Key Points
Question
Are mucus plugs that occlude airways identified on computed tomography scans in patients with chronic obstructive pulmonary disease (COPD) associated with increased all-cause mortality?
Findings
In this observational study that included 4363 patients with COPD, the presence of mucus plugs occluding medium- to large-sized airways (ie, approximately 2- to 10-mm lumen diameter) was significantly associated with higher risk of all-cause mortality (adjusted hazard ratio for mucus plugs affecting 1 to 2 vs 0 lung segments, 1.15; adjusted HR for mucus plugs affecting ≥3 vs 0 lung segments, 1.24).
Meaning
Mucus plugs that occluded medium- to large-sized airways in patients with COPD were associated with increased all-cause mortality.
Abstract
Importance
Airway mucus plugs are common in patients with chronic obstructive pulmonary disease (COPD); however, the association of airway mucus plugging and mortality in patients with COPD is unknown.
Objective
To determine whether airway mucus plugs identified on chest computed tomography (CT) were associated with increased all-cause mortality.
Design, Setting, and Participants
Observational retrospective analysis of prospectively collected data of patients with a diagnosis of COPD in the Genetic Epidemiology of COPD cohort. Participants were non-Hispanic Black or White individuals, aged 45 to 80 years, who smoked at least 10 pack-years. Participants were enrolled at 21 centers across the US between November 2007 and April 2011 and were followed up through August 31, 2022.
Exposures
Mucus plugs that completely occluded airways on chest CT scans, identified in medium- to large-sized airways (ie, approximately 2- to 10-mm lumen diameter) and categorized as affecting 0, 1 to 2, or 3 or more lung segments.
Main Outcomes and Measures
The primary outcome was all-cause mortality, assessed with proportional hazard regression analysis. Models were adjusted for age, sex, race and ethnicity, body mass index, pack-years smoked, current smoking status, forced expiratory volume in the first second of expiration, and CT measures of emphysema and airway disease.
Results
Among the 4483 participants with COPD, 4363 were included in the primary analysis (median age, 63 years [IQR, 57-70 years]; 44% were women). A total of 2585 (59.3%), 953 (21.8%), and 825 (18.9%) participants had mucus plugs in 0, 1 to 2, and 3 or more lung segments, respectively. During a median 9.5-year follow-up, 1769 participants (40.6%) died. The mortality rates were 34.0% (95% CI, 32.2%-35.8%), 46.7% (95% CI, 43.5%-49.9%), and 54.1% (95% CI, 50.7%-57.4%) in participants who had mucus plugs in 0, 1 to 2, and 3 or more lung segments, respectively. The presence of mucus plugs in 1 to 2 vs 0 and 3 or more vs 0 lung segments was associated with an adjusted hazard ratio of death of 1.15 (95% CI, 1.02-1.29) and 1.24 (95% CI, 1.10-1.41), respectively.
Conclusions and Relevance
In participants with COPD, the presence of mucus plugs that obstructed medium- to large-sized airways was associated with higher all-cause mortality compared with patients without mucus plugging on chest CT scans.
This observational retrospective analysis of prospectively collected data examines whether airway mucus plugs identified on chest computed tomography were associated with increased all-cause mortality in patients with chronic obstructive pulmonary disease.
Introduction
Chronic obstructive pulmonary disease (COPD) affects 15.9 million people in the United States and is the fourth leading cause of death.1 Mucus dysfunction, a central pathology in patients with COPD, is characterized by excess mucus production, hypersecretion, and reduced clearance, leading to accumulation in the airways as plugs.2,3 Mucus plugs completely occluding the airways are observed in 25% to 67% of computed tomography (CT) scans of individuals with COPD and are associated with airflow obstruction, lower oxygen saturation, and reduced exercise capacity.4,5 In individuals with COPD, 67% have mucus plugs that persist at 1 year and 73% at 5 years.4,5 Up to 30% of patients with COPD who have mucus plugs on CT report no cough or sputum.5
A prior study that examined lung tissue from patients with advanced-stage COPD who underwent lung volume reduction surgery found that occlusion of small conducting airways (ie, <2-mm lumen diameter) with mucus plugs was associated with early death.6 However, the association between all-cause mortality and mucus plugs in medium- to large-sized airways (ie, approximately 2- to 10-mm lumen diameter) seen on CT scans, and in different stages of COPD, is unknown. Therefore, this study analyzed Genetic Epidemiology of COPD (COPDGene) participants with COPD defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages of mild, moderate, severe, and very severe, and included participants who currently and formerly smoked cigarettes.7,8 It was hypothesized that the presence of mucus plugs occluding medium- to large-sized airways was associated with increased all-cause mortality in individuals with COPD.
Methods
COPDGene Study
COPDGene7 was a multicenter, observational, prospective study designed to identify COPD’s genetic and epidemiologic determinants. COPDGene enrolled non-Hispanic Black and non-Hispanic White participants who were aged 45 to 80 years and smoked 10 pack-years or more of cigarettes (Table 1).7 Participants were excluded if they met any of the following criteria: history of pulmonary disease except for asthma, previous surgical excision of at least 1 lung lobe (or lung volume reduction procedure), active cancer under treatment, suspected lung cancer (large or highly suspicious lung mass), metal in the chest, exacerbation of COPD treated with antibiotics or steroids in the last month, recent eye surgery, myocardial infarction, other cardiac hospitalization, recent chest or abdominal surgery, inability to use albuterol, history of chest radiation therapy, and first- or second-degree relative already enrolled in the study. The participants were enrolled between November 2007 and April 2011, and additional examinations were conducted at year 5 (phase 2) and year 10 (phase 3). Assessments included questionnaires, spirometry, and chest CT imaging. The institutional review board at each participating clinical center approved the COPDGene study, and all participants gave written informed consent.
Table 1. Characteristics of Participants With COPD by Mucus Plug Score Category.
| Characteristic | Mucus plug score category (No. of lung segments with mucus plugs), No. (%) | ||
|---|---|---|---|
| 0 (n = 2585) | 1-2 (n = 953) | ≥3 (n = 825) | |
| Age, median (IQR), y | 62.4 (55.9-68.7) | 63.9 (57.6-70.6) | 65.3 (57.9-71.1) |
| Sex | |||
| Female | 1072 (41.5) | 448 (47.0) | 400 (48.5) |
| Male | 1513 (58.5) | 505 (53.0) | 425 (51.5) |
| Race and ethnicitya | |||
| Non-Hispanic Black | 636 (24.6) | 199 (20.9) | 142 (17.2) |
| Non-Hispanic White | 1949 (75.4) | 754 (79.1) | 683 (82.8) |
| BMI, median (IQR) | 27.6 (24.2-31.9) | 26.8 (23.4-31.0) | 25.5 (22.1-30.1) |
| Current smoker, No. (%) [No.] | 1155 (44.7) [2584] | 383 (40.2) | 347 (42.1) [824] |
| Pack-years of smoking, median (IQR) | 44.3 (33.2-64) | 46.1 (35.0-65.7) | 47.6 (35.0-67.6) |
| Medical history | |||
| Chronic bronchitis | 584 (22.6) | 248 (26.0) | 293 (35.5) |
| Coronary artery disease | 417 (16.1) | 154 (16.2) | 113 (13.7) |
| Asthmab | 406 (15.7) | 168 (17.6) | 187 (22.7) |
| COPD GOLD stage of severityc | |||
| 1 (Mild) | 597 (23.1) | 116 (12.2) | 53 (6.4) |
| 2 (Moderate) | 1250 (48.4) | 389 (40.8) | 248 (30.1) |
| 3 (Severe) | 523 (20.2) | 297 (31.2) | 307 (37.2) |
| 4 (Very severe) | 215 (8.3) | 151 (15.8) | 217 (26.3) |
| BODE index, median (IQR) [No.]d | 2 (0-4) [2525] | 3 (1-5) [924] | 4 (2-6) [803] |
| FEV1, L | 1.8 (1.3-2.4) | 1.4 (0.9-1.9) | 1.1 (0.8-1.6) |
| FEV1, % predicted | 65.0 (47.1-78.4) | 51.9 (36.4-69.7) | 42.1 (29.1-59.4) |
| Emphysema on CT, median (IQR) [No.], %e | 5.6 (1.8-14.9) [2466] | 9.2 (2.8-22.1) [916] | 12.4 (3.4-24.2) [787] |
| Airway wall thickness, median (IQR) [No.], mmf | 2.5 (2.1-2.9) [2466] | 2.7 (2.3-3.1) [916] | 2.9 (2.5-3.3) [787] |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BODE, body mass index, obstruction, dyspnea, and exercise mortality risk score; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in the first second of expiration; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Race and ethnic group were reported by the participant. Only non-Hispanic Black and non-Hispanic White participants were included.
Asthma was defined as current asthma.
GOLD stages were defined with postbronchodilator FEV1 percentage of predicted (pp) values as follows: 1 (mild), FEV1 pp ≥80; 2 (moderate), FEV1 pp ≥50 to <80; 3 (severe) FEV1 pp ≥30 to <50; and 4 (very severe), FEV1 pp <30.
The multidimensional mortality BODE index is composed of 4 factors: BMI, FEV1 percentage predicted, distance walked in 6 minutes, and the modified Medical Research Council dyspnea scale. The BODE index ranges from 0 (lowest risk of death) to 10 (highest risk of death).
Emphysema on CT was measured as percentage of voxels less than −950 Hounsfield units.
Airway wall thickness was measured on CT as the square root of the wall area of an ideal 10-mm-inner-perimeter airway.
CT Assessment
Chest CT scans were performed as part of the COPDGene study protocol and were not based on a clinical indication. Participants underwent volumetric CT examinations, which were reconstructed at submillimeter slice thickness with standard enhancing algorithms, allowing assessment of the bronchial tree in fine detail. Additional information about the COPDGene imaging protocols has been previously described.7 Baseline inspiratory chest CT scans were used to score mucus plugs from September 2018 to April 2022. The CT images were examined with a window width of 1400 Hounsfield units (HU) and level of −500 HU.5
Chest CT scan readers were thoracic radiologists, a pulmonologist, and physicians who underwent training in identification and scoring of mucus plugs and had at least 2 years of experience in lung imaging (A.A.D., J.L.O., S.G., H.P.N., W.D.R., A.Y., S.J.K., K.J., P.P.M., M.A., M.U.A., M.Z., A.N.A., N.L.T., S.S.). The CT readers were blind to the current clinical information. A first reader scored all CT scans for mucus plugs in medium- to large-sized airways (ie, approximately 2- to 10-mm lumen diameter) at lung segment level. A second reader independently scored all CTs that were read as positive for mucus plugs and 20% of the CT scans without mucus plugs. CTs with discrepant mucus plug scores were sent to a third reader.5 The readers used a scoring system based on Netter bronchial anatomy nomenclature, using 18 lung segments.9 A mucus plug was defined as an opacity that completely occluded the lumen of an airway (eFigure 1 in Supplement 1). Lung parenchyma within 2 cm of the costal or diaphragmatic pleura was excluded because the airways in those regions were too small to ascertain occlusive luminal plugs accurately. A final mucus plug score for each study participant was assigned based on the number of lung segments with mucus plugs, ranging from 0 (no mucus plugs seen on CT scan) to 18 (all lung segments with mucus plugs) (eFigure 2 in Supplement 1). The number of lung segments with mucus plugs was averaged for CTs that had more than 1 reading. Then, patients were categorized into 3 groups based on the number of lung segments with mucus plugging on CT scan: 0, 1 to 2, or 3 or more. The readers scored a sample of 20 CTs to measure interreader agreement. The concordance correlation coefficient for interreader agreement of the mucus plug scores was 0.77. In 39 participants, airway lumen size was assessed, following a method detailed in the eMethods in Supplement 1.
Quantitative CT measurements of emphysema and airway disease were performed with Thirona software. Emphysema was defined as the percentage of low attenuation areas under −950 HU, and airway wall thickness was defined as the square root of the wall area of an ideal 10-mm-inner-perimeter airway.
Confirming Mortality and Vitality Status
Mortality was verified using searches of the Social Security Death Index (SSDI) and through the Longitudinal Follow-up (LFU) program of COPDGene.10 Mortality and vital status for participants who were searched via SSDI were back-censored 3 months from the search date to account for the expected lag between the event and its appearance in the SSDI record. For participants evaluated through the LFU program, the dates of the most recent contact, including COPDGene phase 2 and phase 3 dates and dates of LFU surveys between phase visits, were used to calculate the follow-up time. The date of death was ascertained from additional sources, including medical records, published obituaries, and next-of-kin reports.11 Vitality in COPDGene was determined as of August 31, 2022.
Spirometry
Spirometric measures of lung function were performed before and after the administration of albuterol.12
Postbronchodilator forced expiratory volume in the first second of expiration (FEV1) and forced vital capacity (FVC) were expressed as percentages of predicted values.13 COPD was defined as a postbronchodilator FEV1:FVC ratio less than 0.7, and COPD disease severity was classified based on GOLD stages 1 (mild) to 4 (very severe).8
Clinical Assessment
Demographic and clinical data were collected using standardized questionnaires, which are available at http://www.COPDGene.org.7 Race and ethnicity were self-reported and classified as non-Hispanic Black and non-Hispanic White. The included populations are thought to be genetically homogeneous and were deemed eligible for the study, the main goal of which was identifying genetic determinants of COPD. Other groups, such as Hispanic/Latinx individuals, were excluded because of their heterogeneous background. Smoking history included pack-years and current and former smoking status. Body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) was measured in a standardized manner. The body mass index, obstruction, dyspnea, and exercise (BODE) mortality risk score was calculated for each participant.14 Coronary artery disease was defined as a positive answer to any questions about a history of myocardial infarction, angina, coronary disease, angioplasty, or coronary artery bypass grafting surgery.15 Chronic bronchitis was defined as cough and phlegm for at least 3 months per year for at least 2 consecutive years.16 The participants were asked about a history of asthma with the questions, “Have you had asthma?” and “Do you still have it?” If the participant responded yes to both questions, then we considered the participant had a history of current asthma. Exacerbations after enrollment were ascertained with the LFU program of COPDGene.10 In this program, the participants were asked to report their exacerbations every 6 months by telephone contact and web-based surveys. An exacerbation was defined as an increase or new onset of respiratory symptoms (cough, phlegm, dyspnea) treated with antibiotics and/or systemic corticosteroids.17 Additional details are provided in the eMethods in Supplement 1.
Statistical Analysis
Mortality rates were computed with logistic regression. We used Kaplan-Meier analysis to plot mortality probability curves per mucus plug score category (ie, 0, 1-2, ≥3 lung segments with mucus plugs) and a log-rank test to compare groups. The association between mucus plugs and all-cause mortality was assessed with Cox proportional hazard regression analysis. The a priori multivariable model included age, sex, race and ethnicity, BMI, pack-years, smoking status (current vs former), postbronchodilator FEV1, and CT measures of airway wall thickness and emphysema as potential confounders. Additional adjustments included coronary artery disease, chronic bronchitis, current asthma, the number of exacerbations per year over time, and the BODE index. We evaluated proportionality assumptions by multiplying each covariate by the log transform of the follow-up time. The proportionality did not present major violations, and the model fit was adequate. A statistician (W.W.) conducted the analyses using SAS version 9.4 software (SAS Institute). A 2-sided P value less than .05 was used as statistical significance for the main analysis. Additional details are provided in the eMethods in Supplement 1.
Results
Participant Characteristics
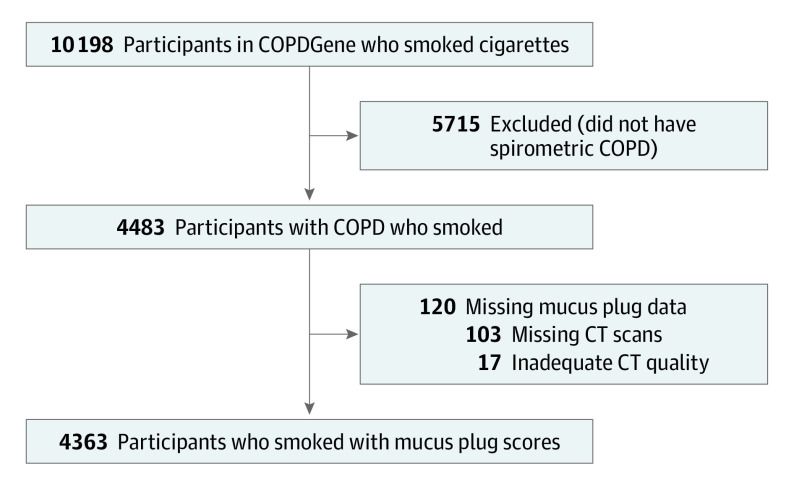
Of the 10 198 participants who smoked in the COPDGene cohort, 4483 were diagnosed with COPD. After exclusion of 120 patients with missing or poor-quality CT scans, 4363 patients were included in this study (Figure 1). Among the 4363 participants, 2585 (59.3%) had a mucus plug score of 0; 953 (21.8%) had a score of 1 to 2, and 825 (18.9%) had a score of 3 or more on their baseline CT scans. eFigure 3 in Supplement 1 shows the mucus plug score distribution of the study participants. Among the 1778 individuals with mucus plugging, the number of lung segments with mucus plugs ranged from 1 to 17, and the median number was 2. In participants with a mucus plug score of 3 or more, the median score was 5 (IQR, 3-7). Compared with participants without mucus plugs, those with mucus plugs in 3 or more lung segments were older and more likely to be female and non-Hispanic White. They also had a greater pack-year history of smoking, more chronic bronchitis, more current asthma, worse BODE index, lower FEV1, greater airway wall thickness, and higher percentage of emphysema on CT scans (Table 1). Participants with 3 or more lung segments with mucus plugs on CT had more COPD exacerbations per year than those without mucus plugs (median, 0.3 [IQR, 0-1.2] vs 0.1 [IQR, 0-0.6]).
Figure 1. COPDGene Participants Who Currently and Formerly Smoked Cigarettes.
COPD indicates chronic obstructive pulmonary disease; COPDGene, Genetic Epidemiology of COPD; and CT, computed tomography.
Association Between Mucus Plug Score and All-Cause Mortality
During a median follow-up of 9.5 years (IQR, 5.0-12.2 years), 1769 participants (40.6%) died. The mortality rate in participants with no mucus plugs was 34.0% (95% CI, 32.2%-35.8%), 46.7% (95% CI, 43.5%-49.9%) in participants with mucus plugs in 1 to 2 lung segments, and 54.1% (95% CI, 50.7%-57.4%) in those with mucus plugs in 3 or more lung segments (Table 2). The mortality probability was highest among participants with mucus plug scores of 3 or more as shown both in unadjusted and adjusted plots (Figure 2). The mortality rates increased across both COPD GOLD stages and mucus plug categories. The lowest 2 mortality rates were in participants with GOLD 1 stage (mild COPD) and mucus plug score category of 0 and 1 to 2, and the highest 2 mortality rates were in those with GOLD 4 stage (very severe COPD) and mucus plug score category of 1 to 2 and 3 or more (Figure 3).
Table 2. Association Between Mucus Plug Score and All-Cause Mortality in Participants With COPD.
| No. | Mucus plug score (No. of lung segments with mucus plugs) | |||||
|---|---|---|---|---|---|---|
| 0 (n = 2585) | 1-2 (n = 953) | ≥3 (n = 825) | ||||
| Mortality rate, % (95% CI) | 34.0 (32.2-35.8) | 46.7 (43.5-49.9) | 54.1 (50.7-57.4) | |||
| Unadjusted mortality rate difference, % (95% CI) | 1-2 vs 0: 12.7 (9.1-16.4) | ≥3 vs 0: 20.1 (16.2-24.0) | ||||
| Model | HR (95% CI) | HR (95% CI) | P value | HR (95% CI) | P value | |
| Unadjusted model | 4363 | 1 [Reference] | 1.51 (1.34-1.69) | <.001 | 1.98 (1.76-2.22) | <.001 |
| Adjusted modela | 4166 | 1 [Reference] | 1.15 (1.02-1.29) | .02 | 1.24 (1.10-1.41) | <.001 |
| Plus coronary artery diseaseb | 4166 | 1 [Reference] | 1.16 (1.03-1.30) | .02 | 1.26 (1.11-1.43) | <.001 |
| Plus chronic bronchitisc | 4166 | 1 [Reference] | 1.15 (1.02-1.30) | .02 | 1.25 (1.10-1.42) | <.001 |
| Plus current asthmad | 4166 | 1 [Reference] | 1.15 (1.02-1.30) | .02 | 1.25 (1.10-1.41) | <.001 |
| Plus exacerbations per yeare | 3759 | 1 [Reference] | 1.10 (0.96-1.25) | .17 | 1.20 (1.05-1.38) | .008 |
| Plus BODE indexf | 4060 | 1 [Reference] | 1.14 (1.01-1.29) | .03 | 1.21 (1.06-1.37) | .004 |
Abbreviations: BODE, body mass index, obstruction, dyspnea, and exercise mortality risk score; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
Estimates are from multivariable Cox models. Adjustment included age (continuous), sex (reference: female), race and ethnicity (reference: non-Hispanic Black), body mass index (BMI, calculated as weight in kilograms divided by height in meters squared; continuous), pack-years of smoking (continuous), current smoking status (reference: former), postbronchodilator forced expiratory volume in the first second of expiration (FEV1; continuous), and computed tomography measures of emphysema (measured as percentage of voxels <−950 Hounsfield units; continuous) and airway wall thickness (measured as the square root of the wall area of an ideal 10-mm-inner-perimeter airway; continuous).
Adjusted HRs included all variables from the adjusted model plus adjustment for coronary artery disease (reference: absence of coronary artery disease).
Adjusted HRs included all variables from the adjusted model plus adjustment for coronary artery disease and chronic bronchitis (reference: absence of chronic bronchitis).
Adjusted HRs included all variables from the adjusted model plus adjustment for coronary artery disease, chronic bronchitis, and history of current asthma (reference: absence of current asthma).
Adjusted HRs included all variables from the adjusted model plus adjustment for coronary artery disease, chronic bronchitis, history of current asthma, and the number of exacerbations per year (continuous).
Adjusted HRs included all variables from the adjusted model except FEV1 and BMI plus adjustment for the BODE index (continuous).
Figure 2. Mortality Plots by Mucus Plug Score Category .
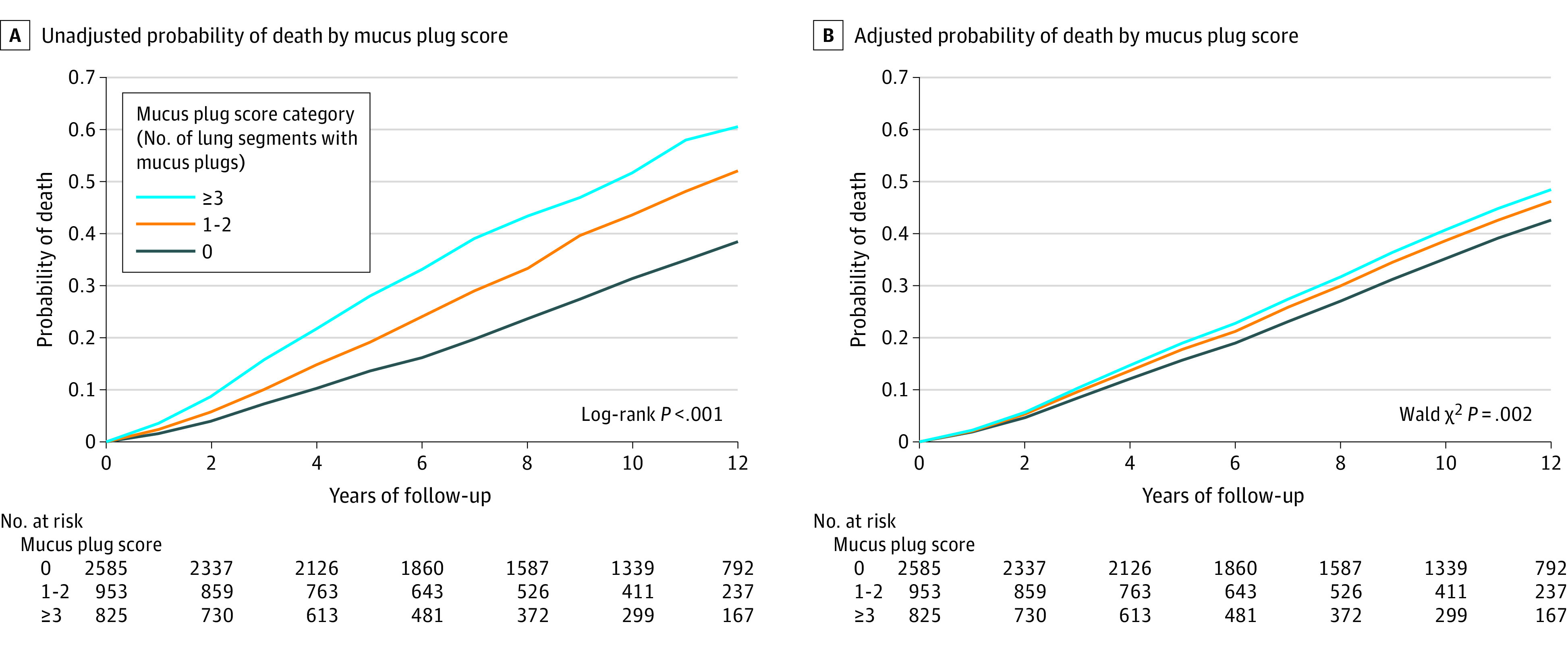
Of the 4363 participants with chronic obstructive pulmonary disease (COPD) included in the analysis, 1769 died from any cause. A, Unadjusted plot included all the 4363 participants with COPD. B, Plot adjusted for age, sex, race and ethnicity, body mass index, smoking status, pack-years of smoking, postbronchodilator forced expiratory volume in 1 second, and computed tomography measures of emphysema and airway wall thickness and included 4166 participants with COPD. The median years of follow-up were 10.3 (IQR, 5.4-12.3), 8.7 (IQR, 5.0-12.0), and 7.1 (IQR, 3.9-11.6) for participants in mucus plug score categories of 0, 1 to 2, and 3 or more, respectively.
Figure 3. All-Cause Mortality by Mucus Plug Score Category and Chronic Obstructive Pulmonary Disease (COPD) Severity (N = 4363).
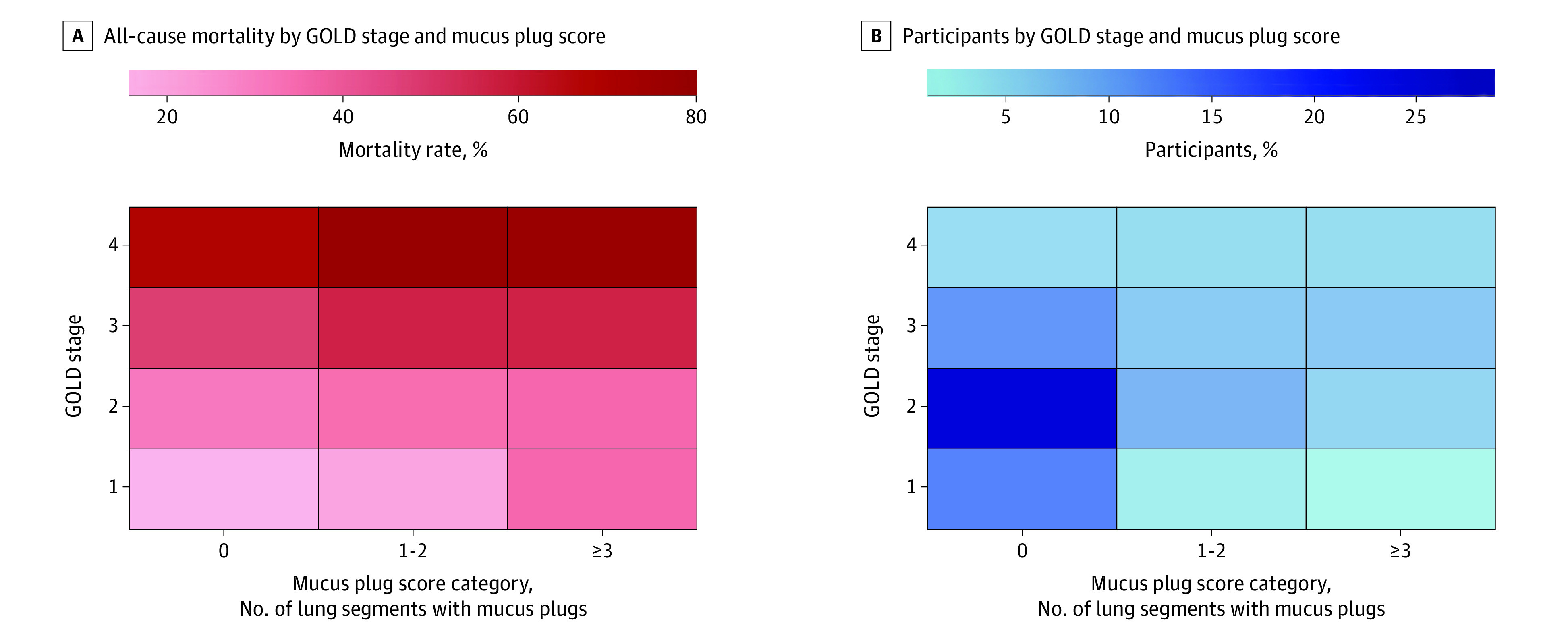
Values represent proportion of all-cause mortality (A) and the percentage of participants (B) by Global Initiative for Chronic Obstructive Lung Disease (GOLD) severity stage of COPD and mucus plug score category. Mortality rate was calculated as the number of participants who died divided by the number of participants (GOLD and mucus plug score category) × 100. GOLD stages were defined with postbronchodilator forced expiratory volume in the first second of expiration (FEV1) percentage of predicted (pp) values as follows: 1 (mild, n = 766), FEV1 pp ≥80; 2 (moderate, n = 1887), FEV1 pp ≥50 to <80; 3 (severe, n = 1127) FEV1 pp ≥30 to <50; and 4 (very severe, n = 583), FEV1 pp <30.
When the model was adjusted for age, sex, race and ethnicity, BMI, pack-years smoked, current smoking status, FEV1, and CT measures of emphysema and airway wall thickness, the presence of mucus plugs was significantly associated with an increased risk of death (score 1-2 vs 0: adjusted hazard ratio [aHR], 1.15 [95% CI, 1.02-1.29]; score ≥3 vs 0: aHR, 1.24 [95% CI, 1.10-1.41]) compared with participants without mucus plugs (Table 2). Additional adjustment for coronary artery disease showed similar results (score 1-2 vs 0: aHR, 1.16 [95% CI, 1.03-1.30]; score ≥3 vs 0: aHR, 1.26 [95% CI, 1.11-1.43]). In the adjusted model plus coronary artery disease and chronic bronchitis, the association between mucus plugs and all-cause mortality did not change substantially (score 1-2 vs 0: aHR, 1.15 [95% CI, 1.02 to 1.30]; score ≥3 vs 0: aHR, 1.25 [95% CI, 1.10-1.42]) (Table 2). Because mucus plugs frequently occur in individuals with asthma,18 and are associated with higher exacerbation rates in individuals who smoke,4 an additional analysis was performed that accounted for the diagnosis of asthma and COPD exacerbations. In the adjusted model described above plus coronary artery disease, chronic bronchitis, and history of current asthma, the association between mucus plugs and all-cause mortality remained significant (score 1-2 vs 0: aHR, 1.15 [95% CI, 1.02-1.30]; score ≥3 vs 0: aHR, 1.25 [95% CI, 1.10-1.41]) (Table 2). When the number of exacerbations per year during follow-up was included in the adjusted model plus coronary artery disease, chronic bronchitis, and current asthma, the association between mucus plugs and all-cause mortality was attenuated (score 1-2 vs 0: aHR, 1.10 [95% CI, 0.96-1.25]; score ≥3 vs 0: aHR, 1.20 [95% CI, 1.05-1.38]) (Table 2). When the adjusted model above (without FEV1 and BMI) was further adjusted for the BODE mortality index, the association between mucus plugs and all-cause mortality was similar (score 1-2 vs 0: aHR, 1.14 [95% CI, 1.01-1.29]; score ≥3 vs 0: aHR, 1.21 [95% CI, 1.06-1.37]) (Table 2).
Discussion
COPD is a heterogeneous and complex condition, and mucus pathology is an underrecognized disease feature.19 Recent studies demonstrated that mucus plugs identified on CT scans are frequent in patients with COPD (25%-67%), persist in most patients at 1-year (67%) and 5-year (73%) follow-up, and closely associated with clinical measures of the disease, including lung function, quality of life, and emphysema on CT.4,5 Further, occlusion of airways by mucus plugging may increase the risk of infection by reducing oxygen diffusion and causing local hypoxia, which potentiates microbial growth.2,20 Mucus plugs occluding multiple bronchi may increase the risk of pneumonia and COPD exacerbations.6 Additionally, the presence of mucus plugs is associated with ventilation/perfusion mismatch, which may lead to respiratory failure, a common cause of death in COPD.21
In this study, the presence of mucus plugs occluding medium- to large-sized airways was associated with increased all-cause mortality in participants with COPD. This result aligned with a study of explanted lung tissue in 101 individuals with advanced COPD,6,22 which found that a higher percentage of mucus occlusion of small conducting airways was associated with early death.6 This study provides new insights into the association of mucus plugging and mortality by evaluating mucus plugging causing complete occlusion of medium- to large-sized airways based on CT imaging and by including individuals with a full spectrum of COPD severity. The association between mucus plugs and all-cause mortality was sustained after adjustment for BMI, pack-years of smoking, FEV1, cardiovascular comorbidity, asthma, CT measures of emphysema and airway wall thickness, and other potential confounders (ie, current smoking status, age, sex, race and ethnicity).
In addition, the association observed between mucus plugs and increased mortality persisted in participants with COPD after adjustment for the diagnosis of chronic bronchitis, which involves chronic mucus hypersecretion and was associated with increased mortality.23 Thus, this study may indicate that CT scan detection of mucus plugs occluding medium- to large-sized airways can provide supplemental information about COPD mortality even when taking into account the diagnosis of chronic bronchitis. An explanation for this finding may be that the CT identification of mucus plugs is more objective than self-reported cough and sputum production, which are the defining symptoms of chronic bronchitis. This hypothesis is supported by the observation that up to 30% of individuals with COPD report no cough nor sputum even when they present with plugs on CT.5 Additionally, occluding mucus plugs on CT may provide additional information about COPD mortality beyond COPD exacerbations because mucus plug formation may be a closer surrogate for underlying mechanisms that lead to death in COPD, such as small airway pathology and ventilation/perfusion mismatch.21 This study also found that mucus plugs were associated with increased mortality after adjusting for the BODE index, which is a validated prediction score for all-cause mortality in patients with COPD.14
A potential implication of detecting occluding mucus plugs on CT scans is that they might provide a target for treatment. Therapies to improve lung function, slow disease progression, and decrease mortality are unmet needs for patients with COPD. Preliminary studies in asthma have suggested that treatment with biologics decreases mucus plugs on CT and that reduction of mucus plugs was associated with improved lung function.24,25 The results of this study and prior studies might be hypothesis-generating to test the clinical value of interventions targeting mucus plugs in COPD.
This study had several strengths, including a large sample size, inclusion of patients with a full spectrum of COPD severity, long-term follow-up, high-quality CT scans, use of multiple blind chest CT scan readers who received training in the detection of mucus plugs, adjustment for multiple potential confounders, and high quality of data collection.
Limitations
This study had several limitations. First, this was an observational study, so conclusions cannot be made that mucus plugging causes death. Second, the COPDGene cohort enrolled only non-Hispanic Black and non-Hispanic White participants so the study findings may not apply to other racial and ethnic groups who were not included. Third, the COPDGene study performed volumetric chest CT scans with submillimetric slice thickness, which might not be routinely acquired in clinical practice.
Fourth, mucus that did not completely occlude the airway lumen was not assessed and the lung segment–based score used might have underestimated the burden of mucus plugs. The underestimation of the burden of the mucus pathology might have modified the magnitude of the association between mucus plugs and all-cause mortality. Fifth, this study only adjusted for cardiovascular and respiratory comorbidities.
Sixth, despite adjustment for multiple potential confounders, there may have been residual and unmeasured confounding factors, such as mucus-related inflammatory sputum biomarkers, that could have affected the observed association between airway mucus plugging and all-cause mortality. Seventh, missing data for some variables, such as CT measures of emphysema and airway wall thickness and exacerbations over time, could have affected the estimates for the association between airway mucus plugs and all-cause mortality.
Conclusions
Among patients with COPD, the presence of mucus plugs that obstruct medium- to large-sized airways is associated with higher all-cause mortality compared with patients without mucus plugging on chest CT scans.
eMethods
eFigure 1. Computed Tomography Sections Showing Airways Completely Occluded With Mucus Plugs
eFigure 2. Illustration of the Mucus Plug Score
eFigure 3. Distribution of Mucus Plug Scores in 4363 Participants With Chronic Obstructive Pulmonary Disease
eReferences
eAppendix. Funding and Acknowledgments
Data Sharing Statement
References
- 1.Sullivan J, Pravosud V, Mannino DM, Siegel K, Choate R, Sullivan T. National and state estimates of COPD morbidity and mortality—United States, 2014-2015. Chronic Obstr Pulm Dis. 2018;5(4):324-333. doi: 10.15326/jcopdf.5.4.2018.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC. Muco-obstructive lung diseases. N Engl J Med. 2019;380(20):1941-1953. doi: 10.1056/NEJMra1813799 [DOI] [PubMed] [Google Scholar]
- 3.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233-2247. doi: 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunican EM, Elicker BM, Henry T, et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med. 2021;203(8):957-968. doi: 10.1164/rccm.202006-2248OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okajima Y, Come CE, Nardelli P, et al. Luminal plugging on chest ct scan: association with lung function, quality of life, and COPD clinical phenotypes. Chest. 2020;158(1):121-130. doi: 10.1016/j.chest.2019.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454-459. doi: 10.1164/rccm.200612-1772OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 9.Netter F. Atlas of Human Anatomy. Medical Education and Publishing, Ciba-Geigy Corp; 1989. [Google Scholar]
- 10.Stewart JI, Moyle S, Criner GJ, et al. ; for the COPDgene Investigators . Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466-472. doi: 10.3109/15412555.2012.690010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe KE, Regan EA, Anzueto A, et al. COPDGene 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384-399. doi: 10.15326/jcopdf.6.5.2019.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Thoracic Society . Standardization of spirometry, 1994 update, American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107-1136. doi: 10.1164/ajrccm.152.3.7663792 [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 14.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 15.Diaz AA, Martinez CH, Harmouche R, et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res. 2018;19(1):62. doi: 10.1186/s12931-018-0771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim V, Crapo J, Zhao H, et al. ; COPDGene Investigators . Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332-339. doi: 10.1513/AnnalsATS.201411-518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowler RP, Kim V, Regan E, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146(4):941-950. doi: 10.1378/chest.13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunican EM, Elicker BM, Gierada DS, et al. ; National Heart Lung and Blood Institute (NHLBI) Severe Asthma Research Program (SARP) . Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997-1009. doi: 10.1172/JCI95693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim V, Evans CM, Dickey BF. Dawn of a new era in the diagnosis and treatment of airway mucus dysfunction. Am J Respir Crit Care Med. 2019;199(2):133-134. doi: 10.1164/rccm.201808-1444ED [DOI] [PubMed] [Google Scholar]
- 20.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317-325. doi: 10.1172/JCI0213870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7(5):375-382. doi: 10.3109/15412555.2010.510160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman A, Fessler H, Martinez F, et al. ; National Emphysema Treatment Trial Research Group . Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075-1083. doi: 10.1056/NEJMoa11798 [DOI] [PubMed] [Google Scholar]
- 23.Fortis S, Shannon ZK, Garcia CJ, et al. Association of nonobstructive chronic bronchitis with all-cause mortality: a systematic literature review and meta-analysis. Chest. 2022;162(1):92-100. doi: 10.1016/j.chest.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 24.Anai M, Yoshida C, Izumi H, et al. Successful treatment with dupilumab for mucus plugs in severe asthma. Respirol Case Rep. 2022;11(1):e01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordenmark L, Emson C, Hellqvist Å, et al. S46 tezepelumab reduces mucus plugging in patients with uncontrolled, moderate-to-severe asthma: the phase 2 CASCADE study. Thorax. 2022;77(suppl 1):A32. doi: 10.1136/thorax-2022-BTSabstracts.52 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Computed Tomography Sections Showing Airways Completely Occluded With Mucus Plugs
eFigure 2. Illustration of the Mucus Plug Score
eFigure 3. Distribution of Mucus Plug Scores in 4363 Participants With Chronic Obstructive Pulmonary Disease
eReferences
eAppendix. Funding and Acknowledgments
Data Sharing Statement