Learning objectives.
After reading this article, the reader should be able to:
-
•
Identify common vascular access sites, catheters and potential mechanical complications relevant to central venous access.
-
•
Describe techniques for appropriate operator training to minimise mechanical complications of central venous access.
-
•
Discuss the advantages and indications for confirmatory modalities for central venous access including ultrasound, manometry, transoesophageal echocardiography, fluoroscopy and chest radiography.
-
•
Anticipate and prepare for potential difficult central venous catheter (CVC) placements.
Key points.
-
•
Central venous catheter placement risks several mechanical complications.
-
•
Appropriate training improves identification and prevention of mechanical complications.
-
•
Identifying difficult vascular access is essential to prepare resources adequately.
-
•
Confirmatory modalities may be used before, during and after CVC placement to reduce the risk of mechanical complications.
-
•
No single technique can confirm or prevent all mechanical complications of CVC placement.
Central venous catheter (CVC) placement is a core skill of clinical anaesthetists in the operating theatre and ICU for short- and longer-term venous access, giving vasoactive medications and fluids, central venous pressure (CVP) monitoring, renal replacement therapy and total parenteral nutrition. It is critical for anaesthetists to understand the complications associated with CVC placement and effective means to reduce the risks. Complications related to access of a central vein may occur at the time of initial placement, after placement, or during maintenance of the catheter. Although catheter-associated infections are a significant source of morbidity and mortality, this article focuses on mechanical complications. Mechanical complications are those primarily associated with initial CVC placement including vascular injury, damage to surrounding structures and catheter malposition. In this article, we review the relevant central venous anatomy and common access sites, describe the characteristics of specific catheters and discuss the presentation and management of specific mechanical complications in adults. We also appraise the data on appropriate training to anticipate difficult catheter placements and prevent mechanical complications. Finally, we explore the use of confirmatory modalities to reduce complications including ultrasound, manometry, fluoroscopy, transoesophageal echocardiography (TOE) and radiography.
Central venous anatomy and access
Central veins
Operators must have comprehensive knowledge of central venous anatomy to successfully select a cannulation site, gain appropriate access and prevent immediate and delayed complications. A complete review of normal and abnormal central venous anatomy is beyond the scope of this article; this has been well reviewed elsewhere.1,2 Briefly, the central veins are classically defined as the superior and inferior venae cavae (SVC and IVC, respectively). The SVC is formed from the junction of the brachiocephalic veins and ends in the right atrium (RA), with the lower half of the vessel lying within the pericardial sac. The IVC is formed by the junction of the common iliac veins and ascends through the abdomen and thoracic cavity to the RA. The goal of central venous cannulation is to place a catheter with the tip positioned within the central lumen of either of these central veins.
Access sites
Although central veins can be accessed surgically via open cutdown procedures, most operators acquire central access percutaneously via the internal jugular vein (IJV), subclavian vein (SCV) and femoral veins (Table 1). Surface anatomical landmarks and knowledge of underlying deep structures help approximate the appropriate site before a cannulation attempt. Peripherally inserted central catheters (PICCs) are alternatives that are commonly inserted with ultrasound guidance via the basilic or cephalic vein in the upper extremity with the catheter tip ending in the SVC.
Table 1.
Comparison of percutaneous access sites for central venous cannulation. SVC, superior vena cava; IVC, inferior vena cava.
| Percutaneous site | Central vein | Advantages | Disadvantages |
|---|---|---|---|
| Internal jugular vein | SVC |
|
|
| Subclavian vein | SVC |
|
|
| Femoral vein | IVC |
|
|
| Peripherally inserted central catheters (PICCs) | SVC |
|
|
Acquired and congenital impediments
Several anatomical and device-associated conditions may impede or increase the difficulty of CVC placement. Mechanically-acquired defects include the presence of pacemaker wires or indwelling catheters such as haemodialysis catheters and chemotherapy ports. When present, these devices narrow the lumen available for catheter placement and potentially distort the normal anatomy. Organically-acquired defects include partial or complete stenosis, mass compression, and thrombosis associated with prior catheter placement. Surface anatomy and venous course may also be compromised by previous cardiac surgery (e.g. a patient with a Glenn shunt), musculocutaneous changes after radiotherapy, neck flaps and burns. Congenital anomalies include the presence of a left-sided SVC that terminates in the coronary sinus; dextrocardia with or without situs inversus; variations in IVC and azygos drainage; and partial anomalous pulmonary venous drainage.
Preoperative evaluation before CVC placement in patients with both acquired and congenital anomalies may entail thorough imaging to evaluate the patency and course of the vessels, including duplex ultrasound, dynamic ultrasound, CT scanning or cardiac magnetic resonance imaging (cMRI). Imaging may reveal anatomical anomalies such as a left-sided SVC or structural anomalies such as narrowed or ligated vessels, vascular repair, grafts or alterations of the normal course of a vessel. Review of surgical records may also reveal interventions that will complicate catheter placement.
Finally, although not a physical impediment per se, a patient with coagulopathy should be managed by the most experienced operator with selection of an easily compressible venous access site such as the femoral or IJV.
Catheter types
In this article we focus on non-tunnelled catheters rather than tunnelled catheters or implanted ports. Factors to consider in choosing a catheter include planned access site, size, planned use(s), duration of planned use(s) and the patient's anatomy and comorbidities.
Multilumen catheters are small-calibre catheters with multiple internal lumens for simultaneous infusion of fluids, nutrition or medications. Multilumen catheters may also be used for CVP monitoring or periodic blood sampling. These catheters should not be used if anticipating rapid transfusion or infusion of large volumes of fluids. The outer diameter of the catheter ranges from 7 to 8.5 French (Fr) calibre and 16–20 cm length with individual lumen sizes of 20–16 G for catheters intended for use in adult patients.
Percutaneous introducers are larger-calibre (9–14 Fr), short (7.5–11 cm), rigid catheters that allow introduction of an additional device for diagnostic or therapeutic purposes such as a pulmonary arterial catheter, temporary pacing wire or multilumen catheters specially designed for use with an introducer. The larger size may also allow rapid administration of fluid if the main lumen is not occupied with another device.
Haemodialysis catheters are larger-calibre (12–15.5 Fr), dual-lumen catheters ranging in length from 16 to 24 cm used for renal replacement therapy. Some of these devices are designed with an additional infusion port.
Peripherally inserted central catheters are small-calibre, long (50–70 cm) catheters intended for long-duration use (1–6 months compared with 7–10 days for non-tunnelled catheters).
Finally, anaesthetists and intensivists are increasingly called upon to assist in establishing access for advanced mechanical circulatory support including extracorporeal membrane oxygenation (ECMO) and ventricular assist devices (VADs). The cannulae used in these procedures are large calibre but range considerably in size and structure depending on the intended use. Dual-lumen venous cannulae include cavoatrial and atriopulmonary cannulae. Cavoatrial cannulae are placed into the IJV and positioned with the inflow from the SVC and IVC and outflow into the RA to provide continuous venovenous (VV) ECMO. Current models include the Medtronic Crescent (24–32 Fr) and the Getinge Avalon (20–31 Fr) catheters. Atriopulmonary cannulae are placed into the IJ vein with inflow from the RA and outflow into the pulmonary artery. They can function to offload the right ventricle and provide VV-ECMO with the addition of an oxygenator. Current models include the LivaTech ProtekDuo (29–31Fr) and the Spectrum Dual-Lumen RA-PA (24–31 Fr) cannulae. Single-lumen venous cannulae (16–27 Fr) are combined with arterial cannulae to accomplish peripheral or central venoarterial (VA) ECMO in patients requiring full haemodynamic support. The choice of cannula depends on the configuration and the ultimate indication for the device. A detailed discussion of the management of mechanical circulatory support is outside the scope of this article.
Anaesthetists may also be called upon to place large calibre (17–19 Fr) percutaneous SVC cannulae to facilitate venous drainage in robotic or minimally invasive cardiac surgeries. Cannulation may pose significant risk to patients given the rigidity and length of the catheters.
Mechanical complications of CVC placement
Central venous catheters are typically placed with a Seldinger or modified Seldinger technique with access of the central vein via needle cannulation, guidewire placement through the needle, skin and blood vessel entry site dilation, insertion, and advancement of the catheter or introducer over the guidewire. Most mechanical complications that would significantly affect the anaesthetist are detected immediately or shortly after the time of catheter insertion. In general, the types of access-related complications for larger CVCs are similar, but complications may be more severe because of the calibre of the catheters.
Table 2 contains a comprehensive summary of mechanical complications including vascular and extravascular complications, and suggested management strategies.3, 4, 5, 6, 7 We excluded estimates of the incidence of specific complications given that most studies exploring this topic pre-date the consistent use of ultrasound. However, a recent multicentre retrospective study in hospitals where real-time ultrasound was standard of practice estimated a 7.7% rate of mechanical complications associated with 12 667 catheter insertions, of which 0.4% were major complications.8 The ASA Closed Claims Project reported that the most common mechanical complications between 1970 and 2000 were guidewire or catheter embolus, cardiac tamponade, carotid artery puncture or cannulation, haemothorax and pneumothorax.9
Table 2.
Common mechanical complications associated with central venous cannulation. IJV internal jugular vein; SCV, subclavian vein; SVC, superior vena cava.
| Complication | Risk factors | Presentation | Management | Prevention |
|---|---|---|---|---|
| Accidental arterial puncture | IJV or femoral placement, close proximity of artery to vein | Bright, pulsatile arterial blood from catheter or manometry, arterial waveform on pressure monitoring, possible symptoms of end-organ ischaemia including cerebrovascular injury | Leave in situ and consult vascular surgery; consider vascular closure device3,4 | Ultrasound and manometry |
| Azygos cannulation | Possibly left-sided placement | Smooth insertion with failure to aspirate blood from port(s), possible pleural effusion or cardiac tamponade | Immediate removal to prevent vascular perforation | Difficult to identify during placement; transoesophageal echocardiography and fluoroscopy could be used |
| Direct vascular injury (e.g. laceration, through-and-through injury, dissection) | Needle or guidewire not positioned within central lumen of vessel, difficult catheter or dilator advancement over guidewire, larger-calibre catheters | Variable presentation depending on degree of vascular injury from asymptomatic to immediate or delayed haematoma, pleural or pericardial effusion, retroperitoneal bleed, and cardiovascular collapse. Must have high index of suspicion with unanticipated bloody pleural or pericardial effusion, unanticipated decline in haematocrit, or unanticipated hypotension | If severe, consult vascular surgeon | Ultrasound |
| Venous air embolism | Spontaneously breathing patient, positioning with access site above right atrium (sitting or reverse Trendelenburg position) | Dyspnoea, chest pain, cardiovascular collapse | Position patient in left lateral decubitus, 100% Fio2, attempt to aspirate air from catheter | Occlude the catheter hub during placement, position with access site inferior to right atrium (e.g. Trendelenburg) |
| Extravascular placement | Lack of confirmatory techniques | Variable depending on location but inability to aspirate blood | Leave in situ and acquire computed tomography scan; consider vascular surgery consult | Ultrasound |
| Pneumothorax | SCV placement, emergent placement, large-calibre catheters, increased number of attempts, left-sided placement | Simple pneumothorax may be asymptomatic, whereas tension pneumothorax manifests with impaired venous filling and cardiovascular collapse | Supportive care, needle decompression and chest tube placement | Ultrasound; seeking non-SCV access site |
| Pericardial effusion | Puncture of carotid, lower SVC, right atrium or right ventricle | Variable from asymptomatic to cardiac tamponade leading to impaired venous filling and haemodynamic collapse | Supportive care, cardiothoracic surgery or interventional cardiology consult to drain pericardial effusion | Ultrasound |
| Chylothorax | Left-sided catheters, SCV placement | Dyspnoea, pleural effusion, milky appearance of pleural fluid | Dietary modification (if small), otherwise thoracic duct embolisation or ligation | Choose right-sided access when able |
| Guidewire embolism or retention | Loss of guidewire control, lack of checklist use5 | Variable from asymptomatic to end-organ ischaemia or vascular damage | Prompt endovascular removal, supportive care; cardiothoracic or vascular surgery may be required to retrieve guidewire | Operator or assistant holds guidewire at all points during procedure; use of systematic counting procedures6; use of locked procedure pack7 |
Prevention of mechanical complications of CVC placement
Central venous catheter placement may be complicated by patient-related, catheter-related, operator-related or environmental factors (Table 3). Patient-related factors can be mitigated by recognition of risk factors for difficult CVC placement with proper escalation to experienced operators, provision of judicious sedative medications and suitable positioning. Catheter-related factors may be unavoidable owing to limited access sites or a specific clinical need (e.g. haemodialysis catheter). Environmental factors are generally fixed, although equipment availability may be addressed as a longer-term quality initiative within an institution. Thus, the best actionable interventions to prevent mechanical complications of CVC placement are operator training and use of procedural and post-placement confirmatory modalities.
Table 3.
Factors associated with increased risk of mechanical complications from central venous cannulation. IJV, internal jugular vein; SCV, subclavian vein.
| Category | Factors |
|---|---|
| Patient-related | Comorbidities including coagulopathies |
| Anatomy (BMI, acquired or congenital anomalies, previous cardiac surgery, pre-existing central venous catheters) | |
| Restlessness, anxiety or lack of cooperation | |
| Positioning | |
| Catheter-related | Site of cannulation (left IJV has greater risk of vascular or chylous injury owing to a sharp turn at brachiocephalic vein; SCV has greater risk of pneumothorax because of proximity to the lung apex; femoral vein has greater risk of haematoma with proximal cannulation or injury) |
| Catheter type, length, and size | |
| Operator-related | Operator experience |
| Lack of operator training | |
| Number of attempts | |
| Lack of use of confirmatory modalities | |
| Environmental factors | Availability of appropriate equipment including confirmatory modalities |
| Emergency placement |
Operator training
Inexperienced operators have a higher rate of complications compared with experienced providers. The definition of ‘experience’ is not consistent in the literature, although one prospective cohort study in the era before ultrasound distinguished experienced providers as those who had performed >50 CVC placements.10 A more recent study, which defined limited operator experience as fewer than 100 CVC placements in a chosen vein, demonstrated that inexperienced operators had a higher risk of both minor and major mechanical complications.8 However, the number of procedures performed during clinical training has also been found not to correlate with competence.11 Furthermore, a 2016 study of CVC placement by experienced providers demonstrated poor adherence to appropriate protocol despite successful cannulations.12 This is likely to be because the traditional method of training through supervised practice does not reliably incorporate training in basic principles including relevant anatomy, identification and deliberate practice of deficient procedural skills, and competency-based evaluations. The number of opportunities for development of skills may also be declining. In the USA, there has been a 38.3% decrease in billing claims for non-tunnelled CVCs placed by anaesthetists in the past decade, with a redistribution to physician assistants or advanced practice nurses.13 Thus, it is even more essential that training and maintenance of competence in CVC placement for anaesthetists should be well designed and comprehensive.
A 2013 evidence-based consensus statement from the World Congress for Vascular Access found 16 recommendations for minimal requirements for CVC training. The authors propose that training should combine didactic or web-based teaching on central venous anatomy, catheter types, ultrasound-guided technique, informed consent, insertion procedure, sterile technique, complications and daily device maintenance with anatomical models or simulation sessions incorporating ultrasound. Simulators may include mannequins, task-trainers or non-human tissue. The authors further recommend objective competency-based assessments with direct supervision by experienced practitioners.14 Objective evaluations include use of checklists, global rating scales, knowledge assessments and first needle-pass success rates.
Multiple studies have confirmed that simulation-based education improves learner performance and comfort in addition to reducing complications.15, 16, 17 A 2015 RCT of interns demonstrated improved protocol adherence with simulation-based training vs traditional training for CVC placement.18 A 2011 meta-analysis suggested that simulation-based education improved learners' performance and attitudes, and reduced the incidence of pneumothorax.19
Failure mode and effects analysis (FMEA) is a method that has been used for cardiopulmonary resuscitation (CPR) education and has been used to develop a formal standardised curriculum for placing CVCs.20 The curriculum focused on decreasing the frequency, diminishing the severity, and improving the early detection of three priority objectives: (1) retained guidewires; (2) improved needle access; and (3) catheter-associated bloodstream infections. The authors found that the number of retained guidewires decreased by more than four-fold after initiation of their curriculum, demonstrating that this method is promising both for curriculum development and long-term assessment of CVC placement proficiency.
In emergency situations, the use of confirmatory modalities including ultrasound may be unavailable and thus multiple international societies continue to recommend separate training in landmark technique in addition to ultrasound technique.21
Operator training should also emphasise the importance of limiting attempts and considering alternative approaches. The incidence of mechanical complications increases six-fold when more than three attempts are made by the same operator.22 In one prospective study of experienced operators, the risk of mechanical complications including failure was 10 times more likely with a second attempt and 40 times more likely with greater than two attempts.23 Thus, we suggest that after one failed attempt, the operator should pursue one of several actions: (1) escalate to a more experienced provider; (2) involve an additional provider for a two-operator approach; (3) optimise the mechanics of the procedure; (4) consider use of an alternate site; (5) consider use of additional confirmatory modalities; or (6) consider collaboration with a procedural team such as interventional radiology or surgery.
Confirmatory modalities
Confirmatory modalities are supplemental steps used to increase the accuracy of venous access, reduce the incidence of catheter malposition and increase safety. Confirmatory modalities can be used before, during and after CVC placement.
The exact position of the catheter tip has been a topic of considerable debate, but it is generally agreed that the tip should sit parallel to the long axis of the central vein to optimise flow and avoid potential vascular wall damage. The optimal final catheter tip position for upper body CVCs is thought to be in the lower one-third of the SVC adjacent to the RA, parallel to the long axis of the vein, outside of the pericardial reflection. For IVC access, the tip of should be past the confluence of the iliac veins. Catheters that are too distal risk perforation of the vessel or RA leading to tamponade, whereas catheters positioned too proximal may increase risk of thrombosis and vascular injury with infusion of irritant medications. Misplaced CVCs may also lead to unreliable medication administration and errors in estimating CVP.
Ultrasound
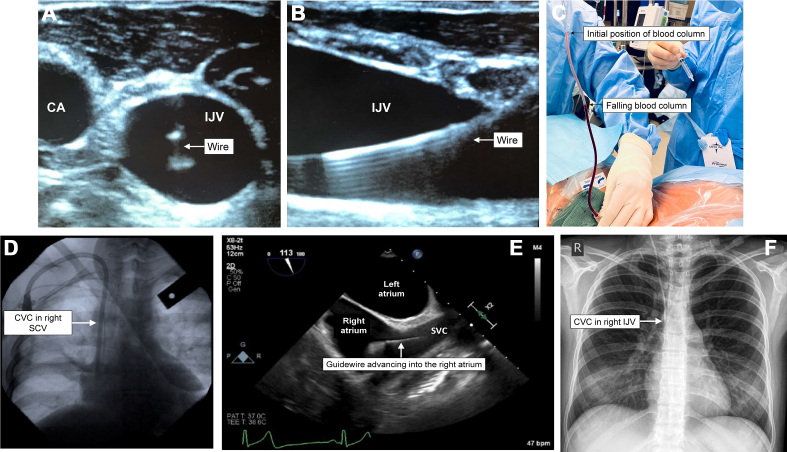
Using ultrasound facilitates visual assessment of the relationships between target veins and neighbouring structures and placement of the wire in the vessel of interest before dilation (Fig 1A and B). Static ultrasound enables operators to identify an appropriate venous vessel with sufficient size and lack of anatomical barriers including neighbouring arteries before preparation and draping for the procedure. It can also facilitate optimal positioning of the patient, such as the degree of Trendelenburg tilt to increase the cross-sectional area of a jugular or subclavian vein or to decrease the overlapping of a jugular vein with the carotid artery.24, 25, 26 However, static ultrasound alone is likely insufficient; meta-analyses of RCTs suggest that static ultrasound improves the success rate of first-insertion attempts but is equivocal to a landmark technique for overall success and arterial puncture rates.27 For IJV access, meta-analyses of RCTs demonstrate that the use of dynamic ultrasound during the procedure increases first-attempt and overall cannulation success rates and dramatically decreases mechanical complications, number of attempts, time per cannulation and inadvertent arterial puncture compared with a landmark approach.28, 29, 30, 31, 32, 33 The evidence for dynamic ultrasound in femoral and SC cannulation is weaker, but overall RCTs suggest benefits in reducing complications and increasing first-attempt success.34, 35, 36, 37, 38
Fig 1.
Confirmatory modalities for successful central venous cannulation including dynamic ultrasound in short-axis (A) and longitudinal (B) views; manometry (C); fluoroscopy (D); transoesophageal echocardiography (E); and chest radiography (F). CA, carotid artery; IJV, internal jugular vein; SVC, superior vena cava.
Transthoracic ultrasound may also be used to rapidly identify mechanical complications of CVC placement. The linear array high-frequency probe may be applied longitudinally on the anterior chest at the midclavicular line between the third and fourth intercostal space to interrogate the presence of ‘lung sliding’, or movement of the parietal and visceral pleura against one another during normal respiration. Lack of lung sliding is highly predictive of a pneumothorax. Transthoracic ultrasound may also be used to localise the guidewire within the SVC via a right supraclavicular fossa window or within the RA via apical or subcostal four-chamber views. A 2017 systematic review and meta-analysis found that bedside ultrasound had a nearly 100% sensitivity and specificity for identifying pneumothorax and could also identify 80% of catheter malpositions, saving almost an hour compared with traditional radiographic identification.39
Manometry
Manual manometry to gravity is a technique where long extension tubing is connected to a catheter or needle within the vessel and blood is aspirated into the tubing with a syringe. The tubing is then held well above the level of the RA If the catheter is appropriately venous, the blood column should decrease as blood drains back into the central vein (Fig 1C). A lack of blood flow may indicate catheter malposition, catheter kinking or high CVP, whereas a significant increase in the column of blood may indicate catheter placement in an artery. The same tubing may also be directly connected to a pressure transducer to confirm a venous waveform. A 2009 retrospective study found that manometry reliably identified arterial puncture and prevented further dilation or catheter placement.40
Fluoroscopy
Fluoroscopy allows for live visualisation of a guidewire before dilation and introduction of CVCs. When combined with contrast, it can be used to delineate venous anatomy, identify the presence of venous stenosis or thrombosis, and diagnose extravenous wire placement (Fig 1D). However, complications may still occur particularly with complex anatomy, operator inexperience or image misinterpretation. Limited evidence is available for use of fluoroscopy during CVC placement, but it is recommended as an alternative technique by international society guidelines and is considered imperative for the placement of the ProtekDuo.27,41
Transoesophageal echocardiography
Transoesophageal echocardiography (TOE) enables reliable confirmation of guidewire placement in the SVC and RA from the site of venous access (Fig 1E). Deep sedation or general anaesthesia is required for TOE. However, the risk–benefit balance is favourable for the placement of certain catheters such as cavoatrial and atriopulmonary catheters, both to avoid mechanical injury and enable precise positioning within the heart and so allow appropriate inflow and outflow. Wire or catheter confirmation with TOE may be difficult in patients with pacemaker wires, pre-existing catheters or conditions that distort the normal cardiac or oesophageal anatomy. As with fluoroscopy, there is limited evidence available to support the routine use of TOE during CVC placement but it is recommended as an alternative technique by international society guidelines.27,41
Radiography
Serial chest radiography may be used in lieu of fluoroscopy to confirm guidewire location before catheter placement. Plain radiographs are also used routinely to check IJV and SCV CVC positioning after placement and to rule out pneumothorax or pleural effusions, although this practice has been questioned in the era of ultrasound-guided placement.42 Catheter position within a vein may be difficult to ascertain given the proximity of several adjacent structures and the limit of a two-dimensional anterior–posterior projection. Typically, the catheter tip should appear adjacent to the upper border of the RA around the level of the carina on the radiograph, approximating the beginning of the pericardial reflection (Fig 1F).
Conclusions and future directions
Using multiple studies cited within this review and society guidelines from the ASA and the Association of Anaesthetists on safe vascular access, we present a suggested algorithm for the prevention and identification of mechanical complications of CVC placement organised by stage within the procedure (Fig 2).27,41 Importantly, no one technique can prevent complications, and thus a multipronged approach combining anticipation of potential problems, appropriate operator training and use of complementary confirmatory modalities is necessary for success.
Fig 2.
Suggested guidelines to prevent or mitigate mechanical central venous catheter complications at each stage of the procedure. CT, computed tomography; CVC, central venous catheter; CXR, chest radiograph; IJV, internal jugular vein; SCV, subclavian vein; TOE, transoesophageal echocardiography.
Future innovations for increasing the safety of CVC placement include ongoing investigations on how to best promote skill retention and appropriate ongoing evaluation for operators. Further work is also needed to provide evidence for the optimal use of checklists to prevent common mechanical complications of CVC placement. Although the majority of evidence for checklists in CVC placement exists for prevention of catheter-associated bloodstream infections, the ASA practice guidelines recommend a checklist for all aspects of CVC placement and maintenance and include an example checklist in their guidelines with specific steps for confirming optimal site selection and venous placement, wire removal, flushing, appropriate suturing and venous confirmation after the procedure.16 Checklists were also included in the final recommendations by multiple teams investigating methods to prevent retained guidewires.5, 6, 7, 8,43 Most recently, Kassis and colleagues5 found that the majority of retained guidewire events occurred in ‘optimal’ patients, under the supervision or directly by an experienced anaesthetist, and during regular working hours under non-emergency circumstances.5 This further highlights the need for universal interventions such as standardised checklists, automated reminders and material counts at the procedure conclusion. We anticipate that these systematic interventions in addition to dedicated operator training and confirmatory modalities will be more necessary than ever to ensure patients' safety as the number of CVCs placed by individual anaesthetists decreases in a multidisciplinary hospital environment.
Ultimately, CVC placement is an essential skill for anaesthetists and intensivists to care for our most vulnerable patients. Operator training and use of confirmatory modalities helps to mitigate the inherent risk of these procedures and identify and manage complications more expediently.
Declaration of Interest
The authors declare that they have no conflicts of interest.
Biographies
Michael G. Fitzsimons MD is Director of the Division of Cardiac Anaesthesia at the Massachusetts General Hospital, Associate Director for Clinical Base Year education and Associate Professor in anaesthesia at Harvard Medical School. Dr Fitzsimons is a nationally known figure in the area of substance use disorders and has published extensively on educational topics including substance use disorders, clinical competency, disabilities, extracorporeal membrane oxygenation (ECMO), mechanical support, and ventricular assist devices.
Elisa C. Walsh MD is a staff anaesthetist and intensivist at the Massachusetts General Hospital and an Instructor in Anaesthesia at Harvard Medical School. Her major interests are graduate medical education, prevention of access-related complications, and thoracic anaesthesia.
Matrix codes: 1H02; 2C04; 3C00
Level 2: 2C04: Support of threatened and failing organ systems. 2D03: Vascular access techniques;
Level 3: 3C00: Adult ICM. 3G00: Cardiothoracic. 3J02: Education and training
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Gibson F., Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 2.Bannon M., Heller Rivera. Anatomic considerations for central venous cannulation. Risk Manag Healthc Policy. 2011;4:27–39. doi: 10.2147/RMHP.S10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilbert M.C., Elkouri S., Bracco D., et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48:918–925. doi: 10.1016/j.jvs.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Makris G.C., Patel R., Little M., et al. Closure devices for iatrogenic thoraco-cervical vascular injuries. Cardiovasc Intervent Radiol. 2017;40:381–387. doi: 10.1007/s00270-016-1506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassis N., Alkukhun L., Kravitz K., et al. Patient, operator, and procedural characteristics of guidewire retention as a complication of vascular catheter insertion. Crit Care Explor. 2023;5 doi: 10.1097/CCE.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vannucci A., Jeffcoat A., Ifune C., Salinas C., Duncan J.R., Wall M. Retained guidewires after intraoperative placement of central venous catheters. Anesth Analg. 2013;117:102–108. doi: 10.1213/ANE.0b013e3182599179. [DOI] [PubMed] [Google Scholar]
- 7.Mariyaselvam M.Z.A., Patel V., Young H.E., Blunt M.C., Young P.J. Central venous catheter guidewire retention: lessons from England’s Never Event Database. J Patient Saf. 2022;18:e387–e392. doi: 10.1097/PTS.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 8.Adrian M., Borgquist O., Kröger T., et al. Mechanical complications after central venous catheterisation in the ultrasound-guided era: a prospective multicentre cohort study. Br J Anaesth. 2022;129:843–850. doi: 10.1016/j.bja.2022.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Domino K.B., Bowdle T.A., Posner K.L., Spitellie P.H., Lee L.A., Cherry F.W. Injuries and liability related to central vascular catheters. Anesthesiology. 2004;100:1411–1418. doi: 10.1097/00000542-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Sznajder J.I., Zveibil F.R., Bitterman H., Weiner P., Bursztein S. Central vein catheterisation: failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146:259–261. doi: 10.1001/archinte.146.2.259. [DOI] [PubMed] [Google Scholar]
- 11.Barsuk J.H., Cohen E.R., Feinglass J., McGaghie W.C., Wayne D.B. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9:201–208. doi: 10.4300/JGME-D-16-00426.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barsuk J.H., Cohen E.R., Nguyen D., et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44:1871–1881. doi: 10.1097/CCM.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 13.Rubin D.S., Apfelbaum J.L., Tung A. Trends in central venous catheter insertions by anesthesia providers: an analysis of the Medicare physician supplier procedure summary from 2007 to 2016. Anesth Analg. 2020;130:1026–1034. doi: 10.1213/ANE.0000000000004530. [DOI] [PubMed] [Google Scholar]
- 14.Moureau N., Lamperti M., Kelly L.J., et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110:347–356. doi: 10.1093/bja/aes499. [DOI] [PubMed] [Google Scholar]
- 15.Barsuk J.H., McGaghie W.C., Cohen E.R., O’Leary K.J., Wayne D.B. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37:2697–2701. [PubMed] [Google Scholar]
- 16.Koh J., Xu Y., Yeo L., et al. Achieving optimal clinical outcomes in ultrasound-guided central venous catheterisations of the internal jugular vein after a simulation-based training program for novice learners. Simul Healthc J Soc Simul Healthc. 2014;9:161–166. doi: 10.1097/SIH.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 17.Soffler M.I., Hayes M.M., Smith C.C. Central venous catheterisation training: current perspectives on the role of simulation. Adv Med Educ Pract. 2018;9:395–403. doi: 10.2147/AMEP.S142605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltan I.D., Shiga T., Gordon J.A., Currier P.F. Simulation improves procedural protocol adherence during central venous catheter placement: a randomised controlled trial. Simul Healthc J Soc Simul Healthc. 2015;10:270–276. doi: 10.1097/SIH.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma I.W.Y., Brindle M.E., Ronksley P.E., Lorenzetti D.L., Sauve R.S., Ghali W.A. Use of simulation-based education to improve outcomes of central venous catheterisation: a systematic review and meta-analysis. Acad Med. 2011;86:1137–1147. doi: 10.1097/ACM.0b013e318226a204. [DOI] [PubMed] [Google Scholar]
- 20.Duncan J.R., Henderson K., Street M., et al. Creating and evaluating a data-driven curriculum for central venous catheter placement. J Grad Med Educ. 2010;2:389–397. doi: 10.4300/JGME-D-10-00007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maizel J., Guyomarc L., Henon P., et al. Residents learning ultrasound-guided catheterisation are not sufficiently skilled to use landmarks. Crit Care. 2014;18:R36. doi: 10.1186/cc13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGee D.C., Gould M.K. Preventing complications of central venous catheterisation. N Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 23.Schummer W., Schummer C., Rose N., Niesen W.D., Sakka S.G. Mechanical complications and malpositions of central venous cannulations by experienced operators: a prospective study of 1794 catheterisations in critically ill patients. Intensive Care Med. 2007;33:1055–1059. doi: 10.1007/s00134-007-0560-z. [DOI] [PubMed] [Google Scholar]
- 24.Sulek C.A., Gravenstein N., Blackshear R.H., Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82:125–128. doi: 10.1097/00000539-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Riopelle J.M., Ruiz D.P., Hunt J.P., et al. Circumferential adjustment of ultrasound probe position to determine the optimal approach to the internal jugular vein: a noninvasive geometric study in adults. Anesth Analg. 2005;100:512–519. doi: 10.1213/01.ANE.0000142115.94440.6C. [DOI] [PubMed] [Google Scholar]
- 26.Kwon M.Y., Lee E.K., Kang H.J., et al. The effects of the Trendelenburg position and intrathoracic pressure on the subclavian cross-sectional area and distance from the subclavian vein to pleura in anesthetized patients. Anesth Analg. 2013;117:114–118. doi: 10.1213/ANE.0b013e3182860e3c. [DOI] [PubMed] [Google Scholar]
- 27.Practice guidelines for central venous access 2020. Anesthesiology. 2020;132:8–43. doi: 10.1097/ALN.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 28.Milling T.J., Rose J., Briggs W.M., et al. Randomised, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33:1764–1769. doi: 10.1097/01.ccm.0000171533.92856.e5. [DOI] [PubMed] [Google Scholar]
- 29.Troianos C.A., Jobes D.R., Ellison N. Ultrasound-guided cannulation of the internal jugular vein: a prospective, randomised study. Anesth Analg. 1991;72:823–826. doi: 10.1213/00000539-199106000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Teichgräber U.K., Benter T., Gebel M., Manns M.P. A sonographically guided technique for central venous access. Am J Roentgenol. 1997;169:731–733. doi: 10.2214/ajr.169.3.9275887. [DOI] [PubMed] [Google Scholar]
- 31.Leung J., Duffy M., Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterisation in the emergency department increases success rates and reduces complications: a randomised, prospective study. Ann Emerg Med. 2006;48:540–547. doi: 10.1016/j.annemergmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Bansal R., Agarwal S.K., Tiwari S.C., Dash S.C. A prospective randomised study to compare ultrasound-guided with nonultrasound-guided double lumen internal jugular catheter insertion as a temporary hemodialysis access. Ren Fail. 2005;27:561–564. doi: 10.1080/08860220500199084. [DOI] [PubMed] [Google Scholar]
- 33.Mallory D.L., McGee W.T., Shawker T.H., et al. Ultrasound guidance improves the success rate of internal jugular vein cannulation. Chest. 1990;98:157–160. doi: 10.1378/chest.98.1.157. [DOI] [PubMed] [Google Scholar]
- 34.Brass P., Hellmich M., Kolodziej L., Schick G., Smith A.F. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterisation. Cochrane Emergency and Critical Care Group. Cochrane Database Syst Rev. 2015;1:CD011447. doi: 10.1002/14651858.CD011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fry W.R., Clagett G.C., O’Rourke P.T. Ultrasound-guided central venous access. Arch Surg. 1999;134:4. doi: 10.1001/archsurg.134.7.738. [DOI] [PubMed] [Google Scholar]
- 36.Gualtieri E., Deppe S.A., Sipperly M.E., Thompson D.R. Subclavian venous catheterisation: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23:692–697. doi: 10.1097/00003246-199504000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Fragou M., Gravvanis A., Dimitriou V., et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomised study. Crit Care Med. 2011;39:1607–1612. doi: 10.1097/CCM.0b013e318218a1ae. [DOI] [PubMed] [Google Scholar]
- 38.Aouad M.T., Kanazi G.E., Abdallah F.W., et al. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg. 2010;111:724–728. doi: 10.1213/ANE.0b013e3181e9c475. [DOI] [PubMed] [Google Scholar]
- 39.Ablordeppey E.A., Drewry A.M., Beyer A.B., et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2017;45:715–724. doi: 10.1097/CCM.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ezaru C.S., Mangione M.P., Oravitz T.M., Ibinson J.W., Bjerke R.J. Eliminating arterial injury during central venous catheterisation using manometry. Anesth Analg. 2009;109:130–134. doi: 10.1213/ane.0b013e31818f87e9. [DOI] [PubMed] [Google Scholar]
- 41.Bodenham Chair A., Babu S., Bennett J., et al. Association of anaesthetists of Great Britain and Ireland: safe vascular access 2016. Anaesthesia. 2016;71:573–585. doi: 10.1111/anae.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chui J., Saeed R., Jakobowski L., et al. Is routine chest X-ray after ultrasound-guided central venous catheter insertion choosing wisely?: a population-based retrospective study of 6,875 patients. Chest. 2018;154:148–156. doi: 10.1016/j.chest.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Mariyaselvam M.Z.A., Catchpole K.R., Menon D.K., Gupta A.K., Young P.J. Preventing retained central venous catheter guidewires. Anesthesiology. 2017;127:658–665. doi: 10.1097/ALN.0000000000001797. [DOI] [PubMed] [Google Scholar]