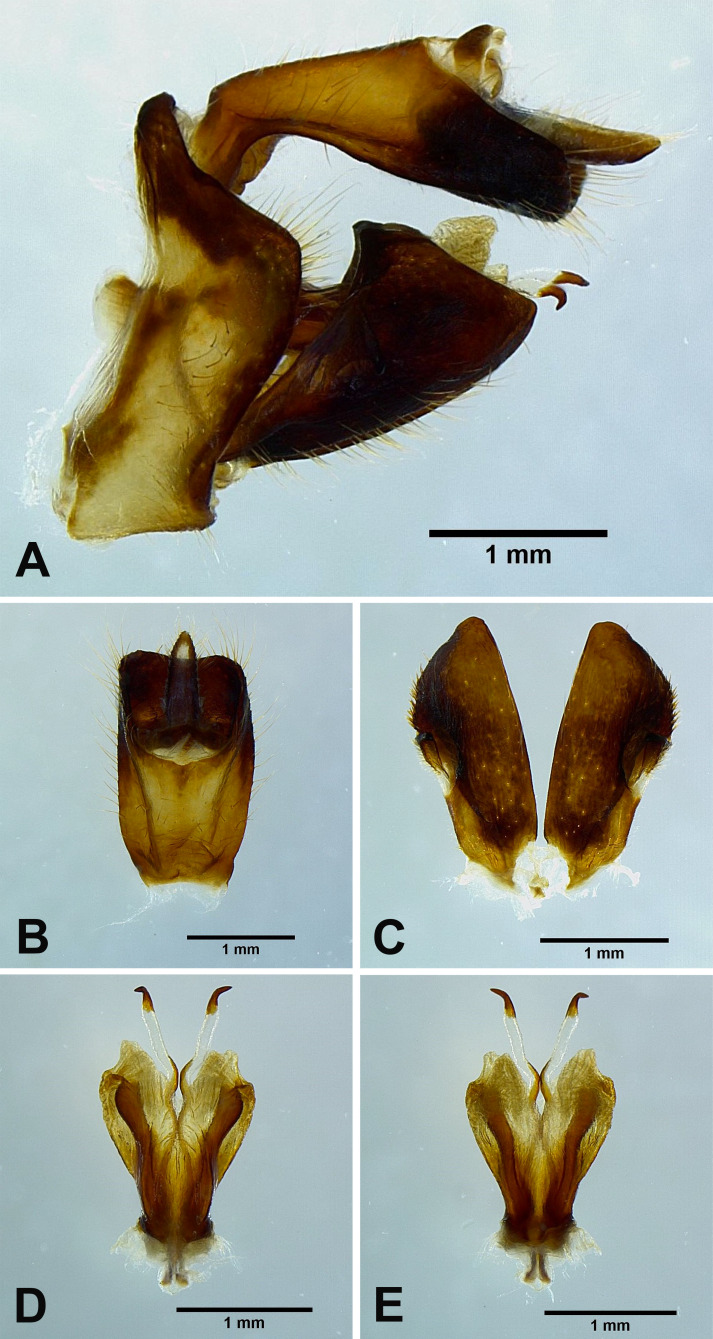
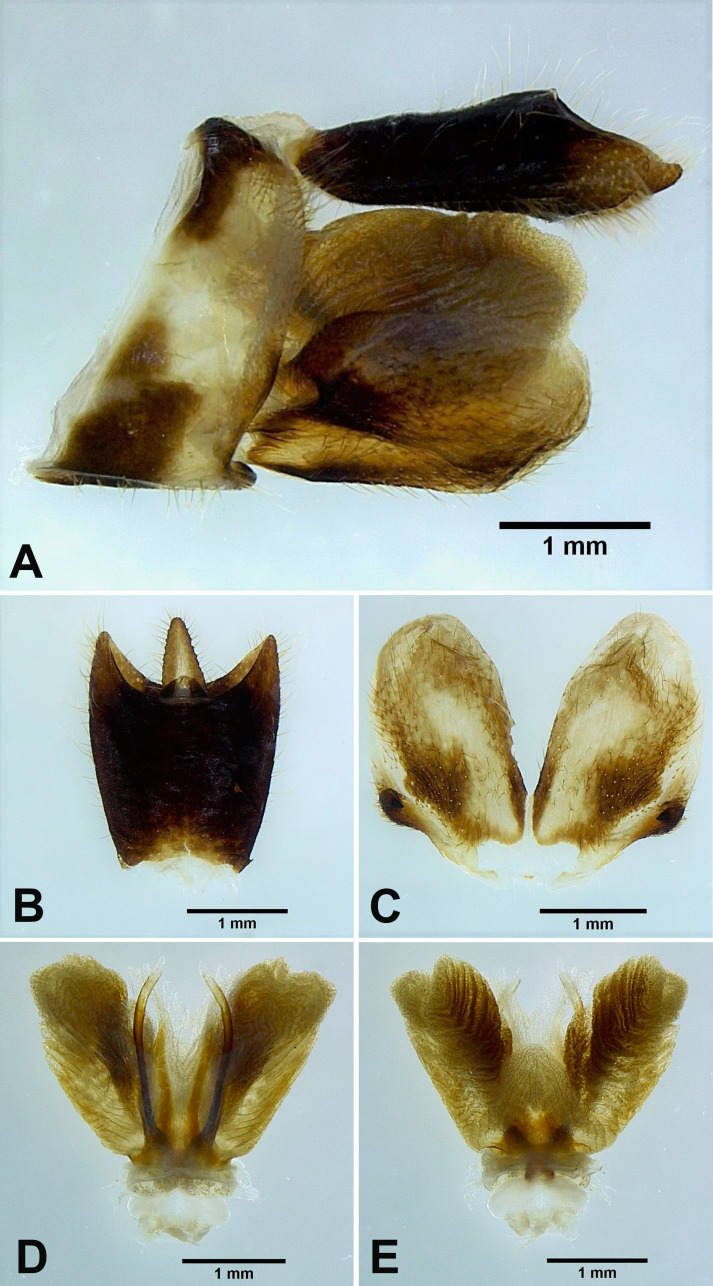
Fig. 2.
Dichoptera similis Schumacher, 1915, male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
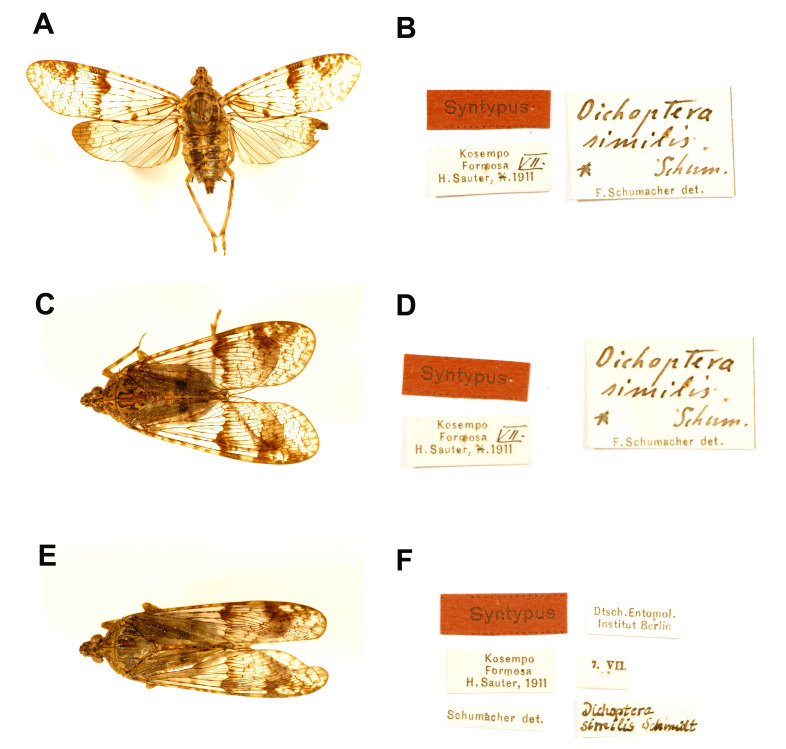
Materials examined: Syntype: male of Dichoptera similis Schumacher, 1915 (examined from photographs, Fig. 3A, B) [Kosempo, Formosa, H. Sauter, VII. 1911] [Dichoptera similis Schum., F. Schumacher det.] [Syntypus] (SDEI); female of Dichoptera similis Schumacher, 1915 (examined from photographs, Fig. 3C, D) [Kosempo, Formosa, H. Sauter, VII. 1911] [Dichoptera similis Schum., F. Schumacher det.] [Syntypus] (SDEI); male of Dichoptera similis Schumacher, 1915 (examined from photographs, Fig. 3E, F) [Kosempo, Formosa, H. Sauter, 1911] [7. VII.] [Dichoptera similis Schmidt] [Schumacher det.] [Syntypus] [Dtsch. Entomol. Institut Berlin] (SDEI).
Fig. 3.
Dichoptera similis Schumacher, 1915, type specimens. A, Syntype. B, Labels. C, Syntype. D, Labels. E, Syntype. F, Labels. (SDEI, photographs from the program “Digitization of Historic Museum Collections of Taiwan Deposited in Foreign Countries” by NMNS).
Other materials: 1 male, Huisun Experimental Forest Station, Nantou, IV-28-1991, leg. N. T. Keng (NCHU); 1 male, same locality, VIII-25-2020, leg. J. A. Liao (NMNS); 1 male, Lanren River, Pingdong, IX-15-2011, leg. Y. C. Lan & Y. H. Peng (NMNS); 1 female, Provincial Rd. No. 20 (166.5 km), Haituan, Taitung, VI-18-2005, leg. J. H. Chen (NMNS); 1 female, Shanping, Liouguei, Kaoshiung, V-25-2002, leg. C. W. Chen (NMNS); 1male, 1 female, Yima Forest-Road, Taitung, XII-16-2020, leg. L. C. Shih (NMNS); 1 female, Dahan Shan (19km), Pingtung, VII-05-2015, leg. B. H. Ho (NTU); 1 male, Fusan Botanical Garden, Ilan, V-08-2021, leg. Y. H. Lin (NTU); 1 male, same locality, V-08-2004, leg. C. F. Lee (NTU); 1 male, same locality, V-08-2003, leg. S. S. Lu (TFRI); 1 female, same locality, IV-27-1995, leg. J. J. Hsiao (TFRI); 1 male, Nanzixianxi, Chiayi, X-16-2007, leg. W. C. Yeh (TFRI); 1 female, same locality, IX-01-2008, leg. Y. M. Chen (TFRI); 1 male, Taimali, Taitung, V-21-2009, leg. Y. M. Lai (TFRI); 1 male, same locality, VI-06-2013, leg. Y. C. Lin (TFRI); 1 male, same locality, VII-11-2013, leg. Y. C. Lin (TFRI).
Host plant: unknown.
Distribution: Taiwan.
Remarks: This species was once classified under the family Dictyopharidae by Metcalf (1946) and has complex characters including body size as Fulgoridae, male genitalia as Dictyopharidae and venation as some species of Tropiduchidae, suggesting that the taxonomic status of this species and related species requires further confirmation.
Genus Limois Stål, 1863
Limois kikuchii Kato, 1932
(Figs. 4, 5)
Limois kikuchii Kato, 1932: 225; Kato, 1933: pl. 3, fig. 3; Metcalf, 1947: 170; Lallemand, 1963: 56; Nagai & Porion, 1996: 22; Chou et al., 1985: 108; Wang et al., 2020: 43.
Type locality: Manchuria [= Northeast China] (Metcalf 1947).
Description: Measurements: body length, male (n = 1) 17.1 mm; tegmen length, male (n = 1) 14.1 mm.
Head: general color reddish brown (Fig. 4A, B, C, D); cephalic process short, protruding upward, ventral margin yellowish green (Fig. 4A, C, D); vertex broader than long, with 1 pair of brown spot on disc and 1 pair of brown subtrapezoid patch along posterior margin, lateral margins medially carinate (Fig. 4A, C); frons longer than broad, with 1 orange patch on each latero-posterior angle, 1 orange patch along middle of posterior margin, and 3 longitudinal carinae extending from ventral side of cephalic process, median carina sometimes indistinct terminally (Fig. 4B, D); antennae brown (Fig. 4C, D); clypeus longer than frons, with 3 orange patches along anterior margin, and 2 orange patches on median carina, 1 near anterior margin and 1 near posterior margin (Fig. 4B); labium elongate, reaching apex of abdomen (Fig. 4B).
Thorax: (Fig. 4A, C) pronotum reddish brown, with 1 pair of brown subquadrangular patch along anterior margin, followed by 1 pair of small brown spot posteriorly and 1 dark brown spot on each lateral area; mesonotum yellowish brown, with 2 pairs of dark brown subquadrangular patch and 1 pair of dark brown spot along anterior margin, 2 big dark brown patches on each lateral side of median carina between lateral carinae, 1 brown subtriangular patch on each lateral area ouside lateral carinae, and 1 pair of dark brown suboval patch on posterior angle.
Tegmina: (Fig. 4A) basal 1/3 with 1 brown oblique irregular band, dividing tegmen into basal colored area and apical hyaline area; basal colored area coral pink, with 1 big brown patch near anterior margin, 2 moderate brown spots along claval suture and numerous small brown spots throughout; apical hyaline area with several brown spots varying in size along anterior margin, 2 moderate brown spots near middle of anterior margin, several brown spots varying in size along apical margin, 1 big brown patch along apico-posterior margin, and numerous small brown spots throughout.
Hindwings: (Fig. 4A, B) basal 1/2 red with 1 big and 1 small brown spot on A1 vein; apical 1/2 hyaline.
Legs: (Fig. 4B, C) general color brown; femora with several yellowish white irregular patches; tibiae with 2 yellowish white rings, metatibiae with 5 lateral spines.
Abdomen: (Fig. 4A, B) general color black, posterior margin of tergites yellow.
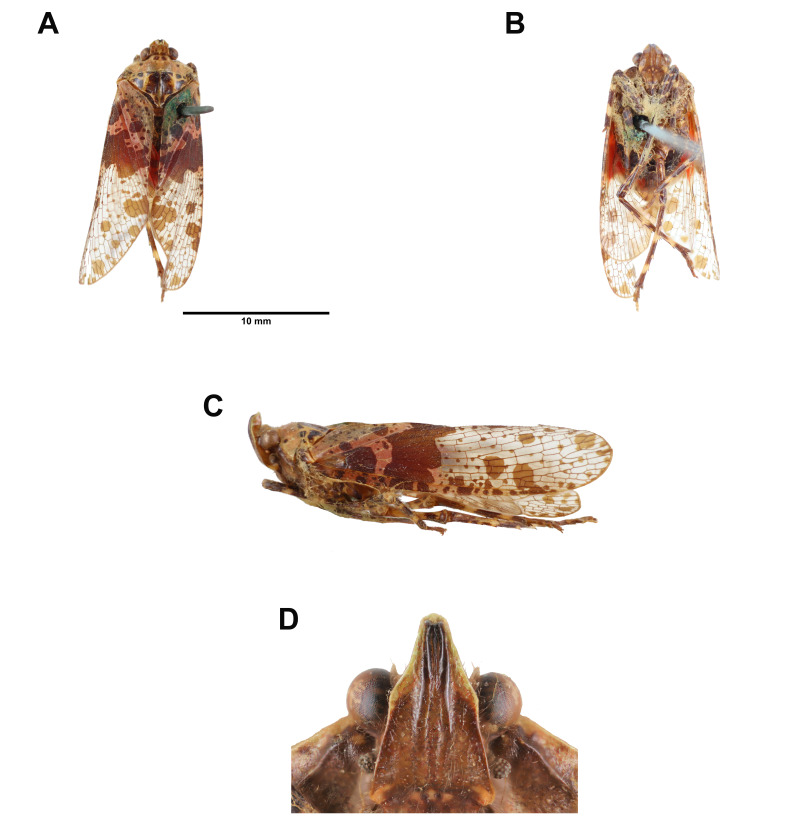
Fig. 4.
Limois kikuchii Kato, 1932, adult male. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale). D, Frons (not to scale).
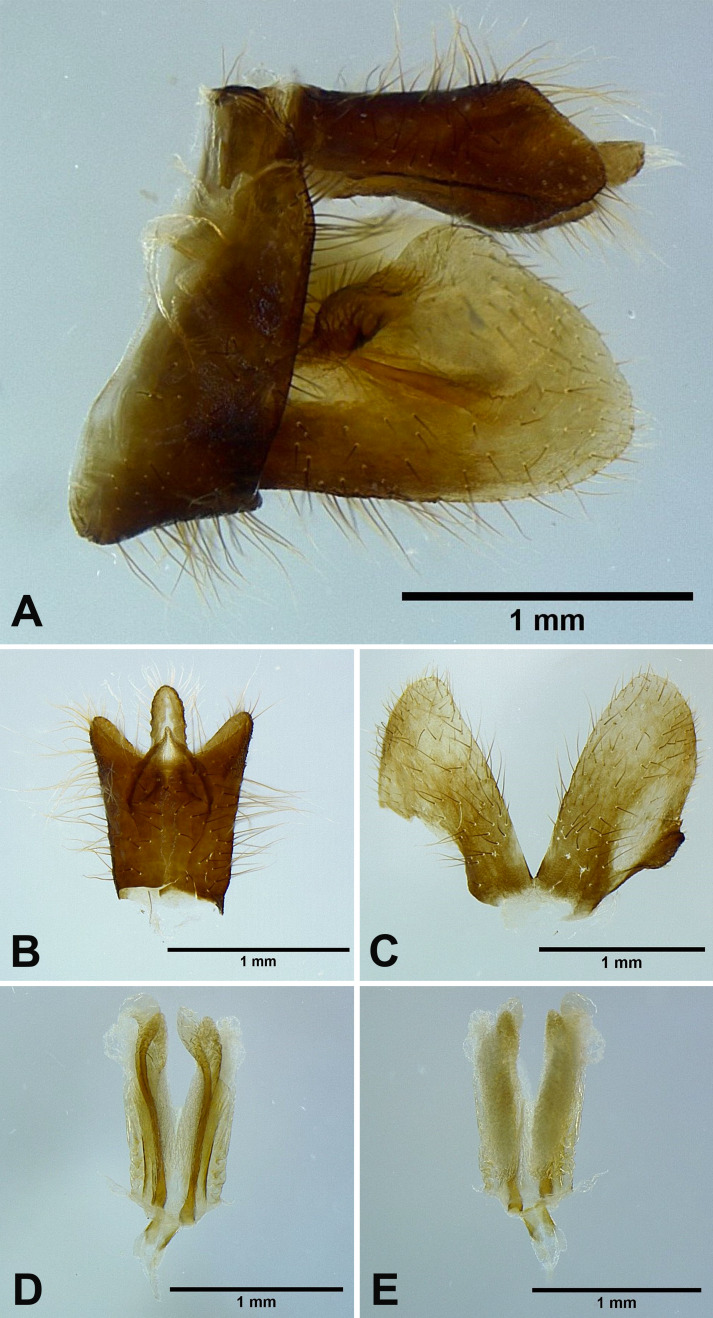
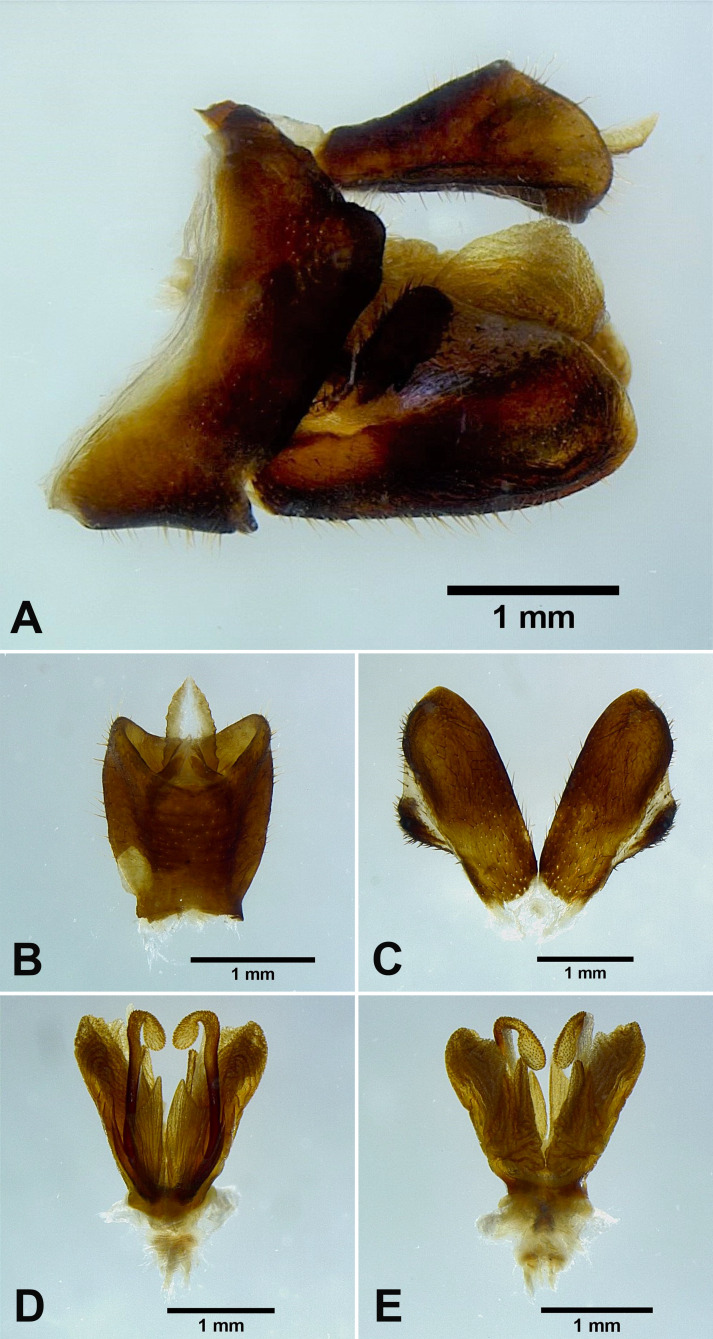
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin slightly convex near ventro-anterior margin, dorso-anterior margin obtuse, dorso-posterior margin angulate, ventro-posterior margin slightly protrudent (Fig. 5A); 10th abdominal segment with ventral margin moderately convex at 1/3 from apex in lateral view (Fig. 5A), apical margin about 1.5 times broader than basal margin, medially concave as “U” shape dorsally and ventrally in dorsal view (Fig. 5B); 11th abdominal sternite about 2 times longer than 11th abdominal tergite (Fig. 5B); genital styles longer than 10th abdominal segment in lateral view (Fig. 5A), suboval with a hook on each lateral margin between 1/3 and 1/2 from base in ventral view (Fig. 5C); lateral phallobasal lobes membranous (Fig. 5D, E); phallobasal conjunctival processes exposed, straight and sclerotized except for apexes, about 6 times longer than sheath, apexes inflated and fin-like (Fig. 5D, E).
Fig. 5.
Limois kikuchii Kato, 1932, male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: 1 male, Ritozan [= Litungshan, Hsinchu], VII-31-1928, leg. S. Issiki (NTU).
Host plant: unknown.
Distribution: Taiwan, China, Korea (Wang et al. 2020).
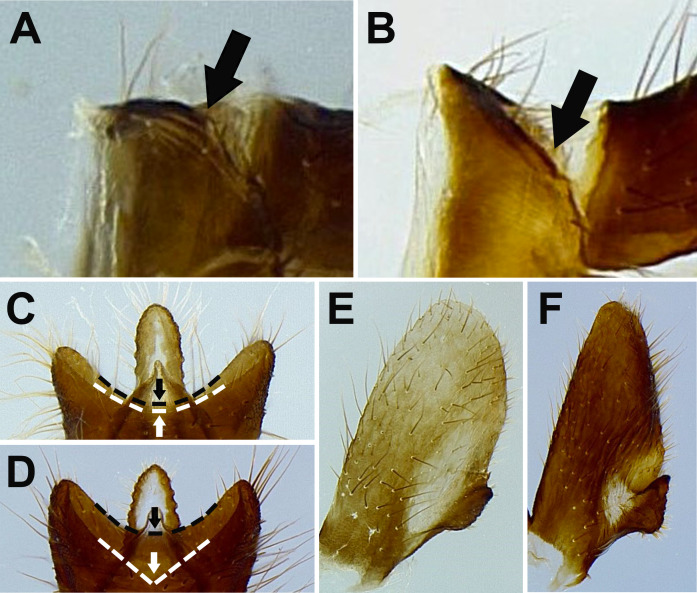
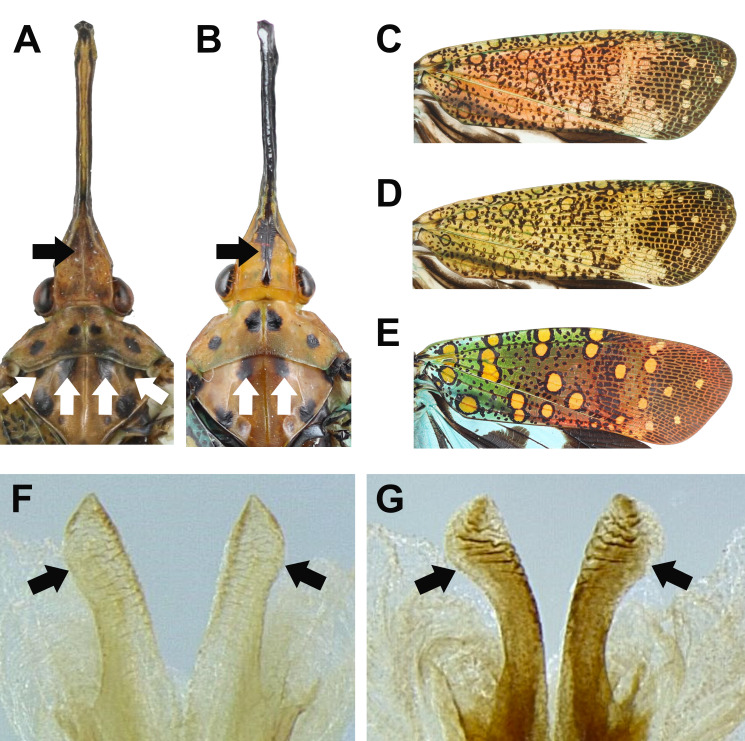
Remarks: This species is similar to Limois westwoodii (Hope, 1843). Wang et al. (2020) argued that the number of longitudinal carinae on frons can be used for distinguishing these two species. However, this character sometimes can be equivocal. Here three new diagnostic characters were proposed based on morphology of male genitalia: (1) L. kikuchii has 9th abdominal segment with dorso-posterior margin angulate in lateral view (Fig. 6A), while L. westwoodii has 9th abdominal segment with dorso-posterior margin rounded in lateral view (Fig. 6B); (2) L. kikuchii has 10th abdominal segment with apical margin medially concave as “U” shape dorsally and ventrally in dorsal view (Fig. 6C), while L. westwoodii has 10th abdominal segment with apical margin medially concave as “V” shape dorsally but slightly concave as “U” shape ventrally in dorsal view (Fig. 6D); (3) L. kikuchii has suboval genital styles in ventral view (Fig. 6E), while L. westwoodii has subtriangular genital styles in ventral view (Fig. 6F).
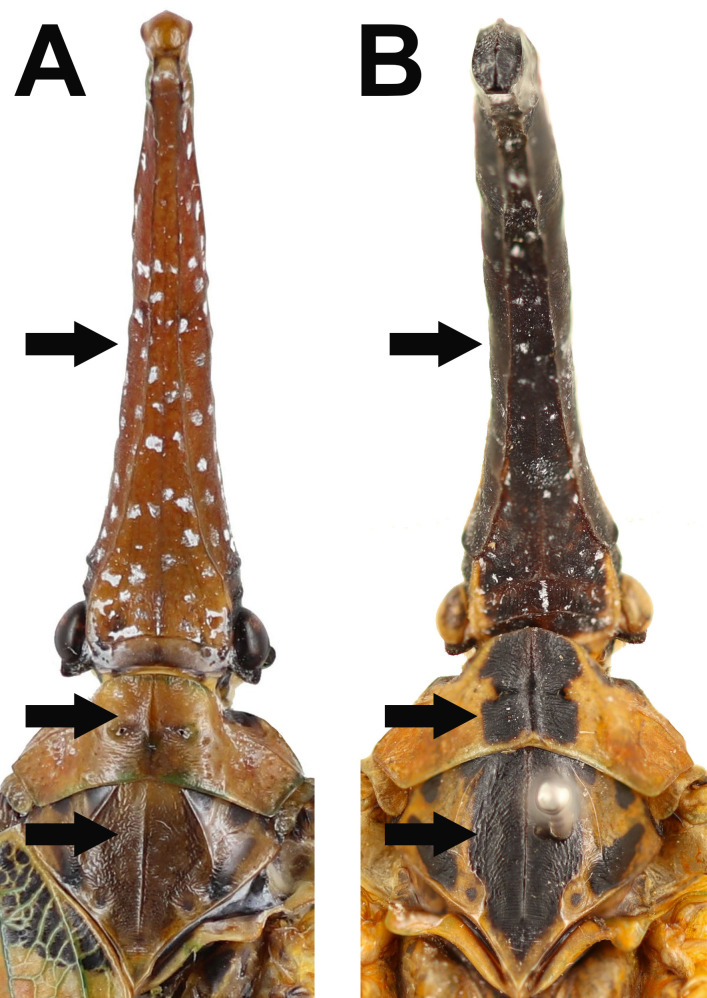
Fig. 6.
Diagnostic characters for Limois kikuchii and L. westwoodii. A–B, Dorso-posterior margin of 9th abdominal segment in lateral view. A, L. kikuchii. B, L. westwoodii. C–D, Apical margin of 10th abdominal segment in dorsal view. C, L. kikuchii. D, L. westwoodii. E–F, Genital styles in ventral view. E, L. kikuchii. F. L. westwoodii.
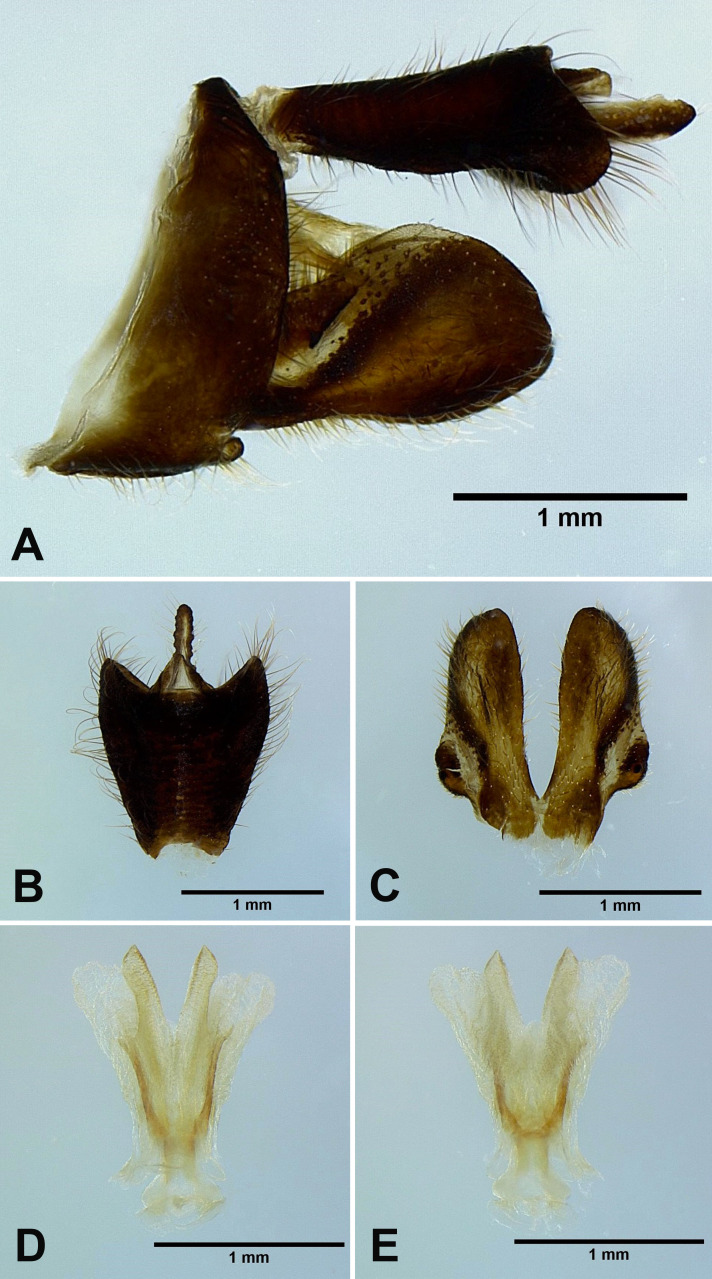
Limois westwoodii (Hope, 1843) new record
(Figs. 7, 8)
Lystra westwoodii Hope, 1843: 133. Limois westwoodii Stål, 1863: 231; Distant, 1906: 200; Metcalf, 1947: 170; Lallemand, 1963: 55; Nagai and Porion, 1996: 22; Wang et al., 2020: 49.
Type locality: Bangladesh (Wang et al. 2020).
Description: Measurements: body length, male (n = 7) 20 mm (17.9–23.3 mm), female (n = 4) 23.2 mm (21.9–25.2 mm); tegmen length, male (n = 7) 17.3 mm (15.7–20.4 mm), female (n = 4) 19.8 mm (18.2–21.9 mm).
Head: general color reddish brown to yellowish green (Fig. 7); cephalic process short, protruding upward and backward (Fig. 7A, C); vertex broader than long, with 1–2 pairs of dark brown spot along posterior margin, lateral margins medially carinate (Fig. 7A, C); frons longer than broad, with 1 yellowish green patch on each latero-posterior angle, 1 yellowish green patch along middle of posterior margin, and 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 7B, D); antennae brown (Fig. 7C, D); clypeus longer than frons, with 2 yellowish green patches on median carina, 1 near anterior margin and 1 near posterior margin (Fig. 7B); labium elongate, reaching apex of abdomen (Fig. 7B).
Thorax: (Fig. 7A, C) general color reddish brown to yellowish green; pronotum with 1 pair of dark brown subquadrangular patch along anterior margin, followed by 1 pair of small brown spot posteriorly, 1 dark brown spot on each lateral area, and 1 pair of dark brown subrectangular patch along posterior margin; mesonotum with 1 pair of dark brown subquadrangular or subtriangular patch along middle of anterior margin between 1 pair of dark brown subsemicircular patch and 1 pair of dark brown spot, numerous small dark brown spots between lateral carinae, and 1 dark brown subtriangular patch and 1 dark brown spot on each lateral area ouside lateral carinae.
Tegmina: (Fig. 7A) basal 1/3 with 1 dark brown oblique irregular band, dividing tegmen into basal colored area and apical hyaline area, sometimes extending to apico-posterior margin; basal colored area yellowish brown to light yellowish green, with 1 big dark brown patch near anterior margin, 1–2 moderate dark brown spots along claval suture and numerous small dark brown spots throughout; apical hyaline area with several brown spots varying in size along anterior margin, 1–2 moderate brown spots near middle of anterior margin, several brown spots varying in size along apical margin, and numerous small brown spots throughout.
Hindwings: (Fig. 7A) basal 1/2 mixed with coral pink and orange, with 1 big and 1 small brown spot on A1 vein; apical 1/2 hyaline; posterior margin dark brown.
Legs: (Fig. 7A, B, C) general color dark brown; femora with several yellowish green irregular patches; tibiae with 2 yellowish white rings, metatibiae with 5–7 lateral spines; mesotarsomeres with 1 yellowish white ring.
Abdomen: (Fig. 7A, B) general color black, posterior margin of tergites yellow to yellowish brown.
Fig. 7.
Limois westwoodii (Hope, 1843), adult male. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale). D, Frons (not to scale).
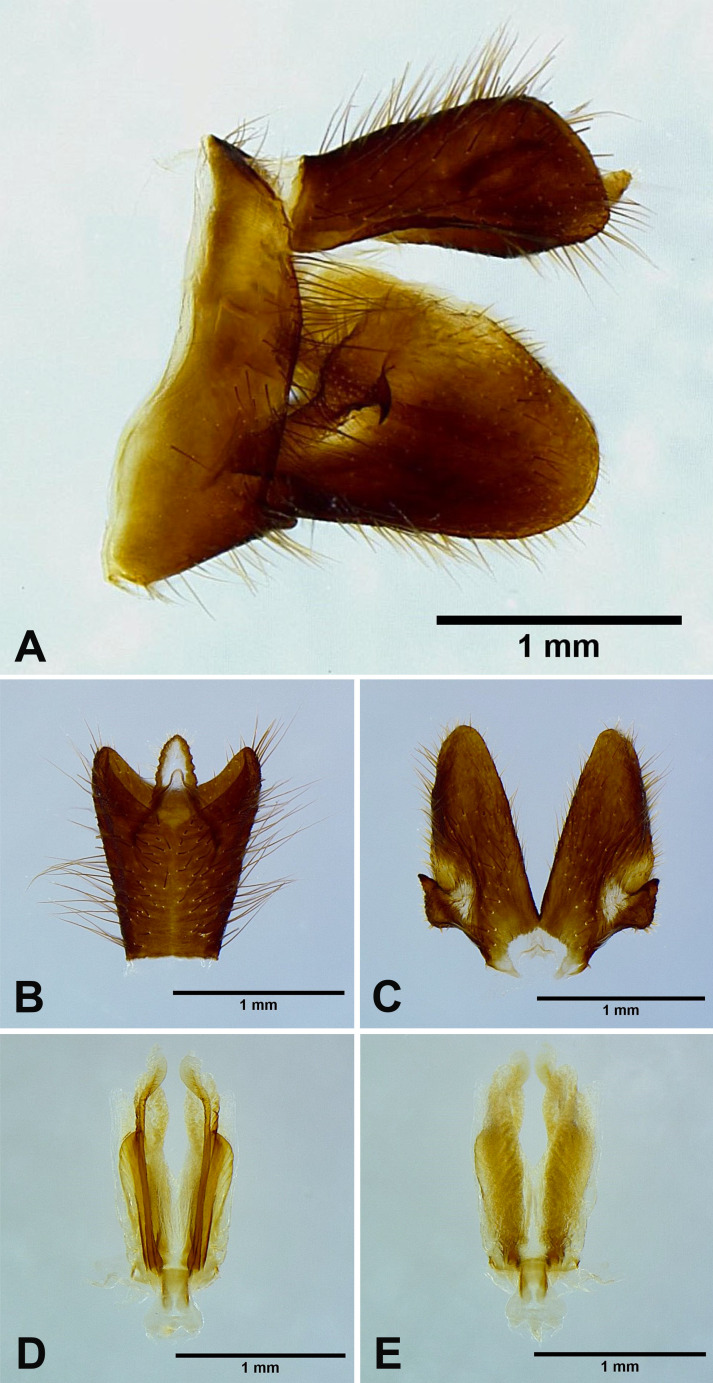
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin slightly convex near ventro-anterior margin, dorso-anterior margin acute, dorso-posterior margin rounded, ventro-posterior margin with a small acute projection (Fig. 8A); 10th abdominal segment with ventral margin slightly convex at 1/3 from apex in lateral view (Fig. 8A), apical margin about 1.5 times broader than basal margin, medially concave as “V” shape dorsally but slightly concave as “U” shape ventrally in dorsal view (Fig. 8B); 11th abdominal sternite about 2 times longer than 11th abdominal tergite (Fig. 8B); genital styles as long as 10th abdominal segment in lateral view (Fig. 8A), subtriangular with a hook on each lateral margin at 1/3 from base in ventral view (Fig. 8C); lateral phallobasal lobes membranous (Fig. 8D, E); phallobasal conjunctival processes exposed, straight and sclerotized except for apexes, about 6 times longer than sheath, apexes inflated and fin-like (Fig. 8D, E).
Fig. 8.
Limois westwoodii (Hope, 1843), male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: Holotype: female of Lystra westwoodii Hope, 1843 (examined from photographs in Wang et al. (2020)), Bangladesh, collecting date unknown, leg. Frederick John Parry (OUMNH).
Other materials: 1 male, Fushan, Wulai, Tapei, X-(14-17)-1996, leg. C. S. Lin & M. L. Chan (NMNS); 1 female, Liyuan, Provincial, Rd. No. 20 (163.5 km), Taitung, IX-02-2002, leg. J. H. Chen (NMNS); 1 male, Yingziking, Ilan, VIII-10-2021, leg. C. F. Lin (NTU); 2 males, Hehuan Yuelin trail (24°10'40.8"N, 121°24'16.2"E), Hualien, X-28-2021, leg. C. F. Lin (NTU); 1 male, Tianxiang, Hualien, II-11-1990, leg. C. F. Lee (NTU); 1 male, Shenmihu, Ilan, X-24-1993, leg. W. I. Chou (TARI); 1 male, Fusan Botanical Garden, Ilan, XII-20-1995, leg. A. Warneke (TFRI); 1 male, same locality, VII-27-1995, leg. Y. J. Chen (TFRI); 1 female, same locality, XI-22-1995, leg. A. Warneke (TFRI); 1 female, same locality, X-05-1994, leg. A. Warneke (TFRI); 1 female, same locality, XI-02-1994, leg. W. T. Jou (TFRI).
Host plant: unknown.
Distribution: Taiwan, Bangladesh, China, Myanmar (Wang et al. 2020).
Remarks: This species is similar to Limois kikuchii but can be distinguished by the morphology of male genitalia. See remarks of L. kikuchii for diagnostic characters. This species has been collected at light traps.
Genus Lycorma Stål, 1863
Lycorma delicatula (White, 1845)
Aphaena delicatula White, 1845: 37.
Lycorma delicatula Stål, 1863: 234; Distant, 1906: 207; Kato, 1933: pl. 5, fig. 3; Metcalf, 1947: 164; Lallemand, 1963: 46; Nagai & Porion, 1996: 21; Chou et al., 1985: 112.
Type locality: Nankin, China (White 1845).
Materials examined: No specimen was examined in this study.
Distribution: Taiwan (?), Bangladesh, China, India, Japan, Korea, USA, Vietnam (Bourgoin 2022). Remarks: This species was recorded from Taiwan by Chou et al. (1985). However, no voucher specimen of Chou et al. (1985) could be examined. In addition, no specimen of this species was found in comprehensive survey and collections of all Taiwanese museums. The record of this species could be considered problematic and was possibly due to misidentification of Lycorma meliae Kato, 1929.
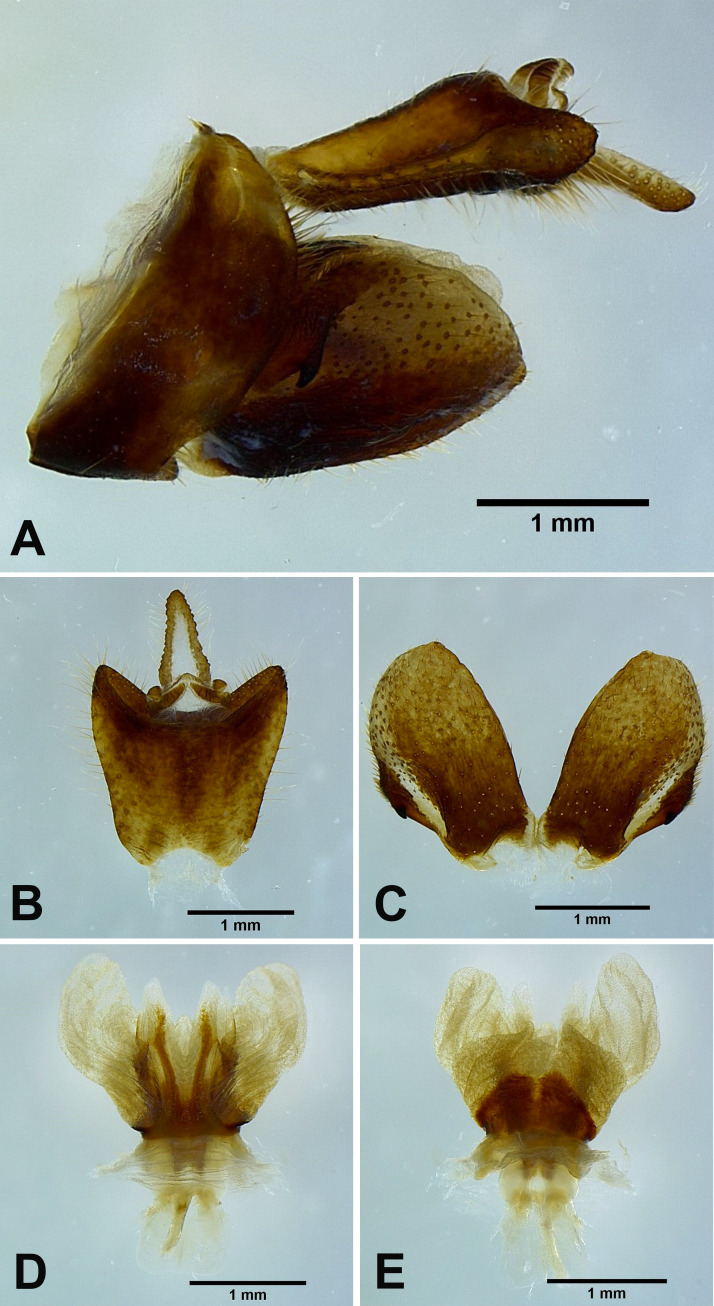
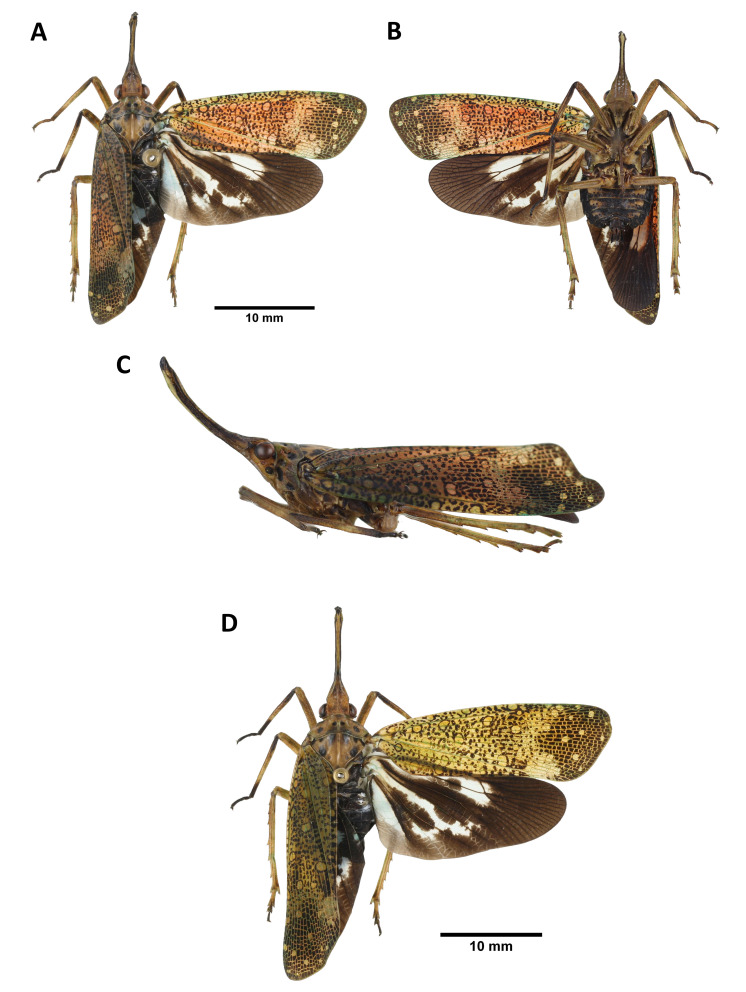
Lycorma meliae Kato, 1929
(Figs. 9, 10, 11, 12)
Lycorma meliae Kato, 1929: 550; Kato, 1933: pl. 3, fig. 5; Metcalf, 1947: 166; Lallemand, 1963: 47; Nagai and Porion, 1996: 21.
Lycorma olivacea Kato, 1929: 550; Kato, 1933: pl. 4, fig. 5; Metcalf, 1947: 166; Lallemand, 1963: 46; Nagai and Porion, 1996: 21. syn. nov.
Type locality: Taihoku, Formosa [= Northern Taiwan].
Description: Measurements: body length, male (n = 20) 25.7 mm (22.2–28.5 mm), female (n = 23) 28.9 mm (26.5–32.2 mm); tegmen length, male (n = 20) 22.3 mm (18.9–25 mm), female (n = 23) 25.3 mm (23.2–27.8 mm).
Head: general color brown to black (Fig. 9A, B, C, D); cephalic process short, protruding upward and backward (Fig. 9A, C, D); vertex broader than long, with 1 pair of dark brown spot on disc and 1 bigger dark brown spot on each latero-posterior angle, lateral margins medially carinate (Fig. 9A, C); frons longer than broad, with 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 9B); antennae marigold yellow (Fig. 9B, C); clypeus longer than frons (Fig. 9B); labium elongate, not reaching apex of abdomen (Fig. 9B).
Thorax: (Fig. 9A, C, D) pronotum brown to black, with 1 pair of dark brown spot on disc and several smaller dark brown spots near each lateral margin; propleuron and prosternum black; mesonotum black.
Tegmina: (Fig. 9A, D) basal 3/5 scarlet red to cerulean blue, with several brown to black spots varying in size, sometimes covered with white powder; apical 2/5 brown to black, with veins colored as basal 3/5 of tegmen.
Hindwings: (Fig. 9A, D) basal 2/3 pink to light blue, with 2–3 brown spots on CuP vein, 3–5 brown spots on A1 vein, and 1 long patch on disc of hindwing, extending to apical brown area, posterior margin pale brown; apical 1/3 brown.
Legs: (Fig. 9A, B, C, D) general color dark brown to black.
Abdomen: (Fig. 9A, B, D) general color black; laterosternites and posterior margin of tergites sometimes marigold yellow.
Fig. 9.
Lycorma meliae Kato, 1929, adult. A–C, Blue female. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale). D, Red female.
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin strongly concave, dorso-anterior margin acute, dorso-posterior margin angulate, ventro-posterior margin with a small acute projection (Figs. 10A, 11A); 10th abdominal segment with ventral margin moderately convex at 1/5 from apex in lateral view (Figs. 10A, 11A), apical margin about 1.5 times broader than basal margin, medially concave as “V” shape dorsally but slightly concave as “U” shape ventrally in dorsal view (Figs. 10B, 11B); 11th abdominal sternite about 2 times longer than 11th abdominal tergite (Figs. 10B, 11B); genital styles longer than 10th abdominal segment in lateral view (Figs. 10A, 11A), suboval with a hook on each lateral margin at 1/2 from base in ventral view (Figs. 10C, 11C); lateral phallobasal lobes membranous (Figs. 10D, E, 11D, E); phallobasal conjunctival processes exposed, straight and sclerotized, about 5 times longer than sheath, apexes inflated, folding backward and downward in ventral view (Figs. 10D, E, 11D, E).
Fig. 10.
Lycorma meliae Kato, 1929, male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Fig. 11.
Lycorma olivacea Kato, 1929 [new junior synonym of Lycorma meliae Kato, 1929], male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: Holotype: female of Lycorma meliae Kato, 1929 (examined from photographs, Fig. 12A, B) [Taihoku, Formosa (July 1924) Col. M. Kato] [Lycorma meliae n. sp. det. M. Kato] [Type No. 169, M. Kato coll.] (UMUT); female of Lycorma olivacea Kato, 1929 (examined from photographs, Fig. 12C, D) [Tattaka, Formosa (X. 1926) Col. S. Issiki] [Lycorma olivacea n. sp. det. M. Kato] [Type No. 170, M. Kato coll.] (UMUT).
Fig. 12.
Lycorma meliae Kato, 1929, type specimens of the synonymized taxa. A–B, Holotype of Lycorma meliae Kato, 1929. A, Holotype. B, Labels. C–D, Holotype of Lycorma olivacea Kato, 1929. C, Holotype. D, Labels. (UMUT, photographs by M. Yago).
Other materials: 2 females, Shinten [= Xindian, New Taipei City], IV-08-1932, leg. M. Chujo (NCHU); 1 female, Kukuan, Taichung, VI-08-1987, leg. W. H. Chen (NCHU); 2 females, Lishan, Taichung, XI-28-1959, collector unknown (NCHU); 1 female, Anmashan, Taichung, VII-03-1972, leg. F. C. Lu (NCHU); 1 female, Xiaowulai, Taoyuan, XI-13-1987, leg. S. C. Tsaur (NCHU); 1 female, Huisun Experimental Forest Station, Nantou, XI-1989, leg. J. T. Yang (NCHU); 1 female, Tianwei, Changhua, II-02-1987, leg. S. R. Yang (NCHU); 1 female, Zhongxing New Village, Nantou, VII-12-1975, leg. C. K. Chang (NCHU); 2 females, Xiaowulai, Taoyuan, VI-20-2002, leg. S. P. Wu (NMNS); 1 female, Baling, Taoyuan, VII-12-1969, leg. B. S. Chang (NMNS); 1 female, Yuanyanghu, Chienshih, Hsinchu, X-22-2004, leg. W. T. Yang (NMNS); 1 female, Piluchi, Jenai, Nantou, XII-04-1991, leg. Y. C. Shiau (NMNS); 1 female, Puli, Nantou, I-16-2010, leg. T. C. Cheng (NMNS); 1 male, Jiujiufeng, Nantou, X-20-1985, leg. C. S. Lin (NMNS); 2 males, Shuangshikou, New Taipei City, VII-07-1982, leg. T. C. Hsu (NTU); 1 male, Xianjiyan, Taipei, VII-06-1986, leg. T. C. Hsu (NTU); 1 female, same locality, V-25-1995, leg. Y. W. Lee (NTU); 1 male, Wulai, New Taipei City, VI-26-1991, leg. H. Yang (NTU); 1 female, same locality, VII-26-1991, leg. Y. T. Chen (NTU); 1 male, 2 females, Taiheizan, Formosa [= Taipingshan, Ilan], VII-09-1933, leg. M. Chujo (NTU); 1 male, 5 females, Shinchiku, Formosa [= Hsinchu], VII-1918, leg. J. Sonan & K. Miyake (NTU); 2 males, 1 female, Yeboshi, Rato-Gun [= Chihtuan, Ilan], VII-07-1933, leg. T. Shiraki (NTU); 4 females, Urai (Taihoku) [= Wulai, New Taipei City], VI-24-1933, leg. K. Kobayashi (NTU); 2 females, Taito [= Taitung], VI-07-1914, leg. I. Nitobe (NTU); 1 female, Shikikun, Formosa [= Skikun, Ilan], VII-11-1933, leg. M. Chujo (NTU); 1 female, Experimental Farm An Kang, College of Bioresources and Agriculture, National Taiwan University, Xindian, New Taipei City, X-19-2009, leg. W. J. Wu (NTU); 1 female, Sanxia, New Taipei City, IV-27-1987, leg. S. J. Peng (NTU); 6 males, 6 females, Shinten [= Xindian, New Taipei City], VI-20-1920, leg. J. Sonan (NTU); 1 female, same locality, VI-20-1920, leg. J. Sonan (NTU); 3 females, same locality, XII-10-1933, leg. S. Sakamoto (NTU); 1 male, Suanlianpi, Ilan, XI-11-1984, leg. C. C. Chiang (NTU); 1 female, Lishan, Taichung, VII-18-1980, leg. T. C. Hsu (NTU); 1 male, Neihu, Taipei, IV-20-1978, leg. S. C. Tsaur (NTU); 1 female, Nanshan, Ilan, VI-24-1988, leg. Y. H. Hsu (NTU); 1 male, Taroko, Hualien, V-24-1993, leg. C. L. Li (NTU); 3 males, 28 females, Paiyang falls, Hualien, XI-18-1989, leg. S. C. Tsaur (NTU); 2 males, 12 females, same locality, XI-18-1989, leg. C. T. Ting (NTU); 5 males, 9 females, same locality, XI-18-1989, leg. S. Fang (NTU); 2 males, 1 female, same locality, XI-21-1989, leg. C. F. Lee (NTU); 1 male, Wulai, New Taipei City, IX-27-1988, leg. C. H. Tzeng (NTU); 1 female, Fuhsing, Taoyuan, VII-(10-11)-1986, leg. K. C. Chou & C. H. Yang (TARI); 1 female, Litungshan, Hsinchu, X-15-1995, leg. W. I. Chou (TARI); 1 female, Cien, Hualien, VIII-18-1993, leg. W. I. Chou (TARI); 1 male, Xindian, New Taipei City, VII-14-2016, leg. W. C. Yeh (TFRI); 1 male, Taipei Botanic Garden, Taipei, VII-15-1995, leg. J. J. Hsiao (TFRI); 1 female, Daxi, Taoyuan, VI-20-2014, leg. S. S. Lu (TFRI); 1 female, Fusan Botanical Garden, Ilan, VI-18-1992, leg. Y. B. Fan (TFRI); 1 male, Sikanshui, Xindian, New Taipei City, XII-19-2006, leg. C. C. Lee (TFRI); 1 female, Wuling, Taichung, IX-26-1994, leg. Z. K. Wang (TFRI); 1 female, Jialuohu, Datong, Ilan, VII-20-2000, leg. W. C. Yeh (TFRI).
Host plant: Melia azedarach L. (Meliaceae) (Kato 1929).
Distribution: Taiwan.
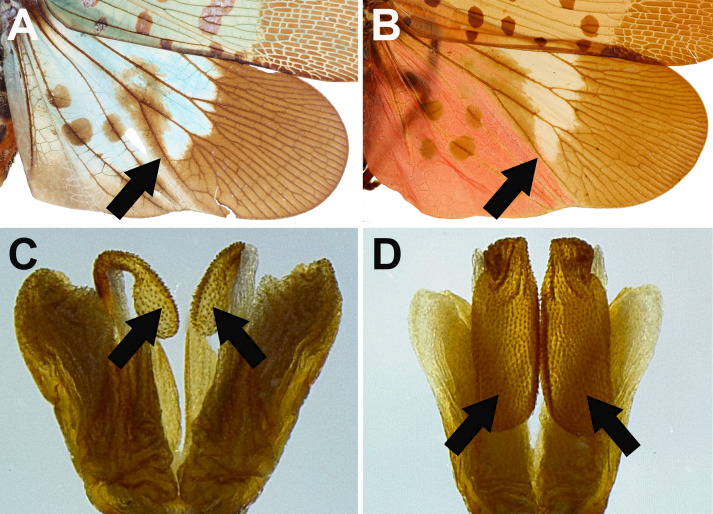
Remarks: The specimens with transitional coloration sometimes look similar to Lycorma delicatula but can be distinguished by the following characters: (1) L. meliae has a long patch extending to apical brown area on disc of hindwings (Fig. 13A), while L. delicatula does not (Fig. 13B); (2) L. meliae has apexes of phallobasal conjunctival processes slightly inflated (Fig. 13C), while L. delicatula has apexes of phallobasal conjunctival processes strongly inflated and enlarged (Fig. 13D). This species performs a series of body coloration transformation during the process of maturation, from scarlet red, pale reddish brown, brown, olivaceous green to cerulean blue. The blue individuals, which were originally named as Lycorma olivacea Kato, 1929, may be actually the mature stage of the species (J. M. Dow, personal communication, February 13, 2020). The morphology of male genitalia supports the view that L. meliae and L. olivacea are the same species (Figs. 10, 11).
Fig. 13.
Diagnostic characters for Lycorma meliae and L. delicatula. A–B, Hindwings in dorsal view. A, L. meliae. B, L. delicatula. C–D, Apexes of phallobasal conjunctival processes in dorsal view. C, L. meliae. D, L. delicatula.
Genus Penthicodes Blanchard, 1845
Penthicodes pulchella (Guérin-Méneville, 1838)
(Figs. 14, 15)
Aphaena pulchella Guérin-Méneville, 1838: 186.
Aphana confuscius White, 1846: 24.
Aphaena io Walker, 1851: 279.
Aphaena confuscius Walker, 1851: 280.
Aphana nigroirrorata Stål, 1854: 244.
Aphaena nigroirrorata Walker, 1858b: 316.
Aphana pulchella Atkinson, 1885: 144; Kato, 1933: pl. 4, fig. 3.
Penthicodes pulchella Distant, 1918: 198; Metcalf, 1947: 130; Lallemand, 1963: 26; Nagai & Porion, 1996: 21; Constant, 2010: 14.
Penthicodes wachsi Schmidt, 1930: 115.
Type locality: Java (Metcalf 1947).
Description: Measurements: body length, male (n = 5) 20.5 mm (18.2–22.5 mm), female (n = 6) 21.9 mm (20.4–24.6 mm); tegmen length, male (n = 5) 17.2 mm (15.8–18.7 mm), female (n = 6) 18.9 mm (17.6–21 mm).
Head: general color brown to dark brown (Fig. 14A, B, C); vertex bumpy, broader than long, with numerous tiny white granules, lateral margins slightly carinate (Fig. 14A, C); frons wrinkled, longer than broad, with 2 weak longitudinal carinae (Fig. 14B); antennae dark brown (Fig. 14B, C); clypeus longer than frons, with numerous tiny pits and tiny white granules (Fig. 14B); labium elongate, reaching apex of abdomen (Fig. 14B).
Thorax: (Fig. 14A, C) general color brown to dark brown; pronotum wrinkled, with several tiny pits near each lateral margin and numerous tiny white granules throughout; mesonotum with numerous small black irregular patches between lateral carinae and numerous tiny white granules throughout.
Tegmina: (Fig. 14A) basal 5/6 brown, with several black spots along anterior margin, numerous black transverse-heart-shaped patches varying in size on longitudinal veins, and numerous tiny dark brown spots throughout; apical 1/6 nearly white, with several small brown spots along apical margin and several dark brown irregular patches varying in size near apical margin, biggest one on apico-posterior angle.
Hindwings: (Fig. 14A) basal 2/3 marigold yellow, with 1 dark brown spot near base, 6–7 dark brown spots on CuP vein, 5–6 dark brown spots on A1 vein, 2–4 dark brown spots between A2 and A3 vein, and 4–6 white spots near apical brown area, basal margin red, posterior margin pale brown; apical 1/3 dark brown, sometimes with white powder on disc.
Legs: (Fig. 14A, B, C) general color dark brown to black; femora with several pale yellowish brown irregular patches; fore-and mesotibiae with 2 pale yellowish brown rings; metatibiae with 4–5 lateral spines.
Abdomen: (Fig. 14A, B) general color black; tergites sometimes red.
Fig. 14.
Penthicodes pulchella (Guérin-Méneville, 1838), adult female. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale).
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin flat, dorso-anterior margin obtuse, dorso-posterior margin rounded, ventro-posterior margin with a small obtuse projection (Fig. 15A); 10th abdominal segment with ventral margin flat in lateral view (Fig. 15A), apical margin nearly as broad as basal margin, medially concave as “V” shape dorsally but weakly concave ventrally in dorsal view (Fig. 15B); 11th abdominal sternite about 2 times longer than 11th abdominal tergite (Fig. 15B); genital styles as long as 10th abdominal segment in lateral view (Fig. 15A), suboval with a hook on each lateral margin at 1/3 from base in ventral view (Fig. 15C); lateral phallobasal lobes membranous (Fig. 15D, E); phallobasal conjunctival processes exposed, straight and sclerotized in basal 2/3, about 7 times longer than sheath, apexes obtuse (Fig. 15D, E).
Fig. 15.
Penthicodes pulchella (Guérin-Méneville, 1838), male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: 1 male, Gandaushi, Nantou, VI-08-1964, leg. S. L. C. (NCHU); 1 male, Kuraru, Formosa [= Kueitzuchiao, Hengchun, Pingtung], VIII-22-1935, leg. Y. Miwa (NTU); 2 males, same locality, III-(12-15)-1931, leg. T. Shiraki (NTU); 1 male, same locality, VII-31-1931, leg. T. Shiraki (NTU); 2 females, Takesaki, Formosa [= Zhuqi, Chiayi], IX-30-1922, leg. M. Kato (NTU); 1 female, Rikiriki [= Lichi, Taitung], XI-20-1930, leg. R. Takahashi (NTU); 1 female, Koshun [= Hengchun, Pingtung], II-08-1921, collector unknown (NTU); 1 female, Horisha [= Puli, Nantou], collecting date unknown, leg. T. Shiraki (NTU); 1 female, Baoli Forest Garden, Pingtung, I-29-2021, leg. K. W. Chan & Y. H. Lin (NTU).
Host plant: Acacia confusa Merr. (Fabaceae) (Kato 1931).
Distribution: Taiwan, Cambodia, China, India, Indonesia, Myanmar, Thailand, Vietnam (Constant 2010).
Remarks: This species has only been recorded in southern Taiwan, while its major host (i.e., Acacia confusa) is widely distributed through Taiwan. It seems that this species is more adapted to tropics or has certain habitat requirements.
Genus Pyrops Spinola, 1839
Pyrops candelaria (Linnaeus, 1758)
(Figs. 16, 17)
Cicada candelaria Linnaeus, 1758: 434.
Laternaria candelaria Linnaeus, 1764: 153; Metcalf, 1947: 187.
Fulgora candelaria Linnaeus, 1767: 703; Butler, 1874: 97; Schmidt, 1905: 350; Lallemand, 1963: 73; Chou et al., 1985: 116.
Pyrops candelaria Spinola, 1839: 233; Nagai & Porion, 1996: 24; Wang et al., 2018: 298.
Hotinus candelaria Amyot & Serville, 1843: 491.
Pyrops candelarius Jacobi, 1905: 435; Liang, 1998: 42.
Type locality: China (Linnaeus 1758).
Description: Measurements: body length, male (n = 6) 35.9 mm (31.3–37.7 mm), female (n = 3) 40.8 mm (39.6–41.9 mm); tegmen length, male (n = 6) 30.9 mm (26.9–32.4 mm), female (n = 3) 35.5 mm (33.8–36.9 mm); cephalic process length, male (n = 6) 14.2 mm (12.7–15.8 mm), female (n = 3) 14.6 mm (13.3–15.9 mm).
Head: cephalic process long, protruding forward and upward, narrowing gradually beyond eyes, dorsal and lateral sides orange to cardinal red with numerous small white powdery spots, ventral side yellowish brown, sometimes with 2 rows of white powdery spots side by side between 2 longitudinal carinae, apex narrowing and compressed laterally (Fig. 16A, B, C); vertex orange to cardinal red, broader than long, sometimes with numerous small white powdery spots, lateral margins slightly carinate (Fig. 16A, C); frons yellowish brown, longer than broad, with 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 16B); genae with 1 black transverse band passing through eye (Fig. 16C); postocular flange black (Fig. 16A); antennae dark brown (Fig. 16B, C); clypeus yellowish brown, longer than frons (Fig. 16B); labium black and elongate, not reaching apex of abdomen (Fig. 16B).
Thorax: (Fig. 16A, C) pronotum chrome yellow to red, with several tiny pits on each lateral area and sometimes small white powdery spots near anterior margin; propleuron and prosternum yellow to red, with 1 black band along anterior margin; mesonotum orange to red, with 2 pairs of dark brown reverse-subtriangular patch along anterior margin, 1 dark brown teardrop-shaped patch on each lateral area outside lateral carinae, and sometimes several small white powdery spots between lateral carinae.
Tegmina: (Fig. 16A) general color black with veins green; basal 1/2 with 4 chrome yellow subquadrangular patches with white border along anterior margin, 1 chrome yellow transverse band with white border behind 1st marginal patch, 6–7 chrome yellow suboval patches with white border behind 2nd and 3rd marginal patch, often connected with each other, and 2 chrome yellow spots behind 4th marginal patch; apical 1/2 with 4–6 chrome yellow spots with white border in a transverse row near disc of tegmen and 4–8 chrome yellow spots varying in size near apical margin, apical spots sometimes with white border.
Hindwings: (Fig. 16A) basal 2/3 chrome yellow; apical 1/3 dark brown.
Legs: (Fig. 16A, B, C) general color orange to red with tibiae and tarsi of forelegs and mesolegs black; metatibiae with 5 lateral spines.
Abdomen: 1st–8th tergites chrome yellow (Fig. 16A); 4th–8th laterosternites and sternites gray with posterior margin chrome yellow (Fig. 16B).
Fig. 16.
Pyrops candelaria (Linnaeus, 1758), adult female. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale).
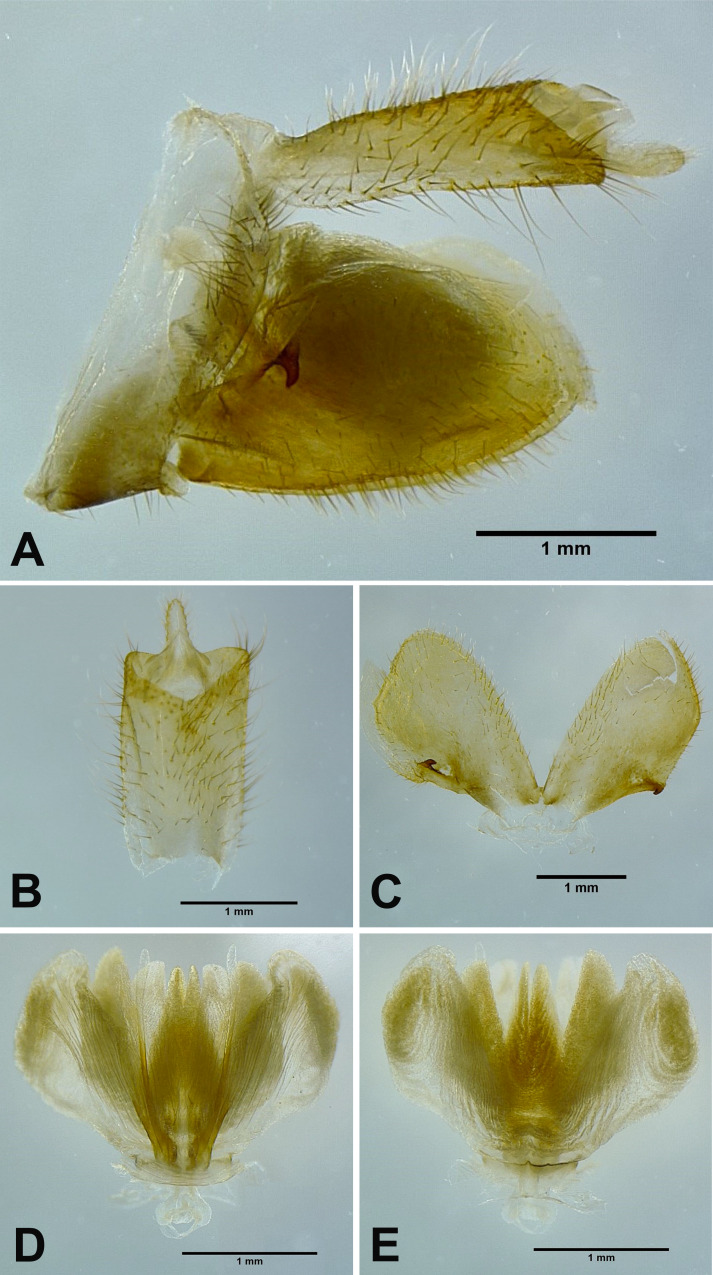
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin flat, dorso-anterior margin obtuse, dorso-posterior margin rounded, ventro-posterior margin with a small obtuse projection (Fig. 17A); 10th abdominal segment with ventral margin flat in lateral view (Fig. 17A), apical margin about 1.5 times broader than basal margin, medially concave as “U” shape dorsally and ventrally in dorsal view (Fig. 17B); 11th abdominal sternite about 4 times longer than 11th abdominal tergite (Fig. 17B); genital styles as long as 10th abdominal segment in lateral view (Fig. 17A), suboval with a hook on each lateral margin at 1/3 from base in ventral view (Fig. 17C); lateral phallobasal lobes membranous (Fig. 17D, E); phallobasal conjunctival processes exposed, straight and sclerotized except for apexes, about 7 times longer than sheath, apexes obtuse (Fig. 17D, E).
Fig. 17.
Pyrops candelaria (Linnaeus, 1758), male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: 1 male, 2 females, Bali (25°8'29.14"N, 121°24'4.03"E), New Taipei City, Dimocarpus longan Lour., III-15-2019, leg. Y. S. Lin (NTU); 1 male, same locality, same host, IV-08-2019, leg. Y. S. Lin (NTU); 1 male, 2 females, same locality, same host, VIII-29-2019, leg. Y. S. Lin (NTU); 3 males, same locality, same host, IX-04-2019, leg. Y. S. Lin (NTU); 1 female, Linkou (25°7'36.19"N, 121°21'4.54"E), New Taipei City, Dimocarpus longan Lour., VIII-12-2021, leg. Y. S. Lin (NTU); 2 males, Kinmen, II-11-2015, leg. C. Chang (TFRI).
Host plant: Dimocarpus longan Lour. (Sapindaceae), Citrus maxima (Burm.) Merr. (Rutaceae), Mangifera indica L. (Anacardiaceae), Triadica sebifera (L.) Small (Euphorbiaceae) (Wang et al. 2000).
Distribution: Taiwan, Cambodia, China, India, Indonesia, Laos, Myanmar, Thailand, Vietnam (Lin et al. 2021).
Remarks: This species is similar to Pyrops spinolae (Westwood, 1842) but can be distinguished by the following characters: (1) P. candelaria has vertex and cephalic process with dorsal side orange to cardinal red (Fig. 18A), while P. spinolae has vertex and cephalic process with dorsal side black (Fig. 18B); (2) P. candelaria has pronotum and mesonotum without 1 black longitudinal band on the median carina (Fig. 18A), while P. spinolae has pronotum and mesonotum with 1 black longitudinal band on the median carina (Fig. 18B). This species was recorded from Taiwan as an invasive species by Lin et al. (2021). To date, the invasive population is still restricted to northern Taiwan.
Fig. 18.
Diagnostic characters for Pyrops candelaria and P. spinolae on their heads and thoraxes in dorsal view. A, P. candelaria. B, P. spinolae.
Pyrops spinolae (Westwood, 1842)
Fulgora spinolae Westwood, 1842: 118; Butler, 1874: 98; Kato, 1929: 548; Kato, 1933: pl. 5, fig. 4; Lallemand, 1963: 76; Schmidt, 1905: 351; Chou et al., 1985: 117.
Pyrops spinolae Schaum, 1850: 64; Jacobi, 1905: 435; Nagai & Porion, 1996: 25; Liang, 1998: 45.
Hotinus spinolae Walker, 1851: 266.
Hotinus nigrirostris Walker, 1858a: 28.
Fulgora nigrirostris Butler, 1874: 98.
Laternaria spinolae Metcalf, 1947: 205.
Laternaria nigrirostris Metcalf, 1947: 201.
Type locality: Mysore and Assam (Westwood 1842).
Materials examined: No specimen was examined in this study.
Distribution: Taiwan (?), Cambodia, China, India, Malaysia, Myanmar, Vietnam (Constant et al. 2016).
Remarks: This species is similar to Pyrops candelaria but can be distinguished by the coloration and markings on the head, pronotum and mesonotum. See remarks of P. candelaria for diagnostic characters. This species was recorded from Taiwan by Kato (1929 1933). However, no voucher specimen collected from Taiwan was found in this study.
Pyrops watanabei (Matsumura, 1913)
(Figs. 19, 20, 21)
Fulgora watanabei Matsumura, 1913: 54; Kato, 1928: 221; Kato, 1933: pl. 4, fig. 1; Lallemand, 1963: 89; Chou et al., 1985: 118.
Fulgora chimara Schumacher, 1915: 129.
Fulgora watanabei var. apicalis Kato, 1928: 221; Kato, 1933: pl. 5, fig. 5.
Laternaria watanabei Metcalf, 1947: 208.
Laternaria watanabei var. formosana Metcalf, 1947: 208.
Hotinus watanabei Matsumura, 1931: 8.
Pyrops watanabei Nagai & Porion, 1996: 26; Liang, 1998: 45; Constant & Pham, 2017: 18.
Type locality: Hoppo, Formosa [= Beipu, Hsinchu, Taiwan] (Matsumura 1913).
Description: Measurements: body length, male (n = 14) 36.8 mm (31.8–40.7 mm), female (n = 10) 43.7 mm (40.3–48 mm); tegmen length, male (n = 14) 32.8 mm (28–36.7 mm), female (n = 10) 38.8 mm (34.9–42.6 mm); cephalic process length, male (n = 14) 10.4 mm (8.1–12.9 mm), female (n = 10) 11.9 mm (10.1–14.7 mm).
Head: general color chrome yellow to marigold yellow (Fig. 19A, B, C, D); cephalic process long, protruding forward and upward, narrowing gradually beyond eyes, dorsal and lateral sides with numerous small white powdery spots, apex strongly inflated (Fig. 19A, B, C, D); vertex broader than long, sometimes covered with white powder, lateral margins slightly carinate (Fig. 19A, C, D); frons longer than broad, with 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 19B); genae with 1 black transverse band passing through eye (Fig. 19C); postocular flange black (Fig. 19A); antennae dark brown (Fig. 19B, C); clypeus longer than frons (Fig. 19B); labium dark brown and elongate, not reaching apex of abdomen (Fig. 19B).
Thorax: (Fig. 19A, C, D) general color chrome yellow; pronotum often covered with white powder; propleuron and prosternum with 1 black band along anterior margin; mesonotum often covered with white powder, with 1 black spot on each lateral area outside lateral carinae.
Tegmina: (Fig. 19A, D) basal 5/6 yellowish white, with 4–5 black patches along anterior margin, 1–3 black patches on clavus, and 4–6 orange spots surrounded by black marking near apical margin; apical 1/6 dark brown, with 2 orange spots with yellowish white border and several yellowish white spots varying in size.
Hindwings: general color white to bluish white with veins yellowish brown (Fig. 19A); apical 1/3 sometimes dark brown (Fig. 19D).
Legs: (Fig. 19A, B, C, D) general color black, often covered with white powder, ventral side of metafemur with 1 chrome yellow long patch.
Abdomen: 1st–8th tergites chrome yellow, often covered with white powder (Fig. 19A, D); 3rd–8th laterosternites and sternites pink (Fig. 19B).
Fig. 19.
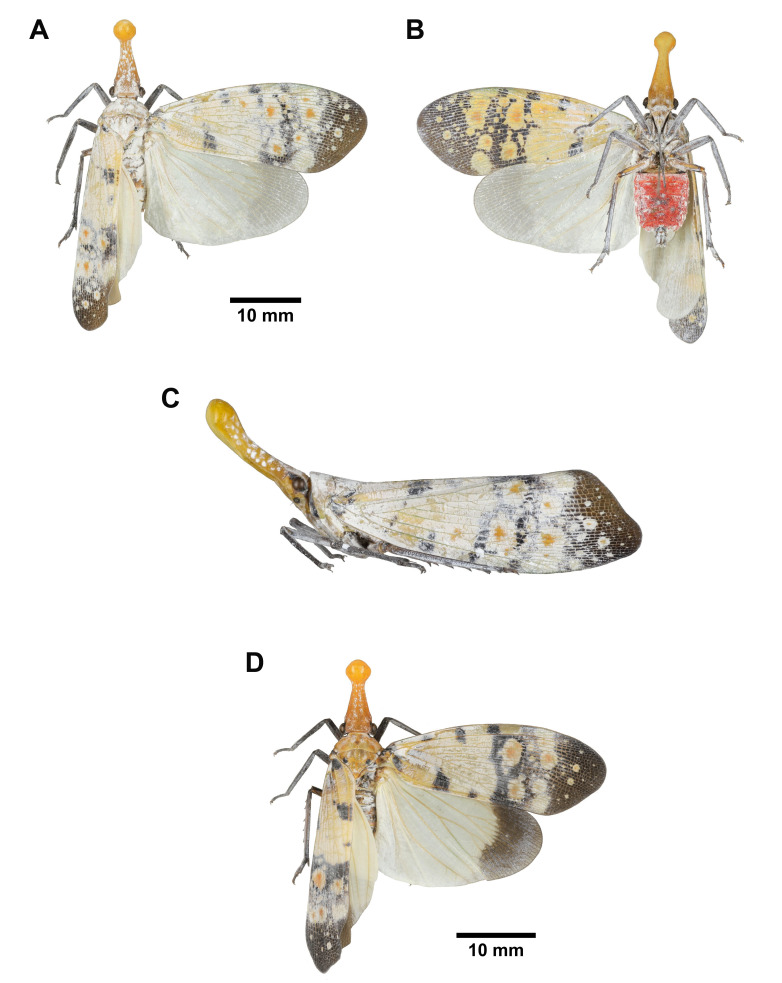
Pyrops watanabei (Matsumura, 1913), adult. A–C, Normal male. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale). D, Variant male.
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin flat, dorso-anterior margin obtuse, dorso-posterior margin rounded, ventro-posterior margin with a small obtuse projection (Fig. 20A); 10th abdominal segment with ventral margin flat in lateral view (Fig. 20A), apical margin about 2 times broader than basal margin, medially concave as “U” shape dorsally and ventrally in dorsal view (Fig. 20B); 11th abdominal sternite about 3 times longer than 11th abdominal tergite (Fig. 20B); genital styles as long as 10th abdominal segment in lateral view (Fig. 20A), suboval with a hook on each lateral margin between 1/3 and 1/2 from base in ventral view (Fig. 20C); lateral phallobasal lobes membranous (Fig. 20D, E); phallobasal conjunctival processes not exposed (Fig. 20D, E).
Fig. 20.
Pyrops watanabei (Matsumura, 1913), male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Materials examined: Lectotype: female of Fulgora (Hotinus) watanabei Matsumura, 1913 (examined from photographs, Fig. 21A, B, C) [Hotinus watanabei Mats.] [Hotinus watanabei Mats.] [Hotinus watanabei det. Matsumura] [Formosa Matsumura /underside: Hoppo, 1st VII ‘07] [Type Matsumura] [Lectotype Fulgora (Hotinus) watanabei Mats. det. A.P. Liang & M. Suwa 1997] (HUIC).
Fig. 21.
Pyrops watanabei (Matsumura, 1913), type specimens of the synonymized taxon, Hotinus watanabei Matsumura, 1913. A. Lectotype. B–C. Labels of lectotype. D. Paralectotype. E. Labels of paralectotype. F. Paralectotype. G. Labels of paralectotype. (HUIC, photographs from the program “Digitization of Historic Museum Collections of Taiwan Deposited in Foreign Countries” by NTU).
Paralectotype: female of of Fulgora (Hotinus) watanabei Matsumura, 1913 (examined from photographs, Fig. 21D, E) [Hoppo] [Paralectotype Fulgora (Hotinus) watanabei Mats. det. A.P. Liang & M. Suwa 1997] (HUIC); female of of Fulgora (Hotinus) watanabei Matsumura, 1913 (examined from photographs, Fig. 21F, G) [Formosa Matsumura /underside: Hoppo, 27 VII ‘07] [Paralectotype Fulgora (Hotinus) watanabei Mats. det. A.P. Liang & M. Suwa 1997] (HUIC).
Syntype: female of of Fulgora chimara Schumacher, 1915 (examined from photographs) [Kosempo, Formosa, H. Sauter, VII. 1911] [Fulgora chimara Schum., F. Schumacher det.] [Syntypus] (SDEI).
Other materials: 3 females, Baoshan Village, Hsinchu, VIII-11-2010, leg. C. T. Tang (NCHU); 1 female, Xiaowulai, Taoyuan, IX-03-1987, leg. K. S. Huang (NCHU); 1 male, Nanshanchi, Jenai, Nantou, VI-(07-08)-1996, collector unknown (NMNS); 2 males, 1 female, Tanshui, Taipei, VII-04-1996, collector unknown (NMNS); 4 males, 1 female, Hsiangshan, Taipei, VII-26-1997, leg. W. I. Chou (NMNS); 2 females, Bishanyan, Taipei, VIII-10-1994, leg. C. Y. Hsiao (NMNS); 1 female, Shinten [= Xindian, New Taipei City], IX-1924, leg. M. Kato (NTU); 1 female, Taihoku, Formosa [= Northern Taiwan], 1928, collector unknown (NTU); 2 males, 1 female, Guizikeng (25°9'6.65"N, 121°29'37.46"E), Beitou, Taipei, Triadica sebifera (L.) Small, VII-16-2019, leg. Y. S. Lin (NTU); 1 male, same locality, same host, VIII-28-2019, leg. Y. S. Lin (NTU); 6 males, 1 female, same locality, same host, VII-26-2020, leg. Y. S. Lin (NTU); 1 female, Bali (25°8'34.61"N, 121°24'22.57"E), New Taipei City, Triadica sebifera (L.) Small, VI-01-2020, leg. Y. S. Lin (NTU); 2 males, same locality, same host, VII-22-2020, leg. Y. S. Lin (NTU); 1 male, Bishanyan, Taipei, VIII-19-1994, leg. W. I. Chou (TARI).
Host plant: Triadica sebifera (L.) Small (Euphorbiaceae) (Kato 1928).
Distribution: Taiwan, China (Wang et al. 2018).
Remarks: Constant and Pham (2017) treated Fulgora watanabei var. apicalis Kato, 1928 as the synonym of this species. The present study agrees with this synonym after comparing their morphology of male genitalia.
Genus Saiva Distant, 1906
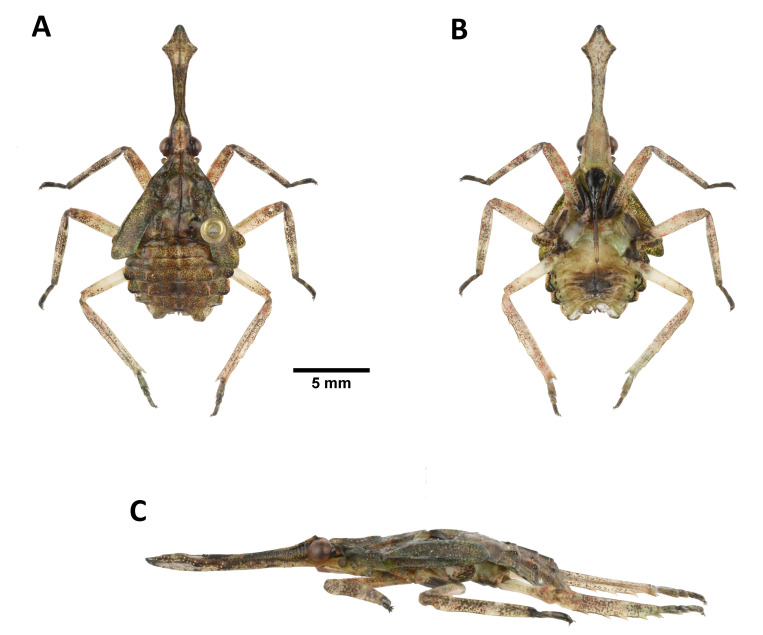
Saiva formosana Kato, 1929
(Figs. 22, 23, 24, 25)
Saiva formosana Kato, 1929: 549; Kato, 1933: pl. 3, fig. 4; Metcalf, 1947: 179; Lallemand, 1963: 67; Nagai & Porion, 1996: 23.
Type locality: Kuraru, Formosa [= Kueitzuchiao, Hengchun, Pingtung, Taiwan].
Description of adult: Measurements: body length, male (n = 7) 23.6 mm (22.3–25.9 mm), female (n = 7) 28.1 mm (26.3–29.3 mm); tegmen length, male (n = 7) 20.0 mm (19.1–21.8 mm), female (n = 7) 23.8 mm (22.3–25.3 mm); cephalic process length, male (n = 7) 6.0 mm (4.6–7.1 mm), female (n = 7) 8.0 mm (6.5–9.1 mm).
Head: general color reddish brown; cephalic process very slender and long, protruding forward and upward, narrowing suddenly beyond eyes, lateral sides black, dorsal and ventral sides yellowish brown and concave (Fig. 22A–D); vertex broader than long, lateral margins slightly carinate (Fig. 22A, C, D); frons longer than broad, with 2 rows of white powdery spots side by side between 2 longitudinal carinae extending from ventral side of cephalic process (Fig. 22B); genae with 1 black transverse band passing through eye (Fig. 22C); antennae black (Fig. 22C); clypeus longer than frons (Fig. 22B); labium dark brown, elongate, not reaching apex of abdomen (Fig. 22B).
Thorax: (Fig. 22A, C, D) general color reddish brown; pronotum with a transverse series of 4 black spots on disc; propleuron with 1 black spot along anterior margin; prosternum with 1 black spot along anterior margin and ventral margin, respectively; mesonotum with 1 pair of black reverse-subtriangular patch between 1 pair of black semicircular patch along anterior margin, 1 black teardrop-shaped patch and 1 small black spot along each lateral side, and 1 black suboval patch on posterior angle.
Tegmina: (Fig. 22A, D) basal 3/4 yellowish green to salmon pink, with several yellow spots with black border and numerous small black spots; apical 1/4 dark brown, with veins and several spots colored as basal 3/4 of tegmen.
Hindwings: (Fig. 22A, D) general color dark brown, with 3 bluish white long bands arising from base, middle one bifurcate at apex, sometimes not connected.
Legs: (Fig. 22A–D) general color yellowish brown to reddish brown; mesotibiae with black rings at apex and base; metatibiae with 6 lateral spines; fore-and mesotarsomeres black; metatarsomeres yellowish brown, sometimes black.
Abdomen: tergites black (Fig. 22A, D); sternites black in disc and yellowish brown in each lateral area with black spots, posterior margins yellowish brown (Fig. 22B).
Fig. 22.
Saiva formosana Kato, 1929, adult. A–C, Red male. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale). D, Green female.
Male genitalia: 9th abdominal segment, in lateral view, with anterior margin flat, dorso-anterior margin obtuse, dorso-posterior margin rounded, ventro-posterior margin with a small obtuse projection (Fig. 23A); 10th abdominal segment with ventral margin slightly concave at 1/3 from apex in lateral view (Fig. 23A), apical margin about 2 times broader than basal margin, medially concave as “U” shape dorsally and ventrally in dorsal view (Fig. 23B); 11th abdominal sternite about 2 times longer than 11th abdominal tergite (Fig. 23B); genital styles as long as 10th abdominal segment in lateral view (Fig. 23A), subtriangular or suboval, with a hook on each lateral margin between 1/3 and 1/2 from base in ventral view (Fig. 23C); ventral pair of lateral phallobasal lobes slightly sclerotized, dorsal pair of lateral phallobasal lobes membranous (Fig. 23D, E); phallobasal conjunctival processes not exposed (Fig. 23D, E).
Fig. 23.
Saiva formosana Kato, 1929, male genitalia. A, Male genitalia, left lateral view. B, 10th–11th abdominal segments, dorsal view. C, Genital styles, ventral view. D, Phallic complex, ventral view. E, Phallic complex, dorsal view.
Description of fifth-instar nymph: Measurements: body length (n = 1) 18.4 mm; cephalic process length (n = 1) 7.0 mm.
Head: cephalic process long, broad and flat, protruding forward, dorsal side greenish brown, with concave on disc, lateral sides greenish brown, ventral side reddish white with numerous small brown spots, apex arrowheaded, with several small white granules along lateral margin (Fig. 24A–C); vertex reddish white, longer than broad, with black band along each lateral side and numerous small brown spots on disc (Fig. 24A); frons reddish white, longer than broad, with 2 longitudinal carinae extending from ventral side of cephalic process and numerous small brown spots (Fig. 24B); genae with 2 black transverse bands passing through eye and 1 orange patch behind eye (Fig. 24C); antennae yellowish brown (Fig. 24A, C); clypeus black, longer than frons (Fig. 24B); labium elongate, basal 2/3 dark brown, apical 1/3 yellowish brown, apex dark brown, not reaching apex of abdomen (Fig. 24B).
Thorax: (Fig. 24A, C) general color olive green, with numerous small brown spots; anterior wing pads olive green, with several yellow spots and numerous small brown spots, extending to 3rd abdominal segment; posterior wing pads white, with small brown spots on apex.
Legs: (Fig. 24A–C) forecoxae and foretrochanters black, other coxae and trochanters dark brown; tibiae and femora reddish white, with numerous small brown spots, fore-and mesotibiae with 2 white rings; hindtarsus with 3 tarsomeres; tarsomeres black, 2nd segments of fore-and mesotarsomeres white.
Abdomen: tergites greenish brown, with numerous small brown spots (Fig. 24A, C); sternites dark brown in disc and yellowish white in each lateral area (Fig. 24B).
Fig. 24.
Saiva formosana Kato, 1929, fifth-instar nymph. A, Habitus, dorsal view. B, Habitus, ventral view. C, Habitus, left lateral view (not to scale).
Materials examined: Holotype: female of Saiva formosana Kato, 1929 (examined from photographs, Fig. 25) [Kuraru, Formosa (Apr. 1925) Col. M. Kato] [Saiva formosana n. sp. det. M. Kato] [Type No. 168, M. Kato coll.] (UMUT).
Fig. 25.
Saiva formosana Kato, 1929, type specimen. A, Holotype. B, Labels. (UMUT, photographs by M. Yago).
Other materials: 1 male, Huisun Experimental Forest Station, Nantou, IV-20-2021, leg. C. A. Liao & J. F. Tsai (NMNS); 1 male, 1 female, Erbazi Botanical Garden (24°56'17.52"N, 121°30'3.34"E), Xindian, New Taipei City, Elaeocarpus decipiens Hemsl. ex F.B. Forbes & Hemsl., IV-29-2020, leg. Y. S. Lin (NTU); 3 males, 1 female, 1 nymph, same locality, same host, V-13-2020, leg. Y. S. Lin (NTU); 3 males, 5 females, same locality, same host, VI-03-2020, leg. Y. S. Lin (NTU); 1 male, 1 female, same locality, same host, V-12-2021, leg. Y. S. Lin (NTU); 1 male, Northern Cross-Island Highway 64K, Ilan, VI-02-1992, leg. C. C. Chen (NTU); 1 male, Mt. Dongmao Trail 3K ((24°10'52.17"N, 120°56'46.59"E)), Kukuan, Taichung, V-01-2022, leg. M. C. Chiu (NTU).
Host plant: Elaeocarpus decipiens Hemsl. ex F.B. Forbes & Hemsl. (Elaeocarpaceae).
Distribution: Taiwan.
Remarks: This species is similar to Saiva gemmata (Westwood, 1848) but can be distinguished by the following characters: (1) S. formosana has vertex without a longitudinal black stripe on the median carina (Fig. 26A), while S. gemmata has vertex with a longitudinal black stripe on the median carina (Fig. 26B); (2) S. formosana has 4 black patches along the anterior margin of mesonotum (Fig. 26A), while S. gemmata has 2 black patches along the anterior margin of mesonotum (Fig. 26B); (3) S. formosana has tegmina with general color yellowish green to salmon pink (some individuals with transitional color) (Fig. 26C, D), while S. gemmata has tegmina with three brilliant color, blue, green and red (Fig. 26E); (4) S. formosana has ventral pair of lateral phallobasal lobes of male genitalia without apexes distinctly inflated (Fig. 26F), while S. gemmata has ventral pair of lateral phallobasal lobes of male genitalia with apexes distinctly inflated (Fig. 26G). The fifth-instar nymphs of this species are similar to those of Pyrops species but can be distinguished by the concave on disc of cephalic process.
Fig. 26.
Diagnostic characters for Saiva formosana and S. gemmata. A–B, Heads and thoraxes in dorsal view. A, S. formosana. B, S. gemmata. C–E, Tegmina in dorsal view. C, S. formosana, red specimen. D, S. formosana, green specimen. E, S. gemmata. F–G, Apexes of ventral pair of lateral phallobasal lobes of male genitalia in ventral view. F, S. formosana. G, S. gemmata.
Genus Zanna Kirkaldy, 1902
Zanna chinensis (Distant, 1893)
Pyrops chinensis Distant, 1893: 444; Kato, 1929: 549; Kato, 1933: pl. 3, fig. 2.
Pyrops distanti Schmidt, 1911: 163.
Zanna chinensis Metcalf, 1947: 249; Lallemand, 1963: 93; Nagai & Porion, 1996: 27; Chou et al., 1985: 113.
Zanna distanti Metcalf, 1947: 250.
Type locality: China (Distant 1893).
Materials examined: No specimen was examined in this study.
Distribution: Taiwan (?), China, India, Vietnam (Bourgoin 2022).
Remarks: This species was recorded from Taiwan (Hengchun) by Kato (1929 1933). However, no voucher specimen collected from Taiwan was found in this study.
DISCUSSION
The present study is the first comprehensive taxonomic study of Fulgoridae from Taiwan, providing detailed descriptions and photos for eight Taiwanese lanternflies. Absolute numbers of lanternfly species occurring in Taiwan remain unclear. Although one new record (Limois westwoodii) is added here, specimens of three other previously recorded species (Pyrops spinolae, Zanna chinensis, and Lycorma deliculata) were not found in either field or museum collections in this study. Kato (1933) documented the records of P. spinolae and Z. chinensis in Taiwan with illustrations of specimens, but the repository of those specimens was not mentioned. These voucher specimens might have been deposited at UMUT (University Museum, University of Tokyo, Japan), where Kato’s collections were preserved. L. delicatula was recorded from Taiwan by Chou et al. (1985), but no information regarding voucher specimens was provided. It is worth mentioning that L. delicatula is somewhat similar to the brown specimens of L. meliae, so it is possible that Chou et al. (1985) misidentified L. meliae as L. delicatula. Unfortunately, this inference could not be verified without a voucher specimen. In addition, Lycorma meliae and Limois kikuchii were not collected in the field during this investigation, though L. meliae used to be widespread around northern Taiwan based on the bulk specimens deposited at museums. Habitat destruction is thought to be one of the potential factors influencing population size of lanternflies since one recorded habitat in Wulai, where a stable population of L. meliae used to exist (Y. F. Hsu, personal communication, January 13, 2020), was found to be destroyed in the recent survey.
The intraspecific polymorphisms on body coloration of lanternflies are prevalent in this study. Saiva formosana was previously described as having pale reddish brown tegmina (Kato 1929; Lallemand 1963). However, yellowish green individuals were found with pale reddish brown ones on the same host plant species from the same locality in this study. In addition, L. meliae and L. olivacea are similar in morphology except for body coloration. The former is red, whereas the latter is blue. A previous science exhibition work found that the newly emerged adults of L. meliae are red in body coloration and will gradually turn blue during the process of maturation. Namely, L. olivacea actually might be the mature stage of L. meliae (J. M. Dow, personal communication, February 13, 2020). The results produced in this study from comparing their holotypes and male genitalia support the idea that these two lanternflies are the same species. The two cases mentioned above suggest the instability of body coloration as a diagnostic character for lanternfly species.
Male genitalia act as a discriminative character not only for clarifying the relationship between individuals of species with body coloration polymorphism but also for distinguishing species similar in appearance. For instance, many Limois species are difficult to identify due to similar body coloration or markings (e.g., L. kikuchii and L. westwoodii). Wang et al. (2020) reviewed all the extant species of the genus Limois and described a new species. Although the authors emphasized the importance of male genitalia, the diagnostic characters they mentioned were mostly body coloration or markings. In this study, three new diagnostic characters for L. kikuchii and L. westwoodii based on the morphology of male genitalia were proposed, including the angle of dorso-posterior margin of 9th abdominal segment in lateral view, the shape of apical margin of 10th abdominal segment in dorsal view, and the shape of genital styles in ventral view.
The life-history of many lanternfly species remains unknown, especially for the nymphal stage. Even the morphology of nymphs for most species is poorly understood by scientists. This might be attributed to the difficulty of collecting and breeding lanternfly nymphs. The present study described the fifth-instar nymph of Saiva formosana for the first time and found its morphology similar to Pyrops spp. Urban and Cryan (2009) first investigated the phylogeny of the family Fulgoridae, and the results indicated that the genus Saiva is closely related to Pyrops. The nymphal morphology of S. formosana might somewhat support this result, while further confirmation of nymphal morphology on the relationships among genera are required.
In the future, further investigation is required for confirming the accuracy of the questionable records. Molecular data should be included for providing additional evidence to support current species delimitation especially within genera Lycorma and Limois. The present study failed to collect Lycorma meliae and Limois kikuchii from the field and instead only examined the museum collections. Citizen science might offer solutions to the difficulties of collecting these two species. Constant et al. (2016) reported a successful case of collaborating with “citizen scientists,” which may allow entomologists to have better understanding of the biodiversity in a certain area and even obtain the biological information about the species. Taiwan seems suitable for conducting citizen science since many Taiwanese hobbyists like to take photos of insects in the field and share them on social media. Perhaps collaborating with citizen scientists is a more efficient approach to study taxa that are difficult to collect and raise such as lanternflies.
CONCLUSIONS
The intraspecific polymorphisms on body coloration prevalently occur within the family Fulgoridae, and thus body coloration is not a stable character for lanternfly species. The present study provided detailed descriptions and photos of six genera and eight species from Taiwan, emphasizing the morphology of male genitalia. The results indicated that male genitalia act as a discriminative character of lanternflies not only for clarifying the relationship between individuals of species with body coloration polymorphism but also for distinguishing species similar in appearance.
Acknowledgments
We are grateful to Dr. Shun-Chern Tsaur (NTU, Taiwan), Yu-Hsiu Lin (NTU, Taiwan; TAMU, USA), Kai-Wei Chan (NTU, Taiwan), Dr. Ming-Chung Chiu (NTU, Taiwan), Ching-Feng Lin (Taiwan), Li-Cheng Shih (Taiwan), Bin-Hong Ho (NCHU, Taiwan), Jian-Yi Cho (NCHU, Taiwan), Yu-Hsiang Ho (NCHU, Taiwan) and Kawin Jiaranaisakul (KU, Thailand) for providing specimens; Dr. Hsin-Ting Yeh (NTU, Taiwan) for providing the information about the habitat of Saiva formosana; Dr. Yu-Feng Hsu (NTNU, Taiwan), Dr. Hsien-Tzung Shih (TARI, Taiwan) and Dr. Hui-Yun Tseng (NTU, Taiwan) for suggestions; Dr. Masaya Yago (UMUT, Japan) and Dr. Mei-Ling Chan (NMNS, Taiwan) for providing photographs of type specimens; Dr. Chi-Wei Tsai (NTU, Taiwan), Dr. Chi-Feng Lee (TARI, Taiwan), Dr. Jing-Fu Tsai (NMNS, Taiwan), Dr. Sheng-Feng Lin (NCHU, Taiwan), and Ling-Mu Jaung (TFRI, Taiwan) for their permission of examining and loaning specimens under their care; Yun-Yin Yeh (TFRI, Taiwan) and Yun-Chen Hsieh (TFRI, Taiwan) for providing photographic equipment. JRL was supported by the YF2022 Japan Society for the Promotion of Science (JSPS KAKENHI n°22P22380) Postdoctoral Fellowships, Japan. The study is supported by grants (MOST108-2621-B-002-005-MY3) from the National Science and Technology Council, Taiwan and grant (09-RA-BQ-09) from the Bureau of Animal and Plant Health Inspection and Quarantine, Taiwan.
Footnotes
Authors’ contributions: All authors drafted and revised the manuscript. YSL and JRL conceived the study, YSL carried out the field collection and taxonomic works, and SFS and CCK were the heads of the present research group and provided funding.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: The specimens were deposited at the museums mentioned in this article.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Abramoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int 11:36–42.
- Amyot C, Serville J. 1843. Deuxième partie. Homoptères. Homoptera Latr. Histoire Naturelle des Insectes. Hémiptères. Librairie encyclopédique de Roret, Paris, 490 pp. (in French)
- Atkinson ET. 1885. Notes on Indian Rhynchota. No. 4. J Proc Asiat Soc Bengal 54:127–158.
- Bartlett CR, O'Brien LB, Wilson SW. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Mem Am Entomol Soc 50:1–287.
- Blanchard E. 1845. Septième ordre les Hémiptères. pp. 424–427. In: Blanchard E (ed.). Histoire des insectes, traitant de leurs moeurs et de leurs métamorphoses en général et comprenant une nouvelle classification fondée sur leurs rapports naturels. Librairie F Savy, Paris. (in French)
- Bourgoin T. 2022. FLOW (Fulgoromorpha Lists on The Web): A world knowledge base dedicated to Fulgoromorpha. Version 8, updated. Available at: http://hemiptera-databases.org/flow/. Accessed 15 May 2022.
- Butler AG. 1874. List of the species of Fulgora, with descriptions of new forms in the collection of the British Museum. Proc Zool Soc Lond 42:97–102. doi:10.1111/j.1096-3642.1874.tb02457.x.
- Chou I, Lu JS, Huang J, Wang SZ. 1985. Economic insect fauna of China Fascicle 36. Homoptera, Fulgoroidea. Beijing: Science Press. (in Chinese)
- Constant J. 2010. The lanternfly genus Penthicodes: key to the species and review of the “Ereosoma group” with two new species and one new subspecies (Hemiptera, Fulgoromorpha, Fulgoridae). Zootaxa 2523:1–26. doi:10.5281/zenodo.196336.
- Constant J, Phauk S, Bourgoin T. 2016. Updating lanternflies biodiversity knowledge in Cambodia (Hemiptera: Fulgoromorpha: Fulgoridae) by optimizing field work surveys with citizen science involvement through Facebook networking and data access in FLOW website. Belg J Entomol 37:1–16.
- Constant J, Pham HT. 2017. Review of the clavatus group of the lanternfly genus Pyrops (Hemiptera: Fulgoromorpha: Fulgoridae). Eur J Taxon 305:1–26. doi:10.5852/ejt.2017.305.
- Distant WL. 1893. On the Homopterous genus Pyrops, with descriptions of two new species. Trans Ent Soc Lond 1893:443–449.
- Distant WL. 1906. The Fauna of British India, including Ceylon and Burma. Rhynchota. Vol. 3: Heteroptera-Homoptera. London: Taylor & Francis.
- Distant WL. 1918. The Homoptera of Indo-China. Ann Mag Nat Hist 1:196–200.
- Du Z, Wu Y, Chen Z, Cao L, Ishikawa T, Kamitani S, Sota T, Song F, Tian L, Cai W. 2021. Global phylogeography and invasion history of the spotted lanternfly revealed by mitochondrial phylogenomics. Evol Appl 14:915–930. doi:10.1111/eva.13170. [DOI] [PMC free article] [PubMed]
- Guérin-Méneville FE. 1838. Crustacés, Arachnides et Insectes. Voyage autour du monde, exécuté par ordre du roi, sur la corvette de sa majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825 par M.L.I. Duperrey 2:1–319. (in French)
- Hope FW. 1843. On some rare and beautiful insects from Silhet, chiefly in the collection of Frederick John Parry, Esq. F.L.S. Trans Linn Soc Lond 19:131–136.
- Jacobi A. 1905. Zur Kenntnis der Cicadenfauna von Tonking. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Thiere 21:425–446. (in German)
- Kato M. 1928. Notes on some Formosan Homoptera, with descriptions of new genus and species. Kontyu, Tokyo Entomological Society 2:221–228. (in Japanese)
- Kato M. 1929. Descriptions of some new Formosan Homoptera. Transactions of the Natural History Society of Formosa 19:540–551. (in Japanese)
- Kato M. 1931. Fulgoridae of Japan. Dobutsugaku Zasshi 43:595–598. (in Japanese)
- Kato M. 1932. Notes on some Homoptera from South Manchurai, collected by Mr. Yukimichi Kikuchi. Kontyu, Tokyo Entomological Society 5:216–229.
- Kato M. 1933. Three Colour Illustrated Insects of Japan. Fascicle 4: Homoptera: Fulgoridae and Others. Koseikaku, 127 pp. (in Japanese)
- Kirkaldy GW. 1902. Memoirs on Oriental Rhynchota. Journal of the Bombay Natural History Society 14:46–58.
- Kozub D, Khmelik V, Shapoval J, Chentsov V, Yatsenko S, Litovchenko B, Starikh V. 2000. Helicon Focus 6.7.1 Pro. Helicon Soft Ltd., Ukraine. [software]
- Lallemand V. 1963. Révision des Fulgoridae (Homoptera). Deuxième partie. Faunes asiatique et Australienne. Mémoires de l’Institut royal des Sciences naturelles de Belgique (2e série) 75:1–99. (in French)
- Latreille PA. 1807. Sectio secunda. Familia quarta. Cicadariae. Cicadaires, pp. 152–168. In: Latreille PA (ed.). Genera Crustaceorum et Insectorum secundum ordinem naturalem in familias disposita, iconibus exemplisque plurimis explicate. A. Koenig, Parisiis et Argentorati.
- Liang AP. 1998. Nomenclatorial notes on the Oriental lanternfly genus Pyrops Spinola (Hemiptera: Fulgoroidea: Fulgoridae). Acta Zootaxonomica Sinica 23:41–47.
- Lin YS, Liao JR, Shiao SF, Ko CC. 2021. Origin and potential expansion of the invasive longan lanternfly, Pyrops candelaria (Hemiptera: Fulgoridae) in Taiwan. Biology 10:678. doi:10.3390/biology10070678. [DOI] [PMC free article] [PubMed]
- Linnaeus C. 1758. II. Hemiptera. pp. 434–457. In: Linné C (ed.). Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio Decima, Reformata. Laurentii Salvii, Holmiae. (in Latin)
- Linnaeus C. 1764. Museum Ludovicae Ulricae Reginae Svecorum, Cothorum, Vandalorumque in quo Animalia Rariora, Exotica, Imprimis Insecta & Conchilia defcribuntur & determinantur. Laurentii Salvii, Holmiae. 720 pp. (in Latin)
- Linnaeus C. 1767. II. Hemiptera. pp. 687–743. In: Linné C (ed.). Systema Naturae per regnae tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio Duodecima, Reformata. Laurentii Salvii, Holmiae. (in Latin)
- Matsumura S. 1913. Thousand Insects of Japan Additamenta. Vol. 1. Keiseisha, Tokyo. (in Japanese)
- Matsumura S. 1931. 6000 Illustrated Insects of Japan Empire. Tokoshoin, Tokyo. (in Japanese)
- Metcalf ZP. 1946. General catalogue of the Hemiptera. Fascicle IV: Fulgoroidea, Part 8: Dictyopharidae. North Carolina State College, Raleigh, NC, USA.
- Metcalf ZP. 1947. General catalogue of the Hemiptera. Fascicle IV: Fulgoroidea, Part 9: Fulgoridae. North Carolina State College, Raleigh, NC, USA.
- Nagai S, Porion T. 1996. Fulgoridae 2: Illustrated Catalogue of Asiatic and Australian Fauna. Sciences Nat, Compiègne.
- O'Brien LB. 1988. New World Fulgoridae. Part 1: genera with elongate head processes. Great Basin Naturalist Memoirs 12:135–170.
- Schaum HR. 1850. Fulgorellae. Erster Section A–G. pp. 58–73. In: Ersch IS & Gruber IG (eds.). Allgemeine Encyklopädie der Wissenschaften und Künste in alphabetischer Folge von genannten Schriftstellern bearbeitet und herausgegeben. Brockhaus Verlag, Mannheim. (in German)
- Schmidt E. 1905. Beitrag zur Kenntnis der Fulgoriden. II. Die im Stettiner Museum vorhandenen Arten des Genus Fulgora Linné. Stett Ent Zeit 66:342–357. (in German)
- Schmidt E. 1911. Neue Fulgoriden. Zool Anz 38:161–171. (in German)
- Schmidt E. 1930. Beitrag zur Kenntnis der Zikaden des indoaustralischen Faunengebietes. Wiener Ent Zeit 47:112–126. (in German)
- Schumacher F. 1915. Homoptera in H. Sauter’s Formosa-Ausbeute. Suppl Ent 4:108–142. (in German)
- Spinola M. 1839. Essai sur les Fulgorelles, sous-tribu de la tribu des Cicadaires, ordre des Rhyngotes. Annales de la Société Entomologique de France 8:133–337. (in French)
- Stål C. 1854. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar. Stockholm: PA Norstedt & Söner, Kongl. Boktryckare. 364 pp. (in Swedish)
- Stål C. 1863. Beitrag zür Kenntnis der Fulgoriden. Stett Ent Zeit 24:230–251. (in German)
- Urban JM, Cryan JR. 2009. Entomologically famous, evolutionarily unexplored: the first phylogeny of the lanternfly family Fulgoridae (Insecta: Hemiptera: Fulgoroidea). Mol Phylogenet Evol 50:471–484. doi:10.1016/j.ympev.2008.12.004. [DOI] [PubMed]
- Walker F. 1851. List of the specimens of Homopterous insects in the collection of the British Museum. Part II. London: Edward Newman.
- Walker F. 1858a. Insecta saundersiana: or characters of undescribed insects in the collection of William Wilson Saunders. London: John Van Voorst, 117 pp.
- Walker F. 1858b. List of the specimens of Homopterous insects in the collection of the British Museum. Supplement. London: Edward Newman, 369 pp.
- Wang GY, Huang J, Huang BK. 2000. Studies on the biology of Fulgora candelaria (L.) (Homoptera: Fulgoridae). Entomological Journal of East China 9:61–65. (in Chinese)
- Wang WQ, Xu SL, Qin DZ. 2018. The lanternfly genus Pyrops Spinola (Hemiptera: Fulgoridae) from China with description of a new species. Entomotaxonomia 40:296–309. doi:10.11680/entomotax.2018031.
- Wang WQ, Xu SL, Constant J, Qin DZ. 2020. Revision of the lanternfly genus Limois Stål (Hemiptera: Fulgoromorpha: Fulgoridae) with description of a new species from China. Eur J Taxon 720:35–61. doi:10.5852/ejt.2020.720.1113.
- Westwood JO. 1842. Insectorum novorum centuria, auctore. Ann Mag Nat Hist 9:118–119. (in Latin)
- Westwood JO. 1848. Order-Homoptera. Section-Trimera. Family-Fulgoridae Leach, pp. 73–74. In: Westwood JO (ed.). The cabinet of oriental entomology. William Smith, London, UK.
- White A. 1845. Descriptions of a new genus and some new species of homopterous insects from the east in the collection of the British Museum. Ann Mag Nat Hist 15:34–37.
- White A. 1846. Descriptions of some apparently new species of orthopterous and homopterous insects. Ann Mag Nat Hist 18:23–26.
- Yang CT, Chang TY. 2000. The External Male Genitalia of Hemiptera (Homoptera-Heteroptera). Shih Way Publishers, Taichung, 746 pp.