Abstract
Background:
Fatty infiltration (FI) of the rotator cuff muscles is correlated with shoulder function and retear rates after rotator cuff repair. High-intensity interval training (HIIT) induces beige adipose tissue to express more uncoupling protein 1 (UCP1) to consume lipids. The beta-3 adrenergic receptor (β3AR) is located on adipocyte membrane and induces thermogenesis.
Purpose:
To test the role of HIIT in improving muscle quality and contractility in a delayed rotator cuff repair mouse model via β3AR.
Study Design:
Controlled laboratory study.
Methods:
Three-month-old C57BL/6J mice underwent a unilateral supraspinatus (SS) tendon transection with a 6-week delayed tendon repair. Mice ran on a treadmill with the HIIT program for 6 weeks after tendon transection or after delayed repair. To study the role of β3AR, SR59230A, a selective β3AR antagonist, was administered to mice 10 minutes before each exercise through intraperitoneal injection. The SS, interscapular brown adipose tissue (iBAT), and subcutaneous inguinal white adipose tissue (ingWAT) were harvested at the end of the 12th week after tendon transection and were analyzed by histology and Western blotting. Tests were performed to assess muscle contractility of the SS.
Results:
Histologic analysis of SS showed that HIIT prevented and reversed muscle atrophy and FI. The contractile tests showed higher contractility of the SS in the HIIT groups than in the no-exercise group. In the HIIT groups, SS, iBAT, and ingWAT all showed increased expression of tyrosine hydroxylase, UCP1, and upregulated β3AR thermogenesis pathway. However, SR59230A inhibited HIIT, suggesting that the effect of HIIT depends on β3AR.
Conclusion:
HIIT improved SS quality and function after delayed rotator cuff repair through a β3AR-dependent mechanism.
Clinical Relevance:
HIIT may serve as a new rehabilitation method for patients with rotator cuff muscle atrophy and FI after rotator cuff repair to improve postoperative clinical outcomes.
Keywords: rotator cuff tear, delayed repair, high-intensity interval training, fatty infiltration
Muscle atrophy and fatty infiltration (FI) are common in patients who have sustained chronic rotator cuff (RC) tears. Intermediate and severe FI (Goutallier stages 2-4) occurs in >30% of patients. 38 Many studies have demonstrated that the amount of atrophy and FI correlates with high retear rates and poor clinical outcomes after surgical repairs.7,12,19 It was historically believed that FI is irreversible, even after surgical repair.21,27
Recent studies have shown the possibility of reversing FI.22,28,48 Laboratory research has shown that brown/beige-like adipose tissue might play an important role in this process since brown adipose tissue (BAT) (see AppendixTable A1 for expansions of specialized terms used in this article) can clear lipids by thermogenesis with uncoupling protein 1 (UCP1). 9 In addition, batokines released by BAT, such as VEGF and IGF1, are beneficial to muscle regeneration. Bryniarski and Meyer 5 tried an intermuscular fat transplant model, and their results showed that brown fat transplant promoted adjacent muscle regeneration, which is possibly achieved with paracrine cross-talk. Lee et al30,31 found that transplantation of beige adipose fibro-adipogenic progenitors (FAPs) was beneficial to muscle regeneration after RC tears. Exosomes secreted by UCP1+ FAPs showed potent promyogenic activity in vitro and in vivo. 11 Meyer et al 39 found epimuscular adipose-derived stem cells from RC. These stem cells tended to increase the expression of beige-selective genes when RC was torn and promoted myogenesis in vitro.
Cold exposure,17,53 diet, 24 exercise,46,53 and some other stimuli activate BAT through the sympathetic nervous system. 40 Noradrenaline released by sympathetic nerve fibers stimulates the beta-3 adrenergic receptor (β3AR) and activates the downstream cAMP-PKA axis. 42 As a result, HSL and UCP1 start hydrolyzing lipid droplets and thermogenesis. 42 High-intensity interval training (HIIT) is an effective method for activating the sympathetic system 45 and losing weight,26,52 including various training models, such as cycling, running, swimming, and other activities. 26 According to some studies, HIIT promotes white fat browning more effectively than moderate-intensity continuous training,37,47 but there is no research about HIIT and FI.
Herein, we aimed to study the effect of HIIT on muscle atrophy and FI with a mouse model of delayed RC tendon repair. 49 We hypothesized that HIIT helps to improve muscle atrophy and FI in RC muscle by activating BAT in muscle through the sympathetic system.
Methods
Experiment Design
The protocol for this animal study received ethics committee approval. To study the effect of HIIT on RC tears, 40 mice were randomly divided into 4 groups of 10 (n = 5 for histological analysis and n = 5 for contractile tests and Western blot) as follows: (1) sham, (2) tendon tear (TT) + delayed repair (DR) + no exercise (NE group), (3) TT + DR + early exercise (EE group), and (4) TT + DR + late exercise (LE group) (Figure 1). All 30 mice outside of the sham group underwent unilateral (right side) supraspinatus (SS) tendon tear followed by delayed repair 6 weeks later. This TT + DR model has been described in our former study. 49 Briefly, after the shoulder was opened for repair, a 0.5-mm tunnel was created through the humerus with p-6 cutting needles (8648G; Ethicon) and the SS tendon was fixed to the anatomic position with No. 7-0 polypropylene sutures (8648G; Ethicon). The sham group and the NE group underwent no exercise during the 12 weeks. The EE group underwent exercise during the 6 weeks between TT and DR and then no exercise after DR. The LE group underwent exercise for 6 weeks from day 3 after DR. To study the role of β3AR, we administered SR59230A (SR), a selective β3AR antagonist, to mice before each HIIT session (Figure 1).
Figure 1.
Flow diagram of experimental design. DMSO, dimethyl sulfoxide; DR, delayed repair; TT, tendon tear.
An additional 40 mice were randomly divided into 4 groups of 10, as follows: (1) TT + DR + EE + dimethyl sulfoxide (DMSO) (EE DMSO group), (2) TT + DR + EE + SR (EE SR group), (3) TT + DR + LE + DMSO (LE DMSO group), and (4) TT + DR + LE + SR (LE SR group). All these mice underwent TT + DR, with SR (2 mg/kg) or 1% DMSO-PBS placebo administered by intraperitoneal injection 10 minutes before each exercise. SR (Sigma-Aldrich) was dissolved in 1% DMSO-PBS buffer.
The mice were housed in cages with a 12-hour dark-light cycle with free access to water and regular chow diet.
Exercise Protocol
HIIT exercise was performed on a motorized mouse treadmill 5 days per week for 6 weeks, according to a protocol slightly modified from that described by Wang et al 47 and Martinez-Huenchullan et al. 37 Before the first exercise, a maximal running capacity (MRC) test was performed on the mice that received surgery (the EE group after TT, the LE group after DR). The HIIT program included 7 rounds (2 minutes at 50% of the MRC followed by 4 minutes at 90% of the MRC). The MRC test was performed every 2 weeks, and the velocity was adjusted accordingly.
Muscle Harvesting and Wet Muscle Weight
All mice were sacrificed 12 weeks after the first surgery. For assessment of muscle atrophy, wet weights of the bilateral SS muscles were measured immediately after harvesting. The percentage change in wet muscle weight was determined with the following equation: ([SSright – SSleft]/SSleft) × 100%. 16
Masson Trichrome Staining, Oil Red O Staining, and Cross-sectional Area
The treated-side SS (n = 5 per group) muscles were flash-frozen and then cryosectioned as described previously. 33 Masson trichrome (G1345; Solarbio) stain was used to assess fibrosis and atrophy in SS muscles. Oil red O (G1260; Solarbio) was used to assess FI in the SS. Fibrosis and FI were assessed as a percentage of the muscle section area. The cross-sectional area (CSA) of myocyte was assessed in the Masson trichrome–stained slides. Slides were covered with 10% glycerol in PBS (for oil red O) or 50% resinene in xylene (for Masson trichrome) and observed on an optical microscope. Cross sections were chosen randomly from the midbellies of the SS. Pictures were analyzed with ImageJ software (National Institutes of Health) as described previously. 33
Immunofluorescence Staining
The treated-side SS (n = 5 per group) cryosection slides were fixed in 4% paraformaldehyde for 30 minutes, rinsed in PBS, placed in 0.1 M glycine (diluted in PBS; Fisher Scientific) for 30 minutes, and washed again in PBS. They were then covered with blocking solution (0.2% Triton X-100; 2% bovine serum albumin in PBS) for 1 hour at room temperature. Primary antibodies against UCP1 (an indicator of BAT activation or white fat browning) (diluted 1:100; NB100-2828; Novus Biologicals), tyrosine hydroxylase (TH) (an indicator of activation of sympathetic nerve fibers) (diluted 1:500; 66334-1-Ig; Proteintech), laminin (diluted 1:500; L9393; Sigma-Aldrich), and perilipin (diluted 1:500; abs137082; Absin) were diluted in a block mix and added to the sections for overnight incubation at 4°C. After a PBS rinse, the sections were incubated with a mixture containing FITC-conjugated (diluted 1:500; SA00003-8, SA00003-2; Proteintech) and Cy3-conjugated (diluted 1:500; SA00009-3, SA00009-1; Proteintech) secondary antibodies at room temperature for 120 minutes. After a PBS rinse for 2 × 10 minutes, the slides were covered with DAPI containing antifade mounting medium.
Western Blot
Total proteins were extracted using RIPA-lysis buffer containing proteinase inhibitors (No. 04693159001; Roche) and phosphatase inhibitors (No. 04906845001; Roche), separated by SDS-PAGE, transferred to PVDF membranes, and analyzed by immunoblotting. Primary antibodies against the following proteins were used: TH (66334-1-Ig; Proteintech), PKA (AF5450; Affinity), p-HSL (AF8026; Affinity), HSL (AF6403; Affinity), p-p38 MAPK (AF4001; Affinity), p38 MAPK (AF6456; Affinity), PGC1α (AF5395; Affinity), UCP1 (NB100-2828; Novus Biologicals), and GAPDH (60004-1-Ig; Proteintech). The antibodies were diluted 1:1000 with PBS. The membranes were then incubated with a peroxidase-conjugated secondary antibody (BS13278 [Bioworld], SA00001-1 [Proteintech], SA00001-3 [Proteintech]), and antibody-specific signals were detected by enhanced chemiluminescence and quantified using an automatic chemiluminescence imaging system (Complex 2000; Nanjing PuoAoXin Biotechnology).
SS Contractile Test
Preparation was performed while the mouse was anesthetized. We exposed the SS and scapular spine and removed the trapezius and deltoid. We sutured around the distal tendon part and proximal part of the SS. Then, we cut the scapula across the scapular spine and cut the humerus. After fixing the 2 sides of SS, we assessed the tetanic contraction force with a BL-420F acquisition system (Chengdu TME Technology). During the test, the muscle was stimulated with parallel wire electrodes (20 V; 100 Hz; 0.6 s), maintained in a bath of Ringer solution (137 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 24 mM NaHCO3, 2 mM CaCl2, 1 mM MgSO4, 11 mM glucose) bubbled with CO2 to maintain pH.
Statistical Analysis
We applied the Dunnett multiple comparison test to determine the significant differences among the sham, NE, EE, and LE groups and applied the Sidak multiple comparison test to determine the significant differences among the EE DMSO, EE SR, LE DMSO, and LE SR groups. All data are shown as the mean ± SD. Statistical differences were determined when P < .05.
Results
SS Muscle Atrophy and FI After HIIT
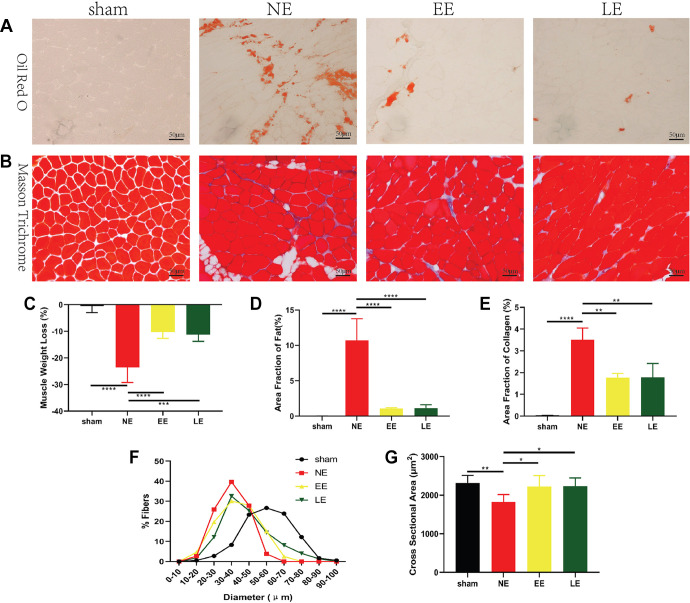
The repaired tendon was found to be intact in all specimens after HIIT in the LE group (AppendixFigure A1). Compared with the sham group, mice in the NE group showed an increased area of FI and collagen, but decreased SS weight and CSA (Figure 2). However, if mice ran with either the early or the late HIIT schedule, their SS quality turned out better than that in the NE groups. The area of FI was 1.05% ± 0.13% for EE (vs 10.7% ± 3.0% for NE; P < .0001) and 1.14% ± 0.44% for LE (vs NE; P < .0001) (Figure 2, A and D). The collagen area was 1.78% ± 0.19% (EE) (vs 3.52% ± 0.54% for NE; P = .003) and 1.79% ± 0.63% (LE) (vs NE; P = .003) (Figure 2, B and E). Muscle weight loss was –10.2% ± 2.4% (EE) (vs –23.5% ± 5.7% for NE; P < .0001) and –11.3% ± 2.5% (LE) (vs NE; P < .001) (Figure 2C). Compared with the NE group (median, 35.75 μm), more myofibers had a larger diameter in the EE group (median, 38.47 μm) and LE group (median, 40.71 μm) (Figure 2, B and F). The mean CSAs of myofibers were 2084 ± 320 μm2 (EE) (vs 1738 ± 291 μm2 for NE; P = .031) and 1903 ± 284 μm2 (LE) (vs NE; P = .026) (Figure 2, B and G). There was no significant difference in FI, collagen area, muscle weight loss, and CSA between the EE and LE groups.
Figure 2.
Histologic staining showing the effect of high-intensity interval training on the supraspinatus (SS). (A) Oil red O staining and (B) Masson trichrome staining of the SS. (C) SS weight loss was lower in the early exercise (EE) and late exercise (LE) groups compared with the no exercise (NE) group. (D) Fatty infiltration in the EE and LE groups was less than that in the NE group. (E) In the EE and LE groups, fibrosis was less than that in the NE group. (F and G) The muscle fiber cross section was larger in the EE and LE groups than in the NE group. Statistically significant difference: *P < .05, **P < .01, ***P < .001, ****P < .0001.
Expression of UCP1 and TH in the SS and Fat Depots After HIIT
Increased UCP1 expression in the EE (vs NE; P < .003) and LE (vs NE; P < .0001) groups indicated browning of adipocytes in SS (Figure 3A). Increased expression of TH in the EE (vs NE; P = .049) and LE (vs NE; P < .003) groups suggested activation of sympathetic fibers (Figure 3B). We also harvested interscapular brown adipose tissue (iBAT) and subcutaneous inguinal white adipose tissue (ingWAT). The results were similar to those in SS. UCP1 and TH expression increased in the EE groups (vs NE: P = .005 for iBAT UCP1; P = .025 for iBAT TH; P = .007 for ingWAT TH) and the LE groups (vs NE: P < .0001 for iBAT UCP1; P < .0001 for iBAT TH; P = .001 for ingWAT UCP1; P < .0001 for ingWAT TH) (Figure 3, C-Fs).
Figure 3.
Immunofluorescence staining showing higher uncoupling protein 1 (UCP1) (red) and tyrosine hydroxylase (TH) (red) expression in the early exercise (EE) and late exercise (LE) groups. In the (A and B) supraspinatus (SS), (C and D) interscapular brown adipose tissue (iBAT), and (E and F) inguinal white adipose tissue (ingWAT), immunofluorescence staining showed higher UCP1 (red) and TH (red) expression in the EE and LE groups compared with the no exercise (NE) group. Statistically significant difference: *P < .05, **P < .01, ****P < .0001. DAPI, 4′,6-diamidino-2-phenylindole; ns, not significant.
Effect of HIIT on FI and β3AR
To study whether β3AR mediated the effect of HIIT, SR and DMSO were administered to mice before exercise. We found that the SS quality of mice injected with SR was worse than that of mice injected with DMSO. Similar to the NE group, SS in the SR groups had more FI (P < .0001 for EE SR vs EE DMSO; P < .0001 for LE SR vs LE DMSO) (Figure 4, A and D). Masson trichrome staining showed more collagen area in the EE SR group (vs EE DMSO; P < .0001) (Figure 4, B and E). SS in the SR groups had less weight (P < .001 for EE SR vs EE DMSO; P < .001 for LE SR vs LE DMSO) (Figure 4C). SR did not induce FI or fibrosis in SS from mice in the sham surgery group (AppendixFigure A2).
Figure 4.
SR59230A (SR) inhibited fatty infiltration and fibrosis decrease. (A) Oil red O staining and (B) Masson trichrome staining of the supraspinatus. (C) Muscle weight loss was greater in the early exercise (EE) SR and late exercise (LE) SR groups. (D) Oil red O staining showed increased fatty infiltration in the EE SR and LE SR groups. (E) Masson trichrome staining showed increased fibrosis in the EE SR group compared with the EE dimethyl sulfoxide (DMSO) group. (F and G) Myofiber showed a decreasing trend in the EE SR and LE SR groups. Statistically significant difference: ***P < .001, ****P < .0001.
UCP1 expression also decreased in the SR groups (P < .0001 for EE SR vs EE DMSO; P < .0001 for LE SR vs LE DMSO) (Figure 5A). However, TH expression was similar whether the mice were injected with SR or DMSO (Figure 5B). In other words, HIIT still activated local sympathetic fibers in the SR groups. In iBAT and ingWAT, just like SS, UCP1 expression decreased if the mice were injected with SR (in iBAT: P < .0001 for EE SR vs EE DMSO, P < .0001 for LE SR vs LE DMSO; in ingWAT: P = .003 for EE SR vs EE DMSO, P < .001 for LE SR vs LE DMSO) (Figure 5, C and E), whereas TH expression had no change (Figure 5, D and F).
Figure 5.
SR59230A (SR) inhibited the expression of uncoupling protein 1 (UCP1) in the (A and B) supraspinatus (SS), (C and D) interscapular brown adipose tissue (iBAT), and (E and F) inguinal white adipose tissue (ingWAT). Immunofluorescence showed lower UCP1 (red) expression in the early exercise (EE) SR and late exercise (LE) SR groups and no change in tyrosine hydroxylase (TH) (red) expression. Statistically significant difference: **P < .01, ***P < .001, ****P < .0001. DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; ns, not significant.
HIIT and the β3AR Thermogenesis Pathway
In total, we obtained proteins from SS, iBAT, and ingWAT in the 8 groups. We found that in the HIIT groups (EE and LE), PKA, p-HSL, p-p38 MAPK, and PGC1α in the β3AR thermogenesis pathway2,32,43 were elevated compared with those in the NE group. In the SR groups (EE SR and LE SR), the expression of these proteins was depressed compared with that in the DMSO groups (EE DMSO and LE DMSO) (Figure 6). A diagram of the β3AR thermogenesis pathway is shown in Figure 7.
Figure 6.
Western blot of critical proteins in the β3AR thermogenesis pathway for the sham (black bars), NE (red bars), EE (yellow bars), and LE (green bars) groups and for the EE DMSO (black bars), EE SR (red bars), LE DMSO (yellow bars), and LE SR (green bars) groups. In the (A and B) supraspinatus (SS), (C and D) interscapular brown adipose tissue (iBAT), and (E and F) inguinal white adipose tissue (ingWAT), for TH, PKA, HSL, p38 MAPK, PGC1α, and UCP1, fold change was calculated as target protein divided by GAPDH. For p-HSL and p-p38 MAPK, fold change was calculated as phosphorylated target protein divided by total target protein. Statistically significant difference: *P < .05, **P < .01, ***P < .001, ****P < .0001. EE, early exercise; LE, late exercise; NE, no exercise; ns, not significant; SR, SR59230A. See AppendixTable A1 for remaining abbreviation expansions.
Figure 7.
Graphic abstract of the β3AR thermogenesis pathway. See AppendixTable A1 for abbreviation expansions.
SS Contractility Caused by HIIT
The tetanic contraction force of SS in the EE (32.9 ± 0.9 mN; P < .001) and LE (36.8 ± 1.5 mN; P < .0001) groups was larger than that in the NE group (24.8 ± 2.6 mN) (Figure 8A). In the SR groups, the tetanic contraction force was depressed to 22.8 ± 1.0 mN (EE SR: P < .001 vs 32.6 ± 3.4 mN for EE DMSO) and 22.2 ± 3.6 mN (LE SR: P < .0001 vs 35.9 ± 3.2 mN for LE DMSO), similar to the values in the NE group (Figure 8B). There was no significant difference in tetanic contraction force between the EE and LE groups.
Figure 8.
Contractile tests of supraspinatus. (A) High-intensity interval training improved the tetanic contraction force in the early exercise (EE) and late exercise (LE) groups. (B) SR59230A (SR) inhibited the improvement of tetanic contraction force. Statistically significant difference: ***P < .001, ****P < .0001. DMSO, dimethyl sulfoxide; NE, no exercise.
Discussion
As expected, we observed significant FI in SS in the delayed RC repair mouse model, and the muscle contraction was weaker than that in the sham group. In this delayed RC repair model, SS degenerates and has significant FI before the RC repair surgery. 49 However, HIIT, a model of exercise, largely reversed FI, atrophy, and the decrease in contractile force of SS after delayed RC repair in mice. If proven to do the same in humans, HIIT could serve as a new rehabilitation treatment for patients with chronic RC tears after surgery.
We also found that HIIT stimulated systemic beige/brown fat dependent on β3AR. Previous studies showed that a group of muscle residential interstitial progenitor cells, named FAPs, are the main source of FI. 34 When mice received β3AR agonist, FAPs and FI tended to adopt a BAT phenotype. 3 HIIT is a novel exercise style to help one lose weight. Some studies show that HIIT activates the sympathetic nervous system and improves metabolic diseases, especially fat metabolism.14,18,36 Previous studies have found that HIIT increased the expression of UCP1 and other metabolism-related genes in subcutaneous adipose tissue, especially in obese mice fed a high-fat diet.37,47 HIIT can be employed in different exercises, such as running, cycling, and swimming. It is controversial whether early active shoulder exercise increases retear rates, 25 but strengthening exercises are usually recommended starting in the 12th week after surgery.29,35 To decrease the retear risk from HIIT, exercise with a low shoulder burden starting in the 12th week after repair may be a safer rehabilitation method.
In this study, we showed that HIIT significantly improved muscle quality and contractile force by reducing muscle atrophy and FI before RC repair and after RC repair. SS in the EE and LE groups had less FI, less fibrosis, wider myofiber, and better contractile force. Clinical research showed that RC muscle quality before surgery is a predictor of clinical outcomes after RC repair. 44 The results in the EE group indicated that HIIT is an option for “pretreatment” to prepare patients for surgical intervention of their RC tears. Interestingly, the RC was torn when the EE group underwent HIIT. This indicates that HIIT prevents FI through something else instead of local muscle exercise. Because of the increased TH and UCP1 expression in the SS and other adipose depots, we attribute the effect to the systemic activation of sympathetic nerve fibers. The results in the LE group challenge the current concept that RC muscle FI is irreversible.
Previous studies have found that exercise affected the metabolism of local muscle. Consitt et al 8 reviewed the impact of endurance and resistance training on skeletal muscle energy metabolism in older adults. An interesting point is that endurance training increases intramuscular triglycerides in older adults just like the athlete’s paradox put forward by Goodpaster et al, 20 while resistance training does not. Intramuscular triglycerides do not equal FI in muscle. Intramuscular triglycerides may come from the adipocytes in muscle or molecules in myocytes. They are explained as energy storage for those who experience endurance training. However, muscles have more glycolytic demand than lipid oxidation demand in resistance training. According to our research, another reason for low intramuscular triglycerides might be consumption by activated FAP in muscle. Effting et al 15 applied a ladder-climbing model in mice in which obesity was induced by a high-fat diet. They found that 8-week exercise increased the phosphorylation of RAC-alpha serine/threonine-protein kinase (Akt) and AMPK to help take up glucose and oxidize fatty acid in the quadriceps. Ladder climbing also reduced the expression of some inflammation genes, such as TNFα and IL1β in the quadriceps muscles of obese mice. A meta-analysis including 12 studies found that exercise improved muscle quality and FI in adults experiencing myosteatosis. 41
The effects of HIIT on the SS were largely attributed to activated UCP1 expression. UCP1 is a marker of BAT. Different from WAT with unilocular morphology, BAT is characterized by multiple lipid droplets and high expression of UCP1. 1 Recent studies have found that a group of unilocular adipose tissue changed to BAT-like morphology after certain stimuli. 53 This adipose tissue was named beige or brite fat tissue, located in ingWAT typically. 53 In activated BAT and beige fat tissue, UCP1 expression increases and consumes free fatty acid quickly. 9 Previous studies confirmed that FI is a kind of beige fat and that UCP1 played a key role in decreasing FI.50,51 Our results showed that HIIT significantly increased the expression of UCP1 and TH. TH is the rate-limiting enzyme in the synthesis of norepinephrine, a sympathetic neurotransmitter. 54 Increased expression of TH indicates activation of local sympathetic nerve fibers,13,54 and increased expression of UCP1 indicates activation of BAT or beige fat. 23 Previous research found some interesting interactions between sympathetic nerves and adipose tissues. Cui et al 10 found that a fat-derived “adipokine,” neurotrophic factor neurotrophin 3, acted on tropomyosin receptor kinase C as a key regulator of sympathetic nerve growth and innervation in adipose tissue. In turn, it helps to activate brown or beige adipocytes. Cao et al 6 found that partial chemical denervation of iBAT sympathetic nerves with 6-OHDA, a selective neurotoxin to sympathetic nerves, activated sympathetic nerve fibers and beige fat browning in ingWAT. In contrast, if ingWAT was also treated with 6-OHDA, no beige fat browning was detected in ingWAT.
To ensure the effect of HIIT is systemic, we also detected TH and UCP1 expression in other beige/brown fat depots by immunofluorescence. Similar results between FI and other beige/brown fat depots indicate that the effect of HIIT on FI is a localized reflection of the systemic effect rather than a result of shoulder exercise. To explore whether the effect is dependent on β3AR, we administered SR, a selective β3AR antagonist, into mice, just 10 minutes before HIIT and investigated the phenotypes of SS atrophy and FI. SR groups showed worse muscle quality and function, similar to those who did not exercise. However, TH expression in SS and other beige/brown depots was high, so HIIT still activated local sympathetic nerve fibers successfully. By comparison, UCP1 expression decreased in the SR groups. Therefore, the effect of HIIT on muscle quality depends on β3AR. We further examined related proteins by Western blotting of the pathway from TH to UCP1. The expression was as expected.
Limitations
Some limitations exist to this study. First, we should consider the difference between mice and humans. Some studies have indicated a low expression of β3AR in human muscle, with it expressing relatively high β2AR instead. 4 It is uncertain whether FI in human SS would reverse in a similar mechanism. Second, we used acute tendon transection in this RC tear model, but chronic RC tears are also present in a large portion of clinical cases. Despite the difference between our model and the clinical situation, the muscle degeneration seen is similar. Third, electrodes were used to stimulate the muscles during HIIT, and such forced intervention may have affected the nervous system. However, in the clinic, patients performing HIIT also need supervision. This supervision may have similar effects on the nervous system. Finally, the mice used here were very young relative to the age of patients in the clinic with common RC degeneration, and the amount of fat in this model was minuscule compared with these patients’ conditions. The young age of the mice might limit the translational implications of the research, but HIIT may serve as an early rehabilitation method for young patients.
Conclusion
In the current study, we discovered that, no matter before repair or immediately after repair, HIIT could improve SS atrophy, FI, and function in a delayed RC repair mice model. The effects might be related to fat browning through the activation of β3AR by excited sympathetic nerve fibers.
Acknowledgment
The authors thank the Medical Functional Experimental Teaching Center of Central South University for providing the BL-420F acquisition system and the Xiangya biobank of Central South University for providing the sample storage space.
Appendix
Table A1.
Abbreviations and Expansions of Specialized Terms Used in the Article
| Abbreviation | Expansion |
|---|---|
| 6-OHDA | 6-Hydroxydopamine |
| AMPK | AMP-activated protein kinase |
| β3AR | Beta-3 adrenergic receptor |
| BAT | Brown adipose tissue |
| cAMP | Cyclic adenosine 3′,5′-monophosphate |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| FAP | Fibro-adipogenic progenitors |
| FITC | Fluorescein isothiocyanate |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HIIT | High-intensity interval training |
| HSL | Hormone-sensitive lipase |
| IGF1 | Insulin-like growth factor 1 |
| IL1β | Interleukin 1 beta |
| iBAT | Interscapular brown adipose tissue |
| ingWAT | Inguinal white adipose tissue |
| MAPK | Mitogen-activated protein kinase |
| PBS | Phosphate-buffered saline |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| p-HSL | Phosphorylated hormone-sensitive lipase |
| PKA | Protein kinase A hormone-sensitive lipase |
| PVDF | Polyvinylidene difluoride |
| RIPA | Radioimmunoprecipitation assay |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| TH | Tyrosine hydroxylase |
| TNFα | Tumor necrosis factor alpha |
| UCP1 | Uncoupling protein 1 |
| VEGF | Vascular endothelial growth factor |
Figure A1.

Repaired tendon kept intact after high-intensity interval training. Hematoxylin-eosin staining of mouse shoulder showed normal structure in the (A) sham group and that scar tissue linked tendon and humerus in the (B) late exercise group. Scar tissue is shown in the circle.
Figure A2.

Oil red O staining showed no fatty infiltration in the supraspinatus of mice that received sham surgery followed by intraperitoneal injection of (A) dimethyl sulfoxide or (B) SR59230A 5 days per week for 6 weeks. Masson trichrome staining showed no obvious fibrosis in the supraspinatus of mice that received sham surgery followed by intraperitoneal injection of (C) dimethyl sulfoxide or (D) SR59230A 5 days per week for 6 weeks.
Footnotes
Final revision submitted January 26, 2023; accepted February 15, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding. This work was supported by grants from the National Natural Science Foundation of China (81902245 to Z.W.), the National Natural Science Foundation of China (82072501 to S.W.), the Hunan Provincial Science and Technology Ministry in China (2021JJ40949 to Z.W.), the Hunan Provincial Health Commission in China (202204073899 to S.W.), and the Hunan Provincial Health Commission in China (202204073792 to Z.W.). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Animal Welfare Committee of Central South University.
References
- 1.Abdullahi A, Jeschke MG. Taming the flames: targeting white adipose tissue browning in Hypermetabolic conditions. Endocr Rev. 2017;38(6):538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akimoto T, Pohnert SC, Li P, et al. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280(20):19587–19593. [DOI] [PubMed] [Google Scholar]
- 3.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104(7):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blondin DP, Nielsen S, Kuipers EN, et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab. 2020;32(2):287–300.e287. [DOI] [PubMed] [Google Scholar]
- 5.Bryniarski AR, Meyer GA. Brown fat promotes muscle growth during regeneration. J Orthop Res. 2019;37(8):1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Q, Jing J, Cui X, Shi H, Xue B. Sympathetic nerve innervation is required for beigeing in white fat. Physiol Rep. 2019;7(6):e14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin P, Kempf JF, Molé D, et al. Ten-year multicenter clinical and MRI Evaluation of isolated supraspinatus repairs. J Bone Joint Surg Am. 2017;99(16):1355–1364. [DOI] [PubMed] [Google Scholar]
- 8.Consitt LA, Dudley C, Saxena G. Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients. 2019;11(11):2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crichton PG, Lee Y, Kunji ER. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie. 2017;134:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui X, Jing J, Wu R, et al. Adipose tissue-derived neurotrophic factor 3 regulates sympathetic innervation and thermogenesis in adipose tissue. Nat Commun. 2021;12(1):5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies MR, Garcia S, Liu M, et al. Muscle-derived beige adipose precursors secrete promyogenic exosomes that treat rotator cuff muscle degeneration in mice and are identified in humans by single-cell RNA sequencing. Am J Sports Med. 2022:50(8):2247–2257. [DOI] [PubMed] [Google Scholar]
- 12.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg. 2014;134(7):985–990. [DOI] [PubMed] [Google Scholar]
- 13.During MJ, Liu X, Huang W, et al. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology. 2015;156(6):2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durrer C, Francois M, Neudorf H, Little JP. Acute high-intensity interval exercise reduces human monocyte toll-like receptor 2 expression in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R529–R538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effting PS, Thirupathi A, Muller AP, et al. Resistance exercise training improves metabolic and inflammatory control in adipose and muscle tissues in mice fed a high-fat diet. Nutrients. 2022;14(11):2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliasberg CD, Dar A, Jensen AR, et al. Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J Bone Joint Surg Am. 2017;99(4):331–341. [DOI] [PubMed] [Google Scholar]
- 17.Finlin BS, Memetimin H, Confides AL, et al. Human adipose beiging in response to cold and mirabegron. JCI insight. 2018;3(15):e121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredrickson G, Barrow F, Dietsche K, et al. Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab. 2021;53:101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. [DOI] [PubMed] [Google Scholar]
- 21.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 22.Hamano N, Yamamoto A, Shitara H, et al. Does successful rotator cuff repair improve muscle atrophy and fatty infiltration of the rotator cuff? A retrospective magnetic resonance imaging study performed shortly after surgery as a reference. J Shoulder Elbow Surg. 2017;26(6):967–974. [DOI] [PubMed] [Google Scholar]
- 23.Herz CT, Kiefer FW. Adipose tissue browning in mice and humans. J Endocrinol. 2019;241(3):R97–R109. [PubMed] [Google Scholar]
- 24.Horie T, Nakao T, Miyasaka Y, et al. MicroRNA-33 maintains adaptive thermogenesis via enhanced sympathetic nerve activity. Nat Commun. 2021;12(1):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyde D, Littlewood C, Mazuquin B, Manning L. Rehabilitation following rotator cuff repair: a narrative review. Phys Ther Rev. 2021;26(4):254–261. [Google Scholar]
- 26.Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–964. [DOI] [PubMed] [Google Scholar]
- 27.Koh KH, Laddha MS, Lim TK, Park JH, Yoo JC. Serial structural and functional assessments of rotator cuff repairs: do they differ at 6 and 19 months postoperatively? J Shoulder Elbow Surg. 2012;21(7):859–866. [DOI] [PubMed] [Google Scholar]
- 28.Lapner PLC, Jiang L, Zhang T, Athwal GS. Rotator cuff fatty infiltration and atrophy are associated with functional outcomes in anatomic shoulder arthroplasty. Clin Orthop Relat Res. 2015;473(2):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134–1142. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, Liu M, Agha O, et al. Beige FAPs transplantation improves muscle quality and shoulder function after massive rotator cuff tears. J Orthop Res. 2020;38(5):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Liu M, Agha O, et al. Beige fibro-adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg. 2020;29(4):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin C, Chen J, Hu M, et al. Sesamol promotes browning of white adipocytes to ameliorate obesity by inducing mitochondrial biogenesis and inhibition mitophagy via β3-AR/PKA signaling pathway. Food Nutr Res. 2021;65. doi:10.29219/fnr.v65.7577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Laron D, Natsuhara K, et al. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94(7):e41. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Ning AY, Chang NC, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longo UG, Carnevale A, Piergentili I, et al. Retear rates after rotator cuff surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2021;22(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magalhães JP, Santos DA, Correia IR, et al. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: a secondary analysis from a 1-year randomized controlled trial. Cardiovasc Diabetol. 2020;19(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Huenchullan SF, Ban LA, Olaya-Agudo LF, et al. Constant-moderate and high-intensity interval training have differential benefits on insulin sensitive tissues in high-fat fed mice. Front Physiol. 2019;10:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95(5):319–324. [DOI] [PubMed] [Google Scholar]
- 39.Meyer GA, Gibbons MC, Sato E, et al. Epimuscular fat in the human rotator cuff is a novel beige depot. Stem Cells Transl Med. 2015;4(7):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulo E, Wu D, Wang Y, et al. Sympathetic inputs regulate adaptive thermogenesis in brown adipose tissue through cAMP-Salt inducible kinase axis. Sci Rep. 2018;8(1):11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez-Velez R, Ezzatvar Y, Izquierdo M, Garcia-Hermoso A. Effect of exercise on myosteatosis in adults: a systematic review and meta-analysis. J Appl Physiol. 2021;130(1):245–255. [DOI] [PubMed] [Google Scholar]
- 42.Schena G, Caplan MJ. Everything you always wanted to know about β3-AR * (* but were afraid to ask). Cells. 2019;8(4):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin JH, Lee SH, Kim YN, et al. AHNAK deficiency promotes browning and lipolysis in mice via increased responsiveness to beta-adrenergic signalling. Sci Rep. 2016;6:23426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin YK, Ryu KN, Park JS, et al. Predictive factors of retear in patients with repaired rotator cuff tear on shoulder MRI. Am J Roentgenol. 2018;210(1):134–141. [DOI] [PubMed] [Google Scholar]
- 45.Silva LRB, Gentil PRV, Rebelo ACS, et al. Exponential model for analysis of heart rate responses and autonomic cardiac modulation during different intensities of physical exercise. R Soc Open Sci. 2019;6(10):190639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92(1):157–191. [DOI] [PubMed] [Google Scholar]
- 47.Wang N, Liu Y, Ma Y, Wen D. High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice. Life Sci. 2017;191:122–131. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Feeley BT, Kim HT, Liu X. Reversal of fatty infiltration after suprascapular nerve compression release is dependent on UCP1 expression in mice. Clin Orthop Relat Res. 2018;476(8):1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Liu X, Davies MR, et al. A mouse model of delayed rotator cuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med. 2018;46(12):2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Liu X, Jiang K, et al. Intramuscular brown fat activation decreases muscle atrophy and fatty infiltration and improves gait after delayed rotator cuff repair in mice. Am J Sports Med. 2020;48(7):1590–1600. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Liu X, Liu M, et al. β(3)-Adrenergic receptor agonist treats rotator cuff fatty infiltration by activating beige fat in mice. J Shoulder Elbow Surg. 2021;30(2):373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–646. [DOI] [PubMed] [Google Scholar]
- 53.Zhang G, Sun Q, Liu C. Influencing factors of thermogenic adipose tissue activity. Front Physiol. 2016;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XH, Tang LY, Wang XY, et al. ADGRA1 negatively regulates energy expenditure and thermogenesis through both sympathetic nervous system and hypothalamus-pituitary-thyroid axis in male mice. Cell Death Dis. 2021;12(4):362. [DOI] [PMC free article] [PubMed] [Google Scholar]