Highlights
-
•
The breast cancer composite score describes breast cancer burden by census tract.
-
•
The composite score is composed of morbidity, mortality, and stage.
-
•
Often area-based cancer prevention programs choose areas by demographics.
-
•
The composite score can aide cancer control programs to select census tracts.
Keywords: Breast neoplasms, Residence characteristics, Prevention & control, Geographic mapping, Epidemiology, Public health
Abstract
Community-based breast cancer prevention efforts often focus on women who live in the same neighborhoods, as they tend to have similar demographic characteristics, health behaviors, and environmental exposures; yet little research describes methods of selecting neighborhoods of focus for community-based cancer prevention interventions. Studies frequently use demographics from census data, or single breast cancer outcomes (e.g., mortality, morbidity) in order to choose neighborhoods of focus for breast cancer interventions, which may not be optimal. This study presents a novel method for measuring the burden of breast cancer among neighborhoods that could be used for selecting neighborhoods of focus. In this study, we 1) calculate a metric composed of multiple breast cancer outcomes to describe the burden of breast cancer in census tracts Philadelphia, PA, USA; 2) map the neighborhoods with the greatest breast cancer burden; and 3) compare census tracts with the highest burden of breast cancer to those with demographics sometimes used for geo-based prioritization, i.e., race and income. The results of our study showed that race or income may not be appropriate proxies for neighborhood breast cancer burden; comparing the breast cancer burden with demographics at the census tract level, we found few overlaps with the highest percentage African American or the lowest median incomes. Agencies implementing community-based breast cancer interventions should consider this method to inform the selection of neighborhoods for breast cancer prevention interventions, including education, screening, and treatment.
1. Introduction
Among women in the United States (US), breast cancer (BC) is the second-most prevalent type of cancer, the most common cause of death from cancer among Hispanic women, and the second most common cause of death from cancer among Whites and most Non-Hispanic subgroups (Breast cancer statistics [Internet]. Centers for Disease Control and Prevention., 2020a). Approximately 1 in 8 women born today in the US will be diagnosed with BC at some point in their lives (American Cancer Society, 2017). Projections for 2022 estimate that there will be 290,560 new cases of invasive BC diagnosed and 43,780 BCE deaths among women in the U.S. (American Cancer Society, 2022). There are dramatic disparities in BC outcomes among African American and White women. Mortality rates are 40% higher among African American women relative to White women (28.4 vs 20.3 per 100,000), despite African American women having a lower incidence rate (126.7 vs 130.8 per 100,000). This disparity is amplified among African American women below 50 years of age, who have twice the mortality rate relative to White women (DeSantis et al., 2019).
At the population level, regular screening, early diagnosis, and timely treatment initiation reduces the mortality and morbidity associated with BC (Richards et al., 1999, Humphrey et al., 2002, Bevers et al., 2018, Siu AL, U.S. Preventive Services Task Force, 2016). To promote early-stage BC diagnosis and treatment initiation, regular screening is recommended by national clinical guidelines (Nelson et al., 2009 Nov, Oeffinger et al., 2015, Siu AL, U.S. Preventive Services Task Force, 2016). The US Preventive Services Task Force recommends biennial screening mammography for women aged 50 to 74 years, whereas any decisions about screening mammography in women younger than 50 years are recommended to be made on an individual basis by weighing the potential benefits and risks (including false-positive results, unnecessary biopsies, overdiagnosis, and overtreatment)(USPSTF, 2022). The American Cancer Society recommends that all women begin screening mammography by age 45, and also recommends giving women aged 40 years and older the choice to start screening mammography (Oeffinger et al., 2015). The Community Preventive Services Task Force recommends multi-component interventions targeting underserved communities in order to boost breast cancer screening rates among populations (Community Prevention Services Task Force, 2016). However, the research describing methods of selecting communities of focus for cancer interventions is scant.
Researchers, health systems, and other organizations conducting cancer prevention interventions choose communities in which to focus their efforts . In urban areas, BC research and cancer prevention interventions often focus on neighborhoods because people who live in the same neighborhoods often have similar demographic characteristics, as place of residence correlates with socioeconomic status and ethnicity (Diez Roux and Mair, 2010). Studies in the peer-reviewed literature use cancer outcome data at the neighborhood level to choose the geographical focus for cancer prevention interventions. Geo-based studies have used BC outcome data by different geographic units, including census block group (MacKinnon et al., 2007), census tract Crabbe et al., 2015, Markossian et al., 2014), city (Sighoko et al., 2018), zip code (Dai and Oyana, 2008), county (Mobley et al., 2017, Scott et al., 2017), and Health Service Area (Tatalovich et al., 2015) among others. Almost all of these studies examined individual cancer outcomes such as mortality, incidence, and/or stage (Huerta et al., 2018, Strömberg et al., 2019).
Individual outcomes such as incidence, tumor stage, and mortality have limitations that restrict their utility for targeting neighborhoods for breast cancer interventions. First, incidence measurement at the census tract level is susceptible to detection bias, whereby areas of high incidence may not be due to disease burden, but may be a function of early detection due to increased opportunity for screening or conversely hesitancy to screen due to medical mistrust . Second, tumor stage can be dependent on local screening resources since screening is noted to detect cancers in earlier stages, as well as the presence or absence of risk factors, and unequal genetic predisposition (as reflected in BC subtypes) by geography (Saini et al., 2019). Third, because mortality occurs at lower frequencies compared with morbidity, analyzing mortality at the neighborhood level can produce unstable results. While small area-estimation using Bayesian hierarchical spatio-temporal methods can be used to deal with unstable rates, generating them is methodologically challenging (Lawson, 2021), and might not be possible for all organizations conducting community-based cancer interventions.
Cancer outcomes do not always drive the selection of neighborhoods for cancer prevention initiatives, as this is sometimes based on neighborhood demographics. This may be due to many reasons including instability of cancer outcome rates due to low numbers of cases or deaths by neighborhood (Beyer et al., 2012), or difficulty accessing the underlying cancer outcome data and relative ease of obtaining neighborhood demographics via the U.S. Census. These demographics may be used to identify underserved communities composed of residents who are at increased risk for cancer and may benefit from cancer prevention interventions. Demographic-focused interventions are very important as the effectiveness of cancer prevention interventions varies dramatically based on age, education, and culture. However, consideration of cancer outcomes would allow area-based prevention interventions to focus on prevention efforts among neighborhood residents living in areas with the greatest BC burden. While BC outcomes at the neighborhood level including stage (Islami et al., 2013, Lin and Wimberly, 2017), incidence (American Cancer Society, 2017, Rushton et al., 2004), and mortality (Rushton et al., 2004, Smith and Madak-Erdogan, 2018) are often correlated with sociodemographic factors, this is not always the case (Brantley-Sieders et al., 2012).
We piloted a novel method to measure the burden of BC within neighborhoods in Philadelphia using an index composed of BC morbidity, American Joint Commission on Cancer (AJCC) stage, and mortality. In addition to other place-and-context-specific considerations, this BC composite score could be used by organizations to help prioritize the neighborhoods chosen for cancer prevention interventions. In this paper, we 1) describe the calculation of the BC composite score; 2) describe the geographic distribution of the neighborhoods in Philadelphia with the greatest burden of BC; and 3) identify if the same neighborhoods with the greatest burden of BC are those with characteristics (high percentage African American and low median household income) that are often used by agencies when deciding geographic areas of focus in U.S. cities.
2. Methods
2.1. Data Sources
We accessed information about cases of BC from the Pennsylvania Cancer Registry (PCR) that were diagnosed during 2005–2014 (N = 11,024). The PCR provides information on cases and deaths due to BC, including grade and stage, and demographics such as age, sex, race, and address. The cases included histologies for invasive breast cancer, with no in-situ cases included. For health outcomes requiring population denominators, we used U.S. census data from the Decennial Census (2010), by Philadelphia Census tracts (CTs). We used CTs to quantify BC-related health outcomes because accurate population data are accessible for CTs and could be used for standardized rate calculations. We used the indirect method in order to age-standardize morbidity and mortality rates by census tract in Philadelphia. We used age-stratified U.S. breast cancer mortality and morbidity rates 2010 Surveillance Epidemiology and End Results (SEER) data as reference data for age-adjustment (NCI, N.D.). We used U.S. Census American Communities Survey (2010–2014) data 5-year estimates to identify inflation adjusted median household income estimates, and the Percent- One Race- Black or African American variable from the 2010 decennial census, to identify income and percentage African American residents, respectively, by CT in Philadelphia. The study was approved by the Thomas Jefferson University Institutional Review Board.
2.2. Sample
We cleaned the cancer registry data, correcting address misspellings. First, we ensured that each patient was included only once, as there were 363 cases with recurring breast cancer at a later date within the time period. Second, we removed cases without residential address information, including PO Boxes. We limited the dataset to Philadelphia residents only, and removed males from the dataset (N = 10,499). After geocoding female BC patients with ArcMap 10.8 offline, via Philadelphia street-centerline files, we matched 10,240 (97.5%) patients to an address in Philadelphia. We aggregated cases by Philadelphia’s 384 CTs. For analysis, we removed census tracts that are primarily industrial in focus and non-residential. We focused only on CTs where 300 or more women ages 20 + resided, based on the 2010 Census, so that sufficient numbers of women could be available to benefit from potential neighborhood-based initiatives aimed to reduce the burden of BC. We did this to ensure that the areas selected were not only identified by BC burden alone, but also considered the volume of people that would benefit from BC prevention interventions.
2.3. Measures
We used information on AJCC stage version 6, the most recent and complete version provided by PCR at the time, to characterize the stage of the diagnosed BC among cases. We created an ordinal variable based on the AJCC stage group display value, representing lower to higher diagnosed stage for each patient. To identify the CTs with the highest percentage of African American residents, we used the Percent-One Race- Black or African American variable from the 2010 decennial census. To identify CTs with the lowest income, we used the inflation-adjusted Median Household Income in dollars 5-year estimates (2010–2014) from the American Communities Survey.
2.4. Statistical analysis
We focused on three BC outcomes to quantify at the CT level: standardized incidence ratio (SIR), standardized mortality ratio (SMR), and mean AJCC stage grouping. Using 2010 SEER data that described the age-stratified BC mortality rates as our reference data, we calculated SIRs and SMRs for each CT. We aggregated the ordinal BC stage grouping for cases by CT to create a mean stage grouping for each CT. For CTs containing 300 or more women ages 20+ (n = 373), we created a BC composite variable by summing the SMR, SIR, and mean stage grouping variables, each centered and scaled by their respective means and standard deviations. Creating the BC composite this way allowed us to assign equal weight to each of the three component variables. We calculated descriptive statistics for each of our outcomes. We mapped CTs with the highest 25 composite scores to identify the locations of neighborhoods with the greatest burden of BC. We then mapped CTs with the 25 highest percentage of individuals that identified as African American, lowest median household income, and then compared these CTs with those exhibiting the highest 25 BC composite scores. Although we generated the BC composite scores for most neighborhoods in Philadelphia, we chose to map CTs with the highest 25 composite scores to highlight a number of neighborhoods that might be useful for cancer prevention organizations, such as a mobile cancer screening program.
3. Results
Incidence: 373 of the 384 CTs in Philadelphia had at least 300 women over 20 years of age. Among the 373 CTs, 372 had at least one case of BC from 2005 to 2014. Among the 373 CTs, the mean number of incident BC cases was 27.39 (Standard Deviation (SD) = 14.1); min = 0, max = 72) and the mean SIR was 0.95 (SD = 0.24; min = 0, max = 1.71).
Mortality: Among the 373 CTs, 343 had at least one BC death. The mean number of deaths among the 373 CTs was 4.05 (SD = 2.85; min = 0, max = 15). The mean SMR was 0.81 (SD = 0.58; min = 0, max = 5.52).
AJCC Stage Grouping: For the 375 CTs with cases, we calculated the mean stage grouping of the cases within the CT. Among the CTs, the (mean) mean stage grouping was 3.45 (SE = 0.03; min = 0, max = 8).
BC Composite Score: After centering and scaling the SIR, SMR, and mean stage grouping variables by their respective means and standard deviations, we summed these measures to calculate the BC composite score for each CT. The mean BC composite score was 0.00 (SD = 2.12; min = -11.22, max = 13.04). We provide a histogram to further describe the distribution of the BC composite score among the 373 CTs (Fig. 1).
Fig. 1.
Distribution of BC Composite Score among Census Tracts in Philadelphia: 2005–2014 (N = 373).
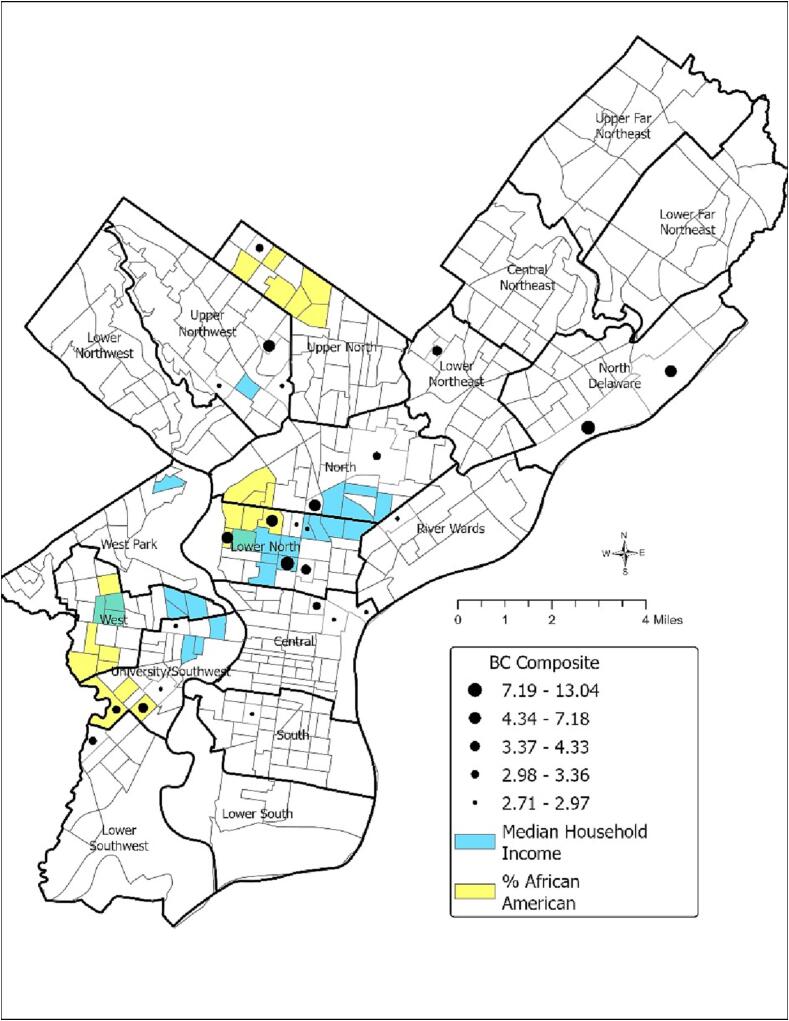
Neighborhood Prioritization and Comparisons: The graduated points in Fig. 2 show the 25 neighborhoods with the highest BC composite scores, representing those with the worst BC outcomes among Philadelphia CTs from 2005 to 2014. All of the CTs with the highest 25 BC composite scores were found in just 10 of the 18 total planning districts in Philadelphia; Upper Northwest, Upper North, Lower Northeast, North Delaware, River Wards, North, Lower North, Central, South, and University Southwest. In fact, 17 of the CTs with the highest BC composite scores were concentrated in four planning districts; Lower North contained 6 CTs, University/Southwest contained 5, and Upper Northwest and Central both contained 3 CTs with the highest BC composite scores.
Fig. 2.
(Color): Census Tracts in Philadelphia with the 25 highest Breast Cancer Composite, 2005–2014; the 25 highest percentage of African American Residents; and the 25 lowest Median Household Incomes.
Fig. 2 also displays the census tracts with the highest percentage of African American residents (yellow) and the lowest median household income (blue), among all 373 CTs. None of the CTs had all three characteristics: among the top 25 BC composite, highest percentage of African Americans, and lowest median household income. Four of the CTs with one of the highest 25 BC composite scores were also CTs with one of the 25 highest percentage of African Americans. Two of the CTs with the highest 25 BC composite scores were also among the CTs with the lowest median household incomes.
Table 1 further characterizes the CTs with the 25 highest BC composite scores, highest percentage of African American residents, and lowest median household incomes in Philadelphia. The mean BC composite score among the 25 highest CTs was 4.41, whereas the mean BC composite scores among the CTs with the highest percentage of African American residents and lowest median incomes were 0.88 and −0.29, respectively. Notably, one of the CTs with the highest BC composite scores did not have a recorded median household income. In the CTs with the highest 25 BC composite scores, the majority of residents were African American (Mean = 69.37%), but the mean percentage was much lower than the mean among those CTs with the highest 25 percent African Americans (Mean = 96.1%). Additionally, the CTs with the highest 25 BC composite scores had a higher average median income ($31,129) compared to the average among CTs with the lowest 25 median household incomes in Philadelphia ($14,079).
Table 1.
Census Tracts in Philadelphia with the Highest Breast Cancer Composite Scores, Highest Percentage (%) of African American Residents and Lowest Median Household Incomes: 2005–2014.
| Highest BC Composite Scores | Highest % African American | Lowest Median Household Income | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Tract | BC Composite Score | % African American | Median Household Income ($) | Tract | BC Composite Score | % African American | Median Household Income ($) | Tract | BC Composite Score | % African American | Median Household Income ($) |
| 1 | 38100 | 13.04 | 26.9 | 25,139 | 26600 | 0.95 | 97.0 | 39,500 | 08802 | −2.98 | 11.5 | 8,993 |
| 2 | 14700 | 12.13 | 43 | 11,578 | 16902 | 1.92 | 96.8 | 21,035 | 09000 | 2.4 | 8.0 | 10,807 |
| 3 | 89100 | 7.18 | 71.7 | – | 15102 | 2.02 | 96.6 | 12,241 | 14700a | 12.13 | 43.0 | 11,578 |
| 4 | 17400 | 5.32 | 91 | 18,319 | 16901 | −0.4 | 96.5 | 25,292 | 08801 | −11.22 | 9.2 | 11,944 |
| 5 | 24800 | 5.3 | 95.3 | 38,048 | 10200 | −0.54 | 96.5 | 13,183 | 14800 | −2.97 | 90.1 | 11,989 |
| 6 | 16800 | 5.23 | 95.8 | 27,574 | 07102 | 1.34 | 96.4 | 28,286 | 15102 | 2.02 | 96.6 | 12,241 |
| 7 | 15101 | 5.09 | 96.1 | 21,000 | 08102 | −2.38 | 96.4 | 32,478 | 16300 | −0.77 | 18.4 | 12,343 |
| 8 | 14600 | 4.33 | 52.2 | 24,833 | 17100 | 0.44 | 96.3 | 28,811 | 16400 | −2.18 | 41.7 | 12,436 |
| 9 | 07000 | 4.33 | 95.4 | 26,685 | 09400 | −1.75 | 96.3 | 16,753 | 19501 | −1.8 | 16.8 | 12,791 |
| 10 | 30501 | 3.9 | 54.2 | 33,814 | 15101a | 5.09 | 96.1 | 21,000 | 13900 | 1.94 | 82.5 | 12,865 |
| 11 | 13100 | 3.36 | 72.9 | 39,868 | 17202 | 0.47 | 96.1 | 21,875 | 10200 | −0.54 | 96.5 | 13,183 |
| 12 | 26000 | 3.27 | 95.1 | 58,947 | 26500 | 0.46 | 96.1 | 36,818 | 10600 | −1.74 | 90.2 | 13,342 |
| 13 | 06500 | 3.25 | 95.7 | 24,350 | 26400 | −1.75 | 96.1 | 39,145 | 16500 | 1.25 | 91.4 | 14,894 |
| 14 | 38300 | 3.13 | 36.2 | 21,250 | 09500 | 0.75 | 96.0 | 16,383 | 17702 | −2.58 | 21.2 | 14,907 |
| 15 | 06400 | 3.06 | 76.3 | 34,153 | 17201 | 0.73 | 96.0 | 21,096 | 10800 | 1.16 | 87.3 | 15,392 |
| 16 | 14200 | 2.97 | 8.3 | 80,154 | 16800a | 5.23 | 95.8 | 27,574 | 15300 | 0.15 | 45.4 | 15,609 |
| 17 | 16702 | 2.94 | 89.1 | 20,565 | 08200 | −0.4 | 95.8 | 38,617 | 17500 | 1.22 | 31.6 | 15,743 |
| 18 | 36700 | 2.89 | 16.1 | 83,086 | 26700 | −1.31 | 95.8 | 32,593 | 16600a | 2.78 | 75.6 | 15,855 |
| 19 | 07400 | 2.89 | 88.1 | 20,317 | 06500a | 3.25 | 95.7 | 24,350 | 10700 | −1.68 | 95.1 | 16,105 |
| 20 | 17800 | 2.86 | 28.9 | 20,484 | 26301 | 1.02 | 95.6 | 40,808 | 12201 | −0.96 | 37.9 | 16,107 |
| 21 | 03100 | 2.8 | 87 | 25,588 | 11200 | −0.25 | 95.6 | 23,936 | 15200 | 0.04 | 94.2 | 16,195 |
| 22 | 16600 | 2.78 | 75.6 | 15,855 | 08101 | 1.51 | 95.5 | 24,759 | 09500 | 0.75 | 96.0 | 16,383 |
| 23 | 23900 | 2.78 | 73.1 | 27,583 | 26100 | −0.22 | 95.5 | 49,156 | 17601 | 0.89 | 11.7 | 16,531 |
| 24 | 09200 | 2.75 | 77.1 | 20,357 | 07000a | 4.33 | 95.4 | 26,685 | 09400 | −1.75 | 96.3 | 16,753 |
| 25 | 24500 | 2.71 | 93.1 | 27,556 | 08302 | 1.45 | 95.4 | 30,075 | 24100 | −2.40 | 80.5 | 16,985 |
| Mean | 4.41 | 69.37 | 31,129 | 0.88 | 96.1 | 27,698 | −0.28 | 58.7 | 14,079 | |||
Also one of the census tracts with the highest 25 BC composite scores.
4. Discussion
This study introduces a new method of measuring the burden of BC at the neighborhood-level using the BC composite score that is composed of BC morbidity, mortality, and AJCC stage grouping. We identified that the burden of BC in Philadelphia was not evenly geographically distributed during 2005–2014, with 17 of the neighborhoods with the greatest burden located in just four planning districts, Lower North, University/Southwest, Upper Northwest and Central. Our results are important for identifying neighborhoods and planning districts that should be the focus for BC prevention efforts. Philadelphia has three major hospital systems—Thomas Jefferson University, University of Pennsylvania, and Temple University (including Fox Chase Cancer Center)—all of which conduct community outreach, education events, and mammography screenings via mobile screening services. The efforts of these outreach and screening programs would benefit from the identification of neighborhoods with the greatest BC burden in Philadelphia so that screening efforts can be targeted.
Our findings show that using the BC composite is an important tool to choose neighborhoods with the greatest burden, compared to decision-making based solely on race or income. On one hand, the planning districts that contained the CTs with the highest BC composite scores were also areas that had high percentages of African Americans and low median household incomes, compared to other planning districts in Philadelphia. However, when comparing the BC composite with demographics at the CT level, we see few overlaps with the highest percentage of African Americans or the lowest median incomes. There was more overlap among CTs with the highest BC composite and highest percentage African American CTs (4) compared with CTs with the highest BC composite and lowest household income (2).
Researchers have clearly identified associations between race, income, and BC outcomes. However, due to multifactorial causal mechanisms for BC, these relationships differ depending upon the BC outcome analyzed. For example, studies show that higher income is associated with higher BC incidence among populations (Lehrer et al., 2016). Primarily this relationship is due to early detection among people with higher income and ease of ability to access to healthcare resources to find cancers earlier when they are more easily treatable. However, the opposite relationship has been identified for income and BC mortality- as income increases, mortality rates decrease and survival time increases (Gordon et al., 1992), although mortality studies have not shown as consistent a relationship as morbidity studies (Akinyemiju et al., 2015). Studies that explore relationships between race and incidence have shown varying results, with associations varying by demographic, environmental exposure, or healthcare access. However, after controlling for confounding factors, researchers have found that BC mortality is higher among Black women compared to White women. Further, studies have identified that race modified the effect of income and 5-year BC survival; as income increased, 5-year survival significantly increased, but only among White women, not Black women (Lehrer et al., 2016). These inconsistent relationships between race, income and individual BC outcomes support the use of our BC composite which combines morbidity, mortality and stage grouping, to characterize neighborhood burden of BC, and as an additional tool to use for neighborhood-based cancer control prioritization.
Our study is not without limitations. First, although we assume that all cases of BC were captured by the PA Cancer Registry, we have no way of confirming the extent to which it is complete. Second, similar to the McIntire et al. (2018) study on prostate cancer, some census tracts in Philadelphia had small numbers of cases and deaths. Using each of these outcomes by themselves to characterize burden of disease, without using statistical procedures to stabilize rates, would not be adequate. However, because we calculated the BC composite as a sum of SIR, SMR, and stage grouping, we bolster the distortion caused by unstable rates at the CT level.
Our findings show that using race and income as a proxy for BC burden is not ideal, considering the lack of overlap between the CTs with the top 25 BC composite and those with the highest percentage of African American residents and lowest household income. In order to measure breast cancer burden, healthcare and public health agencies implementing community-based BC interventions should consider using the novel method of a BC composite as a tool to identify areas of priority for BC prevention interventions, including education, screening, and treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Thank you to Pam Walter for your timely and thorough edits on the manuscript.
This work was supported in part by the NCI Cancer Center Grant P30CA056036 for supporting the Biostatistics core facility and by R01CA227479-S1 (NLS).
Data availability
The authors do not have permission to share data.
References
- Akinyemiju T.F., Genkinger J.M., Farhat M., Wilson A., Gary-Webb T.L., Tehranifar P. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer [Internet]. 2015;15(1):191 doi: 10.1186/s12885-015-1098-z. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society Breast Cancer facts & figures 2019–2020 [Internet]. American Cancer. Society. 2017 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf Available from: [Google Scholar]
- American Cancer Society Cancer facts & figures 2022 [Internet]. American Cancer. Society. 2022 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf Available from: [Google Scholar]
- Berry D.A., Cronin K.A., Plevritis S.K., Fryback D.G., Clarke L., Zelen M., Mandelblatt J.S., Yakovlev A.Y., Habbema J.D.F., Feuer E.J. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med [Internet]. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bevers T.B., Helvie M., Bonaccio E., Calhoun K.E., Daly M.B., Farrar W.B., Garber J.E., Gray R., Greenberg C.C., Greenup R., Hansen N.M., Harris R.E., Heerdt A.S., Helsten T., Hodgkiss L., Hoyt T.L., Huff J.G., Jacobs L., Lehman C.D., Monsees B., Niell B.L., Parker C.C., Pearlman M., Philpotts L., Shepardson L.B., Smith M.L., Stein M., Tumyan L., Williams C., Bergman M.A., Kumar R. Breast Cancer Screening and Diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw [Internet]. 2018;16(11):1362–1389. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- Beyer K.M.M., Tiwari C., Rushton G. Five essential properties of disease maps. Ann Assoc Am Geogr [Internet]. 2012;102(5):1067–1075. doi: 10.1080/00045608.2012.659940. Available from: [DOI] [Google Scholar]
- Brantley-Sieders DM, Fan K-H, Deming-Halverson SL, Shyr Y, Cook RS. Local breast cancer spatial patterning: a tool for community health resource allocation to address local disparities in breast cancer mortality. PLoS One [Internet]. 2012;7(9):e45238. Available from: http://dx.doi.org/10.1371/journal.pone.0045238. [DOI] [PMC free article] [PubMed]
- Breast cancer statistics [Internet]. Centers for Disease Control and Prevention. 2020a. Available from: https://www.cdc.gov/cancer/breast/statistics/.
- Community Prevention Services Task Force. Increasing breast cancer screening: Multicomponent interventions [Internet]. Community Prevention Services Task Force. 2016. Available from: https://www.thecommunityguide.org/sites/default/files/assets/Cancer-Screening-Multicomponent-Breast.pdf.
- Crabbe J.C.F., Gregorio D.I., Samociuk H., Swede H. Secular trends, race, and geographic disparity of early-stage breast cancer incidence: 25 years of surveillance in Connecticut. Am J Public Health [Internet]. 2015;105 Suppl 3(S3):e64–e70. doi: 10.2105/AJPH.2015.302640. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Oyana T.J. Spatial variations in the incidence of breast cancer and potential risks associated with soil dioxin contamination in Midland, Saginaw, and Bay Counties, Michigan, USA. Environ Health. 2008;7(1) doi: 10.1186/1476-069X-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis, CE, Ma, J, Gaudet, MM, Newman, LA, Miller, KD, Goding Sauer, A, et al., 2019. Breast cancer statistics, 2019. CA Cancer J Clin [Internet] 69 (6), 438–51.Available from: 10.3322/caac.21583. [DOI] [PubMed]
- Diez Roux A.V., Mair C. Neighborhoods and health: Neighborhoods and health. Ann N Y Acad Sci [Internet]. 2010;1186(1):125–145. doi: 10.1111/j.1749-6632.2009.05333.x. Available from: [DOI] [PubMed] [Google Scholar]
- Gordon N.H., Crowe J.P., Brumberg D.J., Berger N.A. Socioeconomic factors and race in breast cancer recurrence and survival. Am J Epidemiol [Internet]. 1992;135(6):609–618. doi: 10.1093/oxfordjournals.aje.a116340. Available from: [DOI] [PubMed] [Google Scholar]
- Huerta E.E., Weeks-Coulthurst P., Williams C., Swain S.M. Take care of your neighborhood. Breast Cancer Res Treat [Internet]. 2018;167(1):225–234. doi: 10.1007/s10549-017-4492-1. Available from: [DOI] [PubMed] [Google Scholar]
- Humphrey L.L., Helfand M., Chan B.K.S., Woolf S.H. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med [Internet]. 2002;137(5 Part 1):347–60 doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. Available from: [DOI] [PubMed] [Google Scholar]
- Islami F., Kahn A.R., Bickell N.A., Schymura M.J., Boffetta P. Disentangling the effects of race/ethnicity and socioeconomic status of neighborhood in cancer stage distribution in New York City. Cancer Causes Control [Internet]. 2013;24(6):1069–1078. doi: 10.1007/s10552-013-0184-2. Available from: [DOI] [PubMed] [Google Scholar]
- Lawson A.B. 3rd ed. CRC Press; London, England: 2021. Bayesian disease mapping: Hierarchical modeling in spatial epidemiology, third edition. [Google Scholar]
- Lehrer S., Green S., Rosenzweig K.E. Affluence and breast cancer. Breast J [Internet]. 2016;22(5):564–567. doi: 10.1111/tbj.12630. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wimberly M.C. Geographic variations of colorectal and breast cancer late-stage diagnosis and the effects of neighborhood-level factors. J Rural Health [Internet]. 2017;33(2):146–157. doi: 10.1111/jrh.12179. Available from: [DOI] [PubMed] [Google Scholar]
- U.S. Preventative Services Task Force. Final recommendation statement: Breast cancer: Screening [Internet]. Uspreventiveservicestaskforce.org. [cited 2022 Apr 22]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1.
- MacKinnon JA, Duncan RC, Huang Y, Lee DJ, Fleming LE, Voti L, et al. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Biomarkers Prev [Internet]. 2007;16(4):756–62. Available from: http://dx.doi.org/10.1158/1055-9965.EPI-06-0392. [DOI] [PubMed]
- Markossian T.W., Hines R.B., Bayakly R. Geographic and Racial Disparities in Breast Cancer-Related Outcomes in Georgia. Health Serv Res. 2014;49(2):481–501. doi: 10.1111/1475-6773.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire R.K., Keith S.W., Boamah M., Leader A.E., Glanz K., Klassen A.C., Zeigler-Johnson C.M. A Prostate Cancer Composite Score to Identify High Burden Neighborhoods. Prev Med. 2018 Jul;112:47–53. doi: 10.1016/j.ypmed.2018.04.003. Epub 2018 Apr 4 PMID: 29625131. [DOI] [PubMed] [Google Scholar]
- Mobley L.R., Kuo T.-M., Scott L., Rutherford Y., Bose S. Modeling geospatial patterns of late-stage diagnosis of breast cancer in the US. Int J Environ Res Public Health [Internet]. 2017;14(5) doi: 10.3390/ijerph14050484. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review 1975-2014 [Internet]. Cancer.gov. Available from: https://seer.cancer.gov/archive/csr/1975_2014/results_merged/sect_04_breast.pdf.
- Nelson H.D., Tyne K., Naik A., Bougatsos C., Chan B., Nygren P., Humphrey L. Agency for Healthcare Research and Quality (US); Rockville (MD): 2009 Nov. Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force [Internet] Report No.: 10–05142-EF-1. [PubMed] [Google Scholar]
- Oeffinger K.C., Fontham E.T.H., Etzioni R., Herzig A., Michaelson J.S., Shih Y.-C., Walter L.C., Church T.R., Flowers C.R., LaMonte S.J., Wolf A.M.D., DeSantis C., Lortet-Tieulent J., Andrews K., Manassaram-Baptiste D., Saslow D., Smith R.A., Brawley O.W., Wender R. Breast cancer screening for women at average risk: 2015 Guideline update from the American cancer society. JAMA [Internet]. 2015;314(15):1599. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M.A., Westcombe A.M., Love S.B., Littlejohns P., Ramirez A.J. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet [Internet]. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. Available from: [DOI] [PubMed] [Google Scholar]
- Rushton G., Peleg I., Banerjee A., Smith G., West M. Analyzing geographic patterns of disease incidence: rates of late-stage colorectal cancer in Iowa. J Med Syst [Internet]. 2004;28(3):223–236. doi: 10.1023/b:joms.0000032841.39701.36. Available from: [DOI] [PubMed] [Google Scholar]
- Saini G., Ogden A., McCullough L.E., Torres M., Rida P., Aneja R. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control [Internet]. 2019;30(7):677–686. doi: 10.1007/s10552-019-01180-4. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L., Mobley L.R., Il’yasova D. Geospatial analysis of inflammatory breast cancer and associated community characteristics in the United States. Int J Environ Res Public Health [Internet]. 2017;14(4) doi: 10.3390/ijerph14040404. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sighoko D, Hunt BR, Irizarry B, Watson K, Ansell D, Murphy AM. Disparity in breast cancer mortality by age and geography in 10 racially diverse US cities. Cancer Epidemiol [Internet]. 2018;53:178–83. Available from: http://dx.doi.org/10.1016/j.canep.2018.02.003. [DOI] [PMC free article] [PubMed]
- Siu AL, U.S. Preventive Services Task Force Screening for breast cancer: U.s. preventive Services Task Force recommendation statement. Ann Intern Med [Internet]. 2016;164(4):279–296. doi: 10.7326/M15-2886. Available from: [DOI] [PubMed] [Google Scholar]
- Smith BP, Madak-Erdogan Z. Abstract 3012: Urban neighborhood and residential factors associated with breast cancer in African American women: A systematic review. Cancer Res [Internet]. 2018;78(13 Supplement):3012–3012. Available from: http://dx.doi.org/10.1158/1538-7445.am2018-3012. [DOI] [PMC free article] [PubMed]
- Strömberg U., Peterson S., Holmén A., Holmberg E., Hultcrantz R., Martling A., Nilbert M. Rational targeting of population groups and residential areas for colorectal cancer screening. Cancer Epidemiol [Internet]. 2019;60:23–30. doi: 10.1016/j.canep.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Tatalovich Z., Zhu L., Rolin A., Lewis D.R., Harlan L.C., Winn D.M. Geographic disparities in late stage breast cancer incidence: results from eight states in the United States. Int J Health Geogr [Internet]. 2015;14(1):31 doi: 10.1186/s12942-015-0025-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.