Abstract
Long non-coding RNAs (lncRNAs) are a novel class of non-coding RNA (ncRNA), that have been studied extensively in the field of tumor research in recent years. In the case of tumor-associated lncRNAs, lncRNA cytoskeleton regulator RNA (CYTOR) displays extensive functions in tumorigenesis, including invasion, metastasis, malignant proliferation, glycolysis, and inflammatory response. Moreover, the dysregulation of CYTOR is closely related to clinicopathological characteristics, such as tumor stage, lymph node metastasis and infiltration, and poor prognosis of tumor patients. In this review, we provide a novel strategy to summarize the biological functions and clinical value of CYTOR in tumors through an overview of the literature combined with gene set enrichment analysis. A deeper understanding of the role of CYTOR in tumorigenesis may provide new diagnostic, prognostic and therapeutic markers for human tumors.
Keywords: CYTOR, GSEA, Inflammation, LncRNAs, Therapy, Tumorigenesis
Introduction
With the completion of the Human Genome Project and the arrival of the post-genome era, there is a new understanding of the status of non-coding sequences in the genome. More than 80% of these sequences do not contribute to protein-coding and were considered “junk DNAs”,1, 2, 3 which have now become “hot stars” in the areas of life science and technology. Numerous studies have shown that these “junk DNAs” can generate many non-coding RNAs (ncRNAs), most of which are long noncoding RNAs (lncRNAs) that play an important role in the biological activity of humans.4,5 lncRNAs are a subset of RNAs first found in the eukaryotic cells. They are located in the nucleus or cytoplasm and have a transcription length of 200–100,000 nt without a complete functional open reading frame (ORF), and rarely encode a functional short peptide.6, 7, 8 According to GENCODE analysis (www.gencodegenes.org) by the Ensembl Human Genome Browser (GRCh38, updated version, January 25, 2017), 27,908 transcripts generated by 15,778 genes were defined as lncRNAs. However, the lncRNA disease database (http://www.cuilab.cn/lncrnadisease) showed that only 2947 lncRNAs were related to disease. There are only a few hundred lncRNAs that have been identified as having biological functions because of limited research tools for analyzing lncRNA function and the cell type in which they are active. Therefore, lncRNAs remain a “gold mine” in the field of biological sciences, which researchers urgently need to excavate.
lncRNAs are widely found in prokaryotes and eukaryotes. As organisms increase in complexity, the proportion of lncRNAs in the genome increases accordingly, suggesting that lncRNAs play a pivotal role in biological evolution. In eukaryotes, lncRNAs are widely involved in the regulation of life activities in the form of tissue-specific expression, affecting the progression of disease.9,10 lncRNAs perform multi-spatial, multi-stage, and specific regulation of important genes at epigenetic, transcriptional and post-transcriptional levels, as well as translation and protein modification in the form of initially transcribed RNA or spliced RNA.11,12 They play an important role in basic physiological processes, including development, tissue differentiation, reproduction, and immunity.13,14 Therefore, their dysfunction or abnormal expression is often associated with various human diseases, including tumors. Studies have shown that lncRNAs are closely related to the occurrence and development of tumors and participate in processes including malignant proliferation,15 energy metabolism,16 angiogenesis,17 invasion and metastasis,18,19 inflammatory response,20 and immune escape.21 Cytoskeleton regulator RNA (CYTOR) is a tumor-associated lncRNA molecule discovered in recent years,22 which has been reported to be highly expressed in a variety of tumors.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Meanwhile, CYTOR also participates in the regulation of tumor invasion, metastasis, malignant proliferation, and inflammatory response via epigenetic modification (lncRNA-DNA), competing endogenous RNA (ceRNA) (lncRNA-miRNA), and lncRNA-protein interaction. Recently, a novel bioinformatic analysis software named Gene Set Enrichment Analysis (GSEA) (http://software.broadinstitute.org/gsea/index.jsp) was used to analyze whether a priori defined set of genes showed statistically significant, concordant differences between two biological phenotypes.55 In this review, we provide a novel strategy to summarize the biological functions and clinical value of CYTOR in tumors via the overview of literature combined with GSEA analysis, thereby providing a comprehensive theoretical basis and evidence for CYTOR as a diagnostic marker and therapeutic target for tumors.
Biological characteristics and regulation of CYTOR
Biological characteristics of CYTOR
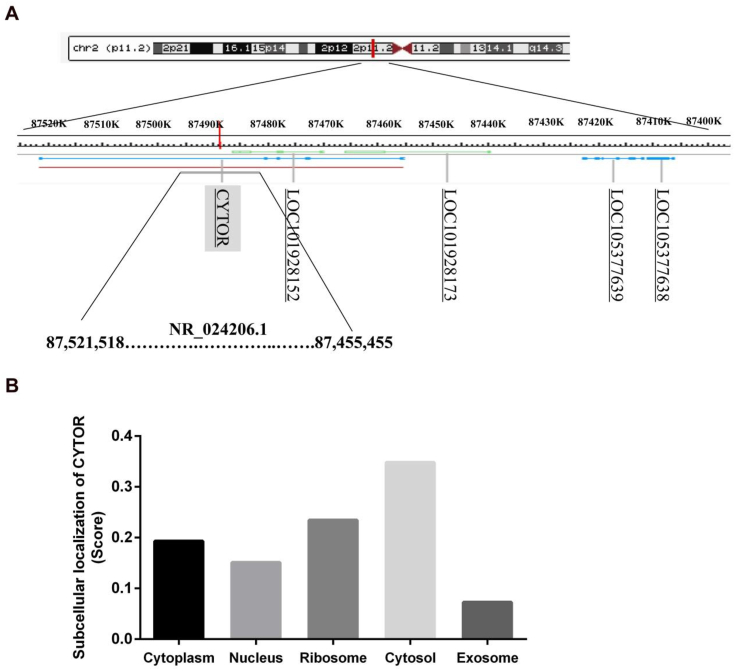
lncRNAs can be divided into five categories including sense lncRNAs, antisense lncRNAs, bidirectional lncRNAs, intragenic lncRNAs, and intergenic lncRNAs according to the relative locations of protein-encoding genes in the genome.56 Based on lncRNA nomenclature,57 the cytoskeleton regulator RNA (CYTOR) was named as long intergenic non-protein coding RNA 152 (LINC00152). CYTOR was homologous with MIR4435-2HG,58 and was a large intergenic non-coding RNA (lincRNA) with a length of 852 bp, located on chromosome 2p11.2 (87455455–87521518) (Fig. 1A). Its nearest protein-coding gene, plasminogen-like B2 (PLGLB2), was more than 100 kb. The Human Genome (hg38) in the Genome Browser of USCS (http://www.genomaize.org/) annotates CYTOR with 14 transcripts, three of which can be transcribed into mature mRNAs (RefSeq: NR_024204, NR_024205, and NR_024206). According to the position and gene information on CYTOR (ID No. ENSG00000222041) from the Ensembl of USCS analysis of the genomic phylogenetic tree, the CYTOR gene sequence is well conserved only in human and macaque genomes, while that in other mammals (e.g., mice, dogs, and sheep) is not complete. The National Center for Biotechnology Information (NCBI) also does not include the corresponding genetic information on human CYTOR gene sequences and other species. In addition, CYTOR belongs to a class of lncRNAs with short half-life. The half-life of CYTOR in neural stem cells (NSCs) is 2.1 h, which affects the expression of surrounding genes.59 CYTOR has the characteristics of most disease-associated lncRNAs and can be stably present in the form of mRNA in tissues, cells, serum, and exosomes.41,60 CYTOR is closely related to physiological processes such as cell differentiation,59 stress response,61 and cell stress.62 For example, Tani et al found that CYTOR was highly expressed in NSCs that were differentiated from human-induced pluripotent stem cells (hiPSCs).59 Moreover, Tani et aldemonstrated that the expression level of CYTOR was responded to the chemical stresses (hydrogen peroxide, cycloheximide, cadmium, or arsenic) in hiPSCs.61 Thus, it results in a series of diseases, including gastritis,63 vascular disease,64 tuberculosis,65 oral diseases,66 tumors,67 and other diseases.
Figure 1.
Biological characteristics of CYTOR. (A) Representative location of CYTOR on chromosome 2p11.2 (87455455–87521518). (B) The subcellular localization of CYTOR was predicted by lncLocator.
lncRNAs exert biological functions mainly in the cells. Therefore, it is helpful to understand and detect the molecular mechanism of the regulatory machinery of CYTOR by analyzing and speculating its location and function at the cellular level. Unlike mRNAs, lncRNAs are widely distributed in the nucleus and cytoplasm,4 and the subcellular localization of lncRNAs is often closely related to their biological functions.68,69 Nötzold et al detected the cellular localization of multiple CYTOR isoforms in HeLa cells and found that more than 70% of CYTOR transcripts were detected in the cytoplasm.22 The distribution of CYTOR in cells was predicted by the lncLocator website of lncRNA localization analysis (www.csbio.sjtu.edu.cn/bioinf/lncLocator) (Fig. 1B).70 CYTOR is distributed in the form of mRNA uniformly in various subcellular locations, including the cytoplasm, nucleus, ribosome, cytosol, and exosomes. Furthermore, CYTOR is mainly distributed in the cytoplasm, especially in the cytosol. Interestingly, the cytoskeleton in the cytosol is an important subcellular structure, which is closely related to cell movement and morphological changes, and provides a favorable framework structure for enzyme reactions in the cytoplasm. In recent years, researchers have found that CYTOR is closely related to cytoskeleton changes, and renamed it based on LINC00152; this is consistent with the fact that CYTOR is mainly located in cytosol. Notably, the distribution ratio of CYTOR from the nucleus to cytoplasm changed with different stimulating factors. Nishizawa et al showed that hypoxia can induce an increase in cytoplasmic localization of CYTOR in colorectal cancer (CRC) cells.33 Moreover, our research group demonstrated that cancer cell density can induce an increase in cytoplasmic expression of CYTOR in CRC cells.71 Therefore, when the cells are under different stress conditions, the subcellular localization of CYTOR also changes, thereby regulating specific biological functions of cells.
Regulatory mechanism of CYTOR
lncRNAs function differently depending on their subcellular location. When lncRNAs are located in the nucleus, they often play a role in regulating epigenetic modification, regulating the expression of downstream target genes through histone modification, and by binding the gene enhancer region.72 When lncRNAs nucleate to the cytoplasm, the expression of the corresponding miRNA target genes and their downstream genes are mainly regulated by the action of ceRNA at the post-transcriptional level or blocking the phosphorylation sites of certain proteins, thereby affecting downstream molecules or signaling pathways.73 However, when lncRNAs are secreted extracellularly in the form of exosomes, they can be transported to target cells in a “cell-to-cell” way, thereby exerting their biological function.74 According to the different functions of lncRNAs, the molecular mechanisms involved can be divided into four categories: 1) Signal archetype: a molecular signal or indicator for transcriptional activity; 2) Scaffold archetype: provides a platform for related molecular components, such as functional proteins or RNAs; 3) Guide archetype: directs the ribonucleoprotein complex to specific targets; 4) Decoy archetype: binds to other regulatory RNAs or proteins and isolates them.75 Therefore, clarifying the subcellular localization of lncRNAs is an important step in the analysis and study of their biological functions.
The regulation of CYTOR can occur in different ways, including epigenetic modifications, ceRNAs, and protein interactions, because of its complex subcellular distribution. Epigenetic modification refers to the heritable change in gene expression caused by a mechanism that does not affect the DNA sequence and is usually related to the regulation of acetylation and methylation of transcription factors. CYTOR is not only regulated by the transcription factors SP148 and β-catenin/TCF4,30 but also by the epigenetic modification of these transcription factors that can affect the expression of CYTOR. CYTOR also transcriptionally regulates a series of downstream genes via binding with other transcription factors, cyclin-dependent kinase inhibitor 1A (CDKN1A, also named p21) and cyclin dependent kinase inhibitor 2B (CDKN2B, also named p15),76 cell cyclin dependent kinase inhibitor 2A (CDKN2A, also named p16),44 IL-24,34 and E-cadherin,76 in the nucleus by recruiting enhancer of zeste homolog 2 (EZH2), thereby affecting their transcription and expression levels.
The ceRNA mechanism involves lncRNAs competitively binding to miRNA by ceRNA, thereby reversing the protein expression level of miRNA target genes and that of their downstream molecules.77,78 Recent studies have shown that the ceRNA mechanism of lncRNAs is involved in the development of various tumors, such as breast cancer,79 CRC,80 gastric cancer (GC),81,82 hepatocellular carcinoma (HCC),83,84 non-small cell lung cancer (NSCLC),85,86 and kidney cancer.87 Since CYTOR is mainly located in the cytoplasm, the important regulatory mechanism of CYTOR is the interaction of ceRNA with miRNAs. CYTOR can also interact with a range of miRNAs, such as miR-139-5p,28,32 miR-103a-3p,35 miR-205,44 miR-4775,45 miR-138,48 miR-125b,54 miR-4767,64 miR-193a-3p,31,88 miR-107,89 miR-612,90 miR-376c-3p,91 miR-632 and miR-185-3p,71 regulating a series of tumor processes.
Aside from regulating epigenetic modification-related proteins, lncRNAs can regulate transcriptional, post-transcriptional, and translational levels of certain proteins through various means of interaction, such as through guide, decoy, or scaffold molecules, thus affecting the expression and activation or silencing of downstream signaling pathways.92,93 Many studies have shown that CYTOR may participate in the regulation of multiple signaling pathways, such as the Wnt/β-catenin,27,30 Notch,32 hypoxia-inducible factor-1 (HIF-1) signaling pathway,33 mTOR,37 MAPK,45 PI3K/AKT,45,48 and epidermal growth factor receptor (EGFR)64,94 in tumorigenesis. However, few studies have demonstrated the specific molecular mechanisms of the interaction between cytoplasmic CYTOR and pathway proteins. Yue et al reported that CYTOR can block β-catenin phosphorylation induced by casein kinase 1 (CK1) by binding to cytoplasmic β-catenin, thereby inducing β-catenin expression in the nucleus to promote invasion and metastasis of CRC.30 Shan et al also found that CYTOR binds to β-catenin and methyltransferase SMYD2, promoting the methylation of β-catenin to maintain its stability, consequently activating the Wnt/β-catenin signaling pathway in GC.27 In addition, Zhou et al showed that CYTOR directly binds to EGFR in GC cells, activating the PI3K/AKT signaling pathway and accelerating cell cycle progression, thereby promoting the proliferation of GC cells.25 Currently, lncRNA-binding proteins are screened mainly by RNA pull-down and CHIRP-seq techniques. However, these have persistent high false-positive rates. Therefore, further studies on targeted drugs that improve the success rate of screening lncRNA-binding proteins are immediately warranted.
Role of CYTOR in the development and progression of tumors
Hanahan and Weinberg published “Hallmarks of cancer: the next generation” in the journal Cell,95 which summarized and clarified 10 characteristics of tumors. lncRNAs are closely associated with the occurrence and development of tumors, and are involved in processes, such as malignant proliferation, angiogenesis, abnormal energy metabolism, invasion, metastasis, radiotherapy and chemotherapy, and immune escape.96,97 CYTOR plays a pivotal role in the occurrence and development of tumors (Fig. 2); however, the comprehensive function of CYTOR remains unknown. In this study,49 the GSE77491 of GEO database was used to identify gene set differences between the two groups (si-CYTOR group vs. si-Ctrl group) in a cancer cell line (Fig. 3). We further revealed the biological role of CYTOR in tumorigenesis, including malignant proliferation, invasion, metastasis, glycolysis, and inflammatory response.
Figure 2.
lncRNA CYTOR in cancer phenotypes. CYTOR contributes to a series of hallmarks of cancer. Selected examples of CYTOR and their molecular partners or genomic targets are shown for malignant proliferation, invasion and metastasis, tumor glycolysis, and inflammatory response.
Figure 3.
Correlation analysis between CYTOR and cytoskeleton-associated gene sets. Gene sets involved with the microtubule-organizing center (A), cytoplasmic microtubule (B), glycolysis (C), hypoxia (D), inflammatory response (E), TNFA signaling (F), chemokine signaling (G), and chemokine binding to receptors (H) as demonstrated by GSEA (analyzing si-CYTOR vs. si-Ctrl MDA-MB-231 cells). ES, enrichment score; NES, normalized enrichment score.
CYTOR and malignant proliferation of tumor cells
The malignant proliferation of tumors is the most common malignant process in tumor development. Tumor cells usually exhibit contact inhibition and proliferation98,99; thus, tumors are also classified as a type of progressive hyperplastic disease.100 Although surgery of a malignant proliferation of tumor is considered effective to begin cancer treatment, the current results regarding its efficacy remain unsatisfactory. Finding key, effective, and broad-spectrum targets for malignant tumor proliferation remain a challenge for scientists.101,102 In recent years, lncRNAs, including CYTOR, have attracted wide attention as targets for tumor proliferation.103 Several studies have reported that CYTOR can participate in multiple gene and signaling pathways related to tumor cell proliferation, thus promoting tumor progression. In the study of GC, Huang et al reported that CYTOR competitively binds to miR-193a-3p in GC cells, thereby promoting the expression of its target gene MCL1 and the proliferation of GC cells.29 In addition, Zhao et al found that knocking out CYTOR in GC cells can block the cell cycle of those cells in the G1 phase.24 Furthermore, Chen et al showed that CYTOR inhibits the expression of the cyclin-dependent kinase inhibitor p15/p21 in the progression from G1 to S phase by recruiting EZH2, thereby promoting the proliferation of GC cells.76 Zhang et al demonstrated that knockdown of CYTOR in lung cancer cells significantly reduced the protein expression of PI3K and AKT and increased p21 expression.94 Cai et al confirmed that CYTOR activates the PI3K/AKT signaling pathway in vitro, ultimately accelerating the cell cycle process and promoting the malignant proliferation of gallbladder cancer cells.48 In addition, Chen et al showed that CYTOR acts as a ceRNA of miR-125b in ovarian cancer to regulate the expression of MCL-1 and MCL-1-mediated mitochondrial apoptosis pathways.54 Wu et al reported that CYTOR promotes tumor growth of triple-negative breast cancer and inhibits apoptosis by inducing the activation of BRCA1/PTEN by promoting the expression of DNA methyltransferases.50
CYTOR, tumor invasion, and metastasis
Tumor invasion and metastasis refer to the process of malignant tumor cells disengaging from the primary tumor site and transferring to secondary tissues or organs via the circulatory system, where they colonize and grow to form secondary tumors. Tumor invasion and metastasis is a complex cascade of dynamic processes, involving epithelial–mesenchymal transition (EMT), hypoxia, angiogenesis, tumor microenvironment, and other mechanisms, as well as changes in cells, including cell adhesion, cytoskeletal reconstruction, extracellular matrix degradation, and formation of cell components and pseudopods.104 Tumor invasion and metastasis is a major problem in the clinical treatment of tumors, affecting the prognosis and survival of patients, and is a major cause of tumor-related death. According to epidemiological statistics, 90% of deaths in cancer patients are due to tumor metastasis, but only approximately 0.02% of tumor cells form distant metastases.105 Therefore, the study of the mechanism of tumor cell invasion and metastasis for better treatment and prevention of tumors is of great significance. lncRNAs are a class of molecules that play an important role in tumor invasion and metastasis,106 and CYTOR is naturally involved as a tumor-associated lncRNA molecule. Deng et al showed that the key protein X of hepatitis B virus (HBV) infection induces the expression of CYTOR, while the high expression of CYTOR inhibits the expression of the epithelial cell marker E-cadherin by binding to EZH2 and promoting the EMT process in liver cancer cells.39
The invasion and metastasis of tumor cells is closely related to the spatial and temporal coordination of the cytoskeleton.107,108 Dynamic reconstruction of the cytoskeleton provides plate-like pseudopods, filopodia, invasive pseudopods, and other special structures for cell invasion,109,110 which enable tumor cells to enter the blood circulation. There is a certain relationship between CYTOR and the skeleton reconstruction of tumor cells. Interestingly, our research group identified that CYTOR is highly expressed in CRC and acts as a competing endogenous RNA sponging with miR-632 and miR-185-3p to regulate the expression of fascin actin-bundling protein 1 (FSCN1), which is an important cell skeleton-associated gene.71 Moreover, we found positive associations between CYTOR and multiple cytoskeleton-associated gene sets, including gene sets involved in the microtubule-organizing center and cytoplasmic microtubule, by analyzing GSE77491 of the GEO database using GSEA software (Fig. 3A, B). This suggests that CYTOR may participate in the regulation of tumor cell invasion and metastasis by regulating the cytoskeleton.
CYTOR and tumor glycolysis
In most solid tumors, hypoxic conditions appear as the tumor volume increases; however, the tumor cells do not stop their malignant biological behavior because of a specific cellular metabolism, called glycolysis-based cellular metabolism.111, 112, 113 Under hypoxic conditions, the glycolytic enzyme activity of tumor cells is strengthened, including hexokinase 2 (HKII), 6-phosphofructokinase subunit alpha (PFK1), pyruvate kinase (PK), and lactate dehydrogenase (LDH),114 while the activity of ATP-producing enzymes in mitochondria is weakened, as is oxidative phosphorylation. Tumor cells maintain the energy supply for biological activities (e.g., cell proliferation, invasion, metastasis, and angiogenesis) by anaerobic glycolysis. However, tumor cells use glycolysis as the primary means of producing ATP even under aerobic conditions, which is known as the Crabtree effect or the anti-Paste effect.115 The expression of HIF-1 in tumor cells was induced in a hypoxic environment,116 which inhibited the oxidative phosphorylation pathway and enhanced the glycolytic pathway in cells.117,118 Effective drugs for targeting tumor cell metabolism are currently lacking; therefore, finding a molecular diagnosis and treatment target related to tumor glycolysis is one strategy for inhibiting tumor growth and controlling tumor progression. There is increasing evidence that lncRNAs play an important role in the process of tumor glycolysis.119, 120, 121 As a key molecule in tumor progression, CYTOR is also closely related to tumor glycolysis. Sun et al reported that CYTOR regulates the expression of miR-139-5p and its downstream target gene protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1) in GC cells, thus promoting their glycolytic process.28 In addition, Nishizawa et al showed that the expression of CYTOR in CRC cells was significantly increased in a hypoxic environment.33 Cai et al found that CYTOR regulates the expression of miR-138 and its downstream target gene HIF-1α as ceRNA, promoting EMT transformation of gallbladder cancer cells.48 HIF-1α is an important molecule regulating tumor glycolysis,116, 117, 118 which implies that there is a relationship between CYTOR, HIF-1α, and tumor glycolysis. Moreover, we found positive associations between CYTOR and multiple energy metabolism-associated gene sets, including gene sets involved in glycolysis and hypoxia, by analyzing GSE77491 of the GEO database using GSEA software (Fig. 3C, D). Thus, the regulatory network between CYTOR, HIF-1α, and tumor glycolysis may provide a theoretical basis for the development of novel tumor-targeted drugs in the future.
CYTOR and inflammatory response
In recent years, tumors and inflammation have been a major focus of research. Tumors affect approximately 25% of humans because of non-resolving inflammation. These studies clarified that non-resolving inflammation accelerates the ‘inflammation–tumor’ reaction chain and eventually leads to the formation of tumors referred to as “non-resolving inflammation-associated tumors”. Non-resolving inflammation is an important biological malignancy and is known as the “seventh characteristic of tumors”.122, 123, 124 In recent years, an increasing amount of evidence has shown that lncRNAs regulate the microenvironment, immune escape, invasion, and metastasis of non-resolving inflammation-associated tumors by affecting important factors related to the occurrence of non-resolving inflammation, such as inflammatory cells, inflammatory factors, and chemokines.125, 126, 127 Recent studies have reported that CYTOR is associated with gastric inflammation,63 hepatitis,39 retinitis pigmentosa,128 and other uncontrollable inflammatory diseases; however, the correlation between CYTOR and uncontrollable inflammatory responses has not been explored via molecular mechanisms. Zhu et al demonstrated that the expression of CYTOR in gastritis tissues infected by Helicobacter pylori increased dramatically in comparison with normal gastric tissue by microarray.63 Deng et al reported that CYTOR expression in patients with HCC with HBV positivity increased and the high expression of CYTOR was associated with HBx, an essential protein of HBV infection.39 In addition, Chen et al found that in lung adenocarcinoma cells, CYTOR inhibits the expression level of interleukin 24 (IL24) by binding to EZH2.34 IL24 is a molecule related to uncontrollable inflammation.129,130 It is worth exploring whether CYTOR can establish a connection with the inflammatory response through IL24. Cai et al reported that CYTOR regulates the AKT2/NF-κB pathway in glioblastoma by competitively binding endogenous miR-612.90 Moreover, while analyzing GSE77491 of the GEO database using GSEA software, we found positive associations between CYTOR and multiple inflammation-associated gene sets, including gene sets involved in inflammatory response, TNFA signaling, chemokine signaling, and chemokine binding to receptors (Fig. 3E–H). In summary, inconspicuous evidences indicate that CYTOR plays an important role in the progression of non-resolving inflammation-associated tumors.
Application of CYTOR in the clinical treatment of tumors
CYTOR and clinicopathological features
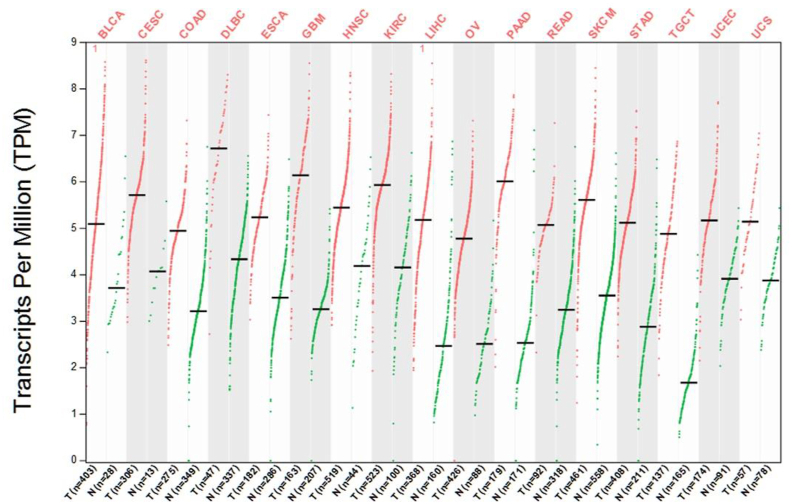
Tumor markers are substances present in malignant tumor tissues or cells, or are produced or secreted by abnormal reactions of malignant tumor cells. They can be used to determine the progress of tumors and monitor the therapeutic effects of drugs.131, 132, 133 Tumor markers play an important role in the early diagnosis of tumors,134 monitoring high-risk populations,135 assessing severity, developing a personalized treatment plan,136 and assessing treatment and prognosis.137 Although lncRNAs are not expressed as high as protein-encoding genes, the former are present in abundance in cells, tissues, blood, and feces, and have a certain stability, specificity, and sensitivity. Therefore, lncRNAs have received considerable attention from researchers as a new tumor marker.138,139 Similar to most tumor-related lncRNA molecules, CYTOR exists stably in tissues, cells, serum,41,60 and exosomes60 in the form of their target mRNA. CYTOR has been reported to be highly expressed in various tumors, such as GC,23, 24, 25, 26, 27, 28, 29 CRC,30, 31, 32, 33 NSCLC,34, 35, 36 HCC,37, 38, 39 pancreatic cancer,40 esophageal cancer,41,42 clear cell renal cell carcinoma,43,44 glioma,45,46 hemangioma,47 gallbladder cancer,48 breast cancer,49,50 tongue squamous cell carcinoma (TSCC),51 head and neck squamous cell carcinoma,52 retinoblastoma,53 and ovarian cancer.54 Interestingly, by analyzing The Cancer Genome Atlas (TCGA) database using the Gene Expression Profiling Interactive Analysis (GEPIA), we also found that CYTOR has abundant expression in various types of tumors (Fig. 4).
Figure 4.
Expression aberration of CYTOR in multiple types of tumors using the GEPIA2 database. Red, tumor samples; grey, normal control samples. BLCA, bladder urothelial carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; LIHC, liver hepatocellular carcinoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; READ, rectum adenocarcinoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma.
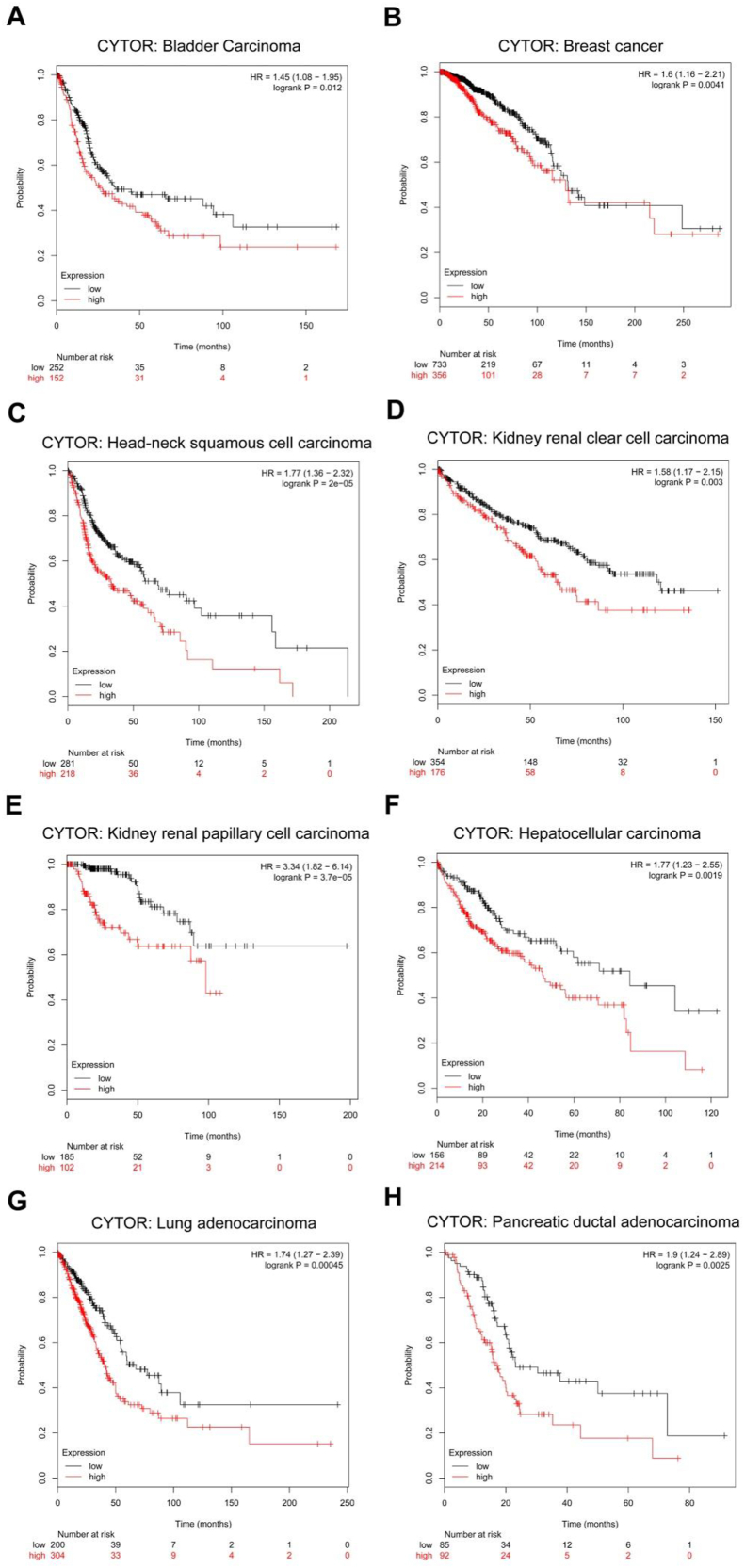
CYTOR is not only highly expressed in tumors, but is also closely related to the clinicopathological features, such as tumor stage, malignancy, infiltration, and poor prognosis of various tumors (Table 1), and has potential tumor marker characteristics. By analyzing TCGA database using the Kaplan–Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=pancancer_rnaseq), we found that high expression of CYTOR was positively correlated with poor survival (Fig. 5). Moreover, Xiong et al constructed the lncRNA expression profile in TSCC using gene chip technology and screened and verified the expression of CYTOR in tongue squamous cell carcinoma, followed by in situ hybridization (ISH) detection of CYTOR expression in paraffin-embedded specimens of tongue squamous cell carcinoma,51 further confirming that high expression of CYTOR is closely related to infiltration, lymph node metastasis, tumor stage, and poor prognosis of TSCC patients. The overall survival time and disease-free survival time of patients with high CYTOR expression were shorter than that of patients with low CYTOR expression. This is similar to the findings of other research teams testing CYTOR on other types of tumors. For example, in GC, increased expression of CYTOR is positively correlated with tumor size, depth of tumor invasion, lymph node metastasis, TNM stage, and adverse survival rate.24,76 Cai et al reported that high levels of CYTOR were positively associated with lymph node invasion and progression of TNM staging in gallbladder carcinoma.9 In addition to CYTOR pathological detection, it can be detected in blood and exosomes as a test indicator.26,60 Yang et al found that people with high CYTOR expression in serum and H. pylori infection were more likely to develop GC.26 In addition, Li et al showed that CYTOR levels in plasma and exosomes of GC patients were significantly higher than those of normal controls, and the expression level of CYTOR in plasma after surgery was significantly lower than that in plasma before surgery.60 CYTOR is an lncRNA molecule with tumor characteristics involved in tumor progression and can be used for early detection and prognosis monitoring of tumors.
Table 1.
Correlation between CYTOR and clinicopathological features of tumors.
| Types of cancer | Sample sources | Dysregulation | Relationship with clinicopathology | Ref. |
|---|---|---|---|---|
| Glioblastoma malignancy | tissue | up | WHO classification | 90 |
| Laryngeal cancer | tissue | up | Lymph node metastasis or an advanced clinical stage | 140 |
| tissue | up | Clinical stage and pathological differentiation degree | 141 | |
| Tongue squamous cell carcinoma | tissue | up | T stage, N stage andTNM stage | 51 |
| Oral squamous cell carcinoma | tissue | up | TNM stage and lymph node metastasis | 142 |
| Esophageal carcinoma | tissue | up | Lymphatic metastasis and advanced pTNM classification | 143 |
| tissue | up | TNM stage and lymph node metastasis | 144 | |
| Papillary thyroid carcinoma | tissue | up | Tumor size, LNM, clinical stage and extrathyroidal extension | 145 |
| Non-small cell lung cancer | tissues, plasma | up | Tumor size and pathological grades | 146 |
| tissue | up | Lymph node metastasis station, remote metastasis and TNM staging | 36 | |
| tissue | up | Tumor volume and lymph node metastases | 147 | |
| tissue | up | TNM stage, larger tumor size and lymph node metastasis | 34 | |
| Hepatocellular carcinoma | tissue | up | Tumor size, HBV infection and tumor number | 39 |
| tissue | up | Tumor size and Edmondson grade | 37 | |
| tissue | up | Differentiation grade, tumor size, TNM stage and tumor capsular | 148 | |
| Gallbladder cancer | tissue | up | Tumor status progression and lymph node invasion | |
| tissue | up | Tumor status progression, lymph node invasion and TNM stage advancement | 48 | |
| Gastric cancer | tissue | up | Clinical stage and lymphatic metastasis | 149 |
| tissue | up | Tumor size | 25 | |
| tissue | up | Tumor invasion depth, lymph node metastasis and TNM stage | 76 | |
| tissue | up | Tumor size | 24 | |
| tissue | up | Invasion | 150 | |
| Colorectal cancer | tissue | up | Pathological grade and lymph node metastases | 151 |
| tissue | up | Tumor stage | 32 | |
| Tissue | Up | Tumor size, tumor grade, tumor node metastasis (TNM) stage and distant metastasis | 71 | |
| Renal cell carcinoma | tissue | up | Lymph node metastasis and higher TNM stage | 44 |
| tissue | up | TNM stage | 43 | |
| Cervical cancer | tissue | up | Histologic grade | 152 |
| Ovarian cancer | tissue | up | Histological grade and clinical stage | 54 |
| Bladder Cancer | tissue | up | Lymph node metastasis and histological grade | 153 |
| Osteosarcoma | tissue | up | Tumor size, TNM stage and clinical stage | 154 |
Figure 5.
Kaplan–Meier analysis showing overall survival (OS) curves of multiple types of tumors patients with different expression of CYTOR. Black graphic lines are representative of tumor patients with low expression of CYTOR; Red graphic lines are representative of tumor patients with high expression of CYTOR.
Prospects for CYTOR in tumor therapy
Although tumor treatment has made great progress and the 5-year survival rate of many cancer patients has been significantly improved, the incidence and mortality of tumors increase annually. Therefore, studies on suitable drug targets for the clinical treatment of tumors remain the main means that improve the therapeutic effect of tumors, delaying the progression of tumors, and improving the survival rate of patients. In recent years, non-coding RNA has played a pivotal role in the study of tumor therapy,155, 156, 157 and several miRNA-related tumor therapeutic drugs have entered clinical trials. The tumor drug MRX34 (NCT01829971), developed by Mirna that targets miR-34, has entered phase I clinical trials of various solid and hematological tumors.158,159 The miR-122-targeted oncology drug Miravirsen (SPC3649) developed by Roche has entered phase II clinical trials for hepatitis C.160, 161, 162 MRG-106, a tumor drug targeting miR-155 developed by miRagen, has entered phase I clinical trials for hematological tumors. The process from basic research to clinical application is a lengthy one. Although miRNA-targeted tumor drugs have entered phase I and phase II clinical trials, it would still take a long way to be considered in clinical treatment. The study of tumor drugs targeting lncRNAs is still at the initial stage. Most of the drugs are currently in animal testing. For example, Arun et al demonstrated that MALAT1 antisense oligonucleotides slow tumor growth and metastasis in a breast cancer mouse model induced by mouse mammary tumor virus-PyMT, and inhibit breast cancer progression in mice.163 In addition, Claes Wahlestedt et al proposed that antagoNAT [antago (natural antisense transcript) oligonucleotides, antagoNAT oligonucleotides] targeting antisense lncRNA is expected to become a new technology to promote the development of tumor-targeted therapeutic drugs.164 There are many lncRNAs with tumor characteristics similar to CYTOR, such as HOTAIR, MALAT1, and AFAP1-AS1. Building a bridge connecting non-coding RNA and tumor therapy to maximize its application value in tumor therapy is a challenge that needs further clinical research.
The development of drugs or inhibitors targeting CYTOR remains unelucidated; however, with the continuous optimization of detection methods, Soares et al used different ISH methods to detect the expression of CYTOR in multiple cell lines.165 It was found that the branched-DNA probe method can detect stronger CYTOR signals. Further clinical verification by this probe may be more conducive to the detection of CYTOR signals in tissues or blood, which may improve the specificity, sensitivity, and accuracy. With the deepening of basic research on CYTOR, the value of its clinical application has attracted increasing attention from researchers. In our laboratory, Xiong et al tested the expression of CYTOR in tissues and serum of patients with TSCC and acquired three Chinese patents (CN106480196A, CN106511368A, and CN106381339A).61 Thus, CYTOR has displayed potential for clinical conversion in various tumor studies. It is believed that in the near future, detection kits and therapeutic drugs targeting CYTOR can be put into clinical research.
Conclusions and future perspectives
Researchers reported approximately 25 years ago that the lncRNA molecule H19 is associated with cancer and fetal growth166,167 and the lncRNA molecule Xist silences the second X chromosome.168 Moreover, these lncRNAs were thought to function only in specific cells. It was not until 2005 that Siepel et al discovered 35,000 ncRNAs through the large-scale project FANTOM,169 after which lncRNAs received renewed attention. However, there has been a disagreement about the function and role of lncRNAs in humans. Bassett et al pointed out that important genetic material should be highly conserved, whereas lncRNA sequences are not highly conserved between species.170 Cabili et al identified lncRNAs in cells using a new fluorescent probe method and performed single-cell imaging of lncRNAs in different human tissues.171 They found that many lncRNAs were not randomly distributed: some were present in the cytoplasm, while some were present in the nucleus, which is contrary to the expectation that lncRNA was a “junk” molecule. Regardless of the final conclusion of the debate, lncRNAs are still a hot “star” in the field of cancer research. With the continuous update and optimization of bioinformatics analysis software as well as the continuous development of high-throughput sequencing technology and molecular experimental technology (especially CRISPR technology172,173), several new lncRNAs have been discovered and identified, especially tumor-associated lncRNAs. These lncRNAs can regulate the expression levels of a series of molecules by interacting with proteins, adsorbing miRNAs, and inducing epigenetic modifications to DNA sequences, thereby participating in the occurrence and development of tumors. The pathogenesis of tumor-associated lncRNAs could be targeted by gene knockout, RNA interference, gene complementation, etc. Thus, the study of lncRNAs can provide new strategies for early tumor detection and treatment. At present, there are two difficulties in research related to lncRNAs: 1) identifying whether the predicted lncRNAs are functional; 2) verifying whether functional lncRNAs are related to the course of disease. Therefore, maximizing the research and application of new tumor-associated lncRNAs is the need of the hour for cancer researchers.
CYTOR, an intergenic lncRNA molecule with a total length of 852 bp, short half-life, and evident tumor characteristics, can be expressed in tissues, cells, serum, and exosomes. It is also involved in tumor invasion and metastasis, malignant proliferation, immune escape, radiotherapy and chemotherapy resistance, formation of microenvironments, and other malignant processes of various tumors. High expression of this molecule is also closely related to clinicopathological features such as staging, infiltration, and poor prognosis, so it is a potential tumor marker and molecular therapeutic target. However, because of the limitations of research methods and strategies, there is still a long way to go from basic research to clinical application for targeted inhibitors of CYTOR. The reasons are as follows: 1) because the sequence of CYTOR is not well conserved among other species; compared with the human genome, the mouse genome does not have a complete CYTOR gene sequence; therefore, it is difficult to construct a CYTOR knockout mouse for corresponding research and drug clinical research; 2) CYTOR has multiple transcripts; therefore, transcript or transcripts that play a regulatory role in the environment remain unclear; 3) CYTOR is located in the nucleus, cytoplasm, cytosol, ribosome, vesicle, and other subcellular locations and organelles, and the distribution of CYTOR changes under different conditions, raising the question of the effect of cellular localization of CYTOR on its biological function 4) The molecular mechanism of CYTOR remains unelucidated, and upstream signal stimulation (such as hypoxia, cell density, and TGFβ stimulating factor) can be accepted, and downstream molecular mechanisms (e. g., interaction with EZH2 for epigenetic modification or adsorption of miRNA to form ceRNA for regulation) can be regulated by CYTOR. Despite these limitations, the etiology and clinical value of CYTOR have been analyzed from both basic and clinical research perspectives. This provides a theoretical basis for CYTOR as a marker in the early diagnosis, prognosis, and treatment of cancer and further promotes the development of basic biological research in clinical settings.
Conflict of interests
The authors declare no competing financial interest.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81974424, 81874133, 81772903 and 81903032), Natural Science Foundation of Hunan Province, China (No. 2018JJ2630 and 2021JJ41013), the Huxiang Young Talent Project, China (No. 2018RS3024), the China Postdoctoral Science Foundation (No. 2020M672520) and the Youth Fund of Xiangya Hospital, China (No. 2018Q011).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yong Liu, Email: liuyongent@csu.edu.cn.
Xin Zhang, Email: xinzhang@csu.edu.cn.
Abbreviations
- BLCA
bladder urothelial carcinoma
- CDKN1A
cell cyclin dependent kinase inhibitor 1A
- CDKN2B
cyclin dependent kinase inhibitor 2B
- ceRNA
competing endogenous RNA
- CESC
cervical squamous cell carcinoma and endocervical adenocarcinoma
- COAD
colon adenocarcinoma
- CRC
colorectal cancer
- CYTOR
cytoskeleton regulator RNA
- DLBC
lymphoid neoplasm diffuse large B-cell lymphoma
- DNMTs
DNA methyltransferases
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- ESCA
esophageal carcinoma
- EZH2
enhancer of zeste homolog 2
- FSCN1
fascin actin-bundling protein 1
- GBM
glioblastoma multiforme
- GC
gastric cancer
- GSEA
gene set enrichment analysis
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HIF-1
hypoxia-inducible factor-1
- HNSC
head and neck squamous cell carcinoma
- ISH
in situ hybridization
- KIRC
kidney renal clear cell carcinoma
- LIHC
liver hepatocellular carcinoma
- lincRNA
large intergenic noncoding RNA
- lncRNAs
long non-coding RNAs
- NCBI
national center for biotechnology information
- ncRNA
non-coding RNA
- NSCLC
non-small cell lung cancer
- ORF
open reading frame
- OS
overall survival
- OV
ovarian serous cystadenocarcinoma
- PAAD
pancreatic adenocarcinoma
- PLGLB2
plasminogen-like B2
- PRKAA1
protein kinase AMP-activated catalytic subunit alpha 1
- READ
rectum adenocarcinoma
- SKCM
skin cutaneous melanoma
- STAD
stomach adenocarcinoma
- TGCT
testicular germ cell tumors
- TNBC
triple-negative breast cancer
- TSCC
tongue squamous cell carcinoma
- UCEC
uterine corpus endometrial carcinoma
- UCS
uterine carcinosarcoma
References
- 1.Ling H., Vincent K., Pichler M., et al. Junk DNA and the long non-coding RNA twist in cancergenetics. Oncogene. 2015;34(39):5003–5011. doi: 10.1038/onc.2014.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie H., Wang Y., Liao Z., et al. The function and mechanism of circular RNAs in gastrointestinal tumours. Cell Prolif. 2020;53(7) doi: 10.1111/cpr.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu X., Sood A.K., Dang C.V., et al. The role of long noncoding RNAs in cancer: the dark matter matters. Curr Opin Genet Dev. 2018;48:8–15. doi: 10.1016/j.gde.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 5.He X., Ou C., Xiao Y., et al. LncRNAs: key players and novel insights into diabetes mellitus. Oncotarget. 2017;8(41):71325–71341. doi: 10.18632/oncotarget.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spizzo R., Almeida M.I., Colombatti A., et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P., Mo Y., Peng M., et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19(1):e22. doi: 10.1186/s12943-020-1147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X., Kuang G., Zuo Y., et al. The role of non-coding RNAs in diabetic nephropathy-related oxidative stress. Front Med. 2021;8 doi: 10.3389/fmed.2021.626423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp F., Mendell J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar C., Chatterjee S., Thum T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation. 2016;134(19):1484–1499. doi: 10.1161/CIRCULATIONAHA.116.023686. [DOI] [PubMed] [Google Scholar]
- 12.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilo F., Zhou M.M., Walsh M.J. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. 2011;71(16):5365–5369. doi: 10.1158/0008-5472.CAN-10-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ørom U.A., Derrien T., Beringer M., et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143(1):46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao C., Zhang T., Zhang D., et al. The long non-coding RNA, SNHG6-003, functions as a competing endogenous RNA to promote the progression of hepatocellular carcinoma. Oncogene. 2017;36(8):1112–1122. doi: 10.1038/onc.2016.278. [DOI] [PubMed] [Google Scholar]
- 16.Peng F., Wang J.H., Fan W.J., et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37(8):1062–1074. doi: 10.1038/onc.2017.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.X., Chen Z.H., Chen D.L., et al. LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37(20):2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sun Z., Ou C., Liu J., et al. YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene. 2019;38(14):2627–2644. doi: 10.1038/s41388-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.He X., Li S., Yu B., et al. Up-regulation of LINC00467 promotes the tumourigenesis in colorectal cancer. J Cancer. 2019;10(25):6405–6413. doi: 10.7150/jca.32216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang T., Li S., Liu J., Yin D., et al. lncRNA-NKILA/NF-κB feedback loop modulates laryngeal cancer cell proliferation, invasion, and radioresistance. Cancer Med. 2018;7(5):2048–2063. doi: 10.1002/cam4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei X., Wang X., Li H. LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/Ido. Int J Biol Macromol. 2018;118(Pt A):24–30. doi: 10.1016/j.ijbiomac.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Nötzold L., Frank L., Gandhi M., et al. The long non-coding RNA LINC00152 is essential for cell cycle progression through mitosis in HeLa cells. Sci Rep. 2017;7(1):e2265. doi: 10.1038/s41598-017-02357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao W.J., Wu H.L., He B.S., et al. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19(23):3658–3664. doi: 10.3748/wjg.v19.i23.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J., Liu Y., Zhang W., et al. Long non-coding RNA linc 00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14(19):3112–3123. doi: 10.1080/15384101.2015.1078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J., Zhi X., Wang L., et al. Linc 00152 promotes proliferation in gastric cancer through the EGFR-dependent pathway. J Exp Clin Cancer Res. 2015;34:e135. doi: 10.1186/s13046-015-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang T., Zeng H., Chen W., et al. Helicobacter pylori infection, H19 and LINC00152 expression in serum and risk of gastric cancer in a Chinese population. Cancer Epidemiol. 2016;44:147–153. doi: 10.1016/j.canep.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Shan Y., Ying R., Jia Z., et al. LINC000152 promotes gastric cancer cell proliferation and metastasis via activating the Wnt/β-catenin signaling pathway. Oncol Res. 2017;25(9):1589–1599. doi: 10.3727/096504017X14897896412027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Sun K., Hu P., Xu F. LINC00152/miR-139-5p regulates gastric cancer cell aerobic glycolysis by targeting PRKAA1. Biomed Pharmacother. 2018;97:1296–1302. doi: 10.1016/j.biopha.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y., Luo H., Li F., et al. LINC00152 down-regulated miR-193a-3p to enhance MCL1 expression and promote gastric cancer cells proliferation. Biosci Rep. 2018;38(3) doi: 10.1042/BSR20171607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Yue B., Liu C., Sun H., et al. A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther. 2018;26(5):1287–1298. doi: 10.1016/j.ymthe.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue B., Cai D., Liu C., et al. LINC00152 functions as a competing endogenous RNA to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol Ther. 2016;24(12):2064–2077. doi: 10.1038/mt.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian Z., Zhang J., Li M., et al. Long non-coding RNA LINC00152 promotes cell proliferation, metastasis, and confers 5-FU resistance in colorectal cancer by inhibiting miR-139-5p. Oncogenesis. 2017;6(11):e395. doi: 10.1038/s41389-017-0008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizawa Y., Konno M., Asai A., et al. Hypoxia stimulates the cytoplasmic localization of oncogenic long noncoding RNA LINC00152 in colorectal cancer. Int J Oncol. 2018;52(2):453–460. doi: 10.3892/ijo.2017.4218. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q.N., Chen X., Chen Z.Y., et al. Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression. Mol Cancer. 2017;16(1):e17. doi: 10.1186/s12943-017-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M., Xue Y., Zheng J., et al. LINC00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol Cancer. 2017;16(1):e110. doi: 10.1186/s12943-017-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Zhang P.P., Wang Y.Q., Weng W.W., et al. LINC00152 promotes cancer cell proliferation and invasion and predicts poor prognosis in lung adenocarcinoma. J Cancer. 2017;8(11):2042–2050. doi: 10.7150/jca.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J., Tang J., Deng L., et al. LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget. 2015;6(40):42813–42824. doi: 10.18632/oncotarget.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan W., Sun Y., Liu L., et al. Circulating lncRNAs serve as diagnostic markers for hepatocellular carcinoma. Cell Physiol Biochem. 2017;44(1):125–132. doi: 10.1159/000484589. [DOI] [PubMed] [Google Scholar]
- 39.Deng X., Zhao X.F., Liang X.Q., et al. LINC00152 promotes cancer progression in hepatitis B virus-associated hepatocellular carcinoma. Biomed Pharmacother. 2017;90:100–108. doi: 10.1016/j.biopha.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Müller S., Raulefs S., Bruns P., et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:e94. doi: 10.1186/s12943-015-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu H.B., Jie H.Y., Zheng X.X. Three circulating lncRNA predict early progress of esophageal squamous cell carcinoma. Cell Physiol Biochem. 2016;40(1–2):117–125. doi: 10.1159/000452529. [DOI] [PubMed] [Google Scholar]
- 42.Yu X., Lin Y., Sui W., et al. Analysis of distinct long noncoding RNA transcriptional fingerprints in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6(3):673–680. doi: 10.1002/cam4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y., Tan C., Weng W.W., et al. Long non-coding RNA LINC00152 is a positive prognostic factor for and demonstrates malignant biological behavior in clear cell renal cell carcinoma. Am J Cancer Res. 2016;6(2):285–299. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y., Liu J., Bai H., et al. Long intergenic non-coding RNA 00152 promotes renal cell carcinoma progression by epigenetically suppressing P16 and negatively regulates miR-205. Am J Cancer Res. 2017;7(2):312–322. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z., Dai J., Liao Y., et al. Knockdown of long noncoding RNA LINC0000125 suppresses cellular proliferation and invasion in glioma cells by regulating MiR-4775. Oncol Res. 2018;26(6):857–867. doi: 10.3727/096504017X15016337254597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W., Wu F., Zhao Z., et al. Long noncoding RNA LINC00152 is a potential prognostic biomarker in patients with high-grade glioma. CNS Neurosci Ther. 2018;24(10):957–966. doi: 10.1111/cns.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Lv R., Zhang L., et al. Long noncoding RNA expression profile of infantile hemangioma identified by microarray analysis. Tumour Biol. 2016;37:15977–15987. doi: 10.1007/s13277-016-5434-y. [DOI] [PubMed] [Google Scholar]
- 48.Cai Q., Wang Z.Q., Wang S.H., et al. Upregulation of long non-coding RNA LINC00152 by SP1 contributes to gallbladder cancer cell growth and tumor metastasis via PI3K/AKT pathway. Am J Transl Res. 2016;8(10):4068–4081. [PMC free article] [PubMed] [Google Scholar]
- 49.Van Grembergen O., Bizet M., de Bony E.J., et al. Portraying breast cancers with long noncoding RNAs. Sci Adv. 2016;2(9) doi: 10.1126/sciadv.1600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Shuang Z., Zhao J., et al. LINC00152 promotes tumorigenesis by regulating DNMTs in triple-negative breast cancer. Biomed Pharmacother. 2018;97:1275–1281. doi: 10.1016/j.biopha.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 51.Yu J., Liu Y., Guo C., et al. Upregulated long non-coding RNA LINC00152 expression is associated with progression and poor prognosis of tongue squamous cell carcinoma. J Cancer. 2017;8(4):523–530. doi: 10.7150/jca.17510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haque S.U., Niu L., Kuhnell D., et al. Differential expression and prognostic value of long non-coding RNA in HPV-negative head and neck squamous cell carcinoma. Head Neck. 2018;40(7):1555–1564. doi: 10.1002/hed.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S., Wen D., Che S., et al. Knockdown of long noncoding RNA 00152 (LINC00152) inhibits human retinoblastoma progression. OncoTargets Ther. 2018;11:3215–3223. doi: 10.2147/OTT.S160428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Chen P., Fang X., Xia B., et al. Long noncoding RNA LINC00152 promotes cell proliferation through competitively binding endogenous miR-125b with MCL-1 by regulating mitochondrial apoptosis pathways in ovarian cancer. Cancer Med. 2018;7(9):4530–4541. doi: 10.1002/cam4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A., Tamayo P., Mootha V.K., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atianand M.K., Caffrey D.R., Fitzgerald K.A. Immunobiology of long noncoding RNAs. Annu Rev Immunol. 2017;35:177–198. doi: 10.1146/annurev-immunol-041015-055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright M.W. A short guide to long non-coding RNA gene nomenclature. Hum Genom. 2014;8(1):e7. doi: 10.1186/1479-7364-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reon B.J., Takao Real Karia B., Kiran M., et al. LINC00152 promotes invasion through a 3'-hairpin structure and associates with prognosis in glioblastoma. Mol Cancer Res. 2018;16(10):1470–1482. doi: 10.1158/1541-7786.MCR-18-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tani H., Okuda S., Nakamura K., et al. Short-lived long non-coding RNAs as surrogate indicators for chemical exposure and LINC00152 and MALAT1 modulate their neighboring genes. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q., Shao Y., Zhang X., et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36(3):2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 61.Tani H., Onuma Y., Ito Y., et al. Long non-coding RNAs as surrogate indicators for chemical stress responses in human-induced pluripotent stem cells. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tani H., Torimura M. Identification of short-lived long non-coding RNAs as surrogate indicators for chemical stress response. Biochem Biophys Res Commun. 2013;439(4):547–551. doi: 10.1016/j.bbrc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Zhu H., Wang Q., Yao Y., et al. Microarray analysis of Long non-coding RNA expression profiles in human gastric cells and tissues with Helicobacter pylori Infection. BMC Med Genom. 2015;8:e84. doi: 10.1186/s12920-015-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teng W., Qiu C., He Z., et al. LINC00152 suppresses apoptosis and promotes migration by sponging miR-4767 in vascular endothelial cells. Oncotarget. 2017;8(49):85014–85023. doi: 10.18632/oncotarget.18777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Wu L., Guo W., et al. Clinical relevance of LINC00152 and its variants in western Chinese tuberculosis patients. Oncotarget. 2017;8(70):115456–115468. doi: 10.18632/oncotarget.23297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Tatakis D.N. Human gingiva transcriptome during wound healing. J Clin Periodontol. 2017;44(4):394–402. doi: 10.1111/jcpe.12669. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y., Yang J., Li Q., et al. LINC00152: a pivotal oncogenic long non-coding RNA in human cancers. Cell Prolif. 2017;50(4):e12349. doi: 10.1111/cpr.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L.L. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 69.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Z., Pan X., Yang Y., et al. The lncLocator: a subcellular localization predictor for long non-coding RNAs based on a stacked ensemble classifier. Bioinformatics. 2018;34(13):2185–2194. doi: 10.1093/bioinformatics/bty085. [DOI] [PubMed] [Google Scholar]
- 71.Ou C., Sun Z., He X., et al. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer. Adv Sci. 2019;7(3) doi: 10.1002/advs.201901380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Q., Hao Q., Prasanth K.V. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet. 2018;34(2):142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 74.He X., Kuang G., Wu Y., Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11(6):e468. doi: 10.1002/ctm2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen W.M., Huang M.D., Sun D.P., et al. Long intergenic non-coding RNA 00152 promotes tumor cell cycle progression by binding to EZH2 and repressing p15 and p21 in gastric cancer. Oncotarget. 2016;7(9):9773–9787. doi: 10.18632/oncotarget.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salmena L., Poliseno L., Tay Y., et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karreth F.A., Pandolfi P.P. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou P., Zhao Y., Li Z., et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5(6):e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang W.C., Fu W.M., Wong C.W., et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou X., Ye F., Yin C., et al. The interaction between miR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem. 2015;36(4):1440–1452. doi: 10.1159/000430309. [DOI] [PubMed] [Google Scholar]
- 82.Liu X.H., Sun M., Nie F.Q., et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun T., Wong N. Transforming growth factor-β-induced long noncoding RNA promotes liver cancer metastasis via RNA-RNA crosstalk. Hepatology. 2015;61(2):722–724. doi: 10.1002/hep.27599. [DOI] [PubMed] [Google Scholar]
- 84.Chen C.L., Tseng Y.W., Wu J.C., et al. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81. doi: 10.1016/j.biomaterials.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 85.Wu H., Zhou C. Long non-coding RNA UCA1 promotes lung cancer cell proliferation and migration via microRNA-193a/HMGB1 axis. Biochem Biophys Res Commun. 2018;496(2):738–745. doi: 10.1016/j.bbrc.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 86.Li S., Mei Z., Hu H.B., Zhang X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J Cell Physiol. 2018;233(9):6679–6688. doi: 10.1002/jcp.26325. [DOI] [PubMed] [Google Scholar]
- 87.Yu G., Yao W., Gumireddy K., et al. Pseudogene PTENP1 functions as a competing endogenous RNA to suppress clear-cell renal cell carcinoma progression. Mol Cancer Therapeut. 2014;13(12):3086–3097. doi: 10.1158/1535-7163.MCT-14-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma P., Wang H., Sun J., et al. LINC00152 promotes cell cycle progression in hepatocellular carcinoma via miR-193a/b-3p/CCND1 axis. Cell Cycle. 2018;17(8):974–984. doi: 10.1080/15384101.2018.1464834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X., Yidayitula Y., Zhao H., et al. LncRNA LINC00152 promoted glioblastoma progression through targeting the miR-107 expression. Environ Sci Pollut Res Int. 2018;25(18):17674–17681. doi: 10.1007/s11356-018-1784-x. [DOI] [PubMed] [Google Scholar]
- 90.Cai J., Zhang J., Wu P., et al. Blocking LINC00152 suppresses glioblastoma malignancy by impairing mesenchymal phenotype through the miR-612/AKT2/NF-κB pathway. J Neuro Oncol. 2018;140(2):225–236. doi: 10.1007/s11060-018-2951-0. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y.H., Fu J., Zhang Z.J., et al. LncRNA-LINC00152 down-regulated by miR-376c-3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res. 2016;8(12):5286–5297. [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Y.H. Crosstalk of lncRNA and cellular metabolism and their regulatory mechanism in cancer. Int J Mol Sci. 2020;21(8):2947. doi: 10.3390/ijms21082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan C., Tang Y., Wang J., et al. Role of long non-coding RNAs in glucose metabolism in cancer. Mol Cancer. 2017;16(1):130. doi: 10.1186/s12943-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y., Xiang C., Wang Y., et al. lncRNA LINC00152 knockdown had effects to suppress biological activity of lung cancer via EGFR/PI3K/AKT pathway. Biomed Pharmacother. 2017;94:644–651. doi: 10.1016/j.biopha.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 95.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 96.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin C., Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28(4):287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 99.Pietras K., Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 100.Balkwill F., Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87(4):401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 101.Semczuk A., Jakowicki J.A. Alterations of pRb1-cyclin D1-cdk4/6-p16(INK4A) pathway in endometrial carcinogenesis. Cancer Lett. 2004;203(1):1–12. doi: 10.1016/j.canlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz G.K., Shah M.A. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23(36):9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 103.Li J., Tian H., Yang J., et al. Long noncoding RNAs regulate cell growth, proliferation, and apoptosis. DNA Cell Biol. 2016;35(9):459–470. doi: 10.1089/dna.2015.3187. [DOI] [PubMed] [Google Scholar]
- 104.Ou C., Sun Z., Li X., et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63. doi: 10.1016/j.canlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 105.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 106.Wei L., Sun J., Zhang N., et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19(1):62. doi: 10.1186/s12943-020-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrie R.J., Yamada K.M. At the leading edge of three-dimensional cell migration. J Cell Sci. 2012;125(Pt 24):5917–5926. doi: 10.1242/jcs.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doyle A.D., Petrie R.J., Kutys M.L., et al. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25(5):642–649. doi: 10.1016/j.ceb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cojoc D., Difato F., Ferrari E., et al. Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components. PLoS One. 2007;2(10) doi: 10.1371/journal.pone.0001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peckham M. How myosin organization of the actin cytoskeleton contributes to the cancer phenotype. Biochem Soc Trans. 2016;44(4):1026–1034. doi: 10.1042/BST20160034. [DOI] [PubMed] [Google Scholar]
- 111.Shi C.Y., Fan Y., Liu B., et al. HIF1 contributes to hypoxia-induced pancreatic cancer cells invasion via promoting QSOX1 expression. Cell Physiol Biochem. 2013;32(3):561–568. doi: 10.1159/000354460. [DOI] [PubMed] [Google Scholar]
- 112.Diaz-Ruiz R., Rigoulet M., Devin A. The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta. 2011;1807(6):568–576. doi: 10.1016/j.bbabio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Alfarouk K.O., Shayoub M.E., Muddathir A.K., et al. Evolution of tumor metabolism might reflect carcinogenesis as a reverse evolution process (dismantling of multicellularity) Cancers. 2011;3(3):3002–3017. doi: 10.3390/cancers3033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Basu D., Lettan R., Damodaran K., et al. Identification, mechanism of action, and antitumor activity of a small molecule inhibitor of hippo, TGF-β, and Wnt signaling pathways. Mol Cancer Therapeut. 2014;13(6):1457–1467. doi: 10.1158/1535-7163.MCT-13-0918. [DOI] [PubMed] [Google Scholar]
- 115.Dell' Antone P. Energy metabolism in cancer cells: how to explain the Warburg and Crabtree effects? Med Hypotheses. 2012;79(3):388–392. doi: 10.1016/j.mehy.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Zeng L., Morinibu A., Kobayashi M., et al. Aberrant IDH3α expression promotes malignant tumor growth by inducing HIF-1-mediated metabolic reprogramming and angiogenesis. Oncogene. 2015;34(36):4758–4766. doi: 10.1038/onc.2014.411. [DOI] [PubMed] [Google Scholar]
- 117.Martinez-Outschoorn U.E., Trimmer C., Lin Z., et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9(17):3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirota K. Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. Cardiovasc Haematol Disord - Drug Targets. 2015;15(1):29–40. doi: 10.2174/1871529X15666150108115553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin A., Li C., Xing Z., et al. The LINK-A lncRNA activates normoxic HIF1α signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18(2):213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng X., Han H., Liu G.P., et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36(22):3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rupaimoole R., Lee J., Haemmerle M., et al. Long noncoding RNA ceruloplasmin promotes cancer growth by altering glycolysis. Cell Rep. 2015;13(11):2395–2402. doi: 10.1016/j.celrep.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ou C., Sun Z., Li S., et al. Dual roles of yes-associated protein (YAP) in colorectal cancer. Oncotarget. 2017;8(43):75727–75741. doi: 10.18632/oncotarget.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ou C., Sun Z., Zhang H., et al. SPLUNC1 reduces the inflammatory response of nasopharyngeal carcinoma cells infected with the EB virus by inhibiting the TLR9/NF-κB pathway. Oncol Rep. 2015;33(6):2779–2788. doi: 10.3892/or.2015.3913. [DOI] [PubMed] [Google Scholar]
- 124.Zhang X., Wei L., Wang J., et al. Suppression colitis and colitis-associated colon cancer by anti-S100a9 antibody in mice. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drak Alsibai K., Meseure D. Tumor microenvironment and noncoding RNAs as co-drivers of epithelial-mesenchymal transition and cancer metastasis. Dev Dynam. 2018;247(3):405–431. doi: 10.1002/dvdy.24548. [DOI] [PubMed] [Google Scholar]
- 126.Magagula L., Gagliardi M., Naidoo J., et al. Lnc-ing inflammation to disease. Biochem Soc Trans. 2017;45(4):953–962. doi: 10.1042/BST20160377. [DOI] [PubMed] [Google Scholar]
- 127.Mathy N.W., Chen X.M. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J Biol Chem. 2017;292(30):12375–12382. doi: 10.1074/jbc.R116.760884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Evans D.R., Green J.S., Johnson G.J., et al. Novel 25 kb deletion of MERTK causes retinitis pigmentosa with severe progression. Invest Ophthalmol Vis Sci. 2017;58(3):1736–1742. doi: 10.1167/iovs.16-20864. [DOI] [PubMed] [Google Scholar]
- 129.Kumari S., Bonnet M.C., Ulvmar M.H., et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity. 2013;39(5):899–911. doi: 10.1016/j.immuni.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 130.Primo M.N., Bak R.O., Schibler B., et al. Regulation of pro-inflammatory cytokines TNFα and IL24 by microRNA-203 in primary keratinocytes. Cytokine. 2012;60(3):741–748. doi: 10.1016/j.cyto.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 131.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. Ca - Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 132.Cooner W.H. Definition of the ideal tumor marker. Urol Clin. 1993;20(4):575–579. [PubMed] [Google Scholar]
- 133.Ishii M. [Definition and classification of tumor marker] Nihon Rinsho. 1996;54(6):1473–1478. [PubMed] [Google Scholar]
- 134.Liu W.H., Ren L.N., Wang X., et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin Oncol. 2015;141(10):1767–1778. doi: 10.1007/s00432-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olivier R.I., Lubsen-Brandsma M.A., Verhoef S., et al. CA125 and transvaginal ultrasound monitoring in high-risk women cannot prevent the diagnosis of advanced ovarian cancer. Gynecol Oncol. 2006;100(1):20–26. doi: 10.1016/j.ygyno.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 136.He Y.W., Zhao M.L., Yang X.Y., et al. Prognostic value of ERCC1, RRM1, and TS proteins in patients with resected non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;75(4):861–867. doi: 10.1007/s00280-015-2714-y. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y.H., Yang Q.C., Lin Y., et al. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine (Baltim) 2014;93(27):e247. doi: 10.1097/MD.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Nie H., He X., et al. The emerging role of super enhancer-derived noncoding RNAs in human cancer. Theranostics. 2020;10(24):11049–11062. doi: 10.7150/thno.49168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ou C., Li X., Li G., et al. WWC3: the bridge linking Hippo and Wnt pathways in lung cancer. J Thorac Dis. 2017;9(8):2315–2316. doi: 10.21037/jtd.2017.08.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng X., Dong S., Sun L., et al. LncRNA LINC00152 promotes laryngeal cancer progression by sponging miR-613. Open Med. 2020;15:240–248. doi: 10.1515/med-2020-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao L., Chi W.W., Cao H., et al. Expression of long-chain non-coding RNA LINC00152 in laryngeal squamous cell carcinoma and its clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33(8):721–725. doi: 10.13201/j.issn.1001-1781.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 142.Li M., Ning J., Li Z., et al. LINC00152 promotes the growth and invasion of oral squamous cell carcinoma by regulating miR-139-5p. OncoTargets Ther. 2018;11:6295–6304. doi: 10.2147/OTT.S168807. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 143.Yang Y., Sun X., Chi C., et al. Upregulation of long noncoding RNA LINC00152 promotes proliferation and metastasis of esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:4643–4654. doi: 10.2147/CMAR.S198905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu D., Gao M., Wu K., et al. LINC00152 facilitates tumorigenesis in esophageal squamous cell carcinoma via miR-153-3p/FYN axis. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108654. [DOI] [PubMed] [Google Scholar]
- 145.Liu J., Tang X., Lv J., et al. LncRNAs SNHG12 and LINC00152 were associated with progression of patients with papillary thyroid carcinoma. Future Oncol. 2019;15(36):4167–4179. doi: 10.2217/fon-2019-0016. [DOI] [PubMed] [Google Scholar]
- 146.Li N., Feng X.B., Tan Q., et al. Identification of circulating long noncoding RNA linc 00152 as a novel biomarker for diagnosis and monitoring of non-small-cell lung cancer. Dis Markers. 2017;2017 doi: 10.1155/2017/7439698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng S., Zhang J., Su W., et al. Overexpression of LINC00152 correlates with poor patient survival and knockdown impairs cell proliferation in lung cancer. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-03043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li J., Wang X., Tang J., et al. HULC and linc 00152 act as novel biomarkers in predicting diagnosis of hepatocellular carcinoma. Cell Physiol Biochem. 2015;37(2):687–696. doi: 10.1159/000430387. [DOI] [PubMed] [Google Scholar]
- 149.Shi Y., Sun H. Down-regulation of lncRNA LINC00152 suppresses gastric cancer cell migration and invasion through inhibition of the ERK/MAPK signaling pathway. OncoTargets Ther. 2020;13:2115–2124. doi: 10.2147/OTT.S217452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pang Q., Ge J., Shao Y., et al. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35(6):5441–5447. doi: 10.1007/s13277-014-1709-3. [DOI] [PubMed] [Google Scholar]
- 151.Chen Z.P., Wei J.C., Wang Q., et al. Long noncoding RNA 00152 functions as a competing endogenous RNA to regulate NRP1 expression by sponging with miRNA206 in colorectal cancer. Int J Oncol. 2018;53(3):1227–1236. doi: 10.3892/ijo.2018.4451. [DOI] [PubMed] [Google Scholar]
- 152.Zheng J.J., Du X.J., Wang H.P., et al. Long non-coding RNA 00152 promotes cell proliferation in cervical cancer via regulating miR-216b-5p/HOXA1 axis. Eur Rev Med Pharmacol Sci. 2019;23(9):3654–3663. doi: 10.26355/eurrev_201905_17789. [DOI] [PubMed] [Google Scholar]
- 153.Xian-Li T., Hong L., Hong Z., et al. Higher expression of linc 00152 promotes bladder cancer proliferation and metastasis by activating the Wnt/β-catenin signaling pathway. Med Sci Mon Int Med J Exp Clin Res. 2019;25:3221–3230. doi: 10.12659/MSM.913944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng L., Hu N., Zhou X. TCF3-activated LINC00152 exerts oncogenic role in osteosarcoma through regulating miR-1182/CDK14 axis. Pathol Res Pract. 2019;215(2):373–380. doi: 10.1016/j.prp.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 155.Arun G., Diermeier S.D., Spector D.L. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med. 2018;24(3):257–277. doi: 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams B.D., Parsons C., Walker L., et al. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Agostini M., Knight R.A. miR-34: from bench to bedside. Oncotarget. 2014;5(4):872–881. doi: 10.18632/oncotarget.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beg M.S., Brenner A., Sachdev J., et al. Safety, tolerability, and clinical activity of MRX34, the first-in-class liposomal miR-34 mimic, in patients with advanced solid tumors. Mol Cancer Therapeut. 2015;14(12 Supplement 2):C43. [Google Scholar]
- 160.Janssen H.L., Reesink H.W., Lawitz E.J., et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 161.Gebert L.F., Rebhan M.A., Crivelli S.E., et al. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42(1):609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Ree M.H., van der Meer A.J., van Nuenen A.C., et al. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43(1):102–113. doi: 10.1111/apt.13432. [DOI] [PubMed] [Google Scholar]
- 163.Arun G., Diermeier S., Akerman M., et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30(1):34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12(6):433–446. doi: 10.1038/nrd4018. [DOI] [PubMed] [Google Scholar]
- 165.Soares R.J., Maglieri G., Gutschner T., et al. Evaluation of fluorescence in situ hybridization techniques to study long non-coding RNA expression in cultured cells. Nucleic Acids Res. 2018;46(1):e4. doi: 10.1093/nar/gkx946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Feil R., Walter J., Allen N.D., et al. Developmental control of allelic methylation in the imprinted mouse Igf2 and H19 genes. Development. 1994;120(10):2933–2943. doi: 10.1242/dev.120.10.2933. [DOI] [PubMed] [Google Scholar]
- 167.Dugimont T., Curgy J.J., Wernert N., et al. The H19 gene is expressed within both epithelial and stromal components of human invasive adenocarcinomas. Biol Cell. 1995;85(2–3):117–124. doi: 10.1016/0248-4900(96)85272-5. [DOI] [PubMed] [Google Scholar]
- 168.Brown C.J., Ballabio A., Rupert J.L., et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 169.Siepel A., Bejerano G., Pedersen J.S., et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15(8):1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bassett A.R., Akhtar A., Barlow D.P., et al. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e3058. doi: 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cabili M.N., Dunagin M.C., Mcclanahan P.D., et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16(1):e20. doi: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu S.J., Horlbeck M.A., Cho S.W., et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355(6320):aah7111. doi: 10.1126/science.aah7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Joung J., Engreitz J.M., Konermann S., et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature. 2017;548(7667):343–346. doi: 10.1038/nature23451. [DOI] [PMC free article] [PubMed] [Google Scholar]