Abstract
Periodontitis is an oral chronic inflammatory disease. Inhibiting tissue destruction and promoting tissue regeneration are important means for the treatment of periodontitis. Metformin not only has hypoglycemic effect but also has anti-inflammatory effect. Metformin has been shown to inhibit oxidative stress and activate autophagy through AMPK/mTOR pathway. High mobility group box 1 (HMGB1) has been implicated in the pathogenesis of many inflammatory diseases including periodontitis, it can participate in the induction of oxidative stress. HMGB1 is an autophagy regulator under oxidative stress, which can activate mTOR pathway. However, it is not clear whether metformin is related to HMGB1 and its mechanism in the process of periodontitis. Cell viability and expression of inflammatory cytokines were clarified by Cell Counting Kit-8, real-time PCR and enzyme-linked immunosorbent assay. Western blot and immunofluorescence were conducted to determine HMGB1 intracellular localization and expression of autophagy-associated proteins in vitro. Experimental periodontitis mice model was induced by administering a ligature. Immunohistochemistry was performed to detect the expression and localization of HMGB1 in vivo. The results of CCK-8, real-time PCR, enzyme-linked immunosorbent assay, Western blot and immunofluorescence showed lipopolysaccharide (LPS) treatment inhibited cell viability, and increased HMGB1 expression at a dose-independent manner. Metformin can reduce the effect of LPS. It also improves autophagy pathway inhibited by LPS and down-regulates mTOR expression. In addition, metformin attenuated alveolar bone resorption induced by ligation. This study provides new evidence for that metformin is a potential drug for the treatment of periodontitis and HMGB1 may be a potential target for periodontal intervention.
Keywords: Autophagy, Metformin, Oxidative stress, Periodontal ligament cells, Periodontitis
Introduction
Periodontal diseases are recognized as chronic infective diseases affecting approximately 20%–50% of the global population.1 Periodontitis is a common periodontal disease, which is a chronic inflammatory disease of tooth supporting tissue caused by bacteria. Periodontitis is usually accompanied by destruction of alveolar bone.2 At the same time, periodontal inflammation may also be caused by changes in bacterial composition, biological disorders and oxidative stress conditions. At present, the treatment of periodontitis includes mechanical debridement, anti-inflammatory drugs and regenerative surgery.1 Periodontal flora and its products can also cause the accumulation of reactive oxygen species (ROS) and oxidative stress in tissues.3 Excessive ROS in periodontal tissue can trigger the release of pro-inflammatory cytokines, the expression of nuclear factor-κ B and the activation of RANKL, and then RANKL promotes the formation of osteoclasts.4 Thus, inflammation control is a priority in periodontitis.
Metformin has been widely used in the treatment of type 2 diabetes because of its hypoglycemic and insulin sensitizing effects, and is recommended as a first-line treatment for all patients with type 2 diabetes.5 In addition, Caballero and Dandona have shown for the first time that metformin can change the state of inflammation without blood glucose control.1 It is reported that metformin has anti-inflammatory effects on human vascular smooth muscle cells and endothelial cells.6,7 In the field of dentistry, metformin reduced the inflammatory response, oxidative stress, and bone loss in ligation-induced periodontitis in rats.8 Our previous studies have shown that metformin can improve the oxidative stress state of cells and increase the osteogenic ability of human periodontal ligament cells (hPDLCs) by reducing the production of reactive oxygen species (ROS).1 Metformin can also activate autophagy through AMPK/mTOR pathway.9 This evidence suggests that metformin can be used as a potential drug for the treatment of periodontal inflammation-related diseases.1 However, the mechanism of metformin inhibiting tissue destruction and its mechanism of reducing oxidative stress is not completely clear.
High mobility group protein 1 (HMGB1) is a nuclear protein which binds to DNA. It is also a cofactor of gene transcription. It has been confirmed that it is involved in a variety of inflammatory responses including periodontitis.10 HMGB1 stimulates the secretion of pro-inflammatory mediators and cytokines such as interleukin-1 β (IL-1 β),11 IL-6 and IL-8 in the extracellular matrix.12 The secretion of inflammatory mediators and cytokines can further promote the positive feedback circulation secretion of HMGB1 in the inflammatory process.13,14 Some studies have found that inflammatory factors such as tumor necrosis factor-α (TNF-α) and IL-1 are usually released in the early stage of etiology, resulting in limited action time of the corresponding antagonists. HMGB1 is a pro-inflammatory factor produced in the later stage of inflammation, so the treatment window of anti-HMGB1 therapy is much wider than that of TNF targeted intervention. HMGB1 antagonists may be more effective in the treatment of periodontitis.15 Studies have shown that, as a redox protein, the function of HMGB1 may depend on its cell location. When the body is stimulated, HMGB1 is released from the nucleus to play a role outside the cell.16, 17, 18 The function of extracellular HMGB1 is closely related to oxidative stress. In periodontitis, stimulated cells accumulate a large amount of ROS due to the imbalance between ROS production and antioxidant activity, which directly reflects the degree of oxidative stress.19 Many studies have pointed out that HMGB1 and ROS regulate each other and show a synergistic effect.20 The accumulation of ROS can promote the redox reaction and up-regulate the nuclear translocation of HMGB1. Extracellular HMGB1 inhibits autophagy, further enhances oxidative stress, and induces apoptosis.21
Our previous studies have shown that metformin can reduce the oxidative stress in human periodontal ligament cells (hPDLCs) through autophagy.1 Our hypothesis is that metformin activates autophagy through AMPK/mTOR pathway, which inhibits HMGB1-induced oxidative stress in periodontal tissue. To test this hypothesis, relationship between metformin and HMGB1 in the occurrence and development of periodontitis is determined.
Materials and methods
Ethics statement
The experimental protocol was approved by the ethics committee of the College of Stomatology, Chongqing Medical University. All healthy volunteers aged from 10 to 20 years with informed consent from guardians donated their teeth for “orthodontic extraction treatment”. All animal experiments in this study were conducted in accordance with the Declaration of Helsinki under protocols reviewed and approved by Ethics Committee of College of Stomatology, Chongqing Medical University.
Cell & treatment schedule
Cell culture
Isolation and culturing of hPDLCs were performed as described previously.1 In short, periodontal ligament tissue was scraped from the middle-third of the root and digested with 3 mg/ml type I collagenase (Sigma, St. Louis, MO, USA) at 37 °C for 30 min. Following full digestion, the periodontal ligament tissue suspension was seeded in α-Minimum Essential Medium (α-MEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 U/ml of penicillin and 100 μg/ml of streptomycin. The culture was incubated at 37 °C in a humidified atmosphere with 95% air and 5% CO2. The cell culture medium was changed every 3 days. hPDLCs were passaged at a ratio of 1:3 when they reached approximately 80% confluence. A pooled cell samples from the third passage were used for further treatment.
Cell treatment
In order to establish the cellular inflammation model, hPDLCs were exposed to LPS (Sigma, St. Louis, MO, USA) for 24 h (h) after reaching approximately 70% confluence and further incubated in culture medium for 8 h hPDLCs were pretreated with metformin 24 h prior to the addition of LPS to investigate its effect on inflammation. hPDLCs was pretreated with Bafilomycin A1 (25 nM) (Solarbio, Beijing, China) for 4 h and 3-MA (10 μM) (Selleck, Houston, TX, USA) for 1 h, and then pretreated with metformin for 24 h hPDLCs was exposed to about 70% fusion of LPS (Sigma, St. Louis, MO, USA) for 24 h to observe the role of autophagy in the mechanism of inflammation. In order to explore the mechanism of mTOR pathway in this experiment, rapamycin (5 μg/mL) (AbMole, Shanghai, China) was used as positive control. hPDLCs was treated with Rapamycin for 24 h. All experiments were performed in triplicate.
Cell viability/Evaluation of cell proliferation
hPDLCs proliferation was evaluated with a Cell Counting Kit-8 (Beyotime Biotechnology, Shanghai, China). The treated cell suspension (5 × 103 cells/well) was seeded on a 96-well plate for 48 h. Next, cells were treated with CCK-8 reagent (Beyotime, Shanghai, China) and incubated for 2 h at 37 °C. Absorbance at 450 nm was measured using a microplate reader (PerkinElmer, USA). All experiments were repeated in triplicate.
Enzyme-linked immunosorbent assay
The levels of IL-6, IL-8 and HMGB1 were measured by commercially available enzyme-linked immunosorbent assay (ELISA) kits from BOSTER Biological Technology (Wuhan, China) and Sangon Biotech (Shanghai, China) according to the manufacturer's instructions.
Real time PCR analysis
Total RNA was extracted from hPDLCs in a six-well plate using RNAiso plus (Takara, Dalian, China). Next, 500 ng of total RNA was used as the template to synthesize cDNA using a 5 × PrimeScript RT Master Mix (Takara, Dalian, China). For PCR amplification, a 10 μg reaction volume was used, comprising 5 μg of 2 × TB Green Fast Mix (Takara, Dalian, China), 0.1 mmol/l of each primer, 1 μl of fivefold diluted cDNA and diethyl pyro carbonate water (DEPC, Sangon Biotech, Shanghai, China). The reaction and detection were conducted using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cycle threshold (Ct) values were collected and normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene. The primer sequences used for IL-6, IL-8, HMGB1 and GAPDH were as follows: IL-6 (F) 5′-GTACATCCTCGACGGCATC-3′, (R) 5′-ACCTCAAACTCCAAAAGACCAG-3′; IL-8 (F) 5′-ATGACTTCCAAGCTGGGCCGTG-3′, (R) 5′-TATGAATTCTCAGCCCTC TTCAAAA-3′; HMGB1 (F) 5′-TATGGCAAAAGCGGACAAGG-3′, (R) 5′-CTTCGCAACATCACCAATGGA-3′; GAPDH (F) 5′-CTTTGGTATCGTGGAAGGACTC-3′, (R) 5′-GTAGAGGCAGGGATGATGTTCT-3′.
Western blot analysis
The total protein content of the cells was isolated using RIPA lysis buffer (Beyotime, Shanghai, China) with 1 mM phenyl methane sulfonyl fluoride (PMSF; Beyotime, Shanghai, China), nuclear protein extraction by nuclear protein extraction kit (Beyotime, Shanghai, China), and protein concentration was measured using a BCA protein assay kit (Beyotime, Shanghai, China). Thirty micrograms of each protein sample were separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Beyotime, Shanghai, China), followed by transfer to a polyvinylidene difluoride membrane (PVDF; Bio-Rad, Hercules, CA, USA). After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies against HMGB1 (1:2000) (Abcam, Cambridge, UK), GAPDH (1:1000) (ZenBio Science, Chengdu, China), PCNA (1:1000) (ZenBio Science, Chengdu, China), LC3 (1:1000) (ZenBio Science, Chengdu, China), mTOR (1:1000) (ZenBio Science, Chengdu, China) overnight at 4 °C, followed by the respective secondary antibodies: peroxidase affinipure goat anti-rabbit IgG (1:3000) (ZenBio Science, Chengdu, China) or peroxidase affinipure goat anti-mouse IgG (1:3000) (ZenBio Science, Chengdu, China). Bands were detected using electrochemiluminescence plus reagent (Bio-Rad, Hercules, CA, USA). Finally, the intensity of these bands was quantified using ImageJ software (Version 1.8.0; National Institutes of Health, USA).
Immunofluorescence
Cells were treated with metformin and LPS to induce the translocation of HMGB1. Following treatment, cells were fixed using 4% paraformaldehyde and blocked with goat serum for 1 h. Cells were then incubated overnight with HMGB1 antibodies (1:200) (Abcam, Cambridge, UK), and subsequently with goat anti-rabbit antibodies (1:400) (BIOSS, Beijing, China) conjugated with Alexa Fluor 555 for 1 h the following day. Cells were stained with DAPI. Images were acquired using an Olympus microscope (Olympus).
Animal & treatment schedule
Animals
Eight-week-old C57BL/6 mice were purchased from Experimental Animal Center of Chongqing Medical University (Chongqing, China). All animals were housed in a specific-pathogen-free animal room of Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences and received water and food ad libitum from the Animal Care Facility Service.
Group allocation
Metformin hydrochloride (Molecular weight 165.63, Sigma–Aldrich, St. Louis, MO, USA) was dissolved in distilled water. Distilled water served as the vehicle treatment. All treatments were administered by oral gavage once daily for 14 days. Eighteen C57BL/6 mice were randomly divided into 3 groups of 6 each. The 3 groups were as follows: Control (no ligature group that received water), Ligature (ligature group that received water), and Ligature + Met (a ligature group treated with 200 mg/kg metformin).
Mouse model of ligature-induced periodontitis
Establishing and inducing the experimental periodontitis model was performed as previously described.22 Except for the Ligature + Met group treated with 200 mg/kg metformin, the distilled water was used as the control in the Control and Ligature groups. All the treatments were given by intragastric administration once a day for 14 days. On the fifth day after intragastric administration, the Ligature and Ligature + Met groups were ligated with a 5-0 silk between the maxillary left first and second molars and knots were tied on both ends to secure the ligature. The distance between the two knots is about 2.5 mm. The ligatures were examined daily to ensure that they remained in place during the experimental period. Periodontal inflammation and bone loss in this model is initiated by massive local accumulation of bacteria on the ligature-treated molars.23 The contralateral side of each mouse was left without ligature treatment. A second set of controls included mice that were not treated with ligatures on either side.
Micro-computed tomography
The upper jaws were dissected and scanned using a high-resolution CT system (Skyscan 1172; Skyscan, Aartselaar, Belgium). Three-dimensional reconstructions were generated using Amira software. Scans were performed using the following scanner settings: X-ray source voltage - 70 kVp; current −114μA, and power -8 W. CT scan (SCANCO Medical AG) settings were high resolution, a voxel size of 15 μm, a slice thickness of 0.01 mm and a FOV/diameter of 30.7 mm, while an integration time of 250 ms Micro-CT was used to assess the loss of alveolar bone. To qualify bone loss, the six-site total cement to enamel junction and alveolar bone crest (CEJ-ABC) distance for the ligature-treated side of each mouse was subtracted from the six-site total CEJ-ABC distance of the contralateral side (no ligature treatment) of the same mouse.
Histologic analysis
Following micro-CT scans, specimens were decalcified in 10% EDTA for at least 1 month, and then blocked parallel to the long axis of left first and second molar on the lingual margin in order to allow subsequent histologic sections to show the interproximal region between the two teeth. Specimens were then dehydrated and embedded in paraffin, and 4 μm sections were coded and stained using the antibodies indicated below.
Immunohistochemistry
Periodontal sections were blocked in 10% normal goat serum, and incubated overnight with primary antibodies against HMGB1 (1:350) (Abcam, Cambridge, UK), at 4 °C and stained with HRP-conjugated secondary antibodies (ZSJQ-BIO, Beijing, China). Diaminobenzidine (DAB, ZSJQ-BIO, Beijing, China) was used as a substrate for color development. All slides were counterstained with hematoxylin. Images of histologically stained sections were obtained using an Olympus microscope (Olympus BX41, Japan).
Statistical analysis
GraphPad Prism software (version 6.0, USA) was used for statistical analyses. Data were presented as the mean ± standard deviation (SD) in all graphs. Data were analyzed using the unpaired Student's t-test in order to compare group pairs or ANOVA for multiple group comparisons. Statistical significance was set at P < 0.05.
Results
LPS inhibited hPDLCs viability and induced inflammation
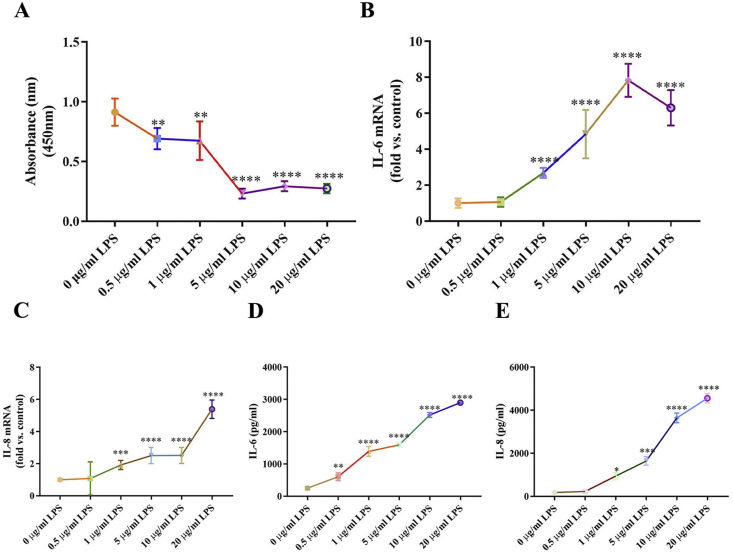
To determine a dosage of LPS that would be adequate to establish the cellular inflammation model, hPDLCs were stimulated with diverse concentrations of LPS, followed by measurements of cell viability. Compared with non-treated cells, cell viability was significantly reduced by 0.5 and 1 μg/ml LPS (Fig. 1A). To further mimic the inflammation status in periodontitis, the RNA level of inflammatory cytokines and its supernatant protein production were detected by real-time PCR and ELISA. LPS markedly increased the inflammatory cytokines of IL-6 and IL-8 both in mRNA (Fig. 1B, C) and protein levels (Fig. 1D, E). IL-6 and IL-8 in LPS-exposed hPDLCs was significantly increased with concentration dependent manner. Accordingly, the LPS concentration that would be best suitable for use in subsequent experiments was ascertained to be 1 μg/ml.
Figure 1.
LPS inhibits hPDLCs viability and induces inflammation. (A) Cell viability detected by the CCK-8 assay at different concentration of LPS. Inflammatory cytokines mRNA expression of IL-6 (B) and IL-8 (C) were examined by real-time PCR. Inflammatory cytokines production of IL-6 (D) and IL-8 (E) were examined by ELISA in culture medium. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
HMGB1 is highly expressed in LPS-induced hPDLCs
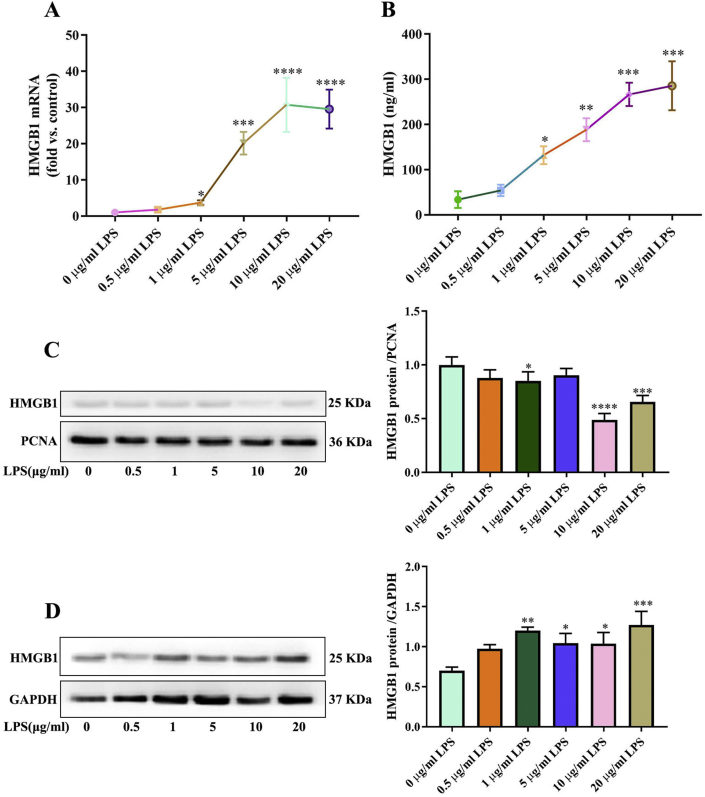
HMGB1 has been implicated in the pathogenesis of inflammatory diseases.24 To determine the mRNA expression levels of HMGB1 in inflammation status, relative quantification was performed by real-time PCR. These data displayed HMGB1 was significantly higher in LPS exposed than in non-stimulated, and the mRNA levels were consistently increased at a concentration-dependent manner (Fig. 2A). HMGB1 protein expression was analyzed by ELISA and Western blotting. Expression levels of HMGB1 protein in supernatants of cells exposed to LPS was significantly higher than with non-stimulated group (Fig. 2B). In the LPS group treated with 1 μg/ml, the expression of total HMGB1 protein increased significantly, but the nuclear HMGB1 protein decreased significantly. The results showed that HMGB1 transferred to the cytoplasm (Fig. 2C, D).
Figure 2.
HMGB1 is highly expressed in LPS-induced hPDLCs. (A) HMGB1 mRNA expression were examined by qPCR. (B) HMGB1 production detected by ELISA in culture medium. Total (D) and nuclear (C) HMGB1 protein expression were examined by Western blot. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
Metformin decreased inflammation cytokines expression in LPS-induced hPDLCs
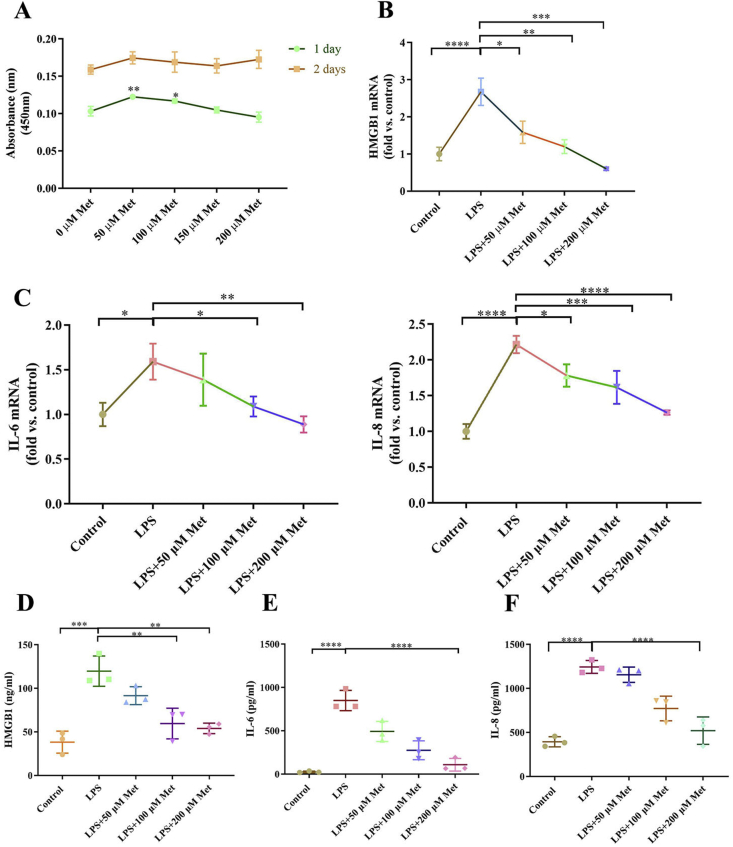
To examine whether metformin exert anti-inflammation effects on LPS-induced inflammation, the cell viability of metformin was first examined. The cell activity was not inhibited at 1 and 2 days after metformin (0–200 μM) exposure (Fig. 3A). To further confirm the protective effect of metformin, hPDLCs were measured by real-time PCR to analyze mRNA expression of the inflammatory cytokines. Metformin attenuated LPS-induced highly HMGB1, IL-6 and IL-8 mRNA expression in a dose-dependent manner, and 200 μM metformin was adequate to significantly reverse inflammation status (Fig. 3B, C). The concentration of HMGB1 in the culture medium of hPDLCs was also increased by LPS. Metformin decreased the levels of HMGB1 in the medium of the cells treated with LPS (Fig. 3D). The ELISA results also showed metformin dramatically down-regulated LPS-influenced IL-6 and IL-8 secreted in supernatant (Fig. 3E, F).
Figure 3.
Metformin alleviates LPS-induced hPDLCs. (A) Cell viability detected by the CCK-8 assay at different concentration of metformin. Inflammatory cytokines mRNA expression of HMGB1 (B), IL-6 and IL-8 (C) were examined by real-time PCR. Inflammatory cytokines production of HMGB1 (D), IL-6 (E) and IL-8 (F) were examined by ELISA in culture medium. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
Metformin inhabited HMGB1 translocation and releasing in LPS-induced hPDLCs
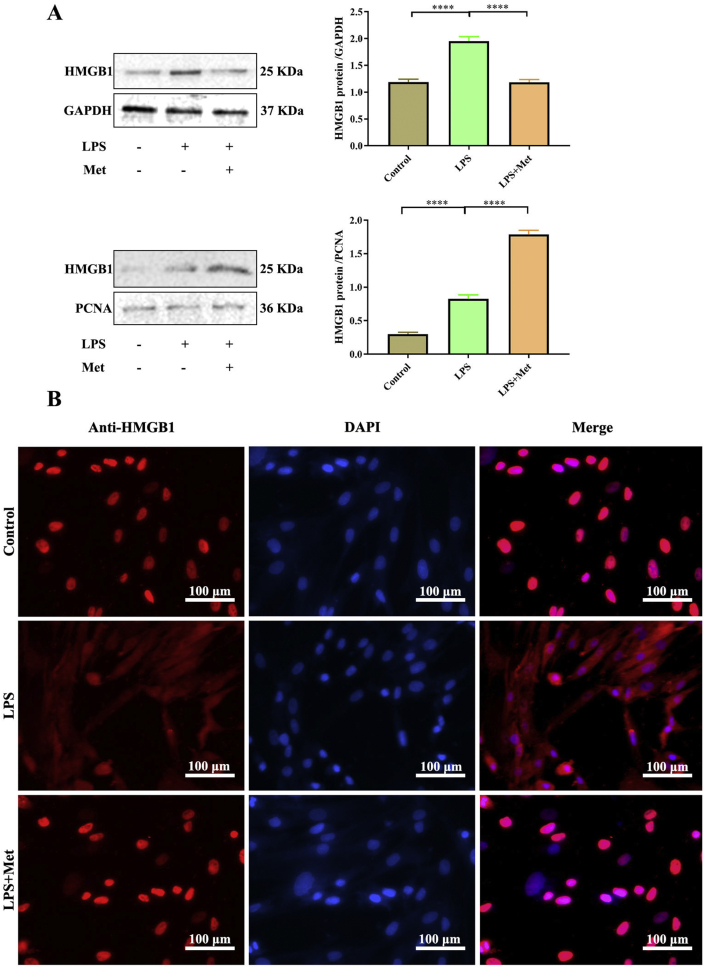
Metformin had an extracellular function that directly interacts with HMGB1 and inhibits its cytokine activity.25 Meanwhile, inhibition of HMGB1 has been demonstrated to ameliorate various diseases in animal models.1 The anti-inflammation effects of metformin and HMGB1 on the inflammatory response were further investigated by Western blot. Protein levels of total HMGB1 were enhanced in LPS exposed and decreased in metformin treatment. Nuclear HMGB1 protein level decreased in LPS exposure group and increased in metformin treatment group. Metformin inhibited the translocation of HMGB1 as demonstrated by the high percentage of HMGB1 expression noted in the nuclei of hPDLCs treated with metformin and LPS (Fig. 4A). Immunofluorescence staining indicated that HMGB1 was localized in the nuclei of normal hPDLCs. However, LPS induced HMGB1 release from the nuclei to the cytoplasm, which was blocked by 200 μM metformin treatment (Fig. 4B).
Figure 4.
Metformin alleviates LPS-induced hPDLCs via inhabiting HMGB1 translocation and releasing. (A) Total and nuclear HMGB1 protein expressions were examined by Western blot. (B) HMGB1 translocation and releasing was determined by immunofluorescence at 400× magnification. Red: HMGB1-staining, Blue: nucleus (DAPI) and Pink: merge of blue and red indicating nuclear localization of HMGB1. Scale bar = 100 μm. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
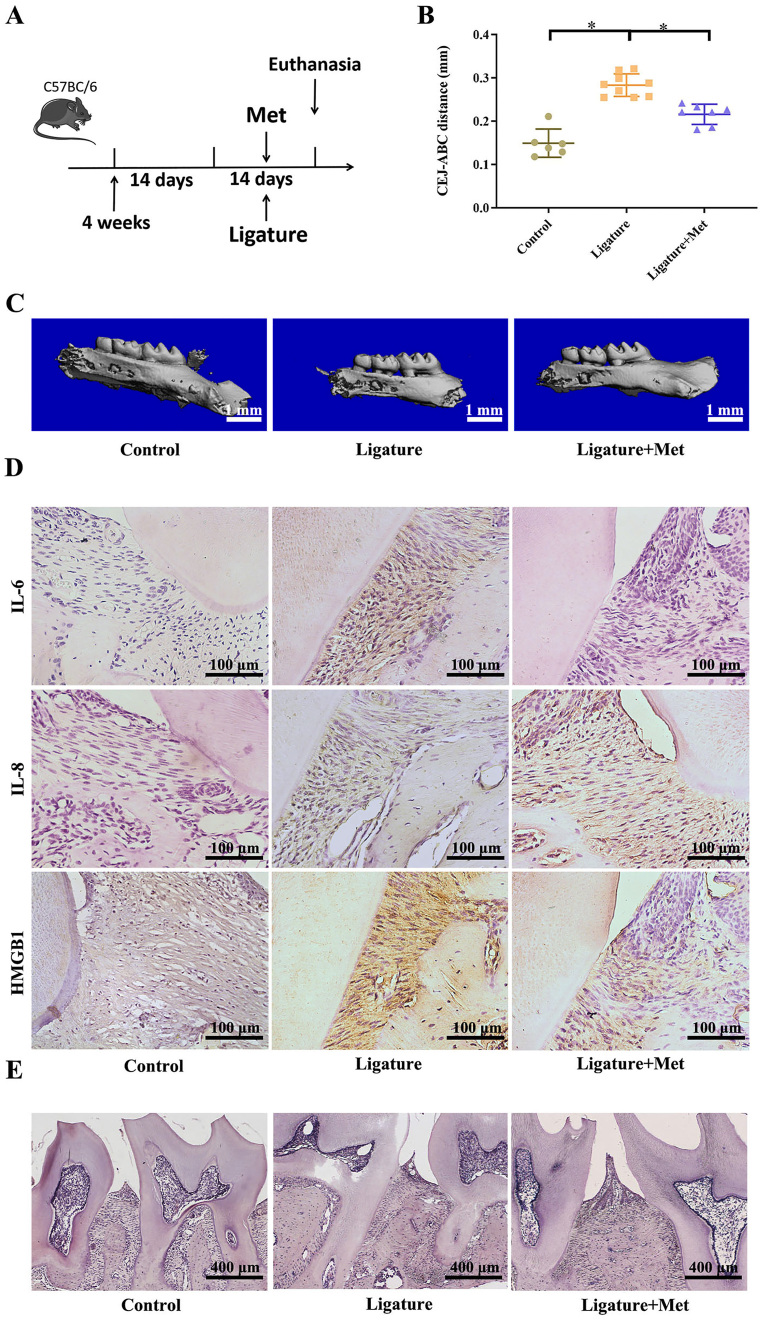
Metformin alleviates ligature induced-periodontitis
To determine relationship between HMGB1 and protective effect of metformin in experimental periodontitis, mice were treated with metformin or vehicle, daily for 14 days (Fig. 5A). Micro-CT imaging showed that CEJ-ABC distance showed that metformin treatment significantly reduced bone resorption in experimental periodontitis (Fig. 5B, C). The results showed that metformin significantly improved the inflammatory state and slowed down bone resorption. In addition, immunohistochemistry staining showed that mice in the ligature group showed significantly elevated expression of HMGB1, IL-6 and IL-8 in extracellular milieu, compared with those in the control, while reduced with metformin treatment (Fig. 5D). However, no significant difference was observed between the control and metformin groups, but the expression of inflammatory factors IL-6 and IL-8 was still higher than that of the control group. Histological analysis of H&E staining showed that bone resorption in the ligature group was increased compared with that of the control group, the infiltration of inflammatory cells was obvious, and the periodontal ligament was widened. However, it was rescued by metformin (Fig. 5E). It indicates that metformin can reduce the expression of inflammatory factors, reduce the release of extracellular inflammatory factors, and have a tendency to inhibit the transfer of inflammatory factors to extracellular cells.
Figure 5.
Metformin alleviates ligature induced-periodontitis. (A) Mice were subjected to ligature insertion for 14 d, with or without metformin treatment (every day, with 200 mg/kg). (B) CEJ-ABC distance. Control (no treatment), Ligature (ligature induced experimental periodontitis), Ligature + Met (experimental periodontitis with metformin treatment). (C) Micro CT image of the alveolar bone. (D) Immunohistochemical staining of HMGB1 at a 400x (scale bar = 100 μm) magnification. (E) Histological analysis of Hype staining showed that the bone resorption in the ligation group was higher than that in the control group. Data are presented as the mean ± SD (n = 6). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
mTOR pathway is involved in the antioxidant stress of metformin
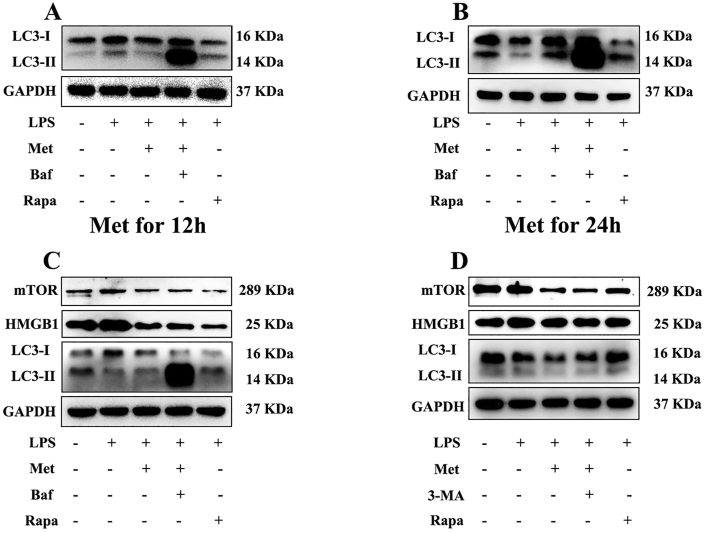
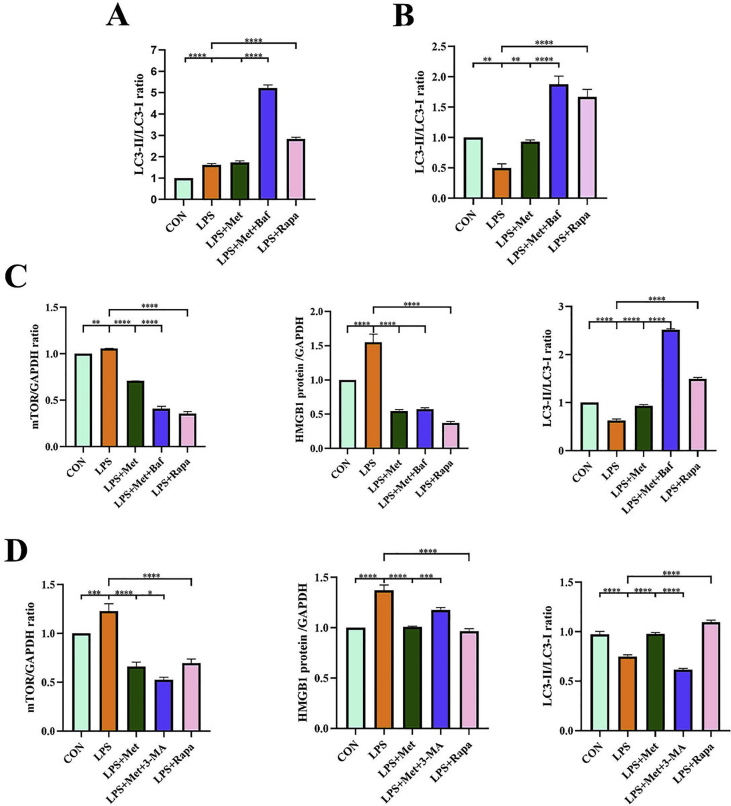
To discuss the role of autophagy in metformin antioxidant stress, we treated hPDLCs with autophagy inhibitors Bafilomycin A1, and then added metformin for 12 h and 24 h, respectively. There was significant difference in the ratio of LC3-II/LC3-I between metformin treatment group and LPS stimulation group in 24 h group. There was no significant statistical difference in the 12-hour treatment group. The results showed that the effect of metformin on autophagy was more obvious at 24 h, indicating that metformin promoted autophagy in a time-dependent manner (Fig. 6A, B; Fig. S1A, B). To further explore whether the mechanism of metformin inhibiting HMGB1 is related to mTOR, we added Rapamycin to the experimental group for positive control. Two autophagy inhibitors 3-MA and Bafilomycin A1 were added to pretreat hPDLCs respectively. Western Blotting results showed that mTOR expression was up-regulated after LPS treatment, mTOR expression was inhibited after metformin treatment, and mTOR expression was restored after adding autophagy inhibitor. This is consistent with the expression trend of HMGB1 (Fig. 6C, D; Fig. S1C, D). These results suggest that the mechanism of metformin inhibiting HMGB1 is related to mTOR-related autophagy.
Figure 6.
mTOR pathway is involved in the antioxidant stress of metformin. (A) hPDLCs was pretreated with autophagy inhibitors 3-MA and Bafilomycin A1, and then treated with metformin for 12 h, then the expression of autophagy related proteins was observed. (B) hPDLCs was pretreated with autophagy inhibitors 3-MA and Bafilomycin A1, and then treated with metformin for 24 h, then the expression of autophagy related proteins was observed. Pretreatment of periodontal ligament stem cells with different autophagy inhibitors bafilomycin a1 (C) and 3-MA (D). Then rapamycin was added to make positive control. Finally, periodontal ligament stem cells were treated with met for 24 h. Autophagy-related proteins were examined by Western blot. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
Discussion
Periodontitis is among the most widespread inflammatory problems encountered worldwide.26 Inflammation plays an important role in the pathogenesis of periodontitis. Excessive release of inflammatory cytokines will accelerate the resorption of alveolar bone and bring difficulties to periodontal tissue regeneration. At present, a number of studies to explore the treatment of periodontitis focus on inflammation control.27 Severe chronic inflammation further causes periodontal tissue destruction. The presence of oral pathogenic bacteria and their products evokes a series of host immune responses that mediate inflammatory events.28 Pro-inflammatory mediators and cytokines such as IL-1 β,11 IL-6 and IL-8 participate in the inflammatory response, stimulate osteoblasts to synthesize and secrete cytokines, and transmit signals to osteoclasts by paracrine or direct connection, resulting in bone resorption.29 Some studies have shown that systemic inflammatory parameters return to values closer to those of good health after periodontal treatment. This provides strong evidence for the effect of periodontitis on the state of the whole body.30 Therefore, periodontitis treatment becomes very important for the maintenance of general health.
Several studies have shown that there is a two-way relationship between periodontitis and diabetes, which aggravates periodontitis.30 Metformin is used in the treatment of type 2 diabetes.31 and is an affordable and well-tolerated drug. In addition to its antidiabetic effects, metformin has also been shown to be a potential drug for the treatment of inflammation-related diseases.8,32 A large number of studies have confirmed that oxidative stress plays a role in periodontal tissue destruction, and excessive ROSs in periodontal tissue can cause tissue destruction. Our previous studies have confirmed that metformin can improve the oxidative stress state of cells in periodontitis, thus partially restoring the osteogenic differentiation ability of hPDLCs.1 This study showed that the expression of inflammatory cytokines in hPDLCs induced by LPS was high, but decreased after metformin treatment. At the same time, these results were also confirmed by micro-CT imaging. CEJ-ABC distance showed that metformin significantly reduced the inflammatory response of experimental periodontitis. The above results suggest that metformin may have a protective effect on periodontal disease through its anti-inflammatory effect. Therefore, we want to further explore the mechanism of metformin in reducing periodontal tissue damage.
As a pro-inflammatory cytokine, HMGB1 has been confirmed to exist in periodontal inflammatory tissues and participate in the induction of oxidative stress in periodontal tissues.10 Previous studies have shown that inflammatory cytokines and bacterial components such as lipopolysaccharide (LPS) can induce the secretion of HMGB1, while HMGB1 is essential for the progression of inflammation.14 Nogueira et al detected a significant increase in the expression of HMGB1 mRNA and protein in hPDLCs, stimulated by LPS in vitro.33 Consistent with this result, our study shows that the expression of HMGB1 in the culture supernatant of hPDLCs stimulated by LPS is significantly increased, the expression of HMGB1 mRNA is enhanced after LPS stimulation, and the expression of inflammation-related cytokines such as IL-6 and IL-8 is also up-regulated. The up-regulation of these inflammatory cytokines promotes the development of the disease.16 The function of HMGB1 can depend on its location.18 When cells are stimulated, HMGB1 in the nucleus is released into the extracellular environment and participates in various inflammatory processes. Studies have shown that in periodontitis, due to the imbalance between the production of ROS and the process of antioxidation, the accumulation of a large amount of ROS. The accumulated ROS directly reflects the level of oxidative stress.19 A large number of studies have pointed out that HMGB1 and ROS regulate each other and show a synergistic effect.20 The accumulation of ROS can form a hyperoxic environment of the tissue, and a large amount of oxidized HMGB1 is released into the extracellular environment, which in turn promotes inflammation.18,34 Extracellular HMGB1 inhibits autophagy, further enhances oxidative stress, and induces apoptosis.21 Furthermore, HMGB1 physically binds to extracellular LPS and subsequently utilizes a RAGE-dependent internalization process to deliver LPS to enhance inflammation activation.35 After LPS exposure, metformin could reduce the up-regulated expression, nuclear and cytoplasmic translocation, and release of HMGB1. Immunohistochemistry showed that metformin inhibited the release of HMGB1 from nucleus to extracellular matrix in experimental periodontitis. It is suggested that metformin can reduce the inflammatory state by reducing the expression of HMGB1 and inhibiting the transfer of inflammatory factors to extracellular. Combined with the conclusions of previous experiments, it is proved that metformin reduces periodontal tissue damage by inhibiting the release of HMGB1. This is also consistent with our previous results that metformin can reduce oxidative stress. Therefore, we believe that HMGB1 is a key factor in the process of oxidative stress in periodontal cells.
Our previous studies have shown that the antioxidant stress mechanism of metformin may related to AMPK pathway and autophagy.1,9 As a key factor in the process of oxidative stress, HMGB1 can cause the change of autophagy.36 There is evidence that HMGB1 plays an important role in inflammation by activating mTOR pathway.37 Therefore, we speculate that metformin weakens the effect of HMGB1 through AMPK/mTOR signal pathway, which mediates the activation of antioxidation.38 By adding early autophagy inhibitor 3-MA and late autophagy inhibitor Baf-A1 to treat hPDLCs, results showed that LC3-II/LC3-I ratio and the expression of HMGB1 was increased or decreased accordingly, which was consistent with the previous studies.39 In our results, the ratio of LC3-II/LC3-I in LPS stimulation group increased after they having metformin treatment for 12 h. This may be due to the expression of HMGB1 promotes the initiation of autophagy in a short time, but cannot sustain the normal flow of autophagy, thus blocking the flux of autophagy.40 However, autophagy is a dynamic process and an autophagy flow, so it is not enough to observe the state of LC3.41 At the same time, it has been proved that HMGB1 has a high affinity with Beclin1 and participates in the expression of p62, which affects the whole process of autophagy.40,42 In order to further confirm the relationship and role of autophagy in metformin in reducing oxidative stress, further research is needed.
Conclusion
This study provides evidence for metformin to reduce the oxidative stress state of periodontitis and tissue destruction by regulating the expression, translocation, and release of HMGB1. It is proved that metformin can activate autophagy through mTOR pathway and then affect HMGB1. Since the translocation and release of HMGB1 play an important role in the pathological process of periodontal disease, our results provide a new idea for the mechanism of metformin in the prevention and treatment of periodontitis and a new perspective for the development of drugs for HMGB1. Nevertheless, there are also deficits in this study. In future studies, the protective role of metformin in periodontitis needs to be further confirmed using HMGB1 knockout mice. What's more, the initiation mechanism of autophagy affected by HMGB1 and the stage of the role of metformin in autophagy flow needs to be explored. As several studies have proved that oxidative stress was higher in diabetic periodontitis and metformin was a drug to fight against type 2 diabetes, metformin may be a useful tool for diabetic periodontitis.
Conflict of interests
The authors declare no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81771082, 31971282), Scientific and Technological Research Program of Chongqing Municipal Education Commission, China (No. KJQN202000417) and Chongqing Graduate Tutor Team, China (2019).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.06.003.
Contributor Information
Jie Li, Email: jieli@hospital.cqmu.edu.cn.
Jinlin Song, Email: songjinlin@hospital.cqmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
Quantifications of western blots in Fig. 6. (A), (B), (C) and (D) HMGB1, mTOR and LC3-II/LC3-I ratio were examined by western blot. Western blot's data are quantified. Data are presented as the mean ± SD (n = 3). ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗, P < 0.0005; ∗∗∗∗, P < 0.00005.
References
- 1.Kuang Y., Hu B., Feng G., et al. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology. 2020;21(1):13–27. doi: 10.1007/s10522-019-09838-x. [DOI] [PubMed] [Google Scholar]
- 2.Cekici A., Kantarci A., Hasturk H., et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000;64(1):57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullon P., Newman H., Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2000;64(1):139–153. doi: 10.1111/j.1600-0757.2012.00455.x. [DOI] [PubMed] [Google Scholar]
- 4.Köse O., Arabaci T., Kizildag A., et al. Melatonin prevents radiation-induced oxidative stress and periodontal tissue breakdown in irradiated rats with experimental periodontitis. J Periodontal Res. 2017;52(3):438–446. doi: 10.1111/jre.12409. [DOI] [PubMed] [Google Scholar]
- 5.Association A.U. Abstracts of the mid-Atlantic section of the American Urological Association 72nd annual meeting. Can J Urol. 2014;21(4):7404–7428. [PubMed] [Google Scholar]
- 6.Ranchoux B., Nadeau V., Bourgeois A., et al. Metabolic syndrome exacerbates pulmonary pypertension due to left heart disease. Circ Res. 2019;125(4):449–466. doi: 10.1161/CIRCRESAHA.118.314555. [DOI] [PubMed] [Google Scholar]
- 7.Tian R., Li R., Liu Y., et al. Metformin ameliorates endotoxemia-induced endothelial pro-inflammatory responses via AMPK-dependent mediation of HDAC5 and KLF2. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1701–1712. doi: 10.1016/j.bbadis.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Araújo A., Pereira A., Medeiros C., et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0183506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z., Gao X., Zhou M., et al. Effect of metformin on human periodontal ligament stem cells cultured with polydopamine-templated hydroxyapatite. Eur J Oral Sci. 2019;127(3):210–221. doi: 10.1111/eos.12616. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q., Guan X., Zuo X., et al. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm Sin B. 2016;6(3):183–188. doi: 10.1016/j.apsb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclerc P., Wähämaa H., Idborg H., et al. IL-1β/HMGB1 complexes promote the PGE2 biosynthesis pathway in synovial fibroblasts. Scand J Immunol. 2013;77(5):350–360. doi: 10.1111/sji.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appavoo E., Hajam I., Muneeswaran N., et al. Synergistic effect of high-mobility group box-1 and lipopolysaccharide on cytokine induction in bovine peripheral blood mononuclear cells. Microbiol Immunol. 2016;60(3):196–202. doi: 10.1111/1348-0421.12350. [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Kwak M., Kim S., et al. The role of high mobility group box 1 in innate immunity. Yonsei Med J. 2014;55(5):1165–1176. doi: 10.3349/ymj.2014.55.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang R., Chen R., Zhang Q., et al. HMGB1 in health and disease. Mol Aspect Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Bloom O., Zhang M., et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Han Y., Yuan F., Deng C., et al. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging(Albany NY) 2019;11(22):10252–10265. doi: 10.18632/aging.102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang D., Kang R., Van Houten B., et al. High mobility group box 1 (HMGB1) phenotypic role revealed with stress. Mol Med. 2014;20:359–362. doi: 10.2119/molmed.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrović A., Bogojević D., Korać A., et al. Oxidative stress-dependent contribution of HMGB1 to the interplay between apoptosis and autophagy in diabetic rat liver. J Physiol Biochem. 2017;73(4):511–521. doi: 10.1007/s13105-017-0574-0. [DOI] [PubMed] [Google Scholar]
- 19.Ying S., Tan M., Feng G., et al. Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress. Theranostics. 2020;10(21):9789–9807. doi: 10.7150/thno.42508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B., Yang N., Mo Z., et al. IL-17A enhances microglial response to OGD by regulating p53 and PI3K/Akt pathways with involvement of ROS/HMGB1. Front Mol Neurosci. 2017;10:e271. doi: 10.3389/fnmol.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Yang Y., Gao H., et al. Trehalose attenuates renal ischemia-reperfusion injury by enhancing autophagy and inhibiting oxidative stress and inflammation. Am J Physiol Ren Physiol. 2020;318(4):F994–F1005. doi: 10.1152/ajprenal.00568.2019. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T., Wu J., Ungvijanpunya N., et al. Smad6 methylation represses NFκB activation and periodontal inflammation. J Dent Res. 2018;97(7):810–819. doi: 10.1177/0022034518755688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves D., Fine D., Teng Y., et al. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35(2):89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S., Choi S., Shin J., et al. High-mobility group box 1 is associated with the inflammatory pathogenesis of Graves' orbitopathy. Thyroid. 2019;29(6):868–878. doi: 10.1089/thy.2018.0285. [DOI] [PubMed] [Google Scholar]
- 25.Horiuchi T., Sakata N., Narumi Y., et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem. 2017;292(20):8436–8446. doi: 10.1074/jbc.M116.769380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds I., Duane B. Periodontal disease has an impact on patients' quality of life. Evid Base Dent. 2018;19(1):14–15. doi: 10.1038/sj.ebd.6401287. [DOI] [PubMed] [Google Scholar]
- 27.Riccia D., Bizzini F., Perilli M., et al. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007;13(4):376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang R., Ho Y., Leung W., et al. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun. 2019;81:63–73. doi: 10.1016/j.bbi.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Wang C.Y., Stashenko P. Characterization of bone-resorbing activity in human periapical lesions. J Endod. 1993;19(3):107–111. doi: 10.1016/S0099-2399(06)80503-0. [DOI] [PubMed] [Google Scholar]
- 30.Herrera D., Molina A., Buhlin K., et al. Periodontal diseases and association with atherosclerotic disease. Periodontol. 2000;83(1):66–89. doi: 10.1111/prd.12302. 2020. [DOI] [PubMed] [Google Scholar]
- 31.Madariaga A., Goodwin P., Oza A. Metformin in gynecologic cancers: opening a new window for prevention and treatment? Clin Canc Res. 2020;26(3):523–525. doi: 10.1158/1078-0432.CCR-19-3645. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Zhang P., Wang Q., et al. Metformin ameliorates experimental diabetic periodontitis independently of mammalian target of rapamycin (mTOR) inhibition by reducing NIMA-related kinase 7 (Nek7) expression. J Periodontol. 2019;90(9):1032–1042. doi: 10.1002/JPER.18-0528. [DOI] [PubMed] [Google Scholar]
- 33.Nogueira A., de Souza J., de Molon R., et al. HMGB1 localization during experimental periodontitis. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/816320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdulmahdi W., Patel D., Rabadi M., et al. HMGB1 redox during sepsis. Redox Biol. 2017;13:600–607. doi: 10.1016/j.redox.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng M., Tang Y., Li W., et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang D., Kang R., Livesey K., et al. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxidants Redox Signal. 2011;15(8):2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arab H., Al-Shorbagy M., Saad M. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. 2021;335 doi: 10.1016/j.cbi.2021.109368. [DOI] [PubMed] [Google Scholar]
- 38.Tang C.J., Xu J., Ye H.Y., et al. Metformin prevents PFKFB3-related aerobic glycolysis from enhancing collagen synthesis in lung fibroblasts by regulating AMPK/mTOR pathway. Exp Ther Med. 2021;21(6) doi: 10.3892/etm.2021.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai H., Ma B., Dai X., et al. Shengma Biejia decoction inhibits cell growth in multiple myeloma by inducing autophagy-mediated apoptosis through the ERK/mTOR pathway. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.585286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J., Yang J., Shen Y., et al. HMGB1 mediates autophagy dysfunction via Perturbing Beclin1-Vps34 complex in dopaminergic cell model. Front Mol Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S., Choi J., Biering S.B., et al. Targeting by AutophaGy proteins (TAG): targeting of IFNG-inducible GTPases to membranes by the LC3 conjugation system of autophagy. Autophagy. 2016;12(7):1153–1167. doi: 10.1080/15548627.2016.1178447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misra M., Dikic I. RNA binding to p62 impacts selective autophagy. Cell Res. 2019;29(7):512–513. doi: 10.1038/s41422-019-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]