Abstract
Background.
Frozen foods have rarely been linked to Listeria monocytogenes illness. We describe an outbreak investigation prompted by both hospital clustering of illnesses and product testing.
Methods.
We identified outbreak-associated listeriosis cases using whole-genome sequencing (WGS), product testing results, and epidemiologic linkage to cases in the same Kansas hospital. We reviewed hospital medical and dietary records, product invoices, and molecular subtyping results. Federal and state officials tested product and environmental samples for L. monocytogenes.
Results.
Kansas officials were investigating 5 cases of listeriosis at a single hospital when, simultaneously, unrelated sampling for a study in South Carolina identified L. monocytogenes in Company A ice cream products made in Texas. Isolates from 4 patients and Company A products were closely related by WGS, and the 4 patients with known exposures had consumed milkshakes made with Company A ice cream while hospitalized. Further testing identified L. monocytogenes in ice cream produced in a second Company A production facility in Oklahoma; these isolates were closely related by WGS to those from 5 patients in 3 other states. These 10 illnesses, involving 3 deaths, occurred from 2010 through 2015. Company A ultimately recalled all products.
Conclusions.
In this US outbreak of listeriosis linked to a widely distributed brand of ice cream, WGS and product sampling helped link cases spanning 5 years to 2 production facilities, indicating longstanding contamination. Comprehensive sanitation controls and environmental and product testing for L. monocytogenes with regulatory oversight should be implemented for ice cream production.
Keywords: listeriosis, whole-genome sequencing, ice cream, food safety
Listeria monocytogenes is primarily transmitted through consumption of contaminated food, such as soft cheeses, deli meats, and in recent years produce [1-3]. Invasive listeriosis is rare, with approximately 600 cases reported annually to the Centers for Disease Control and Prevention (CDC; unpublished data). Illness is typically severe; bacteremia and neurolisteriosis are the most common manifestations, with mortality >30% among these patients [4]. Persons with immunocompromising conditions, the elderly, and pregnant women and their newborns are at highest risk of listeriosis.
In the United States, since 1998 laboratory-confirmed L. monocytogenes isolates from humans, food, and the environment collected by public health and regulatory agencies were characterized using pulsed-field gel electrophoresis (PFGE) and uploaded to PulseNet, the national surveillance network of US public health and regulatory laboratories coordinated by CDC [5]. Over the last 20 years, outbreak investigations have shown that isolates with indistinguishable PFGE patterns are likely to come from the same source, especially when the PFGE pattern is rare.
Since September 2013, the CDC, the US Food and Drug Administration (FDA), the US Department of Agriculture’s Food Safety and Inspection Service, the National Institute for Biotechnology Information (NCBI), and state and local officials have collaborated to perform whole-genome sequencing (WGS) on all L. monocytogenes isolates [6].
Before 2014, ice cream had not been implicated as a source of listeriosis [7]. In February 2015, samples of ice cream collected by the South Carolina Department of Health and Environmental Control (SCDHEC) yielded L. monocytogenes. Concurrently, officials at the Kansas Department of Health and Environment (KDHE) were investigating a listeriosis cluster at a hospital. The CDC, FDA, and state health departments joined the investigations.
METHODS
Case Definition
We defined a confirmed case as invasive listeriosis in a patient whose L. monocytogenes strain, isolated between January 2010 and January 2015, was highly related by WGS to isolates from Company A ice cream or the environment (Figure 1), or the patient was admitted to the same hospital before listeriosis onset as a confirmed case. We defined a suspected case as invasive listeriosis in a patient whose L. monocytogenes isolate had a PFGE pattern indistinguishable from those of Company A ice cream and facility environmental isolates.
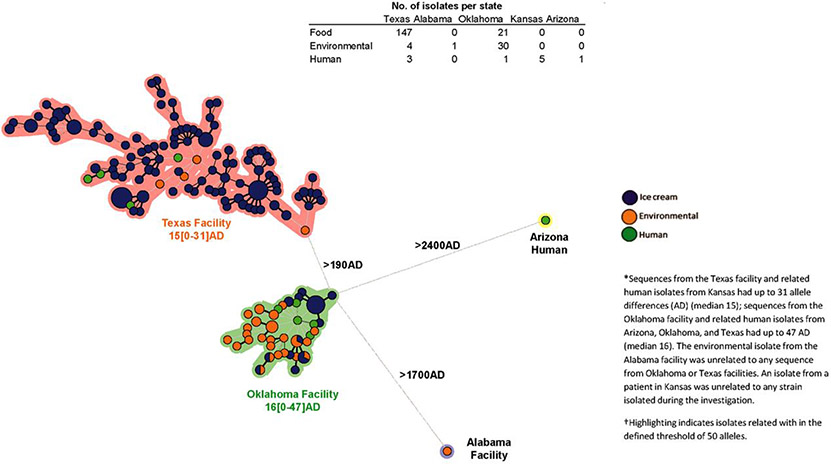
Figure 1.
Minimum spanning tree representing the whole-genome multilocus sequence typing results of Listeria monocytogenes isolates from patients, ice cream products, and manufacturing facilities—United States, January 2010–April 2015. Abbreviation: AD, allele difference.
Epidemiologic Investigation
We reviewed Listeria Initiative (LI) questionnaires, which capture information on the 28 days before listeriosis onset, including ice cream consumption, brand, and hospitalization [8]. We used this period because the time between exposure and illness is almost always ≤28 days [9]. We also reviewed national LI data and patients’ hospital dietary records when available. We recorded limited clinical information when provided. We conducted additional case finding as new laboratory results became available.
Laboratory Methods
We characterized all isolates using PulseNet standardized protocols for PFGE and WGS [10]. We used both whole-genome multilocus sequence typing (wgMLST) using BioNumerics v7.6 [11] and high-quality single-nucleotide polymorphism (hqSNP) analysis to compare genetic relatedness of isolates by each method [6]. All sequenced isolates were uploaded to NCBI (Supplementary Table 1, http://www.ncbi.nlm.nih.gov/bioproject/).
Regulatory Activities
State health and agriculture officials investigated food preparation at the Kansas hospital, collected Company A products from the hospital, and tested them for L. monocytogenes. State regulatory agencies in Alabama, Oklahoma, and Texas and the FDA inspected all 3 Company A manufacturing facilities. They collected environmental swabs throughout manufacturing environments and ice cream products for testing from all facilities. They reviewed records to determine which products were made in specific areas. Samples that yielded L. monocytogenes were characterized using PFGE and WGS.
RESULTS
Investigation of Isolates Possibly Linked to Ice Cream Products Made at Company A’s Texas Facility
In February 2015, SCDHEC isolated L. monocytogenes from ice cream products collected at a Company A distribution center in South Carolina during random product testing. SCDHEC had collected 1 sample each from 2 product types: ice cream bars and ice cream cookie sandwiches. An additional 15 ice cream bars and 15 ice cream cookies, each from a single box, were collected later. Each sample yielded multiple isolates, for a total of 126 L. monocytogenes isolates with 7 two-enzyme PFGE pattern combinations from all 32 positive ice cream products. The products were produced on the same line at Company A’s Texas manufacturing facility; subsequently, the Texas Department of State Health Services collected food and environmental samples at Company A’s Texas facility. Six of 21 ice cream samples yielded L. monocytogenes, among which 4 PFGE patterns were identified; these PFGE patterns were indistinguishable from those of isolates collected at Company A’s South Carolina distribution center.
A PulseNet database search for isolates with PFGE patterns indistinguishable from ice cream isolates identified 14 isolates from 14 patients with listeriosis onset during January 2013–February 2015 (ie, suspect cases). No link to ice cream was discerned based on the limited epidemiologic data available. The CDC contacted KDHE because 4 patients (including 2 with recent illnesses) were from Kansas, a state that typically reports fewer than 5 listeriosis cases per year. Kansas epidemiologists had been investigating these illnesses and an additional case, as all occurred within 13 months in patients who had been in the same hospital for unrelated conditions in the weeks before listeriosis onset.
Four patient isolates were highly related to ice cream isolates by wgMLST (<19 allele differences), all from patients admitted to the Kansas hospital ≤28 days before listeriosis onset. A fifth patient, whose isolate had a different PFGE pattern and was unrelated by WGS, was included in the investigation because the patient was admitted to the same Kansas hospital as the other 4 patients during the same period. LI questionnaire review showed that 2 patients reported consuming ice cream, but Company A was not named. Hospital dietary records from the 28 days before listeriosis onset for 4 patients, including the patient with the dissimilar PFGE pattern, revealed that all 4 had consumed both Company A ice cream cups and hospital-made milkshakes containing Company A single-serving, pre-packaged ice cream “scoops.”
Milkshakes prepared at the Kansas hospital were made with 2 Company A vanilla ice cream scoops (80–85 g/scoop) and 1% milk (118 mL) produced by another company, with chocolate or strawberry syrup added on request [12]. Inspection of the hospital kitchen and milkshake mixing procedures did not identify sanitation deficiencies.
Investigation of Isolates Possibly Linked to Ice Cream Products Made at Company A’s Oklahoma Facility
Kansas health officials collected Company A ice cream cups, but not scoops, because Company A had removed scoops from the hospital and other institutions after SCDHEC isolated L. monocytogenes; this market withdrawal was not publicly announced. The Kansas Department of Agriculture detected L. monocytogenes in 1 chocolate ice cream cup made at Company A’s Oklahoma facility. Its PFGE pattern differed from patterns of isolates from ice cream products made in Texas (Table 1). Six patient isolates in the PulseNet database (covering 1998–2015) shared this PFGE pattern. These were from patients in Texas (4), Arizona (1), and Oklahoma (1) whose specimens were obtained during 2010–2014. Five of the 6 isolates were highly related by wgMLST (<14 allele differences) to the isolate from the ice cream cup; the sixth differed by >1700 alleles from the ice cream cup and was excluded from the investigation. Four patients had completed LI questionnaires; 1 had no ice cream consumption, and data on ice cream consumption were not available for the others. However, all 5 patients had been hospitalized ≤28 days before listeriosis onset at 5 different hospitals that reportedly served Company A ice cream products at the time. Hospital dietary records for these 5 patients were unavailable. However, medical records indicated that 1 patient had consumed sherbet and another consumed milkshakes. Inspection at the Oklahoma facility and additional ice cream sampling yielded 51 isolates (21 ice cream, 30 environmental); all were related by wgMLST.
Table 1.
Listeria monocytogenes Pulsed-Field Gel Electrophoresis Patterns and Serotype Corresponding With Outbreak-Associated Illnesses, Ice Cream Samples, and Environmental Swabs—United States, January 2010–April 2015
| Pulsed-Field Gel Electrophoresis Pattern Number |
Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PulseNet Pattern Name | Serotype | Patients (No.) |
Patient State of Residence (No. Patients) |
Ice Cream (N=168)a |
Company A Production Facility |
||||
|
AscI Restriction Enzyme |
ApaI Restriction Enzyme |
Texas (n=4) |
Oklahoma (n=30) |
Alabama (n=1) |
|||||
| 1 | GX6A16.0026 | GX6A12.0227 | 1/2b | 2 | Kansas (2) | 11 | … | … | … |
| 2 | GX6A16.0020 | GX6A12.0227 | 1/2b | 1 | Kansas | 11 | 1 | … | … |
| 3 | GX6A16.0061 | GX6A12.0026 | 1/2b | 1 | Kansas | 26 | 2 | … | … |
| 4 | GX6A16.0026 | GX6A12.0489 | … | … | … | 8 | … | … | … |
| 5 | GX6A16.0026 | GX6A12.0077 | … | … | … | 3 | … | … | … |
| 6 | GX6A16.0061 | GX6A12.1512 | … | … | … | 1 | … | … | … |
| 7 | GX6A16.0061 | GX6A12.2551 | … | … | … | 3 | … | … | … |
| 8 | GX6A16.0061 | GX6A12.2358 | … | … | … | 1 | … | … | … |
| 9 | GX6A16.0026 | GX6A12.0094 | … | … | … | … | 1 | … | … |
| 10 | GX6A16.0416 | GX6A12.2353 | … | … | … | 4 | … | … | … |
| 11 | GX6A16.0336 | GX6A12.1840 | … | … | … | 17 | … | 19 | … |
| 12 | GX6A16.0336 | GX6A12.2255 | 3b | 5 | Arizona, Oklahoma, Texas (3) | 2 | … | 11 | … |
| 13 | GX6A16.0617 | GX6A12.1840 | … | … | … | 2 | … | … | … |
| 14 | GX6A16.0207 | GX6A12.0511 | … | … | … | … | … | … | 1 |
| 15 | GX6A16.0282 | GX6A12.0355 | 1/2a | 1 | Kansas | … | … | … | … |
Laboratories sequenced 147 ice cream isolates from ice cream products produced in Texas; to conserve resources, pulsed-field gel electrophoresis (PFGE) was performed on 68. PFGE was completed for all 21 ice cream isolates from ice cream products produced in Oklahoma.
Investigation of Isolate Obtained at Company A’s Alabama Facility
After L. monocytogenes was found in ice cream products from Company A’s Texas and Oklahoma facilities, public health officials inspected Company A’s only other production facility, located in Alabama. One environmental sample from this facility yielded L. monocytogenes. No patient isolates in the PulseNet database were indistinguishable from this isolate by PFGE or highly related by WGS. The isolate from the Alabama facility differed by >1900 alleles from isolates linked to the Texas and Oklahoma facilities.
Epidemiologic and Molecular Subtyping Summary
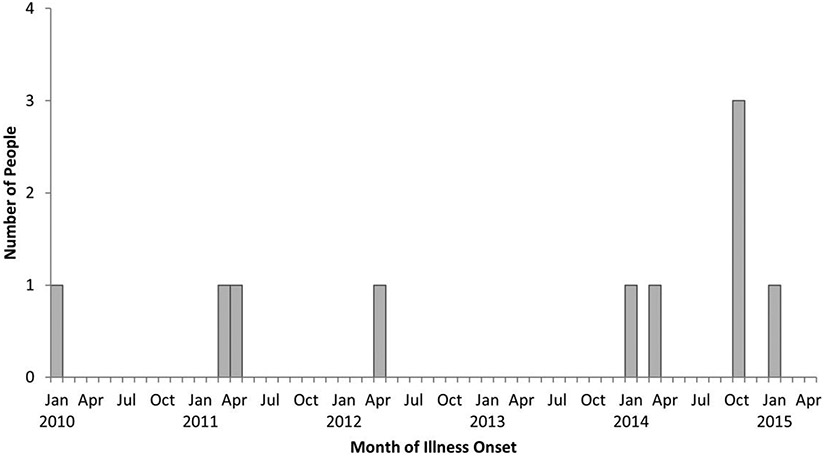
In total, 10 cases were reported from Kansas (5), Texas (3), Arizona, and Oklahoma, with isolation dates from January 2010–January 2015 (Figure 2). All patients were hospitalized during the 28 days before listeriosis onset for other conditions unrelated to listeriosis; 3 deaths were reported. Patients’ ages ranged from 42 to 83 years (median, 71); 5 were women. No cases were pregnancy-associated. Listeria monocytogenes was isolated from the blood of 9 patients and a brain abscess from the other. Underlying condition data were available for 5 patients; 4 had a history of cancer, including 1 on chemotherapy and 2 aged <55 years who had stem cell transplants. The fifth was admitted for upper gastrointestinal hemorrhage, although further clinical information was unavailable. Isolates from 9 cases were highly related by WGS to Company A ice cream or environmental facility isolates. One Kansas patient’s isolate had an unrelated PFGE pattern and WGS; the infection met the case definition because of the hospital link.
Figure 2.
Persons infected with an outbreak strain of Listeria monocytogenes by month of illness onset—United States, January 2010–January 2015 (N = 10).
Testing identified 3 serotypes (1/2a, 1/2b, and 3b) that together had 15 PFGE pattern combinations among patient, food, and environmental L. monocytogenes isolates. Patient isolates had 3 serotypes and 5 PFGE patterns. Most food and environmental isolates from each Company A production facility were highly related to one another by WGS; isolate sequences differed between facilities. Four Kansas patient isolates and 151 Texas facility ice cream and environmental isolates differed by a median of 15 alleles (range, 0–31) by wgMLST and a median of 10 hqSNPs (range, 0–30); they had 9 PFGE patterns (Figure 1). Five patient, 30 environmental, and 21 ice cream isolates associated with the Oklahoma facility differed by a median of 16 alleles (range, 0–47) and 10 hqSNPs (range, 0–30); they had 3 PFGE patterns. Isolate sequences linked to the Texas and Oklahoma facilities differed by a median of 232 alleles (range, 196–263).
Control Measures
On 13 March 2015, Kansas and Texas health departments, CDC, and FDA issued public announcements of the link between human illnesses in Kansas and Company A brand ice cream. These announcements warned consumers to avoid all items produced on the same line as the scoops, bars, and cookie sandwiches that yielded L. monocytogenes [13-15]. That day, Company A issued a public notice that it had withdrawn certain products made at its Texas facility but did not issue a formal recall announcement.
On 23 March 2015, in response to L. monocytogenes detection in the chocolate ice cream cup collected in Kansas, Company A issued a public recall notice of ice cream cups made in Oklahoma. Subsequent inspection of this facility by the Oklahoma Department of Agriculture, Food, and Forestry and FDA noted inadequate testing and cleaning procedures; facility design and construction that allowed for condensate and dripping; equipment storage locations and procedures that failed to protect food-contact surfaces from contamination; employee practices that provided opportunities for condensation and hose water spray to enter ice cream products; and use of wooden pallets and other equipment with mold-like stains [16]. Inspectors collected ice cream and environmental samples; 9 finished product samples and 30 environmental samples yielded L. monocytogenes. On 3 April 2015, Company A voluntarily suspended operations at the Oklahoma facility. On 7 April 2015, Company A expanded its recall to include additional ice cream products made at the Oklahoma facility. On 20 April 2015, Company A publicly recalled all ice cream products.
FDA inspections of the 3 Company A facilities identified a range of deficiencies, including operation that could allow dripping of condensation onto food and food-contact surfaces [16-18].
DISCUSSION
This multistate outbreak of listeriosis was unusual in the type of product implicated and in the type of investigation whereby we identified a multiregional brand of ice cream as the source. Although dairy products, particularly soft cheeses and milk, have commonly been implicated in listeriosis outbreaks, typically from sanitation deficiencies in production facilities [19], frozen dairy products have not. Ice cream and other frozen foods were considered low risk for L. monocytogenes transmission because if contamination occurs, it is usually at low levels and freezing temperatures prevent growth [20]. However, this outbreak was identified within months after the first recognized listeriosis outbreak linked to ice cream, where illnesses in 2 people from a single city were linked to hospital-provided milkshakes made with contaminated ice cream from a small local producer [7]. This investigation resulted in criminal charges against Company A, for which it paid more than $17 million, and against its president [21].
A national investigation would not have been triggered without the opportune finding of contaminated ice cream during product testing conducted by South Carolina officials. The concurrent local Kansas investigation might have remained unsolved, and earlier cases might not have been linked. The ice cream findings altered the typical outbreak investigation chain of events. Rather than a group of illnesses leading to a contaminated food, the contaminated food (ice cream) led to associated illnesses. Similarly, testing by state officials was the impetus for 1 recent investigation [22], and testing by a company prompted another [23]. These outbreak investigations illustrate the value of microbiologic surveillance for product contamination.
Ice cream was not routinely tested for L. monocytogenes because it has been considered to pose little risk for transmission. However, some frozen foods pose a risk, particularly when the product might be consumed partially thawed or after time at room temperature, as with milkshakes. A more recent US multistate outbreak was associated with frozen vegetables [24], and the United Kingdom reported a multicountry outbreak associated with frozen sweet corn [25]; both outbreaks lasted multiple years. After this outbreak, FDA completed targeted L. monocytogenes inspections and environmental sampling at ice cream production facilities [26]. Listeria monocytogenes was detected in 19 (21.3%) of the 89 production facilities sampled, but only on nonfood contact surfaces in all but 1. In response to inspection and sampling results, FDA notified 39 facilities of the need to take voluntary corrective actions, held 7 regulatory meetings, issued 2 warning letters, and suspended an ice cream manufacturer’s registration [27-29]. These outbreaks indicate that L. monocytogenes prevention measures used in production of nonfrozen ready-to-eat foods should be applied to at least some frozen foods.
This outbreak might not have been solved without WGS, which helped show that seemingly sporadic illnesses occurring over several years could be part of a protracted outbreak. In fact, this outbreak provided a key piece of evidence establishing the value of WGS in foodborne outbreak investigations, having since made it the gold standard method in the United States [6, 30]. Most multistate foodborne disease investigations have examined cases that occurred within relatively short periods, usually a few months. Because of PFGE subtyping’s limitations and because most people forget nonrecent food exposures, investigators have been reluctant to include older isolates in investigations. In this investigation, sequencing results provided a high degree of confidence about which patient and food isolates were phylogenetically related. LI questionnaires completed years earlier and patient records allowed investigators to determine not only that patients were hospitalized before illness began but also the specific products they could have consumed while hospitalized. This allowed investigators to include and exclude illnesses spanning 5 years. Such detailed past information is important for listeriosis investigations, because the same strain of L. monocytogenes can persist in food production facilities as a resident pathogen for years, producing illness detected in too few consumers to trigger an outbreak investigation under older methods. Larger numbers of cases, even over multiple years, improve the chance of detecting an epidemiologic or microbiologic link to a source [6].
Although isolates from Kansas patients had different PFGE pattern combinations, 4 of 5 were nearly identical by WGS. Given limited epidemiologic data for some patients, particularly those ill in earlier years, sequencing results were essential in providing stronger evidence that patient and food isolates were associated compared with PFGE alone. Illness in the Kansas hospital patient whose isolate was unrelated by WGS to ice cream isolates may not have been related to ice cream consumption or could have been related given the varying number of PFGE patterns detected.
It is not known why a single hospital was disproportionately affected by an outbreak that involved widely distributed products. Inspection did not identify food handling deficiencies. However, the hospital’s per-capita servings of Company A ice cream were among the highest of all hospitals [12]. That this outbreak and the previous one linked to ice cream involved only patients hospitalized during the 28 days before illness onset is notable. By comparison, only 607 (16%) of 3810 patients with completed LI questionnaires from 2004–2014 had been hospitalized in the 28 days before listeriosis onset (P <.001 by Fisher exact test). Greater susceptibility of hospitalized patients to listeriosis from lower exposure doses might explain this finding, as detailed testing of Company A ice cream following the outbreak revealed frequent (99% of samples tested) but low contamination levels (<20 most probable number/g) [31, 32]. Further, although clinical information was limited, 4 of the 5 patients with available information had a history of malignancy, including 3 with stem cell transplant or active chemotherapy, suggesting that being substantially immunocompromised played a role. Healthcare-associated L. monocytogenes illnesses have been described [33]. For example, in 2010, contaminated diced celery in chicken salad caused an outbreak among patients in multiple Texas hospitals [34]. Ice cream producers should prevent L. monocytogenes contamination because milkshakes provide a calorie-dense source of nutrition for hospitalized patients, and L. monocytogenes growth can begin soon after the milkshake is made [12]. Healthcare facilities should avoid serving foods known to pose an elevated listeriosis risk, including soft cheeses, unheated deli meat, hot dogs, and smoked fish [33, 35, 36], and should discard milkshakes after 2 hours at room temperature.
Listeriosis incidence in the United States has not declined over the past decade [37]. Despite widespread use of WGS since 2013, only 3% of US listeriosis cases during 2015–2017 were traced to a source. Another 13% of cases had isolates in 1 of many clusters of closely related cases for which no source could be determined (CDC, unpublished data). New or improved investigational and analytic approaches are needed to link more illnesses to sources, which could lead to better understanding of the major food sources of listeriosis, resulting in better prevention measures. This investigation and others that relied partly on microbiologic product sampling offer one way to improve. The fact that dairy products, a widening array of produce items, and frozen foods have replaced deli meats and hot dogs as major outbreaks sources indicate that improved process controls and environmental and product sampling should be considered as parts of a comprehensive food safety program. This could result in more investigations, driven initially or partly by finding Listeria-contaminated products or facilities, and improved success in linking illnesses to products. To harness the power of WGS more fully, a robust sampling program needs to be added to classic epidemiologic tools such as patient interviews and standardized questionnaires. Then, a coordinated laboratory, epidemiologic, and regulatory outbreak investigation can stimulate systemic changes to make the food system safer.
Supplementary Material
Acknowledgments.
The authors thank Heidi Dragoo, Jennifer Pistole, Joli Weiss, Linda Gaul, Mandip Kaur, Laura Whitlock, Irina Cody, Sara Belfry, Carrie Williams, Tony Sellars, Cheryl Tarr, Lee Katz, the Alabama Department of Agriculture, the Arizona Department of Health, the Texas Department of State Health Services, the Kansas Department of Agriculture, the Kansas Department of Health and Environment, the Oklahoma Department of Agriculture, Food and Forestry, and the Texas Department of Agriculture.
Financial support.
D. C. H. reports support for the present article from the Centers for Disease Control and Prevention’s (CDC) Epidemiology and Laboratory Capacity for Infectious Diseases (cooperative agreement U50CK000401).
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cartwright EJ, Jackson KA, Johnson SD, Graves LM, Silk BJ, Mahon BE. Listeriosis outbreaks and associated food vehicles, United States, 1998–2008. Emerg Infect Dis 2013; 19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson K, Conrad A, Gould LG, Jackson B. Emergence of listeriosis outbreaks associated with produce in the United States, 1983–2014 [Abstract]. International Association of Food Protection; 25–28 July 2015; Portland, Oregon. P3–233. [Google Scholar]
- 3.Self JL, Conrad A, Stroika S, et al. Multistate outbreak of listeriosis associated with packaged leafy green salads, United States and Canada, 2015–2016. Emerg Infect Dis 2019; 25:1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlier C, Perrodeau É, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 2017; 17:510–9. [DOI] [PubMed] [Google Scholar]
- 5.Halpin JL, Garrett NM, Ribot EM, Graves LM, Cooper KL. Re-evaluation, optimization, and multilaboratory validation of the PulseNet-standardized pulsed-field gel electrophoresis protocol for Listeria monocytogenes. Foodborne Pathog Dis 2010; 7:293–8. [DOI] [PubMed] [Google Scholar]
- 6.Jackson BR, Tarr C, Strain E, et al. Implementation of nationwide real-time whole-genome sequencing to enhance listeriosis outbreak detection and investigation. Clin Infect Dis 2016; 63:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rietberg K, Lloyd J, Melius B, et al. Outbreak of Listeria monocytogenes infections linked to a pasteurized ice cream product served to hospitalized patients. Epidemiol Infect 2016; 144:2728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Listeria (listeriosis) surveillance. Available at: https://www.cdc.gov/listeria/surveillance.html. Accessed 17 August 2016.
- 9.Angelo KM, Jackson KA, Wong KK, Hoekstra RM, Jackson BR. Assessment of the incubation period for invasive listeriosis. Clin Infect Dis 2016; 63:1487–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. PulseNet methods and protocols. 2022. Available at: https://www.cdc.gov/pulsenet/pathogens/protocols. Accessed 4 August 2020.
- 11.Bionumerics. 2022. Available at: https://www.bionumerics.com. Accessed 19 July 2022.
- 12.Chen Y, Allard E, Wooten A, et al. Recovery and growth potential of Listeria monocytogenes in temperature abused milkshakes prepared from naturally contaminated ice cream linked to listeriosis outbreak. Front Microbiol 2016; 7: 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Multistate outbreak of listeriosis linked to Blue Bell Creameries products. Available at: https://www.cdc.gov/listeria/outbreaks/ice-cream-03-15/index.html. Accessed 4 August 2020.
- 14.Kansas Department of Health and Environment. Five Kansans ill from listeriosis outbreak linked to Blue Bell Creameries. Available at: https://www.kdhe.ks.gov/CivicAlerts.aspx?AID=344. Accessed 4 August 2020.
- 15.Texas Department of State Health Services. Illnesses prompt advisory about certain Blue Bell products. Available at: https://dshs.texas.gov/news/releases/20150313.aspx. Accessed 4 August 2020.
- 16.US Food and Drug Administration. Blue Bell Creameries, LP, Broken Arrow, OK: 483 Issued April/23/2015. Available at: https://www.fda.gov/media/91871/download. Accessed 2 February 2018. [Google Scholar]
- 17.US Food and Drug Administration. Blue Bell Creameries, LP, Sylacauga, AL, 483 Issued April/30/2015. Available at: https://www.fda.gov/media/91865/download. Accessed 31 May 2022. [Google Scholar]
- 18.US Food and Drug Administration. Blue Bell Creameries, LP, Brenham, TX, 483 Issued May/01/2015. Available at: https://www.fda.gov/media/92059/download. Accessed 31 May 2022. [Google Scholar]
- 19.Jackson K, Gould L, Hunter JC, Kucerova Z, Jackson B. Listeriosis outbreaks associated with soft cheeses, United States, 1998–2014. Emerg Infect Dis 2018; 24: 1116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Guidance for industry: control of Listeria monocytogenes in refrigerated or frozen ready-to-eat foods; draft guidance. Available at: https://www.regulations.gov/document/FDA-2008-D-0096-0001. Accessed 28 June 2016.
- 21.US Department of Justice. Blue Bell Creameries agrees to plead guilty and pay $19.35 million for ice cream listeria contamination—former company president charged. Available at: https://www.justice.gov/opa/pr/blue-bell-creameries-agrees-plead-guilty-and-pay-1935-million-ice-cream-listeria. Accessed 1 November 2021.
- 22.Gottlieb SL, Newbern EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis 2006; 42:29–36. [DOI] [PubMed] [Google Scholar]
- 23.Heiman KE, Garalde VB, Gronostaj M, et al. Multistate outbreak of listeriosis caused by imported cheese and evidence of cross-contamination of other cheeses, USA, 2012. Epidemiol Infect 2016; 144:2698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Multistate outbreak of listeriosis linked to frozen vegetables (final update). Available at: https://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html#:~:text=FoodSafety.gov%20website%20.-,CDC%20recommends%20that%20consumers%20do%20not%20eat%2C%20and%20restaurants%20and,This%20outbreak%20investigation%20is%20over. Accessed 17 August 2016.
- 25.McLauchlin J, Aird H, Amar C, et al. An outbreak of human listeriosis associated with frozen sweet corn consumption: investigations in the UK. Int J Food Microbiol 2021; 338:108994. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. Inspection and environmental sampling of ice cream production facilities for Listeria monocytogenes and Salmonella FY 2016–17. Available at: https://www.fda.gov/food/sampling-protect-food-supply/inspection-and-environmental-sampling-ice-cream-production-facilities-listeria-monocytogenes-and Accessed 25 October 2020.
- 27.US Food and Drug Administration. Warning letter Friendly’s Manufacturing and Retail LLC MARCS-CMS 590821—NOVEMBER 22, 2019. Available at: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/friendlys-manufacturing-and-retail-llc-590821-11222019 Accessed 25 October 2020.
- 28.US Food and Drug Administration. Warning letter Velvet Ice Cream Company MARCS-CMS 575444—MAY 06, 2019. Available at: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/velvet-ice-cream-company-575444-05062019. Accessed 25 October 2020.
- 29.US Food and Drug Administration. FDA suspends food facility registration of Working Cow Homemade, Inc. Available at: https://www.fda.gov/food/alerts-advisories-safety-information/fda-suspends-food-facility-registration-working-cow-homemade-inc. Accessed 25 October 2020.
- 30.Brown E, Dessai U, McGarry S, Gerner-Smidt P. Use of whole-genome sequencing for food safety and public health in the United States. Foodborne Pathog Dis 2019; 16:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouillot R, Klontz KC, Chen Y, et al. Infectious dose of Listeria monocytogenes in outbreak linked to ice cream, United States, 2015. Emerg Infect Dis 2016; 22:2113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YI, Burall LS, Macarisin D, et al. Prevalence and level of Listeria monocytogenes in ice cream linked to a listeriosis outbreak in the United States. J Food Prot 2016; 79:1828–32. [DOI] [PubMed] [Google Scholar]
- 33.Silk BJ, McCoy MH, Iwamoto M, Griffin PM. Foodborne listeriosis acquired in hospitals. Clin Infect Dis 2014; 59:532–40. [DOI] [PubMed] [Google Scholar]
- 34.Gaul LK, Farag NH, Shim T, Kingsley MA, Silk BJ, Hyytia-Trees E. Hospital-acquired listeriosis outbreak caused by contaminated diced celery—Texas, 2010. Clin Infect Dis 2013; 56:20–6. [DOI] [PubMed] [Google Scholar]
- 35.Cutro SR, Dean R, Phillips MS. Implementation of a restricted foods policy at a large academic medical center. Infect Control Hosp Epidemiol 2014; 35:749–51. [DOI] [PubMed] [Google Scholar]
- 36.Lund BM. Microbiological food safety and a low-microbial diet to protect vulnerable people. Foodborne Pathog Dis 2014; 11:413–24. [DOI] [PubMed] [Google Scholar]
- 37.Tack DM, Ray L, Griffin PM, et al. Preliminary incidence trends of infections with pathogens transmitted commonly through food—and Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR Morb Mortal Wkly Rep 2020; 69:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.