Abstract
Purpose of Review:
Sex dimorphism in Parkinson’s disease (PD) is an ostensible feature of the neurological disorder, particularly as men are 1.5-2 times more likely to develop PD than women. Clinical features of the disease, such as presentation at onset, most prevalent symptoms, and response to treatment are also affected by sex. Despite these well-known sex differences in PD risk and phenotype, the mechanisms that impart sex dimorphisms in PD remain poorly understood.
Recent Findings:
As PD incidence is influenced by environmental factors, an intriguing pattern has recently emerged in research studies suggesting a male-specific vulnerability to dopaminergic neurodegeneration caused by neurotoxicant exposure, with relative protection in females. These new experimental data have uncovered potential mechanisms that provide clues to the source of sex differences in dopaminergic neurodegeneration and other PD pathology such as alpha-synuclein toxicity.
Summary:
In this review, we discuss the emerging evidence of increased male sensitivity to neurodegeneration from environmental exposures. We examine mechanisms underlying dopaminergic neurodegeneration and PD-related pathologies with evidence supporting the roles of estrogen, SRY expression, the vesicular glutamate transporter VGLUT2, and the microbiome as prospective catalysts for male vulnerability. We also highlight the importance of including sex as a biological variable, particularly when evaluating dopaminergic neurotoxicity in the context of PD.
Keywords: Parkinson’s disease, environment, toxicant, dopamine, sex differences
Introduction
A recent focus on biological observations of sex dimorphism in Parkinson’s disease (PD) confirms what individuals with this disorder already know; PD affects men and women differently. From the male-to-female incidence ratio (~1.5:1), to symptomatic presentation, disease progression, and response to treatment, sexual dimorphism in PD is a central factor in the disease [1-3]. Assessing biological sex in the risk for PD is a complicated task, as both genetic and hormonal sex characteristics likely contribute to intrinsic PD risk [1]. Furthermore, PD risk is influenced by exogenous factors such as environmental exposures (e.g., pesticides, metals, solvents, pathogens, head trauma, diet, lifestyle) [4], which may disproportionately affect individuals with traditionally or culturally held gender roles such as farming, mining, or heavy industry. However, with spurious links of PD risk to occupation titles [5], it is unclear what role exposure plays in sex dimorphism for dopaminergic neurotoxicity and ultimately neurodegeneration.
Emerging evidence from experimental studies indicates that males may have a biologic and specific vulnerability to neurotoxicants, or consequently, that females display a relative resilience to environmental factors linked to PD. While the protective effect of estrogen is often quoted in this context [6, 7], distinct cellular mechanisms that appear to be separate from the hormonal system and conserved across species may also drive neuroprotection from toxicant exposure [8]. In this context, we discuss the epidemiology, recent experimental model systems, and biological mechanisms that demonstrate male and female differences in vulnerability to environmental exposures that produce dopaminergic neurodegeneration.
Sex differences in PD risk from environmental exposure - evidence from human epidemiology
The first indications that environmental exposure may influence sex dimorphism in PD became apparent in human epidemiology studies that assessed PD risk and occupation [9-11]. From these, an anecdote developed that occupations traditionally held by men were more likely to result in exposure to known neurotoxicants that increase risk for the disease. For example, historically male-dominated fields such as welding, industrial machining, and farming produce high exposure risk to contaminants including manganese and other heavy metals, solvents, or pesticides, respectively [11]. However, population-specific influences also may play a role in PD risk, as PD incidence is between 1.5-2 times higher in men than women in Western populations but is more equivalent at 1:1.04 between sexes in Japan [12]. Though this may be due to unknown intrinsic risk factors within the population, other biological, environmental, and cultural factors cannot be ruled out; for example, tobacco smoking, which is inversely associated with PD risk [13], is highly prevalent in Asia and more common in men [14].
Of the relatively few epidemiological studies focused on PD incidence from environmental factors that specifically compare sex, there is empirical evidence for male vulnerability to PD from toxicant exposure (Table 1). For example, in Denmark, males with occupations correlated to high levels of pesticide exposure experience a higher hospitalization rate ratio for onset of PD symptoms than women who also hold equivalent occupations [15]. In addition, a French epidemiology study showed that males may experience more severe symptoms following exposure than females [10]. Men were twice as likely to experience cognitive decline and depressive symptoms after occupational exposure to pesticides [10]. There are also data supporting a greater tolerance to longer exposure windows in females while still maintaining a lower PD incident ratio than males [11]. In participants who maintained occupational exposure for a period of at least 2 or 3 years, men were almost two times more likely to develop PD than women [11]. Despite the prolonged exposure, women maintained a lower risk indicating that female neuroprotection is persistent throughout exposure.
Table 1.
Epidemiological studies identifying PD risk by sex as a result of environmental or occupational exposure.
| Region | Risk Assessment | PD Risk | Exposure | Exposure reporting method | Reference |
|---|---|---|---|---|---|
| Southwest France | Adjusted relative risk (RR) |
↑ Male: RR 5.6 (95% CI: 1.5, 21.6) Female: No risk effect |
Pesticides: nonspecific | Occupational history questionnaire. Pesticide exposure was assessed by a panel of experts. | Baldi et al 2002 [10] |
| India | Adjusted odds ratio (OR) |
↑ Male: OR 1.98 (95% CI: 1.34–2.92) Female: No risk effect |
Well water consumption | Patient survey | Behari et al 2001 [22] |
| Denmark | Age-standardized hospitalization ratio (SHR) |
↑ Male: Increased SHR Female: No risk effect |
Pesticides: non specific | Population registration database, first-time hospitalization for PD. Pesticide exposure was classified by occupational registration. | Tuchsen et al 2000 [9] |
| Sweden | Standard incidence ratios (SIR) for hospitalization for PD |
↑ Male: Increased SIR in 5 occupational categories Female: Increased SIR in 1 occupational category |
Occupational exposures | Population registration database, diagnoses, and hospital admissions. Exposure estimated by occupation. Socioeconomic status. | Li et al 2009 [11] |
In contrast, some epidemiological studies show no correlation between sex and toxicant-induced PD incidence or show the reverse with a female preponderance [16-18]. While the source of this variability is unclear, the lack of or difficulty quantifying environmental exposure in epidemiological studies may be at least partially to blame [19, 20]. For example, many studies rely on participant questionnaires to estimate the level of exposure based on reported occupational and residential history, which naturally induces a margin of error. This is further amplified by a reported gender effect in recall bias, as women are statistically more likely to correctly recall information, potentially influencing exposure recall bias in retrospective cohort studies [21]. Furthermore, recall of past exposures or using occupation as a proxy for potential exposures cannot provide dose, time of exposure, or report multiple exposures either simultaneously or over the lifespan. Thus, the reports of male vulnerability to toxicant-induced PD, despite the limitations of exposure assessment in retrospective cohort studies, may further strengthen this connection.
Sex differences in PD risk from environmental exposure - evidence from experimental studies
There are numerous and varied toxicant models of parkinsonian neurodegeneration, and most reproduce some, but not all, phenotypic symptoms and pathologies of the human disease [23-25]. To investigate sex dimorphism in PD, in vivo mammalian models provide a valuable experimental tool as primary sex characteristics and hormone expression are present, and systemic toxicant metabolism can be approximated with a high degree of accuracy [24, 26]. However, most animal models used to measure PD-related neurodegeneration as a result of neurotoxicant exposure, environmental contaminant or otherwise, have historically been conducted in male rodents. Thus, the first several decades of animal modeling in basic PD research largely overlooked any sexual dimorphisms in vulnerability to neurotoxicant exposure. As recent efforts have increased to include sex as a biological variable [27], an intriguing pattern has emerged – female animals are less vulnerable to dopaminergic neurodegeneration from environmental toxicants. To this end, in the section below we discuss sex differences in dopaminergic neurotoxicity from experimental studies with environmental toxicants associated with PD risk, summarized in Table 2.
Table 2.
Summarized sex differences in neurotoxicity of rodents exposed to environmental PD toxicants. Intraperitoneal (IP), subcutaneous (SC).
| Toxicant | Animal Model | Sex differences | References |
|---|---|---|---|
| Rotenone |
Rats 2.8 – 3.6 mg/kg (IP) daily |
Males significantly more vulnerable to nigrostriatal dopaminergic neurodegeneration from rotenone (2.8 mg/kg) than females. Females displayed reduced accumulation of α-syn within dopaminergic neurons of the SN. |
De Miranda et al 2019 [32] |
| Dieldrin |
Mice 0.3 mg/kg in diet every three days (neurodevelopment) |
Males showed increased traversal time and number of steps on the challenging beam. Males lose 5% more ST dopamine than females. Males show increased striatal dopamine turnover at 6 months. Males show 15% more TH neuron loss than females. |
Gezer et al 2020 [37] |
| Paraquat |
Mice Prenatal: 0.3 mg/kg paraquat, or 1 mg/kg maneb Postnatal: 5 mg/kg paraquat, or 30 mg/kg maneb (SC) |
Male-specific locomotor deficits and striatal neurochemistry alterations with prenatal maneb and adulthood paraquat. Males selectively vulnerable to 2nd hit exposure. |
Barlow et al 2004 [39] |
|
Mice 2.19x104 ± 3.32x103 particles/cm3 inhaled |
Males displayed incorrect olfactory discrimination. | Anderson et al. 2021 [40] | |
|
Drosophila 10 or 20 mM in diet |
Males showed symptoms 12 hours before females. Males become nearly completely incapacitated at 24 hr and females never experience total immobilization. |
Chaudhuri et al. 2007 [41] | |
|
Drosophila 0.25 μM – 25 mM in diet |
Males showed increased lethality. Males showed increased superoxide dismutase (SOD) activity. |
Krůček et al. 2015 [42] | |
| Manganese |
Mice 1 g MnCl2 / 1 kg sediment |
Males displayed increased beam traversal time. Males displayed decreased time on rotarod. |
Freeman et al 2020 [60] |
|
Mice 50 mg/kg (SC) |
Striatal MSN spine density increased in females, decreased in males one day post exposure, but reversed following three weeks of exposure. | Madison et al. 2011 [61] |
Rotenone
Rotenone administration in adult rats causes the selective neurodegeneration of dopaminergic neurons in the substantia nigra (SN) and their terminal projections to the dorsolateral striatum (ST) [28-31]. Most published data from parkinsonian modeling with rotenone is within male, aged, inbred or outbred rat strains (e.g. Lewis, Sprague Dawley) using systemic dosing of rotenone at 2.8 mg/kg, a concentration that provides the most reproducible neurodegenerative effects [31]. In contrast, when female rats of the same age and strain (Lewis) were exposed to the same dose (2.8 mg/kg) and scheduling of rotenone treatment, they exhibited virtually no discernable dopaminergic pathology [32]. In fact, female Lewis rats required a higher rotenone dose than males (up to 3.6 mg/kg) to produce equivalent dopaminergic pathology within the SN – a dose that is acutely lethal to male rats.
In addition to the overall lack of dopaminergic neurodegeneration, female rats exposed to higher doses of rotenone (3.2 and 3.6 mg/kg) also exhibited a reduction in overall PD-related pathology. For example, endogenous α-synuclein accumulation in dopaminergic neurons, a hallmark of rotenone-induced neuropathology, was significantly lower in female animals compared to male counterparts [32]. This was postulated to be at least partially influenced by a sex-dependent preservation of macroautophagy, as lysosomes within dopaminergic neurons of female rats were less impaired than those in male rats exposed to rotenone [29]. While the mechanisms underlying female resilience toward rotenone-induced protein aggregation are unclear, other model systems of α-synuclein toxicity support a similar male-specific vulnerability. For example, a study using 3K mice, a tetramer-abrogating mutant of the E46K α-synuclein mutation, showed that male animals were more vulnerable to α-synuclein-induced motor deficits and dopaminergic neuropathology than females [33]. Moreover, male animals treated with an estrogen compound (10b-17β-dihydroxyestra-1,4-dien-3-one) showed dopaminergic neuroprotection against α-synuclein tetramers [34]. Whether estrogen is protective against environmental neurotoxicant-induced α-synuclein aggregation remains to be specifically assessed. Nonetheless, α-synuclein aggregation influenced by environmental exposures, such as pesticides, may underlie male-specific vulnerability to neurodegeneration.
Dieldrin
In addition to rotenone, male-specific vulnerability to motor dysfunction was observed in a study with the organochlorine dieldrin, a pesticide implicated in PD risk that produces selective degeneration of dopaminergic neurons from the SN in rodents [35, 36]. These data showed that developmental exposure to 0.3 mg/kg dieldrin in wildtype (C57Bl/6J) mice, followed by α-synuclein pre-formed fibril (PFF) intrastriatal injection, resulted in male-specific motor deficits and striatal dopamine turnover [37]. As the dieldrin exposure occurred only during perinatal periods – breeding, gestation, and lactation of the dam – this study demonstrated that developmental exposure may direct sex dimorphisms in neurodegenerative disease later in life. In particular, developmentally exposed males may have elevated vulnerability toward secondary exposures or risk factors (e.g., α-synuclein) that influence dopaminergic neurotoxicity [37].
Interestingly, Gezer and colleagues showed that developmental dieldrin exposure did not alter the amount of phosphorylated, aggregated α-synuclein in dopaminergic neurons (pSer129-αSyn) in male or female animals [37]. However, differential gene expression and DNA methylation alterations based on sex were observed. These findings suggest that sex-specific gene regulatory pathways are likely altered by developmental exposure to pesticides and may provide insight into mechanisms involved in lifetime risk of dopaminergic neurodegeneration. For example, differentially expressed genes relating to inflammatory and adaptive immune responses were observed in a sex-specific pattern [37], which could drive microglial or inflammatory activation in the brain in sexually divergent mechanisms: one that is relatively resilient in females, or one that is relatively susceptible in males. These results reveal a critical need to examine how sex-specific gene regulation is influenced by environmental contaminant exposure during development, shaping risk for neurodegeneration in adulthood.
Paraquat
Paraquat represents a key environmental risk factor associated with PD, as it is a widely used herbicide in the United States that elevates risk for the disease over two-fold (Odds Ratio 2.27, 95% CI: 0.91, 5.7) [20]. Despite epidemiological evidence supporting a link between paraquat and PD risk, there are inconsistencies in the amount of nigrostriatal degeneration produced in animal models of paraquat exposure [38]. However, experimental data support a male-specific vulnerability to dopaminergic neurotoxicity from paraquat in mice. For example, a study investigating prenatal and adulthood paired exposures to pesticides found that prenatal exposure to maneb (1 mg/kg) followed by adult exposure to paraquat caused a 50% reduction in striatal dopamine levels and a 40% increase in dopamine turnover in male rats [39]. In contrast, female rats showed no reduction in motor function nor changes to striatal neurochemistry. Interestingly, motor dysfunction in males was not correlated with prenatal paraquat or maneb exposure alone, but were induced after a second paraquat exposure as adults [39]. These data suggest that male offspring could be more vulnerable to developmental exposures than females, resulting in elevated PD risk in adulthood. In addition to developmental exposure, paraquat also caused preferential neurotoxicity in adult male animals; mice exposed to 2.19x104 ± 3.32x103 particles/cm3 of paraquat via inhalation induced male-specific deficits in olfactory discrimination [40]. Finally, studies performed in Drosophila showed that male flies exposed to paraquat displayed earlier and more severe symptoms than females [41, 42], suggesting that sex-dependent vulnerability to paraquat may be conserved across species.
Manganese
In addition to pesticides, heavy metals are linked to PD risk [43, 44] and represent common environmental contaminants as components of air pollution and as drinking water [45, 46]. Although manganese is a necessary dietary component, it is commonly sampled in the environment at concentrations of over twice the recommended limit by the World Health Organization [46]. Excess manganese exposure is associated with midbrain and developmental neurotoxicity, as well as risk for PD [47, 48]. Moreover, manganism, a disorder cause by overexposure to manganese, presents with motor symptoms similar to idiopathic PD [49-52]. Manganese appears to induce dopaminergic neurotoxicity via multiple mechanisms; mitochondrial dysfunction and oxidative stress, glial-driven neuroinflammation, and protein misfolding, transmission, and accumulation [48, 53-55]. There is also evidence that manganese catalyzes the auto-oxidation of dopamine [56, 57], providing a possible environmental link between dopamine oxidation and PD pathogenesis [58, 59].
Manganese also appears to induce sex-specific parkinsonian symptoms in experimental models of exposure. For example, a study investigating the interaction between manganese and groundwater sediment found that exposure to 1 g manganese chloride per 1 kg sediment caused male and female rats to underperform on motor behavior assessments such as rotarod, cylinder, and beam tests [60]. However, male rats displayed worse performance in all motor assessments compared to females. An important feature of this study was the use of manganese in sediment, which more accurately mimics environmental conditions, suggesting that sex-specific effects observed in this experimental model may be more reflective of human exposure [60]. In a separate study, FVB mice exposed to 50 mg/kg manganese subcutaneously displayed sex differences in medium spiny neuron (MSN) morphology within the striatum, where total spine density was increased in female mice, but significantly decreased in males one day post exposure [61]. As striatal MSNs are involved in dopaminergic signaling that coordinates motor movement, these data indicate that sexually dimorphic neurotoxicity from manganese exposure could be responsible for some of the reported sex differences in motor sequelae [62].
Putative biological mechanisms for sex-dependent vulnerability to environmental toxicants associated with PD risk
Estrogen
Estrogen is the most widely studied sex hormone in mechanisms of PD pathogenesis, with over 40 years of experimental and clinical research investigating its role in neurodegeneration [63-65]. In addition, numerous studies have investigated estrogen for its potentially protective role in toxicant exposure-induced PD as estrogen increases the production of dopamine in both the striatum and the SN [6, 7, 66-68]. For example, ovariectomized female rats injected with 6-OHDA that are then treated with estrogen were protected against the toxin-induced motor defects [69]. Epidemiological studies corroborate these data. One such study evaluated the risk of parkinsonism with oophorectomy in women prior to the onset of menopause [70]. They found that oophorectomy increased the risk of developing parkinsonian-like neurological evaluations by 1.68 times. The study also found that this risk increased with the earlier age of oophorectomy [70].
Estrogen treatment appears to reduce mitochondrial oxidative stress in cultured neurons (e.g., PC-12 cells), as well as in male and female rat brain tissue [71, 72], making it an attractive candidate to protect against environmental contaminants that cause neurotoxicity. For example, several studies show that estrogen is protective against manganese [73-75], a potent inducer of oxidative stress, including in rat primary neurons and astrocytes, which were protected against manganese induced toxicity in vitro by 17beta-estradiol as well as the estrogen modulator tamoxifen [76]. Estrogen and tamoxifen treatment were also neuroprotective against dopaminergic neurodegeneration following manganese injection in the striatum of mice, in part due to a reduction of oxidative stress as well as glutamate mediated excitotoxicity [75]. Despite this strong mechanistic evidence, it remains unclear if estrogen-mediated protection against manganese induced dopaminergic neurotoxicity is conserved in humans. A recent epidemiological study of parkinsonism in a manganese exposed population in South Africa did not report sex differences in risk for PD-related motor symptoms (UPDRS subsection part 3 scores) [77]. However, as females appear to absorb more manganese than males [78], resulting in higher blood manganese levels in humans [79], the similar rate of parkinsonism between sexes among exposed populations suggests that female resiliency to manganese could be partially occluded.
There is also evidence that estrogen plays a role in α-synuclein accumulation. As previously mentioned, 3K transgenic mice that amplify the E46K α-synuclein mutation, displayed worsened male vulnerability to α-synuclein accumulation, motor deficits, and dopaminergic neurodegeneration [33]. However, treatment with 10b-17β-dihydroxyestra-1,4-dien-3-one (DHED), that increases brain-specific estrogen, was protective against α-synuclein driven neurodegeneration in 3K male mice. Much speculation remains about the mechanism by which estrogen may protect against α-synuclein accumulation, but some data seem to support destabilizing effects of estrogens against α-synuclein fibrils [80], though the influence of estrogen on autophagy is likely a key factor as well [81].
Hormone and/or estrogen replacement therapy (HRT/ERT) has been extensively studied in PD risk, progression, and management of symptoms, with conclusions ranging from beneficial to negative effects [65, 82-86]. Additionally, there are significant risks associated with long term use of certain postmenopausal HRT, including coronary heart disease and breast cancer [87]. While there are potential methods to avoid off-target effects, such as selective estrogen receptor modulators or other tissue or cell specific estrogen targets, [33, 69], there is little consensus on the precise mechanism(s) and neuroprotection conveyed by estrogen. Future work to understand the potential neuroprotective efficacy of estrogen as a potential intervention in PD risk from neurotoxicants in exposed populations will be an important translational link between experimental studies and human health.
SRY
The expression of the sex determining region of the Y chromosome (SRY) is present within a subset of dopaminergic neurons of the ventral midbrain in males, and is completely absent in individuals with no Y chromosome [88]. SRY is a transcription factor that functions as the initial switch to begin the cascade for male sex development [89]. However, SRY also appears to be involved in gene transcription of dopaminergic neuron-related proteins such as tyrosine hydroxylase, monoamine oxidase-A, and dopamine decarboxylase [88, 90]. An emerging hypothesis suggests that SRY expression itself may elevate dopaminergic neuron susceptibility to exogenous neurotoxicants, providing a source for intrinsic male-specific vulnerability in the dopamine system [19]. This was demonstrated in rats exposed to 6-hydroxydopamine (6-OHDA) or rotenone, where knockdown of SRY using antisense oligonucleotides (ASOs) in male animals was protective against nigrostriatal neurodegeneration caused by either neurotoxicant [19]. In addition, cultured ventral midbrain neurons from male mouse pups on embryonic day 13.5, prior to gonad development, were more vulnerable to rotenone toxicity than female neurons [90], suggesting that female resilience to neurotoxicants is at least partially due to non-hormonal factors.
There is also evidence that SRY expression may influence the neuroprotective effects of estrogen. Overexpression of the SRY gene in mouse Neuro 2A (N2A) cells decreased estrogen receptor beta (ERβ) expression, but increased the dopamine metabolizing enzyme monoamine oxidase (MAO) [90]. Conversely, recent data suggests that estradiol signaling though ERα during neurodevelopment shapes neuronal gene expression that drives sexual dimorphism in the mouse brain [91]. This bidirectional effect of gene expression and hormone influence on neuronal steroid receptors likely contributes to sex and cell specific vulnerability to environmental factors. In addition, exposure to neurotoxicants during development or puberty may further influence the complex interaction between genetically encoded sex characteristics, hormone regulation, and dopaminergic vulnerability, as observed in dieldrin and paraquat rodent studies [37, 39].
VGLUT2
In addition to SRY, a subset of dopamine neurons also express vesicular glutamate transporter 2 (VGLUT2), which packages glutamate into vesicles and is necessary and sufficient for glutamate release [92-95]. Because a greater proportion of dopamine neurons express VGLUT2 in the ventral tegmental area [96, 97], which is relatively protected in PD compared to the SN [98, 99], an association between dopamine neuron VGLUT2 expression and resilience to PD was hypothesized. Indeed, multiple animal PD models have observed a role of VGLUT2 in mediating dopamine neuron vulnerability [100-102], and in a rotenone exposure model of PD, VGLUT2 expression conferred greater dopamine neuron survival [103]. These findings concur with a recent study of human PD brain tissue, which observed that upregulation of VGLUT2 in dopamine neurons is associated with survival [104]. Importantly, analysis of dopamine neuron VGLUT2 expression in Drosophila (Drosophila VGLUT, abbreviated dVGLUT), rats, and humans found a consistently higher level of VGLUT2 expression in females compared to males [8], suggesting VGLUT2 may play a role in mediating the sex difference in vulnerability to neurotoxicant-induced PD.
Though the precise mechanism behind VGLUT2-associated dopamine neuroprotection has not been uncovered, multiple clues provide insight on its role in neuron resilience. Conditional knockout of VGLUT2 in dopamine neurons decreases expression of brain-derived neurotrophic factor (BDNF) and its receptor, TrkB, and viral rescue of VGLUT2 restores BDNF and TrkB expression [101]. Additionally, lentiviral-mediated upregulation of VGLUT2 in dopamine neurons promotes axonal outgrowth in vitro, and treatment with glial cell-derived neurotrophic factor (GDNF) increases dopamine neuron VGLUT2 expression [102].
A more direct mechanism of VGLUT2-associated neuroprotection may have been uncovered via the discovery of activity-dependent vesicular synergy in glutamate/dopamine co-releasing neurons [105]. In this study of both flies and mice, cell depolarization led to an increase in dopamine loading into synaptic vesicles, and this increase was driven by vesicle hyperacidification driven by dVGLUT/VGLUT2 [105]. Thus, activity-dependent dopamine loading via VGLUT2 may remove dopamine from the cytosol, where it can cause oxidative stress through its metabolites [106]. VGLUT2 may also confer protection through a non-vesicular function, as work in Drosophila has demonstrated that after localization to the plasma membrane during exocytosis, dVGLUT can modulate proton efflux from nerve terminals [107], thus presenting another mechanism by which VGLUTs may alter oxidative stress in PD. Finally, protection may be conferred by glutamate rather than VGLUT2, as glutamate can be converted to α-ketoglutarate, allowing it to participate in the TCA cycle in times of stress to maintain mitochondrial function [108]. While more work is needed to determine the precise mechanism of neuroprotection, VGLUT2 expression and glutamate co-release may impact mitochondrial function and oxidative stress, thus serving as an intriguing candidate for mediating sex differences in PD vulnerability.
Microbiome
The microbiome has emerged as a central factor in numerous chronic physiological and psychiatric diseases, with growing evidence linking microbiome alterations with PD risk and progression. In addition, individuals who received truncal vagotomy have reduced PD risk [109], possibly as a result of decreased retrograde α-synuclein, pathogen, and/or toxicant trafficking from the enteric nervous system to the central nervous system [110, 111]. There is also a premise that gut permeability, driven by intestinal pathology or proinflammatory gut microbes, induces systemic and local neuroinflammation that influences neurodegeneration [112]. Gut microbiome signatures of individuals with PD indicate a proinflammatory environment, with a reduction in short chain fatty acid (SCFA)-producing bacteria, and elevation of opportunistic microbes that interact with the mucosal immune system to promote cytokine production [113]. While it remains unclear what triggers microbiome or gastrointestinal alterations in individuals with PD, growing clinical and experimental evidence suggest the gut-brain axis plays a role in disease pathogenesis [114-116].
Along with data from human fecal microbiomes from familial PD cases, sex differences have been observed in the gut microbiome of toxicant-treated animals as well. One such study evaluated the effects of manganese on the gut microbiome in mice exposed to manganese in drinking water which caused a decrease in microbial phylogenetic diversity in a sex-dependent manner [117]. In addition, male and female animals displayed different alterations in relative abundance of genera often associated with those in the PD gut microbiome. For example, the genus Lactobacillius was enriched only in manganese-treated female mice. In addition, DNA repair genes were upregulated in the gut microbiota of manganese-exposed females and downregulated in males, as well as aminotransferase and prephenate dehydratase genes (involved in phenylalanine synthesis), which were increased in female but decreased in male microbiota. The study also found that bacterial gene transcription of the gene encoding glutamate decarboxylase was significantly decreased in the gut microbiome of male mice [117]. As glutamate decarboxylase catalyzes glutamate to gamma-aminobutyric acid (GABA), these data suggest that toxicant exposure may induce a sex-specific influence on GABA production within the gut microbiome, which may in turn modulate the gut-brain axis [118].
Under normal conditions, significant differences in the gut microbiome between males and females are inconsistent, particularly in human studies [119]. Thus, as the gastrointestinal system is a primary site for environmental contaminant exposure [120], sex-dependent responses to toxicant-induced changes in gut microbiota may influence disease pathogenesis. However, direct gastrointestinal exposure to neurotoxicants may not be necessary to induce sex-dependent changes in the gut microbiome. Treatment with synthetic dopaminergic neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) via intramuscular injection in non-human primates decreased gut microbiome genus diversity in males but increased genus diversity in females [121]. Though MPTP is not an environmental contaminant, these data suggest that routes of exposure beyond the gastrointestinal system (e.g., inhalation, dermal), may still influence gut-brain interaction in a sex-dependent manner. Interestingly, some studies indicate that changes in microbiome influence circulating sex hormone concentration and metabolism, which supports the interplay between toxicant exposure, microbiome, and hormone regulation in neurodegeneration [122, 123]. Future studies involving toxicant exposure, sex, and the gut microbiome in PD pathogenesis will be critical to assess this complex relationship.
Conclusions
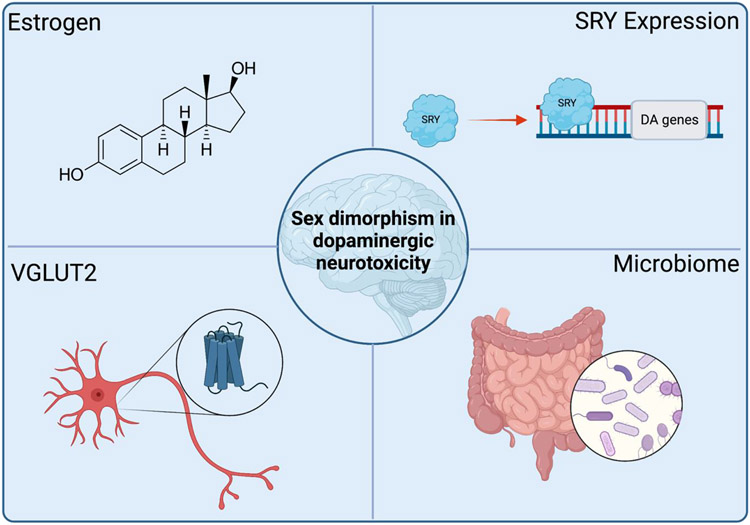
In this focused review, we have highlighted the limited available epidemiological and experimental data that support sex differences in environmental toxicant-induced neurodegeneration, which represents a component of a broader sex dimorphism in PD topic (see Gillies et al., 2014, Picillo et al., 2017 [1, 62]). The data reviewed here support a male-specific dopaminergic vulnerability and/or increased neuroprotection in females specific to neurotoxicant exposures associated with PD risk†. Though numerous potential mechanisms underlying the observed sex differences exist, there is evidence that estrogen plays a major protective role in female protection from neurotoxicant exposures (Figure 1). In addition, emerging data suggest that genetic sex characteristics, VGLUT2 expression, and microbiome influences are potential mechanisms that provide female resilience to toxicants associated with dopaminergic neurodegeneration. Given the sexual dimorphism in PD risk, clinical presentation, and progression, experimental models demonstrating male-specific vulnerability to environmental toxicants in PD is a critical but largely understudied research area. Future studies to address these mechanisms will be essential for prevention and appropriate therapeutic and/or symptomatic treatment for men and women with this neurodegenerative disease.
Figure 1.
Summary of identified physiological mechanisms involved in female resilience to dopaminergic neurodegeneration caused by environmental toxicant exposure. Figure created with Biorender.com.
Acknowledgements:
This work was supported by research grants from the National Institute of Environmental Health Sciences (R00ES029986, BRD), National Institute of Neurological Disorders and Stroke (F31NS118811, SAB), National Institute on Aging (R21AG068607, ZF), National Institute on Alcohol Abuse and Alcoholism (R21AA028800, ZF), National Institute of Diabetes and Digestive and Kidney Diseases (R01DK124219, ZF), and the Commonwealth of Pennsylvania (PA-HEALT, ZF).
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
Competing Interests: The authors have no competing interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Gender-related terms for human studies within this review were reflective of clinical and epidemiological reports, where “male/female, men/women” are categorized by available published data.
References
- 1.Gillies GE, et al. , Sex differences in Parkinson's disease. Frontiers in neuroendocrinology, 2014. 35(3): p. 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurado-Coronel JC, et al. , Sex differences in Parkinson’s disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics. Frontiers in Neuroendocrinology, 2018. 50: p. 18–30. [DOI] [PubMed] [Google Scholar]
- 3.Haaxma CA, et al. , Gender differences in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry, 2007. 78(8): p. 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caudle WM, et al. , Industrial toxicants and Parkinson's disease. Neurotoxicology, 2012. 33(2): p. 178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner CM, et al. , Occupation and Risk of Parkinsonism A Multicenter Case-Control Study. Archives of Neurology, 2009. 66(9): p. 1106–1113. [DOI] [PubMed] [Google Scholar]
- 6.Disshon KA and Dluzen DE, Estrogen as a neuromodulator of MPTP-induced neurotoxicity: effects upon striatal dopamine release. Brain Res, 1997. 764(1-2): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 7.Arvin M, et al. , Estrogen modulates responses of striatal dopamine neurons to MPP(+): evaluations using in vitro and in vivo techniques. Brain Res, 2000. 872(1-2): p. 160–71. [DOI] [PubMed] [Google Scholar]
- 8.Buck SA, et al. , Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging cell, 2021. 20(5): p. e13365–e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tüchsen F and Astrup Jensen A, Agricultural work and the risk of Parkinson's disease in Denmark, 1981-1993. Scandinavian Journal of Work, Environment & Health, 2000(4): p. 359–362. [DOI] [PubMed] [Google Scholar]
- 10.Baldi I, et al. , Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol, 2003. 157(5): p. 409–14. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Sundquist J, and Sundquist K, Socioeconomic and occupational groups and Parkinson's disease: a nationwide study based on hospitalizations in Sweden. Int Arch Occup Environ Health, 2009. 82(2): p. 235–41. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H, et al. , Female preponderance of Parkinson's disease in Japan. Neuroepidemiology, 2002. 21(6): p. 292–6. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y and Wang Y-J, Tobacco smoking and the reduced risk of Parkinson disease. Neurology, 2020. 94(20): p. 860–861. [DOI] [PubMed] [Google Scholar]
- 14.Yang B-Y and Dong G-H, Tobacco Smoking in Asia—A Public Health Threat. JAMA Network Open, 2019. 2(3): p. e191471–e191471. [DOI] [PubMed] [Google Scholar]
- 15.Tüchsen F and Jensen AA, Agricultural work and the risk of Parkinson's disease in Denmark, 1981-1993. Scand J Work Environ Health, 2000. 26(4): p. 359–62. [DOI] [PubMed] [Google Scholar]
- 16.Marttila RJ and Rinne UK, Epidemiology of Parkinson's disease in Finland. Acta Neurol Scand, 1976. 53(2): p. 81–102. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Nishikawa S, and Takahashi K, Epidemiology of Parkinson's disease in a Japanese city. Arch Neurol, 1983. 40(3): p. 151–4. [DOI] [PubMed] [Google Scholar]
- 18.Schoenberg BS, Anderson DW, and Haerer AF, Prevalence of Parkinson's disease in the biracial population of Copiah County, Mississippi. Neurology, 1985. 35(6): p. 841–5. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, et al. , Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proceedings of the National Academy of Sciences, 2019. 116(33): p. 16577–16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.•. Costello S, et al. , Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol, 2009. 169(8): p. 919–26. Key study that found elevated risk for Parkinson's disease from paraquat and maneb exposure.
- 21.Baer A, Trumpeter N, and Weathington B, differences in memory recall. Modern Psychological Studies, 2006. 12(1): p. 5. [Google Scholar]
- 22.Behari M, et al. , Risk factors of Parkinson's disease in Indian patients. J Neurol Sci, 2001. 190(1-2): p. 49–55. [DOI] [PubMed] [Google Scholar]
- 23.Blesa J and Przedborski S, Parkinson's disease: animal models and dopaminergic cell vulnerability. Front Neuroanat, 2014. 8: p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez TN and Greenamyre JT, Toxin models of mitochondrial dysfunction in Parkinson's disease. Antioxid Redox Signal, 2012. 16(9): p. 920–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meredith GE and Rademacher DJ, MPTP mouse models of Parkinson's disease: an update. J Parkinsons Dis, 2011. 1(1): p. 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blais EM, et al. , Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nature Communications, 2017. 8(1): p. 14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shansky RM and Murphy AZ, Considering sex as a biological variable will require a global shift in science culture. Nature Neuroscience, 2021. 24(4): p. 457–464. [DOI] [PubMed] [Google Scholar]
- 28.Betarbet R, Sherer TB, and Greenamyre JT, Animal models of Parkinson's disease. Bioessays, 2002. 24(4): p. 308–18. [DOI] [PubMed] [Google Scholar]
- 29.Caboni P, et al. , Rotenone, deguelin, their metabolites, and the rat model of Parkinson's disease. Chem Res Toxicol, 2004. 17(11): p. 1540–8. [DOI] [PubMed] [Google Scholar]
- 30.Greenamyre JT, et al. , Lessons from the rotenone model of Parkinson's disease. Trends Pharmacol Sci, 2010. 31(4): p. 141–2; author reply 142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.•. Cannon JR, et al. , A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis, 2009. 34(2): p. 279–90. A fundamental study in the rotenone model of dopaminergic neurodegeneration.
- 32.De Miranda BR, et al. , Sex Differences in Rotenone Sensitivity Reflect the Male-to-Female Ratio in Human Parkinson’s Disease Incidence. Toxicological Sciences, 2019. 170(1): p. 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajsombath MM, et al. , Female Sex and Brain-Selective Estrogen Benefit alpha-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice. J Neurosci, 2019. 39(38): p. 7628–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajsombath MM, et al. , Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice. The Journal of Neuroscience, 2019. 39(38): p. 7628–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrigan FM, et al. , Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health A, 2000. 59(4): p. 229–34. [DOI] [PubMed] [Google Scholar]
- 36.Kanthasamy AG, et al. , Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology, 2005. 26(4): p. 701–19. [DOI] [PubMed] [Google Scholar]
- 37.•. Gezer AO, et al. , Developmental exposure to the organochlorine pesticide dieldrin causes male-specific exacerbation of alpha-synuclein-preformed fibril-induced toxicity and motor deficits. Neurobiol Dis, 2020. 141: p. 104947. Key experimental study evaluating developmental exposure to dieldrin and sex-specific PD risk following α-synuclein exposure in adulthood.
- 38.Smeyne RJ, et al. , Assessment of the Effects of MPTP and Paraquat on Dopaminergic Neurons and Microglia in the Substantia Nigra Pars Compacta of C57BL/6 Mice. PLoS One, 2016. 11(10): p. e0164094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlow BK, et al. , A fetal risk factor for Parkinson's disease. Dev Neurosci, 2004. 26(1): p. 11–23. [DOI] [PubMed] [Google Scholar]
- 40.•. Anderson T, et al. , Paraquat Inhalation, a Translationally Relevant Route of Exposure: Disposition to the Brain and Male-Specific Olfactory Impairment in Mice. Toxicological Sciences, 2021. 180(1): p. 175–185. Inhaled exposured to paraquat in mice produced male-specific vulnerability in olfaction, with a relevant dose and route of exposure.
- 41.Chaudhuri A, et al. , Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci, 2007. 27(10): p. 2457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krůček T, et al. , Effect of low doses of herbicide paraquat on antioxidant defense in Drosophila. Arch Insect Biochem Physiol, 2015. 88(4): p. 235–48. [DOI] [PubMed] [Google Scholar]
- 43.Raj K, et al. , Metals associated neurodegeneration in Parkinson's disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett, 2021. 753: p. 135873. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery EB Jr., Heavy metals and the etiology of Parkinson's disease and other movement disorders. Toxicology, 1995. 97(1-3): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 45.Howe PD, H. MM, Dobson S, Manganese and its compounds: environmental aspects, I.P.o.C. Safety, Editor. 2004, United Nations Environment Program, International Labor Organization, World Health Organization: Monks Wood, United Kingdom. [Google Scholar]
- 46.Chowdhury S, et al. , Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Science of The Total Environment, 2016. 569-570: p. 476–488. [DOI] [PubMed] [Google Scholar]
- 47.•. Aschner M, et al. , Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med, 2009. 11(4): p. 252–66. Comprehensive review on manganese neurotoxicity and mechanisms of dopaminergic neurodegeneration.
- 48.Neal AP and Guilarte TR, Mechanisms of lead and manganese neurotoxicity. Toxicol Res (Camb), 2013. 2(2): p. 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth JA, et al. , The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology, 2013. 35: p. 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harischandra DS, et al. , Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Frontiers in Neuroscience, 2019. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zoni S and Lucchini RG, Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Current opinion in pediatrics, 2013. 25(2): p. 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claus Henn B, et al. , Early postnatal blood manganese levels and children's neurodevelopment. Epidemiology (Cambridge, Mass.), 2010. 21(4): p. 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitazawa M, et al. , Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther, 2002. 302(1): p. 26–35. [DOI] [PubMed] [Google Scholar]
- 54.Filipov NM, Seegal RF, and Lawrence DA, Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci, 2005. 84(1): p. 139–48. [DOI] [PubMed] [Google Scholar]
- 55.Harischandra DS, et al. , Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci Signal, 2019. 12(572). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen X-M and Dryhurst G, Iron- and Manganese-Catalyzed Autoxidation of Dopamine in the Presence of l-Cysteine: Possible Insights into Iron- and Manganese-Mediated Dopaminergic Neurotoxicity. Chemical Research in Toxicology, 1998. 11(7): p. 824–837. [DOI] [PubMed] [Google Scholar]
- 57.Sistrunk SC, Ross MK, and Filipov NM, Direct effects of manganese compounds on dopamine and its metabolite Dopac: an in vitro study. Environ Toxicol Pharmacol, 2007. 23(3): p. 286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hastings TG, The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson's disease. J Bioenerg Biomembr, 2009. 41(6): p. 469–72. [DOI] [PubMed] [Google Scholar]
- 59.Burbulla LF, et al. , Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science, 2017. 357(6357): p. 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freeman DM, et al. , Manganese-induced Parkinsonism in mice is reduced using a novel contaminated water sediment exposure model. Environmental Toxicology and Pharmacology, 2020. 78: p. 103399. [DOI] [PubMed] [Google Scholar]
- 61.Madison JL, et al. , Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology, 2011. 32(6): p. 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Picillo M, et al. , The relevance of gender in Parkinson's disease: a review. J Neurol, 2017. 264(8): p. 1583–1607. [DOI] [PubMed] [Google Scholar]
- 63.Zárate S, Stevnsner T, and Gredilla R, Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Frontiers in Aging Neuroscience, 2017. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandy S, Estrogen and Neurodegeneration. Neurochemical Research, 2003. 28(7): p. 1003–1008. [DOI] [PubMed] [Google Scholar]
- 65.Bustamante-Barrientos FA, et al. , The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Frontiers in Cellular Neuroscience, 2021. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDermott JL, Liu B, and Dluzen DE, Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol, 1994. 125(2): p. 306–11. [DOI] [PubMed] [Google Scholar]
- 67.Dluzen DE, McDermott JL, and Liu B, Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem, 1996. 66(2): p. 658–66. [DOI] [PubMed] [Google Scholar]
- 68.Dluzen D, Estrogen decreases corpus striatal neurotoxicity in response to 6-hydroxydopamine. Brain Res, 1997. 767(2): p. 340–4. [DOI] [PubMed] [Google Scholar]
- 69.Baraka AM, et al. , The possible role of estrogen and selective estrogen receptor modulators in a rat model of Parkinson's disease. Life Sci, 2011. 88(19-20): p. 879–85. [DOI] [PubMed] [Google Scholar]
- 70.Rocca WA, et al. , Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology, 2008. 70(3): p. 200–9. [DOI] [PubMed] [Google Scholar]
- 71.Azevedo RB, et al. , Regulation of antioxidant enzyme activities in male and female rat macrophages by sex steroids. Braz J Med Biol Res, 2001. 34(5): p. 683–7. [DOI] [PubMed] [Google Scholar]
- 72.Razmara A, et al. , Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res, 2007. 1176: p. 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee E, et al. , Estrogen attenuates manganese-induced glutamate transporter impairment in rat primary astrocytes. Neurotox Res, 2013. 23(2): p. 124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee ES, et al. , Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem, 2009. 110(2): p. 530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.•. Pajarillo E, et al. , 17β-estradiol and tamoxifen protect mice from manganese-induced dopaminergic neurotoxicity. Neurotoxicology, 2018. 65: p. 280–288. Comphrehensive in vivo study that showed estrogen was neuroprotective against manganese induced dopaminergic neurodegeneration.
- 76.Lee ES, et al. , Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol Sci, 2009. 110(1): p. 156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Racette BA, et al. , Severity of parkinsonism associated with environmental manganese exposure. Environmental Health, 2021. 20(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finley JW, Johnson PE, and Johnson LK, Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am J Clin Nutr, 1994. 60(6): p. 949–55. [DOI] [PubMed] [Google Scholar]
- 79.Oulhote Y, Mergler D, and Bouchard MF, Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environmental Health, 2014. 13(1): p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirohata M, et al. , Anti-aggregation and fibril-destabilizing effects of sex hormones on α-synuclein fibrils in vitro. Experimental Neurology, 2009. 217(2): p. 434–439. [DOI] [PubMed] [Google Scholar]
- 81.Xiang J, et al. , How does estrogen work on autophagy? Autophagy, 2019. 15(2): p. 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dye RV, et al. , Hormone Replacement Therapy and Risk for Neurodegenerative Diseases. International Journal of Alzheimer’s Disease, 2012. 2012: p. 258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lundin JI, et al. , Formulations of hormone therapy and risk of Parkinson's disease. Mov Disord, 2014. 29(13): p. 1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song YJ, et al. , The Effect of Estrogen Replacement Therapy on Alzheimer's Disease and Parkinson's Disease in Postmenopausal Women: A Meta-Analysis. Front Neurosci, 2020. 14: p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, et al. , Hormone replacement therapy and Parkinson's disease risk in women: a meta-analysis of 14 observational studies. Neuropsychiatric disease and treatment, 2014. 11: p. 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu M, et al. , Postmenopausal hormone therapy and Alzheimer's disease, dementia, and Parkinson's disease: A systematic review and time-response meta-analysis. Pharmacol Res, 2020. 155: p. 104693. [DOI] [PubMed] [Google Scholar]
- 87.Rossouw JE, et al. , Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama, 2002. 288(3): p. 321–33. [DOI] [PubMed] [Google Scholar]
- 88.Czech DP, et al. , The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem, 2012. 122(2): p. 260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fechner PY, The role of SRY in mammalian sex determination. Acta Paediatr Jpn, 1996. 38(4): p. 380–9. [DOI] [PubMed] [Google Scholar]
- 90.Tao Q, et al. , Gender segregation in gene expression and vulnerability to oxidative stress induced injury in ventral mesencephalic cultures of dopamine neurons. Journal of Neuroscience Research, 2012. 90(1): p. 167–178. [DOI] [PubMed] [Google Scholar]
- 91.Gegenhuber B, et al. , Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawano M, et al. , Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol, 2006. 498(5): p. 581–92. [DOI] [PubMed] [Google Scholar]
- 93.Berube-Carriere N, et al. , The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol, 2009. 517(6): p. 873–91. [DOI] [PubMed] [Google Scholar]
- 94.Root DH, et al. , Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Sci Rep, 2016. 6: p. 30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hnasko TS, et al. , Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron, 2010. 65(5): p. 643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morales M and Root DH, Glutamate neurons within the midbrain dopamine regions. Neuroscience, 2014. 282: p. 60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poulin JF, et al. , Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci, 2018. 21(9): p. 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Surmeier DJ, Obeso JA, and Halliday GM, Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci, 2017. 18(2): p. 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sulzer D and Surmeier DJ, Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord, 2013. 28(6): p. 715–24. [DOI] [PubMed] [Google Scholar]
- 100.Steinkellner T, et al. , Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J Clin Invest, 2018. 128(2): p. 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen H, et al. , Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice. Proc Natl Acad Sci U S A, 2018. 115(49): p. E11532–E11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kouwenhoven WM, et al. , VGluT2 Expression in Dopamine Neurons Contributes to Postlesional Striatal Reinnervation. J Neurosci, 2020. 40(43): p. 8262–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buck SA, et al. , VGLUT2 Is a Determinant of Dopamine Neuron Resilience in a Rotenone Model of Dopamine Neurodegeneration. J Neurosci, 2021. 41(22): p. 4937–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steinkellner T, et al. , Dopamine neurons exhibit emergent glutamatergic identity in Parkinson's disease. Brain, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aguilar JI, et al. , Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT. Neuron, 2017. 95(5): p. 1074–1088 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hastings TG, Lewis DA, and Zigmond MJ, Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A, 1996. 93(5): p. 1956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossano AJ, et al. , Na(+) /H(+) exchange via the Drosophila vesicular glutamate transporter mediates activity-induced acid efflux from presynaptic terminals. J Physiol, 2017. 595(3): p. 805–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Divakaruni AS, et al. , Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J Cell Biol, 2017. 216(4): p. 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu B, et al. , Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology, 2017. 88(21): p. 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.•. Borghammer P and Van Den Berge N, Brain-First versus Gut-First Parkinson's Disease: A Hypothesis. J Parkinsons Dis, 2019. 9(s2): p. S281–s295. Comprehensive review on brain and/or body origin of Parkinson's disease.
- 111.Phillips RJ, et al. , Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease? Neuroscience, 2008. 153(3): p. 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Houser MC and Tansey MG, The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? npj Parkinson's Disease, 2017. 3(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.•. Wallen ZD, et al. , Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. npj Parkinson's Disease, 2020. 6(1): p. 11. Demonstrates a Parkinson's disease gut microbiome "signature" that is distinct from healthy controls.
- 114.Aho VTE, et al. , Gut microbiota in Parkinson's disease: Temporal stability and relations to disease progression. EBioMedicine, 2019. 44: p. 691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen H, et al. , Environmental triggers of Parkinson's disease – Implications of the Braak and dual-hit hypotheses. Neurobiology of Disease, 2022. 163: p. 105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toh TS, et al. , Gut microbiome in Parkinson's disease: New insights from meta-analysis. Parkinsonism Relat Disord, 2022. 94: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 117.Chi L, et al. , Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol, 2017. 331: p. 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duranti S, et al. , Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Scientific reports, 2020. 10(1): p. 14112–14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim YS, et al. , Sex Differences in Gut Microbiota. The world journal of men's health, 2020. 38(1): p. 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tu P, et al. , Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics, 2020. 8(1): p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joers V, et al. , Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson's disease: A case series. Neurobiol Dis, 2020. 144: p. 105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baker JM, Al-Nakkash L, and Herbst-Kralovetz MM, Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas, 2017. 103: p. 45–53. [DOI] [PubMed] [Google Scholar]
- 123.Parida S and Sharma D, The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells, 2019. 8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]