Abstract
Aims
Reimers migration percentage (MP) is a key measure to inform decision-making around the management of hip displacement in cerebral palsy (CP). The aim of this study is to assess validity and inter- and intra-rater reliability of a novel method of measuring MP using a smart phone app (HipScreen (HS) app).
Methods
A total of 20 pelvis radiographs (40 hips) were used to measure MP by using the HS app. Measurements were performed by five different members of the multidisciplinary team, with varying levels of expertise in MP measurement. The same measurements were repeated two weeks later. A senior orthopaedic surgeon measured the MP on picture archiving and communication system (PACS) as the gold standard and repeated the measurements using HS app. Pearson’s correlation coefficient (r) was used to compare PACS measurements and all HS app measurements and assess validity. Intraclass correlation coefficient (ICC) was used to assess intra- and inter-rater reliability.
Results
All HS app measurements (from 5 raters at week 0 and week 2 and PACS rater) showed highly significant correlation with the PACS measurements (p < 0.001). Pearson’s correlation coefficient (r) was constantly over 0.9, suggesting high validity. Correlation of all HS app measures from different raters to each other was significant with r > 0.874 and p < 0.001, which also confirms high validity. Both inter- and intra-rater reliability were excellent with ICC > 0.9. In a 95% confidence interval for repeated measurements, the deviation of each specific measurement was less than 4% MP for single measurer and 5% for different measurers.
Conclusion
The HS app provides a valid method to measure hip MP in CP, with excellent inter- and intra-rater reliability across different medical and allied health specialties. This can be used in hip surveillance programmes by interdisciplinary measurers.
Cite this article: Bone Jt Open 2023;4(5):363–369.
Keywords: Cerebral palsy, Hip dislocation, Hip subluxation, Migration percentage, HipScreen, Validity, Reliability, hips, Radiographs, orthopaedic surgeons, Hip displacement, Intraclass correlation coefficient (ICC), Pearson’s correlation coefficient, pelvis, Paediatric orthopaedic, physiotherapists
Introduction
Cerebral palsy (CP) is a major cause of physical disability in high-income countries, with a prevalence of 1.5 to 1.6/1,000 live births and probably higher prevalence in low- and middle-income countries.1 Hip displacement is the second most common musculoskeletal deformity in CP with an incidence of 35% (nearly 1 in 3) and more common in nonambulatory children.2 Without treatment, hips can exhibit progressive lateral displacement in a rate that is dependent upon age, CP subtype, and Gross Motor Function Classification System (GMFCS) level.2,3 If hip migration exceeds 46%, displacement will not regress spontaneously and ultimately hip dislocation will ensue,4 accompanied by flattening of the femoral head by the abductor muscles,5 cartilage degeneration, and/or arthritis.6 Hip dislocation in CP carries a high morbidity as it can cause pain, sitting difficulties, decubitus ulcers, impaired mobility, difficult perineal care, pelvic obliquity, and secondary scoliosis. Although early hip subluxation may be painless, severe subluxation and dislocation are more often painful.7 Hence, effective proactive treatment should be implemented early to avoid these consequences later.
In an attempt to decrease the rate of hip dislocation, hip surveillance programmes were developed internationally, entailing serial clinical and radiological examinations done by physical and/or occupational therapists with the aim of timely referral for orthopaedic assessment and management. Sweden launched the first programme (CPUP) in 1994, followed by Australia in 1997. Norway (CPOP) in 2006, Denmark (CPUP) in 2010, Iceland (CPUP) in 2012, Scotland (CPIPS) in 2013, and others also followed. Surveillance programmes have significantly reduced the rate of hip dislocation and subsequent salvage surgeries by offering early recognition and treatment for hip migration.8-10 This notion is evident when the rate of dislocation was compared in countries where no surveillance is adopted, e.g. Norway before screening,9 Finland,11 and Arab countries.12
An effective hip surveillance programme consists of early detection of CP cases, serial clinical and radiological examinations, followed by prompt referral to specialist orthopaedic surgeons when required.3 Due to the effectiveness of hip surveillance in CP, it has been suggested that it could be extended to non-CP diagnoses in children with limited ambulation13 and beyond skeletal maturity following neurosurgical or orthopaedic interventions, or in cases of pelvic obliquity, progressive scoliosis, deteriorating gait, and/or worsening migration percentage (MP).14,15
Radiological examination for hip screening is done on a standardized anteroposterior (AP) pelvis radiograph showing both hips with the patient supine, symmetrical pelvis, neutral hip add/abduction, and flat lumbar spine.16 MP, as described by Reimers,17 is considered the most important single measure to quantitatively assess femoral head cover by acetabular roof, and is the keystone for hip screening.3 It has the advantage of being relatively easy to measure, minimally affected by the degree of femoral rotation,18 valid, and reliable.19 MP is measured by a clinician who is trained to do so, usually a radiologist, orthopaedic surgeon, or specialist physiotherapist.3
With the advent of technology, several smartphone apps have been developed to make the process easier and more time-efficient. The earliest of these apps was introduced in Sweden (CPUP) aiming to quantify the risk of hip dislocation based on age, GMFCS level, MP, and head-shaft angle.20 Kulkarni et al21 followed by developing the HipScreen app, which offers an easy way of measuring MP. This app has the potential to help the frontline hip screeners making measurements using a mobile phone device.
The aim of our study is twofold: first, to assess validity of HS app measurements by comparing it to the gold standard in everyday practice which is picture archiving and communication system (PACS) measurements, and second, to assess inter- and intrarater reliability by comparing HS app measurements done two weeks apart by five different interdisciplinary measurers with variable experience.
Methods
Upon consultation with our Institutional Review Board, ethical approval was not required for this study. Sample size was calculated using PASS program version 15 (NCSS, USA), setting type-1 error (α) at 0.05 and power at 80%. Calculations produced a sample size of 35 hips. Taking into account a 10% drop-out rate, our study included 40 hips (20 pelvis radiographs).
In total, 100 standardized pelvis radiographs were identified from our surveillance programme and, after taking each fifth radiograph, we had 20 radiographs (40 hips) that were circulated to the study participants. We excluded radiographs with no hip migration (contained hips) or a completely dislocated hip in either one or both sides. In cases where the fifth radiograph was excluded, the next in the sequence was checked for inclusion. Radiographs were anonymized and copied to two PowerPoint slides (PP1 and PP2) with different orders. The classic method of measurement, referencing the lateral border of the acetabulum, was used throughout the whole study, as it was proved to be more reliable than the modified method, referencing the lateral edge of the sourcil (Figure 1).22,23
Fig. 1.
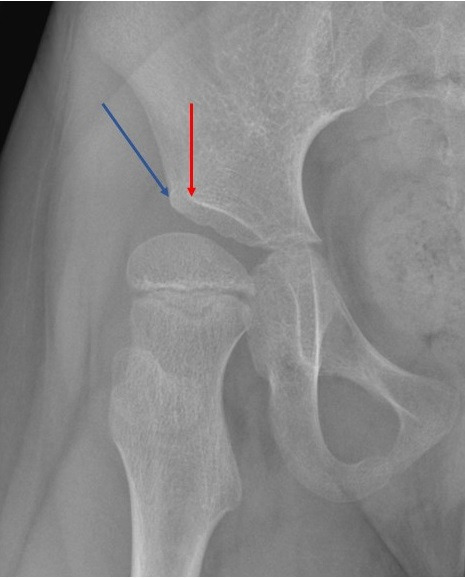
Anteroposterior radiograph of right hip. Red arrow indicates the lateral edge of the sourcil used in the modified method, while the blue arrow indicates the lateral edge of the acetabulum used in the classic method.
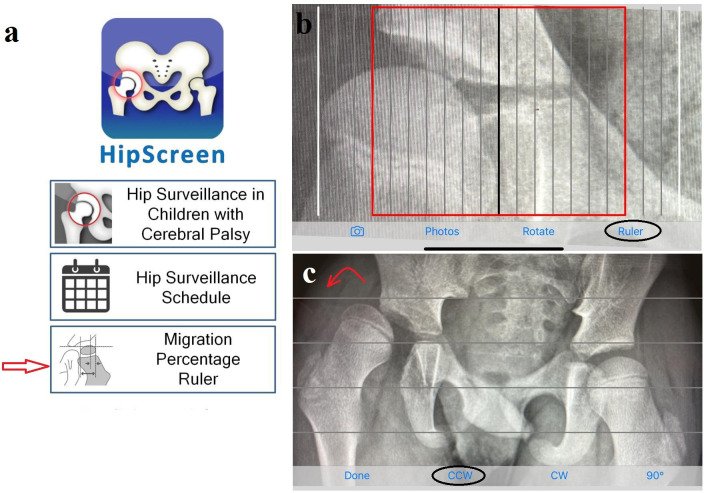
The HS app is available free of charge and compatible with the vast majority of commonly used smartphone operating systems. It measures the MP by dividing the femoral head ossific nucleus into ten partitions, each representing 10%. It allows correction of pelvic obliquity by rotating the whole radiograph either clockwise or counter-clockwise until Hilgenreiner’s line is perfectly horizontal (Figure 2). Study participants were instructed to watch the online tutorial videos,21 followed by a training session organized by the senior author (MK), an experienced orthopaedic surgeon familiar with using the HS app, along with PACS, in his daily practice. As per Kulkarni et al,24 all measures were rounded to the nearest 5%.
Fig. 2.
a) Home screen of the HipScreen app, with red arrow pointing to the ruler function. b) Ruler with the red line indicating 30% migration percentage. c) Rotate function to correct pelvic obliquity; in this radiograph counter-clockwise (CCW) is needed (red curved arrow). CW, clockwise.
Five interdisciplinary measurers in a tertiary referral centre with different levels of expertise in radiological measurements (Paediatric orthopaedic Consultant ‘PC1’, Senior Paediatric Orthopaedic Fellow ‘PF’, Senior Physiotherapist ‘SP’, Senior Radiographer ‘SR’, and Paediatrician in Neurodisability ‘PN’) measured the MP using the HS app on PP1. Measurements were repeated two weeks later, on PP2 slides, aiming to assess inter- and intrarater reliability. A different Paediatric Orthopaedic Consultant ‘PC2’ measured the Reimers MP on the same radiographs, first on PACS (gold-standard measure), then by using the app on PP1, to assess validity. Subsequently, measurements were put in the correct order and used for statistical analysis.
Statistical analysis
Pearson’s correlation coefficient was used to calculate correlation between measurements. Analysis was performed using SPSS statistical package version 25 (IBM, USA). A p-value < 0.05 was considered statistically significant.
Results
All HS app measurements (from five raters at week 0 and week 2) were correlated with PACS measurements, which was considered the gold standard in everyday practice. Correlation coefficient (Pearson’s coefficient r) was consistently over 0.9, suggesting a high correlation. Each correlation was highly significant (p < 0.001). The rater of the PACS measurements (PC2) also correlated with his own HS app measurements at a highly significant level (r = 0.936; p < 0.001). The correlation of HS app measurements of all raters to each other also suggests a high validity (r > 0.874) at a highly significant level (p < 0.001) (Table I).
Table I.
Validity assessment, correlating all HipScreen app measurements to picture archiving and communication system measurement, besides correlating HipScreen app measurements to each other.
| r W0 | PACS | PC2 app | PC1 | PF | SP | SR | PN |
|---|---|---|---|---|---|---|---|
| PACS | 1 | 0.936 | 0.962 | 0.959 | 0.965 | 0.925 | 0.940 |
| PC2 app | 1 | 0.979 | 0.963 | 0.980 | 0.963 | 0.971 | |
| PC1 | 1 | 0.970 | 0.969 | 0.946 | 0.945 | ||
| PF | 1 | 0.984 | 0.934 | 0.960 | |||
| SP | 1 | 0.942 | 0.973 | ||||
| SR | 1 | 0.955 | |||||
| PN | 1 | ||||||
| r W2 | |||||||
| PACS | 1 | 0.936 | 0.939 | 0.952 | 0.965 | 0.904 | 0.919 |
| PC2 app | 1 | 0.964 | 0.977 | 0.974 | 0.944 | 0.956 | |
| PC1 | 1 | 0.943 | 0.947 | 0.906 | 0.942 | ||
| PF | 1 | 0.970 | 0.906 | 0.949 | |||
| SP | 1 | 0.909 | 0.947 | ||||
| SR | 1 | 0.901 | |||||
| PN | 1 |
W0: week 0, W2: week 2, r = Pearson’s correlation coefficient.
PACS, picture archiving and communication system; PC, Paediatric orthopaedic Consultant; PF, Senior Paediatric Orthopaedic Fellow; PN, Paediatrician in Neurodisability; SP, Senior Physiotherapist; SR, Senior Radiographer.
Intraclass correlation (ICC) was used to assess inter-rater reliability (reliability across raters) and intrarater reliability (reliability across time, test and retest after two weeks). Values less than 0.5 indicate poor reliability, values between 0.5 to 0.75 indicate moderate reliability, values between 0.75 to 0.9 indicate good reliability, and values above 0.9 indicate excellent reliability. Both inter- and intrarater reliability were excellent (ICC > 0.9) (Table II).
Table II.
Intraclass correlation for inter- and intrarater reliability; values for all raters at W0 compared to themselves at W2 and to other raters at W2.
| ICC | PC1 W2 | PF W2 | SP W2 | SR W2 | PN W2 |
|---|---|---|---|---|---|
| PC1 W0 | 0.984 | 0.989 | 0.985 | 0.948 | 0.976 |
| PF W0 | 0.984 | 0.984 | 0.978 | 0.964 | 0.968 |
| SP W0 | 0.983 | 0.987 | 0.988 | 0.954 | 0.973 |
| SR W0 | 0.962 | 0.962 | 0.963 | 0.970 | 0.951 |
| PN W0 | 0.966 | 0.981 | 0.980 | 0.951 | 0.973 |
| SEM | |||||
| PC1 W0 | 1.55 | 1.17 | 1.45 | 2.51 | 1.72 |
| PF W0 | 1.46 | 1.66 | 1.76 | 2.09 | 1.99 |
| SP W0 | 1.50 | 1.27 | 1.30 | 2.36 | 1.83 |
| SR W0 | 2.25 | 2.18 | 2.29 | 1.91 | 2.46 |
| PN W0 | 2.13 | 1.54 | 1.68 | 2.44 | 1.83 |
| 95% CI | |||||
| PC1 W0 | 3.03 | 2.29 | 2.85 | 4.92 | 3.38 |
| PF W0 | 2.86 | 3.24 | 3.45 | 4.09 | 3.90 |
| SP W0 | 2.95 | 2.49 | 2.55 | 4.63 | 3.58 |
| SR W0 | 4.41 | 4.26 | 4.48 | 3.74 | 4.83 |
| PN W0 | 4.17 | 3.02 | 3.29 | 4.78 | 3.58 |
CI, confidence interval; ICC, intraclass correlation coefficient; PC, Paediatric orthopaedic Consultant; PF, Senior Paediatric Orthopaedic Fellow; PN, Paediatrician in Neurodisability; SEM, standard error of measurement; SP, Senior Physiotherapist; SR, Senior Radiographer.
Confidence interval (CI) and the standard error of measurement (SEM) are illustrated in Table II. SEM stayed under 2% for the same rater and went up to 2.51% between raters. In a 95% CI of normally distributed values [score ± (1.96*SEM)] for repeated measurements, the deviation of each specific measurement was less than 4% MP for one rater and less than 5% for different raters. For example, if any trained clinician measures 40% MP with the HS app and repeats the measurement 100 times, in 95 of these, measurement will be 40% (standard deviation 3.67%) [40 ± (1.96*1.87)].
The results therefore demonstrate that measurement of hip MP using the HS app is a valid method compared to the gold standard (PACS measurement). In addition, it has excellent inter and intrarater reliability. Accurate repeatability of the measurements also adds to its reliability.
Discussion
MP, as first described by Reimers,17 is currently the gold standard for radiological assessment of hip displacement in CP and the basis for hip surveillance. Measuring MP should be done with caution, as there are many factors that can decrease the accuracy of the measures such as patient positioning,25 defining the lateral border of the acetabulum,26 and clinician’s experience. When MP measurements were done by five different clinicians with different expertise, Demir et al27 found excellent inter- and intrarater reliability. However, Miller et al28 concluded that radiological reporting of hip images of CP children was inadequate to detect hip displacement. They advised educating radiologists to make the report of any value. These contradictory results highlight the importance of training all raters on the accurate measurement of MP. All raters in this study were trained in a standardized manner by the lead author (MK) and watched videos instructing them in the use of the HS app.21
Davids29 reported hip surveillance in CP to be one of the most important advances in the care of CP children in the last three decades. However, its implementation represents a challenge to decentralized healthcare systems and those with limited resources. Development of an easy method, like the HS app, for measuring MP may help alleviate some of these obstacles. Further, in addition to measuring MP, the HS app provides an easy access to educational material on the proper technique of obtaining hip radiographs, interpretation of radiographs, clinical and functional assessments, and different surveillance programme guidelines.21
In our study, we aimed to assess the validity and reliability of the HS app in measuring MP in CP hips. Correlating the app measures, from five different raters at two different timepoints (ten measures), to PACS measures showed highly significant correlation (p < 0.001) and confirmed validity of the app measurement. We got the same results when PACS measurements were correlated to HS app measures done by the same person. HS app measures of all raters correlated to each other, which adds to validity. Inter- and intrarater reliability were both excellent. In their systematic review, Pons et al19 reported good to excellent reliability of radiological MP measurement based on seven studies. The standard error of measurement (SEM) was assessed in three of these studies.25,30,31 It ranged from 5.8% to 12.9% for single measurer and 11% to 22% for different measurers. When compared to our results, HS app has better reliability and lower SEM, being < 4% for single measurer and < 5% for different measurers.
Kulkarni et al24 compared inter- and intrarater reliability of three methods of measuring MP, namely PACS, computer-aided digital tool, and the HS app. They found excellent reliability of all methods. Our results match their conclusion. In their study, the mean absolute difference in HS app measurements was not significant (2.2% for single rater, 3.6% between raters). This similarly echoes our results of SEM < 4% and < 5% for single and multiple measurers, respectively. The measurers in Kulkarni et al’s24 study were two paediatric orthopaedic surgeons and one paediatric orthopaedic fellow, but in our study, we included other professionals involved in the care of CP patients. Kulkarni et al24 also investigated the time efficiency of their three methods of measurements, and found that HS app was more efficient than the PACS measurement.
Our study has some limitations. Images were transferred from PACS to PowerPoint slides, which may have affected the quality of the images and hence the measurements. Raters used different phones with different camera specifications and different software, which again may have affected the results. However, all raters involved in this study have had experience in the diagnosis and management of hip subluxation in CP, which may have led to more accurate results. Assessing reliability of measurements by first line screeners in remote regions will be the scope of another study. The effect of training prior to the study, together with watching the tutorial videos is not known,21 but some of the variation noted in other studies may reflect a lack of consistent and structured training between measurers. The authors believe that using the HS app, following a short and standardized training session, allows clinicians from varying disciplines, including orthopaedic surgeons, surgical trainees, radiographers, physiotherapists, and paediatricians, to reliably and repeatably measure the hip MP on pelvic radiographs of children with CP.
HS app is a valid method of measurement of MP of the hip in CP. It has excellent inter- and intrarater reliability and can be used on a wide scale, particularly in hip surveillance programmes by interdisciplinary measurers.
Take home message
- HipScreen app is a readily accessible and an easy method in measurement of hip migration in cerebral palsy.
- It is a valid method, with excellent inter- and intrarater reliability across different medical and allied health specialties.
- It provides an easy alternative to frontline hip screeners.
Author contributions
J. Amen: Conceptualization, Data curation, Methodology, Writing – original draft.
O. Perkins: Data curation, Formal analysis, Project administration, Writing – original draft.
J. Cadwgan: Conceptualization, Methodology, Data curation, Supervision.
S. J. Cooke: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing.
K. Kafchitsas: Conceptualization, Data curation, Formal analysis, Methodology, Funding aquisition.
M. Kokkinakis: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
None declared.
Data sharing
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
Acknowledgements
We would like to thank Senior Specialist Paediatric Physiotherapist Anita Patel (AP) and Superintendent Radiographer Lucy Clough (LC) for all their work with the radiographic measurements.
Ethical review statement
Given the nature of the study, the IRB approval was not required.
Open access funding
The open access fee for this article was funded by the Department of Orthopedics and Traumatology, University of Mainz, Germany.
Follow J. Amen @JohnFHAmen
Follow S. J. Cooke @stevecookeortho
Supplementary material
Tables showing the actual measurements before statistical analysis.
© 2023 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
John Amen, Email: john.amen@nhs.net, john_amen@yahoo.com.
Oliver Perkins, Email: oliver.perkins2@nhs.net.
Jill Cadwgan, Email: Jill.Cadwgan@gstt.nhs.uk.
Stephen J. Cooke, Email: stephen.cooke@uhcw.nhs.uk.
Konstantinos Kafchitsas, Email: kafchitsas@googlemail.com.
Michail Kokkinakis, Email: Michail.Kokkinakis@gstt.nhs.uk.
References
- 1. McIntyre S, Goldsmith S, Webb A, et al. Global prevalence of cerebral palsy: A systematic analysis. Dev Med Child Neurol. 2022;64(12):1494–1506. doi: 10.1111/dmcn.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soo B, Howard JJ, Boyd RN, et al. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88(1):121–129. doi: 10.2106/JBJS.E.00071. [DOI] [PubMed] [Google Scholar]
- 3. Terjesen T. The natural history of hip development in cerebral palsy. Dev Med Child Neurol. 2012;54(10):951–957. doi: 10.1111/j.1469-8749.2012.04385.x. [DOI] [PubMed] [Google Scholar]
- 4. Wordie SJ, Bugler KE, Bessell PR, Robb JE, Gaston MS. Hip displacement in children with cerebral palsy. Bone Joint J. 2021;103-B(2):411–414. doi: 10.1302/0301-620X.103B2.BJJ-2020-1528.R1. [DOI] [PubMed] [Google Scholar]
- 5. Beck M, Woo A, Leunig M, Ganz R. Gluteus minimus-induced femoral head deformation in dysplasia of the hip. Acta Orthop Scand. 2001;72(1):13–17. doi: 10.1080/000164701753606626. [DOI] [PubMed] [Google Scholar]
- 6. Ulusaloglu AC, Asma A, Rogers KJ, Shrader MW, Miller F, Howard JJ. Femoral Head Deformity Associated With Hip Displacement in Nonambulatory Cerebral Palsy: Results at Skeletal Maturity. J Pediatr Orthop. 2023;43(3):156–161. doi: 10.1097/BPO.0000000000002333. [DOI] [PubMed] [Google Scholar]
- 7. Ramstad K, Terjesen T. Hip pain is more frequent in severe hip displacement: a population-based study of 77 children with cerebral palsy. J Pediatr Orthop B. 2016;25(3):217–221. doi: 10.1097/BPB.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 8. Hägglund G, Andersson S, Düppe H, Lauge-Pedersen H, Nordmark E, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy. The first ten years of a population-based prevention programme. J Bone Joint Surg Br. 2005;87-B(1):95–101. [PubMed] [Google Scholar]
- 9. Hägglund G, Alriksson-Schmidt A, Lauge-Pedersen H, Rodby-Bousquet E, Wagner P, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014;96-B(11):1546–1552. doi: 10.1302/0301-620X.96B11.34385. [DOI] [PubMed] [Google Scholar]
- 10. Wordie SJ, Robb JE, Hägglund G, Bugler KE, Gaston MS. Hip displacement and dislocation in a total population of children with cerebral palsy in Scotland. Bone Joint J. 2020;102-B(3):383–387. doi: 10.1302/0301-620X.102B3.BJJ-2019-1203.R1. [DOI] [PubMed] [Google Scholar]
- 11. Jeglinsky I, Alriksson-Schmidt AI, Hägglund G, Ahonen M. Prevalence and treatment of hip displacement in children with cerebral palsy in Finland. J Child Orthop. 2022;16(2):128–135. doi: 10.1177/18632521221089439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassanein SMA, El-Sobky TA. Towards creation of national cerebral palsy registries in Arab countries: what is missing? World J Pediatr. 2022;18(3):222–224. doi: 10.1007/s12519-021-00510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tillmann R, Maizen C, Bijlsma P, Firth G. Cerebral palsy integrated pathway (CPIP) of hip surveillance, for children with non- cerebral palsy diagnosis? Physiotherapy. 2020;107:e208–e209. doi: 10.1016/j.physio.2020.03.307. [DOI] [Google Scholar]
- 14. Wynter M, Gibson N, Willoughby KL, Love S, Kentish M, Thomason P. National hip surveillance working group. Australian hip surveillance guidelines for children with cerebral palsy: 5‐Year review. Dev Med Child Neurol. 2015;(9):808–820. doi: 10.1111/dmcn.12754. [DOI] [PubMed] [Google Scholar]
- 15. Asma A, Ulusaloglu AC, Shrader MW, Miller F, Rogers KJ, Howard JJ. Hip displacement after triradiate cartilage closure in nonambulatory cerebral palsy: Who needs continued radiographic surveillance? J Bone Joint Surg Am. 2023;105-A(1):27–34. doi: 10.2106/JBJS.22.00648. [DOI] [PubMed] [Google Scholar]
- 16. Dobson F, Boyd RN, Parrott J, Nattrass GR, Graham HK. Hip surveillance in children with cerebral palsy. Impact on the surgical management of spastic hip disease. J Bone Joint Surg Br. 2002;84-B(5):720–726. doi: 10.1302/0301-620x.84b5.12398. [DOI] [PubMed] [Google Scholar]
- 17. Reimers J. The Stability of the Hip in Children: A Radiological Study of the Results of Muscle Surgery in Cerebral Palsy. Acta Orthopaedica Scandinavica. 1980;51(sup184):1–100. doi: 10.3109/ort.1980.51.suppl-184.01. [DOI] [PubMed] [Google Scholar]
- 18. Reimers J, Bialik V. Influence of femoral rotation on the radiological coverage of the femoral head in children. Pediatr Radiol. 1981;10(4):215–218. doi: 10.1007/BF01001585. [DOI] [PubMed] [Google Scholar]
- 19. Pons C, Rémy-Néris O, Médée B, Brochard S. Validity and reliability of radiological methods to assess proximal hip geometry in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2013;55(12):1089–1102. doi: 10.1111/dmcn.12169. [DOI] [PubMed] [Google Scholar]
- 20.No authors listed AppAdvice - CPUP Hip Score. [25 April 2023]. https://appadvice.com/app/cpup-hip-score/1047761003 date last. accessed.
- 21.No authors listed HipScreen. Shriners Hospital for Children. [25 April 2023]. https://www.hipscreen.org date last. accessed.
- 22. Kim SM, Sim EG, Lim SG, Park ES. Reliability of hip migration index in children with cerebral palsy: the classic and modified methods. Ann Rehabil Med. 2012;36(1):33–38. doi: 10.5535/arm.2012.36.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wek C, Chowdhury P, Smith C, Kokkinakis M. Is the Gothic Arch a reliable radiographic landmark for migration percentage in children with cerebral palsy? J Child Orthop. 2020;14(5):397–404. doi: 10.1302/1863-2548.14.200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kulkarni VA, Davids JR, Boyles AD, Cung NQ, Bagley A. Reliability and efficiency of three methods of calculating migration percentage on radiographs for hip surveillance in children with cerebral palsy. J Child Orthop. 2018;12(2):145–151. doi: 10.1302/1863-2548.12.170189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cliffe L, Sharkey D, Charlesworth G, Minford J, Elliott S, Morton RE. Correct positioning for hip radiographs allows reliable measurement of hip displacement in cerebral palsy. Dev Med Child Neurol. 2011;53(6):549–552. doi: 10.1111/j.1469-8749.2011.03970.x. [DOI] [PubMed] [Google Scholar]
- 26. Miller S, Habib E, Bone J, et al. Inter-rater and Intrarater Reliabilities of the Identification of a “Gothic Arch” in the Acetabulum of Children With Cerebral Palsy. J Pediatr Orthop. 2021;41(1):6–10. doi: 10.1097/BPO.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demir N, Demirel M, Turna Ö, Yildizlar D, Demirbaş Ö, Sağlam Y. Effect of clinician’s experience and expertise on the inter- and intra-observer reliability of hip migration index in children with cerebral palsy: A STROBE-compliant retrospective study. Medicine (Baltimore) 2021;100(10):e24538. doi: 10.1097/MD.0000000000024538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller SD, Coates J, Bone JN, Farr J, Mulpuri K. A Review of Radiology Reports From Hip Surveillance Radiographs for Children With Cerebral Palsy. J Pediatr Orthop. 2022;42(7):e742–e746. doi: 10.1097/BPO.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 29. Davids JR. Management of Neuromuscular Hip Dysplasia in Children With Cerebral Palsy: Lessons and Challenges. J Pediatr Orthop. 2018;38 Suppl 1:S21–S27. doi: 10.1097/BPO.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 30. Faraj S, Atherton WG, Stott NS. Inter- and intra-measurer error in the measurement of Reimers’ hip migration percentage. J Bone Joint Surg Br. 2004;86-B(3):434–437. doi: 10.1302/0301-620x.86b3.14094. [DOI] [PubMed] [Google Scholar]
- 31. Parrott J, Boyd RN, Dobson F, et al. Hip displacement in spastic cerebral palsy: repeatability of radiologic measurement. J Pediatr Orthop. 2002;22(5):660–667. [PubMed] [Google Scholar]