Abstract
Tryptophanyl-tRNA synthetase (TrpRS) links tryptophan to tRNATrp, thereby playing an indispensable role in protein translation. Unlike most class I aminoacyl-tRNA synthetases (AARSs), TrpRS functions as a homodimer. Herein, we captured an ‘open-closed’ asymmetric structure of Escherichia coli TrpRS (EcTrpRS) with one active site occupied by a copurified intermediate product and the other remaining empty, providing structural evidence for the long-discussed half-of-the-sites reactivity of bacterial TrpRS. In contrast to its human counterpart, bacterial TrpRS may rely on this asymmetric conformation to functionally bind with substrate tRNA. As this asymmetric conformation is probably a dominant form of TrpRS purified from bacterial cells, we performed fragment screening against asymmetric EcTrpRS to support antibacterial discovery. Nineteen fragment hits were identified, and 8 of them were successfully cocrystallized with EcTrpRS. While a fragment named niraparib bound to the L-Trp binding site of the ‘open’ subunit, the other 7 fragments all bound to an unprecedented pocket at the interface between two TrpRS subunits. Binding of these fragments relies on residues specific to bacterial TrpRS, avoiding undesired interactions with human TrpRS. These findings improve our understanding of the catalytic mechanism of this important enzyme and will also facilitate the discovery of bacterial TrpRS inhibitors with therapeutic potential.
Graphical Abstract
Graphical Abstract.

Crystal structures of Escherichia coli TrpRS reveal an “open-closed” asymmetric conformation which may be important for tRNA binding, and chemical fragments can bind to distinct pockets on the asymmetric TrpRS.
INTRODUCTION
Aminoacyl-tRNA synthetases (AARSs) faithfully attach amino acids to their cognate tRNAs, and the ribosomes then use the produced aminoacyl-tRNAs as substrates to synthesize polypeptides based on the genetic information brought in mRNA (1). Proteins are generally synthesized with 20 proteinogenic amino acids; consequently, the AARS family mainly contains 20 members, each for a specific amino acid. All the AARS members are indispensable for protein translation, and their dysfunction disturbs cell viability and induces various diseases (2,3). On the other hand, inhibition of AARSs using small molecules is a promising strategy for treating multiple diseases, such as microbial infections (4,5), human cancers (6), fibrosis (7,8), and osteoporosis (9,10). To date, the AARS inhibitors mupirocin, tavaborole and halofuginone have been used in the clinic or as veterinary drugs (11–13), and more inhibitors are being evaluated at preclinical and clinical stages (14,15).
Tryptophanyl-tRNA synthetase (TrpRS) is the AARS member responsible for charging tRNATrp with L-tryptophan (L-Trp). TrpRS belongs to class I AARSs, and it is further grouped into subclass Ic together with tyrosyl-tRNA synthetase (TyrRS). Similar to other class I AARSs, TrpRS contains a Rossmann fold (RF) aminoacylation domain (AD) (16). Notably, TrpRSs from prokaryotes and eukaryotes show obvious sequence and structural differences at their active sites on AD and have developed distinct mechanisms to recognize the substrate L-Trp. The indole nitrogen of substrate L-Trp is coordinated by hydrogen bonding with a tyrosine residue located in a β-strand when binding to eukaryotic TrpRS but with an aspartate residue in an α-helix when binding to bacterial TrpRS (17). In contrast, the active site residues forming specific hydrogen-bonding interactions with amino acid substrates are usually conserved throughout evolution in other AARSs (17). Moreover, the charging of tRNATrp was also reported to be kingdom-specific that bacterial and eukaryotic TrpRSs can only catalyze their own tRNATrp substrates (18). Therefore, TrpRS is considered an attractive drug target for developing bacterial-selective inhibitors for fighting microbial infections (19). For example, two natural products, indolmycin and chuangxinmycin, have been shown to inhibit bacterial TrpRS at the nanomolar level with almost no undesired binding to human cytoplasmic TrpRS (HcTrpRS) (20,21). However, unfortunately, both inhibitors failed to enter the clinical use due to insufficient permeability or narrow antibacterial spectrum, and inhibitors against bacterial TrpRS with new scaffolds and novel mechanisms are highly desired.
Different to most class I AARSs, TrpRSs in both prokaryotes and eukaryotes are homodimers (22). Previous studies have revealed a half-of-the-sites reaction mechanism for HcTrpRS that once an intermediate product tryptophanyl adenylate (TrpAMP) is formed in one subunit, the second subunit can no longer efficiently produce TrpAMP (23). Despite the existence of obvious differences between human and bacterial TrpRSs at their active sites, the half-of-the-sites reactivity was also proposed for bacterial TrpRS because it was found to prefer to bind only one ATP at a time (24,25). Dozens of structures of TrpRS from various bacteria have been determined so far, but they all form symmetric conformations in which both subunits of bacterial TrpRS bind with the same ligands or both remain empty (25–28). Notably, bacterial TrpRS incubated with high concentrations of ATP or ATP + L-Trp was crystallized in a ‘closed-closed’ symmetric conformation with ATP or TrpAMP bound in both active sites (25,27), which somewhat conflicts with the proposed half-of-the-sites reactivity. There are not yet direct structural data to validate the half-of-the-sites catalytic mode for bacterial TrpRS, explain why bacterial TrpRS uses this catalytic mode, and, more importantly, explore the implications of this catalytic mode in drug discovery.
In this study, TrpRS from Escherichia coli (EcTrpRS) was crystallized in an ‘open-closed’ asymmetric state with a copurified intermediate product TrpAMP bound to the ‘closed’ subunit. The structural modelling and binding assay revealed that only this ‘open-closed’ asymmetric EcTrpRS is suitable for functionally binding of the substrate tRNATrp, giving a biological relevance to using half of the sites in the catalysis of bacterial TrpRS. Considering that the asymmetric EcTrpRS may represent the dominant conformation of TrpRS in bacterial cells, we used it as a template to screen chemical fragments. Nineteen fragment hits were identified, and the binding modes of 8 fragments to asymmetric EcTrpRS were successfully clarified by cocrystal structures, providing valuable information for inhibitor design in the future.
MATERIALS AND METHODS
Protein preparation
The coding sequence of full-length EcTrpRS (UniProt ID P00954) was amplified from the genomic DNA of the E. coli K12 strain and cloned into the pET20b (+) plasmid (Novagen) with a hexahistidine tag at its C-terminus. BL21 (DE3) cells transformed with the EcTrpRS-pET20b (+) plasmid were grown in Luria-Bertani (LB) medium supplemented with 100 mg/l ampicillin at 37°C until the OD600 = 0.6–0.8. Then, 0.15 mM isopropyl-β-d-thiogalactoside (IPTG) was added, and the bacterial cells were further cultured at 16°C for 12 h. Cells were harvested using centrifugation, resuspended in wash buffer (400 mM NaCl, 50 mM Tris–HCl pH 8.0, 5% glycerol and 20 mM imidazole), and broken by sonication. The cell lysate was loaded onto a Ni-NTA column (Qiagen) pre-equilibrated with wash buffer. The impurities were washed away with 20 columns of wash buffer, and then the target protein was eluted using elution buffer (400 mM NaCl, 50 mM Tris–HCl pH 8.0, 5% glycerol, and 100 mM imidazole). The target protein was concentrated and further purified using HiLoad 16/60 Superdex 200 pg (GE healthcare) with running buffer (200 mM NaCl, 20 mM Tris–HCl pH 8.0, and 5% glycerol). Protein purity was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS−PAGE). The target protein was finally concentrated to 60 mg/ml in storage buffer (50 mM NaCl, 2 mM Tris–HCl pH 8.0, and 5% glycerol) and stored at -80°C before use. Staphylococcus aureus TrpRS (SaTrpRS, residues 1–329) and N-terminal truncated HcTrpRS (residues 48–471) were expressed and purified similarly to EcTrpRS.
Crystallography
EcTrpRS was crystallized using the sitting-drop vapor-diffusion method. Each crystallization drop consisted of 1 μl EcTrpRS protein (30 mg/ml) and 1 μl reservoir solution (0.16 M ammonium sulfate, 0.1 M HEPES pH 7.5, 25% PEG3350) and was equilibrated against 100 μl of reservoir solution at 18°C for 1–3 days to allow crystals to grow. Large crystals were immersed in cryoprotectant solution (reservoir solution supplemented with 20% ethylene glycol) for a few seconds and then flash-frozen in liquid nitrogen. For growing cocrystals of the EcTrpRS·fragment complexes, the selected fragments (2–5 mM) were mixed with EcTrpRS (30 mg/ml) and incubated on ice for 30 min before setting up crystallization drops.
The diffraction data were collected at 100 K at the BL19U1 beamline of the National Facility for Protein Sciences Shanghai (NFPSS), the BL02U1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF) and an in-house Xcalibur Nova single-crystal diffractometer, and processed using XDS (29) and CrysAlisPro software (Agilent Technologies UK Ltd). Structures were solved using the program Molrep (30) by the molecular replacement method with the ‘open-open’ EcTrpRS structure (PDB code 5V0I) as the search model. Coot (31) and Refmac5 (32) were used iteratively to refine the structural models. The stereochemical quality of the final models was assessed using MolProbity (33). The statistics of data collection and structural refinements are listed in Supplementary Table S1. The coordinates and structural factors of the structures described in this paper have been deposited in the Protein Data Bank (PDB) under the accession codes 8I1W (TrpAMP), 8I4I (TrpAMP and L-Trp), 8I27 (TrpAMP and M1-67), 8I2A (TrpAMP and M1-109), 8I1Z (TrpAMP and M1-158), 8I2C (TrpAMP and M2-54), 8I2J (TrpAMP and M2-140), 8I1Y (TrpAMP and M3-108), 8I2L (TrpAMP and chlorzoxazone), and 8I2M (TrpAMP and niraparib).
Fluorescence-based thermal shift assay
Ligand binding usually stabilizes a protein during its thermal denaturation process, resulting in a positive shift of the protein melting temperature compared to the apo protein (34). The fluorescence-based thermal shift assay (TSA) was employed for fragment screening against asymmetric EcTrpRS bound with a molecule of TrpAMP. The 20 μl reactions, consisting of 150 mM NaCl, 100 mM MES pH 6.5, 2 μg EcTrpRS, 4 × SYPRO orange fluorescence dye (Sigma-Aldrich) and 1 mM of one of the tested fragments, were prepared in the 96-well plates (Life Technologies) on ice. The reaction without adding any fragment was used as the blank control. The plates were incubated at 25°C for 10 min and then heated from 25°C to 95°C at a rate of 1°C/min. The fluorescence intensity was recorded every 30 s using a StepOne Plus™ RT-PCR equipment. The melting temperatures (Tm) of EcTrpRS with and without fragments were calculated using StepOne™ software v2.3. The average Tm values of triplicate assays were used. A fragment was considered as a positive hit when the ΔTm between EcTrpRS with and without adding this fragment was greater than 1°C. Furthermore, a parallel fragment screening against the ‘open-open’ EcTrpRS (prepared by dialysis of the ‘open-closed’ asymmetric EcTrpRS) was performed as a control.
Preparation of tRNATrp
For the ATP consumption assay, E. coli tRNATrp was prepared by overexpression in E. coli. The DNA sequence encoding E. coli tRNATrp (CCA) (tRNAdb ID tdbD00011300) was synthesized and inserted into the pET20b (+) vector between the T7 promoter and T7 terminator through homologous recombination. The transformed E. coli BL21 (DE3) cells were cultured in LB medium until the OD600 reached ∼0.6, and 1 mM IPTG was added to induce the overexpression of tRNATrp (CCA) at 30°C for 16 h. The tRNATrp (CCA) was extracted from cell pellets using RNAiso Plus (TakaRa) and chloroform, and precipitated from aqueous fractions using isopropanol. The tRNA pellet after centrifugation was redissolved using a buffer consisting of 20 mM Tris–HCl pH 8.0 and 1 mM EDTA, and loaded onto a HiTrap Q XL (GE healthcare). The column was eluted with a linear gradient of NaCl (0–1.0 M) supplemented with 20 mM Tris–HCl pH 8.0 and 10 mM MgCl2. The fractions containing tRNA were concentrated to 10 mg/ml and stored at –80°C in a buffer consisting of 10 mM HEPES pH 7.5 and 10 mM MgCl2.
Transfer of L-Trp from the intermediate product TrpAMP to the 3’ end of tRNATrp will trigger a state transition of EcTrpRS. Thus, an A76-truncated tRNATrp was prepared with in vitro T7 polymerase transcription and used in the EMSA to test the binding of tRNATrp to EcTrpRS at different states. The DNA template of E. coli tRNATrp(ΔA76) (CCA) with a T7 promoter fused at the 5’ end was synthesized using PCR with primer 1 (5'-TAATACGACTCACTATAAGGGGCGTAGTTCAATTGGTAGAGCACCGGTCTCCAAAACC-3') and primer 2 (5'-GGCAGGGGCGGAGAGACTCGAACTCCCAACACCCGGTTTTGGAGACCGGTGCT-3'). These two primers covered the full sequence of E. coli tRNATrp(ΔA76) (CCA) and partially overlapped with each other (underlined nucleotides), and primer 1 also contained a T7 promoter sequence (nucleotides in bold). The PCR product was further amplified by the second round of PCR with primer 3 (5'-TAATACGACTCACTATAAGGGGCGTAG-3') and primer4 (5'- GGCAGGGGCGGAGAGACTCGA-3'), and the product was then used as the DNA template for the in vitro T7 transcription assay without additional purification. The last two nucleotides at the 5’ terminus of primer 4 (nucleotides in italics) were methylated at their 2’-hydroxyl groups to reduce nontemplated nucleotide addition by the T7 RNA polymerase (35). The in vitro transcription reaction mixture contained 200 mM Tris–HCl pH 8.0, 20 mM MgCl2, 2 mM spermidine, 10 mM DTT, 4 mM ATP, 4 mM UTP, 4 mM CTP, 4 mM GTP, 50 μg/ml template DNA, and 1 μM T7 polymerase. After incubation at 37°C for 3–4 h, the transcripts were denatured at 95°C for 5 min. The tRNA transcripts were purified using 10% PAGE supplemented with 8 M urea. The tRNA band was cut, and tRNA was extracted from the gel using 0.5 M ammonium acetate and precipitated by ethanol. The tRNA was redissolved in a buffer consisting of 20 mM Tris–HCl pH 8.0 and 1 mM EDTA, heated at 65 °C for 5 min, and then refolded by slowly cooling to room temperature after the addition of 10 mM MgCl2. The refolded tRNA was concentrated to 10 mg/ml, aliquoted, and stored at –80°C for future use.
ATP consumption assay
The inhibition of the aminoacylation activity of EcTrpRS by different ligands was measured using an ATP consumption assay (36). EcTrpRS was incubated with 1 mM of each ligand for 20 min on ice, and then substrates were added to initiate the reactions. The 10 μl reactions, consisting of 50 nM EcTrpRS, 4 μM ATP, 10 μM L-Trp, 0.4 mg/ml E. coli tRNATrp (CCA) (prepared by overexpression in E. coli), 30 mM HEPES pH 7.5, 150 mM NaCl, 30 mM KCl, 40 mM MgCl2, 1 mM DTT and 0.1% BSA, were incubated in a 384-well microplate at 37°C for 10 min. Then, 10 μl of Kinase-Glo® Reagent (Promega) was added to each well to measure the amount of remaining ATP. After 10 min of incubation, the luminescence (L) was read on a FlexStation 3 multimode microplate reader (Molecular Devices). The luminescence intensity of the sample without any inhibitor was used as Lmin, and the luminescence intensity of the sample without EcTrpRS was used as Lmax. The inhibitory rate of a ligand against EcTrpRS was calculated as inhibitory rate = (L – Lmin)/(Lmax – Lmin) × 100%. The reactions were each repeated three times. For niraparib, its inhibitory rates at different concentrations (31.25, 62.5, 125, 250, 500 and 1000 μM) were measured, and then its IC50 was calculated by fitting a does-response curve using GraphPad Prism 8 software.
Isothermal titration calorimetry (ITC) assay
The binding affinities of ligands to EcTrpRS were measured by using a MicroCal VP-ITC microcalorimeter (MicroCal). The proteins and ligands were prepared in PBS buffer (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4). The titration assays were performed at 15°C with 0.4 μl for the first injection and 2 μl each for the next 19 injections, and the interval between two injections was 120 s. The disassociation constants (Kd) were determined by fitting the calorimetric data to the one-site binding model using MicroCal PEAQ-ITC analysis software, and the errors of Kd values represented the curving fitting errors. All of the above ITC experiments were repeated at least twice, and titrations of ligands to HcTrpRS and SaTrpRS were also performed for comparison.
Construction of the EcTrpRS·tRNATrp complex models
The docking of tRNATrp to asymmetric EcTrpRS was based on a cocrystal structure of HcTrpRS bound with two tRNATrp molecules (PDB code 2DR2) (18). To build the EcTrpRS·tRNATrp binding model I, one tRNATrp molecule was removed from the HcTrpRS·tRNATrp complex, and the remaining HcTrpRS·tRNATrp complex was then overlaid on EcTrpRS by aligning the AD of its subunit bound with the tRNATrp acceptor stem with the AD of the subClosed of EcTrpRS. Two α-helices (Y100-R106 and R158-A166) on the AD of EcTrpRS recognized the acceptor stem of tRNATrp as their corresponding helices did in the HcTrpRS·tRNATrp complex, and the CCA end of tRNATrp was shown to approach TrpAMP in the active site of subClosed to receive the L-Trp. We kept the interactions between the tRNATrp acceptor stem and subClosed and slightly rotated tRNATrp to move its anticodon arm towards the CTD of subOpen of EcTrpRS. To build the EcTrpRS·tRNATrp binding model II, the HcTrpRS·tRNATrp complex was first overlaid on the subOpen of EcTrpRS, and then the anticodon arm of tRNATrp was rotated towards the CTD of subClosed of EcTrpRS. However, in binding model II, the anticodon binding site of the subClosed of EcTrpRS was unable to reach the tRNATrp anticodon triplets because the distance between the anticodon binding site of subClosed and acceptor stem binding site of subOpen of EcTrpRS is shorter than the distance between their corresponding binding elements on tRNATrp, suggesting that tRNATrp cannot functionally bind to EcTrpRS in binding model II.
Electrophoretic mobility shift assay
An electrophoretic mobility shift assay (EMSA) was performed to explore the differences in the binding of tRNATrp to EcTrpRS in different states. As shown in the crystal structure, the two subunits of purified EcTrpRS mainly adopted the ‘open-closed’ asymmetric conformation. The ‘open-open’ EcTrpRS was prepared via dialysis. Briefly, the purified ‘open-closed’ asymmetric EcTrpRS was diluted to 1 mg/ml, loaded onto a dialysis bag (cut-off molecular weight of 3000 Da), and dialyzed against 1 L dialysis buffer (300 mM NaCl, 50 mM Tris–HCl pH 9.0, 5% glycerol and 30 mM L-Trp) twice at 8°C to remove the copurified TrpAMP. The ‘closed-closed’ EcTrpRS was prepared by incubating EcTrpRS with 2 mM ATP and 2 mM L-Trp on ice for 30 min as described in the literature (27).
EcTrpRS at different states was diluted to 10 μM, 20 μM, 40 μM and 80 μM in the buffer consisting of 30 mM sodium cacodylate pH 6.5, 40 mM MgCl2, 150 mM NaCl, 30 mM KCl, 2 mM DTT and 10% glycerol, and incubated with tRNATrp(ΔA76) (5 μM) at 4°C for 30 min. The mixtures were loaded onto a 5% native polyacrylamide gel and electrophoresed at a voltage of 80 V for 2 h on ice. The gel was dyed with GelRed and then with Coomassie brilliant blue R-250.
RESULTS
An ‘open-closed’ asymmetric structure supports the long-discussed half-of-the-sites reactivity of bacterial TrpRS
In this study, a crystal structure of full-length EcTrpRS·TrpAMP was solved at 1.80 Å resolution with R/Rfree = 18.6%/22.2% (Supplementary Table S1). The asymmetric unit contains two EcTrpRS subunits that form a functional homodimer. Each EcTrpRS subunit consists of a Rossmann fold aminoacylation domain (AD, residues 1–85, 140–183 and 299–334), a connecting polypeptide 1 domain [CP1, residues 86–139; CP1 was combined with AD in some previous studies (37)] and a C-terminal α-helical domain (CTD, residues 184–298) (Figure 1A).
Figure 1.
The ‘open-closed’ asymmetric structure of EcTrpRS. (A) The domain organization of EcTrpRS. (B) The overall structure of homodimeric EcTrpRS in an asymmetric conformation with a molecule of the intermediate product TrpAMP bound at the active site cavity of the closed subunit (subClosed). EcTrpRS is presented as a cartoon model and colored according to (A), and TrpAMP is presented as a sphere. (C) Superposition of subOpen and subClosed by aligning their ADs.
Intriguingly, two subunits of this EcTrpRS dimer adopt different conformations. A copurified tryptophanyl adenylate (TrpAMP), the intermediate product of the two-step tRNA tryptophanylation reaction, was observed to bind to one of the two active sites of EcTrpRS (Figure 1B and Supplementary Figure S1A). The crystal structure of EcTrpRS in complex with L-Trp and AMP has been determined (PDB code 5V0I) (Supplementary Figure S1B). The L-Trp moiety of TrpAMP interacts with EcTrpRS similarly to the substrate L-Trp, except that the side chain of Tyr128 rotates downwards (∼5.3 Å for the phenolic hydroxyl) to interact with the α-amino group of the L-Trp moiety of TrpAMP (Supplementary Figure S1C). Compared with the AMP molecule, the AMP portion of TrpAMP moves approximately 6.0 Å to attach to the L-Trp moiety, and the class I AARS signature motifs TIGN (equal to HIGH in other class I AARSs) and KMSKS of EcTrpRS shift together with the AMP part of TrpAMP towards the active site (Supplementary Figure S2A). The shift of these two motifs couples with an inwards rotation of the CTD around a short hinge linker located at the beginning of the CTD (residues 184–188) (37), which makes the TrpAMP-bound subunit a closed active site cavity and a more compact overall conformation (named subClosed) compared with EcTrpRS bound with L-Trp and AMP (Supplementary Figure S2A). In contrast, the active site cavity of the other subunit of EcTrpRS dimer remains empty, and this empty subunit displays an open conformation (named subOpen) similar to that when L-Trp and AMP bind (Supplementary Figure S2B). Aligning subOpen and subClosed by superimposing their ADs shows that the anticodon binding site at the distal end of the CTD of subClosed moves ∼6.9 Å towards the active site compared with that of subOpen (Figure 1C). Thus, this EcTrpRS dimer is in an ‘open-closed’ asymmetric conformation unique to all the reported structures of bacterial TrpRS.
Production of TrpAMP requires the simultaneous binding of both substrates L-Trp and ATP to the active site cavity. We tried to supplement the asymmetric EcTrpRS with excessive substrates (either 2 mM L-Trp or 2 mM ATP) during crystallization. Only L-Trp, but not ATP, was successfully cocrystallized with the asymmetric EcTrpRS in the active site cavity of its subOpen (Supplementary Figure S3 and Supplementary Table S1). We also employed isothermal titration calorimetry (ITC) assays to compare the differences in affinities of both substrates between binding to ‘open-closed’ asymmetric EcTrpRS and binding to apo EcTrpRS (prepared by dialysis). The binding of TrpAMP in one subunit of EcTrpRS caused little change in the affinity of L-Trp to the rest active site in the other subunit, but it significantly decreased the affinity of ATP compared with apo EcTrpRS (Figure 2A–D). These results suggested that EcTrpRS formed this ‘open-closed’ asymmetric conformation because it is unfavorable to bind with an additional ATP, which is consistent with the previous findings that Bacillus stearothermophilus TrpRS prefers to bind only one substrate ATP at a time (25). However, it is unclear how the two active sites of the bacterial TrpRS dimer communicate with each other in regard to ATP binding. A short CP1 sequence (residues Gln109 to Ser114) is disordered in subOpen but forms an α-helix in TrpAMP-bound subClosed (Figure 1B). This short α-helix could contribute to stabilizing the conformation of the KMSKS loop, a loop important for ATP binding (Supplementary Figure S4). This short CP1 sequence in two TrpRS subunits may affect each other by involving in a unified large hydrophobic interaction network (Supplementary Figure S4), which provides a possible way to bridge two ATP binding sites of the TrpRS dimer.
Figure 2.
Binding affinities of substrates L-Trp and ATP to EcTrpRS in asymmetric and apo states as measured by isothermal titration calorimetry. (A, B) ITC measurements of L-Trp to ‘open-closed’ asymmetric EcTrpRS and ‘open-open’ apo EcTrpRS showed similar binding affinities, revealing that occupying one active site by the intermediate product TrpAMP does not affect the binding of L-Trp to the other active site. (C, D) ITC measurements of ATP to ‘open-closed’ EcTrpRS and ‘open-open’ EcTrpRS showed that once an active site binds with a TrpAMP, the second active site could not recruit ATP efficiently.
The asymmetric conformation of EcTrpRS is needed for the functional docking of substrate tRNATrp
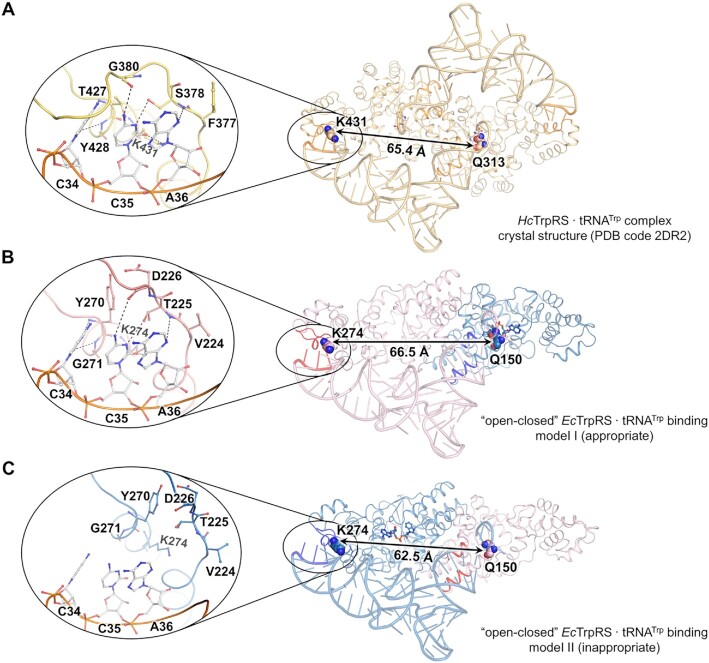
The kingdom-specific recognition of bacterial and eukaryotic tRNATrp molecules by their corresponding TrpRSs could be reversed by just switching their discriminator nucleotide N73 on tRNATrp molecules or the N73 interacting residues on their corresponding TrpRSs (38). Thus, the overall structure and the major interactions with TrpRS (for both the anticodon and acceptor stem) should be similar between bacterial and eukaryotic tRNATrp. The complex structures of HcTrpRS bound with tRNATrp have been solved (PDB codes: 2DR2 and 2AZX), revealing a cross-subunit tRNA binding mode typically observed in class II AARSs (18,39). In this binding mode, the acceptor stem of tRNATrp is approached through its major groove side by two α-helices on the AD of one HcTrpRS subunit, and the anticodon of tRNATrp is recognized by the anticodon binding site located at the distal end of the other subunit (Figure 3A) (18,39).
Figure 3.
The binding mode of EctRNATrp asks TrpRS to apply the half-of-the-sites reactivity. (A) Crystal structure of the HcTrpRS·tRNATrp·Trp complex (PDB code 2DR2). (B, C) Docking of tRNATrp to ‘open-closed’ EcTrpRS in two opposite ways·tRNATrp·TrpAMP complex. The distance between the anticodon binding site of subClosed and acceptor stem binding site of subOpen of EcTrpRS is shorter than the corresponding distance observed in cocrystal structure of HcTrpRS·tRNATrp complex, suggesting that tRNATrp cannot functionally bind to EcTrpRS in this way. In the enlarged views of tRNA anticodon binding sites, the tRNA anticodon binding sites are shown as orange (A), pink (B) and skyblue (C) sticks, respectively, while the anticodon nucleotides as white sticks.
We modeled tRNATrp to asymmetric EcTrpRS based on the cocrystal structure of the HcTrpRS·tRNATrp complex. We found that the distance between the acceptor stem binding helices of subClosed and the anticodon binding site of subOpen perfectly matches the distance between their corresponding interacting elements on tRNATrp (Figure 3B), suggesting that tRNATrp is able to bind to EcTrpRS in this way. In contrast, because of the closed conformation induced by TrpAMP, the distance between the acceptor stem binding helices and anticodon binding site of subClosed is approximately 4.0 Å shorter [the distance between the Cα atoms of K274 and Q150, two residues corresponding to K431 and Q313 in HcTrpRS that play central roles in recognizing the anticodon and acceptor stem of tRNATrp, respectively (18)] than that of subOpen (Supplementary Figure S5). When the acceptor stem of tRNATrp was docked onto the AD of subOpen, its anticodon, particularly nucleotide C34, stretched to outside of the anticodon binding site of subClosed, suggesting that tRNATrp could not functionally bind to EcTrpRS in this way (Figure 3C). For similar reasons, two tRNATrp could be modeled onto ‘open-open’ EcTrpRS, but no tRNATrp could be modeled onto ‘closed-closed’ EcTrpRS (Supplementary Figure S5).
Notably, the potential flexibility and conformation changes of tRNATrp, which are hard to predict, were not taken into account for the above docking model. Thus, to examine the binding models, we performed an electrophoretic mobility shift assay (EMSA) to detect the binding of tRNATrp to EcTrpRS in different states. While the transfer of L-Trp from TrpAMP to tRNATrp will change the state of EcTrpRS, the E. coli tRNATrp without A76 (EctRNATrp(ΔA76)) was used in the experiments. The results showed that tRNATrp(ΔA76) was shifted in a does-dependent manner by the apo (‘open-open’ conformation; prepared by dialysis) and the asymmetric (‘open-closed’ conformation) EcTrpRS. However, the shift of tRNATrp(ΔA76) by EcTrpRS bound with two TrpAMPs [prepared by incubating EcTrpRS with high concentrations of ATP and L-Trp as described (27)] was not observed (Figure 4), indicating that the ‘closed-closed’ conformation of EcTrpRS is unfavorable for tRNA binding and that the binding of tRNATrp to EcTrpRS requires at least one subunit to adopt the open conformation. Thus, interestingly, although the half-of-the-sites reactivity of bacterial TrpRS is apparently due to the unfavourable binding of the second ATP (the first step of the aminoacylation reaction catalyzed by TrpRS), our structural analysis and binding assay suggested that the functional binding of tRNATrp (the second step of the tRNA aminoacylation reaction) requires the asymmetric conformation of TrpRS and therefore provides possible selection pressure for evolving a half-of-the-sites catalytic mode.
Figure 4.
Electrophoretic mobility shifts of EctRNATrp(ΔA76) with EcTrpRS in different conformations. tRNATrp(ΔA76) was shifted in a does-dependent manner by the ‘open-open’ and ‘open-closed’ EcTrpRSs but not by the ‘closed-closed’ EcTrpRS, suggesting that capturing a substrate EctRNATrp by EcTrpRS requires at least one subunit of EcTrpRS to adopt an open conformation, which agrees with the half-of-the-sites reactivity. The gel was dyed with GelRed and then with Coomassie brilliant blue R-250.
Fragment screening against the asymmetric state EcTrpRS
The fact that EcTrpRS purified from E. coli cells was crystallized as the ‘open-closed’ conformation suggested that this asymmetric state with the intermediate product bound at one of the two active sites might be one of the major states of TrpRS in bacterial cells. Thus, this asymmetric conformation of EcTrpRS provides a valuable new template for discovering TrpRS inhibitors. Fragment screening has been widely used to discover drug leads with novel scaffolds as well as to identify new druggable pockets (40,41). We employed the fluorescence-based thermal shift assay (TSA) to screen a library of 1628 fragments against asymmetric EcTrpRS to identify potential building blocks for TrpRS inhibitors (42). As a control, a parallel fragment screening assay was also performed against apo EcTrpRS (prepared by dialysis). Ligand binding could enhance the melting temperature (Tm) of a protein during its thermal denaturation process, and tighter binders usually cause larger positive shifts of Tm (34). We considered the fragments (at a final concentration of 1 mM) that caused ΔTm (ΔTm = Tm(Frg) − Tm(apo)) values of EcTrpRS greater than 1.0°C (approximately threefold of the s.d. of triplicate measurements of the Tm of EcTrpRS) as positive hits.
Finally, 19 potential hits were identified for the asymmetric EcTrpRS and 30 hits for the apo EcTrpRS (Figure 5A and Supplementary Figure S6). Many of these fragment hits contain a moiety similar to indole, the side chain of substrate L-Trp of EcTrpRS, which supports the reliability of our fragment screening assay. Notably, all 19 hits bound to the asymmetric EcTrpRS are included in the 30 hits for apo EcTrpRS, suggesting that chemicals targeting to the asymmetric bacterial TrpRS have great potential to also bind to apo TrpRS, which would result in more complete inhibition of TrpRS in bacterial cells.
Figure 5.
Fragment screening against EcTrpRS using a fluorescence-based thermal shift assay. (A) Schematic illustration of the TSA-based fragment screening process. A fragment was identified as a binder of EcTrpRS if it increased the Tm value of EcTrpRS by > 1.0°C. Fragments were screened in parallel against both the ‘open-open’ EcTrpRS and ‘open-closed’ EcTrpRS. Thirty fragments were identified to bind the ‘open-open’ EcTrpRS, including 19 fragments that could bind the ‘open-closed’ EcTrpRS. (B) The representative thermal melting curves of ‘open-closed’ EcTrpRS in the presence of the eight fragments whose binding modes have later been determined by cocrystal structures with EcTrpRS.
Then, we selected the 19 hits bound to asymmetric EcTrpRS to grow the cocrystals with EcTrpRS. Through efforts, eight cocrystal structures were determined at resolutions between 1.78 and 2.65 Å (Supplementary Figure S5 and Supplementary Table S1). To our surprise, while a fragment named niraparib bound to the L-Trp binding site, the other seven fragments, although three of them contained indole-like aromatic heterocyclic structures, all bound to an unprecedented pocket buried at the interface between the two subunits of EcTrpRS.
Seven fragments reveal a new binding pocket at the dimeric interface of TrpRS
In this study, seven fragments were observed to bind to an unprecedented pocket buried at the dimeric interface between the CP1 domains of two EcTrpRS subunits (Figure 6). This pocket is also observed in bacterial TrpRS in ‘open-open’ and ‘closed-closed’ states (PDB codes: 5V0I and 7ELT), and fragment binding does not significantly change the volume and the shape of this buried pocket, as predicted using DoGSiteScorer (43). The CP1 domains of both subunits contribute equal residues (Ala89, Gly92, Trp93, Asn96, Asp127, Val130 and Leu131) to build this pocket, and this pocket is generally symmetric in shape and shows both hydrophobic and hydrophilic characteristics (Figure 6A). Site-directed mutations were performed on these pocket-surrounding residues, and EcTrpRS variants with D127A, V130A or L131A mutations were successfully expressed and purified. TSA results showed that the fragments caused significantly smaller ΔTm values to these EcTrpRS variants than to wild-type EcTrpRS (Supplementary Figure S7), confirming the specific binding of the fragments to this pocket.
Figure 6.
Binding of seven fragments to the dimeric interface of ‘open-closed’ EcTrpRS. (A) An unprecedented pocket at the dimeric interface of EcTrpRS. This pocket is built by several hydrophobic and hydrophilic residues (Ala89, Gly92, Trp93, Asn96, Asp127, Val130 and Leu131) from both subunits. Binding of fragments chlorzoxazone (B), M1-67 (C), M1-109 (D), M1-158 (E), M2-54 (F), M2-140 (G) and M3-108 (H) to this pocket. Residues from subOpen and subClosed are colored dark blue and light blue, respectively. The hydrogen bonds are shown as black dashes.
Chlorzoxazone is a fragment-like small molecule drug used to relieve pain and stiffness caused by muscle spasms, strains and sprains. The residues Asn96 from subOpen and Asp127 from subClosed could form hydrogen bonds with the nitrogen of the oxazolone group of chlorzoxazone. In addition, two Trp93 residues from both subOpen and subClosed contribute hydrophobic contacts and π–π interactions to stabilize chlorzoxazone (Figure 6B). The binding modes of the other six fragments in this pocket are similar to chlorzoxazone, particularly for the hydrophobic interactions contributed by Trp93 from both subunits (Figure 6C–H). Remarkably, although this pocket is generally symmetric in shape, however, all the seven fragments bind to it in an asymmetric way that the electron density maps clearly show a single orientation for each fragment in the pocket. Probably, the binding of TrpAMP caused a slight conformational difference in the CP1 domain between subOpen and subClosed. We used an ITC assay to test the binding of these fragments to EcTrpRS in different states, and the fragment M1-109 gave a detectable heat signal. Consistently, M1-109 showed a slightly better binding affinity to ‘open-closed’ (26.1 ± 4.4 uM) than ‘open-open’ (46.0 ± 10.5 uM) EcTrpRS (Supplementary Figure S8A-B).
The CP1 domain of HcTrpRS adopts a similar structure to EcTrpRS, and a buried pocket also exists at the dimeric interface of HcTrpRS (Supplementary Figure S9A). However, sequence alignments revealed that the CP1 sequences in forming this buried pocket are vary widely between bacterial and eukaryotic TrpRSs (Supplementary Figure S9B). The ITC results showed that M1-109 loses affinity to HcTrpRS (Supplementary Figure S8C, D). Thus, these fragment hits are likely specific to bacterial TrpRS.
The CP1 domain not only serves as a connecting bridge between the two subunits, but also plays crucial roles in forming the L-Trp binding pocket and interacting with the tRNA acceptor arm (22,44). Specially, the important fragment-binding residue Trp93 (numbered as EcTrpRS) is highly conserved in all the aligned bacterial TrpRSs except for Mycoplasma genitalium TrpRS in which Trp is replaced by a similar residue Tyr (Supplementary Figure S8). Mutation of Trp93 to Ser resulted in insoluble expression of EcTrpRS, consistent with a previous study on the W93F mutation of Bacillus subtilis TrpRS (45). Thus, Trp93 and other fragment-binding residues on the CP1 domain likely play roles in the structure and function of bacterial TrpRS. We then tested whether these fragments could affect the catalytic activity of bacterial TrpRS. However, none of these small fragments could significantly inhibit the activity of EcTrpRS in the tRNA-dependent ATP consumption assay (data not shown). The development of potent drug-like molecules based on these fragments is needed to evaluate the druggability of this pocket.
The unique and bacterial selective binding mode of niraparib at the active site
Niraparib is a fragment-like small inhibitor against poly ADP-ribose polymerase (PARP), and it is clinically used for treating multiple human cancers including recurrent epithelial ovarian, fallopian tube and primary peritoneal cancer (46). In this study, the cocrystal structure of niraparib bound to the asymmetric EcTrpRS was determined to 2.10 Å resolution (Figure 7A and Supplementary Table S1), and a niraparib molecule was unambiguously modeled into the active site cavity of subOpen according to the electron density map (Figure 7B).
Figure 7.
The binding mode and selectivity of niraparib to bacterial TrpRS. (A) Chemical structure of niraparib. (B) The binding mode of niraparib at the active site of EcTrpRS. An annealed omit electron density map of niraparib calculated with Fourier coefficients 2Fo – Fc and contoured at 1.0 σ. (C) The binding mode of L-Trp in EcTrpRS (PDB code 5V0I). (D) Structural explanation for the insensitivity of HcTrpRS to niraparib. In (B–D), the hydrogen bonds are shown as black dashes, and salt bridges as yellow dashes. (E–H) ITC experiments showed that niraparib could potently bind to EcTrpRS (E) and SaTrpRS (F), and this binding was specifically blocked by the high concentration of substrate L-Trp (G). In contrast, niraparib did not bind to HcTrpRS (H).
The structure of niraparib could be simply described as linearly linked indazole, phenyl and piperidinyl rings (Figure 7A). The phenyl and piperidinyl rings bind to a cleft formed by residues Gln11, His45, Val49, Leu125 and Tyr128 of EcTrpRS mainly through hydrophobic interactions, and the backbone oxygen of Ala46 forms a water-mediated hydrogen bond with the nitrogen of the piperidinyl ring (Figure 7B). The indazole moiety, a structure similar to the indole group of L-Trp, occupies the L-Trp binding pocket surrounded by hydrophobic residues Phe7, Val42, Met132, Ile136 and Val144. Importantly, the specific recognition of the side chain of L-Trp by EcTrpRS involves a key hydrogen bonding interaction between Asp135 and the nitrogen of the indole moiety (Figure 7C), and this interaction is conserved in the binding of L-Trp to all bacterial TrpRSs (17). Notably, the natural products indolmycin (PDB code: 5DK4) and chuangxinmycin (PDB code: 7CKI) both use an indole-like structure to occupy the L-Trp binding site of bacterial TrpRS, and both form key hydrogen bonding interactions with this conserved aspartate residue, similar to what substrate L-Trp does (20,21). However, this interaction does not exist between niraparib and EcTrpRS. Instead, residues Gly9 and Gln150 of EcTrpRS form three hydrogen bonds with the nitrogen and carboxamide group of niraparib from the opposite side (Figure 7B). Thus, niraparib represents a new mechanism by which chemicals inhibit the L-Trp binding site of bacterial TrpRS, providing a potential way to overcome drug resistance (47).
The key residues for recognizing the indole group of L-Trp in HcTrpRS are different from those in bacterial TrpRS (23). While neither the binding of substrate L-Trp nor inhibitor niraparib causes a significant conformation change to EcTrpRS, the binding of L-Trp to HcTrpRS uses an induced fit mechanism in which the AIDQ motif (corresponding to GEDQ in bacterial TrpRS) moves towards the active site to interact with L-Trp and induces an overall closed conformation of HcTrpRS (23). This closed L-Trp binding pocket of HcTrpRS cannot accommodate indolmycin, which results in an approximately 1000-fold weaker affinity of indolmycin to HcTrpRS than to bacterial TrpRS (20). Here, when niraparib was modeled into the L-Trp binding site of HcTrpRS (PDB code: 2QUH), it clashed with residues Gln284 and Glu199 (Figure 7D). Consistently, niraparib exhibited enzyme inhibitory activity to bacterial TrpRS (IC50 = 148 μM) (Supplementary Figure S10), but not HcTrpRS, in the tRNA-denpendent ATP consumption assays, implying that niraparib is unlikely to interfere with protein biosynthesis in human cells through inhibiting HcTrpRS. While the IC50 values are associated with the conditions in which the assays have been carried out, we then used the ITC assays to directly measure the binding affinities of niraparib to bacterial TrpRS. The results showed that niraparib has affinities around 20 μM to TrpRSs from both E. coli (gram-negative) and S. aureus (gram-positive, SaTrpRS) (Figure 7E and F), a value about twofold better than the substrate L-Trp (Figure 2A and B) and better than the affinities of typical fragment hits binding to their targets (40). Notably, this binding of niraparib to bacterial TrpRS is specific to the L-Trp binding site as it could be blocked by adding high concentration of L-Trp (Figure 7G). In contrast, niraparib showed no significant binding to HcTrpRS (Figure 7H). The unique and bacteria-specific binding mode makes niraparib a valuable starting point for developing TrpRS-targeted antibacterials via a fragment-based drug discovery (FBDD) strategy.
DISCUSSION
The half-of-the-sites reactivity in bacterial TrpRS and other AARSs
The half-of-the-sites reactivity for homodimeric enzymes refers to a phenomenon in which only one subunit, despite both subunits having the same sequence, is active at a time, and this phenomenon has been frequently observed in many homooligomeric enzymes (48). Most AARSs in class II and TrpRS, TyrRS and MetRS in class I function as homodimers (49). In addition, heterotratetrameric bacterial GlyRS and PheRS are organized as two equal protomers, each containing an active site (50). Previous studies revealed that the two active sites in some oligomeric AARSs, such as TyrRS, LysRS-II, AspRS, HisRS and ProRS (51–55), are not simultaneously active, exhibiting the half-of-the-sites activity similar to that observed in TrpRS. Notably, because crystallization drops contain high concentrations of AARSs and ligands, in most cases, the ligands could be pushed into both active sites, although one of them is less active. For example, TyrRS binds only one molecule of L-Tyr or tRNATyr and forms only one molecule of tyrosyl adenylate (TyrAMP) per dimer in solution, showing clear half-of-the-sites activity (56,57). However, TyrRS in crystals forms an artificial symmetric dimer bound with two molecules of L-Tyr, TyrAMP or tRNATyr (58,59). Thus, the ‘open-closed’ asymmetric structure of EcTrpRS determined in this study provides a good opportunity for understanding the structural state associated with the half-of-the-sites reactivity.
TrpRS and TyrRS are the only two members of subclass Ic AARSs, and the differentiation between them was suggested occurring at a late stage during the development of genetic codes (17). Impressively, evidences have shown that the active and inactive sites are randomly selected from the two active sites of the TyrRS dimer, and once selected, no interconversion between active and inactive sites could be detected over time (49,51). Thus, the half-of-the-sites reactivity of TyrRS is likely caused by its preexisting inherent asymmetry. The underlying mechanism for TrpRS is not such clear. The asymmetric binding of 7 fragments at the dimer interface supports a potential preexisting asymmetry for EcTrpRS (Figure 6), but the unfavorable binding of the second ATP but not L-Trp (Figure 2) suggested that it might be the strong negative cooperation between two ATP binding sites causes the half-of-the-sites reactivity in bacterial TrpRS. Interestingly, regardless of how bacterial TrpRS achieves the half-of-the-sites reactivity, our structural analysis and binding assays suggested that the ‘open-closed’ asymmetric conformation is necessary for the functional binding of tRNATrp (Figure 3), which may provide the evolutionary pressure for driving the formation of the half-of-the-sites reactivity in bacterial TrpRS.
Implications of dimer stabilization effects of the fragments buried at the interface
In this study, seven fragments were observed to enter the pocket buried at the dimeric interface of EcTrpRS, suggesting a monomer-dimer exchange of EcTrpRS in solution. Once EcTrpRS in different states was diluted to 3.5 μM and loaded onto gel-filtration column, both ‘open-closed’ and ‘closed-closed’ EcTrpRSs (bound with one or two TrpAMP molecules respectively) dominantly existed as dimers, but apo EcTrpRS formed almost equal dimer and monomer peaks (Supplementary Figure S11). Incubation with 200 μM chlorzoxazone could increase the dimer peak and decrease the monomer peak for apo EcTrpRS, suggesting that binding of these fragments to the dimer interface did not disrupt but actually facilitated stabilization of the EcTrpRS dimer. Considering that dimerization is necessary for the cross-subunit binding of tRNATrp to TrpRS (39), these fragments may help to maintain the activity of bacterial TrpRS when the TrpRS concentration is low in vivo.
Charcot-Marie-Tooth disease (CMT) is one of the most common inherited neuropathies without available clinical treatment. AARSs are the largest known protein family associated with the etiology of CMT, and mutations in seven human cytoplasmic AARSs including TrpRS have been reported to cause CMT via either the loss-of-function mechanism (e.g. mutations weaken the tRNA charging activity of AARSs) or the gain-of-function mechanism (e.g. gain of new protein-protein interactions toxic to neuron cells) (60,61). Consequently, different therapeutic strategies have been proposed. Overexpression of the corresponding substrate tRNA was shown to rescue protein synthesis levels and attenuate CMT caused by AARS mutations (62). For the second mechanism, an example is that overexpression of vascular endothelial growth factor (VEGF, a natural ligand of the axon guidance receptor neuropilin1) could compete off the pathogenic interaction between neuropilin1 and mutant GlyRS and rescue the CMT phenotype in a mouse model (63). Notably, it has been proven that most CMT mutations of AARSs are related to the weakened dimer (61,64). Dimer disassociation likely plays an important role in both the loss-of-function and gain-of-function mechanisms of the development of AARS-associated CMT diseases (61,65). In this study, gel filtration assays revealed that even wild-type EcTrpRS has the potential to dissociate from dimer to monomer, especially in the apo state. Fragment screening and crystallography identified seven chemical fragments that are buried between two subunits of EcTrpRS. Importantly, insertion of these chemicals at the dimer interface did not disrupt the dimerization nor impair enzyme activity (at least for these small fragments), but actually helped to stabilize the dimer according to gel filtration assays (Supplementary Figure S11). Probably, searching for small molecules that stabilize human AARS dimers [e.g. molecules binding to the buried pocket at the dimeric interface of HcTrpRS (Supplementary Figure S8A)] could be an alternative strategy for developing drugs to treat related human diseases.
In conclusion, this study determined an ‘open-closed’ EcTrpRS structure with a copurified TrpAMP bound in subClosed, providing not only direct structural evidence for the long-discussed half-of-the-sites reactivity of bacterial TrpRS but also a possible reason why bacterial TrpRS requires this half-of-the-sites reactivity. Fragment screening identified 19 hits against ‘open-closed’ asymmetric EcTrpRS. With eight cocrystal structures, we clarified the unique binding mode of niraparib at the active site, and also identified an unprecedented pocket at the dimeric interface of bacterial TrpRS. These findings improve the understanding of the structural and catalytic mechanism of bacterial TrpRS and will help AARS-based antimicrobial discovery in the future.
DATA AVAILABILITY
Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession numbers 8I1W, 8I4I, 8I27, 8I2A, 8I1Z, 8I2C, 8I2J, 8I1Y, 8I2L and 8I2M.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of beamlines BL19U1 at National Facility for Protein Sciences Shanghai (NFPSS) and BL02U1 at Shanghai Synchrotron Radiation Facility (SSRF), Shanghai, People's Republic of China, for assistance during X-ray diffraction data collection.
Author contributions: M.X. and H.Z. designed the research. M.X., K.X., Z.L. and Y.Y. performed the experiments. B.C. and L.J. contributed to data analysis and crystal structure refinements. M.X. and H.Z. wrote the paper. H.Z. supervised this research. All authors approved the final version of the manuscript.
Contributor Information
Manli Xiang, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Kaijiang Xia, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Bingyi Chen, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Zhiteng Luo, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Ying Yu, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Lili Jiang, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
Huihao Zhou, Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery and Research Center for Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, Guangdong, 510006, China.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China [22177140, 22207133]; Guangdong Basic and Applied Basic Research Foundation [2023A1515012453, 2023B1515040006, 2023A1515012936, 2021A1515110117]; China Postdoctoral Science Foundation [2021TQ0390]; Guangdong-Hong Kong-Macao research team project of the Guangdong Basic and Applied Basic Research Foundation [2022B1515130008]; Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery [2019B030301005]. Funding for open access charge: National Natural Science Foundation of China.
Conflict of interest statement. None declared.
REFERENCES
- 1. Ibba M., Soll D.. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000; 69:617–650. [DOI] [PubMed] [Google Scholar]
- 2. Jiang L., Jones J., Yang X.L.. Human diseases linked to cytoplasmic aminoacyl-tRNA synthetases. Enzymes. 2020; 48:277–319. [DOI] [PubMed] [Google Scholar]
- 3. Yao P., Fox P.L.. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol. Med. 2013; 5:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai Z., Chen B., Yu Y., Guo J., Luo Z., Cheng B., Xu J., Gu Q., Zhou H.. Design, synthesis, and proof-of-concept of triple-site inhibitors against aminoacyl-tRNA synthetases. J. Med. Chem. 2022; 65:5800–5820. [DOI] [PubMed] [Google Scholar]
- 5. Pang L., Weeks S.D., Van Aerschot A.. Aminoacyl-tRNA synthetases as valuable targets for antimicrobial drug discovery. Int. J. Mol. Sci. 2021; 22:1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung Y., Yoon I., Han J.M., Kim S.. Functional and pathologic association of aminoacyl-tRNA synthetases with cancer. Exp. Mol. Med. 2022; 54:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu J., Subbaiah K.C.V., Xie L.H., Jiang F., Khor E.S., Mickelsen D., Myers J.R., Tang W.H.W., Yao P.. Glutamyl-prolyl-tRNA synthetase regulates proline-rich Pro-fibrotic protein synthesis during cardiac fibrosis. Circ. Res. 2020; 127:827–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou H., Sun L., Yang X.L., Schimmel P.. ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature. 2013; 494:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woo J.T., Kawatani M., Kato M., Shinki T., Yonezawa T., Kanoh N., Nakagawa H., Takami M., Lee K.H., Stern P.H.et al.. Reveromycin A, an agent for osteoporosis, inhibits bone resorption by inducing apoptosis specifically in osteoclasts. Proc. Natl. Acad. Sci. U.S.A. 2006; 103:4729–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen B., Luo S., Zhang S., Ju Y., Gu Q., Xu J., Yang X.L., Zhou H.. Inhibitory mechanism of reveromycin A at the tRNA binding site of a class I synthetase. Nat. Commun. 2021; 12:1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silvian L.F., Wang J., Steitz T.A.. Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science. 1999; 285:1074–1077. [PubMed] [Google Scholar]
- 12. Rock F.L., Mao W., Yaremchuk A., Tukalo M., Crépin T., Zhou H., Zhang Y.K., Hernandez V., Akama T., Baker S.J.et al.. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007; 316:1759–1761. [DOI] [PubMed] [Google Scholar]
- 13. Gill J., Sharma A.. Prospects of halofuginone as an antiprotozoal drug scaffold. Drug Discov. Today. 2022; 27:2586–2592. [DOI] [PubMed] [Google Scholar]
- 14. Kwon N.H., Fox P.L., Kim S.. Aminoacyl-tRNA synthetases as therapeutic targets. Nat. Rev. Drug Discov. 2019; 18:629–650. [DOI] [PubMed] [Google Scholar]
- 15. Tenero D., Derimanov G., Carlton A., Tonkyn J., Davies M., Cozens S., Gresham S., Gaudion A., Puri A., Muliaditan M.et al.. First-time-in-human study and prediction of early bactericidal activity for GSK3036656, a potent leucyl-tRNA synthetase inhibitor for tuberculosis treatment. Antimicrob. Agents Chemother. 2019; 63:e00240-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ribas de Pouplana L., Schimmel P.. Aminoacyl-tRNA synthetases: potential markers of genetic code development. Trends Biochem. Sci. 2001; 26:591–596. [DOI] [PubMed] [Google Scholar]
- 17. Yang X.L., Otero F.J., Skene R.J., McRee D.E., Schimmel P., Ribas de Pouplana L.. Crystal structures that suggest late development of genetic code components for differentiating aromatic side chains. Proc. Natl. Acad. Sci. U.S.A. 2003; 100:15376–15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen N., Guo L., Yang B., Jin Y., Ding J.. Structure of human tryptophanyl-tRNA synthetase in complex with tRNATrp reveals the molecular basis of tRNA recognition and specificity. Nucleic Acids Res. 2006; 34:3246–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu Y., Liu Y., Shen N., Xu X., Xu F., Jia J., Jin Y., Arnold E., Ding J.. Crystal structure of human tryptophanyl-tRNA synthetase catalytic fragment: insights into substrate recognition, tRNA binding, and angiogenesis activity. J. Biol. Chem. 2004; 279:8378–8388. [DOI] [PubMed] [Google Scholar]
- 20. Williams T.L., Yin Y.W., Carter C.W. Jr. Selective inhibition of bacterial tryptophanyl-tRNA synthetases by indolmycin is mechanism-based. J. Biol. Chem. 2016; 291:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan S., Lv G., Feng X., Wu G., Jin Y., Yan M., Yang Z.. Structural insights into the specific interaction between Geobacillus stearothermophilus tryptophanyl-tRNA synthetase and antimicrobial Chuangxinmycin. J. Biol. Chem. 2022; 298:101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sever S., Rogers K., Rogers M.J., Carter C. Jr, Söll D.. Escherichia coli tryptophanyl-tRNA synthetase mutants selected for tryptophan auxotrophy implicate the dimer interface in optimizing amino acid binding. Biochemistry. 1996; 35:32–40. [DOI] [PubMed] [Google Scholar]
- 23. Shen N., Zhou M., Yang B., Yu Y., Dong X., Ding J.. Catalytic mechanism of the tryptophan activation reaction revealed by crystal structures of human tryptophanyl-tRNA synthetase in different enzymatic states. Nucleic Acids Res. 2008; 36:1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acchione M., Guillemette J.G., Twine S.M., Hogue C.W., Rajendran B., Szabo A.G.. Fluorescence based structural analysis of tryptophan analogue-AMP formation in single tryptophan mutants of Bacillus stearothermophilus tryptophanyl-tRNA synthetase. Biochemistry. 2003; 42:14994–15002. [DOI] [PubMed] [Google Scholar]
- 25. Retailleau P., Huang X., Yin Y., Hu M., Weinreb V., Vachette P., Vonrhein C., Bricogne G., Roversi P., Ilyin V.et al.. Interconversion of ATP binding and conformational free energies by tryptophanyl-tRNA synthetase: structures of ATP bound to open and closed, pre-transition-state conformations. J. Mol. Biol. 2003; 325:39–63. [DOI] [PubMed] [Google Scholar]
- 26. Retailleau P., Weinreb V., Hu M., Carter C.W. Jr. Crystal structure of tryptophanyl-tRNA synthetase complexed with adenosine-5' tetraphosphate: evidence for distributed use of catalytic binding energy in amino acid activation by class I aminoacyl-tRNA synthetases. J. Mol. Biol. 2007; 369:108–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y., Xu Y., Yue Y., Wang H., Cui Y., Pan M., Zhang X., Zhang L., Li H., Xu M.et al.. Investigate natural product indolmycin and the synthetically improved analogue toward antimycobacterial agents. ACS Chem. Biol. 2022; 17:39–53. [DOI] [PubMed] [Google Scholar]
- 28. Ilyin V.A., Temple B., Hu M., Li G., Yin Y., Vachette P., Carter C.W. Jr. 2.9 A crystal structure of ligand-free tryptophanyl-tRNA synthetase: domain movements fragment the adenine nucleotide binding site. Protein Sci. 2000; 9:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kabsch W. XDS. Acta Crystallogr. D. Biol. Crystallogr. 2010; 66:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vagin A., Teplyakov A.. Molecular replacement with MOLREP. Acta Crystallogr. D. Biol. Crystallogr. 2010; 66:22–25. [DOI] [PubMed] [Google Scholar]
- 31. Emsley P., Lohkamp B., Scott W.G., Cowtan K.. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 2010; 66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murshudov G.N., Vagin A.A., Dodson E.J.. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. Biol. Crystallogr. 1997; 53:240–255. [DOI] [PubMed] [Google Scholar]
- 33. Chen V.B., Arendall W.B. 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C.. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 2010; 66:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niesen F.H., Berglund H., Vedadi M.. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007; 2:2212–2221. [DOI] [PubMed] [Google Scholar]
- 35. Kao C., Zheng M., Rüdisser S.. A simple and efficient method to reduce nontemplated nucleotide addition at the 3 terminus of RNAs transcribed by T7 RNA polymerase. RNA. 1999; 5:1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi J., Cai Z., Qiu H., Lu F., Luo Z., Chen B., Gu Q., Xu J., Zhou H.. Fragment screening and structural analyses highlight the ATP-assisted ligand binding for inhibitor discovery against type 1 methionyl-tRNA synthetase. Nucleic Acids Res. 2022; 50:4755–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han G.W., Yang X.L., McMullan D., Chong Y.E., Krishna S.S., Rife C.L., Weekes D., Brittain S.M., Abdubek P., Ambing E.et al.. Structure of a tryptophanyl-tRNA synthetase containing an iron-sulfur cluster. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010; 66:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jia J., Chen X.L., Guo L.T., Yu Y.D., Ding J.P., Jin Y.X.. Residues Lys-149 and Glu-153 switch the aminoacylation of tRNA(Trp) in Bacillus subtilis. J. Biol. Chem. 2004; 279:41960–41965. [DOI] [PubMed] [Google Scholar]
- 39. Yang X.L., Otero F.J., Ewalt K.L., Liu J., Swairjo M.A., Köhrer C., RajBhandary U.L., Skene R.J., McRee D.E., Schimmel P.. Two conformations of a crystalline human tRNA synthetase-tRNA complex: implications for protein synthesis. EMBO J. 2006; 25:2919–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Erlanson D.A., Fesik S.W., Hubbard R.E., Jahnke W., Jhoti H.. Twenty years on: the impact of fragments on drug discovery. Nat. Rev. Drug Discov. 2016; 15:605–619. [DOI] [PubMed] [Google Scholar]
- 41. Huang X., Guo J., Liu Q., Gu Q., Xu J., Zhou H.. Identification of an auxiliary druggable pocket in the DNA gyrase ATPase domain using fragment probes. Medchemcomm. 2018; 9:1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pantoliano M.W., Petrella E.C., Kwasnoski J.D., Lobanov V.S., Myslik J., Graf E., Carver T., Asel E., Springer B.A., Lane P.et al.. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen. 2001; 6:429–440. [DOI] [PubMed] [Google Scholar]
- 43. Volkamer A., Kuhn D., Rippmann F., Rarey M.. DoGSiteScorer: a web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics. 2012; 28:2074–2075. [DOI] [PubMed] [Google Scholar]
- 44. Jia J., Xu F., Chen X., Chen L., Jin Y., Wang D.T.. Two essential regions for tRNA recognition in Bacillus subtilis tryptophanyl-tRNA synthetase. Biochem. J. 2002; 365:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hogue C.W., Doublié S., Xue H., Wong J.T., Carter C.W. Jr, Szabo A.G.. A concerted tryptophanyl-adenylate-dependent conformational change in Bacillus subtilis tryptophanyl-tRNA synthetase revealed by the fluorescence of Trp92. J. Mol. Biol. 1996; 260:446–466. [DOI] [PubMed] [Google Scholar]
- 46. Nakai H., Matsumura N.. Individualization in the first-line treatment of advanced ovarian cancer based on the mechanism of action of molecularly targeted drugs. Int. J. Clin. Oncol. 2022; 27:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vecchione J.J., Sello J.K.. A novel tryptophanyl-tRNA synthetase gene confers high-level resistance to indolmycin. Antimicrob. Agents Chemother. 2009; 53:3972–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wielgus-Kutrowska B., Grycuk T., Bzowska A.. Part-of-the-sites binding and reactivity in the homooligomeric enzymes - facts and artifacts. Arch. Biochem. Biophys. 2018; 642:31–45. [DOI] [PubMed] [Google Scholar]
- 49. Ward W.H., Fersht A.R.. Asymmetry of tyrosyl-tRNA synthetase in solution. Biochemistry. 1988; 27:1041–1049. [DOI] [PubMed] [Google Scholar]
- 50. Ju Y., Han L., Chen B., Luo Z., Gu Q., Xu J., Yang X.L., Schimmel P., Zhou H.. X-shaped structure of bacterial heterotetrameric tRNA synthetase suggests cryptic prokaryote functions and a rationale for synthetase classifications. Nucleic Acids Res. 2021; 49:10106–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward W.H., Fersht A.R.. Tyrosyl-tRNA synthetase acts as an asymmetric dimer in charging tRNA. A rationale for half-of-the-sites activity. Biochemistry. 1988; 27:5525–5530. [DOI] [PubMed] [Google Scholar]
- 52. Hughes S.J., Tanner J.A., Hindley A.D., Miller A.D., Gould I.R.. Functional asymmetry in the lysyl-tRNA synthetase explored by molecular dynamics, free energy calculations and experiment. BMC Struct. Biol. 2003; 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larson E.T., Kim J.E., Napuli A.J., Verlinde C.L., Fan E., Zucker F.H., Van Voorhis W.C., Buckner F.S., Hol W.G., Merritt E.A.. Structure of the prolyl-tRNA synthetase from the eukaryotic pathogen Giardia lamblia. Acta Crystallogr. D. Biol. Crystallogr. 2012; 68:1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guth E., Farris M., Bovee M., Francklyn C.S.. Asymmetric amino acid activation by class II histidyl-tRNA synthetase from Escherichia coli. J. Biol. Chem. 2009; 284:20753–20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmitt E., Moulinier L., Fujiwara S., Imanaka T., Thierry J.C., Moras D.. Crystal structure of aspartyl-tRNA synthetase from Pyrococcus kodakaraensis KOD: archaeon specificity and catalytic mechanism of adenylate formation. EMBO J. 1998; 17:5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jakes R., Fersht A.R.. Tyrosyl-tRNA synthetase from Escherichia coli. Stoichiometry of ligand binding and half-of-the-sites reactivity in aminoacylation. Biochemistry. 1975; 14:3344–3350. [DOI] [PubMed] [Google Scholar]
- 57. Fersht A.R., Ashford J.S., Bruton C.J., Jakes R., Koch G.L., Hartley B.S.. Active site titration and aminoacyl adenylate binding stoichiometry of aminoacyl-tRNA synthetases. Biochemistry. 1975; 14:1–4. [DOI] [PubMed] [Google Scholar]
- 58. Brick P., Bhat T.N., Blow D.M.. Structure of tyrosyl-tRNA synthetase refined at 2.3 A resolution. Interaction of the enzyme with the tyrosyl adenylate intermediate. J. Mol. Biol. 1989; 208:83–98. [DOI] [PubMed] [Google Scholar]
- 59. Yaremchuk A., Kriklivyi I., Tukalo M., Cusack S.. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 2002; 21:3829–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang H., Zhou Z.W., Sun L.. Aminoacyl-tRNA synthetases in Charcot-Marie-Tooth disease: a gain or a loss?. J. Neurochem. 2021; 157:351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wei N., Zhang Q., Yang X.L.. Neurodegenerative Charcot-Marie-Tooth disease as a case study to decipher novel functions of aminoacyl-tRNA synthetases. J. Biol. Chem. 2019; 294:5321–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zuko A., Mallik M., Thompson R., Spaulding E.L., Wienand A.R., Been M., Tadenev A.L.D., van Bakel N., Sijlmans C., Santos L.A.et al.. tRNA overexpression rescues peripheral neuropathy caused by mutations in tRNA synthetase. Science. 2021; 373:1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. He W., Bai G., Zhou H., Wei N., White N.M., Lauer J., Liu H., Shi Y., Dumitru C.D., Lettieri K.et al.. CMT2D neuropathy is linked to the neomorphic binding activity of glycyl-tRNA synthetase. Nature. 2015; 526:710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bervoets S., Wei N., Erfurth M.L., Yusein-Myashkova S., Ermanoska B., Mateiu L., Asselbergh B., Blocquel D., Kakad P., Penserga T.et al.. Transcriptional dysregulation by a nucleus-localized aminoacyl-tRNA synthetase associated with Charcot-Marie-Tooth neuropathy. Nat. Commun. 2019; 10:5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morant L., Erfurth M.L., Jordanova A.. Drosophila models for Charcot-Marie-Tooth neuropathy related to aminoacyl-tRNA synthetases. Genes (Basel). 2021; 12:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and structure factors for the reported crystal structures have been deposited with the Protein Data Bank under accession numbers 8I1W, 8I4I, 8I27, 8I2A, 8I1Z, 8I2C, 8I2J, 8I1Y, 8I2L and 8I2M.