Abstract
Background & Aims:
Transoral Incisionless Fundoplication (TIF) using EsophyX device is a minimally invasive endoscopic fundoplication technique. Our study aimed to assess the efficacy of TIF for atypical GERD symptoms in patients with chronic or refractory GERD.
Methods:
A systematic search of 4 major databases was performed. All original studies assessing atypical GERD using a validated symptom questionnaire (the Reflux Symptom Index (RSI) were included. The RSI score was assessed pre-and post-TIF at 6- and 12-month follow-up. The data on technical success rate, adverse events, proton pump inhibitor (PPI) use, and patient satisfaction were also collected. Only TIF procedures currently in practice using EsophyX device, i.e., TIF 2.0 and TIF with concomitant hiatal hernia repair (cTIF), were included in the review.
Results:
A total of 10 studies (564 patients) were included. At 6- and 12- month follow-up, there was 15.72 (95% CI 12.15 to 19.29) and 14.73 points (95% CI 11.74 to 17.72) mean reduction of RSI score post-TIF, respectively, with a technical success rate of 99.5% and a pooled adverse event rate of 1%. At both time intervals, more than two-thirds of the patients were satisfied with their health condition and roughly three-fourths of the patients were off daily PPI.
Conclusions:
Our study shows that TIF using the EsophyX device is safe and effective in reducing atypical GERD symptoms at 6- and 12-month follow-up. It improves patient-centered outcomes and can be a minimally invasive therapeutic option for patients suffering from atypical GERD symptoms on chronic medical therapy.
Introduction:
Gastroesophageal Reflux Disease (GERD) is a common condition that profoundly impacts patients’ quality of life. The prevalence of the disease in North America ranges from 18.1% - 27.8%.1 GERD can present with both typical (including heartburn, regurgitation and bloating) and extraesophageal or atypical (including cough, hoarseness, excess throat mucus, breathing difficulty and globus sensation) symptoms. Atypical GERD is a diagnostic challenge as only about 43% of these patients present with typical gastrointestinal symptoms.2 In addition to being a diagnostic challenge, it also poses a significant financial burden on the health care system. The estimated annual cost for atypical GERD is $50 Billion, five times the cost for typical GERD. This high healthcare cost is attributed to lack of gold standard diagnostic test, delayed diagnosis, prolonged use of acid-suppressive medications, and lack of effective therapies.3
The first line therapy for atypical GERD is medical treatment predominantly with proton pump inhibitors (PPI) and lifestyle intervention. However, the evidence in support of PPI for atypical GERD is inconsistent. For example, in 2019, a systematic review found out that only 3 out of 9 systematic reviews/metanalysis on this topic showed the superiority of PPI over placebo in improving atypical GERD symptoms.4 [NO_PRINTED_FORM]On the other hand, surgical anti-reflux treatment with laparoscopic Nissen Fundoplication has shown efficacy for both typical and atypical GERD symptoms.5,6 However, it is more invasive and associated with unwanted long-term side effects like dysphagia, uncontrolled flatulence, and gas bloating.7,8
Transoral Incisionless Fundoplication (TIF) is a minimally invasive, endoscopic fundoplication technique. It has demonstrated long-term efficacy and safety profile in patients suffering from chronic or refractory GERD.9,10 TIF was introduced in 2005 as a novel endoscopic fundoplication technique and approved by Food and Drug Administration (FDA) in 2007. It has evolved and further improved over the years. TIF 2.0 utilizes the EsophyX device (EndoGastric Solutions, Inc., Redmond, WA, USA) to restore the valve at the gastroesophageal (GE) junction. It is anatomically and functionally similar to fundoplication but is less invasive and has a low rate of adverse events.11,12 Currently in use TIF procedures include TIF 2.0 and concomitant TIF or cTIF (TIF 2.0 with hiatal hernia repair) for hiatal hernia > 2cm. The efficacy of TIF against typical GERD has been extensively reported in systematic reviews,10,12,13 but our understanding of its effectiveness against atypical GERD symptoms is limited.
This study aims to perform a systematic review and meta-analysis to evaluate the efficacy of the TIF 2.0 procedure for atypical GERD symptoms. (For the purpose of this systematic review, we will use TIF for TIF 2.0 procedure interchangeably).
Methods and Materials:
This review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) by the University of York with registration ID: CRD42021237931
Search Strategy and Study Selection:
Following MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines,14 we conducted a systematic search of published literature in four major databases: PubMed (NCBI), Embase (Elsevier), Web of Science Core Collection (Clarivate Analytics), and the Cochrane Central Register of Controlled Trials (Wiley). It was developed and run with the assistance of a Librarian (PAB), with the last search performed on October 8th, 2021. No geographic, language or date limits were applied (Supplementary Figure 1).
The study selection process involved two phases: Title & Abstract screening and Full Text Review. Two independent investigators conducted each step (MH & UH). Conflicts were then resolved by consensus discussion between the independent investigators and, when necessary, by the senior reviewer (CT).
Inclusion and Exclusion Criteria:
There were predefined Inclusion and Exclusion criteria.
Inclusion:
We included all retrospective and prospective study designs that assessed pre-and post-TIF atypical GERD symptoms with a minimum of at least 6 month follow-up. We included all adult patients over 18 years having chronic or refractory GERD undergoing the TIF 2.0 or cTIF procedures using the EsophyX device without any body mass index or hiatal hernia size limitation.
Exclusion:
We excluded studies reporting patients who underwent the procedure using an older technique than TIF 2.0. We also excluded patients who underwent TIF using any device other than the EsophyX device.
Data Extraction and Quality Assessment:
The data extraction and quality assessment were conducted by two independent investigators (MH & UH) separately, followed by cross-check of the data. Conflicts were then resolved by consensus discussion between the independent investigators and, when necessary, by a third reviewer (CT). The data extraction sheet broadly included detailed information for reported patient characteristics, study characteristics, procedure details, adverse events, and effectiveness outcomes. For Quality assessment of eligible studies, we utilized Cochrane Risk of Bias assessment tools for randomized and non-randomized studies and the Institute of Health Economics (IHE) quality appraisal tool for case series.15,16
Outcome Measures:
Our primary outcome of interest was TIF’s efficacy in patients with chronic or refractory atypical GERD symptoms, measured by a validated scoring system called Reflux Symptom Index (RSI). The RSI is a 9-item questionnaire developed and validated for assessing atypical GERD symptoms, also referred to as symptoms resulting from Laryngopharyngeal Reflux (LPR) including hoarseness, throat clearing, excess throat mucus, and cough. Each item on RSI has a scale that ranges from 0 (‘No Problem’) to 5 (‘Severe Problem’). The maximum total score can be 45, with a normality threshold of 13.17 The secondary outcomes included pre-and post-TIF procedure Proton Pump Inhibitor (PPI) usage and patient satisfaction level. Patient satisfaction was assessed in the studies as part of the GERD-Health Related Quality of Life (GERD-HRQL) validated questionnaire.
Statistical Analysis:
The primary outcome of RSI score was a continuous variable, reported as either mean/standard deviation or median/interquartile range. We performed a meta-analysis of mean differences within-person of RSI scores pre-and post-TIF. We stratified our analysis based on the follow-up time at 6 month and 12 month to decrease inter-study differences and get precise estimates at the given follow-up time. Due to small number of studies available and in order to be especially careful not to make an incorrect inference of significance due to failure to recognize heterogeneity, a random effects model was used for each meta-analytic model. The secondary outcome of PPI use and patient satisfaction were proportions. We meta-analyzed pooled proportions for secondary outcomes pre- and post-TIF separately. Forest plots were generated to show a graphical display of individual study results and the weighted average or magnitude of their combined effect. Wherever applicable, we calculated the data points from available information following the Cochrane Handbook guide.18 For missing or incomplete information, we reached out to principal authors of respective studies. For overlapping patient populations derived from the same registry data,19 raw data were requested from their industry sponsor to calculate outcomes to prevent inaccurate estimation of precision. The I2 statistic was used to calculate heterogeneity, and wherever applicable, causes of increased heterogeneity were further investigated. Sensitivity analysis was also performed for exploring increased heterogeneity by plotting effect sizes with or without each study. A subgroup analysis was designated a priori, specifically, the analysis focused on those patients with hiatal hernia size >2 cm who underwent cTIF. Funnel plots were generated for visual assessment of publication bias. It is recognized that there was multiple testing of outcome data arising from individual studies. The main results for the RSI scores are the primary finding and require no correction of p-values; other results should be considered as secondary findings with their p-values taken as descriptive only. As such, all p-values are presented without correction for multiple testing. All statistical analysis was performed using Stata software (MP/17.0).
Results:
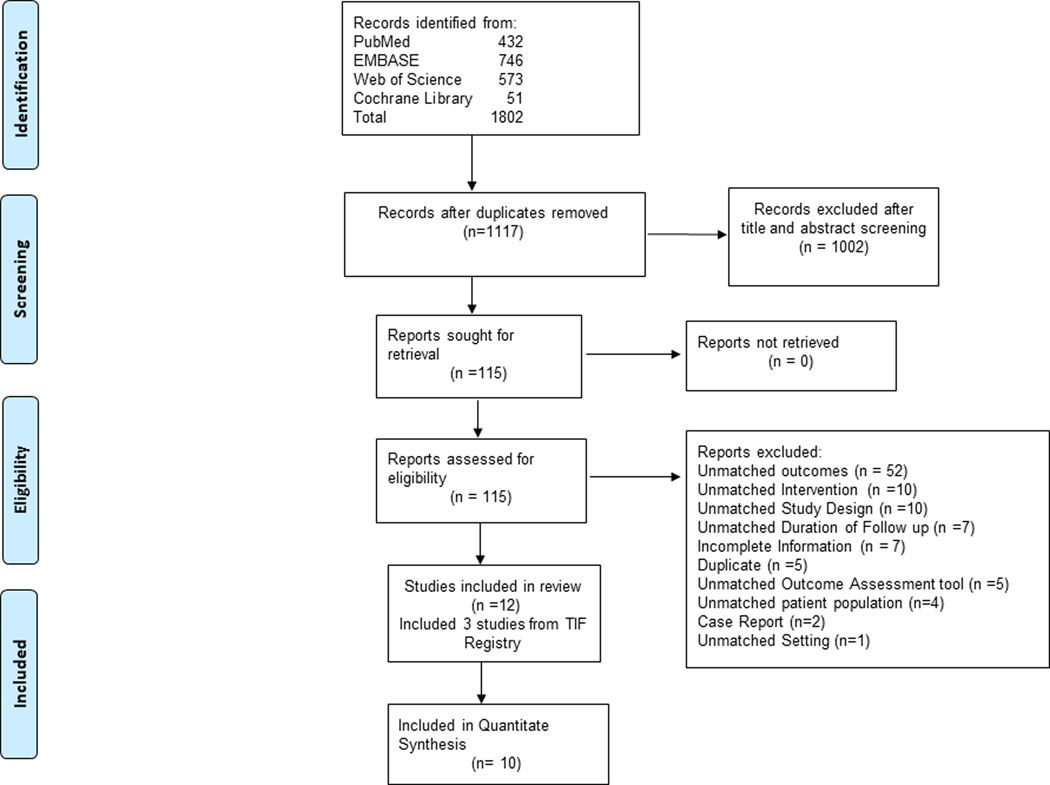
The PRISMA flow diagram depiction of the study selection process is shown in Figure 1. Our systematic database search retrieved 1,117 unique records. Following title and abstract screening, we retrieved 115 articles for full-text review. A total of 10 studies met our predefined eligibility criteria following full text review.20–31 For the three studies derived from the same data registry (TIF Registry), given the concern for overlapping patient population, we retrieved raw data from the industry sponsor of the data registry to extract results and presented as a single entity, “TIF Registry”.20–22
Figure 1:
PRISMA Flowchart
Study and Patient Characteristics:
All eligible studies were conducted between 2008 and 2021 and included one Randomized Control Trial, 4 Prospective, and 5 Retrospective observational studies. The characteristics of these studies are summarized in Table 1a. A total of 564 patients were included in our study, having information about validated atypical GERD symptom score (RSI) from a cohort of 740 patients undergoing TIF procedure in the eligible studies. Mean age and BMI were 57.0 ± 2.3 and 27.7 ± 1.7 respectively. There was a female predominance in the entire cohort (60%). The average GERD symptom and PPI use duration was 8.8 ± 1.9 years from 6/10 reporting studies and 7 ± 1.2 years from 5/10 reporting studies, respectively. A total of 287 patients had a hiatal hernia size 2 cm in the entire cohort. The characteristics of the patients are summarized in Table 1a and Table 1b.
Table 1a:
Study and Patient Characteristics:
| Author, Year | Cohort Location | Study Design | Study Duration | Total patients (n) | Atypical GERD patients (n) | Age (yr) | Female, n (%) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| Bell et al. 2012 (TIF Registry) | USA, Multicenter | Prospective | Jan 2010-Feb 2011 | 100 | 51 | 53 (18–75) | 65 (65) | 26.4 (18–35.1) |
| Wilson et al. 2014 (TIF Registry) | USA, Multicenter | Prospective | Jan 2010-Feb 2011 | 100 | 51 | 53 (18–75) | 65 (65) | 26.4 (18–35.1) |
| Bell et al. 2014 (TIF Registry) | USA, Multicenter | Prospective | Jan 2010-Jun 2012 | 158 | 124 | 58.5 (19–90) | 112 (71) | . |
| Trad et al, 2014 (TEMPO Trial) | USA, Multicenter | RCT | 2012–2013 | 60 | 39 | 54.8 (35.7–73.3) | 20 (51) | 28.9 (20.5–34.9) |
| Bell et al. 2011 | USA, Single Center | Retrospective | Nov 2008-Oct 2009 | 37 | 32 | 58 (20–81) | 21 (57) | 25.5 (15.9–36.1) |
| Trad et al. 2012 | USA, Single Center | Retrospective | May 2008-Jun 2010 | 28 | 27 | 57 (23–77) | 14 (50) | 25.7 (18.3–36.4) |
| Barnes et al. 2011 | USA, Multicenter | Retrospective | Nov 2008-Dec 2009 | 110 | NR | 60 (21–87) | 81 (74) | 27.5 (19–47.9) |
| Janu et al. 2019 | USA, Multicenter | Prospective | NR | 99 | NR | 55 | 54 (55) | 30 |
| Ihde et al. 2011 | USA, Multicenter | Retrospective | Nov 2009-Jun2010 | 42 | NR | 54 (21–72) | 23 (55) | 20.8–51.7 |
| Ihde et al. 2019 | USA, Single Center | Retrospective | Oct 2015- Dec 2017 | 97 | 29 | 59 | ~60 (61) | 28 |
| Choi et al. 2020 | USA, Single Center | Prospective | Jan 2018-Jul 2020 | 60 | 12 | 59.3 (27–77) | 28 (47) | 30 (19.8–36) |
| Snow et al. 2021 | USA, Multicenter | Prospective | Apr 2019– 2021 | 49 | 49 | 54.4 (13.5) | 31 (63) | 27.5 (4.3) |
Abbreviations: RCT: randomized control trial; NR: not reported
Table 1b:
Study and Patient Characteristics.
| Author, Year | Hiatal Hernia | HH ≤ 2cm | HH ≥ 2cm | Duration of Symptoms (yr) | Duration of PPI use (yr) |
|---|---|---|---|---|---|
| Bell et al. 2012 (TIF Registry) | 43/51 | 42 | 1 | 9 (1–35) | 7 (1–20) |
| Wilson et al. 2014 (TIF Registry) | 43/51 | 42 | 1 | 9 (1–35) | 7 (1–20) |
| Bell et al. 2014 (TIF Registry) | 113/158 | NR | NR | . | . |
| Trad et al, 2014 (TEMPO Trial) | 36/39 | 36 | 0 | 10 (2–50) | 7 (1–25) |
| Bell et al. 2011 | 25/37 | NR | 12 | NR | NR |
| Trad et al. 2012 | 21/28 | 21 | 0 | 5 (1–20) | 5 (1–11) |
| Barnes et al. 2011 | 70/110 | 51 | 19 | 9 (1–35) | 8 (1–25) |
| Janu et al. 2019 | 99 | 0 | 99 | 10 (1–30) | NR |
| Ihde et al. 2011 | NR | NR | 18 | 10 (1–30) | NR |
| Ihde et al. 2019 | NR | NR | 29 | NR | 8 |
| Choi et al. 2020 | 60/60 | 0 | 60 | NR | NR |
| Snow et al. 2021 | 23/49 | 26 | 23 | NR | NR |
Abbreviations: HH: hiatal hernia
Risk of Bias:
All observational studies were assessed using the Institute of Health Economics (IHE) quality appraisal tool for case series.16 We had a total of 11 observational studies for quality assessment, which included 3 studies derived from the same data registry. (Table 1a)19,21,32 All studies were of acceptable quality with compliance of 14 items on the quality appraisal tool. In addition, we also used Newcastle-Ottawa Quality Assessment Scale (NOS) for all observational studies.33 The NOS indicated that all articles included in the review had a low risk of bias (score of 5 or more out of 8 items on study selection, comparability, and outcome). We had 2 reports from the same Randomized Control Trial (TEMPO Trial), which were assessed using the Cochrane Risk of Bias assessment tool.15 The study was funded by EndoGastric Solutions, and there was evidence of detection and performance bias. But the overall risk of bias was judged to be low.
Procedure details:
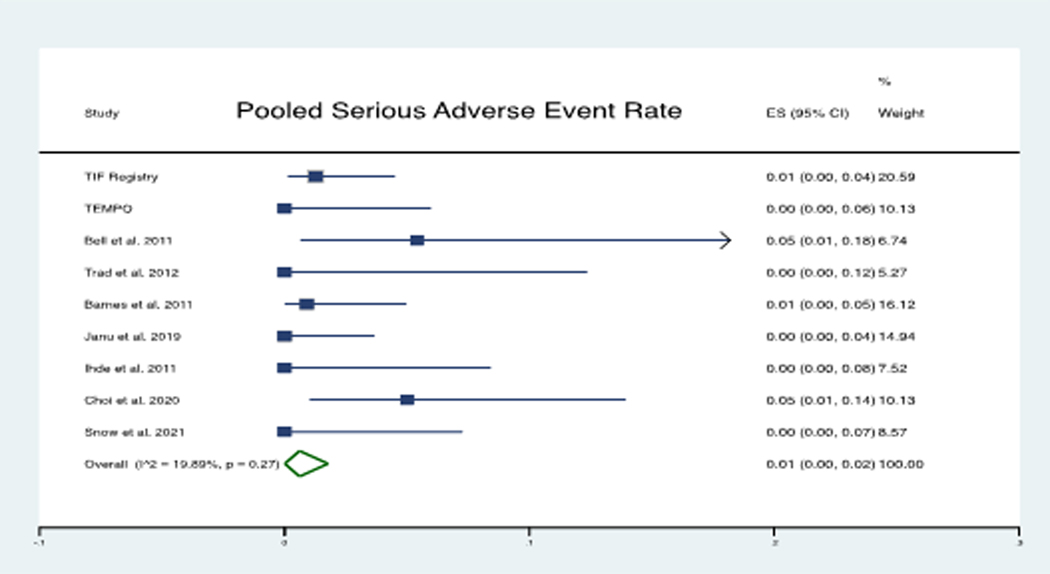
The immediate technical success rate of the procedure was 99.5%. The rate of pooled serious adverse event was 1% (Figure 2). A total of 9 serious adverse events included 3 superficial esophageal tears, 2 gastrointestinal bleeding episodes, 1 hematoma formation, 1 esophageal perforation, 1 postop fever with thrush, and 1 postop mediastinal abscess formation. All serious adverse events were immediately addressed, and there was no mortality reported. Out of 287 patients with hiatal hernia size 2 cm, 255 were from 5 studies that reported outcomes of cTIF.27–31 The mean time to perform the TIF procedure was 51 ± 14.8 minutes. The mean time to perform cTIF was 96 ± 42 minutes, reported only in one of the 4 studies.30 The details of the procedure and follow-up time periods are reported in Table 2.
Figure 2:
Forest plot of pooled Serious Adverse Event Rate: The pooled weighted average of adverse event rate was 0.01 (1%)
Table 2:
Procedure Details and Duration of follow up
| Author, Year | cTIF | Procedure time (min) | Technical Success (%) | Major Adverse events (n) | Follow up |
|---|---|---|---|---|---|
| Bell et al. 2012 (TIF Registry) | No | 42 (21–85) | 100 | 0 | 6 M |
| Wilson et al. 2014 (TIF Registry) | No | 42 (21–85) | 100 | 0 | 12 M |
| Bell et al. 2014 (TIF Registry) | No | NR | 98.7 | 2 (Small Esophageal tears) | 6 M, 12 M, 24 M, 36 M |
| Trad et al, 2014 (TEMPO Trial) | No | 38 (20–68) | 100 | 0 | 6 M, 12 M |
| Bell et al. 2011 | No | 75 (45–110) | 100 | 2 (Mediastinal Abscess POD 6, Bleeding from traumatic dislodgment of helical screws) | 6 M |
| Trad et al. 2012 | No | 55 | 100 | 0 | 12 M-24 M |
| Barnes et al. 2011 | No | 45 (21–122) | 99.2 | 1 (Hematoma formation) | 6 M |
| Janu et al. 2019 | Yes | NR | 100 | 0 | 6 M, 12 M |
| Ihde et al. 2011 | Yes | NR | 97.9 | 1 (Esophageal Perforation) | 6 M |
| Ihde et al. 2019 | Yes | NR | NR | NR | 6 M-12 M |
| Choi et al. 2020 | Yes | 90 | 100 | 3 (Esophageal mucosal tear, GI bleed + Ileus, Thrush + fever) | 6 M, 12 M |
| Snow et al. 2021 | Yes | NR | 100 | 0 | 6 M |
Abbreviations: M: month; NR: not reported
Atypical GERD Symptoms Pre- and Post-TIF:
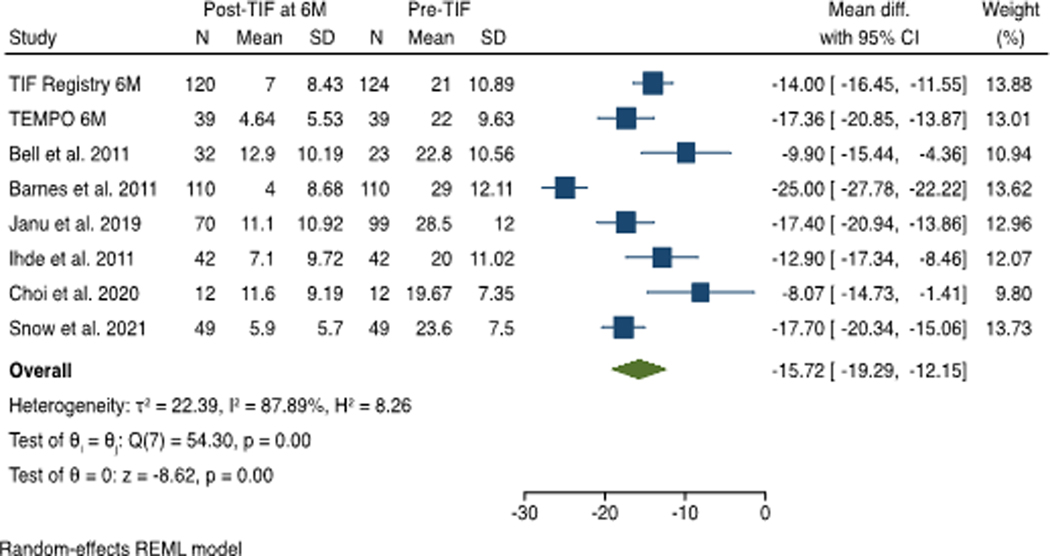
At 6 month follow up, a total of 474 patients’ data was available from 8 studies. The mean RSI score post-TIF procedure decreased below the normality threshold of 13 for all studies. The RSI score decreased after the TIF procedure compared with the pre-TIF score with a mean difference of −15.72 (95% CI −12.15 to −19.29), favoring the TIF procedure. There was considerable heterogeneity among the studies, with an I2 statistic of 88% using the random-effects model. (Figure 3)
Figure 3:
Effect Estimate of Reflux Symptom Index (RSI) score at 6 Month Post-TIF: The mean RSI score for each individual study post-TIF decreased below the normality threshold of 13 and the magnitude of their combined effect pre- and post-TIF was 15.72 on reduction of RSI score.
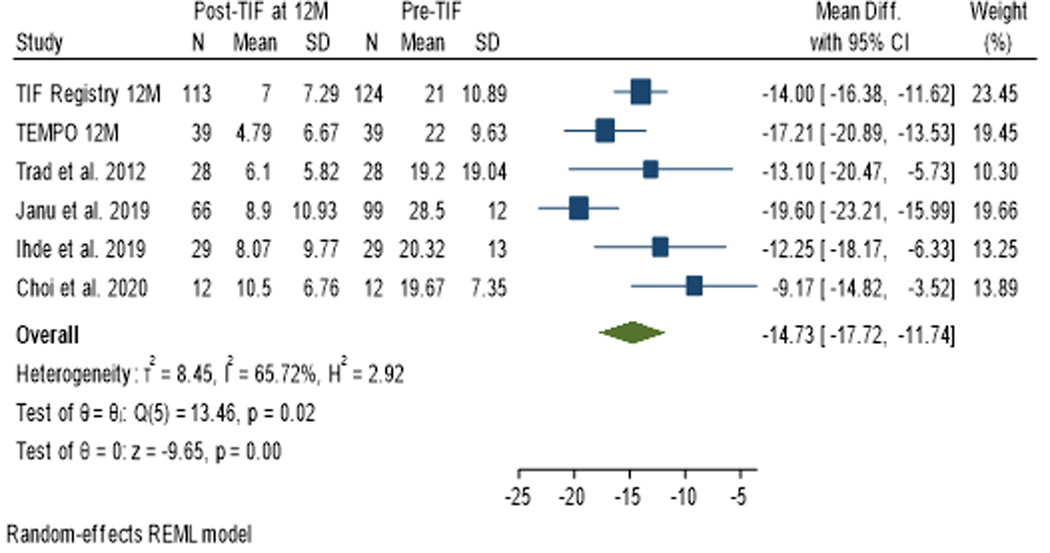
At 12 month follow up, a total of 287 patients’ data was available from 6 studies. The mean RSI score post-TIF procedure decreased below the normality threshold of 13 for all studies. The RSI score decreased after the TIF procedure compared with the pre-TIF score with a mean difference of −14.73 (95% CI −11.74 to −17.72), favoring the TIF procedure. The studies had substantial heterogeneity, with an I2 statistic of 66% using the random-effects model. (Figure 4)
Figure 4:
Effect Estimate of Reflux Symptom Index (RSI) score at 12 Month Post TIF: The mean RSI score for each individual study post-TIF decreased below the normality threshold of 13 and the magnitude of their combined effect pre- and post-TIF was 14.73 on reduction of RSI score.
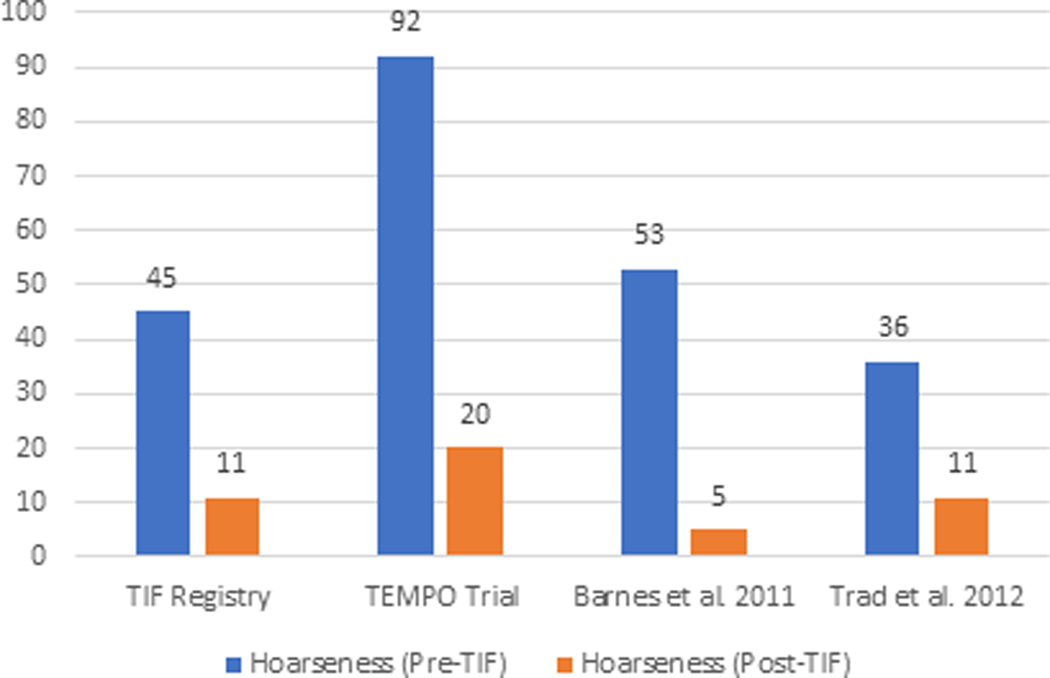
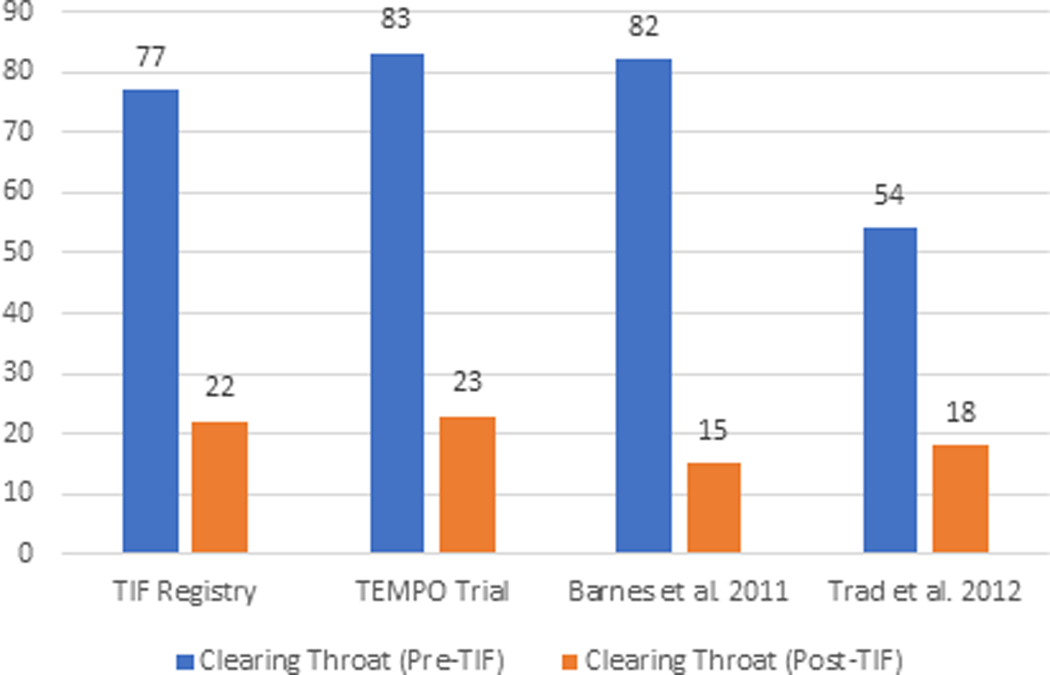
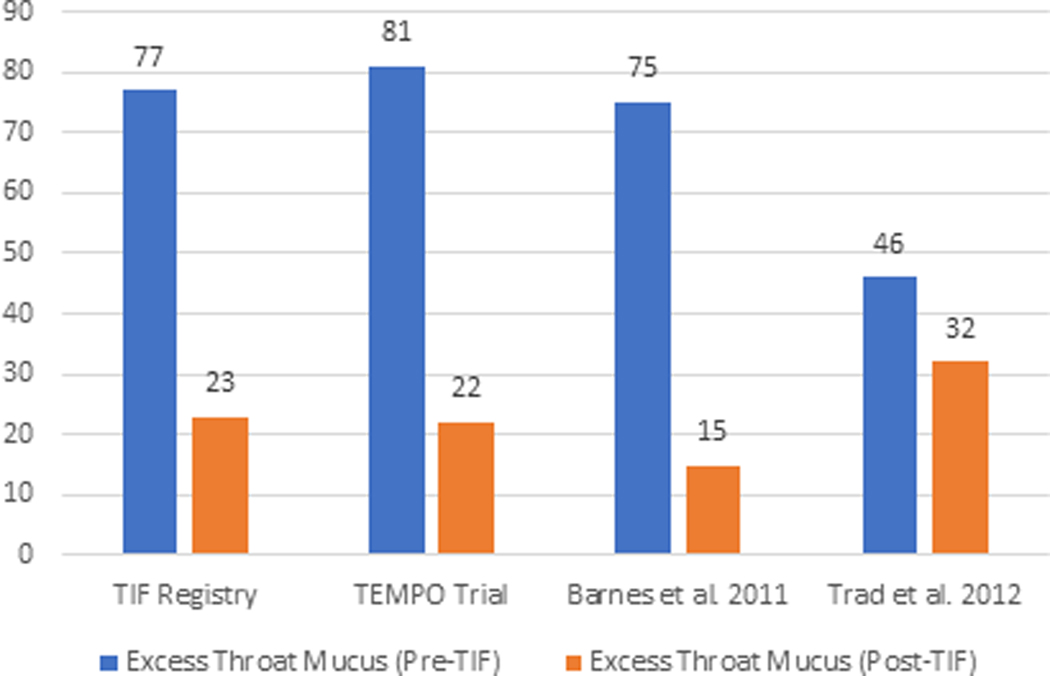
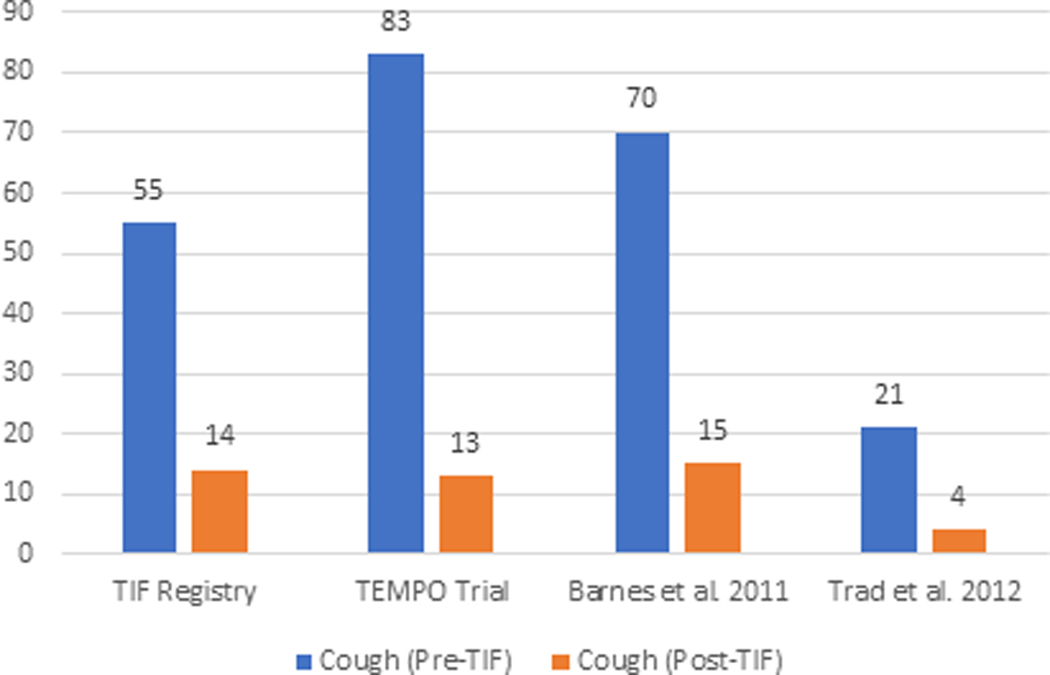
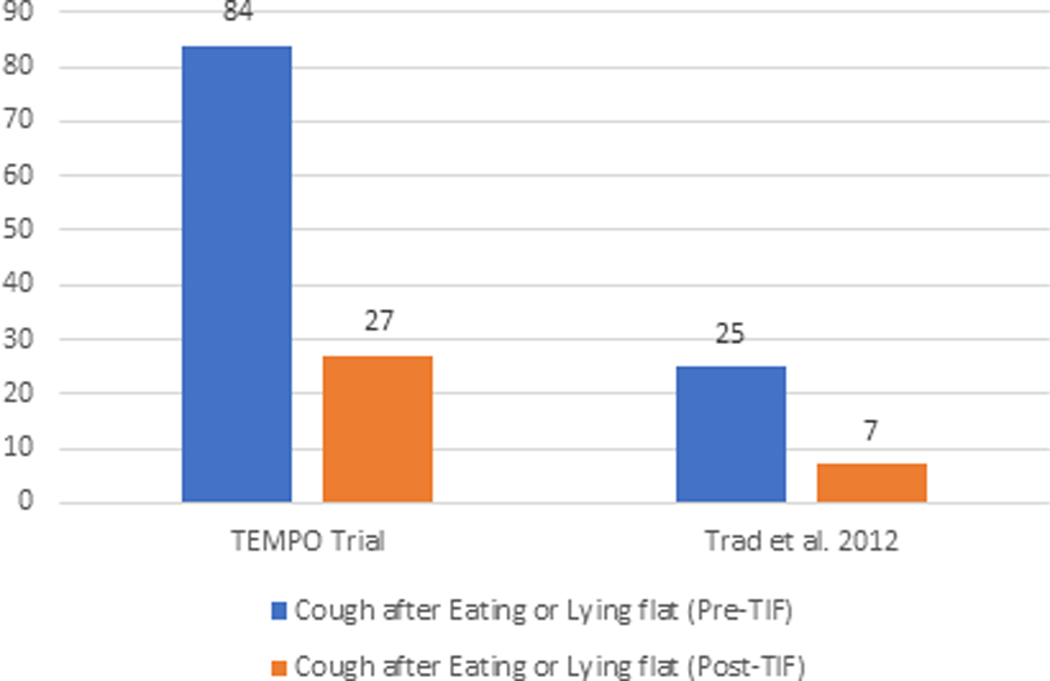
The elimination of individual daily troublesome atypical GERD symptoms was variably reported in the included studies. It is assessed using the individual question of the RSI score questionnaire on a Likert scale from 0 to 5 with an effectiveness endpoint of score 2. The atypical GERD symptoms include hoarseness, clearing throat, excess throat mucus, chronic cough, cough after eating or lying flat, globus sensation, breathing difficulty, and difficulty swallowing. There was a significant reduction in the proportion of patients with each atypical GERD symptom post-TIF, as shown in Figure 5.
Figure 5 (A-H):
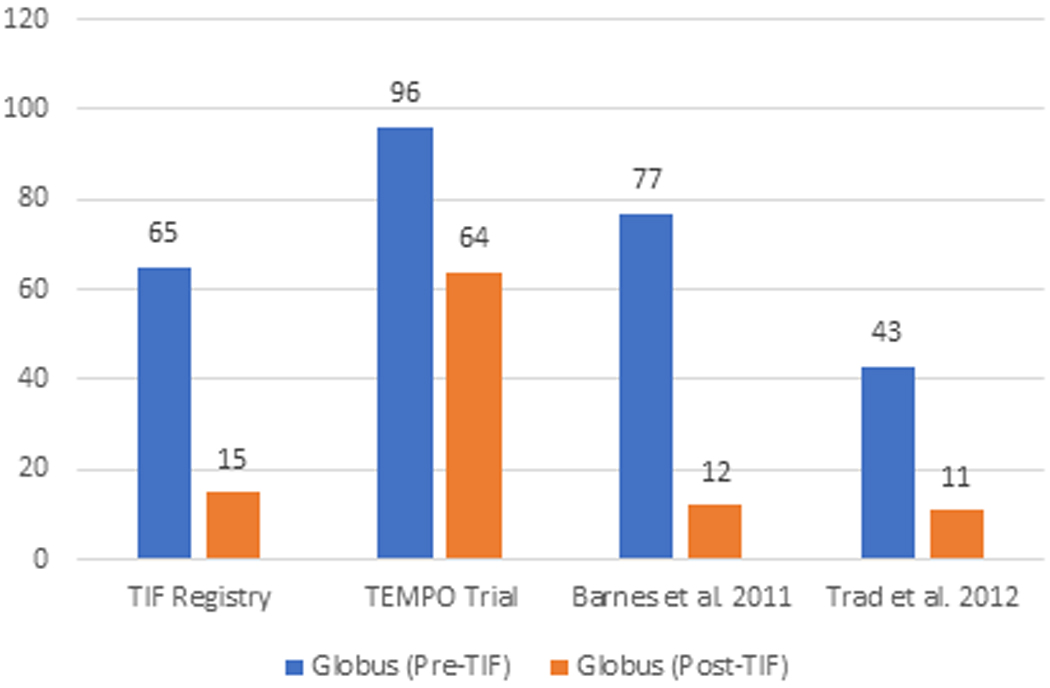
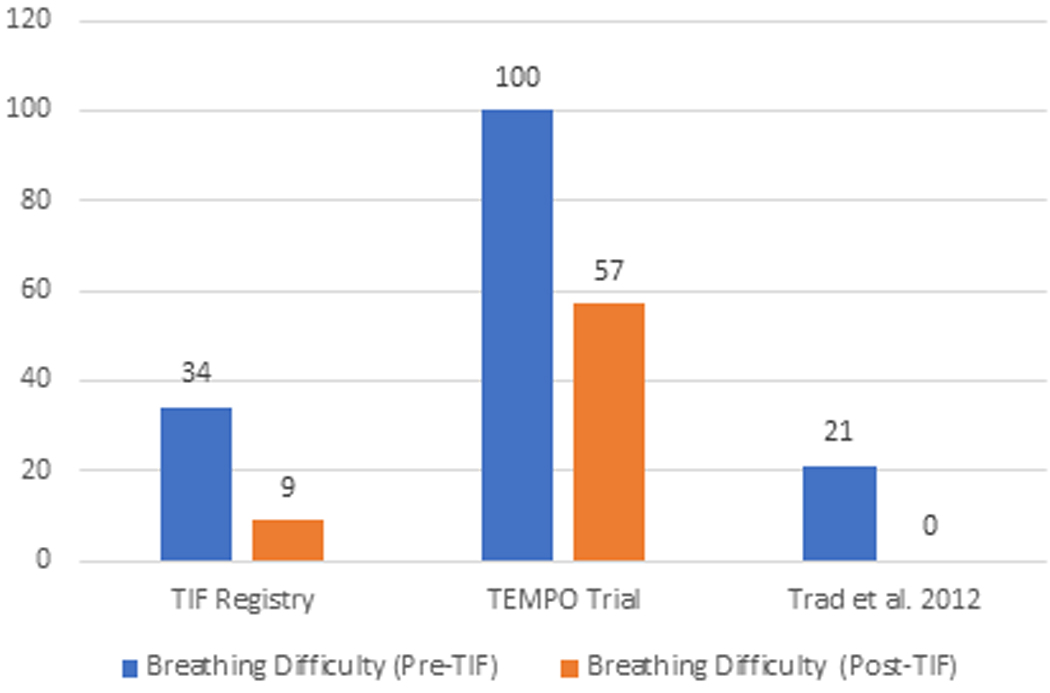
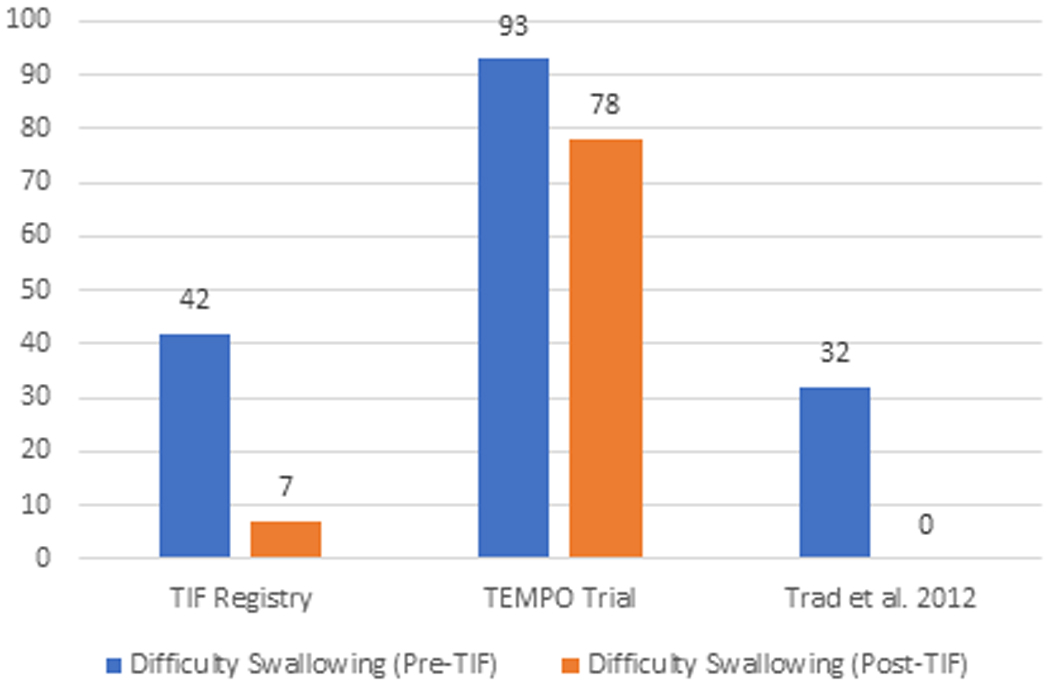
Elimination of Daily Troublesome atypical GERD symptoms (%). 5A: Hoarseness 5B: Clearing Throat 5C: Excess Throat Mucus 5D: Cough 5E: Cough after eating or lying flat 5F: Globus sensation 5G: Breathing difficulty 5H: Difficulty Swallowing
PPI Usage Pre and Post TIF:
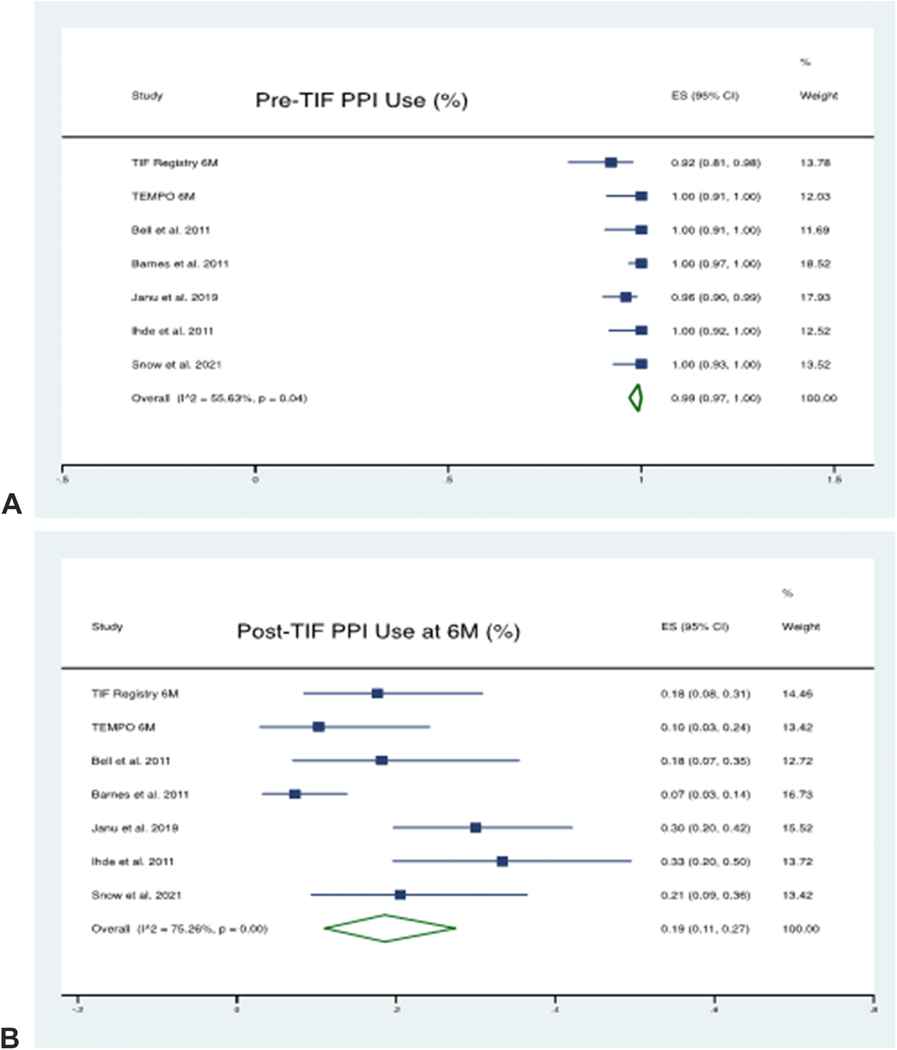
At 6 month follow-up, a total of 384 patients’ data was available from 7 studies. The pooled proportion of patients using PPI Pre-TIF was 99% (95% CI 97% - 100%), which was reduced to 19% (95% CI 11% - 27%) post-TIF. There was considerable heterogeneity between the studies with an I2 statistic of 75% using the random-effects model.
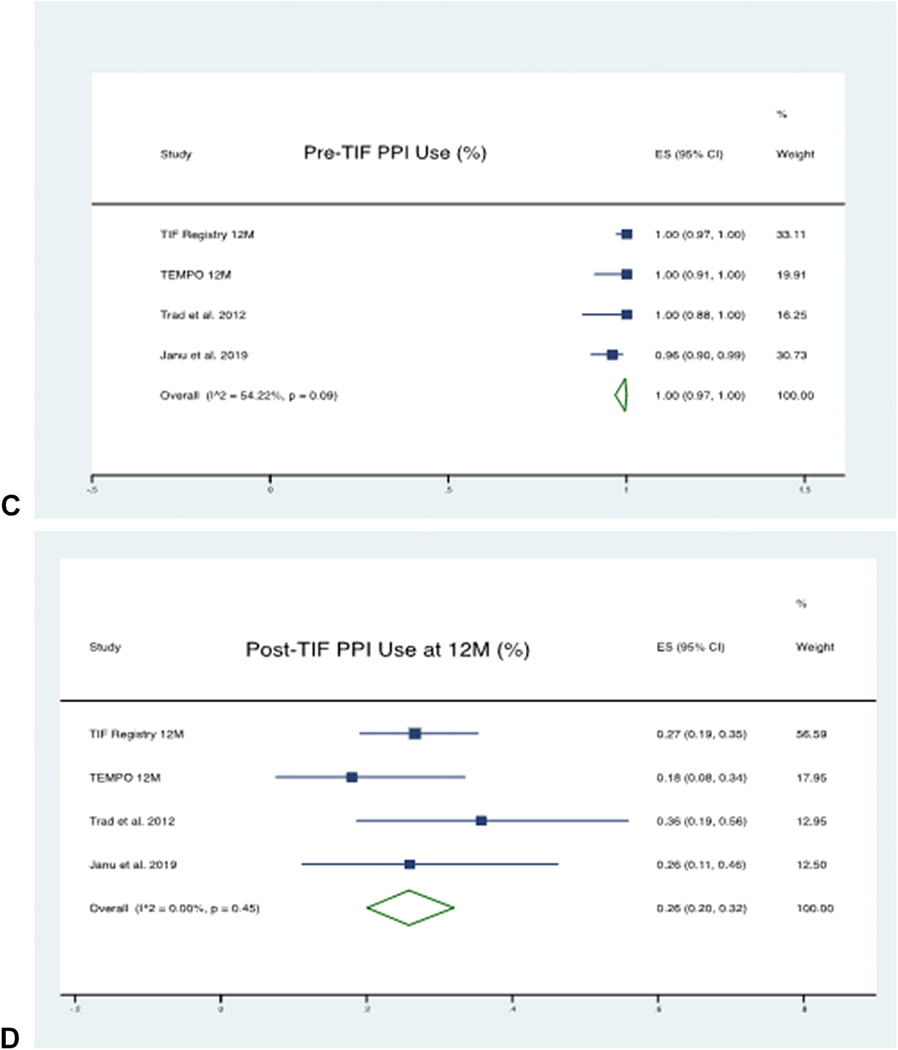
At 12 month follow-up, a total of 218 patients’ data was available from 4 studies. The pooled proportion of patients using PPI Pre-TIF was 100% (95% CI 97% - 100%), which was reduced to 26% (95% CI 20% - 32%) post-TIF. There was no significant heterogeneity between the studies with an I2 statistic of 45% using the random-effects model. The forest plots depicting PPI therapy cessation are shown in Figure 6.
Figure 6 (A-D):
Forest plots of PPI Use Pre and Post TIF at 6 and 12 Month. 6A: Pre-TIF PPI Use (For 6 Month) 6B: Post-TIF PPI Use (At 6 Month) 6C: Pre-TIF PPI Use (For 12 Month) D: Post-TIF PPI Use (At 12 Month)
Patient Satisfaction Pre and Post TIF:
At 6 month follow up, a total of 392 patients’ data was available from 7 studies. The pooled proportion of patients satisfied with their health condition at baseline was 4% (95% CI 2% - 8%) which was improved post-TIF to 73% (95% CI 67% - 79%). There was no significant heterogeneity between the studies with an I2 statistic of 38% using a random-effects model.
At 12 month follow-up, a total of 190 patients’ data was available from 3 studies. The pooled proportion of patients satisfied with their health condition at baseline was 11% (95% CI 3% - 21%) which was significantly improved post-TIF to 75% (95% CI 61% - 87%). There was substantial heterogeneity between the studies with an I2 statistic of 76% using the random-effects model. The forest plots with patient satisfaction are shown in Supplementary Figure 2.
Subgroup Analysis:
A predefined subgroup analysis was performed for patients with atypical GERD symptoms and hiatal hernia size > 2 cm who underwent cTIF. Out of 5 studies reporting outcomes of cTIF,27–31 one study was excluded from the subgroup analysis due to incomplete data information.31
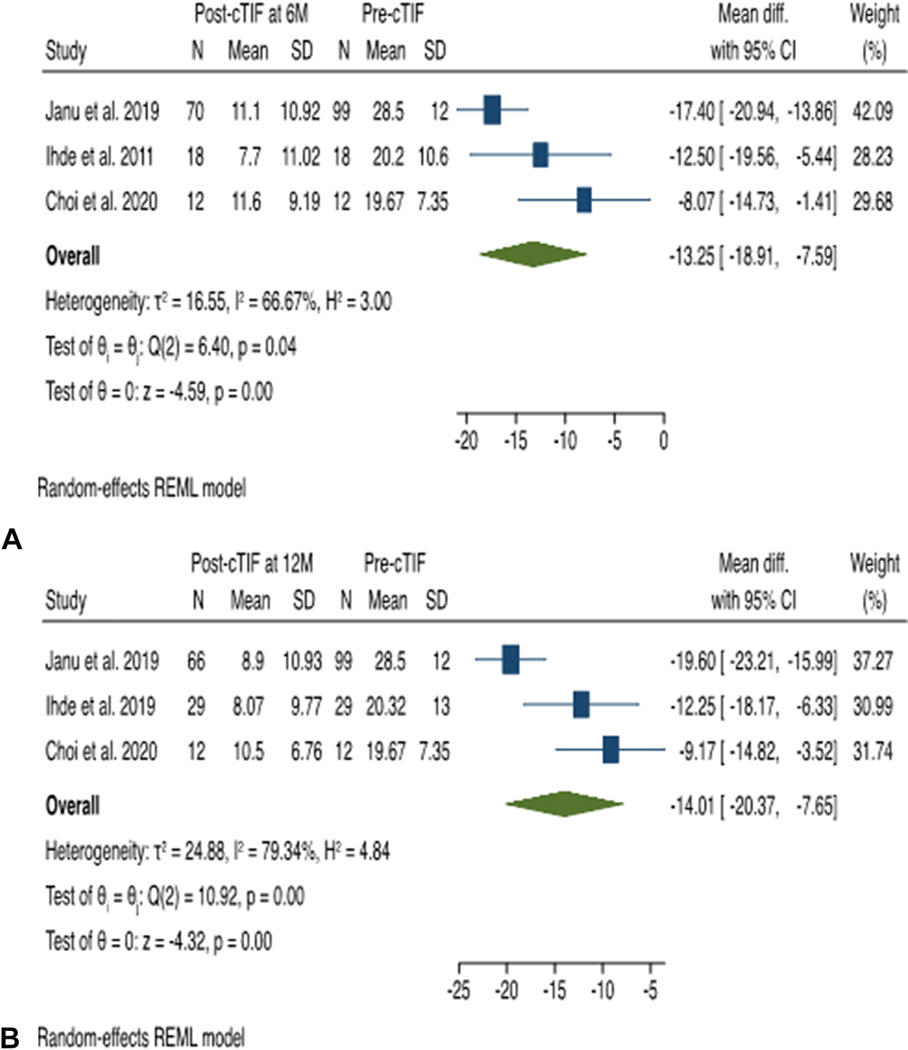
At 6 month follow-up, a total of 100 patients’ data were available from 3 studies. The mean RSI score post-cTIF procedure decreased below the normality threshold of 13 for all studies. The RSI score decreased after cTIF procedure compared with pre-cTIF with a mean difference of −13.25 (95% CI −7.59 to −18.91), in favor of the cTIF procedure. The studies had moderate heterogeneity, with an I2 statistic of 56% using the random-effects model.
At 12 month follow-up, a total of 107 patients’ data were available from 3 studies. The mean RSI score post-cTIF procedure decreased below the normality threshold of 13 for all studies. The RSI score decreased after cTIF procedure compared with pre-cTIF with a mean difference of −14.01 (95% CI −7.65 to −20.37), in favor of the cTIF procedure. There was considerable heterogeneity among the studies, with an I2 statistic of 79% using the random-effects model. (Figure 7)
Figure 7 (A & B):
Effect Estimate of Reflux Symptom Index (RSI) score of cTIF. 7A: At 6 Month 7B: At 12 Month
Heterogeneity and Publication Bias:
All results with moderate to considerable heterogeneity were further investigated. We did not find any explainable causes of heterogeneity following the Cochrane Handbook guide.18 A sensitivity analysis was also performed, omitting one study sequentially on a plot that did not change the effect as the 95% CI overlapped. The sensitivity analysis for the primary outcome of RSI score at 6, and 12 month post-TIF is shown in Supplementary Figures 3A & 3B.
Funnel plots to assess publication bias showed visual asymmetry, but it was difficult to distinguish chance from real asymmetry as there were less than 10 studies for each meta-analysis in our systematic review.18 For the same reason, further statistical assessment of this visual asymmetry with Egger’s test was not performed. In addition to chance, moderate to considerable heterogeneity in the studies can explain the visual asymmetry observed in Funnel plots.34 The funnel plots for the primary outcome of RSI score at 6, and 12 month post-TIF are shown in Supplementary Figures 4A & 4B.
Discussion:
Atypical GERD or laryngopharyngeal reflux (LPR), or extraesophageal reflux symptoms represent a disorder that lacks a widely available effective therapy despite posing a significant healthcare burden.3 Our systematic review and meta-analysis of 10 studies containing data of 564 patients demonstrate that TIF is effective in controlling subjective atypical GERD symptoms and patient-centered outcomes at 6- and 12-month intervals. The results were comparable at both time intervals with a reduction of mean RSI score of ~15 points at 6 month and 14.73 points at 12-month post-TIF, respectively. The mean RSI score for each study at both time intervals also crossed the normality threshold of 13 for the RSI score. In addition, there was a significant improvement in patient-centered outcomes. Nearly all patients were on daily PPI for years at the start of the study, but only 19% at 6 month and 26% at 12 month were using daily or occasional PPI following the TIF procedure. More than two-thirds of the patients were satisfied with their health condition after the procedure at 6 month, which was maintained at a one-year follow-up. Persistently high patient satisfaction rate at both time intervals highlights the clinical significance of these results. The feasibility and safety of the procedure were evident from high technical success (99.5%) and low adverse event rate (1.0%).
There have been several well-done meta-analyses which have focused on typical GERD and have maybe looked at other devices or procedures.10,13,35–37 However, this meta-analysis is distinct as it focuses on atypical GERD and only includes currently in practice TIF procedures. Nonetheless our results can be compared with previously published literature. In 2013, Wendling et al., while addressing the overall impact of TIF on GERD indices, reported outcomes from 4 studies with an average follow-up of 7.6 month demonstrating a mean reduction of RSI score of 19.1 (24.5 at baseline to 5.4 after TIF). The pooled complication rate across all studies included in the review was 3.2%.38 Similarly, a subgroup analysis in a study by McCarty et al. in 2018 assessed the efficacy of TIF for refractory GERD finding a mean reduction of RSI score of 14.28 in patients with atypical GERD symptoms and an average follow-up of 15.8 month. They calculated a pooled serious adverse event rate of 2%.9 Despite having a higher number of patients with hiatal hernia size 2 cm who underwent cTIF procedure (129 vs. 18 each), our results show a better safety profile with a pooled adverse event rate of 1.0%. This may be because we included patients with atypical GERD representing different population. Additionally, we only included patients who underwent TIF 2.0. TIF 2.0 has been in use since 2008, but the actual transition from TIF 1.0 to TIF 2.0 happened in 2010–2011. Both of these techniques and the design of the EsophyX device used in them are distinctly different, so combining their outcomes for effectiveness and safety can lead to incorrect conclusions.11
In 2017, the FDA allowed modification of the TIF device Instructions for Use (IFU), permitting TIF to be performed with hiatal hernia repair, similar to surgical fundoplication for this patient population. This procedure is known as cTIF. Studies published in 2019 or after frequently included patients with and without hiatal hernia > 2cm who underwent cTIF, which was less often performed before 2019. We performed a subgroup analysis in our review for patients who underwent cTIF. The results were comparable to the overall patient-centered outcomes of our primary analysis. These results suggest that careful selection of patients for the type of TIF procedure (TIF vs. cTIF) may have contributed to these outcomes. Hiatal hernia size has been demonstrated as a predictor of response following TIF.39,40 The results of our subgroup analysis further support the evidence behind correcting hiatal hernia 2 cm before TIF.
Our study should be interpreted with its limitations. First, the study did not assess objective outcomes following the TIF procedure. There is limited consensus on the utility of objective testing for the diagnosis of atypical GERD and symptom scores are often relied upon. Therefore, our analysis focused on patient-centered outcomes that directly affect patients’ quality of life. Second, the study reports a systematic review of short-term results against atypical GERD symptoms following the TIF procedure. The paucity of data available on the long-term effectiveness of TIF against atypical GERD limited our scope to short-term follow-up. However, it is relevant to point out that the long-term outcomes of the TEMPO trial at 3 and 5 years have demonstrated that TIF provides sustained long-term effectiveness against atypical GERD.41,42 Third, most of the included studies were observational studies. Lastly, high heterogeneity despite efforts to address it, remains a major limitation of our study.
Despite some limitations, our study has several strengths. First, we believe that our effect estimates are informative and more precise than previous studies.9,38 It is because we stratified studies on their follow-up time and avoided overlapping populations by getting results from raw data of studies derived from the same registry.19,21,22,32 More importantly, we have systematically summarized all available data on currently in practice TIF procedures using EsophyX device, namely TIF 2.0 and cTIF. Prior techniques like ELF or TIF 1.0 have not been in use for more than 10 years. Thus, it provides more accurate estimates for the efficacy and tolerability of the procedures available in practice.
In conclusion, the endoscopic fundoplication technique of TIF 2.0 using the EsophyX device is safe and effective in reducing atypical symptoms of GERD and improving patient-centered outcomes. It has the potential to be a minimally invasive treatment option for patients with chronic or refractory atypical GERD who have either failed or want to avoid chronic medical therapy.
Supplementary Material
Supplementary Figure 1: Database Searches
Supplementary Figure 2 (A-D): Patient Satisfaction Pre and Post TIF at 6 and 12 Month. A: Pre-TIF Patient Satisfaction (For 6 Month) B: Post-TIF Patient Satisfaction (At 6 Month) C: Pre-TIF Patient Satisfaction (For 12 Month) D: Post-TIF Patient Satisfaction (At 12 Month)
Supplementary Figure 3 (A & B): Sensitivity Analysis of RSI score at 6, and 12 month post-TIF. A: At 6 Month (1 = TIF Registry 6M; 2 = TEMPO 6M; 3 = Bell et al. 2011; 4 = Barnes et al. 2011; 5 = Janu et al. 2019; 6 = Ihde et al. 2011; 7 = Choi et al. 2020; 8 = Snow et al. 2021) B: At 12 Month (1 = TIF Registry 12M; 2 = TEMPO 12M; 3 = Trad et al. 2012; 4 = Janu et al. 2019; 5 = Ihde et al. 2019; 6 = Choi et al. 2020)
Supplementary Figure 4 (A & B): Funnel plots of RSI score at 6, and 12 month post-TIF. A: At 6 Month B: At 12 Month
ACRONYMS
- GERD
Gastroesophageal Reflux Disease
- TIF
Transoral Incisionless Fundoplication
- RSI
Reflux Symptom Index
- PPI
Proton Pump Inhibitor
- cTIF
TIF with concomitant hiatal hernia repair
- CI
Confidence Interval
- FDA
Food and Drug Administration
- GE
gastroesophageal
- PROSPERO
Prospective Register of Systematic Reviews
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- IHE
Institute of Health Economics
- LPR
Laryngopharyngeal Reflux
Footnotes
Conflict of Interest:
MH: None
JGB: None
UH: None
CB: None
PAB: None
PJ: has received research support from Apollo Endosurgery, Fractyl and GI Dynamics, has served as a consultant to Endogastric Solutions and GI Dynamics, has received an honorarium from Obalon Therapeutics, and has received in-kind support from USGI Medical.
CCT: is a consultant and received research support from Apollo Endosurgery, Boston Scientific, Fujifilm, GI Dynamics, Lumendi, Olympus, USGI Medical. Consultant for Medtronic and Fractyl. Institutional research grants from Aspire Bariatrics and ERBE. General partner for BlueFlame Healthcare. Founder/Consultant/Board Member for Envision Endoscopy, Enterasense and GI Windows.
References:
- 1.El-Serag HB, Sweet S, Winchester CC. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koufman JA. The Otolaryngologic Manifestations of Gastroesophageal Reflux Disease (GERD): A Clinical Investigation of 225 Patients Using Ambulatory 24-Hour pH Monitoring and an Experimental Investigation of the Role of Acid and Pepsin in the Development of Laryngeal. Laryngoscope. 1991;101(4):1–78. doi: 10.1002/lary.1991.101.s53.1 [DOI] [PubMed] [Google Scholar]
- 3.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. American Journal of Gastroenterology. 2013;108(6):905–911. doi: 10.1038/ajg.2013.69 [DOI] [PubMed] [Google Scholar]
- 4.Spantideas N, Drosou E, Bougea A, AlAbdulwahed R. Proton Pump Inhibitors for the Treatment of Laryngopharyngeal Reflux. A Systematic Review. Published online 2019. doi: 10.1016/j.jvoice.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Catania RA, Kavic SM, Scott Roth J, et al. Laparoscopic Nissen Fundoplication Effectively Relieves Symptoms in Patients with Laryngopharyngeal Reflux. doi: 10.1007/s11605-007-0318-5 [DOI] [PubMed] [Google Scholar]
- 6.CJ W, MB H, K B, GN P, PC B, JA K. Fundoplication for laryngopharyngeal reflux disease. J Am Coll Surg. 2004;199(1):23–30. doi: 10.1016/J.JAMCOLLSURG.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 7.Ciovica R, Gadenstätter M, Klingler A, Lechner W, Riedl O, Schwab GP. Quality of Life in GERD Patients: Medical Treatment Versus Antireflux Surgery. doi: 10.1016/j.gassur.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 8.Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: The LOTUS randomized clinical trial. JAMA - Journal of the American Medical Association. 2011;305(19):1969–1977. doi: 10.1001/jama.2011.626 [DOI] [PubMed] [Google Scholar]
- 9.McCarty TR, Itidiare M, Njei B, Rustagi T. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: A systematic review and meta-analysis. Endoscopy. 2018;50(7):708–725. doi: 10.1055/a-0576-6589 [DOI] [PubMed] [Google Scholar]
- 10.Testoni S, Hassan C, Mazzoleni G, et al. Long-term outcomes of transoral incisionless fundoplication for gastro-esophageal reflux disease: systematic-review and meta-analysis. Endosc Int Open. 2021;09(02):E239–E246. doi: 10.1055/a-1322-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihde GM. The evolution of TIF: transoral incisionless fundoplication. Therap Adv Gastroenterol. 2020;13:1–16. doi: 10.1177/1756284820924206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerson L, Stouch B, Lobonåiu A. Transoral incisionless fundoplication (tif 2.0): A meta-analysis of three randomized, controlled clinical trials. Chirurgia (Romania). 2018;113(2):173–184. doi: 10.21614/chirurgia.113.2.173 [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Chen S, Zhao H, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc. 2017;31(3):1032–1044. doi: 10.1007/s00464-016-5111-7 [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/JAMA.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.JAC S, J S, MJ P, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. doi: 10.1136/BMJ.L4898 [DOI] [PubMed] [Google Scholar]
- 16.Institute of Health Economics | About Methodology Development. Accessed September 2, 2021. https://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about
- 17.Belafsky PC, Postma GN, Koufman JA. Validity and Reliability of the Reflux Symptom Index (RSI). Journal of Voice. 2002;16(2):274–277. doi: 10.1016/S0892-1997(02)00097-8 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (Editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane, 2021. Available from Www.Training.Cochrane.Org/Handbook. [Google Scholar]
- 19.=Transoral Incisionless Fundoplication (TIF) Registry Study for Treatment of Gastroesophageal Reflux Disease (GERD) - Full Text View - ClinicalTrials.gov. Accessed August 15, 2021. https://clinicaltrials.gov/ct2/show/NCT01118585
- 20.Bell RCW, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215(6):794–809. doi: 10.1016/j.jamcollsurg.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 21.Bell RCW, Fox MA, Barnes WE, et al. Univariate and multivariate analyses of preoperative factors influencing symptomatic outcomes of transoral fundoplication. Surg Endosc. 2014;28(10):2949–2958. doi: 10.1007/s00464-014-3557-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson EB, Barnes WE, Mavrelis PG, et al. The effects of transoral incisionless fundoplication on chronic GERD patients: 12-month prospective multicenter experience. Surg Laparosc Endosc Percutan Tech. 2014;24(1):36–46. doi: 10.1097/SLE.0b013e3182a2b05c [DOI] [PubMed] [Google Scholar]
- 23.Trad KS, Simoni G, Barnes WE, et al. Efficacy of transoral fundoplication for treatment of chronic gastroesophageal reflux disease incompletely controlled with high-dose proton-pump inhibitors therapy: A randomized, multicenter, open label, crossover study. BMC Gastroenterol. 2014;14(1):1–12. doi: 10.1186/1471-230X-14-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell RCW, Freeman KD. Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc. 2011;25(6):1975–1984. doi: 10.1007/s00464-010-1497-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trad KS, Turgeon DG, Deljkich E. Long-term outcomes after transoral incisionless fundoplication in patients with GERD and LPR symptoms. Surg Endosc. 2012;26(3):650–660. doi: 10.1007/s00464-011-1932-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes WE, Hoddinott KM, Mundy S, Williams M. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov. 2011;18(2):119–129. doi: 10.1177/1553350610392067 [DOI] [PubMed] [Google Scholar]
- 27.Janu P, Shughoury AB, Venkat K, et al. Laparoscopic Hiatal Hernia Repair Followed by Transoral Incisionless Fundoplication With EsophyX Device (HH + TIF): Efficacy and Safety in Two Community Hospitals. Surg Innov. 2019;26(6):675–686. doi: 10.1177/1553350619869449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihde GM, Besancon K, Deljkich E. Short-term safety and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease. Am J Surg. 2011;202(6):740–747. doi: 10.1016/j.amjsurg.2011.06.035 [DOI] [PubMed] [Google Scholar]
- 29.Ihde GM, Pena C, Scitern C, Brewer S. pH scores in hiatal repair with transoral incisionless fundoplication. Journal of the Society of Laparoendoscopic Surgeons. 2019;23(1). doi: 10.4293/JSLS.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi AY, Roccato MK, Samarasena JB, et al. Novel Interdisciplinary Approach to GERD: Concomitant Laparoscopic Hiatal Hernia Repair with Transoral Incisionless Fundoplication. J Am Coll Surg. 2021;232(3):309–318. doi: 10.1016/j.jamcollsurg.2020.11.021 [DOI] [PubMed] [Google Scholar]
- 31.Snow GE, Dbouk M, Akst LM, et al. Response of Laryngopharyngeal Symptoms to Transoral Incisionless Fundoplication in Patients with Refractory Proven Gastroesophageal Reflux: https://doi-org.ezp-prod1.hul.harvard.edu/101177/00034894211037414. 2021;(0):0.doi:10.1177/00034894211037414 [DOI] [PubMed]
- 32.Bell RCW, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215(6):794–809. doi: 10.1016/j.jamcollsurg.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 33.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Accessed September 4, 2022. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 34.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Online). 2011;343(7818):1–8. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 35.Chandan S, Mohan BP, Khan SR, et al. Clinical efficacy and safety of magnetic sphincter augmentation (MSA) and transoral incisionless fundoplication (TIF2) in refractory gastroesophageal reflux disease (GERD): a systematic review and meta-analysis. Endosc Int Open. 2021;9(4):E583. doi: 10.1055/A-1352-2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie P, Yan J, Ye L, et al. Efficacy of different endoscopic treatments in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Surg Endosc. 2021;35(4):1500–1510. doi: 10.1007/S00464-021-08386-1/TABLES/2 [DOI] [PubMed] [Google Scholar]
- 37.Gawron AJ, Bell R, Abu Dayyeh BK, et al. Surgical and endoscopic management options for patients with GERD based on proton pump inhibitor symptom response: recommendations from an expert U.S. panel. Gastrointest Endosc. 2020;92(1):78–87.e2. doi: 10.1016/J.GIE.2020.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendling MR, Melvin WS, Perry KA. Impact of transoral incisionless fundoplication (TIF) on subjective and objective GERD indices: A systematic review of the published literature. Surg Endosc. 2013;27(10):3754–3761. doi: 10.1007/s00464-013-2961-0 [DOI] [PubMed] [Google Scholar]
- 39.Barnes WE, Hoddinott KM, Mundy S, Williams M. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov. 2011;18(2):119–129. doi: 10.1177/1553350610392067 [DOI] [PubMed] [Google Scholar]
- 40.Testoni PA, Vailati C, Testoni S, Corsetti M. Transoral incisionless fundoplication (TIF 2.0) with EsophyX for gastroesophageal reflux disease: Long-term results and findings affecting outcome. Surg Endosc. 2012;26(5):1425–1435. doi: 10.1007/s00464-011-2050-1 [DOI] [PubMed] [Google Scholar]
- 41.Trad KS, Fox MA, Simoni G, et al. Transoral fundoplication offers durable symptom control for chronic GERD: 3-year report from the TEMPO randomized trial with a crossover arm. Surg Endosc. 2017;31(6):2498–2508. doi: 10.1007/s00464-016-5252-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trad KS, Barnes WE, Prevou ER, et al. The TEMPO trial at 5 years: Transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov. 2018;25(2):149–157. doi: 10.1177/1553350618755214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Database Searches
Supplementary Figure 2 (A-D): Patient Satisfaction Pre and Post TIF at 6 and 12 Month. A: Pre-TIF Patient Satisfaction (For 6 Month) B: Post-TIF Patient Satisfaction (At 6 Month) C: Pre-TIF Patient Satisfaction (For 12 Month) D: Post-TIF Patient Satisfaction (At 12 Month)
Supplementary Figure 3 (A & B): Sensitivity Analysis of RSI score at 6, and 12 month post-TIF. A: At 6 Month (1 = TIF Registry 6M; 2 = TEMPO 6M; 3 = Bell et al. 2011; 4 = Barnes et al. 2011; 5 = Janu et al. 2019; 6 = Ihde et al. 2011; 7 = Choi et al. 2020; 8 = Snow et al. 2021) B: At 12 Month (1 = TIF Registry 12M; 2 = TEMPO 12M; 3 = Trad et al. 2012; 4 = Janu et al. 2019; 5 = Ihde et al. 2019; 6 = Choi et al. 2020)
Supplementary Figure 4 (A & B): Funnel plots of RSI score at 6, and 12 month post-TIF. A: At 6 Month B: At 12 Month