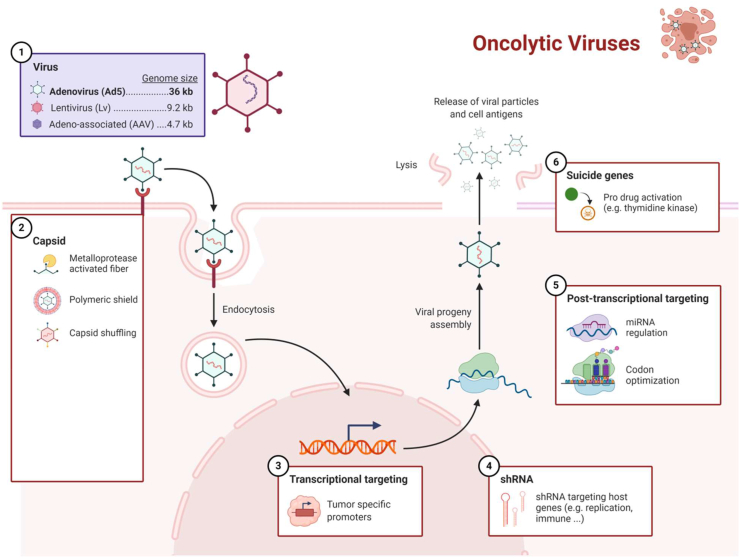
Abstract
Breast cancer, an unceasingly occurring neoplasm, is one of the major determinants of mortality in women. Several ineffective attempts have been pursued using with conventional therapies against breast cancer. Resistance to existing therapies and their respective debilitating adverse effects have led research toward a new era of cancer treatment using viruses. Virotherapy constitutes a developing treatment modality with multiple mechanisms of therapeutic activity in which the viruses can be directly oncolyticand can express transgenes or induce host immune response against tumor cells. Several different DNA- and RNA-containing viruses have been considered for virotherapy of breast cancer including adenovirus, herpes virus, vaccinia, reovirus, Newcastle Disease virus, measles virus and vesicular stomatitis virus. This review aims to summarize the viro-therapeutical agents against breast malignancies. Key Scientific Concepts of Review: In this review paper, we proposed a new strategy to virus's combinatorial treatments using several kinds of transgenes and drugs. These recombinant viruses have provided evidence of treatment efficacy against human breast cancer.
Keywords: Adenovirus, Breast cancer, Herpes virus, Measles virus, Newcastle disease virus, Reovirus, Vaccinia, Vesicular stomatitis virus, Virotherapy
Background
Breast cancer is the most frequently diagnosed malignancy in women,1, 2, 3, 4, 5, 6, 7 of whom it is one of the most prevalent cause of cancer related death worldwide.4,5,8 Since traditional therapies have limited ability to treat breast tumors, especially when metastatic,9,10 an alternative and more efficacious treatment approach is strongly recommended.10
The biological cycle of viruses involves cell infection, replication and subsequent cell death with release of a progeny of virions11 (Fig. 1). This vital feature of viruses has initiated the oncolytic virotherapy era for cancer treatment.12, 13, 14 This novel therapeutic strategy was first introduced in the early twentieth century15 and administered to cancer patients in 1996 in the first clinical trial16 resulting in cancer remission following viral infection.17
Figure 1.
Schematic view of cancer cell infection and lysis by oncolytic virus (OV).
An oncolytic virus (OV) is a natural or modified replication-selective virus infecting target tumor cells yet relatively neglecting normal cells.13,14,18, 19, 20, 21, 22, 23 This serendipitous feature indicates the large therapeutic potential of OVs thanks to great amplification inside tumor cells and concurrent clearance from normal cells. No severe adverse effects and no deaths have been recorded with virotherapy so far.14 In recent years the significant progress of molecular biology prompted the viral properties to be exploited in cancer therapy,11 using a wide variety of DNA and RNA OVs.21 Oncolytic virotherapy initially was not a successful method due to the inability to confine virulence exclusively to cancer cells.19 Characteristics of cancer cells including permanent division regardless of growth suppressors and DNA damage stress along with no cellular apoptosis and no proper response to host immune system have provided a suitable environment for OVs.21 The Interferon-beta signal pathway is deemed a deteriorated protective mechanism of cancer cells against viral infection, thereby facilitating virus replication inside tumor cells (Fig. 2).19
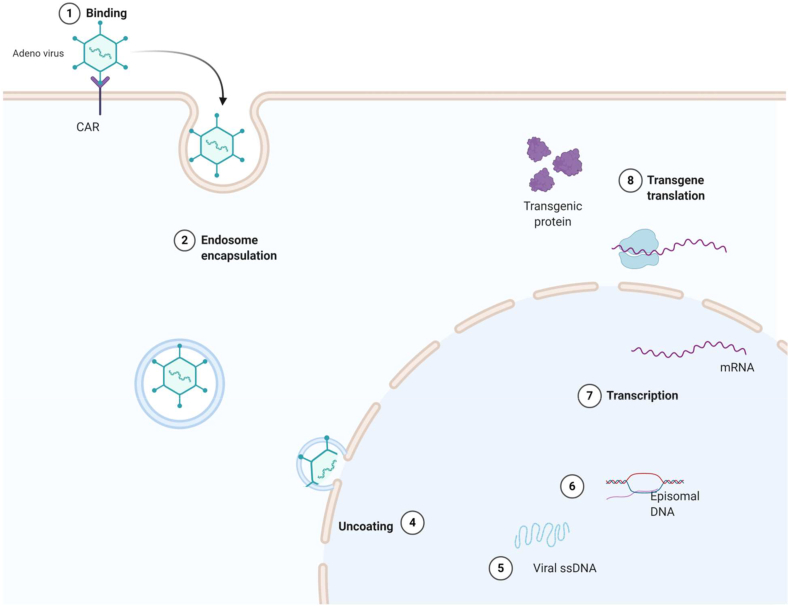
Figure 2.
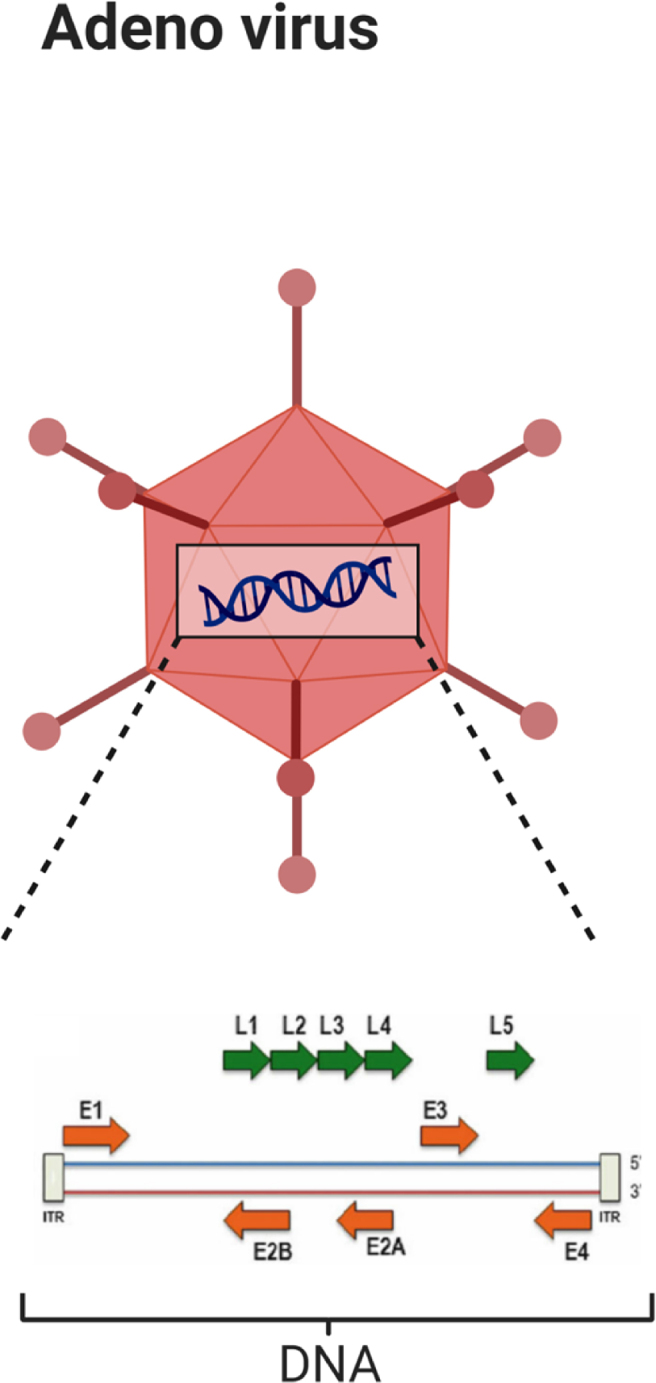
Schematic view of adenovirus.
OVs take advantage of the impaired cell signaling pathways to replicate properly in tumor cells.22 Oncolytic virus therapy is rather favorable compared to conventional therapeutic modalities because it is capable of overcoming the resistance against traditional treatment methods21; hence, low toxicity21,22 and a reasonable dose enhancement within tumor cells over time make OV therapy an appealing antineoplastic strategy.21
Oncolytic virus therapy has a dual mechanism of action13,19, 20, 21,23:
-
1)
Oncotoxic activity of the virus; and
-
2)
Systemic immunogenicity induced in host toward the OVs and tumor cells
The efficacy of OVs has proven both in vitro and in vivo by intravenous/systemic and local administration.11 The intravenous (I.V.) has been recognized as the simplest treatment route to deliver OV 21,24 though the virus neutralization by a robust antibody or the complement cascade, is a concrete concrete limitation of this procedure. To overcome this obstacle, cell carriers or delivery vehicles can be used to prevent virus recognition by the immune system and facilitate its delivery to the tumor.21,22 These vehicles include mesenchymal stem cells, monocytes, T cells and even autologous tumor cells.22
This paper examines the characteristics of viruses potential candidates for oncolytic therapy against breast cancer.
Discussion
DNA viruses
Adenovirus
Adenovirus is a small,25 nonenveloped,16,26, 27, 28 linear,28 double-stranded DNA virus16,26, 27, 28, 29 with a genome size varying from 26 to 45 kb in size,27 used in various studies as a safe OV4,27, 28, 29 (Fig. 2). Approximately 50 serotypes of adenoviruses have been identified so far11,26,27 with the serotype 2 and 5 tested most extensively in cancer trials.28,30, 31, 32 Serotype 5 has been introduced as an efficacious vector for breast cancer therapy.33 Adenovirus has low pathogenicity in humans,12,27,28,32 causing mild infections in the respiratory tract,27,28,32 gastro-intestinal (GI) tract and ophthalmic cavity.27 Adenoviruses are grouped into two categories:
-
1)
Replicative or oncolytic or replication-competent adenoviruses; and
-
2)
Non-replicative adenoviruses, result of removing E1-E3 genes.34
Adenovirus enters targets cells via receptor-mediated endocytosis35 by primary attachment with Coxsackie–adenovirus receptor (CAR). The entrance of the virus is mediated by the capsid arginine–glycine–aspartic acid motif interaction with αV integrins.27,29,30,32,35,36 The higher CAR expression, the higher number of target cells are infected with adenovirus. Moreover, there is an inverse correlation between tumor stage and aggressivity with CAR expression,31 which is low in various tumors including breast malignancies.4 CAR is down-regulated by excessive activity of the RAS-MAPK pathway in various types of cancers29; In contrast, histone deacetylase inhibitors (HDIs) known as a type of antineoplastic agents, upregulate CAR expression.22 Therefore, various signals can control CAR expression.33 Although transduction of tumor with viruses is almost dependent on CAR expression, the extracellular matrix (ECM) plays a crucial role in the infectivity of cancer cells31 (Fig. 3).
Figure 3.
Schematic representation of adenovirus binding and internalization via CAR and αV integrins, respectively.
Oncolytic adenoviruses have been grouped into four specific classes so far.37 Oncorine or H10119 or ONYX-015 (dl1520) enrolled as the first OV with genetic modification in humans.11,16 ONYX-015 is a replicative adenovirus with deletion in the E1-b region,11,16,19,27,32,37 resulting in the suppression of E1B-55kd expression.28 E1B-55kd is a p53-inhibitory protein and is selective for tumors with abolished p53 function11,37 since interfering the function of p53 is a condition for virus replication.16 The gene of interest is engineered into the deleted region27 (Table 1).
Table 1.
Summary of modified adenoviruses applied on breast malignancies.
| Applied virus | Design features | Benefit and limitation of the therapy | References |
|---|---|---|---|
| Ad5–Δ24–GMCSF | Featured by GMCSF gene and retargeted towards integrin | Disease stabilization in 3 of 7 patients with breast and colorectal cancer | 39 |
| Ad.dcn | Expressing human decorin gene | Oncolysis of metastatic breast cancer | 40 |
| ad5/IFN | Armed with human IFN gene | Oncolysis of human breast cancer xenografts | 28 |
| Ad5/3-Δ24 and Ad5.pk7-Δ24 | Capsid modification of Ad | Oncolysis of CD44+CD24−/low breast cancer cells in vitro and in vivo | 41, 42, 43 |
| Ad5ERE2 | Inserting a pS2 promoter section into the E1a and E4 promoters of Ad5 | Oncolysis of breast epithelial cells | 37 |
| Ad.DF3-E1 | Regulation of the E1A gene expression with MUC1 promoter | Oncolysis of breast cancer cells in vitro | 44 |
| CNHK600-IL24 | Armed with IL-24 gene | Oncolysis of breast tumor cells in vitro and in vivo | 45 |
Despite promising advances in viral therapy, some limitations feature adenoviruses as anti-cancer treatment, including38:
-
1)
Inefficacy of virus to reach metastatic lesions;
-
2)
Poor distribution in tumor lesions;
-
3)
Narrow target cancer cell spectrum;
-
4)
Difficulties in monitoring the transgenes being carried.
Although OVs do not necessarily need transgenes to perform as anti-cancer agents - unlike non_oncolytic viruses -, arming them with functional immunogenic transgenes including cytokines or prodrug-activating enzymes has the potential to strengthen their tumoricidal activity.16 Granulocyte macrophage-colony stimulating factor (GM-CSF) is a therapeutic transgene that has been introduced into the genome of several OVs.19 The antineoplastic activity of GM-CSF is attributed to T cell activation.16 Adenovirus vectors are the most frequently utilized viruses in gene therapy against malignant tumors,33 as for instance Ad5–Δ24–GMCSF, an oncolytic adenovirus armed with GM-CSF. The integrin retargeted form of this recombinant virus was tested on 7 patients with breast and colorectal cancer, 3 of whom showing disease stabilization.39 Furthermore, in a study by Yang et al40 an adenovirus encoding for decorin gene yielded promising antitumor results in a metastatic breast cancer model originating from MDA-MB-231 cells. In another study human interferon consensus gene was inserted into the genome of an oncolytic adenovirus providing encouraging results in inducing regression of human breast cancer xenografts.28 Cancer stem cells (CSC) are one of the key factors in cancer initiation, relapse, metastasis formation and resistance to treatment.3 Two types of modified OVs Ad5/3-Δ24 and Ad5.pk7-Δ24 showed promising results against breast cancer stem cells both in vitro and in vivo.41, 42, 43 Tissue- or tumor cell-specific promoters proved promising in developing potential therapeutic viruses, providing additional tissue selectivity.16,37 A conditionally replicative adenovirus named Ad5ERE2 was developed to target epithelial breast cells by inserting a pS2 promoter section with two estrogen response elements into the E1a and E4 promoters of parental Ad5 with the aim to regulate E1a and E4 genes expression.37 Since approximately 70% breast malignancies are considered to be estrogen-receptor positive,7,33,37 estrogen can activate this promoter thereby stimulating virus replication and enhancement in cancer therapy.37 On the other hand, an adenovirus was developed by regulating E1A gene expression with MUC1 promoter. MUC1 is an antigen being expressed aberrantly in approximately 80% of primary breast neoplasms. MUC1 promoter showed efficient gene transduction and expression of the viral gene in MUC1-expressing breast cancer cells.44 The antitumor effect of IL-24 has also been eye-catching since it causes cell cycle arrest when transduced with adenovirus. An Ad5 armed with IL-24 gene named CNHK600-IL24 provided potential effects on apoptosis and immune response stimulation, resulting in considerable disruption of breast tumor cells both in vitro and in vivo45 (Table 1).
HSV
Herpes simplex virus (HSV) is an enveloped, double-stranded DNA virus11,34,35 with a large linear genome of about 152 Kb,11,35 used in cancer therapy as a delivery vector for oncolysis.34 HSV-1 is more advantageous over other OVs due to46:
-
1)
A wider spectrum of target cells;
-
2)
Native oncotropism of the virus;
-
3)
Large genome with high capacity for transgenes;
-
4)
Availability of antiviral medicines in case of unfavorable replications;
-
5)
Avoidance of unwanted mutations
HF10 is a spontaneously attenuated form of HSV-1 that was tested in a pre-clinical study on breast cancer-bearing mice, showing prolonged survival rate in treated mice.1,34,47 These encouraging findings led to a clinical trial testing HF10 on six breast cancer patients, achieving a 30–40% tumor regression.47 Another pilot clinical trial investigating the effect of HF10 on tumor micro-environment of six patients with recurrent breast cancer showed potent antitumor immunity.1
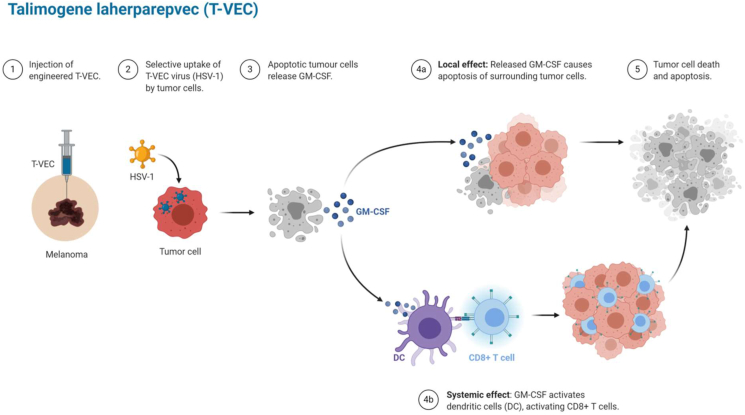
Talimogene laherparepvec (T-VEC), also known as or IMLYGIC48 or OncoVEX GM-CSF49 - a cancer therapeutic drug approved in the USA, Europe and Australia-was the first OV tested in humans.13,20 T-VEC is a live20 modified intralesional49 oncolytic HSV-1 with replacement of the herpes simplex virus ICP34.5 and ICP47 genes with two copies of the GM-CSF gene.13,47 The removal of the ICP34.5 gene limits the neuro-pathogenicity of HSV-1,13,20 enhancing the tumor-induced immunity with a long-term immunological memory thanks to GM-CSF factor.13,19,34 T-VEC was clinically tested in a phase 1 clinical trial in 2006 on 30 patients with various types of metastatic tumors, including patients with head and neck malignancies, colorectal cancer, refractory breast cancer and melanoma. There was no relation between antibody titers, severity of adverse effects and therapeutic responses in the latter clinical trial. Tumors injected with T-VEC showed size reduction and an inflammatory response was observed in 14 out of 19 biopsies. There was no evidence of necrosis in non-cancerous cells in support of tumor-specificity of the virus.49,50 In another phase 1 clinical trial on 14 breast cancer patients treated with OncoVEX GM-CSF, a reasonable efficacy and safety was detected in injected and metastatic lesions.51 G47Δ is a further oncolytic HSV-1 which proved as effective treatment against breast cancer patients19,46 (Fig. 4).
Figure 4.
Schematic view of T-VEC and oncolytic HSV-1 efficacy in tumor cell.
As mentioned above, arming viruses with immunogenic factors is a strategy to enhance their viro-therapeutical potential.51 In this regard, a γ134.5-deleted HSV-1 expressing IL-12 displayed more effective oncolytic activity against breast cancer with brain metastases compared to an oHSV without inserted genes.2 Furthermore, the insertion of an inhibitor of growth 4 (Ing4) (HSV1716Ing4) into an oHSV yielded high replication of virus in breast cancer cells in vivo. Combinational therapies have shown beneficial results in improving onco-selectivity and onco-toxicity of OVs.51 A synergic effect of oHSV HF10 and bevacizumab was observed in a pre-clinical study of human breast cancer xenografts.52 Furthermore, a potent antitumor effect and increased apoptosis of breast cancer cells both in vitro and in vivo studies was detected when paclitaxel and oHSV G47Δ were applied together53 (Table 2).
Table 2.
Summary of herpes simplex viruses applied on breast malignancies.
| Applied virus | Design features | Benefit and limitation of the therapy | References |
|---|---|---|---|
| HF10 | Naturally mutated HSV-1 | Prolonged survival rate in breast cancer-bearing mice | 47 |
| 30–100% regression of tumor in breast cancer patients | |||
| Oncolysis in 6 patients with recurrent breast tumor | 1 | ||
| HF10 + bevacizumab | Combination therapy | Oncolysis in human breast carcinoma xenografts | 52 |
| T-VEC or IMLYGIC or OncoVEXGM-CSF | Deletion of the herpes simplex virus ICP34.5 and ICP47 genes, armed with two copies of GM-CSF gene replaced in ICP34.5 gene region | Oncolysis in 30 patients with various types of metastatic tumors, including breast cancer | 49,50 |
| Reduction in tumor size | |||
| Oncolysis in regional and distant lesions in 14 breast cancer patients | 51 | ||
| M002 | γ134.5-deleted HSV-1 expressing IL-12 | Oncolysis against breast cancer with brain metastases | 2 |
| HSV1716Ing4 | Insertion of an inhibitor of growth 4 into an oHSV | Oncolysis in breast cancer cells in vivo | 51 |
| oHSV + Doxorubicin | Combination therapy | Oncolysis in a subcutaneous syngeneic model | 51 |
| oHSV + Mitoxantrone | Combination therapy | Increasing immune response and oncolysis in an immunocompetent model | 51 |
| HER2-retargeted oncolytic HSV | Generated by modification of single-chain antibody (scFv) to HER-2 in gD | >50% reduction in metastatic breast cancer in NSG mice | 56 |
| oHSV + Losartan | Combination therapy | Oncolysis by increasing oHSV dissemination within tumors | 51 |
| HSV-15PGDH | oHSV-1 expressing 15-prostaglandin dehydrogenase | Oncolysis and prevention of lung metastasis in breast cancer-bearing mice | 58 |
| HSV-1 1716 | Deletion of ICP34.5 | Oncolysis of primary breast tumors in a mouse model and reduction of lung metastasis | 59 |
| oHSV KM100 | ICP0 mutation of HSV-1 | Long term tumor regression in a breast cancer model with increased survival rate | 60 |
| G47Δ | A G207 with ICP47 and the US11 promoter deletions | Oncolysis of both primary and secondary breast tumors in brain | 61 |
| 9-fold reduction in lung metastasis of breast cancer models | 62 | ||
| oncolysis of breast cancer stem cells in vitro and in vivo | 3 | ||
| G47Δ + paclitaxel | A G207 with ICP47 and the US11 promoter deletions accompanied by paclitaxel | Increased apoptosis in breast cancer cells both in vitro and in vivo | 53 |
| Increased oncolysis against NCSCs and CSCs | 63 | ||
| G47Δ + mannitol | Combination therapy | Oncolysis and increased survival rate in nude mice bearing intracerebral human breast tumors | 24 |
| NV1042 | Insertion of a murine interleukin (IL)-12 in NV1023 | Oncolysis of peripheral breast tumors | 61 |
| NV1066 | Removing single-copy of ICP-4, ICP-0, and g134.5 on HSV-1 | Oncolysis of TNBC cell lines by targeting the MEK/MAPK pathway | 64 |
Another chemo-therapeutic drug, doxorubicin, was reported to elicit enhanced antitumor activity when combined with a Type-2 oHSV in a subcutaneous syngeneic model. Moreover, combining an oHSV with the chemo-therapeutic drug mitoxantrone increased neutrophils and CD8+ T cell infiltration, leading to enhanced immunity response and survival rate in an immuno-competent model.51 Moreover, the cytolytic effect of EGFR-CAR NK-92 cells was synergized in co-therapy with oHSV-1 both in vitro and in a mouse model bearing intracranial breast tumor54 (Table 2). Since 10–20% of breast cancers develop brain metastases, intra-carotid injection of oHSV G47Δ after blood brain barrier disruption followed by mannitol administration was undertaken on nude mice bearing intra-cerebral human breast tumors, significantly increasing their survival.24 Breast cancer is the most common cause of metastasis in meninges - affecting 5–8% of breast cancer patients -, with devastating impact on the respective survival. oHSV-1 showed promising therapeutic effect against meningeal metastatic from breast tumors.55 Furthermore, metastatic breast malignancies reduced by more than half with (I.V.) delivery of fully HER2-retargeted oHSV using mesenchymal stromal cells (MSCs) as a carrier in NSG mice.56
Several agents have been used to increase the viro-therapy effect against breast cancer.51 For instance, HDIs proved to increase oHSV replication in breast cancer cell lines than in normal breast cells.57 Some agents used to treat other diseases could also be used as combination therapy approach for breast cancer. For instance, losartan drug showed to increase oHSV dissemination within tumors by inhibiting collagen I synthesis from mammary carcinoma-associated fibroblasts in vitro. Though metastatic lesions are still a challenge in breast cancer treatment, I.V. delivery as well as local injection of some viruses provided promising perspectives.51 An oHSV expressing 15-prostaglandin dehydrogenase hampered the tumor growth and the development and accumulation of tumor cells in the lungs of mice treated by intra-lesional administration.58 In some cases, cancer therapy yielded long-lasting anti-neoplastic immune response.51 The HSV-1 1716 mutant, which is another version of ICP34.5-deleted HSV,11 was used to target primary tumors of an immuno-competent mouse model, showing moderate reduction of tumor growth rate, prolonged survival and a decreased rate of lung metastases.59 In an immunocompetent animal model, the injection of an ICP0 mutant HSV-1 virus designated oHSV KM100 into flank tumors yielded long-term tumor regression in 80% cases, averting tumor relapse and enhancing the survival rate.60
Two families of oHSV, the G207 and NV1020 series, were tested as breast cancer therapies in a mouse model, showing that third generation oHSV vector G47Δ, a G207, with deletions of ICP47 and US11 promoters, is efficacious against both primary and metastatic tumors of the brain, with vector NV1042 containing IL-12 being highly effective in the periphery.61 The efficacy of I.V. adminstration of oHSV G47Δ was also assessed on secondary lung tumors originating from breast cancer in nude mice, showing a 9-fold decrease rate of lung metastasis than in control mice.62 Oncolytic HSV G47Δ are seemingly effective in treating breast malignancies both in vitro and in vivo.3 Likewise, oHSV G47Δ was successfully directed towards CSCs in vitro, diminishing their propensity of self-renewal and inducing regression of BALB/c mice tumor xenografts. Furthermore, a synergic effect of paclitaxel against NCSCs and CSCs was achieved.63 OVs can take advantage of activated signaling pathways in cancer cells.64 Triple-negative breast cancer (TNBC) is a sub-division of breast neoplasms with considerably high relapse rate accompanied by poor health outcomes of the respective patients.Triple negative breast cancer (TNBC) accounts for approximately 15% of breast cancers65,66 and over-expressed levels of MEK/MAPK - a protein chain signaling from a surface cell receptor to the DNA in the nucleus of the cell - have been identified in TNBC. The effect of a genetically engineered HSV-1 (NV1066) on TNBC cell lines targeted the MEK/MAPK pathway and effectively treated TNBC64 (Table 2).
Since hypoxia affects 40% of breast cancers, whose cells become unresponsive to chemotherapy, oHSV vectors may be useful to treat hypoxic cancer cells. The concentration of HSV-1-derived OV was estimated to be nine-fold higher in hypoxic breast cancer cells when compared with normoxic counterparts.67
Vaccinia
Vaccinia virus is an enveloped,11,16,28,34 linear,16,68 double-stranded DNA virus11,16,28,34,68 with a large genome size of approximately 200 Kb11,28,35 belonging to the genus orthopoxvirus and the poxvirus family.68 Vaccinia virus is a genetically modified,69 cytoplasmically replicating virus,11,16,34,35,48,68,70 being appealing for oncolytic virotherapy71 in the past 20 years.68 The excision of two genes, including vaccinia growth factor (VGF) and thymidine kinase (TK) genes renders vaccinia virus exclusively selective for tumor.69,72 The unique features of vaccinia to fight tumors are:
- 1)
-
2)
Hematogenous dissemination with a reasonable stability and efficiency,14,34 differently from adenovirus and HSV38;
- 3)
-
4)
High immunogenic property28;
- 5)
-
6)
Elevated tumor tropism.34
Furthermore, vaccinia enters tumor cells via a unique way called apoptotic bleb mimicry a receptor-independent entry considered an advantage of vaccinia against other OVs.14 In a study with four breast cancer patients, the administration of 3 × 107 PFU of vaccinia within tumors was well tolerated and showed selective oncolytic features.69 JX-594 (also Pexa-Vec) is a vaccinia virus used as the first engineered oncolytic vaccinia virus for oncolytic therapy of cancer cells.19,68 JX-594 has a large transgene capacity14 and is a thymidine kinase gene deleted mutant armed with a human GM-CSF gene and a Lac-Z gene19,38 under the control of a synthetic early/late promoter and the p7.5 promoter, respectively.14 Although vaccinia is a virus with innate tumor cell specificity,38 the removal of the TK gene and the insertion of the above mentioned genes effectively limits virus replication in cancer cells.14,39 Low doses of JX-594 (<1 PFU/cell) proved beneficial in killing tumor cells, including breast cancer cells.14 The potency of vaccinia virus GLV-1h68 strain was assessed in breast cancer stem-like cells (CSC) and xenografts.74 GLV-1h68 was injected I.V. into human breast GI-101A cancer xenografts in nude mice to evaluate its oncolytic feature and replication capacity, showing complete regression and suppression of tumors, thanks to a reasonable tumor targeting, entry and amplification capacity.71 For several years, recombinant vaccinia viruses have been enrolled in gene delivery as well as cancer vaccines. Furthermore, there is evidence that vaccination against small-pox decreases the risk of some malignancies as melanoma and breast cancer due to cross-immunity against HERV-K oncogenes.75,76 Targeting vascular endothelial growth factor proved an advantageous therapeutic approach for breast cancer patients. With this view, a type of vaccinia virus was developed by arming it with a gene against vascular endothelial growth factor (VEGF). The anti-vasculature property of the virus yielded high anti-tumor activity in Triple negative breast cancer (TNBC) xenografts in an orthotopic murine model.65 An oncolytic vaccinia virus with CXCR4 antagonizing property was also designed against tumor vasculature. CXCR4 antagonizing resulted in anti-angiogenesis and tumor regression in TNBC syngenic mice with primary tumors. This oncolytic vaccinia virus also effectively prevented the metastasis risk and increased overall survival of mice.34,75 Since TNBC is an invasive breast cancer, complete margin-free surgical resection is recommended to enhance patients' survival.77 A recombinant vaccinia virus carrying the human sodium iodide symporter (hNIS) designated GLV_1h153 targeted positive surgical margins after resection of TNBC in a murine model.77 To augment the oncolysis effect of OVs, several other therapeutical agents involved in treatment of other diseases are also being employed against breast cancer.51 An oncolytic vaccinia virus named GLV-1h153 expressing the hNIS gene and combined with radionuclide I targeted orthotopic xenografts of MDA-M-231 cells in TNBC. Tumor regression was six-fold higher in the treatment group as compared to controls treated with parental virus.78 Furthermore, vaccinia virus VG9-IL-24 with the insertion of IL-24 was targeted against human breast cancer cells and xenografts, showing cell lysis and increased apoptosis.70 A rather promising double recombinant vaccinia virus was also created by inserting a combination of lactopin (human milk protein with anti-tumor properties) and GM-CSF genes. The VV-GMCSF-Lact exhibited remarkable suppression activity of tumor growth in breast cancer cells and xenografts.48 The efficacy and safety of a vaccinia virus recombined with tumor-associated antigen MUC1 and IL-2 genes was tested in breast cancer patients, showing no adverse effects and long-term T cells in tumor biopsies.34 Furthermore, a prime-boost immunization with a vector carrying tumor-associated antigen and co-stimulatory factors elicited significant anti-tumoral immune response. Taking into account all this evidence, PANVAC-VF, a cancer vaccine therapy delivered by two combining viral vectors encoding MUC1 and CEA antigens with human T-cell co-stimulatory molecules was developed against breast and ovarian cancers, yielding significant efficacy in limiting the tumor burden.39,79 Moreover, the median progression-free survival was 7.9 months in metastatic breast cancer patients treated with co-therapy of PANVAC and docetaxel and 3.9 months in the docetaxel-only treated group, respectively34,68 (Table 3).
Table 3.
Summary of modified vaccinia viruses applied on breast malignancies.
| Applied virus | Design features | Benefit and the limitation of the therapy | References |
|---|---|---|---|
| JX-594 (Pexa-Vec) | Deletion of a thymidine kinase gene plus arming with a human GM-CSF gene and a Lac-Z gene | Oncolysis of tumor cells including breast cancer cells | 14 |
| GLV-1h68 | – | Oncolysis of breast cancer stem-like cells (CSC) in cell line and xenografts | 74 |
| Oncolysis of human breast GI-101A cancer xenografts in nude mice | 71 | ||
| GLV-1h164 | Armed with a gene against VEGF | Oncolysis in TNBC xenografts in an orthotopic murine model | 65 |
| OVV-CXCR4-A | Armed with CXCR4 antagonist gene | Oncolysis in TNBC syngeneic mice with primary tumors | 34,75 |
| GLV_1h153 | Armed with the human sodium iodide symporter (hNIS) gene | Oncolysis of positive surgical margins after resection in TNBC murine model | 76 |
| GLV_1h153 + radionuclide I | Combination therapy | Six fold higher oncolysis in an orthotopic xenografts of MDA-M-231 cells in TNBC | 78 |
| VG9-IL-24 | Vaccinia virus strain Guang9 armed with IL-24 gene | Oncolysis of human breast cancer cells and xenografts | 70 |
| VV-GMCSF-Lact | Armed with lactopin and GM-CSF genes | Oncolysis in breast cancer cells and xenografts | 48 |
| VV-MUC1-IL-2 (TG-1031) | Armed with the tumor-associated antigen MUC1 and IL-2 genes | Potent oncolysis in advanced inoperable breast cancer recurrences | 34 |
| PANVAC-VF | Combined with human T-cell costimulatory molecules | Oncolysis of breast and ovarian cancers in limited tumor burden | 39,79 |
| PANVAC + docetaxel | Vaccinia prime, fowlpox boost | Combination therapy: Increased median progression-free survival in metastatic breast cancer patients | 34,68 |
RNA viruses
RNA viruses, featured by double-stranded RNA, are alternative prominent agents for viro-therapy against a wide range of cancers.80 Protein Kinase R (PKR), an interferon-inducible double stranded RNA-activated enzyme, plays a key role in protection against viral infection by promoting apoptosis, hence defects of PKR and/or interferon signaling pathway lead to selective replication of tumor cells and consequent viral propagation.16
Reovirus
REOvirus (Respiratory Enteric Orphan virus)81 is a non-enveloped, double-stranded, RNA-containing virus16,28,81, 82, 83 belonging to genus Orthoreoviridae16 and Reoviridae family.83 REOvirus is considered a benign virus16,28,81, 82, 83, 84 causing mild enteric and respiratory tract infections in humans.81,82,84 Reovirus is considered a naturally occurring OV28,43 and experimental investigations showed its ability to infect several cancer cell types including brain,43,84 breast,28,43,80,82,84 bladder,43 colon,28,43,80,82,84 glioma,80,82 ovaries,28,43,80,82,84 lymphoid,28,43,82 pancreatic,82,84 prostatic84 and spinal43 cancer cells. A reovirus like HSV-1 84 has an inherent propensity to infect cells with activated RAS pathway,11,16,47 featuring 30% of human malignancies.16,84 Whilst in normal cells PKR is phosphorylated and viral gene activation is not facilitated, in cancer cells the activation of RAS-pathway leads to a lytic infection.16 RAS pathways can be frequently activated by factors other than mutations of RAS itself.83,84 Although RAS mutations are infrequently observed in breast cancers,82,85 overexpression of pathways downstream of RAS leading to aberrant RAS activation is considered to be associated with breast tumor growth.82 Other mutations may occur in malignancies, including the activation of c-erbB gene, which is responsible for encoding EGF-R. C-erbB-2/HER2/neu is overexpressed in ovarian83,86 and breast cancers.85,86 Studies have shown that cells with an activated neu can be infected by reovirus.83 On the other hand, reovirus was introduced as a promising therapeutic agent for breast cancer with high RAS activity regardless of HER2 overexpression. No correlation was observed between sensitivity to reovirus and HER2 expression.82 Src is a family of non-receptor tyrosine kinases also implicated in breast cancer by activating the RAS pathway and inducing cell infection by reovirus.83 Additionally, reovirus takes advantage of the immune system stimulation by activating dendritic cells to combat cancer.17,81 By presenting the antigen, dendritic cells collaborate with reovirus to initiate innate and adaptive immune system responses against the virus regulating also their magnitude.17 Hence, reovirus proved capable of inducing a systemic effect even by intralesional injection.51 TRAIL (TNF-related apoptosis-inducing ligand) is a potential therapeutic ligand implicated in apoptosis. Research on HEK293 cell lines, lung cell lines (A157/H549) and breast malignancies (MDA231/ZR75-1) have shown that reovirus-infected cells are more susceptible to death by exogenous TRAIL.17 By contrast, immuno-suppression promoted reoviral-induced oncolysis in immune-competent animal models.84
Since cancer-initiating cells are resistant to commonly used cancer treatment, CSC may be targeted, as pointed out by a study on human breast cancer xenografts using an oncolytic reovirus, showing significant tumor regression and reduction of the CSC population equalizing the reduction of non-CSC tumor cells.43 To increase the treatment efficacy of reovirus, a study by Mostafa et al was done.
A study on murine breast cancer cells was undertaken to increase the treatment efficacy of reovirus by synergizing its oncolytic effect with the immune checkpoint blockade feature of PD-L1, showing promising results in terms of tumor regression and improved survival rate.87 Several studies have confirmed the oncolytic property of reovirus. In a study on six breast cancer cell lines, Hs578Bst normal mammary gland epithelial cell line (the control) showed no infection, upholding the concept of normal cells spared from reovirus infection differently from tumorous cells.17 Moreover, the oncolytic capacity of reovirus was tested on 8 severe combined immunodeficient (SCID) mice models whose hind flank was implanted with v-erbB–transformed NIH-3T3 fibroblasts, undergoing tumor regression in 6 of them 12 months following intra-lesion injection of the virus in tumor xenografts.83 The same outcome was achieved also against xenografts of other human cancer cell lines (breast, colorectal and prostate malignancies).83 Similar to other OVs, the efficacy of reovirus may be weakened by circulating antibodies, as confirmed by a phase I study on immunocompetent mice, whose tumors relapsed three weeks after an initial growth inhibition following systemic administration of reovirus, with the concomitant rise of serum anti-reoviral antibodies. In the latter study the maximum anti-reoviral antibody concentration was reached on day 7 for 36% mice and day 14 in 61% of them, suggesting that the systemic administration of reovirus should be fast, frequent and at high doses during the first week of treatment, before the rise of antiretroviral antibodies19 (Table 4).
Table 4.
Summary of reoviruses applied on breast malignancies.
| Applied virus | Design features | Benefit and the limitation of the therapy | References |
|---|---|---|---|
| Reovirus | – | Oncolysis of human breast cancer xenografts by targeting cancer stem cells (CSCs) | 43 |
| Oncolysis of six breast cancer cell lines and sparing the control Hs578Bst normal mammary gland epithelial cell line | 17 | ||
| Oncolysis of breast cancer cell lines as tumor xenografts on SCID mice models | 83 | ||
| Reovirus | Combined with PD-1 blockade | Oncolysis of murine breast cancer cells with increasing survival rate | 86 |
| Reovirus + topoisomerase inhibitors | Combination therapy | Potent oncolysis in triple-negative breast cancer patients | 66 |
| Pelareorep (Reolysin®) | Wild form of reovirus | Applied on 2 breast cancer patients that resulted in partial oncolysis with 34% tumor shrinkage in one of them | 47 |
| Increased median overall survival (OS) in 74 advanced breast cancer patients | 69 | ||
| REO-010 | Combination therapy of Reolysin + Docetaxel | Reasonable oncolysis of advanced tumors including breast cancer patients | 34 |
| REO-009 | Combination therapy of Reolysin + Gemcitabine | Partial oncolysis of advanced tumors including breast cancer patients | 34 |
A combination of topoisomerase inhibitors as DNA-damaging agents with reovirus yielded promising therapeutic effects in breast cancer therapy.66
Pelareorep69 (Reolysin®) is a wild form of reovirus34 administered for the first time in the USA to 2 patients affected by breast cancer and 16 other solid tumor-bearing patients to evaluate the maximum dose tolerated. A low toxicity resolving over time was observed with Reolysin, along with a partial response in one breast cancer, whose size shrank by 34%.47 Furthermore, is administration of Reolysin with docetaxel was also performed in a phase 1 clinical trial (REO-010) on patients affected by advanced tumors including breast cancer, exhibiting safe as well as reasonably effective antitumor response. A partial anti-tumor response was also observed in another phase I trial (REO-009) on co-therapy with Reolysin and gemcitabine in patients affected with advanced tumors.34 Finally, the synergistic effect of pelareorep combined with paclitaxel was evaluated in a phase II trial on 74 advanced breast cancer patients, showing a significantly improved median overall survival in the group undergoing combinatory therapy.69
Measles
Measles virus (MV) is an enveloped9,15,88 single-stranded RNA virus15 belonging to the genus Morbillivirus9,15 and the family of Paramyxoviruses,9,15,88 featured by a long non-segmented genome15,88 of 16 kb size15 (Table 5).
Table 5.
Summary of measles viruses applied on breast malignancies.
| Applied virus | Design features | Benefit and the limitation of the therapy | References |
|---|---|---|---|
| Measles | – | Managing pleural effusions in breast cancer patients by direct intrapleural and IV administration | 51,88 |
| MV-s-NAP and MV-lambda-NAP | Armed with helicobacter-pylori neutrophil-activating gene | Oncolysis in breast cancer metastases | 15,93 |
| rMV-SLAMblind | Engineered toward SLAM blindness | Oncolysis of PVRL4-positive breast cancer cells | 9 |
| rMV-BNiP3 | Armed with BNiP3 gene | Oncolysis in breast cancer cells in vitro | 8 |
| Measles + Aurora A kinase inhibitor alisertib | Combination therapy | Oncolysis in aggressive breast cancer cell lines and xenografts | 89,93 |
| Measles + small molecule inhibitor of the Rho family | Combination therapy | Oncolysis of breast cancer cells in vitro and in vivo | 51 |
| MV-m-uPA and MV-h-uPA | Designed as stromal-selective | Oncolysis of breast cancer in both syngeneic and xenotransplant models | 90 |
The anti-cancer property of MV was first discovered in 1949 by regression of Hodgkin's lymphoma following infection by wild type MV.89 The oncolytic MV is an attenuated vaccine strain derived from the Edmonston-B (MV-Edm) vaccine lineage,89 proving reasonable safety and potency as anti-cancer treatment both in vitro and in vivo preclinical studies.90 MV replicates totally in the cytoplasm,9 using CD46, CD150 and nectin-4 receptors to enter the cell. CD150 or signal lymphocyte-activation molecule (SLAM) is merely used by wild-type MVs 15 but CD150 also is found both in wild type and vaccine strains,9 hence SLAM-positive cells are the main targets of MVs.9 CD46, represented only in attenuated types of MVs,9,15 is a highly-expressed receptor in variety of cancers including breast malignancies91 and its density is a good determinant of targeted selectivity for attenuated MV.22,91 Nectin-4 receptor or poliovirus-receptor-like-4 (PVRL-4) is a transmembrane glycoprotein on epithelial cells featuring both wild types and attenuated MVs.9,15,89 Nectin-4 receptor, a reliable indicator of viral spread into human airways,9,15,89 is an up-regulated biomarker of breast tumor,9,89,92 ovarian and lung cancer.89,92 Cell to cell fusion of infected cells forming syncytia is an exclusive characteristics of the infection by MV leading to apoptosis.15
Attenuated MV strains look promising for oncolysis of human breast cancer xenografts.88,93 Tumor cell-specificity of MV is merely related to high expression of CD46 on cancer cells compared to non-cancerous normal cells.88 Expression of transgenes such as helicobacter-pylori neutrophil-activating protein by MVs showed effective antitumor immunity in breast cancer metastases.15,93
Direct intra-pleural and I.V. administration of MV proved effective as an oncolytic approach to manage pleural effusions in breast cancer.51,88 An engineered MV named rMV-SLAMblind, with mutations leading blindness to SLAM, was designed to infect breast cancer cells while sparing SLAM-positive lymphoid cells. This recombinant MV, which is both SLAM-negative and CD46-negative, was recognized to have a relevant effect on PVRL4-positive breast cancer cells9 (Table 5).
The oncolytic property of MV was also enhanced by insertional modifications, although a pro-apoptotic gene named BNiP3 was inserted into the MV genome (rMV-BNiP3) to make an armed MV proving effective to kill and induce apoptosis of breast cancer cells.8 Since combinational strategies maximize the oncolytic activity of OVs, the MV-mediated cytolysis has been increased by co-treatment with heat shock protein 90 (HSP90) inhibitors.51,89 Co-therapy of MV with geldanamycin (GA) as a HSP90 inhibitor enhanced the virus activity in several tumor cell lines.22 Moreover, the combination of Aurora A kinase inhibitor alisertib with MV, boosted the therapeutic effects of MV in aggressive breast cancer cell lines and xenografts.89,93 Additionally, the combinatorial effect of paclitaxel with oncolytic MV showed significant tumor growth inhibition.51 These satisfactory results pushed to investigate combinatorial strategies to maximize the effect of MVs against cancers,93 for instance by targeting cytoskeleton components. A small molecule inhibitor of the Rho family proved effective to improve the oncolytic effect of MV against breast cancer cells both in vitro and in vivo.51 Tumor micro-environment (TME) components are one of the key factors in viro-therapy efficiency. Tumor-associated macrophages (TAMs) are one of these components potentiating the antitumor property of OVs.94 The biological effect of OVs on TME plays a crucial role on tumor progression90 and factors targeting the tumor micro-environment are more effective.51 A stromal-selective MV, directed towards breast cancer cells in both syngeneic and xenotransplant models, mediated prevention of tumor progression and extended survival when compared with the control group90 (Table 5).
Newcastle disease virus (NDV)
Newcastle disease is a viral avian disease caused by a virus named Newcastle Disease Virus (NDV), firstly reported in Indonesia and England95 (Table 6). NDV is an enveloped,28,96 non-segmented, single-stranded RNA virus with a genome size of 15.9 kb95 belonging to the genus Rubulavirus and the family Paramyxoviridae.95,96 Oncolysis by NDV, an appealing anti-cancer agent with minimal side effects,97 was pioneered by Cassel et al in the 1960s98, 99, 100, 101 with NDV 73-T,98 a lytic strain98 replicating in cancerous cells and inducing syncytia formation and apoptosis.80,98 It is reported that the apoptotic mechanism of NDV 73-T strain uses the extrinsic death pathway based upon enhanced secretion of INF-alpha and TNF-alpha by peripheral blood mononuclear cells (PBMCs).98 Many studies on NDV 73-T strain against human tumor xenografts of neuroblastoma, fibrosarcoma, epidermoid, colon, lung, breast, prostate and subcutaneous IMR-32 neuroblastoma have shown significant tumor regression.28,80 A purified NDV AF2240 strain elicited apoptotis in 50% of cancer cells MDA-MB-231 in vitro, inhibiting also their proliferation, while it had no detrimental effect on non-cancerous human umbilical endothelial cells HUVECs and human epithelial breast cells line Hs578Bst. NDV-AF2240 replicates more efficiently in MDA-MB-231 breast cancer cells than in MCF-7 cells.102
Table 6.
Summary of Newcastle Disease Viruses applied on breast malignancies.
| Applied virus | Design features | Benefit and the limitation of the therapy | References |
|---|---|---|---|
| NDV AF2240 | – | Oncolysis in 50% of human breast cancer cell line MDA-MB-231 in vitro with no effect on noncancerous cells (HUVECs and Hs578Bst) | 97 |
| Oncolysis of MDA-MB-231 breast cancer cells compared to MCF-7 cells | 102 | ||
| NDV-PV701 | Naturally attenuated strain | Oncolysis and complete tumor regression in seventy-nine patients with advanced solid tumors including breast cancer | 98 |
| rNDV/IL2 | Armed with IL-2 | Oncolysis of several human cell lines including human mammary carcinoma cell line MCF-7, the human colon adenocarcinoma cell line HT29, and human Jurkat cell line | 98 |
| ATV-NDV | An autologous NDV-modified tumor vaccine | Increase host immunogenicity and oncolysis of various types of cancers including breast cancer | 100,103 |
| Increased survival rate, especially in early-stage breast cancer patients | 103 | ||
| NDV + radiofrequency hyperthermia (RHT) + dendritic cell (DC) vaccination | Combination therapy | Increased survival rate and quality of life in patients with secondary malignancy of breast in the liver | 106 |
| NDV + glycolysis inhibitor 2-Deoxyglucose (2-DG) | Combination therapy | Oncolysis of in vitro mouse and human breast cancer cell cultures and xenografts | 107 |
Several pre-clinical studies have been conducted with native and recombinant NDV.103 In a phase I pre-clinical study, the systemic administration of a naturally attenuated strain of NDV (PV701) to 79 patients with advanced solid tumors achieved complete tumor regression in several types of cancers with different origins, including epithelial derived cancers (breast, colon, lung, and prostate) and cancers with neuro-ectodermal and mesenchymal origins, with minimal toxicity even at high doses of NDV-PV70198 (Table 6). Therefore, NDV can be administered at high doses, and when the infusion time is extended from 1 to 3 h, the NDV toxicity progressively reduces.16 The immunogenicity of NDV can be amplified by adding IL-2 and GM-CSF recombinant genes to the viral coding sequence.22 In vitro studies with recombinant NDV, especially rNDV/IL2, have shown robust anti-cancer activity against several human cell lines including human mammary carcinoma cell line MCF-7, human colon adenocarcinoma cell line HT29, and human Jurkat cell line.98 Treating mice bearing tumor xenografts with NDV/IL2 also showed T-cell infiltration and tumor regression. Furthermore, the insertion of GM-CSF as an incorporated therapeutic gene activates the innate immunity amplifying the production of interferon and increasing the anti-neoplastic properties of NDV.104
Various clinical trials were performed on autologous tumor cell vaccine NDV (ATV-NDV) following inactivation of patient-derived tumor cells by irradiation,103,105 increasing the production of INF a/b and IL-2 thereby stimulating host immunogenicity. Considerable anti-cancer outcomes and increased survival were obtained by using ATV-NDV on various types of cancers, including breast, colorectal, ovarian, renal cell, head and neck and glial tumors.100,103
In end-stage breast and ovarian cancer patients and especially in early-stage breast cancer, two or more administrations of ATV-NDV vaccination significantly increased the survival rate of cancer patients.103 Schirrmacher indicated that the efficacy of ATV-NDV seems to vary by quality of the vaccine, showing about 36% higher five-year survival rate in high-grade breast cancer patients administered with high-quality vaccine than low-quality one.105
Combination of radiofrequency hyperthermia (RHT) with virotherapy by systemic oncolytic NDV and dendritic cell (DC) vaccination led to expansion of tumor-reactive memory T-cells resulting in longer survival (>66 months) in comparison to counterparts receiving conventional treatment. Furthermore, stable disease and a high quality of life were elicited.106
Since glucose uptake is higher in cancer cells compared to normal counterparts, targeting glucose metabolism is considered a rational strategy to reduce tumor growth. The combination of NDV and a glycolysis inhibitor 2-Deoxyglucose (2-DG) can have a synergic effect in vitro in mouse and human breast cancer cell cultures and xenografts.107
Vesicular stomatitis virus (VSV)
Vesicular stomatitis virus (VSV) is an enveloped,108, 109, 110, 111 negative, single-stranded RNA genome81,111,112 of 11 Kb size113 belonging to the genus Vesiculovirus of the Rhabdoviridae family.80,112 The five proteins encoded by VSV include the envelope glycoprotein (G), matrix (M) protein, nucleocapsid (N) protein, large polymerase (L) and phosphoprotein (P).111, 112, 113 Glycoprotein G cooperates with the infectious property of VSV in most mammalian cells.113
VSV is a another promising candidate for viro-therapy against malignant tumors108, 109, 110 (Table 7). Since it is highly sensitive to Interferon, VSV is safe for normal human cells but has oncolytic effect on cancer cells, featured by a malfunctioning antiviral immune response with diminished interferon signaling.22,81,114,115
Table 7.
Summary of vesicular stomatitis viruses applied on breast malignancies.
| Applied virus | Design features | Benefit and the limitation of the therapy | References |
|---|---|---|---|
| VSV-CD:UPRT + 5FC | Armed with the cytosine deaminase/uracil phosphoribosyl-transferase suicide gene and combined with prodrug 5-fluorocytosine | Increased oncolysis of non-infected bystander cells in vitro including breast MCF7 cells on tumor-bearing mice | 116 |
| VSV + VSe | Combination therapy of VSV with virus-sensitizer molecules | Enhanced VSV replication in VSV-resistant breast cancer cell lines | 117 |
| rVSV-IL4 | Armed with IL-4 gene | Oncolysis of breast and melanoma tumor xenografts | 112 |
| VSV-S-GP | The entire G gene on VSV was altered to a modified glycoprotein of Sindbis virus | High affinity towards human epidermal growth factor receptors overexpressed on breast cancer cells on mice and oncolysis of tumor | 113 |
| [A pseudotyped VSV] | The G gene of VSV was pseudotyped with the glycoprotein of a chimeric Sindbis virus containing the Fc region of a synthetic immunoglobulin G (IgG) targeting Staphylococcus aureus protein A | Targeted human epidermal growth factor receptor 2 (Her2/neu) in breast cancer | 112 |
| rrVSV-GMCSF | Armed with a mouse GM-CSF | Increased T cell response and faster tumor elimination by targeting her2 receptor-positive breast cancer cells | 112 |
| VSV-TK + ganciclovir | Combination therapy of an armed VSV with the prodrug ganciclovir | Oncolysis of melanoma or breast malignancy in immunocompetent mice | 113 |
| VSV (M51R)-LacZ | Containing the M51R mutation in the matrix protein gene | Oncolysis of metastatic lesions of experimental breast cancer-bearing mice | 114 |
The rapid replication kinetics of VSV (8–12 h) ensures an oncolytic activity before the production of neutralizing antibodies by the host 114. The combination of VSV with HDIs decreases the interferon-mediated antiviral effect, thereby enhancing the anticancer activity of VSV against several kinds of tumors.22
The combinational therapy of prodrug 5-fluorocytosine (5FC) with recombinant VSV encoding for cytosine deaminase/uracil phosphoribosyl-transferase suicide gene increased the anti-tumor activity of VSV and the bystander killing of uninfected cells in tumor bearing mice.116 Furthermore, in a virus/cell-based assay virus-sensitizer molecules were used to enhance VSV replication in VSV-resistant breast cancer cell lines.117
Immunotherapy can have a synergic effect on oncolysis induced by OVs. In this regard, recombinant VSV with the insertion of IL-4 gene (rVSV-IL4) functioned as a potent anti-tumor agent against breast and melanoma tumor xenografts.112
Several studies have been conducted restricting the replication of VSVs exclusively to tumor cells113 Generating viruses targeting receptors over-expressed in cancer cells is a promising means to enhance the their respective oncolytic function.51 In a mice study, the pseudotyped virus (VSV–S-GP) obtained by replacing the entire G gene of VSV with a modified glycoprotein of Sindbis virus, showed a high affinity towards human epidermal growth factor receptors overexpressed on breast cancer cells, reducing tumor bulk.113 Likewise, another pseudotyped virus replacing the G gene of VSV with the glycoprotein of a chimeric Sindbis virus containing the Fc region of a synthetic immunoglobulin G (IgG) targeting Staphylococcus aureus protein A showed high affinity against human epidermal growth factor receptor 2 (Her2/neu) of breast cancer cells.112 HER2 receptor-positive breast cancer cells were also targeted by another recombinant VSV with an insertion of mouse GM-CSF, showing increased T cell response and faster tumor elimination.112
To increase the bystander effect of VSV therapy, a VSV armed with the herpes thymidine kinase (TK) gene accompanied by the prodrug ganciclovir was administered to melanoma or breast malignancies in immuno-competent mice, significantly increasing the oncolytic and immunogenic activity of VSV.113
However, the virus activity can also be diminished by various factors and type I Interferon immunity was reportedly stimulated by macrophages surrounding various tumor cell lines (including breast tumors), giving these cells a VSV resistant status118 (Table 7).
Tamoxifen, an anti-estrogen receptor drug being used in estrogen receptor-positive breast cancers proved a stimulatory effect on macrophage activation and type I interferon-mediated immunity response. Consequently, pre-treatment with tamoxifen showed suppressive effects on VSV replication both in vitro and in vivo.119 Aside from in situ onco-toxicity of VSV, distant metastasis of breast adenocarcinoma are also responsive to VSV infection.120 In this regard, a matrix protein mutant VSV was administered systemically to experimental breast cancer-bearing mice, selectively replicating within metastatic lesions and manifesting effective oncolytic outcomes.114 There is evidence that CD4+ T-cells are crucial elements against tumor cells and OV immunotherapy by recombinant VSV generates a long-lasting immune memory in T cells compared to other conventional therapies121 (Table 7).
Conclusion
Viro-therapy is relatively a novel promising treatment for a wide range of human diseases, including cancer. Oncolytic viruses have led to integrated advances allowing more efficient and translatable therapies with very favorable risk–benefit ratio. Oncolytic viruses (e.g., Ad, NDV, HSV, reovirus, etc.) or alternative viruses (e.g., measles, poliovirus, VSV, vaccinia, etc.) can be tested against human cancers.
Non-engineered wild-type virus strains and innovative recombinant selectivity-enhanced viruses have shown limited success over the past 60 years in clinical trials as the first and second generation of oncolytic viruses, respectively. The use of third generation transgene-delivering armed oncolytic viruses has gained prominent appeal in clinical settings since their potency against tumor treatment is increased by engineering additional factors to viruses.
A growing body of evidence indicates that the development of new class of drugs (i.e., viral-based therapy) in combination with other therapies or approaches (e.g. checkpoint blockade immunotherapy [Opdivo, Keytruda, etc.]) can be applied to deal with the heterogeneous nature of the tumors. Combinatorial treatments of oncolytic viruses with several kinds of transgenes and drugs can achieve highly potent viro-therapeutical effect, especially against human breast cancer. Combinatorial therapies in preclinical trials can also combine chemotherapeutical and oncolytic viruses as well as oncolytic viruses and immunomodulating agents. Oncolytic virus immunotherapy will offer new class of drugs to clinicians to optimize cancer immunotherapy.
Author contributions
M. J., S.T., L.C., N.G.H., M.K., H.N., M.M., M.M., S.H.Z., M.K.H., M.I., S.F., and R.A.S., were responsible for drafting the manuscript, reading, writing and editing supervision and contributed equally to the development of the manuscript and its revision. All authors read and approved the final manuscript.
Conflict of interests
Authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Mohammad Javanbakht, Email: mhmjvbt81@gmail.com.
Sanaz Tahmasebzadeh, Email: sanaz_th91@yahoo.com.
References
- 1.Sahin T.T., Kasuya H., Nomura N., et al. Impact of novel oncolytic virus HF10 on cellular components of the tumor microenviroment in patients with recurrent breast cancer. Cancer Gene Ther. 2012;19(4):229–237. doi: 10.1038/cgt.2011.80. [DOI] [PubMed] [Google Scholar]
- 2.Cody J.J., Scaturro P., Cantor A.B., et al. Preclinical evaluation of oncolytic δγ(1)34.5Herpes simplex virus expressing interleukin-12 for therapy of breast cancer brain metastases. Int J Breast Cancer. 2012;2012:628697. doi: 10.1155/2012/628697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Zeng W., Huang Y., et al. Treatment of breast cancer stem cells with oncolytic Herpes simplex virus. Cancer Gene Ther. 2012;19(10):707–714. doi: 10.1038/cgt.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranki T., Särkioja M., Hakkarainen T., et al. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer. 2007;121(1):165–174. doi: 10.1002/ijc.22627. [DOI] [PubMed] [Google Scholar]
- 5.Bramante S., Koski A., Liikanen I., et al. Oncolytic virotherapy for treatment of breast cancer, including triple-negative breast cancer. Oncoimmunology. 2016;5(2) doi: 10.1080/2162402X.2015.1078057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garza-Morales R., Gonzalez-Ramos R., Chiba A., et al. Temozolomide enhances triple-negative breast cancer virotherapy in vitro. Cancers. 2018;10(5):E144. doi: 10.3390/cancers10050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lypova N., Lanceta L., Gibson A., et al. Targeting palbociclib-resistant estrogen receptor-positive breast cancer cells via oncolytic virotherapy. Cancers. 2019;11(5):E684. doi: 10.3390/cancers11050684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lal G., Rajala M.S. Combination of oncolytic measles virus armed with BNiP3, a pro-apoptotic gene and paclitaxel induces breast cancer cell death. Front Oncol. 2019;8:676. doi: 10.3389/fonc.2018.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T., Yoneda M., Kuraishi T., et al. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013;20(3):338–347. doi: 10.1038/gt.2012.44. [DOI] [PubMed] [Google Scholar]
- 10.Li L., Xie X., Luo J., et al. Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol Ther. 2012;20(12):2326–2334. doi: 10.1038/mt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins L.K., Lemoine N.R., Kirn D. Oncolytic biotherapy: a novel therapeutic platform. Lancet Oncol. 2002;3(1):17–26. doi: 10.1016/s1470-2045(01)00618-0. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Navarro J., Curiel D.T. Conditionally replicative adenoviral vectors for cancer gene therapy. Lancet Oncol. 2000;1:148–158. doi: 10.1016/s1470-2045(00)00030-9. [DOI] [PubMed] [Google Scholar]
- 13.Rehman H., Silk A.W., Kane M.P., et al. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parato K.A., Breitbach C.J., Le Boeuf F., et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aref S., Bailey K., Fielding A. Measles to the rescue: a review of oncolytic measles virus. Viruses. 2016;8(10):E294. doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghi M., Martuza R.L. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 17.Maitra R., Ghalib M.H., Goel S. Reovirus: a targeted therapeutic: progress and potential. Mol Cancer Res. 2012;10(12):1514–1525. doi: 10.1158/1541-7786.MCR-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne S.H., Negrin R.S., Contag C.H. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311(5768):1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 19.Fukuhara H., Ino Y., Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohlhapp F.J., Kaufman H.L. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22(5):1048–1054. doi: 10.1158/1078-0432.CCR-15-2667. [DOI] [PubMed] [Google Scholar]
- 21.Chiocca E.A., Rabkin S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.L., Tumilasci V.F., Singhroy D., et al. The emergence of combinatorial strategies in the development of RNA oncolytic virus therapies. Cell Microbiol. 2009;11(6):889–897. doi: 10.1111/j.1462-5822.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 23.Pol J., Buqué A., Aranda F., et al. Trial Watch-Oncolytic viruses and cancer therapy. OncoImmunology. 2015;5(2) doi: 10.1080/2162402X.2015.1117740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R., Martuza R.L., Rabkin S.D. Intracarotid delivery of oncolytic HSV vector G47Delta to metastatic breast cancer in the brain. Gene Ther. 2005;12(8):647–654. doi: 10.1038/sj.gt.3302445. [DOI] [PubMed] [Google Scholar]
- 25.Hermiston T.W., Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002;9(12):1022–1035. doi: 10.1038/sj.cgt.7700542. [DOI] [PubMed] [Google Scholar]
- 26.Yang C., Chen H., Yu L., et al. Inhibition of FOXM1 transcription factor suppresses cell proliferation and tumor growth of breast cancer. Cancer Gene Ther. 2013;20(2):117–124. doi: 10.1038/cgt.2012.94. [DOI] [PubMed] [Google Scholar]
- 27.Young L.S., Searle P.F., Onion D., et al. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208(2):299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 28.Everts B., van der Poel H.G. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12(2):141–161. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 29.Bauerschmitz G.J., Barker S.D., Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review) Int J Oncol. 2002;21(6):1161–1174. [PubMed] [Google Scholar]
- 30.Ring C.J. Cytolytic viruses as potential anti-cancer agents. J Gen Virol. 2002;83(Pt 3):491–502. doi: 10.1099/0022-1317-83-3-491. [DOI] [PubMed] [Google Scholar]
- 31.Shayakhmetov D.M., Li Z.Y., Ni S., et al. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62(4):1063–1068. [PubMed] [Google Scholar]
- 32.Vorburger S.A., Hunt K.K. Adenoviral gene therapy. Oncologist. 2002;7(1):46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 33.Lucas A., Kremer E.J., Hemmi S., et al. Comparative transductions of breast cancer cells by three DNA viruses. Biochem Biophys Res Commun. 2003;309(4):1011–1016. doi: 10.1016/j.bbrc.2003.08.101. [DOI] [PubMed] [Google Scholar]
- 34.Asad A.S., Moreno Ayala M.A., Gottardo M.F., et al. Viral gene therapy for breast cancer: progress and challenges. Expert Opin Biol Ther. 2017;17(8):945–959. doi: 10.1080/14712598.2017.1338684. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z.S., Thorne S.H., Bartlett D.L. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785(2):217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jounaidi Y., Doloff J.C., Waxman D.J. Conditionally replicating adenoviruses for cancer treatment. Curr Cancer Drug Targets. 2007;7(3):285–301. doi: 10.2174/156800907780618301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez-Alcoceba R., Pihalja M., Wicha M.S., et al. A novel, conditionally replicative adenovirus for the treatment of breast cancer that allows controlled replication of E1a-deleted adenoviral vectors. Hum Gene Ther. 2000;11(14):2009–2024. doi: 10.1089/10430340050143435. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.H., Oh J.Y., Park B.H., et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14(3):361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Cawood R., Hills T., Wong S.L., et al. Recombinant viral vaccines for cancer. Trends Mol Med. 2012;18(9):564–574. doi: 10.1016/j.molmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y., Xu W., Neill T., et al. Systemic delivery of an oncolytic adenovirus expressing decorin for the treatment of breast cancer bone metastases. Hum Gene Ther. 2015;26(12):813–825. doi: 10.1089/hum.2015.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eriksson M., Guse K., Bauerschmitz G., et al. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24−/low cells. Mol Ther. 2007;15(12):2088–2093. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- 42.Short J.J., Curiel D.T. Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther. 2009;8(8):2096–2102. doi: 10.1158/1535-7163.MCT-09-0367. [DOI] [PubMed] [Google Scholar]
- 43.Marcato P., Dean C.A., Giacomantonio C.A., et al. Oncolytic reovirus effectively targets breast cancer stem cells. Mol Ther. 2009;17(6):972–979. doi: 10.1038/mt.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurihara T., Brough D.E., Kovesdi I., et al. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Investig. 2000;106(6):763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu W., Wei L., Zhang H., et al. Oncolytic adenovirus armed with IL-24 inhibits the growth of breast cancer in vitro and in vivo. J Exp Clin Cancer Res. 2012;31(1):51. doi: 10.1186/1756-9966-31-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varghese S., Rabkin S.D. Oncolytic Herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9(12):967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 47.McCrudden C.M., McCarthy H.O. Current status of gene therapy for breast cancer: progress and challenges. Appl Clin Genet. 2014;7:209–220. doi: 10.2147/TACG.S54992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kochneva G., Sivolobova G., Tkacheva A., et al. Engineering of double recombinant vaccinia virus with enhanced oncolytic potential for solid tumor virotherapy. Oncotarget. 2016;7(45):74171–74188. doi: 10.18632/oncotarget.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington K.J., Puzanov I., Hecht J.R., et al. Clinical development of talimogene laherparepvec (T-VEC):a modified Herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev Anticancer Ther. 2015;15(12):1389–1403. doi: 10.1586/14737140.2015.1115725. [DOI] [PubMed] [Google Scholar]
- 50.Hu J.C., Coffin R.S., Davis C.J., et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic Herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 51.Cody J.J., Hurst D.R. Promising oncolytic agents for metastatic breast cancer treatment. Oncolytic Virother. 2015;4:63–73. doi: 10.2147/OV.S63045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan G., Kasuya H., Sahin T.T., et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int J Cancer. 2015;136(7):1718–1730. doi: 10.1002/ijc.29163. [DOI] [PubMed] [Google Scholar]
- 53.Zeng W.G., Li J.J., Hu P., et al. An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncol Rep. 2013;29(6):2355–2361. doi: 10.3892/or.2013.2359. [DOI] [PubMed] [Google Scholar]
- 54.Chen X., Han J., Chu J., et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764–27777. doi: 10.18632/oncotarget.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuruppu D., Tanabe K.K. HSV-1 as a novel therapy for breast cancer meningeal metastases. Cancer Gene Ther. 2015;22(10):506–508. doi: 10.1038/cgt.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leoni V., Gatta V., Palladini A., et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget. 2015;6(33):34774–34787. doi: 10.18632/oncotarget.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cody J.J., Markert J.M., Hurst D.R. Histone deacetylase inhibitors improve the replication of oncolytic Herpes simplex virus in breast cancer cells. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker J.D., Sehgal I., Kousoulas K.G. Oncolytic Herpes simplex virus 1 encoding 15-prostaglandin dehydrogenase mitigates immune suppression and reduces ectopic primary and metastatic breast cancer in mice. J Virol. 2011;85(14):7363–7371. doi: 10.1128/JVI.00098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas D.L., Fraser N.W. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8(4):543–551. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 60.Hummel J.L., Safroneeva E., Mossman K.L. The role of ICP0-Null HSV-1 and interferon signaling defects in the effective treatment of breast adenocarcinoma. Mol Ther. 2005;12(6):1101–1110. doi: 10.1016/j.ymthe.2005.07.533. [DOI] [PubMed] [Google Scholar]
- 61.Liu R., Varghese S., Rabkin S.D. Oncolytic Herpes simplex virus vector therapy of breast cancer in C3(1)/SV40 T-antigen transgenic mice. Cancer Res. 2005;65(4):1532–1540. doi: 10.1158/0008-5472.CAN-04-3353. [DOI] [PubMed] [Google Scholar]
- 62.Wang J., Hu P., Zeng M., et al. Oncolytic herpes simplex virus treatment of metastatic breast cancer. Int J Oncol. 2012;40(3):757–763. doi: 10.3892/ijo.2011.1266. [DOI] [PubMed] [Google Scholar]
- 63.Zeng W., Hu P., Wu J., et al. The oncolytic herpes simplex virus vector G47Δ effectively targets breast cancer stem cells. Oncol Rep. 2013;29(3):1108–1114. doi: 10.3892/or.2012.2211. [DOI] [PubMed] [Google Scholar]
- 64.Gholami S., Chen C.H., Gao S., et al. Role of MAPK in oncolytic Herpes viral therapy in triple-negative breast cancer. Cancer Gene Ther. 2014;21(7):283–289. doi: 10.1038/cgt.2014.28. [DOI] [PubMed] [Google Scholar]
- 65.Gholami S., Marano A., Chen N.G., et al. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014;148(3):489–499. doi: 10.1007/s10549-014-3180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodríguez Stewart R.M., Berry J.T.L., Berger A.K., et al. Enhanced killing of triple-negative breast cancer cells by reassortant reovirus and topoisomerase inhibitors. J Virol. 2019;93(23):e01411–e01419. doi: 10.1128/JVI.01411-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fasullo M., Burch A.D., Britton A. Hypoxia enhances the replication of oncolytic Herpes simplex virus in p53- breast cancer cells. Cell Cycle. 2009;8(14):2194–2197. doi: 10.4161/cc.8.14.8934. [DOI] [PubMed] [Google Scholar]
- 68.Guo Z.S., Lu B., Guo Z., et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. 2019;7(1):6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eissa I.R., Bustos-Villalobos I., Ichinose T., et al. The Current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers. 2018;10(10):E356. doi: 10.3390/cancers10100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L., Fan J., Ding Y., et al. Target therapy with vaccinia virus harboring IL-24 for human breast cancer. J Cancer. 2020;11(5):1017–1026. doi: 10.7150/jca.37590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q., Yu Y.A., Wang E., et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67(20):10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 72.Zeh H.J., Downs-Canner S., McCart J.A., et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23(1):202–214. doi: 10.1038/mt.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alemany R. A smart move against cancer for vaccinia virus. Lancet Oncol. 2008;9(6):507–508. doi: 10.1016/S1470-2045(08)70136-0. [DOI] [PubMed] [Google Scholar]
- 74.Wang H., Chen N.G., Minev B.R., et al. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J Transl Med. 2012;10:167. doi: 10.1186/1479-5876-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gil M., Seshadri M., Komorowski M.P., et al. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cegolon L., Salata C., Weiderpass E., et al. Human endogenous retroviruses and cancer prevention: evidence and prospects. BMC Cancer. 2013;13:4. doi: 10.1186/1471-2407-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gholami S., Chen C.H., Belin L.J., et al. Vaccinia virus GLV-1h153 is a novel agent for detection and effective local control of positive surgical margins for breast cancer. Breast Cancer Res. 2013;15(2):R26. doi: 10.1186/bcr3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gholami S., Chen C.H., Lou E., et al. Vaccinia virus GLV-1h153 in combination with 131I shows increased efficiency in treating triple-negative breast cancer. FASEB J. 2014;28(2):676–682. doi: 10.1096/fj.13-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohebtash M., Tsang K.Y., Madan R.A., et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17(22):7164–7173. doi: 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russell S.J. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9(12):961–966. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- 81.Kim M., Chung Y.H., Johnston R.N. Reovirus and tumor oncolysis. J Microbiol. 2007;45(3):187–192. [PubMed] [Google Scholar]
- 82.Hata Y., Etoh T., Inomata M., et al. Efficacy of oncolytic reovirus against human breast cancer cells. Oncol Rep. 2008;19(6):1395–1398. [PubMed] [Google Scholar]
- 83.Norman K.L., Lee P.W. Reovirus as a novel oncolytic agent. J Clin Investig. 2000;105(8):1035–1038. doi: 10.1172/JCI9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirasawa K., Nishikawa S.G., Norman K.L., et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63(2):348–353. [PubMed] [Google Scholar]
- 85.Yang W.Q., Senger D.L., Lun X.Q., et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Ther. 2004;11(21):1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- 86.Campadelli-Fiume G., Petrovic B., Leoni V., et al. Retargeting strategies for oncolytic Herpes simplex viruses. Viruses. 2016;8(3):63. doi: 10.3390/v8030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mostafa A.A., Meyers D.E., Thirukkumaran C.M., et al. Oncolytic reovirus and immune checkpoint inhibition as a novel immunotherapeutic strategy for breast cancer. Cancers. 2018;10(6):E205. doi: 10.3390/cancers10060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iankov I.D., Msaouel P., Allen C., et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122(3):745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Msaouel P., Opyrchal M., Dispenzieri A., et al. Clinical trials with oncolytic measles virus: current status and future prospects. Curr Cancer Drug Targets. 2018;18(2):177–187. doi: 10.2174/1568009617666170222125035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jing Y., Chavez V., Ban Y., et al. Molecular effects of stromal-selective targeting by uPAR-retargeted oncolytic virus in breast cancer. Mol Cancer Res. 2017;15(10):1410–1420. doi: 10.1158/1541-7786.MCR-17-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson B.D., Nakamura T., Russell S.J., et al. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 92.Fujiyuki T., Yoneda M., Amagai Y., et al. A measles virus selectively blind to signaling lymphocytic activation molecule shows anti-tumor activity against lung cancer cells. Oncotarget. 2015;6(28):24895–24903. doi: 10.18632/oncotarget.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iankov I.D., Kurokawa C.B., D'Assoro A.B., et al. Inhibition of the aurora A kinase augments the anti-tumor efficacy of oncolytic measles virotherapy. Cancer Gene Ther. 2015;22(9):438–444. doi: 10.1038/cgt.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan D.Q., Zhang L., Ohba K., et al. Macrophage response to oncolytic paramyxoviruses potentiates virus-mediated tumor cell killing. Eur J Immunol. 2016;46(4):919–928. doi: 10.1002/eji.201545915. [DOI] [PubMed] [Google Scholar]
- 95.Omar A.R., Ideris A., Ali A.M., et al. An overview on the development of Newcastle disease virus as an anti-cancer therapy. Malays J Med Sci. 2003;10(1):4–12. [PMC free article] [PubMed] [Google Scholar]
- 96.Schirrmacher V., Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmad U., Ahmed I., Keong Y.Y., et al. Inhibitory and apoptosis-inducing effects of Newcastle disease virus strain AF2240 on mammary carcinoma cell line. BioMed Res Int. 2015;2015:127828. doi: 10.1155/2015/127828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ravindra P.V., Tiwari A.K., Sharma B., et al. Newcastle disease virus as an oncolytic agent. Indian J Med Res. 2009;130(5):507–513. [PubMed] [Google Scholar]
- 99.Jamal M.H., Ch'ng W.C., Yusoff K., et al. Reduced Newcastle disease virus-induced oncolysis in a subpopulation of cisplatin-resistant MCF7 cells is associated with survivin stabilization. Cancer Cell Int. 2012;12(1):35. doi: 10.1186/1475-2867-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fiola C., Peeters B., Fournier P., et al. Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int J Cancer. 2006;119(2):328–338. doi: 10.1002/ijc.21821. [DOI] [PubMed] [Google Scholar]
- 101.Schirrmacher V., van Gool S., Stuecker W. Breaking therapy resistance: an update on oncolytic Newcastle disease virus for improvements of cancer therapy. Biomedicines. 2019;7(3):E66. doi: 10.3390/biomedicines7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Othman F., Ideris A., Motalleb G., et al. Oncolytic effect of Newcastle disease virus AF2240 strain on the MCF-7 breast cancer cell line. Cell J. 2010;12(1):17–24. [Google Scholar]
- 103.Zamarin D., Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7(3):347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janke M., Peeters B., de Leeuw O., et al. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Ther. 2007;14(23):1639–1649. doi: 10.1038/sj.gt.3303026. [DOI] [PubMed] [Google Scholar]
- 105.Lam H.Y., Yeap S.K., Rasoli M., et al. Safety and clinical usage of Newcastle disease virus in cancer therapy. J Biomed Biotechnol. 2011;2011:718710. doi: 10.1155/2011/718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schirrmacher V., Stücker W., Lulei M., et al. Long-term survival of a breast cancer patient with extensive liver metastases upon immune and virotherapy: a case report. Immunotherapy. 2015;7(8):855–860. doi: 10.2217/imt.15.48. [DOI] [PubMed] [Google Scholar]
- 107.Al-Shammari A.M., Abdullah A.H., Allami Z.M., et al. 2-deoxyglucose and Newcastle disease virus synergize to kill breast cancer cells by inhibition of glycolysis pathway through Glyceraldehyde3-phosphate downregulation. Front Mol Biosci. 2019;6:90. doi: 10.3389/fmolb.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergman I., Whitaker-Dowling P., Gao Y., et al. Vesicular stomatitis virus expressing a chimeric Sindbis glycoprotein containing an Fc antibody binding domain targets to Her2/neu overexpressing breast cancer cells. Virology. 2003;316(2):337–347. doi: 10.1016/j.virol.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 109.Bergman I., Whitaker-Dowling P., Gao Y., et al. Preferential targeting of vesicular stomatitis virus to breast cancer cells. Virology. 2004;330(1):24–33. doi: 10.1016/j.virol.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 110.Ahmed M., Brzoza K.L., Hiltbold E.M. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J Virol. 2006;80(5):2194–2205. doi: 10.1128/JVI.80.5.2194-2205.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janelle V., Brassard F., Lapierre P., et al. Mutations in the glycoprotein of vesicular stomatitis virus affect cytopathogenicity: potential for oncolytic virotherapy. J Virol. 2011;85(13):6513–6520. doi: 10.1128/JVI.02484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bishnoi S., Tiwari R., Gupta S., et al. Oncotargeting by vesicular stomatitis virus (VSV): advances in cancer therapy. Viruses. 2018;10(2):E90. doi: 10.3390/v10020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hastie E., Grdzelishvili V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93(Pt 12):2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ebert O., Harbaran S., Shinozaki K., et al. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12(4):350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 115.Gao Y., Whitaker-Dowling P., Griffin J.A., et al. Treatment with targeted vesicular stomatitis virus generates therapeutic multifunctional anti-tumor memory CD4 T cells. Cancer Gene Ther. 2012;19(4):282–291. doi: 10.1038/cgt.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leveille S., Samuel S., Goulet M.L., et al. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18(6):435–443. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- 117.Diallo J.S., Le Boeuf F., Lai F., et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18(6):1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y.P., Suksanpaisan L., Steele M.B., et al. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci Rep. 2013;3:2375. doi: 10.1038/srep02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cham L.B., Friedrich S.K., Adomati T., et al. Tamoxifen protects from vesicular stomatitis virus infection. Pharmaceuticals. 2019;12(4):E142. doi: 10.3390/ph12040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barber G.N. VSV-tumor selective replication and protein translation. Oncogene. 2005;24(52):7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 121.Gao Y., Bergman I. Potent antitumor T-cell memory is generated by curative viral oncolytic immunotherapy but not curative chemotherapy. Anticancer Res. 2018;38(12):6621–6629. doi: 10.21873/anticanres.13029. [DOI] [PubMed] [Google Scholar]