Abstract
In this review, we provide a historical perspective on probiotic use in preterm infants. We review recent data on the treatment effects of probiotics on necrotizing enterocolitis, sepsis, and mortality. We highlight guidance statements from professional societies and organizations, discussing key points within the context of the currently available evidence from both randomized trials and cohort studies. Finally, we summarize experiences from several North American centers that have reported on the routine use of probiotics, including our center. Our goal is to highlight some of the considerations and complexities surrounding routine probiotics use in preterm infants.
Introduction
Probiotics are live microorganisms, that, when consumed in the appropriate amount, confer health benefits to the host1. Probiotic supplementation in preterm infants has been extensively studied for its use in the prevention of necrotizing enterocolitis (NEC), a devastating gastrointestinal disease that primarily affects preterm infants, causing significant morbidity and mortality2. In pre-clinical models of NEC, probiotic supplementation reduced the risk of developing NEC-like injury by close to 50%3. Through in vitro and in vivo studies, we have learned that probiotics can improve the health of the immature intestine through several mechanisms, including enhancement of the intestinal barrier, production of short-chain fatty acids such as butyrate, competition with pathologic bacteria, down-regulation of pro-inflammatory genes, upregulation of cytoprotective genes and regulation of cellular immunity4.
History of Probiotic Use in Preterm Infants
One of the initial reports of routine probiotic administration to preterm infants was in 19995. Dr. Angela Hoyos reported that daily administration of Lactobacillus acidophilus (L. acidophilus) and Bifidobacterium infantis (B. infantis) to all infants admitted to a neonatal intensive care unit (NICU) lead to a significant reduction in NEC and NEC-associated mortality5. Since that time over 10,000 preterm infants have been randomized in trials to assess the effects of probiotics administration in preterm infants6. There is significant methodological heterogeneity between each trial, with regards to the population studies and product used. Even with this heterogeneity, pooled risk ratios favor the use of probiotics for the prevention of NEC, mortality, and late-onset sepsis4. Additionally, effect estimates for probiotic administration on the risk of NEC demonstrate low statistical heterogeneity (I2 <20%). Despite the breadth of pre-clinical and clinical evidence supporting the use of probiotics for the prevention of NEC in preterm infants, the routine administration of probiotics to preterm infants continues to be controversial. Two important factors that mitigate routine use are the lack of an FDA-approved pharmaceutical-grade probiotic in the United States and concerns for risks of probiotic-associated sepsis7. Some of these concerns are highlighted in statements from professional societies, that we discuss in detail below.
Statements from Professional Societies
Several professional societies have published statements and guidance on the use of probiotics in preterm infants. In 2019, the Canadian Pediatric Society (CPS) published a statement in which they recommended using caution when considering the use of probiotics in preterm infants. CPS encouraged the promotion of breastfeeding and stated that probiotic administration may be considered in preterm infants who are at risk for NEC and have a birthweight greater than 1 kilogram (kg)8. In 2020, the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published consensus-based guidance for the use of probiotics in preterm infants. In this report, ESPGHAN recommended that if probiotics are used, the product should be manufactured according to current good manufacturing practices, hospital laboratories should be able to detect probiotic bacteremia and clinicians should be prepared to share the potential risks and benefits of probiotics with the parents of preterm infants9. The panel also conditionally recommended the use of Lactobacillus rhamnosus GG (LGG) with a dose of 1 × 109 colony forming units (CFUs) to 6 × 109 CFUs or a combination of B infantis, Bifidobacterium lactis (B. lactis) and Streptococcus thermophilus (S. thermophilus) with a dose of 3–3.5 × 108 CFUs for each strain (low certainty of evidence)9. Additionally, the panel highlighted the lack of evidence for optimal initiation and length of treatment9.
In 2020, the American Gastroenterological Association published recommendations on the use of specific probiotics in preterm infants for the prevention of NEC10. Specifically, they recommended using a combination of Lactobacillus species and Bifidobacterium species such as L rhamnosus ATCC 53103 and B infants; or L casei and B breve; or L rhamnosus, L acidophilus, L casei, B infants, B bifidum and B longum; or L acidophilus and B infants; or L acidophilus and B bifidum; or L rhamnosus ATCC 53103 and B longum Reuter ATCC BAA-999; or L acidophilus, B bifidum, B lactis and B longum; or B lactis or L reuteri or L rhamnosus10. This recommendation was based on moderate/high-level evidence10.
In 2021, the American Academy of Pediatrics (AAP) Committee on Fetus and Newborn published a statement on the use of probiotics in preterm infants. In this statement, the AAP did not recommend universal administration of probiotics to preterm infants, especially those with a birth weight of less than 1 kg. This statement was based on several concerns including the lack of an FDA-approved pharmaceutical-grade product in the United States, the potential risk for harm in this population (e.g. sepsis), and conflicting evidence on efficacy and safety7. Four key points in the statement included:
Emphasis on the uncertainty of evidence and recommendations against universal use in preterm infants.
Clinicians should discuss the risks and benefits with parents and develop local guidelines.
The potential for contamination should be recognized and mitigated.
Centers should monitor outcomes.
In August of 2021, the NEC Society released a Statement on Probiotics (https://necsociety.org/probiotics/). The statement advised that “probiotics can be considered as a strategy to help reduce the risks of NEC and death in very low birth weight infants (VLWBs).” Consistent with the AAP, the statement also emphasized the importance of informing families about the risk and benefits of probiotics and the collection of data to track and understand the effect of probiotics for centers adopting use. Additionally, the NEC Society provides resources for clinicians, such as a probiotic information sheet for parents.
Evidence from RCT and observational studies
In the section below, we place the aforementioned guidance statements within the context of available data.
Randomized Trials
Since 2002, over 56 RCTs which include over 10,000 preterm infants have been conducted to assess the effect of routine probiotics administration in preterm infants16. Bin-Nun et al conducted an RCT in Israel, with 145 preterm infants, in which the mixture of B infantis, S thermophilus, and B bifidus lead to a reduction in the incidence of NEC11. Dani et al conducted an RCT in Italy which included 585 preterm infants in which supplementation with LGG resulted in a reduction in the incidence of NEC12. Additionally, an RCT with 367 preterm infants in Taiwan evaluated L acidophilus and B infantis supplementation in preterm infants and found a reduction in NEC13. The PiPS trial was a large RCT of Bifidobacterium breve supplementation in very preterm infants which was conducted in the United Kingdom. There were 1315 preterm infants randomized. This trial found no difference in outcomes of NEC (RR 0.93; 95% CI, 0.68–1.27), sepsis (RR 0.97; 95% CI, 0.73–1.29) or mortality (RR 0.93; 95% CI, 0.67–1.3) before hospital discharge14. It is important to note that in every study site in this trial (24 hospitals), there were patients randomized to the control group who became colonized by the probiotic being studied. By 2 weeks of life, 20% of patients in the control group were colonized with B breve, and by 36 weeks post-menstrual age (PMA), 49% had been colonized. This contamination may have diminished the results toward the null. The ProPrems trial was a large RCT conducted in Australia and New Zealand which included 1099 very low birth weight infants who had significant exposure to a human milk diet. In this RCT, supplementation with B infantis, S thermophiles, and B lactis was evaluated. This trial found no difference in late-onset sepsis with probiotic supplementation but did find that the incidence of NEC decreased by over half (RR 0.46; 95% CI, 0.23–0.93) among infants randomized to receive probiotics, compared to placebo. However, in a prespecified subgroup analysis, among infants who were born before 28 weeks gestation with a birth weight of less than 1 kg, estimates suggested less efficacy of probiotic supplementation on the rate of NEC15. While there is significant heterogeneity between these trials, several systematic reviews, and meta-analyses that have evaluated these RCTs have found that probiotic supplementation favors the prevention of NEC in preterm infants4,16–19. For instance, one systematic review reported that the cumulative pooled risk ratio for NEC, strongly favors probiotic administration for the prevention of NEC (RR 0.54; 95% CI, 0.45–0.65), mortality (RR 0.76; 95% CI, 0.65–0.89) and invasive infection (RR 0.89; 95% CI, 0.82–0.97)16 (Figure 1).
Figure 1. Effects of Probiotics on NEC, Sepsis, and Mortality.
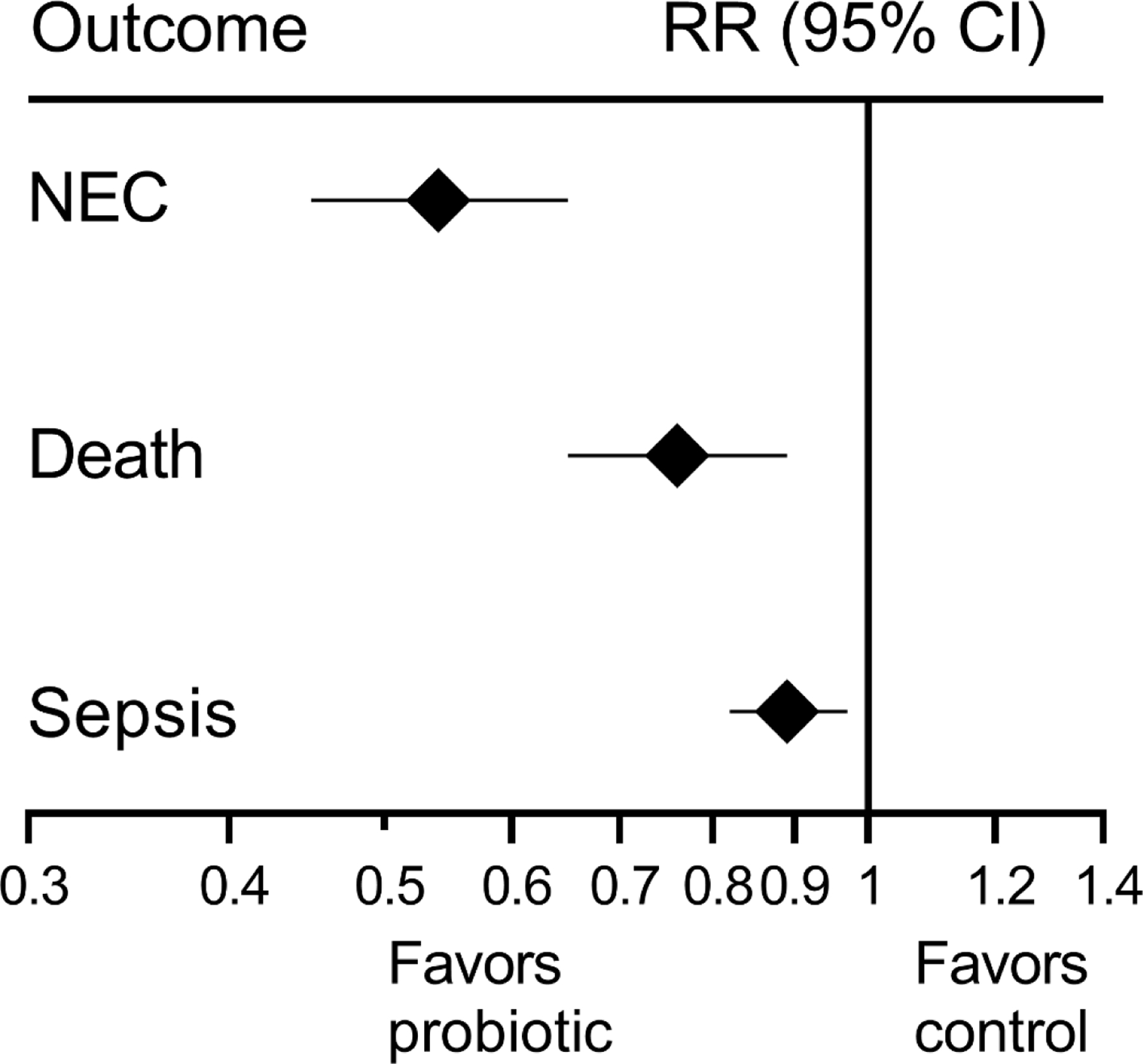
Data are from a meta-analysis of RCT by Sharif et al.16
Observational Studies
In addition to RCTs, it is important to evaluate observational studies. This is because treatments demonstrating efficacy in an RCT may not show effectiveness when implemented in a routine clinical setting. A systematic review and meta-analysis of 30 good quality non-randomized studies which included over 77,000 infants in 18 different countries found that probiotic supplementation reduced the risk of NEC (OR 0.6; 95% CI, 0.5–0.73)20. There may also be differences in outcomes based on the type of probiotic used---whether it is a single strain or multiple strain21.
Routine Use of Probiotics
Variability in Probiotic Use within Large Cohorts Around the World
There is substantial variability in the use of probiotics in NICUs worldwide. A survey from 2016 estimates that approximately 14% of NICUs in the United States use probiotics6. While a report from a large collaborative database estimates that 10% of extremely preterm infants in the United States receive probiotics during their NICU admission6. In Canada, 21% of infants with gestational age less than 29 weeks receive probiotics22. In the United Kingdom, 12% of NICUs use probiotics23, while 68% of NICUS in Germany24 and 100% of NICUs in New Zealand report the use of probiotics25. These data may not reflect current practice.
Experiences of Selected Centers
Below are four examples of protocols and experiences of implementing probiotic supplementation from NICUs in North America, which are provided as examples of protocols used and associated results.
Sunnybrook Health Sciences Centre NICU in Toronto, Ontario, Canada, is a tertiary level, 42-bed unit which cares for approximately 300 very low birth weight infants each year. Between 2003–2014, the rate of NEC among VLBW infants was about 5%. This NICU began the administration of Lactobacillus reuteri DSM 17938 suspension (BioGaia, Ferring, Stockholm, Sweden) to infants <33 weeks’ gestation. Patients received probiotic supplementation from the first day of life or the first day of admission (for outborn patients). In addition, these infants were fed an exclusive human milk diet with either maternal breastmilk or donor breastmilk and a feeding protocol was used to advance enteral feeds. NEC rates decreased from 4.4% to 1.7%. There was no reduction in mortality and no adverse events occurred26. Adherence to guidelines was tracked and was ~100%.
Oregon Health & Science University NICU in Oregon, Washington, United States, a level-IV NICU. The unit began supplementation with B infants EVC001 (Evivo; Evolve BioSystem) to infants with a birthweight <1500 grams after their third day of life. Infants received 8 billion CFU of B infantis suspended in 0.5 ml of medium-chain triglyceride oil daily via a gastric tube before morning feed. From June 2018 to July 2019 probiotic supplementation was started at feeding volumes of 80–100 ml/kg/day and in August 2019 the protocol was revised, and probiotic supplementation was started on the second day of trophic feeding. B infantis was given daily until 34 weeks post menstrual age or for a minimum of 2 weeks, whichever was longer. In addition, these infants received a human milk diet and their feedings were advanced based on an established feeding protocol.27 The probiotic product was produced in a dedicated facility as a Food for Special Dietary Use under US FDA guidelines. Pathogen testing and heavy metal analysis were performed by an independent third-party laboratory. The strain identity of each lot was confirmed by whole genome sequencing and a shelf-life testing program ensured the product contained 8 billion CFU per dose at the end of shelf life27. The incidence of NEC decreased from 11% to 2.7%, comparing pre- and post-probiotic supplementation periods. Additionally, NEC-related mortality decreased from 2.7% to 0%. These authors also found a similar reduction in NEC incidence and risk for ELBW infants27.
University of Utah Medical Center NICU, Salt Lake City, Utah, United States, a 52 bed, level III unit that admits approximately 185 preterm infants born less than 33 weeks gestation per year. Infants with a birthweight < 1500 grams or gestational ages between 24 0/7 weeks and 33 0/7 weeks, age ≥ 72 hours old and tolerating over 6 ml per day of human milk for 24 hours, received Ultimate Flora Baby Probiotic (Renew Life, Palm Harbor, Florida, USA) until a corrected gestational age of 36 0/7 weeks. Infants with lethal anomalies or significant gastrointestinal anomalies did not receive probiotic supplementation. Ultimate Flora Baby Probiotic contains four Bifidobacteria species (Bifidobacterium breve HA-129, Bifidobacterium bifidum HA-132, Bifidobacterium infants HA-117, Bifidobacterium longum HA-135) and Lactobacillus rhamnosus HA-111, for a total of 4 billion CFU per gram28. As described, safety measures included preparation outside of patient care areas, use of gloves when handling suspension, and nursing staff required to perform hand hygiene after probiotic administration. The microbiology lab within the hospital was able to culture Lactobacillus and Bifidobacterium species from pediatric blood culture specimens. The annual rate of NEC decreased from 7% to 2%. Among infants born < 30 weeks’ gestation, the annual NEC rate decreased from 10% to 2%. There was no change in the rate of surgical NEC. There were no cases of probiotic sepsis. There was one case of Lactobacillus paracasei and Candida lusitaniae sepsis in a patient born at 26 1/7 weeks’ gestation who had received three doses of probiotic and had a bowel perforation, this infant survived to discharge.
Emory University Midtown Neonatal Intensive Care Unit, Atlanta, Georgia, United States, a level-III NICU with 48 beds. Implementation of probiotic supplementation began in 2014 with Culturelle (LGG) at a dose of 2.5–5 × 109 CFU per day. The use of LGG supplementation was not associated with a reduction in NEC and there were no episodes of Lactobacillus sepsis, as previously reported29. Following this initial effort, antimicrobial stewardship through the Vermont Oxford Network iNICQ was pursued and probiotic supplementation was changed to Lactobacillus reuteri (BioGaia, Ferring, Stockholm, Sweden), due to ease of administration. The target population was infants with a birthweight < 1500 grams and gestation age <34 weeks with no congenital gastrointestinal anomalies. Supplementation with L reuteri occurred once a day once an infant began enteral feedings. The neonatal pharmacist prepared a patient-specific oral syringe of L reuteri and delivered it to the NICU within a plastic bag. Nurses used gloves at all times and washed their hands before and after handling the syringe with probiotics. Nurses did not touch vascular access devices or administer intravenous medications during times of probiotic administration. Probiotic syringes were discarded immediately following administration and surfaces were cleaned with hospital-grade wipes. Routine blood cultures are able to detect Lactobacillus in the local microbiology lab. The incidence of NEC decreased from a baseline of 13.2% to 5.6%, with a special cause reduction (8 points below the prior mean). There were no cases of Lactobacillus-sepsis. A summary of the implementation of L. reuteri supplementation within the context of quality improvement efforts to prevent NEC is shown in Figure 2.
Figure 2. Probiotic supplementation as part of quality improvement efforts to reduce NEC.
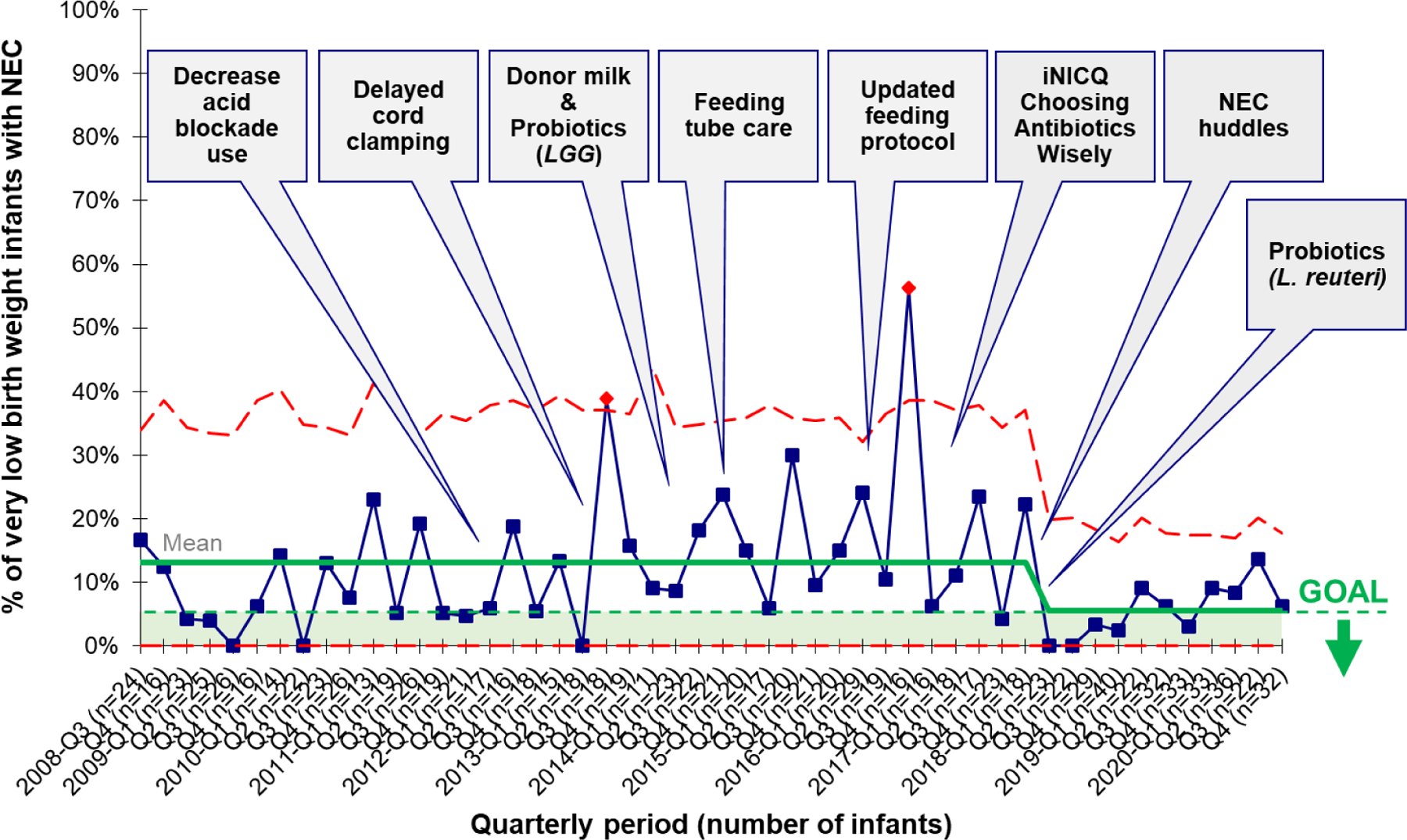
Data from 1089 very low birth weight infants at Emory University Hospital Midtown from 2008 through 2020. The annotated statistical process control chart shows 3 sigma control limits (red dotted line) along with the mean incidence of NEC (solid green line), with individual data points (blue boxes) showing quarterly incidence. The goal denotes a target NEC incidence of < 5%. There was a reduction in the incidence of NEC from 13.2% to 5.6%, meeting special cause rules.
Potential for contamination
Quality of product
In the United States, probiotics are manufactured as dietary supplements. Thus, the product itself may have variations in the CFUs and/or in the actual strain of bacteria within each product. In one study, 16 different commercially available probiotic products were analyzed to assess whether the probiotic product matched the bacterial species listed on the label30. Using culture and polymerase chain reaction, the authors only found 1 out of 16 products matched their label30. This study also found significant variability in the composition of the product, with one product not containing any of the species listed30. An expert panel convened by the International Scientific Association for Probiotics and Prebiotics expressed concern that when probiotics are used with the intent to treat or prevent disease, the products administered must meet a higher standard of regulation7. While probiotics, which are consumed with the purpose of supporting a healthy intestinal microbiome, can be regulated like dietary supplements, this panel recommended that probiotics given to treat or prevent disease should meet the following regulatory requirements: a defined strain of bacteria, proof that microbe can be delivered at an efficacious dose at the end of shelf-life and a risk/benefit assessment based on RCT to meet regulatory standards for drugs7.
Cases of contamination
There is concern for the risk of contamination of the probiotic product leading to serious infection in this vulnerable population. There have been several cases of probiotic-associated sepsis among infants receiving probiotics12. Thus, it is important for facilities that implement probiotic supplementation to develop guidelines to ensure safety measures31 and for parents to be aware of these risks. When implementing probiotic supplementation, it is important to monitor the effects of this therapy. Centers should decide what parameters they would like to follow and implement a system to monitor these outcomes. We highlighted select safety measures among reports of probiotic use from 4 centers. If a unit chooses not to implement probiotic supplementation, then the following outcomes are still important to assess the incidence of NEC. Furthermore, there are many interventions that can help to decrease the rate of NEC such as promotion of breastfeeding, reduction of antibiotics when an infection is unlikely, elimination of acid-suppressing medications, and the use of a feeding protocol31 (Figure 2), which should be pursued regardless of whether probiotic supplementation is implemented.
Conclusion
In conclusion, the use of probiotics to prevent NEC in preterm infants has been extensively studied. More clinical trials will likely not lead to a difference in pooled outcomes, given the large number of studies to date32. The decision of whether to implement probiotic supplementation is complex and will vary based on many factors such as a center’s baseline NEC incidence and the status of other efforts to decrease the risk of NEC, such as the promotion of a human milk diet. While the lack of a pharmaceutical grade FDA regulated product is a valid concern, tens of thousands of infants have received probiotic products in trials and cohort studies with data demonstrating its beneficial effects and a reduction in the incidence of NEC, mortality, and late-onset sepsis. Thus, if a unit’s NEC rate is particularly high, the benefit of initiating probiotic supplementation may outweigh the risks. Since the decision to pursue routine use of probiotics is a complex one, it is critical to involve multiple stakeholders, including the perspectives of families, as routine use is considered.
Acknowledgments
Supported in part by NIH K12 HD072245 to Maria Estefania Barbian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The authors report no conflicts of interest.
References
- 1.Hill C et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11, 506–514, doi: 10.1038/nrgastro.2014.66 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Patel RM et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 372, 331–340, doi: 10.1056/NEJMoa1403489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athalye-Jape G, Rao S & Patole S Effects of probiotics on experimental necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Res 83, 16–22, doi: 10.1038/pr.2017.218 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Patel RM & Underwood MA Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 27, 39–46, doi: 10.1053/j.sempedsurg.2017.11.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyos AB Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis 3, 197–202, doi: 10.1016/s1201-9712(99)90024-3 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan S, Lau C, Akbari H, Hoyen C & Walsh MC Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J Perinatol 36, 1106–1111, doi: 10.1038/jp.2016.144 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Poindexter B,Committee On, F. & Newborn. Use of Probiotics in Preterm Infants. Pediatrics 147, doi: 10.1542/peds.2021-051485 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Marchand V Using probiotics in the paediatric population. Paediatr Child Health 17, 575–576, doi: 10.1093/pch/17.10.575 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Akker CHP et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 70, 664–680, doi: 10.1097/MPG.0000000000002655 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Su GL et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 159, 697–705, doi: 10.1053/j.gastro.2020.05.059 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Bin-Nun A et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147, 192–196, doi: 10.1016/j.jpeds.2005.03.054 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Dani C et al. Lactobacillus Sepsis and Probiotic Therapy in Newborns: Two New Cases and Literature Review. AJP Rep 6, e25–29, doi: 10.1055/s-0035-1566312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HC et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115, 1–4, doi: 10.1542/peds.2004-1463 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Costeloe K et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess 20, 1–194, doi: 10.3310/hta20660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs SE et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132, 1055–1062, doi: 10.1542/peds.2013-1339 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX & McGuire W Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev 10, CD005496, doi: 10.1002/14651858.CD005496.pub5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dermyshi E et al. The “Golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 112, 9–23, doi: 10.1159/000454668 (2017). [DOI] [PubMed] [Google Scholar]
- 18.AlFaleh K & Anabrees J Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev, CD005496, doi: 10.1002/14651858.CD005496.pub4 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Sawh SC, Deshpande S, Jansen S, Reynaert CJ & Jones PM Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ 4, e2429, doi: 10.7717/peerj.2429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh M & Patole S Prophylactic Probiotic Supplementation for Preterm Neonates-A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv Nutr 12, 1411–1423, doi: 10.1093/advances/nmaa164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang HY et al. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS One 12, e0171579, doi: 10.1371/journal.pone.0171579 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B et al. Probiotics for preterm infants: A National Retrospective Cohort Study. J Perinatol 39, 533–539, doi: 10.1038/s41372-019-0315-z (2019). [DOI] [PubMed] [Google Scholar]
- 23.Duffield SD & Clarke P Current use of probiotics to prevent necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 104, F228, doi: 10.1136/archdischild-2018-316199 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Denkel LA et al. Protective Effect of Dual-Strain Probiotics in Preterm Infants: A Multi-Center Time Series Analysis. PLoS One 11, e0158136, doi: 10.1371/journal.pone.0158136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer MP et al. Probiotics for Prevention of Severe Necrotizing Enterocolitis: Experience of New Zealand Neonatal Intensive Care Units. Frontiers in Pediatrics 8, doi: 10.3389/fped.2020.00119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolnitsky A et al. A Quality Improvement Intervention to Reduce Necrotizing Enterocolitis in premature infants with Probiotic Supplementation. Pediatr Qual Saf 4, e201, doi: 10.1097/pq9.0000000000000201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobias J et al. Bifidobacteriumlongum subsp. infantis EVC001 Administration Is Associated with a Significant Reduction in the Incidence of Necrotizing Enterocolitis in Very Low Birth Weight Infants. J Pediatr 244, 64–71 e62, doi: 10.1016/j.jpeds.2021.12.070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekhon MK, Grubb PH, Newman M & Yoder BA Implementation of a probiotic protocol to reduce rates of necrotizing enterocolitis. J Perinatol 39, 1315–1322, doi: 10.1038/s41372-019-0443-5 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Kane AF, Bhatia AD, Denning PW, Shane AL & Patel RM Routine Supplementation of Lactobacillus rhamnosus GG and Risk of Necrotizing Enterocolitis in Very Low Birth Weight Infants. J Pediatr 195, 73–79 e72, doi: 10.1016/j.jpeds.2017.11.055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis ZT et al. Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr Res 79, 445–452, doi: 10.1038/pr.2015.244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbian ME, Buckle R, Denning PW & Patel RM To start or not: Factors to consider when implementing routine probiotic use in the NICU. Early Hum Dev 135, 66–71, doi: 10.1016/j.earlhumdev.2019.05.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razak A, Patel RM, Gautham KS. Use of Probiotics to Prevent Necrotizing Enterocolitis: Evidence to Clinical Practice. JAMA Pediatr 175(8):773–774. doi: 10.1001/jamapediatrics.2021.1077. (2021) [DOI] [PubMed] [Google Scholar]


