Specialized therapeutic delivery, or use of pharmaceuticals and other biomaterials to target specific parts of the body or diseased tissue, has long been sought as an ideal way of treating human diseases. A recent article published in Nature Biomedical Engineering revealed an innovative strategy to engineer nucleus-free human mesenchymal stem cells (MSCs) for targeted delivery of therapeutics to disease site.1 MSCs have emerged as promising vehicles of therapeutic delivery.2,3 MSCs are undifferentiated pluripotent stem cells derived from areas such as bone marrow and adipose tissue.4,5 MSCs are sought after for their chemotaxis, or ability to home towards a chemical stimulus, and capacity for modification with elements such as chemoattractant receptors and adhesion molecules.1 These properties allow for site-specific and minimally-invasive therapeutic administration and treatment.
Despite these benefits, MSC usage has been limited by the size and variability of the cells. When administered through intravenous (IV) injection, MSCs are often trapped at small capillary beds (namely in the lungs) due to their large size. This means they do not always reach their target sites and instead accumulate in potentially hazardous areas.2 Additionally, MSCs may secrete undesirable byproducts and are known to differentiate unpredictably in vivo.1 Other closely-related vessels, such as cell-free delivery vehicles (CFDVs), avoid these pitfalls. However, CFDVs often lack chemotactic abilities and the resources to move to designated sites.1
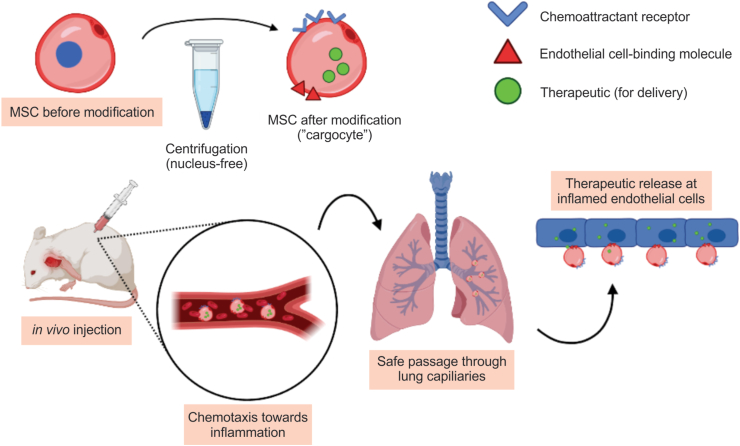
In this recent article, Wang et al worked to overcome some of these drawbacks while preserving the beneficial qualities of MSCs.1 Their solution involves removing the nuclei from MSCs in a process known as enucleation. Enucleated MSCs are then equipped with chemoattractant receptors and endothelial-cell-binding molecules. The authors hypothesized that these so-called “cargocytes” would retain many properties of unmodified MSCs while avoiding the aforementioned risks by adopting some characteristics of other CFDVs (Fig. 1).
Figure 1.
“Cargocyte” production through centrifugation and the attachment of additional molecules, enucleating the cells and optimizing them for delivery (top). Cells are then injected in vivo where they migrate through the bloodstream to the target site (bottom).
The authors first tested whether cargocytes would retain homing and mRNA transcription properties of MSCs. They transfected both hTERT-immortalized adipose-derived MSCs (hT-MSCs) and their derived cargocytes with Gaussia luciferase mRNA. Transfected cargocytes secreted the resultant proteins at levels comparable to regular hT-MSCs, suggesting that cargocytes can still home and, if given exogenous mRNA, can secrete therapeutic proteins as expected.
The next experiments revealed that cargocytes migrate towards chemoattractant molecules without issue. The authors also found that they can successfully equip cargocytes with chemoattractant receptors (such as CXCR4) to aid in migration. Further analysis supported that cargocytes are more deformable than their MSC counterparts. This makes it easier to fit through small channels, including capillary beds that typically pose issues for nucleated MSCs. We see already that cargocytes offer advantages over traditional MSC usage.
Adhesion to target endothelial cells involves two steps: initiation of adhesion and activation of cell integrins. The authors identified that attaching endothelial-cell-binding molecules allows cargocytes to complete both of these steps, a function usually unfeasible for other CFDVs. This is essential for optimized targeting of injured tissue. For example, injured tissue often secretes glycoprotein ligands E− and P-selectin. These molecules are also compatible with cargocytes, allowing cargocytes to migrate towards their targets with greater specificity.
Cargocytes are clearly equipped to target and home towards sites of inflammation. Now the question becomes how well they ameliorate these targets in vivo after IV injection. Due to their deformability, cargocytes could migrate towards injured areas without trapping in the lungs or surrounding tissue in mouse models. They also successfully administered therapeutics on site, relieving acute inflammation and pancreatitis in other models (Fig. 1).
In conclusion, Wang et al demonstrated an alternative to traditional MSC usage in therapeutic delivery, creating a CFDV that retains the essential characteristics of unmodified MSCs. The so-called “cargocyte” circumvents other issues involved with MSCs by increasing deformability and allowing for successful therapeutic administration with less unpredictability. This breakthrough represents an important stepping stone as we search for less invasive and more site-specific ways of delivering therapeutics. Nonetheless, much remains to be learned about how efficaciously the “cargocyte” system would work in preclinical models and/or clinical studies, and whether the system can be engineered to treat non-inflammation diseases.
Conflict of interests
The authors declare no conflict of interests.
Funding
The reported work was supported in part by research grants from the National Institutes of Health (No. CA226303 to TCH and No. DE030480 to RRR). This project was also supported in part by The University of Chicago Cancer Center Support Grant (No. P30CA014599) and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (No. 5UL1TR002389). TCH was also supported by the Mabel Green Myers Research Endowment Fund and The University of Chicago Orthopaedics Alumni Fund. Funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Wang H., Alarcón C.N., Liu B., et al. Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue. Nat Biomed Eng. 2022;6(7):882–897. doi: 10.1038/s41551-021-00815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger T.E.G., Thorek D.L.J., Denmeade S.R., Isaacs J.T., Brennen W.N. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651–663. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvinova L.S., Shupletsova V.V., Khaziakhmatova O.G., et al. Human mesenchymal stem cells as a carrier for a cell-mediated drug delivery. Front Bioeng Biotechnol. 2022;10:796111. doi: 10.3389/fbioe.2022.796111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastegar F., Shenaq D., Huang J., et al. Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cell. 2010;2(4):67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]