Abstract
Background
Depression is the leading cause of global disability and can develop following the change in body image and functional capacity associated with stoma surgery. However, reported prevalence across the literature is unknown. Accordingly, we performed a systematic review and meta-analysis aiming to characterise depressive symptoms after stoma surgery and potential predictive factors.
Methods
PubMed/MEDLINE, Embase, CINAHL and Cochrane Library were searched from respective database inception to 6 March 2023 for studies reporting rates of depressive symptoms after stoma surgery. Risk of bias was assessed using the Downs and Black checklist for non-randomised studies of interventions (NRSIs), and Cochrane RoB2 tool for randomised controlled trials (RCTs). Meta-analysis incorporated meta-regressions and a random-effects model. Registration: PROSPERO, CRD42021262345.
Results
From 5,742 records, 68 studies were included. According to Downs and Black checklist, the 65 NRSIs were of low to moderate methodological quality. According to Cochrane RoB2, the three RCTs ranged from low risk of bias to some concerns of bias. Thirty-eight studies reported rates of depressive symptoms after stoma surgery as a proportion of the respective study populations, and from these, the median rate across all timepoints was 42.9% 42.9% (IQR: 24.2–58.9%). Pooled scores for respective validated depression measures (Hospital Anxiety and Depression Score (HADS), Beck Depression Inventory (BDI), and Patient Health Questionnaire-9 (PHQ-9)) across studies reporting those scores were below clinical thresholds for major depressive disorder according to severity criteria of the respective scores. In the three studies that used the HADS to compare non-stoma versus stoma surgical populations, depressive symptoms were 58% less frequent in non-stoma populations. Region (Asia–Pacific; Europe; Middle East/Africa; North America) was significantly associated with postoperative depressive symptoms (p = 0.002), whereas age (p = 0.592) and sex (p = 0.069) were not.
Conclusions
Depressive symptoms occur in almost half of stoma surgery patients, which is higher than the general population, and many inflammatory bowel disease and colorectal cancer populations outlined in the literature. However, validated measures suggest this is mostly at a level of clinical severity below major depressive disorder. Stoma patient outcomes and postoperative psychosocial adjustment may be enhanced by increased psychological evaluation and care in the perioperative period.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-023-04871-0.
Keywords: Depression, Stoma surgery, Mood, Patients, Nurses
Introduction
Depression is the leading cause of disability worldwide [1] and has an estimated lifetime prevalence of greater than 10% globally [2]. Since the disease can present in various ways [3], many who experience depressive symptoms may go undiagnosed [4]. Both psychosocial and biological stressors are implicated in the complex pathophysiology underlying depression. However, with evidence-based psychotherapy and appropriate pharmacological intervention, prognosis can be favourable [5]. To increase the likelihood of positive outcomes, low socioeconomic and other marginalized populations experiencing depressive symptoms must be identified and provided effective care. This is of particular importance as an association between depression and multiple forms of inequality, including gender [6], and socioeconomic position has been observed [7]. Further, to avoid healthcare inequity, it is imperative that when patients with stomas are from potentially marginalised populations, such as those of minority ethnic backgrounds, they have their sociocultural needs addressed [8], and receive comprehensive and integrative care within a biopsychosocial model [9].
Surgery is a common medical need, with the average person undergoing multiple operations across their lifetime [10]. Patients that experience considerable change in body image or functional capacity following surgery can develop depressive symptoms in the postoperative period [4]. Stoma surgery is frequently conducted, most commonly to treat either cancer, inflammatory bowel disease, or diverticular disease. As stoma surgery and the resultant ostomy bag represent both psychological and biological stressors, the mental health of these patients may be affected during this postoperative psychosocial adjustment period [11, 12]. It is estimated that around 25% of stoma patients experience clinically significant psychological symptoms after surgery [12].
A comprehensive analysis of the rates and factors associated with risk of increased depressive symptoms across the international literature of depressive symptoms after stoma surgery has not been conducted. In particular, descriptions of clinical severity and perioperative change in depressive symptoms, and effect of age, sex, geographic region, type of stoma, stoma permanency, surgical pathology, and postoperative time, have not been investigated within a single review. Accordingly, to inform the biopsychosocial clinical care of patients undergoing stoma surgery and living with stomas worldwide, we performed this systematic literature review and meta-analysis aiming to characterise rates of depressive symptoms after stoma surgery, describe clinical severity and perioperative change in these symptoms, and also identify potential predictive factors such as age, sex, geographic region, type of stoma, stoma permanency, surgical pathology, and time after surgery.
Methods
The methods protocol for this study was generated prior to its conduct. The protocol was prospectively submitted for registration with PROSPERO (number CRD42021262345), and follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) [13] and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) [14] reporting guidelines.
Search strategy and selection criteria
The population, intervention, comparator group, outcome (PICO) framework was used to formulate the research question and inclusion criteria [15]. The population comprised patients of all ages undergoing surgery resulting in a stoma in any country. The intervention was surgery producing a stoma. There was no overall comparator group, however comparisons were made between various sub-populations of stoma patients. Outcomes included measures of depressive symptoms. Editorials, perspectives, letters, and conference abstracts were considered inappropriate for analysis, and were excluded.
PubMed (incorporating MEDLINE), Embase, CINAHL, and the Cochrane Library were searched from database inception to 6 March 2023 for studies of any design and in any setting that reported rates of depressive symptoms after surgery resulting in a stoma. Publications from any country were included. The search strategies were designed to include DSM-5 requirements for major depressive disorder of either depressed mood or anhedonia [3], and are detailed within Additional file 1: Appendix 1. Searches were not limited by language and no publication restrictions were implemented. During the process of searching, 14 full-texts could not be obtained.
Data extraction
Two reviewers independently screened titles and abstracts, reviewed full texts, and extracted data using a standard extraction form. Screening of titles and abstracts was facilitated via a web application (Rayyan, Qatar Computing Research Institute, Ar-Rayyan, Qatar) [16]. Disagreements were resolved by consensus. The extracted data included research design, study setting, population characteristics, intervention characteristics, comparator characteristics, timeframe for follow-up, quantitative and qualitative outcomes, source of funding and reported conflicts of interest, methodological quality information, and other information relevant to the review questions. Data were synthesised in narrative and tabular formats. The primary outcome was rates of depressive symptoms after stoma surgery. Effect on depressive symptoms was investigated for age, sex, region, before versus after stoma surgery, stoma versus no stoma populations, colostomy versus ileostomy, permanent versus temporary stoma, surgical pathology, and time after surgery.
Data analysis
Data analyses were performed using Stata Statistical Software: Release 15.1 College Station, TX: StataCorp LP. To evaluate heterogeneity, we used the I2 statistic (with I2 > 50% indicating significant heterogeneity) and Cochran’s Q p value (with p < 0.05 indicating significant heterogeneity). A random-effects model was used throughout. A p value of < 0.05 denoted statistical significance. A Funnel plot was constructed for each variable to test for publication bias. An Egger’s Test was performed for each variable to test for small study effects. A variable was included in the meta-analysis if ≥ 2 articles meeting inclusion criteria reported sufficient data for that variable.
Prevalence of depressive symptoms (with 95% confidence intervals (CIs)) were calculated for each study, and all studies were combined within a Forest plot. Predictors of mean age, male sex, and region of procedure (with 95% confidence intervals) were presented in a Forest plot. Within this study, regions were split into four groups: Asia–Pacific; Europe; Middle East/Africa; and North America. Meta-regressions were performed incorporating the prevalence of depression versus these respective predictors. Odds of experiencing depressive symptoms before and after stoma surgery, and for stoma versus non-stoma patients, were calculated for each respective study (with 95% CIs), then all respective studies were combined within a Forest plot. Mean differences regarding depressive symptoms for stoma versus non-stoma patients (measured via the Hospital Anxiety and Depression Scale (HADS)) [17], and colostomy versus ileostomy patients, were calculated for each respective study (with 95% CIs), and all respective studies were combined within a Forest plot.
Methodological quality was independently assessed by two reviewers using validated tools. The Downs and Black checklist [18] was used for risk of bias assessment for included non-randomised studies of interventions (NRSIs). This checklist evaluates risk of confounding and selection bias, methods used to ascertain exposures and outcomes, and selection of the reported results from among multiple measurements or analyses of specified outcomes. The subsections within this checklist incorporate measurements of study reporting, external validity, internal validity, bias, confounding, and statistical power. Within the original version of the Downs and Black checklist that was used, no specific scoring system or cut-offs are specified, with the independent reviewers conducting the critical appraisal left to make an overall assessment of the study’s methodological quality and risk of bias based on a total score out of 32 [18]. Version 2 of the Cochrane tool for assessing risk of bias in randomised trials [19] was used to critically appraise any included randomised controlled trials (RCTs).
Results
Study characteristics
Searches identified a total of 7,020 records (6,032 unique reports), from which 333 full-text articles were retrieved and 68 of these studies were included (Fig. 1). Median sample size across all studies was 66 (IQR: 38.8–187). A list of studies excluded at full-text review, with justification of exclusion for each potentially relevant study, can be found in Additional file 1: Appendix 2. The characteristics of the included studies are outlined in Table 1.
Fig. 1.
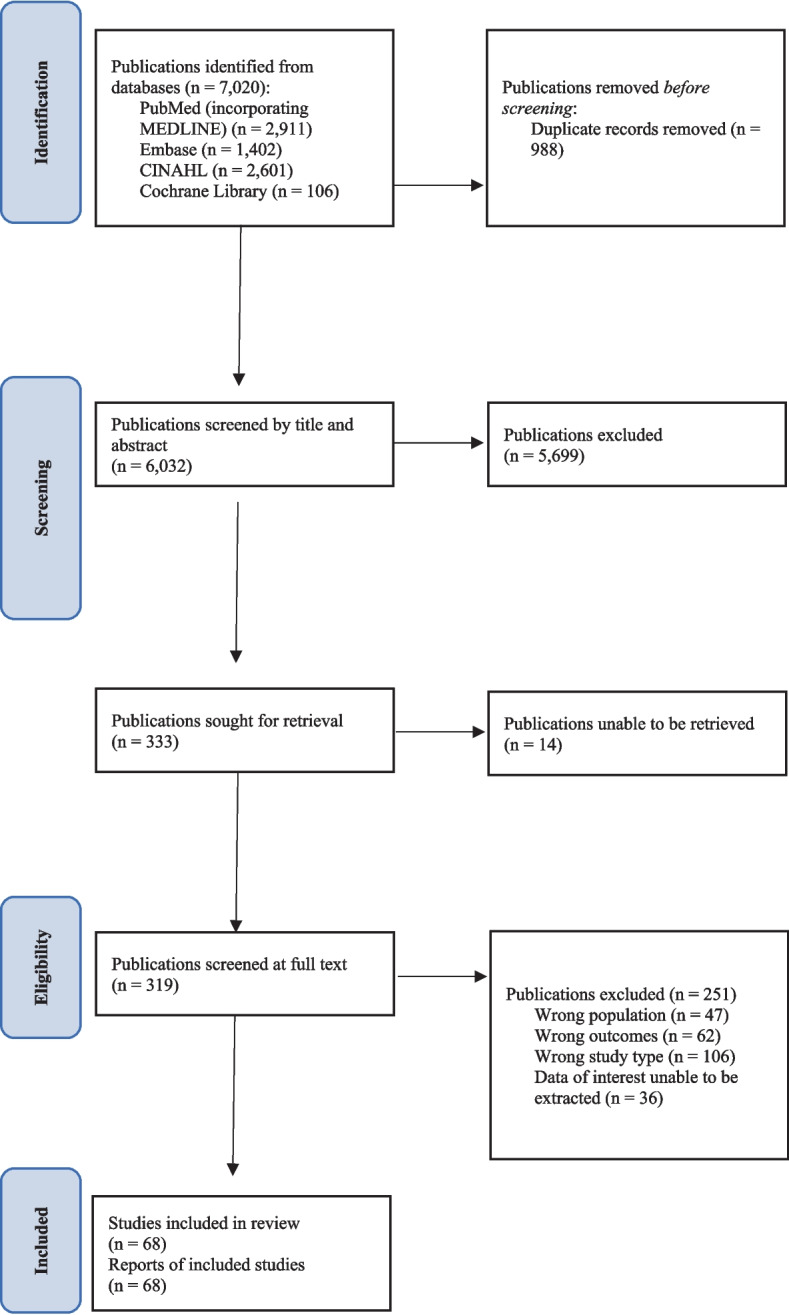
Study selection
Table 1.
Characteristics of included studies reporting depressive symptoms after stoma surgery
| Author | Year | Country | Design | Sample Size | Measure of Depressive Symptoms | Pathology | Stoma Type(s) |
|---|---|---|---|---|---|---|---|
| Abdalla [20] | 2016 | USA | Survey | 398 | PROMIS | IBD | NS |
| Ananthakrishnan [21] | 2013 | USA | Retrospective cohort | 158 | ICD-9 | IBD | NS |
| Anaraki [22] | 2012 | Iran | Survey | 102 | Non-validated | Cancer, IBD, polyps, necrosis, trauma, peritonitis, obstruction, fistula, congenital, radiation | Ileostomy, colostomy, urostomy |
| Anaraki [23] | 2012 (2) | Iran | Survey | 102 | Non-validated | Cancer, IBD, polyps, necrosis, trauma, peritonitis, obstruction, fistula, congenital, radiation | Ileostomy, colostomy, urostomy |
| Armbruster [24] | 2018 | USA | Survey | 41 | Center for Epidemiologic Studies-Depression Scale | Cancer | Colostomy |
| Bahayi [25] | 2018 | Turkey | Retrospective case–control | 50 | Beck Depression Inventory | Colorectal cancer | Colostomy, ileostomy |
| Barisic [26] | 2011 | Serbia | Prospective cohort | 45 | Depression scale of Faecal Incontinence quality of life scale | Rectal cancer | Ileostomy |
| Bau [27] | 2001 | France | Survey | 19 | Non-validated | Fecal incontinence | Urostomy |
| Blackwell [28] | 2020 | UK | Retrospective cohort | 401 | New antidepressant use postoperatively | Crohn’s disease | NS |
| Bossema [29] | 2011 | Netherlands | Survey | 62 | Hospital Anxiety and Depression Scale | Rectal cancer | NS |
| Bullen [30] | 2012 | Australia | Comparative cohort | 22 | Hospital Anxiety and Depression Scale | Colorectal cancer, IBD | NS |
| Chaudhri [31] | 2005 | UK | Randomised Controlled trial | 21 | Hospital Anxiety and Depression Scale | NS | Ileostomy, colostomy |
| Chen [32] | 2013 | USA | Survey | 22 | University of Washington Quality of Life Instrument | Head and neck cancer | Tracheostomy or stoma dependent |
| Coggrave [33] | 2012 | UK | Survey | 91 | Hospital Anxiety and Depression Scale | Spinal cord injury | NS |
| Colquhoun [34] | 2006 | USA | Comparative cohort | 39 | Depression Scale of Fecal Incontinence Quality of Life Score | Fecal incontinence | Colostomy |
| Cotrim [35] | 2008 | Portugal | Comparative cohort | 46 | Hospital Anxiety and Depression Scale | Colorectal cancer | NS |
| Davidson [36] | 2016 | Ireland | Survey | 256 | Non-validated | Cancer, IBD | Ileostomy |
| Davis [37] | 2020 | India | Survey | 55 | Non-validated | Cancer and NS | Ileostomy, colostomy |
| Geng [38] | 2017 | China | Survey | 729 | Hospital Anxiety and Depression Scale | NS | Colostomy, ileostomy, urostomy |
| Gonzalez [39] | 2016 | Sweden | Survey | 545 | Non-validated | Rectal cancer | Colostomy |
| Grant [40] | 2011 | USA | Survey and qualitative | 51 | Non-validated | Colorectal cancer | NS |
| Holzer [41] | 2005 | International | Survey | 257 | Depression Scale of Faecal Incontinence Quality of Life Score | Rectal cancer | Colostomy |
| Hong [42] | 2014 | South Korea | Survey | 65 | Beck Depression Inventory | Cancer, IBD, intestinal obstruction, colon perforation | Ileostomy, colostomy |
| Hornbrook [43] | 2011 | USA | Survey | 284 | Psychological domain of City of Hope Quality of Life | Colorectal cancer | NS |
| Iqbal [44] | 2018 | UK | Survey | 24 | Hospital Anxiety and Depression Scale | Chronic constipation | Ileostomy, colostomy |
| Jayarajah [45] | 2017 | Sri Lanka | Survey | 41 | Patient Health Questionnaire-9 | NS | Ileostomy, colostomy |
| Jayarajah [46] | 2017 (2) | Sri Lanka | Survey | 43 | Non-validated | NS | Ileostomy, colostomy |
| Jin [47] | 2019 | China | Survey | 67 | Hospital Anxiety and Depression scale | Rectal cancer | Colostomy |
| Karakayali [48] | 2016 | Turkey | Comparative cohort | 21 | Fecal Incontinence Quality of Life | Radiation-induced recto-vaginal fistula | Ileostomy |
| Kaltikangas-Jaryinen [49] | 1983 | Finland | Prospective cohort | 66 | Beck Depression Inventory | Colorectal cancer, ulcerative colitis | Colostomy |
| Kaltikangas-Jaryinen [50] | 1984 | Finland | Prospective cohort | 66 | Beck Depression Inventory | Colorectal cancer, ulcerative colitis | Colostomy, ileostomy |
| Ketterer [51] | 2021 | Australia | Survey | 280 | Non-validated | Bowel cancer and NS | Colostomy, ileostomy, urostomy |
| Knowles [52] | 2013 | Australia | Survey | 83 | Hospital Anxiety and Depression Scale | IBD | Colostomy, ileostomy |
| Knowles [53] | 2013 (2) | Australia | Survey | 31 | Hospital Anxiety and Depression Scale | Crohn’s disease | NS |
| Knowles [54] | 2014 | Australia | Survey | 150 | Hospital Anxiety and Depression scale | IBD, cancer, diverticular disease, and NS | Ileostomy, colostomy |
| Koc [55] | 2022 | Turkey | Randomised Controlled Trial | 214 | Hospital Anxiety and Depression Scale | IBD, cancer, polyposis syndromes, perianal benign diseases | Ileostomy, colostomy |
| Krouse [56] | 2009 | USA | Survey | 246 | Non-validated | Rectal cancer | Colostomy, ileostomy |
| Krouse [57] | 2016 | USA | Survey | 38 | Hospital Anxiety and Depression scale | Rectal or bladder cancer | Colostomy, ileostomy, urostomy |
| Lamb [58] | 2019 | UK | Survey | 19 | Non-validated | NS | NS |
| Leminski [59] | 2021 | Poland | Comparative cohort | 95 | Hospital Anxiety and Depression Scale | Bladder cancer | Uterostomy, ileostomy |
| Lim [60] | 2019 | Singapore | Randomised Controlled Trial | 24 | Hospital Anxiety and Depression Scale | Colorectal cancer | Ileostomy, colostomy |
| Liu [61] | 2021 | China | Case–control | 63 | Hamilton Depression Rating Scale | Colorectal cancer | Colostomy |
| Lowe [62] | 2019 | UK | Survey | 92 | Patient Health Questionnaire-9 | NS | Colostomy, ileostomy, urostomy |
| MacDonald [63] | 1985 | UK | Qualitative study | 265 | Interviews | Rectal cancer | Colostomy |
| Mohamed [64] | 2021 | USA | Qualitative study | 30 | Interviews | Bladder and colorectal cancer | Colostomy, ileostomy, urostomy |
| Mols [65] | 2014 | Netherlands | Survey | 407 | Hospital Anxiety and Depression Scale | Rectal cancer | NS |
| Norton [66] | 2005 | UK | Survey | 66 | Hopital Anxiety and Depression Scale | Fecal incontinence | Colostomy |
| Park [67] | 2018 | South Korea | Survey | 217 | Center for Epidemiological Studies Depression Scale | NS | NS |
| Portier [68] | 2005 | France | Observational cohort | 18 | Depression scale of Fecal Incontinence Quality of Life | Rectal and anal cancer | Colostomy |
| Powell-Chandler [69] | 2020 | UK | Survey | 371 | Patient Health Questionnaire-9 | NS | NS |
| Rafiei [70] | 2017 | Iran | Survey | 70 | Depression, Anxiety, Stress Scale 21 | NS | Colostomy, ileostomy |
| Rafiei [71] | 2019 | Iran | Survey | 70 | Depression, Anxiety, Stress Scale 21 | NS | Colostomy, ileostomy |
| Ramer [72] | 1992 | USA | Observational cohort | 12 | Depression dimension of Brief Symptom Inventory | NS | Colostomy |
| Reese [73] | 2014 | USA | Survey | 25 | Center for Epidemiologic Studies Depression Scale-Short Form | Colorectal cancer | Colostomy, ileostomy |
| Repic [74] | 2018 | Serbia | Survey | 67 | Depression scale of Quality of Life Questionnaire for a Patient with an Ostomy | NS | Colostomy, ileostomy, urostomy |
| Richbourg [75] | 2007 | USA | Survey | 43 | Non-validated | NS | Colostomy, ileostomy, urostomy |
| Rud [76] | 2022 | Denmark | Survey | 178 | Major Depression Inventory | IBD, colorectal cancer, chronic constipation, surgical complications, ischemic bowel disease, familial adenomatous polyposis, diverticulitis, incarceration | Ileostomy |
| Sceats [77] | 2020 | USA | Retrospective cohort | 1965 | ICD-9 and ICD-10 codes | IBD | NS |
| Sharpe [78] | 2011 | Australia | Comparative cohort | 34 | Hospital Anxiety and Depression Scale | Colorectal cancer | NS |
| Shrestha [79] | 2022 | Nepal | Prospective cohort | 116 | Hospital Anxiety and Depression Scale | Cancer, IBD, trauma, complication of radiotherapy, bowel obstruction, intestinal tuberculosis | Ileostomy, colostomy, urostomy |
| Sivero [80] | 2022 | Italy | Retrospective cohort | 12 | Non-validated | Colorectal cancer | Colostomy |
| Song [81] | 2020 | China | Comparative cohort | 148 | Hospital Anxiety and Depression Scale | Colorectal cancer | NS |
| Ssewanyana [82] | 2021 | Uganda | Survey | 51 | Patient Health Questionnaire-9 | Intestinal obstruction, colorectal cancer, penetrating abdominal trauma, aganglionic colon and NS | Colostomy, ileostomy |
| Thomas [83] | 1987 | UK | Observational interview | 68 | Non-validated | Bowel cancer, IBD, diverticular disease | NS |
| Wang [84] | 2018 | China | Comparative cohort | 231 | Non-validated | Rectal cancer | Sigmoidostomy |
| Williams [85] | 1983 | UK | Comparative cohort | 38 | Leeds Self Assessment of Depression Scale | Rectal cancer | Colostomy |
| Wirsching [86] | 1975 | Germany | Case–control | 214 | Non-validated | Rectal cancer | Colostomy |
| Zewude [87] | 2021 | Ethiopia | Prospective cohort | 64 | City of Hope Quality of Life – Ostomy Questionnaire | Cancer, obstruction, trauma | Colostomy, ileostomy |
IBD Inflammatory bowel disease, NS Not specified
Depressive symptoms after stoma surgery
Of the included studies, 44 reported rates of depressive symptoms after stoma surgery as a proportion of the respective study populations. Median sample size within these studies was 76.5 (IQR: 43–214.8). From these populations, the median rate of depressive symptoms after stoma surgery was 42.9% (IQR: 24.2–58.9%) across all timepoints after surgery. Overall results of the meta-analyses that were conducted, including the Egger’s tests for small study effects and meta-regressions, are summarised in Table 2.
Table 2.
Results of Egger’s test for small study effects and meta-regressions
| Test | Dataset | Outcome | Predictor | Comparison | Mean difference (95% CI) | Comparison P value | Global P value |
|---|---|---|---|---|---|---|---|
| Egger | Age | Prevalence of depressive symptoms | 0.635 | ||||
| Meta-regression | Age | Prevalence of depressive symptoms | Age | -0.003 (-0.128, 0.007) | 0.592 | ||
| Egger | Age | Age | 0.001 | ||||
| Egger | Gender | Prevalence of depressive symptoms | 0.930 | ||||
| Meta-regression | Gender | Prevalence of depressive symptoms | Proportion Male | 0.410 (-0.034, 0.854) | 0.069 | ||
| Egger | Gender | Gender | 0.224 | ||||
| Egger | Region | Prevalence of depressive symptoms | 0.041 | ||||
| Meta-regression | Region | Prevalence of depressive symptoms | Region | Europe vs Asia–Pacific | 0.037 (-0.115, 0.188) | 0.629 | 0.002 |
| Middle East / Africa vs Asia–Pacific | 0.364 (0.162, 0.566) | 0.001 | |||||
| North America vs Asia–Pacific | -0.033 (-0.220, 0.154) | 0.722 | |||||
| Egger | Before versus after stoma surgery | Odds of depressive symptoms | 0.229 | ||||
| Egger | Other: Stoma versus No stoma | Odds of depressive symptoms | 0.352 | ||||
| Egger | HADS: Stoma versus No stoma | Mean difference of depressive symptoms | 0.461 | ||||
| Egger | Colostomy versus Ileostomy | Mean difference of depressive symptoms | Did not converge |
Depressive symptoms were measured using both validated and non-validated tools across the 68 included studies. The most common validated measures that were used were the Hospital Anxiety and Depression Score (HADS, total score of 21) [17], the Beck Depression Inventory (BDI, total score of 60) [88], and the Patient Health Questionnaire-9 (PHQ-9, total score of 27) [89]; these were used to measure postoperative depressive symptoms in 17, 4, and 4 studies, respectively. Of the included studies, 17 studies measured postoperative depressive symptoms using the HADS and reported extractable mean or median scores across the study cohorts [29–31, 33, 35, 38, 44, 47, 52–54, 57, 60, 65, 66, 78, 81]. For the 14 studies that reported mean HADS scores, the median reported mean HADS score was 5.1 (IQR: 4.5–7.0) [29–31, 35, 38, 44, 47, 52–54, 57, 60, 78, 81]. The other three studies utilising the HADS reported median scores, and the median reported median HADS score was 3 (IQR: 2.75–3) [33, 65, 66]. These HADS scores are within the normal range (0–7) for the HADS scoring criteria, and thus did not meet the clinical threshold for major depressive disorder [17]. Of the included studies, four used the PHQ-9 as a measure of depressive symptoms, however none reported raw PHQ-9 scores, merely rates of depressive symptoms within the study population [45, 62, 69, 82]. Of the four included studies that used the BDI, all reported mean scores [25, 42, 49, 50]. For these, the median reported mean BDI score was 10.0 (IQR: 5.7–14.0), which was within the normal range of the scoring criteria (0–10), whereas the upper quartile falls into the mild mood disturbance range (11–16) [88].
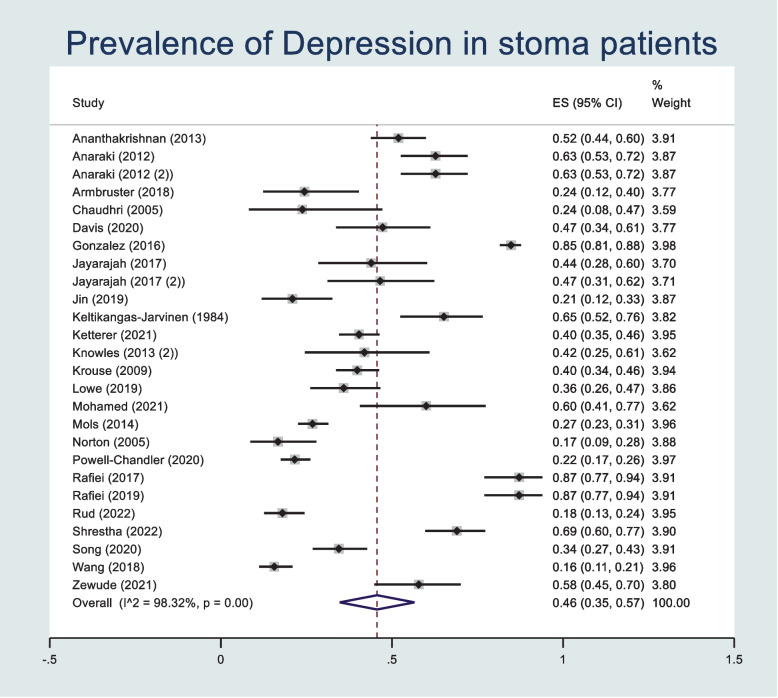
Effect of age
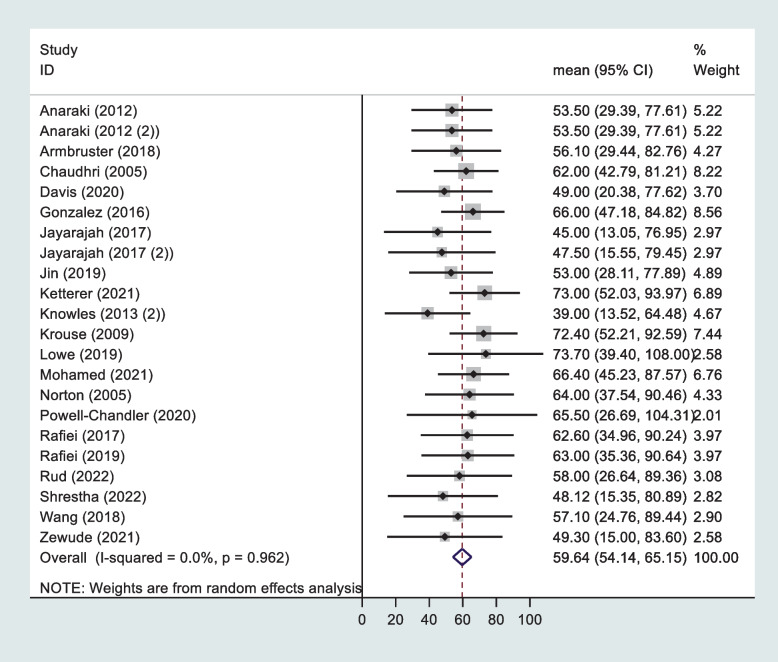
The prevalence of postoperative depressive symptoms was pooled across 27 studies reporting extractable data regarding age using a random effects meta-analysis model. Heterogeneity in the study estimates was assessed using the I-squared statistic (98.3%) and Cochran's Q p value (< 0.001) which showed significant heterogeneity. The overall mean prevalence of depressive symptoms within these studies was 0.46 (95% confidence interval (CI): 0.35, 0.57) (Fig. 2). A Funnel plot shows possible publication bias (Supplementary Fig. 1), however the Egger’s Test does not suggest small study effects (p = 0.635). Means and standard deviations of age were pooled across 22 studies using a random effects meta-analysis model (Fig. 3). Heterogeneity in the study estimates was assessed using the I-squared statistic (0%) and Cochran's Q p value (0.962) which showed no heterogeneity. The overall mean age is 59.6 (95% CI: 54.1, 65.2). A Funnel plot indicated publication bias and an Egger test indicated small study effects (p = 0.001) (Supplementary Fig. 2). A meta-regression was performed to assess the association between prevalence of postoperative depressive symptoms and age as a predictor. No statistically significant association was found (p = 0.592).
Fig. 2.
Forest plot showing prevalence of depressive symptoms after stoma surgery in studies reporting patient age
Fig. 3.
Forest plot summarising mean age and standard deviations in studies with data regarding effect of age on depressive symptoms after stoma surgery
Effect of sex
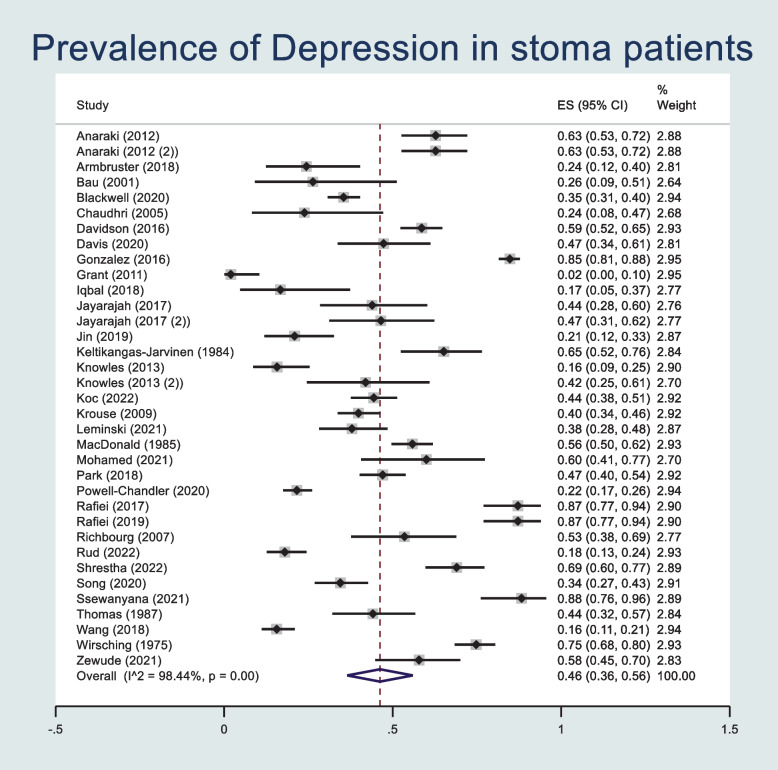
The prevalence of postoperative depressive symptoms was pooled across 35 studies reporting extractable data regarding sex using a random effects meta-analysis model. Heterogeneity in the study estimates was assessed using the I-squared statistic (98.44%) and Cochran’s Q p value (< 0.001) which showed significant heterogeneity, however this was less relevant as a random effects model was used. The overall mean prevalence of depressive symptoms within these studies was 0.46 (95% confidence interval (CI): 0.36, 0.56) (Fig. 4). A Funnel plot shows possible publication bias (Supplementary Fig. 3), however Egger’s Test does not suggest small study effects (p = 0.930). Prevalence of male sex was pooled across the 35 studies using a random effects meta-analysis model (Supplementary Fig. 4). Heterogeneity in the study estimates was assessed using the I-squared statistic (91.21%) and Cochran’s Q p value (< 0.001) which showed significant heterogeneity, however this was less relevant as a random-effects model was used. The overall mean proportion of male sex within the study populations was 0.55 (95% CI: 0.50, 0.60). A Funnel plot indicated publication bias and an Egger test indicated no small study effects (p = 0.226) (Supplementary Fig. 5). A meta-regression was performed to assess the association between prevalence of postoperative depressive symptoms and sex as a predictor. No statistically significant association was found (p = 0.069).
Fig. 4.
Forest plot showing prevalence of depressive symptoms after stoma surgery in studies reporting patient sex
Effect of geographic region
The prevalence of postoperative depressive symptoms was pooled across 44 studies reporting extractable data regarding region using a random effects meta-analysis model. Heterogeneity in the study estimates was assessed using the I-squared statistic (98.6%) and Cochran’s Q p value (< 0.001) which showed significant heterogeneity, however this was less relevant as a random effects model was used. The overall mean prevalence of depressive symptoms within these studies was 0.44 (95% confidence interval (CI): 0.36, 0.52) (Supplementary Fig. 6). A Funnel plot shows possible publication bias (Supplementary Fig. 7) and Egger’s Test does not suggest small study effects (p = 0.041). Meta-regressions were performed to assess the association between prevalence of depressive symptoms and region as a predictor. Within this, study regions were split into four groups: Asia–Pacific; Europe; Middle East/Africa; and North America. There was a statistically significant association between region and postoperative depressive symptoms (p = 0.002). When comparing Europe versus Asia–Pacific regions, the mean difference was 0.037 (-0.115, 0.188), and this was not statistically significant (p = 0.629). When comparing Middle-East/Africa versus Asia–Pacific regions, the mean difference was 0.364 (0.162, 0.566), and this was statistically significant (p = 0.001). When comparing North America versus Asia–Pacific regions, the mean difference was -0.033 (-0.220, 0.154), and this was not statistically significant (p = 0.722).
Before versus after stoma surgery
The odds of experiencing depressive symptoms before versus after stoma surgery were pooled across four studies providing extractable data using a random-effects meta-analysis model. Within these studies, follow-up ranged up to one year after stoma surgery [47]. Heterogeneity in the study estimates was assessed using the I-squared statistic (0%) and Cochran’s Q p value (0.592) which showed no heterogeneity. The overall difference in odds of experiencing depressive symptoms was 24% greater after stoma surgery versus before (1.24, 95% CI: 0.95, 1.62) (Supplementary Fig. 8). A Funnel plot showed no possibility of publication bias (Supplementary Fig. 9), and an Egger’s test indicated no small study effects (0.229).
Stoma versus non-stoma populations
Across four eligible studies, stoma and non-stoma populations were directly compared with regards to odds of experiencing postoperative depressive symptoms. Heterogeneity in the study estimates was assessed using the I-squared statistic (79%) and Cochran’s Q p value (0.003) which showed significant heterogeneity, however this was less relevant as a random-effects model was used. The overall difference in odds of experiencing postoperative depressive symptoms was 27% greater in non-stoma surgical population versus stoma surgical populations (1.27, 95% CI: 1.14, 1.40) (Supplementary Fig. 10). A Funnel plot showed a small possibility of publication bias (Supplementary Fig. 11), and an Egger’s test indicated no small study effects (0.352).
Mean differences in postoperative depressive symptoms, as measured by the HADS [17] in stoma versus non-stoma populations were pooled across three studies providing extractable data using a random-effects meta-analysis model. Heterogeneity in the study estimates was assessed using the I-squared statistic (74.6%) and Cochran’s Q p value (0.02) which showed significant heterogeneity, however this was less relevant as a random-effects model was used. The overall mean difference in postoperative depressive symptoms was 58% less in non-stoma surgical population versus stoma surgical populations (0.42, 95% CI: 0.20, 0.65) (Supplementary Fig. 12). A Funnel plot showed no possibility of publication bias (Supplementary Fig. 13), and an Egger’s test indicated no small study effects (0.461).
Colostomy versus Ileostomy
Mean differences in postoperative depressive symptoms in colostomy versus ileostomy populations were pooled across two studies providing extractable data using a random-effects meta-analysis model. Heterogeneity in the study estimates was assessed using the I-squared statistic (0%) and Cochran’s Q p value (0.804) which showed no heterogeneity. The overall mean difference in postoperative depressive symptoms was 51% less in ileostomy populations versus colostomy populations (0.49, 95% CI: 0.15, 0.84) (Supplementary Fig. 14). A Funnel plot showed no possibility of publication bias (Supplementary Fig. 15), and an Egger’s test did not converge.
Permanent versus temporary stoma
Amongst the included studies, four compared depressive symptoms in cohorts of permanent versus temporary stomas, all using different measures of depression. Blackwell et al. found higher rates of depressive symptoms requiring antidepressant medication in those with permanent stomas versus those with temporary stomas (37.1% versus 33.5%) [28]. Davis et al. found that those with permanent stomas had significantly greater depressive symptom severity than those with temporary stomas (p = 0.023) [37]. Hong et al. compared BDI scores in the two populations, however did not find a statistically significant difference (p = 0.276) [42]. In contrast, Knowles et al. found greater depression severity scores in those with a temporary stoma versus those with a permanent stoma, however it was not stated whether this difference was statistically significant [53].
Effect of surgical pathology
Of the included studies, three compared depressive symptoms in populations with different surgical pathologies [20–22]. Abdalla et al. measured depressive symptoms using the Patient-Reported Outcomes Measurement Information System (PROMIS) in stoma patients who underwent surgery for Crohn’s disease that was active versus in remission, and found greater depression severity in those with active disease [20]. Ananthakrishnan et al. compared rates of depression in cohorts of Crohn’s disease and ulcerative colitis patients, and found higher rates in those with Crohn’s disease (65.9% versus 47%, respectively) [21]. Anaraki et al. compared rates of depression in cancer versus non-cancer patients, and did not find a statistically significant difference (p = 0.19) [22].
Effect of time after surgery
Of the included studies, three reported longitudinal data characterising changes in depressive symptoms with time after surgery [24, 30, 79]. Armbruster et al. measured depressive symptoms using the Center for Epidemiologic Studies-Depression Scale, whereas Bullen et al. and Shrestha utilised the HADS. Armbruster et al. reported depressive symptoms preoperatively, 6 months postoperatively, and 12 months postoperatively in a population of women undergoing pelvic exenterations, and found a change with time that was not significant (p = 0.78) over the three respective time points [24]. Bullen et al. reported depressive symptoms preoperatively and 3 months postoperatively in a population of patients undergoing surgery for colorectal cancer or inflammatory bowel disease, and found that stoma patients experienced significantly greater levels of depressive symptoms throughout the study and increased depression severity with time [30]. Shrestha et al. investigated a range of patients with a stoma and found that proportions of abnormal depressive symptoms within the study population decreased relative to their time from stoma surgery: 71.4% at 2 months to 1 year, 65.1% at 2–5 years, and 58.3% at greater than 6 years [79].
Risk of bias
The 65 included NRSIs that were critically appraised using the Downs and Black checklist [18] were critically appraised by the two independent reviewers to be of low to moderate methodological quality overall. The individual breakdown of these scores can be found in the Additional file 1. Mean scores, representing the mean of the average of the two reviewer scores, were calculated for each category. Calculated means were as follows: total mean score 18.7 out of 32 (SD: 2.9, range 11–25.5); reporting sub-scale mean score 8.4 out of 11 (SD: 1.3, range 4.5–10.5); external validity sub-scale mean score 1.0 out of 3 (SD: 0.8, range 0–3); bias sub-scale mean score 4.3 out of 7 (SD: 0.5, range 3–5); confounding sub-scale mean score 2.5 out of 6 (SD: 0.8, range 0.5–4); power sub-scale mean score 4.7 out of 5 (SD: 0.9, range 1–5). The three RCTs that were critically appraised using version 2 of the Cochrane tool for assessing risk of bias in randomised trials [19] were found to range between having some concerns of risk of bias to low risk of bias [31, 55, 60].
Discussion
To our knowledge, this is the first systematic literature review and meta-analysis to characterize depressive symptoms after stoma surgery and identify potential predictive factors. Depressive symptoms occur in almost half (43% across all timepoints) of stoma surgery patients, however mostly occur at a level of severity below the DSM-5 clinical threshold for major depressive disorder, based on pooled scores of validated depression measures (HADS, BDI, PHQ-9) and their associated severity criteria [3]. Given that this rate of depressive symptoms is significantly higher than the reported rates (under 20%) in the general population, both before and after COVID-19 [90], and also many inflammatory bowel disease [91] and colorectal cancer [92] populations presented in the peer-reviewed literature, it is likely that a proportion of this figure is a direct result of stoma surgery itself, however it is also likely that another proportion is as a result of having and managing a stoma in the short and long-term, and the psychosocial modifications that accompany this. Pooled scores for respective validated depression measures (HADS, BDI, and PHQ-9) across studies reporting those scores were below clinical thresholds for major depressive disorder according to severity criteria of the respective scores. Geographic region was significantly predictive of experiencing postoperative depressive symptoms, whereas age and sex were not. Odds of experiencing depressive symptoms were greater after stoma surgery versus before, and were less frequent in non-stoma surgical populations versus stoma surgical populations for studies conducting this comparison and using the validated HADS measure. However, of those experiencing depression after surgery, depressive symptoms were greater in non-stoma versus stoma populations. Ileostomy patients were less likely to experience postoperative depressive symptoms compared with colostomy patients, as were those with a permanent stoma versus a temporary stoma. Higher rates of depressive symptoms were reported after stoma surgery for patients with active Crohn’s disease versus those without, which may be considered surprising given that stomas are expected to improve quality of life in many of these cases, particularly in Crohn’s disease patients who fail to respond to medical therapy. The evidence for the association between time since surgery and depressive symptoms is mixed, with three studies reporting no association, a positive association, and a negative association respectively [24, 30, 79]. This may be considered surprising as many of the patient populations within these studies involved those with colorectal cancer, as there may be expected to be a period of post-traumatic growth (enduring positive psychological change as a result of adversity, trauma, or challenging life circumstances) [93] after undergoing life events such as stoma surgery, and it could be hypothesised that these patients may be experiencing depressive symptoms that vary depending on their response to their cancer, as opposed to the stoma itself. Methodological quality varied across the included studies as assessed by the Downs and Black and Cochrane RoB 2.0 checklists. According to Downs and Black checklist, the 65 NRSIs were of low to moderate methodological quality. According to Cochrane RoB2, the three RCTs ranged from low risk of bias to some concerns of bias.
The pathophysiology of depression comprises both psychosocial and biological components, and there is strong evidence of a causal link between environmental milieu and depressive symptoms [4]. Psychological adjustment before and after stoma surgery can be conceptualised within Engel’s biopsychosocial model [9] as an aggregation of stressors that patients experience and must manage: physical symptoms of their illness, diagnosis, the informed decision to have surgery, undergoing surgery, and then managing the resultant stoma bag and its associated implications. A considerable biological toll is exacted from these patients as they have been burdened with a significant chronic disease, and undergo major surgery to produce a stoma. The significant morbidity that usually follows stoma surgery is well established [94]. Most of the recent literature in this area relates to the psychosocial changes that occur after surgery [11, 12, 95, 96]. Patients likely undergo a form of grieving as they experience a loss of self-concept during psychological adjustment to their stoma [97]; losing elements of their independence, body-image, self-worth, hobbies, relationships, and intimacy. Despite historical advances in stoma appliances and stomal nursing care, the psychosocial adjustment required by patients is still considerable [98]. In addition to developing stoma care self-efficacy, learning to accept the necessity of the stoma bag and its care despite prominent social stigma and also maintaining interpersonal relationships may be challenging for stoma surgery patients [99]. Throughout the initial adjustment after surgery, strength of social supports may be as important as access to stoma care. Further, cost-effective methods of enhancing shared decision-making may improve patient adherence to management plans as with other forms of chronic disease [100].
Although there were insufficient data to be evaluated within this present study, the effect of socioeconomic status, city versus rural setting, urgency of stoma surgery, cultural differences, strength of familial and community supports, and participation in stoma support groups should be explored in future research for the benefit of stoma care. Given that psychological adjustment to stoma surgery encompasses a wide range of biological, psychological, and socioeconomic factors, it is likely that vulnerable populations will face inequalities in health outcomes [101]. The authors hypothesise that this may provide an explanation for the significant difference between geographical region in occurrence of depressive symptoms after stoma surgery that was found in the present study. Separate to patients who have undergone stoma surgery, variation at a country level in depressive symptoms has been well described in the prominent literature, with individual-level factors, socioeconomic and sociocultural inequality, and population characteristics being identified as having significant association with observed differences [102, 103]. Given the importance of patient demographics, and sociocultural and socioeconomic milieu, on the care and biopsychosocial outcomes of patients who have undergone stoma surgery, it is intuitive that depressive symptoms in this patient population would vary significantly according to geographical region when all of these associated factors also vary significantly according to geographical region. Of note within the present study’s results, although geographic region was found to be a factor that was significantly associated with depressive symptoms after stoma surgery, in direct comparisons between regions, only the Middle East / Africa versus Asia–Pacific was found to be significantly different with regards to depressive symptoms (differences in Europe versus Asia–Pacific and North America versus Asia–Pacific comparisons were not statistically significant). These findings could potentially reflect sociocultural differences and differences in approaches to the perioperative biopsychosocial care of patients undergoing stoma surgery between the two geographical regions. Going forward, these findings should be evaluated through robustly designed studies and findings integrated within evidence-based care that span multiple geographic regions and patient populations.
The greater incidence and severity of mood disorders following colostomy is likely attributable to a myriad of social and biological factors. From a biological perspective, two key concepts have emerged over the past decade which may contribute to the development of mood disorders: inflammation and the microbiome. Under homeostatic conditions, bidirectional communication exists between the resident microbiome and the gut-brain axis, with the microbiome releasing neuroactive molecules including short-chain fatty acids, neurotransmitters (e.g. GABA), hormones, and immune modulators. In addition, the microbiome synthesizes precursors for several neurotransmitters (e.g. L-tryptophan) [104]. These microbiome-derived molecules influence brain development, psychology, and in turn behaviour [105]. Insults which disrupt or alter the microbiome's composition (and hence the type of molecules released) have been associated with alterations in behaviour and the development of mood disorders in humans and animals [104]. Furthermore, patients with depression have shown gut microbiome dysbiosis [106]. Since surgical resection of the colon is a significant insult that unavoidably modifies the microbiome's composition (due to perioperative interventions [bowel preparation and antibiotics] and the surgery itself [resection, transplant, stoma formation]) [107, 108], and the current results demonstrate an increased prevalence of mood disorders following surgery, it is interesting to speculate whether microbiome modifications contribute to the development of mood disorders in these patients, and whether interventions aimed at modifying the microbiome may improve their condition.
Inflammatory processes are implicated in the pathophysiology of depression [1]. The bidirectional relationship between depression and inflammation has changed across evolutionary time, potentially explaining greater rates of depression in the more sanitary environments of modern societies [109]. About a quarter of patients with depression likely display evidence of low-grade inflammation, with over half of patients having mildly elevated C-Reactive Protein levels [110]. Stoma surgery is most frequently conducted in the treatment of colorectal cancer or inflammatory bowel disease. For both diseases, inflammation is key within the pathophysiology [111, 112]. While the psychosocial changes that occur in patients’ lives after stoma surgery are important to the considerable rates of postoperative depressive symptoms, it is possible that there is also a biological component of inflammation within the multifaceted pathogenesis [4]. Further research comparing depressive symptoms after surgery in patient populations with inflammatory diseases versus those without, is needed to clarify the role that inflammation plays in these patients’ postoperative psychological symptoms and whether non-steroidal anti-inflammatory drugs may have utility [113].
This study’s findings add to the growing evidence that mental health issues occur after stoma surgery, and echo the need for greater prioritisation within clinical care [11, 12, 95, 96]. Going forward, perioperative protocols should be modified to optimise psychological adjustment following significant surgery-induced changes in functional capacity or body image. Recent studies have indicated that increased access to psychological support may be beneficial for certain stoma surgery populations [114]. Along with preoperative workup of biological risks that could worsen surgical and anaesthetic outcomes, more rigorous psychological evaluation should be conducted before surgery to screen for individuals vulnerable to worse psychological outcomes. After surgery occurs, psychological evaluation must be undertaken in the immediate postoperative period so that longer-term adjustment can be optimised and evidence-based psychotherapy or pharmacotherapy implemented if appropriate. Given that most of the postoperative depressive symptoms characterised in this study did not reach the clinical threshold for major depressive disorder [3], a multidisciplinary approach should be undertaken early in this patient population to prevent progression past this threshold.
This study has several limitations that are inherent to studies of this kind. Both validated and non-validated measures of depressive symptoms were used within the included studies, and this may have added bias to subsequent findings. The included studies varied widely in their methodological quality, and in their study design ranging from qualitative studies to RCTs; both these factors increase the risk of bias within the conclusions. However, the large evidence base and rigorous adherence to internationally accepted reporting guidelines [13, 14] that we employed should improve the reliability of this study's findings. The diagnostic definition of major depressive disorder within the DSM-5 is multifaceted, reflecting the reality that depression can present in various ways [3]. Accordingly heterogeneity was found regarding the definition of depression in many of the included studies. To minimise any possible resultant bias, our review search strategies were designed in accordance with the DSM-5 definition of major depressive disorder, and emphasis was given to raw rates of depressive symptoms within stoma surgery populations as opposed to their severity.
Conclusion
Depressive symptoms occur in almost half of stoma surgery patients, however mostly occur at a level of severity below the DSM-5 clinical threshold for major depressive disorder. Geographic region may be predictive of these postoperative depressive symptoms. Type of stoma, stoma permanency, and surgical pathology may influence the development of depressive symptoms after stoma surgery. Perioperative care may be enhanced by increased psychological evaluation to screen for vulnerable individuals in the preoperative period, and detect depressive symptoms in the postoperative period so that psychosocial adjustment can be optimised via appropriate psychotherapy. Future research should investigate depressive symptoms before versus after stoma surgery, and sub-analysed and controlled for different pathology indications for surgery.
Supplementary Information
Additional file 1: Appendix 1. PRISMAChecklist. Appendix 2. Studies included at full-text review. Supplementary Figure 1. Funnel plot summarising publication bias for studies with data regarding effect of age on depressive symptoms after stoma surgery. Supplementary Figure 2. Funnel plot summarising publication bias for studies with data regarding mean age and standard deviations in studies with data regarding effect of age on depressive symptoms after stoma surgery. Supplementary Figure 3. Funnel plot summarising publication bias for studies with data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 4. Forest plot summarising prevalence of male sex in studies with data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 5. Funnel plot summarising publication bias for studies with data regarding prevalence of male sex and data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 6. Forest plot showing prevalence of depressive symptoms after stoma surgery in studies reporting by region. Supplementary Figure 7. Funnel plot summarising publication bias for studies with data regarding effect of region on depressive symptoms after stoma surgery. Supplementary Figure 8. Forest plot summarising the odds of experiencing depressive symptoms before versus after stoma surgery. Supplementary Figure 9. Funnel plot summarising publication bias in studies reporting odds of experiencing depressive symptoms before versus after stoma surgery. Supplementary Figure 10. Forest plot summarising the odds of experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 11. Funnel plot summarising publication bias in studies reporting data regarding the odds of experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 12. Forest plot summarising mean differences in experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 13. Funnel plot summarising publication bias in studies reporting data regarding mean differences in experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 14. Forest plot summarising the mean differences in experiencing depressive symptoms after surgery in colostomy versus ileostomy patients. Supplementary Figure 15. Funnel plot summarising publication bias in studies reporting data regarding the mean differences in experiencing depressive symptoms after surgery in colostomy versus ileostomy patients. Supplementary Table 1. Risk of bias assessment using the Downs and Black Checklist for 65 non-randomised studies of interventions included in the present systematic review. Supplementary Table 2. Overall risk of bias assessment using the Cochrane RoB 2.0 checklist for three randomised controlled trials included in the present systematic review.
Acknowledgements
None.
Authors’ contributions
Joshua G. Kovoor = conception and design, acquisition of data, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content, final approval of the version to be published. Jonathan Henry W. Jacobsen= conception and design, acquisition of data, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content, final approval of the version to be published. Brandon Stretton= acquisition of data, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Stephen Bacchi= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Aashray K. Gupta= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Brayden Claridge= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Matthew V. Steen= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Ameya Bhanushali= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Lorenz Bartholomeusz= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Suzanne Edwards= analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Gayatri P. Asokan= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Gopika Asokan= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Amanda McGee= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Christopher D. Ovenden= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Joseph N. Hewitt= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Markus I. Trochsler= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Robert T. Padbury= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Seth W. Perry= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Ma-Li Wong= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Julio Licinio= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Guy J. Maddern= conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published. Peter J. Hewett = conception and design, analysis and interpretation of data, revising the article critically for important intellectual content, final approval of the version to be published.
Funding
This study did not have funding.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
No human or animal participants were involved in this study, and thus no formal ethics approval was required.
Consent for publication
No participants were involved in this study, and thus no consent for publication was required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci Rep. 2018;8(1):2861. doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5) Arlington, VA, USA: American Psychiatric Publishing; 2013. [Google Scholar]
- 4.Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 5.Kraus C, Kadriu B, Lanzenberger R, Zarate CA, Jr, Kasper S. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 2019;9(1):127. doi: 10.1038/s41398-019-0460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracke P, Delaruelle K, Dereuddre R, Van de Velde S. Depression in women and men, cumulative disadvantage and gender inequality in 29 European countries. Soc Sci Med. 2020;267:113354. doi: 10.1016/j.socscimed.2020.113354. [DOI] [PubMed] [Google Scholar]
- 7.Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 8.Cooper-Gamson L. Are we bridging the gap? A review of cultural diversity within stoma care. British Journal of Nursing. 2017;26(17):S24–S28. doi: 10.12968/bjon.2017.26.17.S24. [DOI] [PubMed] [Google Scholar]
- 9.Engel GL. The clinical application of the biopsychosocial model. J Med Philos. 1981;6(2):101–123. doi: 10.1093/jmp/6.2.101. [DOI] [PubMed] [Google Scholar]
- 10.Lee PH, Gawande AA. The number of surgical procedures in an American lifetime in 3 states. J Am Coll Surg. 2008;207(3):S75.
- 11.Ayaz-Alkaya S. Overview of psychosocial problems in individuals with stoma: A review of literature. Int Wound J. 2019;16(1):243–249. doi: 10.1111/iwj.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White CA, Hunt JC. Psychological factors in postoperative adjustment to stoma surgery. Ann R Coll Surg Engl. 1997;79(1):3–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-12. [DOI] [PubMed]
- 15.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:1–6. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Abdalla MI, Sandler RS, Kappelman MD, Martin CF, Chen W, Anton K, Long MD. The Impact of Ostomy on Quality of Life and Functional Status of Crohn's Disease Patients. Inflamm Bowel Dis. 2016;22(11):2658–2664. doi: 10.1097/MIB.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, Gainer VS, Cai T, Perez RG, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, et al. Similar risk of depression and anxiety following surgery or hospitalization for Crohn's disease and ulcerative colitis. Am J Gastroenterol. 2013;108(4):594–601. doi: 10.1038/ajg.2012.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anaraki F, Vafaie M, Behboo R, Maghsoodi N, Esmaeilpour S, Safaee A. Clinical profile and post-operative lifestyle changes in cancer and non-cancer patients with ostomy. Gastroenterol Hepatol Bed Bench. 2012;5(Suppl 1):S26–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Anaraki F, Vafaie M, Behboo R, Maghsoodi N, Esmaeilpour S, Safaee A. Quality of life outcomes in patients living with stoma. Indian J Palliat Care. 2012;18(3):176–180. doi: 10.4103/0973-1075.105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armbruster SD, Sun CC, Westin SN, Bodurka DC, Ramondetta L, Meyer LA, et al. Prospective assessment of patient-reported outcomes in gynecologic cancer patients before and after pelvic exenteration. Gynecol Oncol. 2018;149(3):484–90. [DOI] [PMC free article] [PubMed]
- 25.Bahayi K, Attaallah W, Yardımcı S, Bulut H, Özten E. Depression, anxiety, sexual dysfunction and quality of life in patients with ileostomy or colostomy. Turk J Colorectal Dis. 2018;28(2):69–75. doi: 10.4274/tjcd.87369. [DOI] [Google Scholar]
- 26.Barisic G, Markovic V, Popovic M, Dimitrijevic I, Gavrilovic P, Krivokapic Z. Function after intersphincteric resection for low rectal cancer and its influence on quality of life. Colorectal Dis. 2011;13(6):638–643. doi: 10.1111/j.1463-1318.2010.02244.x. [DOI] [PubMed] [Google Scholar]
- 27.Bau MO, Younes S, Aupy A, Bernuy M, Rouffet MJ, Yepremian D, Lottmann HB. The Malone antegrade colonic enema isolated or associated with urological incontinence procedures: evaluation from patient point of view. J Urol. 2001;165(6 Pt 2):2399–2403. doi: 10.1016/S0022-5347(05)66214-3. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell J, Saxena S, Jayasooriya N, Petersen I, Hotopf M, Creese H, Bottle A, Pollok RCG, Group P-IS: Stoma Formation in Crohn's Disease and the Likelihood of Antidepressant Use: A Population-Based Cohort Study. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed]
- 29.Bossema ER, Seuntiens MW, Marijnen CA, Baas-Thijssen MC, van de Velde CJ, Stiggelbout AM. The relation between illness cognitions and quality of life in people with and without a stoma following rectal cancer treatment. Psychooncology. 2011;20(4):428–434. doi: 10.1002/pon.1758. [DOI] [PubMed] [Google Scholar]
- 30.Bullen TL, Sharpe L, Lawsin C, Patel DC, Clarke S, Bokey L. Body image as a predictor of psychopathology in surgical patients with colorectal disease. J Psychosom Res. 2012;73(6):459–463. doi: 10.1016/j.jpsychores.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhri S, Brown L, Hassan I, Horgan AF: Preoperative intensive, community-based vs. traditional stoma education: a randomized, controlled trial. Dis Colon Rectum 2005, 48(3):504–509. [DOI] [PubMed]
- 32.Chen AM, Daly ME, Vazquez E, Courquin J, Luu Q, Donald PJ, Farwell DG. Depression among long-term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Head Neck Surg. 2013;139(9):885–889. doi: 10.1001/jamaoto.2013.4072. [DOI] [PubMed] [Google Scholar]
- 33.Coggrave MJ, Ingram RM, Gardner BP, Norton CS. The impact of stoma for bowel management after spinal cord injury. Spinal Cord. 2012;50(11):848–852. doi: 10.1038/sc.2012.66. [DOI] [PubMed] [Google Scholar]
- 34.Colquhoun P, Kaiser R, Jr, Efron J, Weiss EG, Nogueras JJ, Vernava AM, 3rd, Wexner SD. Is the quality of life better in patients with colostomy than patients with fecal incontience? World J Surg. 2006;30(10):1925–1928. doi: 10.1007/s00268-006-0531-5. [DOI] [PubMed] [Google Scholar]
- 35.Cotrim H, Pereira G. Impact of colorectal cancer on patient and family: implications for care. Eur J Oncol Nurs. 2008;12(3):217–226. doi: 10.1016/j.ejon.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Davidson F. Quality of life, wellbeing and care needs of Irish ostomates. Br J Nurs. 2016;25(17):S4–S12. doi: 10.12968/bjon.2016.25.17.S4. [DOI] [PubMed] [Google Scholar]
- 37.Davis D, Ramamoorthy L, Pottakkat B. Impact of stoma on lifestyle and health-related quality of life in patients living with stoma: A cross-sectional study. J Educ Health Promot. 2020;9:328. doi: 10.4103/jehp.jehp_256_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng Z, Howell D, Xu H, Yuan C. Quality of Life in Chinese Persons Living With an Ostomy: A Multisite Cross-sectional Study. J Wound Ostomy Continence Nurs. 2017;44(3):249–256. doi: 10.1097/WON.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez E, Holm K, Wennstrom B, Haglind E, Angenete E. Self-reported wellbeing and body image after abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2016;31(10):1711–1717. doi: 10.1007/s00384-016-2628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant M, McMullen CK, Altschuler A, Mohler MJ, Hornbrook MC, Herrinton LJ, Wendel CS, Baldwin CM, Krouse RS. Gender differences in quality of life among long-term colorectal cancer survivors with ostomies. Oncol Nurs Forum. 2011;38(5):587–596. doi: 10.1188/11.ONF.587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holzer B, Matzel K, Schiedeck T, Christiansen J, Christensen P, Rius J, et al. Study Group for Quality of Life in Rectal Cancer. Do geographic and educational factors influence the quality of life in rectal cancer patients with a permanent colostomy? Dis Colon Rectum. 2005;48(12):2209–16. [DOI] [PubMed]
- 42.Hong KS, Oh BY, Kim EJ, Chung SS, Kim KH, Lee RA. Psychological attitude to self-appraisal of stoma patients: prospective observation of stoma duration effect to self-appraisal. Ann Surg Treat Res. 2014;86(3):152–160. doi: 10.4174/astr.2014.86.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornbrook MC, Wendel CS, Coons SJ, Grant M, Herrinton LJ, Mohler MJ, Baldwin CM, McMullen CK, Green SB, Altschuler A, et al. Complications among colorectal cancer survivors: SF-6D preference-weighted quality of life scores. Med Care. 2011;49(3):321–326. doi: 10.1097/MLR.0b013e31820194c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqbal F, van der Ploeg V, Adaba F, Askari A, Murphy J, Nicholls RJ, Vaizey C. Patient-Reported Outcome After Ostomy Surgery for Chronic Constipation. J Wound Ostomy Continence Nurs. 2018;45(4):319–325. doi: 10.1097/WON.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 45.Jayarajah U, Samarasekera DN. Psychological Adaptation to Alteration of Body Image among Stoma Patients: A Descriptive Study. Indian J Psychol Med. 2017;39(1):63–68. doi: 10.4103/0253-7176.198944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayarajah U, Samarasekera DN. A cross-sectional study of quality of life in a cohort of enteral ostomy patients presenting to a tertiary care hospital in a developing country in South Asia. BMC Res Notes. 2017;10(1):75. doi: 10.1186/s13104-017-2406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y, Zhang J, Zheng MC, Bu XQ, Zhang JE. Psychosocial behaviour reactions, psychosocial needs, anxiety and depression among patients with rectal cancer before and after colostomy surgery: A longitudinal study. J Clin Nurs. 2019;28(19–20):3547–3555. doi: 10.1111/jocn.14946. [DOI] [PubMed] [Google Scholar]
- 48.Karakayali FY, Tezcaner T, Ozcelik U, Moray G. The Outcomes of Ultralow Anterior Resection or an Abdominoperineal Pull-Through Resection and Coloanal Anastomosis for Radiation-Induced Recto-Vaginal Fistula Patients. J Gastrointest Surg. 2016;20(5):994–1001. doi: 10.1007/s11605-015-3040-8. [DOI] [PubMed] [Google Scholar]
- 49.Keltikangas-Jarvinen L, Loven EL. Stability of personality dimensions related to cancer and colitis ulcerosa: preliminary report. Psychol Rep. 1983;52(3):961–962. doi: 10.2466/pr0.1983.52.3.961. [DOI] [PubMed] [Google Scholar]
- 50.Keltikangas-Jarvinen L, Loven E, Moller C. Psychic factors determining the long-term adaptation of colostomy and ileostomy patients. Psychother Psychosom. 1984;41(3):153–159. doi: 10.1159/000287803. [DOI] [PubMed] [Google Scholar]
- 51.Ketterer SN, Leach MJ, Fraser C. Factors Associated With Quality of Life Among People Living With a Stoma in Nonmetropolitan Areas. Nurs Res. 2021;70(4):281–288. doi: 10.1097/NNR.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knowles SR, Cook SI, Tribbick D. Relationship between health status, illness perceptions, coping strategies and psychological morbidity: a preliminary study with IBD stoma patients. J Crohns Colitis. 2013;7(10):e471–478. doi: 10.1016/j.crohns.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Knowles SR, Wilson J, Wilkinson A, Connell W, Salzberg M, Castle D, Desmond P, Kamm MA. Psychological well-being and quality of life in Crohn's disease patients with an ostomy: a preliminary investigation. J Wound Ostomy Continence Nurs. 2013;40(6):623–629. doi: 10.1097/01.WON.0000436670.56153.7b. [DOI] [PubMed] [Google Scholar]
- 54.Knowles SR, Tribbick D, Connell WR, Castle D, Salzberg M, Kamm MA. Exploration of health status, illness perceptions, coping strategies, and psychological morbidity in stoma patients. J Wound Ostomy Continence Nurs. 2014;41(6):573–580. doi: 10.1097/WON.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 55.Koc MA, Akyol C, Gokmen D, Aydin D, Erkek AB, Kuzu MA. Effect of Prehabilitation on Stoma Self-Care, Anxiety, Depression, and Quality of Life in Patients With Stomas: A Randomized Controlled Trial. Dis Colon Rectum. 2023;66(1):138–147. doi: 10.1097/DCR.0000000000002275. [DOI] [PubMed] [Google Scholar]
- 56.Krouse RS, Herrinton LJ, Grant M, Wendel CS, Green SB, Mohler MJ, Baldwin CM, McMullen CK, Rawl SM, Matayoshi E. Health-related quality of life among long-term rectal cancer survivors with an ostomy: manifestations by sex. J Clin Oncol. 2009;27(28):4664. doi: 10.1200/JCO.2008.20.9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krouse RS, Grant M, McCorkle R, Wendel CS, Cobb MD, Tallman NJ, Ercolano E, Sun V, Hibbard JH, Hornbrook MC. A chronic care ostomy self-management program for cancer survivors. Psychooncology. 2016;25(5):574–581. doi: 10.1002/pon.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamb KA, Lodge J. Managing symptoms associated with the redundant distal colon and rectum: a pilot scoping exercise. Gastrointest Nurs. 2019;17(1):20–24. doi: 10.12968/gasn.2019.17.1.20. [DOI] [Google Scholar]
- 59.Lemiński A, Kaczmarek K, Bańcarz A, Zakrzewska A, Małkiewicz B, Słojewski M: Educational and Psychological Support Combined with Minimally Invasive Surgical Technique Reduces Perioperative Depression and Anxiety in Patients with Bladder Cancer Undergoing Radical Cystectomy. Int J Environ Res Public Health 2021, 18(24). [DOI] [PMC free article] [PubMed]
- 60.Lim SH, Chan SWC, Chow A, Zhu L, Lai JH, He HG. Pilot trial of a STOMA psychosocial intervention programme for colorectal cancer patients with stomas. J Adv Nurs. 2019;75(6):1338–1346. doi: 10.1111/jan.13973. [DOI] [PubMed] [Google Scholar]
- 61.LIU Y, NI L: Construction and Application of Remote Continuing Care Model for Colorectal Cancer Patients in the Internet Era. Indian Journal of Pharmaceutical Sciences 2021:177–181.
- 62.Lowe BG, Alsaleh E, Blake H. Assessing physical activity levels in people living with a stoma. Nurs Stand. 2019;35(1):70–77. doi: 10.7748/ns.2019.e11278. [DOI] [PubMed] [Google Scholar]
- 63.MacDonald LD, Anderson HR. The health of rectal cancer patients in the community. Eur J Surg Oncol. 1985;11(3):235–241. [PubMed] [Google Scholar]
- 64.Mohamed NE, Shah QN, Kata HE, Sfakianos J, Given B. Dealing With the Unthinkable: Bladder and Colorectal Cancer Patients' and Informal Caregivers' Unmet Needs and Challenges in Life After Ostomies. Semin Oncol Nurs. 2021;37(1):151111. doi: 10.1016/j.soncn.2020.151111. [DOI] [PubMed] [Google Scholar]
- 65.Mols F, Lemmens V, Bosscha K, van den Broek W, Thong MS. Living with the physical and mental consequences of an ostomy: a study among 1–10-year rectal cancer survivors from the population-based PROFILES registry. Psycho–Oncology. 2014;23(9):998–1004. doi: 10.1002/pon.3517. [DOI] [PubMed] [Google Scholar]
- 66.Norton C, Burch J, Kamm MA. Patients' views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48(5):1062–1069. doi: 10.1007/s10350-004-0868-5. [DOI] [PubMed] [Google Scholar]
- 67.Park S, Jang IS, Kim YS. Risks for depression among ostomates in South Korea. Jpn J Nurs Sci. 2018;15(3):203–209. doi: 10.1111/jjns.12197. [DOI] [PubMed] [Google Scholar]
- 68.Portier G, Bonhomme N, Platonoff I, Lazorthes F. Use of Malone antegrade continence enema in patients with perineal colostomy after rectal resection. Dis Colon Rectum. 2005;48(3):499–503. doi: 10.1007/s10350-004-0802-x. [DOI] [PubMed] [Google Scholar]
- 69.Powell-Chandler A, Boyce K, James O, Scourfield L, Torkington J, Bisson J, Cornish JA, Group PTM. Psychological sequelae of colonic resections. Colorectal Dis. 2020;22(8):945–51. [DOI] [PubMed]
- 70.Rafiei H, Hoseinabadi-Farahani MJ, Aghaei S, Hosseinzadeh K, Naseh L, Heidari M. The prevalence of psychological problems among ostomy patients: A cross-sectional study from Iran. Gastrointest Nurs. 2017;15(2):39–44. doi: 10.12968/gasn.2017.15.2.39. [DOI] [Google Scholar]
- 71.Rafiei H, Hosseinzadeh K, Hoseinabadi-Farahani MJ, Naseh L, Razaghpoor A, Aghaei S, Mazroei A. The relationship between psychological health and spiritual wellbeing in Iranian stoma patients. Gastrointest Nurs. 2019;17(Sup5):S18–S22. doi: 10.12968/gasn.2019.17.Sup5.S18. [DOI] [Google Scholar]
- 72.Ramer L. Self-image changes with time in the cancer patient with a colostomy after operation. J ET Nurs. 1992;19(6):195–203. doi: 10.1097/00152192-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Reese JB, Finan PH, Haythornthwaite JA, Kadan M, Regan KR, Herman JM, Efron J, Diaz LA, Jr, Azad NS. Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status. Support Care Cancer. 2014;22(2):461–468. doi: 10.1007/s00520-013-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Repic GB, Ivanović S, Stanojević Č, Trgovčević S: Psychological and spiritual well-being aspects of the quality of life in colostomy patients. Vojnosanitetski pregled 2018, 75(6).
- 75.Richbourg L, Thorpe JM, Rapp CG. Difficulties experienced by the ostomate after hospital discharge. J Wound Ostomy Continence Nurs. 2007;34(1):70–79. doi: 10.1097/00152192-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 76.Rud CL, Baunwall SMD, Bager P, Dahlerup JF, Wilkens TL, Tøttrup A, Lal S, Hvas CL. Patient-Reported Outcomes and Health-Related Quality of Life in People Living With Ileostomies: A Population-Based. Cross-Sectional Study Dis Colon Rectum. 2022;65(8):1042–1051. doi: 10.1097/DCR.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sceats LA, Dehghan MS, Rumer KK, Trickey A, Morris AM, Kin C. Surgery, stomas, and anxiety and depression in inflammatory bowel disease: a retrospective cohort analysis of privately insured patients. Colorectal Dis. 2020;22(5):544–553. doi: 10.1111/codi.14905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharpe L, Patel D, Clarke S. The relationship between body image disturbance and distress in colorectal cancer patients with and without stomas. J Psychosom Res. 2011;70(5):395–402. doi: 10.1016/j.jpsychores.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Shrestha S, Siwakoti S, Shakya U, Shakya R, Khadka S. Quality of Life, Anxiety and Depression among Clients with Ostomy Attending Selected Stoma Clinics. J Nepal Health Res Counc. 2022;20(2):383–391. doi: 10.33314/jnhrc.v20i02.3978. [DOI] [PubMed] [Google Scholar]
- 80.Sivero L, Bottone M, Siciliano S, Volpe S, Maione R, Chini A, Pollastro M, Iovino S, Sivero S. Post-operative oncological and psychological evaluation of patients with colostomy for colorectal cancer. Ann Ital Chir. 2022;93:435–438. [PubMed] [Google Scholar]
- 81.Song L, Han X, Zhang J, Tang L. Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer. Psychooncology. 2020;29(4):796–802. doi: 10.1002/pon.5352. [DOI] [PubMed] [Google Scholar]
- 82.Ssewanyana Y, Ssekitooleko B, Suuna B, Bua E, Wadeya J, Makumbi TK, Ocen W, Omona K. Quality of life of adult individuals with intestinal stomas in Uganda: a cross sectional study. Afr Health Sci. 2021;21(1):427–436. doi: 10.4314/ahs.v21i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas C, Madden F, Jehu D. Psychological effects of stomas--I. Psychosocial morbidity one year after surgery. J Psychosom Res. 1987;31(3):311–316. doi: 10.1016/0022-3999(87)90050-X. [DOI] [PubMed] [Google Scholar]
- 84.Wang P, Liang J, Zhou H, Wang Z, Shi L, Zhou Z. Extraperitoneal sigmoidostomy: a surgical approach with less complications and better functions for abdominoperineal resection of rectal cancer. Int J Colorectal Dis. 2018;33(1):41–46. doi: 10.1007/s00384-017-2931-4. [DOI] [PubMed] [Google Scholar]
- 85.Williams NS, Johnston D. The quality of life after rectal excision for low rectal cancer. Br J Surg. 1983;70(8):460–462. doi: 10.1002/bjs.1800700805. [DOI] [PubMed] [Google Scholar]
- 86.Wirsching M, Druner HU, Herrmann G. Results of psychosocial adjustment to long-term colostomy. Psychother Psychosom. 1975;26(5):245–256. doi: 10.1159/000286938. [DOI] [PubMed] [Google Scholar]
- 87.Zewude WC, Derese T, Suga Y, Teklewold B. Quality of Life in Patients Living with Stoma. Ethiop J Health Sci. 2021;31(5):993–1000. doi: 10.4314/ejhs.v31i5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 89.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van der Velden PG, Hyland P, Contino C, von Gaudecker H-M, Muffels R, Das M. Anxiety and depression symptoms, the recovery from symptoms, and loneliness before and after the COVID-19 outbreak among the general population: Findings from a Dutch population-based longitudinal study. PLoS ONE. 2021;16(1):e0245057. doi: 10.1371/journal.pone.0245057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. 2016;87:70–80. doi: 10.1016/j.jpsychores.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Mols F, Schoormans D, de Hingh I, Oerlemans S, Husson O. Symptoms of anxiety and depression among colorectal cancer survivors from the population-based, longitudinal PROFILES Registry: Prevalence, predictors, and impact on quality of life. Cancer. 2018;124(12):2621–2628. doi: 10.1002/cncr.31369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayawickreme E, Infurna FJ, Alajak K, Blackie LE, Chopik WJ, Chung JM, Dorfman A, Fleeson W, Forgeard MJ, Frazier P. Post-traumatic growth as positive personality change: Challenges, opportunities, and recommendations. J Pers. 2021;89(1):145–165. doi: 10.1111/jopy.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shellito PC. Complications of abdominal stoma surgery. Dis Colon Rectum. 1998;41(12):1562–1572. doi: 10.1007/BF02237308. [DOI] [PubMed] [Google Scholar]
- 95.Brown H, Randle J. Living with a stoma: a review of the literature. J Clin Nurs. 2005;14(1):74–81. doi: 10.1111/j.1365-2702.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 96.Manderson L. Boundary breaches: the body, sex and sexuality after stoma surgery. Soc Sci Med. 2005;61(2):405–415. doi: 10.1016/j.socscimed.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 97.Kübler-Ross E. On Death and Dying. London: Tavistock Publications Limited; 1969. [Google Scholar]
- 98.Burch J: Stoma appliances and accessories: getting it right for the patient. Br J Nurs 2014, 23(17):S4, S6, S8–10. [DOI] [PubMed]
- 99.Simmons KL, Smith JA, Bobb KA, Liles LL. Adjustment to colostomy: stoma acceptance, stoma care self-efficacy and interpersonal relationships. J Adv Nurs. 2007;60(6):627–635. doi: 10.1111/j.1365-2648.2007.04446.x. [DOI] [PubMed] [Google Scholar]
- 100.Kovoor JG, McIntyre D, Chik WWB, Chow CK, Thiagalingam A: Clinician-Created Educational Video Resources for Shared Decision-making in the Outpatient Management of Chronic Disease: Development and Evaluation Study. JMIR 2021, (in press). [DOI] [PMC free article] [PubMed]
- 101.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 102.Rai D, Zitko P, Jones K, Lynch J, Araya R. Country-and individual-level socioeconomic determinants of depression: multilevel cross-national comparison. Br J Psychiatry. 2013;202(3):195–203. doi: 10.1192/bjp.bp.112.112482. [DOI] [PubMed] [Google Scholar]
- 103.Melgar N, Rossi M. A cross-country analysis of the risk factors for depression at the micro and macro levels. Am J Econ Sociol. 2012;71(2):354–376. doi: 10.1111/j.1536-7150.2012.00831.x. [DOI] [Google Scholar]
- 104.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 106.Limbana T, Khan F, Eskander N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus. 2020;12(8):e9966. doi: 10.7759/cureus.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krezalek MA, Skowron KB, Guyton KL, Shakhsheer B, Hyoju S, Alverdy JC. The intestinal microbiome and surgical disease. Curr Probl Surg. 2016;53(6):257–293. doi: 10.1067/j.cpsurg.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, Eisen JA. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc Natl Acad Sci U S A. 2009;106(40):17187–17192. doi: 10.1073/pnas.0904847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49(12):1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Friedrich M, Pohin M, Powrie F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity. 2019;50(4):992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 113.Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 114.Polidano K, Chew-Graham CA, Farmer AD, Saunders B. Access to Psychological Support for Young People Following Stoma Surgery: Exploring Patients' and Clinicians' Perspectives. Qual Health Res. 2021;31(3):535–549. doi: 10.1177/1049732320972338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. PRISMAChecklist. Appendix 2. Studies included at full-text review. Supplementary Figure 1. Funnel plot summarising publication bias for studies with data regarding effect of age on depressive symptoms after stoma surgery. Supplementary Figure 2. Funnel plot summarising publication bias for studies with data regarding mean age and standard deviations in studies with data regarding effect of age on depressive symptoms after stoma surgery. Supplementary Figure 3. Funnel plot summarising publication bias for studies with data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 4. Forest plot summarising prevalence of male sex in studies with data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 5. Funnel plot summarising publication bias for studies with data regarding prevalence of male sex and data regarding effect of sex on depressive symptoms after stoma surgery. Supplementary Figure 6. Forest plot showing prevalence of depressive symptoms after stoma surgery in studies reporting by region. Supplementary Figure 7. Funnel plot summarising publication bias for studies with data regarding effect of region on depressive symptoms after stoma surgery. Supplementary Figure 8. Forest plot summarising the odds of experiencing depressive symptoms before versus after stoma surgery. Supplementary Figure 9. Funnel plot summarising publication bias in studies reporting odds of experiencing depressive symptoms before versus after stoma surgery. Supplementary Figure 10. Forest plot summarising the odds of experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 11. Funnel plot summarising publication bias in studies reporting data regarding the odds of experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 12. Forest plot summarising mean differences in experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 13. Funnel plot summarising publication bias in studies reporting data regarding mean differences in experiencing depressive symptoms after surgery in stoma versus non-stoma populations. Supplementary Figure 14. Forest plot summarising the mean differences in experiencing depressive symptoms after surgery in colostomy versus ileostomy patients. Supplementary Figure 15. Funnel plot summarising publication bias in studies reporting data regarding the mean differences in experiencing depressive symptoms after surgery in colostomy versus ileostomy patients. Supplementary Table 1. Risk of bias assessment using the Downs and Black Checklist for 65 non-randomised studies of interventions included in the present systematic review. Supplementary Table 2. Overall risk of bias assessment using the Cochrane RoB 2.0 checklist for three randomised controlled trials included in the present systematic review.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.