Abstract
Atherosclerosis, the underlying pathophysiological basis of cardiovascular disease, has been recognized as a lipid-driven chronic inflammatory disease. Sterol carrier protein 2 (SCP-2) is a 13-kDa non-specific lipid-transfer protein expressed by various tissues and cells, such as liver, heart, vascular smooth muscle cells (VSMCs), and macrophages. SCP-2 has an extensive role in cardiovascular and metabolic diseases. Recently, SCP-2 was reported to promote the development of atherosclerosis by regulating lipid metabolism and peroxidation, endocannabinoid metabolism, vascular inflammation, and fatty acid metabolism. In this review, we summarized the recent advances regarding the role of SCP-2 in the pathogenesis of atherosclerosis and tried to provide a rationale for future investigation and a better understanding of the biological functions of SCP-2 in atherosclerotic cardiovascular disease.
Keywords: Atherosclerosis, Fatty acid metabolism, Lipid metabolism, SCP-2, Vascular inflammation
Abbreviations
- 2-AG
2-arachinoylglicerol
- 3′UTR
3′ untranslated region
- ABCA1
ATP binding cassette transporter A1
- ac-LDL
acetylated-LDL
- ACAT1
acyl-CoA: cholesterol acyltransferase 1
- AEA
N-arachidonoylethanolamine
- ATF-2
activating transcription factor-2
- cAMP
cyclic adenosine monophosphate
- ChOOH
Cholesterol hydroperoxide
- FOXO3a
Forkhead box O3a
- HDL-C
high-density lipoprotein cholesterol
- HR-3
hormone receptor 3
- LDL-C
low-density lipoprotein cholesterol
- miR-15a
microRNA-15a
- NKT
natural killer T
- NPC1L1
Niemann-Pick C1-like 1
- PLOOH
phospholipid hydroperoxide
- RCT
reverse cholesterol transport
- ROS
reactive oxygen species
- SCP-2
sterol carrier protein 2
- SF-1
steroidogenic factor 1
- SR-A
scavenger receptor class A
- THAP
thanatos-associated protein
- TICE
transintestinal cholesterol efflux
- VLDL
very low-density lipoprotein
- VSMCs
vascular smooth muscle cells
- WHO
World Health Organization
Introduction
Cardiovascular disease has long been regarded as the most common cause of disability and premature death across the world. Recently, the World Health Organization (WHO) reported that cardiovascular disease accounts for >17 million deaths per year and this figure will rise to >23 million by the year 2030.1 Atherosclerosis is the major pathological basis of most cardiovascular diseases, such as peripheral artery disease, myocardial infarction, hypertension, and ischemic stroke.2, 3, 4 It is widely recognized that atherosclerosis is a lipid-driven chronic inflammatory disease, which is characterized by foam cell formation and atheromatous plaques in medium- and large-sized arteries.5, 6, 7 Although statins therapy is effective in improving plasma lipid profile in patients with cardiovascular disease, the residual cardiovascular risk is still representing a challenge worthy of attention.8,9 Therefore, it is necessary to develop new promising therapeutic strategies aiming to target critical molecules involved in the pathogenesis of atherosclerosis.
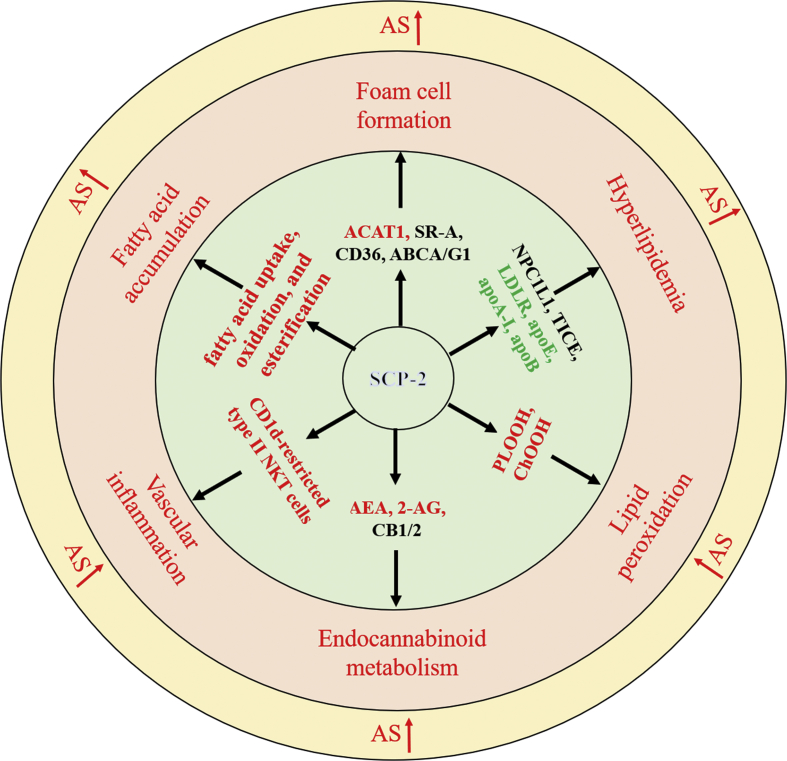
Sterol carrier protein-2 (SCP-2), also called non-specific lipid-transfer protein, plays an important role in the intracellular transport and metabolism of lipids, such as phospholipids, fatty acids, and cholesterol.10, 11, 12 Several lines of evidence suggest that SCP-2 is involved in the development of myocardial infarction and diabetes mellitus.13, 14, 15 Of note, there is increasing evidence that SCP-2 is implicated in the occurrence and development of atherosclerosis through multiple mechanisms (Fig. 1). In the present review, we summarized the current knowledge about the pathophysiological and etiological role of SCP-2 in atherosclerosis to provide an important framework for future research and therapy.
Figure 1.
SCP-2-mediated regulation and the major cardiometabolic risk factors of atherosclerosis. Abbreviations: ACAT1, acyl-CoA: cholesterol acyltransferase 1; SR-A, scavenger receptor class A; ABCA/G1, ATP binding cassette transporter A/G1; NPC1L1, Niemann-Pick C1-like 1; TICE, transintestinal cholesterol efflux; LDLR, low-density lipoprotein receptor; PLOOH, phospholipid hydroperoxide; AEA, N-arachidonoylethanolamine; ChOOH, Cholesterol hydroperoxide; 2-AG, 2-arachinoylglicerol; CB1/2, cannabinoid receptor 1/2; AS, atherosclerosis. Arrows in red: promote; text in red: proatherogenic changes; text in green: antiatherogenic changes; text in black word: un-certain effects.
Structure and function of SCP-2
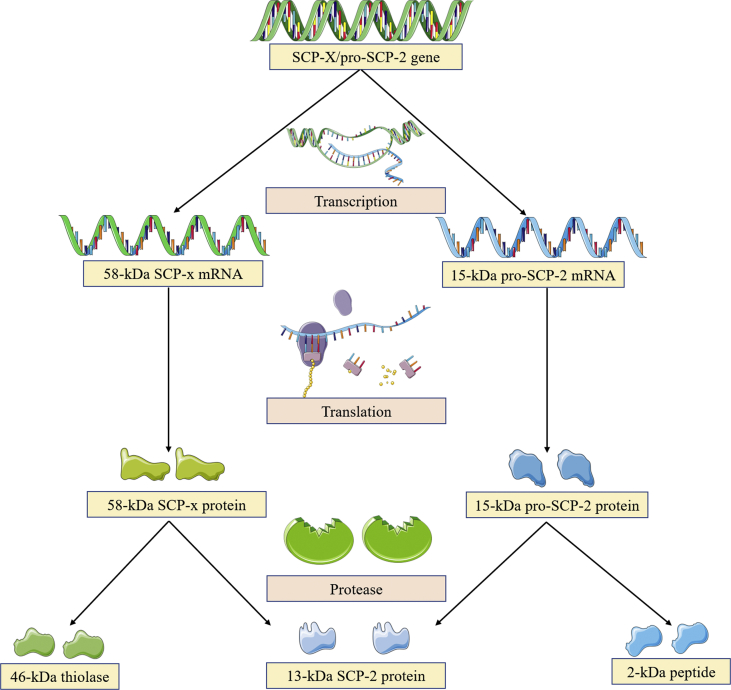
The SCP-2 enzyme gene has two start sites, which encode proteins sharing a common 13-kDa SCP-2 domain at the C-terminus: I) One site codes for a precursor 15-kDa pro-SCP-2 protein (no activity), which is post-translationally completely cleaved into the mature 13-kDa SCP-2 and a 2-kDa peptide.16 II) A second site codes for a full-length 58-kDa SCP-x protein that is partially post-translationally processed to yield a 46-kDa protein and a 13-kDa SCP-216 (Fig. 2). Human SCP-2 is located on chromosome 1p32.3 and contains 123 amino acids and 18 exons. SCP-x/SCP-2 is comprised of at least four important domains that have been identified and associated with specific functions. First, the 46-kDa protein located at the N-terminal presequence of the 58-kDa SCP-x is a 3-ketoacyl-CoA thiolase functioning in the oxidation of branched chain fatty acids.17,18 Also, this thiolase is most likely to split in the peroxisome during the transition between SCP-2 presequence and mature sequence. Second, the N-terminal presequence (20 amino acids) present in 15-kDa pro-SCP-2 significantly regulates the tertiary and secondary structure of SCP-2 and strengthens its intracellular targeting encoded by the C-terminal peroxisomal targeting sequence.19,20 This may explain the observation that up to half of the total SCP-2 protein is located outside of peroxisomes.16 Third, the 32 amino acids at the N-terminal of 13-kDa SCP-2 protein form an amphipathic α-helix segment, one side of which is a membrane-binding domain. Of note, the amino acid residues with positive charges on one side of the amphiphilic α-helix enable SCP-2 to bind to the surface of membranes containing anionic phospholipids.21 Fourth, the helix D and β-strands 4,5 along with the hydrophobic faces of the amphipathic N-terminal α-helix in the 13-kDa SCP-2 protein form a ligand-binding cavity able to accommodate multiple types of lipids (e.g., cholesterol, isoprenoids, fatty acyl CoAs, phospholipids, and fatty acids).20,22 Despite molecular details that comprise the fatty acid binding site in the SCP-2 binding pocket have been identified, the specific amino acid residues interacting with other bound ligands are still unknown.23,24
Figure 2.
The SCP-x/pro-SCP-2 gene encodes two 13-kDa SCP-2 precursors. The 13-kDa SCP-2 gene can be produced by two sites. One site codes for a precursor 15-kDa pro-SCP-2 protein that undergoes completely post-translationally cleaved to the mature 13-kDa SCP-2 and a 2-kDa peptide. Another site codes for a full-length 58-kDa SCP-x protein, which is partially post-translationally processed into yield a 46-kDa protein and a 13-kDa SCP-2 protein.
Numerous studies demonstrated that SCP-2 promotes the transfer of lipid (e.g., cholesterol, phospholipid) from other intracellular membranes to mitochondria, as well as lipid transfer from the outer to the inner mitochondrial membrane.25,26 SCP-2 can facilitate intermembrane lipid transfer by directly interacting with the lipid in the membrane and enhancing its desorption from the membrane.27 Besides, SCP-2 also stimulates the exchange of oxidized derivatives,28,29 glycosphingolipids and gangliosides,30 and fatty acids28,31 between vesicles or membranes. The underlying mechanism of SCP-2-mediated lipid transfer enhancement has been well described by a collisional model, which involves lipid binding and membrane interaction.31 The interaction of SCP-2 protein with vesicles has been studied by membrane surface pressure,32 rotational correlation time,33 and circular dichroism.31,34 Of note, SCP-2-mediated lipid transport and membrane interaction depend on vesicle composition, concentration, curvature, and charge, especially small highly-curved negatively charged vesicles.20,35 Intriguingly, this characteristic has been found in SCP-2 from yeast to mammals, which may be related to the fact that the majority of SCP-2 protein possesses a positively charged surface patch.36,37 Thus, electrostatic interaction is crucial for SCP-2 to interact with the membrane, as many other protein-membrane interactions have reported.38 Taken together, understanding the structure and function of SCP-2 can help to provide a better understanding of its biological roles in lipid metabolism and atherogenesis.
Tissue and intracellular distribution of SCP-2
The 13-kDa SCP-2 protein is ubiquitously present in almost all mammalian tissues. However, the relative content of SCP-2 varies in different tissues. The 13-kDa SCP-2 protein was found in highest abundance in tissues involved in cholesterol transport and oxidation, such as liver, intestine, heart, testis, ovary, and adrenal.14,39 Also, most SCP-2 in the vascular system is expressed in endothelial cells, macrophages, and vascular smooth muscle cells (VSMCs).40, 41, 42 Intracellular localization of SCP-2 has been well established by exploring the distribution of SCP-2/SCP-x gene products in transfected cells overexpressing the 13-kDa SCP-2 and 15-kDa pro-SCP-2.19,43,44 Overexpression of 15-kDa pro-SCP-2 leads to a marked localization in peroxisomes, but nearly half are localized in extraperoxisomal sites.19 By contrast, overexpression of 13-kDa SCP-2 not only results in SCP-2 being predominantly extraperoxisomal in the cytoplasm, but also in other organelles such as endoplasmic reticulum, lysosomes, and mitochondria,19,45 suggesting that SCP-2 may have a broad-scale lipid trafficking activity. The differential distribution of these gene products is owing to the stronger exposure of the C-terminal peroxisome targeting sequence (AKL) of 15-kDa pro-SCP-2 to aqueous buffers while that of the mature 13-kDa SCP-2 is slightly exposed.20 Notably, the presence of the N-terminal presequence (20 amino acids) in 15-kDa pro-SCP-2 changes the conformation of the protein, thereby significantly enhancing the aqueous exposure of the C-terminal AKL peroxisome targeting sequence.19,20 Therefore, 15-kDa pro-SCP-2 likely interacts with the peroxisomal membrane AKL receptor and then participates in the translocation of 15-kDa pro-SCP-2 into the peroxisome matrix. The relative amount of SCP-2 in cytoplasm vs. peroxisomes may be due to the extent of cleavage of the 20-amino acid N-terminal presequence of 15-kDa pro-SCP-2 before entering the peroxisomal matrix. However, the specific protease responsible for this cleavage and the intracellular localization of this protease remains unclear.
Regulation of SCP-2
SCP-2 expression can be positively and negatively regulated by numerous factors (Table 1). Forkhead box O3a (FOXO3a), an important transcription factor belonging to the Forkhead box O family, is closely implicated with neointima formation and atherogenesis.46,47 It has been reported that FOXO3a positively regulates SCP-2 at the level of promoter activity in human colon carcinoma cells.48 The orphan nuclear receptors, hormone receptor 3 (HR-3) and steroidogenic factor 1 (SF-1), have been identified as key transcription factors that promote SCP-2 expression by directly binding to its promoter regions.49,50 SCP-2 is also transcriptionally upregulated by cyclic adenosine monophosphate (cAMP).51 Furthermore, SF-1 is required for cAMP-induced regulation of the SCP-2 gene.49
Table 1.
The regulatory factors of SCP-2.
| Regulators | Cells/Tissues | Mechanisms | Functions | Ref. |
|---|---|---|---|---|
| Stimulators | ||||
| FOXO3a | Human colon carcinoma cells | Binds SCP-2 promoter region | Protects against H2O2/Cu2+-induced oxidative damage | 48 |
| SF-1 | Luteal cells | Binds SCP-2 promoter region | Promotes steroid hormone production | 49 |
| HR-3 | Aag-2 cells | Binds SCP-2 promoter region | Promotes sterol trafficking | 50 |
| cAMP | Grs-21 cells, Immature female Sprague–Dawley rats | SF-1 | Promotes steroid hormone production | 51 |
| Inhibitors | ||||
| ATF-2 | Aedes aegypti | Binds SCP-2 promoter region | Plays a negative role in development and growth | 52 |
| THAP | Aedes aegypti | Binds SCP-2 promoter region | Plays a negative role in development and growth | 52 |
| miR-15a | DF1 cells, Chicken breast muscle tissue | Targets the 3′UTR of SCP-2 mRNA | Promotes the differentiation of intramuscular preadipocytes | 53 |
| miR-1285-5p | Type 2 diabetes mellitus clinical samples | Promotes the hypermethylation of SCP-2 promoter region | A promising biomarkers and therapeutic target for type 2 diabetes mellitus | 54 |
| Gemfibrozil | Male Sprague–Dawley rats | Unknown | Plays a hypolipidemic role | 55 |
In addition, a number of factors have been shown to negatively regulate SCP-2 expression. Activating transcription factor-2 (ATF-2) and thanatos-associated protein (THAP) were reported to antagonistically regulate SCP-2 transcriptional activity.52 SCP-2 also undergoes post-transcriptional modulation. Overexpression of microRNA-15a (miR-15a) significantly reduces SCP-2 expression in chicken intramuscular adipocytes.53 Furthermore, luciferase assay showed that miR-15a inhibits SCP-2 translation by targeting the 3′ untranslated region (3′UTR) of SCP-2 mRNA.53 SCP-2 is also identified as a direct target of miR-1285-5p.54 MiR-1285-5p was reported to be involved in decreasing the expression of SCP-2 through hypermethylation of the promoter region.54 Besides, SCP-2 gene expression is translationally or post-translationally downregulated by gemfibrozil, a widely used hypolipidemic drug.55 However, the exact molecular mechanism of translational or post-translational regulation of SCP-2 remains poorly understand.
Roles of SCP-2 in the pathogenesis of atherosclerosis
Atherosclerosis is a complex and multifactorial disease involving numerous pathophysiological processes. Besides, atherosclerosis is also associated with obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD).56,57 SCP-2 expression is significantly altered in mice models of high-fat diet-induced obesity and NAFLD as well as streptozotocin-treated diabetic rat,58, 59, 60, 61 suggesting a potential role of SCP-2 in these diseases despite the precise mechanisms are largely unknown. Importantly, SCP-2 has been shown to aggravate atherosclerosis by promoting lipid metabolic disorder, lipid peroxidation, vascular inflammation, endocannabinoid metabolism, and fatty acid metabolism.
SCP-2 in cholesterol metabolism
Lipid metabolic disorder, especially hyperlipidemia, is an important risk factor for atherosclerosis. Generally, high-density lipoprotein cholesterol (HDL-C) is thought to be atheroprotective, but very low-density lipoprotein (VLDL), triglyceride, and low-density lipoprotein cholesterol (LDL-C) are proatherogenic. It was estimated that a 1 mg/dL increment in circulating HDL-C levels is associated with a reduced risk of cardiovascular events in women (3%) and men (2%).62 Of note, deficiency of SCP-2 in LDLR−/− mice exhibit a significant increase in HDL-C levels and a reduction in VLDL, LDL-C, and triglyceride levels,63 suggesting an important role of SCP-2 in the development of hyperlipidemia.
The accumulation of foam cells (cholesterol-laden macrophages) in the arterial walls plays a key role in the pathogenesis of atherosclerosis. Cholesterol accumulated in macrophages contributes to the formation of foam cells. In contrast, cholesterol efflux from macrophage foam cells to HDL particles is the first step in reverse cholesterol transport (RCT) that inhibits the development of hyperlipidemia and atherosclerosis.64,65 It has been reported that acetylated-LDL (ac-LDL)-stimulated foam cells exhibit significantly increased SCP-2 mRNA and protein levels,42,66 suggesting that SCP-2 plays a crucial role in foam cell formation. The formation of foam cells is attributed to decreased cholesterol efflux, enhanced cholesterol uptake, and increased cholesterol esterification. Acyl-CoA: cholesterol acyltransferase 1 (ACAT1) is a pivotal endoplasmic reticulum membrane-spanning enzyme that catalyzes the synthesis of cholesterol esters from free cholesterol in macrophages.67,68 Of note, SCP-2 promotes ACAT1-catalyzed cholesterol esterification in mouse peritoneal macrophages and aorta.42,69 Scavenger receptor class A (SR-A) and CD36 belong to the members of the scavenger receptor family, which are mainly responsible for macrophage cholesterol uptake. There is growing evidence that the knockdown of SR-A and CD36 dramatically inhibits foam cell formation and hypercholesterolemia.70, 71, 72 Importantly, numerous studies suggest that overexpression of SCP-2 enhances cholesterol uptake.43,73 However, whether SCP-2 increases macrophage cholesterol uptake via regulating SR-A and CD36 expression is unknown. In addition, overexpression of SCP-2 was found to inhibit HDL-mediated cholesterol efflux.74 Conversely, SCP-2 gene ablation strikingly increases cholesterol efflux to HDL.75 However, the molecular mechanism by which SCP-2 suppresses macrophage cholesterol efflux is still largely unknown. ATP binding cassette (ABC) transporter A1 (ABCA1) and G1 (ABCG1), two important transmembrane proteins, reduce foam cell formation and protect against atherosclerosis by mediating cholesterol efflux to HDL particles.76,77 Thus, it is highly possible that SCP-2 inhibits cholesterol efflux by regulating the expression of ABCA1 and/or ABCG1.
In addition to macrophages, VSMCs are another important source of foam cells in atherosclerosis. In human coronary atherosclerotic lesions, VSMCs contribute about 50% of foam cells and exhibit lower expression of ABCA1 in the intimal of early and advanced plaques.78 Besides, in apoE−/− mice fed a western diet, approximately 70% of atheroma foam cells are derived from VSMCs, accompanied by decreased ABCA1 expression.79 Of note, the expression of SCP-2 mRNA and protein is markedly increased in VSMC-derived foam cells.41 Given that SCP-2 is a negative regulator of cholesterol efflux. These findings suggest that SCP-2 may inhibit cholesterol efflux from VSMC-derived foam cells by the regulation of ABCA1 expression.
The liver is an important organ for cholesterol metabolism. It has been reported that hepatic overexpression of SCP-2 plays a detrimental role in lipid metabolism.80 It can reduce circulating HDL-C and increase LDL-C concentrations, which contributes to the development of hyperlipidemia and atherosclerosis. Furthermore, these changes are linked to reduced hepatic LDLR, apoE, apoA-I, and apoB expression.80 Moreover, SCP-2 overexpression in the mouse liver significantly enhances hepatobiliary cholesterol secretion.61,81 In contrast, knockout of SCP-2 diminishes the ability to secrete cholesterol to bile acid for biliary excretion.82, 83, 84 It is well known that ABCG5 and ABCG8 are two important half-transporters mediating hepatobiliary elimination, contributing to 70%–90% of biliary cholesterol secretion.85,86 However, several studies suggest that the knockout or overexpression of SCP-2 has no effect on hepatic levels of ABCG5/G8 protein or mRNA.61,87,88 Recent studies have suggested that transintestinal cholesterol efflux (TICE) is an alternative nonbiliary pathway for cholesterol excretion.89,90 In this process, hepatic cholesterol can be directly trafficked to the proximal small intestine via enterocytes. Thus, SCP-2 may promote hepatobiliary cholesterol secretion via the TICE pathway. Although SCP-2 has an active role in the regulation of hepatobiliary cholesterol secretion, the total bile acid pool size remains unchanged.80 This can be explained by the enterohepatic circulation of bile acids and biliary cholesterol. Hepatic overexpression of SCP-2 significantly enhances intestinal cholesterol absorption, which leads to the recycling of cholesterol back into the blood.80 Conversely, SCP2 deficiency inhibits the absorption of intestinal cholesterol and reduces circulating cholesterol levels.63 Therefore, the increased hepatobiliary cholesterol secretion is in turn reabsorbed into the blood by the intestine. Of note, intestinal Niemann-Pick C1-like 1 (NPC1L1) is known as a pivotal transporter responsible for intestinal cholesterol absorption, and its expression is positively associated with the risk of hyperlipidemia and atherosclerosis.91, 92, 93 Thus, NPC1L1 may be a key target for SCP-2 to increase intestinal cholesterol absorption. Taken together, these studies suggest that SCP-2 promotes the development of hyperlipidemia by the regulation of hepatic cholesterol metabolism as well as intestinal cholesterol absorption.
SCP-2 in fatty acid metabolism
Fatty acids are identified as important components in cell membranes, and they are required for energy storage, generation of signaling molecules, and membrane proliferation. Accumulating evidence has suggested that abnormal fatty acid metabolism plays a key role in the etiology of atherosclerosis.94,95 Increased fatty acid accumulation, especially in macrophages and endothelial cells, accelerates atherogenesis by significantly aggravating vascular dysfunction, promoting inflammation, and increasing lipid accumulation in the arterial walls.96, 97, 98 SCP-2 is closely associated with fatty acid metabolism. It has been reported that SCP-2 binds fatty acyl CoAs99 and fatty acids100,101 with high affinity. Furthermore, overexpression of SCP-2 enhances cellular fatty acid uptake and stimulates fatty acid oxidation.11,102 In addition, SCP-2 also stimulates fatty acid esterification into the triacylglycerol fraction, which promotes cellular lipid dysfunction.103 Taken together, these data suggest that SCP-2 may promote the development of atherosclerosis by regulating fatty acid metabolism.
SCP-2 in lipid peroxidation
It is well known that oxidative stress plays a significant role in the development of atherosclerosis and some of its effects are mediated via lipid oxidation.104 In particular, lipid peroxidation is known as a free-radical process in which oxidants such as reactive oxygen species (ROS) attack unsaturated lipids, producing a variety of oxidation products.105,106 Cholesterol hydroperoxide (ChOOH) and phospholipid hydroperoxide (PLOOH) are the major oxidation products of lipid peroxidation, which are actively involved in the inflammatory responses, apoptosis, and mitochondrial damage in atherosclerosis by interacting with endothelial cells, VSMCs, and macrophages.107, 108, 109 Notably, it has been reported that mouse fibroblast transfectant clones overexpressing SCP-2 are substantially more sensitive to apoptotic killing induced by ChOOH than vector controls.110 In striking contrast, two SCP-2 inhibitors, SCPI-3 and SCPI-1, significantly reduce ROS accumulation and protect against oxidative killing in ChOOH-treated cells.110 Furthermore, cellular SCP-2 promotes the intermembrane transfer of ChOOH and PLOOH under oxidative stress conditions, thereby greatly enhancing peroxide-induced mitochondrial damage and apoptosis.26,110,111 Mechanistically, SCP-2 facilitates translocation via binding to donor/acceptor membranes and nonspecifically reducing the association/dissociation energy of lipid monomers.26,32 The donor membrane has properties that contribute to SCP-2-enhanced PLOOH and ChOOH transfer, including increased net negative charge exerted by phosphatidylserine and increased phospholipid unsaturation. Thus, these findings suggest that SCP-2 acts as a crucial molecular regulator in the regulation of lipid peroxidation and may be a promising target in the pathogenesis of atherosclerosis.
SCP-2 in endocannabinoid metabolism
The endocannabinoid system consists of endocannabinoids, such as 2-arachinoylglicerol (2-AG) and N-arachidonoylethanolamine (AEA), and types 1 and 2 G-protein coupled cannabinoid receptors (CB1 and CB2). Endocannabinoids are arachidonic acid derivatives that play a crucial physiological role in atherosclerotic cardiovascular disease by CB1 and CB2 activation and signaling.112,113 A growing number of studies have demonstrated that enhanced endocannabinoid system activation promotes atherogenesis through multiple effects, including regulation of macrophage cholesterol metabolism, vascular inflammation, leukocyte recruitment, and consequently atherosclerotic plaque stability.114, 115, 116, 117 Moreover, elevated levels of circulating endocannabinoids are also prevalent in human and mouse atherosclerosis.117,118 Of note, SCP-2 has been identified as an important binding protein of AEA and 2-AG, which can promote their cellular uptake and accumulation.119,120 In contrast, loss of SCP-2 was reported to decrease serum levels of free arachidonic acid, thereby attenuating the availability of arachidonic acid uptake by cells and downstream synthesis of 2-AG and AEA.121 Thus, these studies suggest that SCP-2 promotes atherosclerosis by regulating endocannabinoid metabolism. However, whether SCP-2 regulates endocannabinoid metabolism by activating CB1 and/or CB2 is still largely unknown.
SCP-2 in vascular inflammation
Atherosclerosis has long been regarded as a progressive chronic inflammatory disease of the vascular wall. Vascular inflammation not only promotes lipid metabolism dysregulation but also contributes to plaque rupture and the onset of cardiovascular events.122,123 Type II natural killer T (NKT) cells are CD1d-restricted and immunoregulatory T cells, and the disorder of these cells is involved in vascular inflammation and atherosclerosis-associated immune response.124,125 More recently, proinflammatory CD1d-restricted type II NKT cells reactive with the endogenous SCP-2 peptide have been reported to be implicated in vascular inflammation in rats.126,127 The SCP-2 peptide can activate CD1d-restricted type II NKT cells to produce pro-inflammatory cytokines and thereby enhance vascular inflammation.126,127 Of note, endothelial cells, macrophages, and VSMCs are the major effector cells involving vascular inflammation and atherosclerosis.128, 129, 130 Thus, further studies are necessary to investigate whether SCP-2 promotes vascular inflammation by directly regulating these effector cells.
Conclusions and future directions
SCP-2 is an important lipid binding protein with multiple biological functions and has attracted growing interest in recent years. SCP-2 plays a critical role in the development of atherosclerosis through its involvement in the regulation of lipid metabolic disorder, vascular inflammation, lipid peroxidation, endocannabinoid metabolism, and fatty acid metabolism (Table 2).
Table 2.
The potential role of SCP-2 in the pathogenesis of atherosclerosis.
| SCP-2 expression | Cells/tissues | Mechanisms | Effects | AS | Ref. |
|---|---|---|---|---|---|
| Knockout | Global | Intestinal cholesterol absorption↓, hepatic triglyceride/VLDL secretion↓ | Hyperlipidemia↓ | ↓ | 63 |
| Overexpression | Rat peritoneal macrophages | ACAT1↑ | Cholesterol esterification↑ | ↑ | 42,66 |
| Overexpression | Rat aorta | ACAT1↑ | Cholesterol esterification↑ | ↑ | 69 |
| Overexpression | Mouse L-cell fibroblasts | – | Cholesterol uptake↑ | ↑ | 43,73 |
| Overexpression | Mouse L-cell fibroblasts | – | Cholesterol efflux↓ | ↑ | 74 |
| Knockdown | Primary mouse hepatocytes | – | Cholesterol efflux↑ | ↓ | 75 |
| Overexpression | VSMCs | – | Lipid accumulation↑ | ↑ | 41 |
| Overexpression | Liver | LDLR, apoE, apoA-I, and apoB↓, Intestinal cholesterol absorption↑ | Hyperlipidemia↑ | ↑ | 80 |
| Overexpression | Liver | – | Hepatobiliary cholesterol secretion↑ | ↑ | 61,81 |
| Overexpression | Liver | PLOOH and ChOOH transfer↑ | Lipid peroxidation↑ | ↑ | 26 |
| Suppression | Mouse L-cell fibroblasts | ChOOH transfer and uptake↓ | Lipid peroxidation↓ | ↓ | 110 |
| Overexpression | Rat hepatoma cells | ChOOH transfer↑ | Lipid peroxidation↑ | ↑ | 111 |
| Overexpression | HEK 293 Cells | AEA and 2-AG uptake/accumulation↑ | Endocannabinoid accumulation↑ | ↑ | 119,120 |
| Knockout | Global | AEA and 2-AG accumulation↓ | Endocannabinoid accumulation↓ | ↓ | 121 |
| Overexpression | Vascular endothelial cells | CD1d-restricted type II NKT cells↑ | Vascular inflammation↑ | ↑ | 126,127 |
| Overexpression | Mouse L-cell fibroblasts | – | fatty acid uptake, oxidation, and esterification↑ | ↑ | 11,102 |
–, Not determined; AS, atherosclerosis.
Ongoing studies of SCP-2 will be needed to elucidate its exact role in atherosclerosis. Although many studies have established the harmful effects of enhanced expression, it is currently difficult to speculate on the most effective strategy to inhibit the expression of SCP-2 in atherosclerosis. Moreover, more work is required to clarify how to most effectively target SCP-2, either by transcriptional/post-transcriptional regulation, or by post-translational modification. Currently, most of our knowledge about the role of SCP-2 in the pathogenesis of atherosclerosis has been learned from studies performed in vitro and rodent models with genetic modifications and/or dietary treatments. Nevertheless, rodent models are insufficient to reflect the complex heterogeneity of pathological changes that occur during the progression of atherosclerosis in humans. In contrast, large-animal models can offer striking advantages, including their marked metabolic, physiological, biochemical, and genetic similarities to humans. Thus, they may be better models and helpful for bridging the gap between basic research and prudent clinical usage. In addition, there are also several crucial questions that require to be answered in future research. 1) Pyroptosis, a pro-inflammatory programmed cell death, plays a crucial role in the development of atherogenesis.131, 132, 133 Does SCP-2 promote atherosclerosis by regulating pyroptosis? 2) Macrophage polarization is also known as a pivotal determinant of inflammation and is closely related to the progression of atherosclerosis.134,135 However, there is very little known about the role of SCP-2 in the regulation of macrophage polarization. 3) Does a specific and efficient method to counteract the detrimental actions of SCP-2 on atherosclerosis exist in vivo? Finally, it is of importance to explore whether SCP-2 inhibits the whole-body RCT process. In summary, understanding these sorts of questions will undoubtedly provide insightful knowledge about the underlying mechanisms and accelerate the development of SCP-2-targeted therapy.
Author contributions
Conception and design: all authors; Manuscript writing: Can Xu; Collection and assembly of data: Can Xu and Heng li; Final approval of manuscript: all authors.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Sciences Foundation of China (No. 81770461) and the Science and Technology Project of Hengyang City, China (No. hkf201947206).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Heng Li, Email: hliwilling@163.com.
Chao-Ke Tang, Email: tangchaoke@qq.com.
References
- 1.Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen N.B., Krefman A.E., Labarthe D., et al. Cardiovascular health trajectories from childhood through middle age and their association with subclinical atherosclerosis. JAMA Cardiol. 2020;5(5):557–566. doi: 10.1001/jamacardio.2020.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scannapieco F.A., Bush R.B., Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8(1):38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Anand S.S., Yusuf S., Vuksan V., et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356(9226):279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 5.Maguire E.M., Pearce S.W.A., Xiao Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc Pharmacol. 2019;112:54–71. doi: 10.1016/j.vph.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Bäck M., Yurdagul A., Jr., Tabas I., et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi: 10.1038/s41569-019-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMahon S., Sharpe N., Gamble G., et al. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation. 1998;97(18):1784–1790. doi: 10.1161/01.cir.97.18.1784. [DOI] [PubMed] [Google Scholar]
- 8.Sampson U.K., Fazio S., Linton M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atherosclerosis Rep. 2012;14(1):1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols G.A., Philip S., Reynolds K., et al. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes Metabol. 2019;21(2):366–371. doi: 10.1111/dom.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N.C., Fan J., Papadopoulos V. Sterol carrier protein-2, a nonspecific lipid-transfer protein, in intracellular cholesterol trafficking in testicular leydig cells. PLoS One. 2016;11(2):e0149728. doi: 10.1371/journal.pone.0149728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starodub O., Jolly C.A., Atshaves B.P., et al. Sterol carrier protein-2 localization in endoplasmic reticulum and role in phospholipid formation. Am J Physiol Cell Physiol. 2000;279(4):C1259–C1269. doi: 10.1152/ajpcell.2000.279.4.C1259. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E.J., Stiles T., Schroeder F. Sterol carrier protein-2 expression alters phospholipid content and fatty acyl composition in L-cell fibroblasts. J Lipid Res. 2000;41(5):788–796. [PubMed] [Google Scholar]
- 13.Seidelmann S.B., Li L., Shen G.Q., et al. Identification of a novel locus for triglyceride on chromosome 1p31-32 in families with premature CAD and MI. J Lipid Res. 2008;49(5):1034–1038. doi: 10.1194/jlr.M700576-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Shao M., Zhang X., et al. The effect of Chinese medicine on lipid and glucose metabolism in acute myocardial infarction through PPARγ pathway. Front Pharmacol. 2018;9:1209. doi: 10.3389/fphar.2018.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean M.P., Warden K.J., Sandhoff T.W., et al. Altered ovarian sterol carrier protein expression in the pregnant streptozotocin-treated diabetic rat. Biol Reprod. 1996;55(1):38–46. doi: 10.1095/biolreprod55.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Gallegos A.M., Atshaves B.P., Storey S.M., et al. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40(6):498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 17.Seedorf U., Ellinghaus P., Roch Nofer J. Sterol carrier protein-2. Biochim Biophys Acta. 2000;1486(1):45–54. doi: 10.1016/s1388-1981(00)00047-0. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder F., Atshaves B.P., McIntosh A.L., et al. Sterol carrier protein-2: new roles in regulating lipid rafts and signaling. Biochim Biophys Acta. 2007;1771(6):700–718. doi: 10.1016/j.bbalip.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder F., Frolov A., Starodub O., et al. Pro-sterol carrier protein-2: role of the N-terminal presequence in structure, function, and peroxisomal targeting. J Biol Chem. 2000;275(33):25547–25555. doi: 10.1074/jbc.M000431200. [DOI] [PubMed] [Google Scholar]
- 20.Martin G.G., Hostetler H.A., McIntosh A.L., et al. Structure and function of the sterol carrier protein-2 N-terminal presequence. Biochemistry. 2008;47(22):5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H., Ball J.M., Billheimer J.T., et al. The sterol carrier protein-2 amino terminus: a membrane interaction domain. Biochemistry. 1999;38(40):13231–13243. doi: 10.1021/bi990870x. [DOI] [PubMed] [Google Scholar]
- 22.Stolowich N.J., Petrescu A.D., Huang H., et al. Sterol carrier protein-2: structure reveals function. Cell Mol Life Sci. 2002;59(2):193–212. doi: 10.1007/s00018-002-8416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer D.H., Lovell S., Thoden J.B., et al. The structural determination of an insect sterol carrier protein-2 with a ligand-bound C16 fatty acid at 1.35-A resolution. J Biol Chem. 2003;278(40):39085–39091. doi: 10.1074/jbc.M306214200. [DOI] [PubMed] [Google Scholar]
- 24.Stolowich N.J., Frolov A., Atshaves B., et al. The sterol carrier protein-2 fatty acid binding site: an NMR, circular dichroic, and fluorescence spectroscopic determination. Biochemistry. 1997;36(7):1719–1729. doi: 10.1021/bi962317a. [DOI] [PubMed] [Google Scholar]
- 25.Gallegos A.M., Schoer J.K., Starodub O., et al. A potential role for sterol carrier protein-2 in cholesterol transfer to mitochondria. Chem Phys Lipids. 2000;105(1):9–29. doi: 10.1016/s0009-3084(99)00128-0. [DOI] [PubMed] [Google Scholar]
- 26.Vila A., Levchenko V.V., Korytowski W., et al. Sterol carrier protein-2-facilitated intermembrane transfer of cholesterol- and phospholipid-derived hydroperoxides. Biochemistry. 2004;43(39):12592–12605. doi: 10.1021/bi0491200. [DOI] [PubMed] [Google Scholar]
- 27.Woodford J.K., Colles S.M., Myers-Payne S., et al. Sterol carrier protein-2 stimulates intermembrane sterol transfer by direct membrane interaction. Chem Phys Lipids. 1995;76(1):73–84. doi: 10.1016/0009-3084(95)02436-m. [DOI] [PubMed] [Google Scholar]
- 28.Viitanen L., Nylund M., Eklund D.M., et al. Characterization of SCP-2 from Euphorbia lagascae reveals that a single Leu/Met exchange enhances sterol transfer activity. FEBS J. 2006;273(24):5641–5655. doi: 10.1111/j.1742-4658.2006.05553.x. [DOI] [PubMed] [Google Scholar]
- 29.Girotti A.W., Kriska T. Binding and cytotoxic trafficking of cholesterol hydroperoxides by sterol carrier protein-2. Methods Mol Biol. 2015;1208:421–435. doi: 10.1007/978-1-4939-1441-8_30. [DOI] [PubMed] [Google Scholar]
- 30.Bloj B., Zilversmit D.B. Accelerated transfer of neutral glycosphingolipids and ganglioside GM1 by a purified lipid transfer protein. J Biol Chem. 1981;256(12):5988–5991. [PubMed] [Google Scholar]
- 31.Falomir Lockhart L.J., Burgardt N.I., Ferreyra R.G., et al. Fatty acid transfer from Yarrowia lipolytica sterol carrier protein 2 to phospholipid membranes. Biophys J. 2009;97(1):248–256. doi: 10.1016/j.bpj.2009.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Amerongen A., Demel R.A., Westerman J., et al. Transfer of cholesterol and oxysterol derivatives by the nonspecific lipid transfer protein (sterol carrier protein 2): a study on its mode of action. Biochim Biophys Acta. 1989;1004(1):36–43. doi: 10.1016/0005-2760(89)90209-9. [DOI] [PubMed] [Google Scholar]
- 33.Gadella T.W., Jr., Bastiaens P.I., Visser A.J., et al. Shape and lipid-binding site of the nonspecific lipid-transfer protein (sterol carrier protein 2): a steady-state and time-resolved fluorescence study. Biochemistry. 1991;30(22):5555–5564. doi: 10.1021/bi00236a031. [DOI] [PubMed] [Google Scholar]
- 34.Huang H., Gallegos A.M., Zhou M., et al. Role of the sterol carrier protein-2 N-terminal membrane binding domain in sterol transfer. Biochemistry. 2002;41(40):12149–12162. doi: 10.1021/bi0260536. [DOI] [PubMed] [Google Scholar]
- 35.Huang H., Ball J.M., Billheimer J.T., et al. Interaction of the N-terminus of sterol carrier protein 2 with membranes: role of membrane curvature. Biochem J. 1999;344 Pt 2(Pt 2):593–603. [PMC free article] [PubMed] [Google Scholar]
- 36.Burgardt N.I., Ferreyra R.G., Falomir-Lockhart L., et al. Biophysical characterisation and urea-induced unfolding of recombinant Yarrowia lipolytica sterol carrier protein-2. Biochim Biophys Acta. 2009;1794(8):1115–1122. doi: 10.1016/j.bbapap.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.García F.L., Szyperski T., Dyer J.H., et al. NMR structure of the sterol carrier protein-2: implications for the biological role. J Mol Biol. 2000;295(3):595–603. doi: 10.1006/jmbi.1999.3355. [DOI] [PubMed] [Google Scholar]
- 38.Mulgrew-Nesbitt A., Diraviyam K., Wang J., et al. The role of electrostatics in protein-membrane interactions. Biochim Biophys Acta. 2006;1761(8):812–826. doi: 10.1016/j.bbalip.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Vahouny G.V., Chanderbhan R., Kharroubi A., et al. Sterol carrier and lipid transfer proteins. Adv Lipid Res. 1987;22:83–113. doi: 10.1016/b978-0-12-024922-0.50007-2. [DOI] [PubMed] [Google Scholar]
- 40.Nishioka Y., Sonoda T., Shida H., et al. Detection of autoreactive type II NKT cells: a pilot study of comparison between healthy individuals and patients with vasculitis. Cytometry A. 2018;93(11):1157–1164. doi: 10.1002/cyto.a.23618. [DOI] [PubMed] [Google Scholar]
- 41.Kraemer R., Pomerantz K.B., Kesav S., et al. Cholesterol enrichment enhances expression of sterol-carrier protein-2: implications for its function in intracellular cholesterol trafficking. J Lipid Res. 1995;36(12):2630–2638. [PubMed] [Google Scholar]
- 42.Hirai A., Kino T., Tokinaga K., et al. Regulation of sterol carrier protein 2 (SCP2) gene expression in rat peritoneal macrophages during foam cell formation. A key role for free cholesterol content. J Clin Invest. 1994;94(6):2215–2223. doi: 10.1172/JCI117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atshaves B.P., Petrescu A.D., Starodub O., et al. Expression and intracellular processing of the 58 kDa sterol carrier protein-2/3-oxoacyl-CoA thiolase in transfected mouse L-cell fibroblasts. J Lipid Res. 1999;40(4):610–622. [PubMed] [Google Scholar]
- 44.Seedorf U., Brysch P., Engel T., et al. Sterol carrier protein X is peroxisomal 3-oxoacyl coenzyme A thiolase with intrinsic sterol carrier and lipid transfer activity. J Biol Chem. 1994;269(33):21277–21283. [PubMed] [Google Scholar]
- 45.Keller G.A., Scallen T.J., Clarke D., et al. Subcellular localization of sterol carrier protein-2 in rat hepatocytes: its primary localization to peroxisomes. J Cell Biol. 1989;108(4):1353–1361. doi: 10.1083/jcb.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H., Fellows A., Foote K., et al. FOXO3a (forkhead transcription factor O subfamily member 3a) links vascular smooth muscle cell apoptosis, matrix breakdown, atherosclerosis, and vascular remodeling through a novel pathway involving MMP13 (matrix metalloproteinase 13) Arterioscler Thromb Vasc Biol. 2018;38(3):555–565. doi: 10.1161/ATVBAHA.117.310502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park D.W., Baek K., Kim J.R., et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1- mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009;41(3):171–179. doi: 10.3858/emm.2009.41.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dansen T.B., Kops G.J., Denis S., et al. Regulation of sterol carrier protein gene expression by the forkhead transcription factor FOXO3a. J Lipid Res. 2004;45(1):81–88. doi: 10.1194/jlr.M300111-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Lopez D., Shea-Eaton W., McLean M.P. Characterization of a steroidogenic factor-1-binding site found in promoter of sterol carrier protein-2 gene. Endocrine. 2001;14(2):253–261. doi: 10.1385/ENDO:14:2:253. [DOI] [PubMed] [Google Scholar]
- 50.Vyazunova I., Lan Q. Yellow fever mosquito sterol carrier protein-2 gene structure and transcriptional regulation. Insect Mol Biol. 2010;19(2):205–215. doi: 10.1111/j.1365-2583.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rennert H., Amsterdam A., Billheimer J.T., Strauss J.F., 3rd Regulated expression of sterol carrier protein 2 in the ovary: a key role for cyclic AMP. Biochemistry. 1991;30(47):11280–11285. doi: 10.1021/bi00111a013. [DOI] [PubMed] [Google Scholar]
- 52.Peng R., Fu Q., Hong H., et al. THAP and ATF-2 regulated sterol carrier protein-2 promoter activities in the larval midgut of the yellow fever mosquito, Aedes aegypti. PLoS One. 2012;7(10):e46948. doi: 10.1371/journal.pone.0046948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G., Fu S., Chen Y., et al. microRNA-15a regulates the differentiation of intramuscular preadipocytes by targeting ACAA1, ACOX1 and SCP2 in chickens. Int J Mol Sci. 2019;20(16):4063. doi: 10.3390/ijms20164063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai L., Li J., Panagal M., et al. Methylation dependent microRNA 1285-5p and sterol carrier proteins 2 in type 2 diabetes mellitus. Artif Cells Nanomed Biotechnol. 2019;47(1):3417–3422. doi: 10.1080/21691401.2019.1652625. [DOI] [PubMed] [Google Scholar]
- 55.Baum C.L., Kansal S., Davidson N.O. Regulation of sterol carrier protein-2 gene expression in rat liver and small intestine. J Lipid Res. 1993;34(5):729–739. [PubMed] [Google Scholar]
- 56.Targher G., Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Ramos R., Comas-Cufí M., Martí-Lluch R., et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362:k3359. doi: 10.1136/bmj.k3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozawa S., Honda A., Kajiwara N., et al. Induction of peroxisomal lipid metabolism in mice fed a high-fat diet. Mol Med Rep. 2011;4(6):1157–1162. doi: 10.3892/mmr.2011.560. [DOI] [PubMed] [Google Scholar]
- 59.McLean M.P., Billheimer J.T., Warden K.J., et al. Differential expression of hepatic sterol carrier proteins in the streptozotocin-treated diabetic rat. Endocrinology. 1995;136(8):3360–3368. doi: 10.1210/endo.136.8.7628371. [DOI] [PubMed] [Google Scholar]
- 60.McLean M.P., Nanjo K., Irby R.B., et al. Reduced hepatic sterol carrier protein-2 expression in the streptozotocin treated diabetic rat. Endocrine. 1995;3(8):563–571. doi: 10.1007/BF02953020. [DOI] [PubMed] [Google Scholar]
- 61.Atshaves B.P., McIntosh A.L., Martin G.G., et al. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res. 2009;50(7):1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon D.J., Probstfield J.L., Garrison R.J., et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 63.He H., Wang J., Yannie P.J., et al. Sterol carrier protein-2 deficiency attenuates diet-induced dyslipidemia and atherosclerosis in mice. J Biol Chem. 2018;293(24):9223–9231. doi: 10.1074/jbc.RA118.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis G.F., Rader D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96(12):1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 65.Bellanger N., Orsoni A., Julia Z., et al. Atheroprotective reverse cholesterol transport pathway is defective in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2011;31(7):1675–1681. doi: 10.1161/ATVBAHA.111.227181. [DOI] [PubMed] [Google Scholar]
- 66.Tokinaga K., Hirai A., Kino T., et al. Increase in the content of sterol carrier protein 2 (SCP2) during foam cell formation of rat peritoneal macrophages. Ann N Y Acad Sci. 1995;748:571–574. doi: 10.1111/j.1749-6632.1994.tb17363.x. [DOI] [PubMed] [Google Scholar]
- 67.Dove D.E., Su Y.R., Zhang W., et al. ACAT1 deficiency disrupts cholesterol efflux and alters cellular morphology in macrophages. Arterioscler Thromb Vasc Biol. 2005;25(1):128–134. doi: 10.1161/01.ATV.0000148323.94021.e5. [DOI] [PubMed] [Google Scholar]
- 68.Dove D.E., Su Y.R., Swift L.L., et al. ACAT1 deficiency increases cholesterol synthesis in mouse peritoneal macrophages. Atherosclerosis. 2006;186(2):267–274. doi: 10.1016/j.atherosclerosis.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Ban T., Hirai A., Kino T., et al. Sterol carrier protein2 (SCP2)-like protein in rat aorta. Artery. 1991;18(2):54–70. [PubMed] [Google Scholar]
- 70.Manning-Tobin J.J., Moore K.J., Seimon T.A., et al. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29(1):19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamura D.M., Pennathur S., Pasichnyk K., et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol. 2009;20(3):495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yazgan B., Sozen E., Karademir B., et al. CD36 expression in peripheral blood mononuclear cells reflects the onset of atherosclerosis. Biofactors. 2018;44(6):588–596. doi: 10.1002/biof.1372. [DOI] [PubMed] [Google Scholar]
- 73.Moncecchi D., Murphy E.J., Prows D.R., Schroeder F. Sterol carrier protein-2 expression in mouse L-cell fibroblasts alters cholesterol uptake. Biochim Biophys Acta. 1996;1302(2):110–116. doi: 10.1016/0005-2760(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 74.Atshaves B.P., Starodub O., McIntosh A., et al. Sterol carrier protein-2 alters high density lipoprotein-mediated cholesterol efflux. J Biol Chem. 2000;275(47):36852–36861. doi: 10.1074/jbc.M003434200. [DOI] [PubMed] [Google Scholar]
- 75.Storey S.M., Atshaves B.P., McIntosh A.L., et al. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2010;299(1):G244–G254. doi: 10.1152/ajpgi.00446.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singaraja R.R., Brunham L.R., Visscher H., Kastelein J.J., Hayden M.R. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol. 2003;23(8):1322–1332. doi: 10.1161/01.ATV.0000078520.89539.77. [DOI] [PubMed] [Google Scholar]
- 77.Baldán A., Pei L., Lee R., et al. Impaired development of atherosclerosis in hyperlipidemic Ldlr-/- and ApoE-/- mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol. 2006;26(10):2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 78.Allahverdian S., Chehroudi A.C., McManus B.M., et al. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129(15):1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Dubland J.A., Allahverdian S., et al. Smooth muscle cells contribute the majority of foam cells in ApoE (apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39(5):876–887. doi: 10.1161/ATVBAHA.119.312434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanlungo S., Amigo L., Mendoza H., et al. Sterol carrier protein 2 gene transfer changes lipid metabolism and enterohepatic sterol circulation in mice. Gastroenterology. 2000;119(6):1708–1719. doi: 10.1053/gast.2000.20198. [DOI] [PubMed] [Google Scholar]
- 81.Amigo L., Zanlungo S., Miquel J.F., et al. Hepatic overexpression of sterol carrier protein-2 inhibits VLDL production and reciprocally enhances biliary lipid secretion. J Lipid Res. 2003;44(2):399–407. doi: 10.1194/jlr.M200306-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Klipsic D., Landrock D., Martin G.G., et al. Impact of SCP-2/SCP-x gene ablation and dietary cholesterol on hepatic lipid accumulation. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G387–G399. doi: 10.1152/ajpgi.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin G.G., Atshaves B.P., Landrock K.K., et al. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest Liver Physiol. 2014;307(11):G1130–G1143. doi: 10.1152/ajpgi.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J., Bie J., Ghosh S. Intracellular cholesterol transport proteins enhance hydrolysis of HDL-CEs and facilitate elimination of cholesterol into bile. J Lipid Res. 2016;57(9):1712–1719. doi: 10.1194/jlr.M069682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Liu X., Pijut S.S., et al. The combination of ezetimibe and ursodiol promotes fecal sterol excretion and reveals a G5G8-independent pathway for cholesterol elimination. J Lipid Res. 2015;56(4):810–820. doi: 10.1194/jlr.M053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H., Yu X.H., Ou X., et al. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog Lipid Res. 2021;83:101109. doi: 10.1016/j.plipres.2021.101109. [DOI] [PubMed] [Google Scholar]
- 87.Martin G.G., Landrock D., Landrock K.K., et al. Relative contributions of L-FABP, SCP-2/SCP-x, or both to hepatic biliary phenotype of female mice. Arch Biochem Biophys. 2015;588:25–32. doi: 10.1016/j.abb.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin G.G., Atshaves B.P., Landrock K.K., et al. Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Arch Biochem Biophys. 2015;580:41–49. doi: 10.1016/j.abb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Velde A.E., Brufau G., Groen A.K. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21(3):167–171. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
- 90.Jakulj L., van Dijk T.H., de Boer J.F., et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metabol. 2016;24(6):783–794. doi: 10.1016/j.cmet.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Davis H.R., Jr., Hoos L.M., Tetzloff G., et al. Deficiency of niemann-pick C1 like 1 prevents atherosclerosis in ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2007;27(4):841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 92.Altmann S.W., Davis H.R., Jr., Zhu L.J., et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 93.Davies J.P., Scott C., Oishi K., et al. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280(13):12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 94.Ménégaut L., Jalil A., Thomas C., et al. Macrophage fatty acid metabolism and atherosclerosis: the rise of PUFAs. Atherosclerosis. 2019;291:52–61. doi: 10.1016/j.atherosclerosis.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Wakil S.J., Abu-Elheiga L.A. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009;50:S138. doi: 10.1194/jlr.R800079-JLR200. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneider J.G., Yang Z., Chakravarthy M.V., et al. Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem. 2010;285(30):23398–23409. doi: 10.1074/jbc.M110.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh A., Gao L., Thakur A., et al. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24(1):50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H., Yang Y., Yang M., et al. Pigment epithelial-derived factor deficiency accelerates atherosclerosis development via promoting endothelial fatty acid uptake in mice with hyperlipidemia. J Am Heart Assoc. 2019;8(22):e013028. doi: 10.1161/JAHA.119.013028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frolov A., Cho T.H., Billheimer J.T., et al. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271(50):31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- 100.Schroeder F., Myers-Payne S.C., Billheimer J.T., et al. Probing the ligand binding sites of fatty acid and sterol carrier proteins: effects of ethanol. Biochemistry. 1995;34(37):11919–11927. doi: 10.1021/bi00037a033. [DOI] [PubMed] [Google Scholar]
- 101.Stolowich N., Frolov A., Petrescu A.D., et al. Holo-sterol carrier protein-2. (13)C NMR investigation of cholesterol and fatty acid binding sites. J Biol Chem. 1999;274(50):35425–35433. doi: 10.1074/jbc.274.50.35425. [DOI] [PubMed] [Google Scholar]
- 102.Atshaves B.P., Storey S.M., Schroeder F. Sterol carrier protein-2/sterol carrier protein-x expression differentially alters fatty acid metabolism in L cell fibroblasts. J Lipid Res. 2003;44(9):1751–1762. doi: 10.1194/jlr.M300141-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Murphy E.J., Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochim Biophys Acta. 1997;1345(3):283–292. doi: 10.1016/s0005-2760(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 104.Zhong S., Li L., Shen X., et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. 2019;144:266–278. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 105.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors. 2008;34(2):171–180. doi: 10.1002/biof.5520340208. [DOI] [PubMed] [Google Scholar]
- 106.Requena J.R., Fu M.X., Ahmed M.U., et al. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11(Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 107.Bai T., Li M., Liu Y., et al. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe T., Pakala R., Katagiri T., et al. Lipid peroxidation product 4-hydroxy-2-nonenal acts synergistically with serotonin in inducing vascular smooth muscle cell proliferation. Atherosclerosis. 2001;155(1):37–44. doi: 10.1016/s0021-9150(00)00526-8. [DOI] [PubMed] [Google Scholar]
- 109.Korytowski W., Wawak K., Pabisz P., et al. Impairment of macrophage cholesterol efflux by cholesterol hydroperoxide trafficking: implications for atherogenesis under oxidative stress. Arterioscler Thromb Vasc Biol. 2015;35(10):2104–2113. doi: 10.1161/ATVBAHA.115.306210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kriska T., Pilat A., Schmitt J.C., et al. Sterol carrier protein-2 (SCP-2) involvement in cholesterol hydroperoxide cytotoxicity as revealed by SCP-2 inhibitor effects. J Lipid Res. 2010;51(11):3174–3184. doi: 10.1194/jlr.M008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kriska T., Levchenko V.V., Korytowski W., et al. Intracellular dissemination of peroxidative stress. Internalization, transport, and lethal targeting of a cholesterol hydroperoxide species by sterol carrier protein-2-overexpressing hepatoma cells. J Biol Chem. 2006;281(33):23643–23651. doi: 10.1074/jbc.M600744200. [DOI] [PubMed] [Google Scholar]
- 112.Montecucco F., Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci. 2012;33(6):331–340. doi: 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Pacher P., Mukhopadhyay P., Mohanraj R., et al. Modulation of the endocannabinoid system in cardiovascular disease: therapeutic potential and limitations. Hypertension. 2008;52(4):601–607. doi: 10.1161/HYPERTENSIONAHA.105.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guillamat-Prats R., Rami M., Herzig S., et al. Endocannabinoid signalling in atherosclerosis and related metabolic complications. Thromb Haemostasis. 2019;119(4):567–575. doi: 10.1055/s-0039-1678738. [DOI] [PubMed] [Google Scholar]
- 115.Pacher P., Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009;31(1):63–77. doi: 10.1007/s00281-009-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molica F., Burger F., Thomas A., et al. Endogenous cannabinoid receptor CB1 activation promotes vascular smooth-muscle cell proliferation and neointima formation. J Lipid Res. 2013;54(5):1360–1368. doi: 10.1194/jlr.M035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sugamura K., Sugiyama S., Nozaki T., et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119(1):28–36. doi: 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- 118.Lenglet S., Thomas A., Soehnlein O., et al. Fatty acid amide hydrolase deficiency enhances intraplaque neutrophil recruitment in atherosclerotic mice. Arterioscler Thromb Vasc Biol. 2013;33(2):215–223. doi: 10.1161/ATVBAHA.112.300275. [DOI] [PubMed] [Google Scholar]
- 119.Liedhegner E.S., Vogt C.D., Sem D.S., et al. Sterol carrier protein-2: binding protein for endocannabinoids. Mol Neurobiol. 2014;50(1):149–158. doi: 10.1007/s12035-014-8651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hillard C.J., Huang H., Vogt C.D., et al. Endocannabinoid transport proteins: discovery of tools to study sterol carrier protein-2. Methods Enzymol. 2017;593:99–121. doi: 10.1016/bs.mie.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin G.G., Seeger D.R., McIntosh A.L., et al. Sterol carrier protein-2/sterol carrier protein-x/fatty acid binding protein-1 ablation impacts response of brain endocannabinoid to high-fat diet. Lipids. 2019;54(10):583–601. doi: 10.1002/lipd.12192. [DOI] [PubMed] [Google Scholar]
- 122.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mason J.C., Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36(8):482–489c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tupin E., Nicoletti A., Elhage R., et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199(3):417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Y., Kanellakis P., Hosseini H., et al. A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE-/- mice by reducing lesion necrosis and inflammation. Cardiovasc Res. 2016;109(2):305–317. doi: 10.1093/cvr/cvv259. [DOI] [PubMed] [Google Scholar]
- 126.Nishioka Y., Yamaguchi M., Kawakami A., et al. Type II natural killer T cells that recognize sterol carrier protein 2 are implicated in vascular inflammation in the rat model of systemic connective tissue diseases. Am J Pathol. 2017;187(1):176–186. doi: 10.1016/j.ajpath.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 127.Nishioka Y., Masuda S., Tomaru U., et al. CD1d-restricted type II NKT cells reactive with endogenous hydrophobic peptides. Front Immunol. 2018;9:548. doi: 10.3389/fimmu.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raman P., Khanal S. Leptin in atherosclerosis: focus on macrophages, endothelial and smooth muscle cells. Int J Mol Sci. 2021;22(11):5446. doi: 10.3390/ijms22115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cochain C., Zernecke A. Macrophages in vascular inflammation and atherosclerosis. Pflügers Archiv. 2017;469(3–4):485–499. doi: 10.1007/s00424-017-1941-y. [DOI] [PubMed] [Google Scholar]
- 130.Menon P., Fisher E.A. Immunostaining of macrophages, endothelial cells, and smooth muscle cells in the atherosclerotic mouse aorta. Methods Mol Biol. 2015;1339:131–148. doi: 10.1007/978-1-4939-2929-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meng Q., Li Y., Ji T., et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J Adv Res. 2021;28:149–164. doi: 10.1016/j.jare.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.He X., Fan X., Bai B., et al. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res. 2021;165:105447. doi: 10.1016/j.phrs.2021.105447. [DOI] [PubMed] [Google Scholar]
- 133.Fidler T.P., Xue C., Yalcinkaya M., et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592(7853):296–301. doi: 10.1038/s41586-021-03341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Colin S., Chinetti-Gbaguidi G., Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262(1):153–166. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 135.Mantovani A., Garlanda C., Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29(10):1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]