Abstract
The retirement phase is an opportunity to integrate healthy (nutrition/exercise) habits into daily life. We conducted this systematic review to assess which nutrition and exercise interventions most effectively improve body composition (fat/muscle mass), body mass index (BMI), and waist circumference (WC) in persons with obesity/overweight near retirement age (ages 55–70 y). We conducted a systematic review and network meta-analysis (NMA) of randomized controlled trials, searching 4 databases from their inception up to July 12, 2022. The NMA was based on a random effects model, pooled mean differences, standardized mean differences, their 95% confidence intervals, and correlations with multi-arm studies. Subgroup and sensitivity analyses were also conducted. Ninety-two studies were included, 66 of which with 4957 participants could be used for the NMA. Identified interventions were clustered into 12 groups: no intervention, energy restriction (i.e., 500–1000 kcal), energy restriction plus high-protein intake (1.1–1.7 g/kg/body weight), intermittent fasting, mixed exercise (aerobic and resistance), resistance training, aerobic training, high protein plus resistance training, energy restriction plus high protein plus exercise, energy restriction plus resistance training, energy restriction plus aerobic training, and energy restriction plus mixed exercise. Intervention durations ranged from 8 wk to 6 mo. Body fat was reduced with energy restriction plus any exercise or plus high-protein intake. Energy restriction alone was less effective and tended to decrease muscle mass. Muscle mass was only significantly increased with mixed exercise. All other interventions including exercise effectively preserved muscle mass. A BMI and/or WC decrease was achieved with all interventions except aerobic training/resistance training alone or resistance training plus high protein. Overall, the most effective strategy for nearly all outcomes was combining energy restriction with resistance training or mixed exercise and high protein. Health care professionals involved in the management of persons with obesity need to be aware that an energy-restricted diet alone may contribute to sarcopenic obesity in persons near retirement age.
This network meta-analysis is registered at https://www.crd.york.ac.uk/prospero/ as CRD42021276465.
Keywords: obesity, overweight, retirement, network meta-analysis, caloric restriction, resistance training, body composition, body mass index, fasting
Statement of significance.
This comprehensive network meta-analysis uniquely focuses on people near retirement age which have a great potential for implementing healthy nutrition and exercise habits during this transition phase. The methodology allowed an indirect comparison to be included in the statistical model, therefore further enhancing the significance and coverage of the model.
Introduction
Overweight and obesity are serious disorders with prevalence rates among older Europeans of about 60% and 20%, respectively [1]. In the United States, the obesity prevalence is even higher at around 40% [2]. These rates have steadily increased worldwide over the last 40 y in men and women [[2], [3], [4], [5]]. Its high prevalence and serious social, economic, and health consequences make it one of the major global health problems [[6], [7], [8]]. Obesity is a major risk factor for several diseases, including type 2 diabetes mellitus, coronary artery disease, cerebral vascular disease, arterial hypertension, dyslipidemia, and several types of cancer. All of these conditions contribute to a reduction in both the quality of life and life expectancy [7,8]. For example, an increase in a society’s BMI by 2 points shortens the life expectancy by 0.7 to 1 y [9]. Furthermore, obesity is accompanied by burdens such as falls, disability, or care dependency, especially in older adults [7,8,10].
Obesity is characterized by excessive fat accumulation [11] that often occurs during the process of aging. This especially occurs in persons aged 45 to 70 y, with a weight peak observed at middle age, i.e., 50 to 65 y of age [8,[12], [13], [14]]. Aging is accompanied not only by a gradual increase in body fat (BF) stores but also a decrease in muscle mass, muscle function, and water retention. Simultaneously suffering from obesity and the progression of the aging process can lead to sarcopenic obesity, a condition that combines the loss of muscle mass, strength, and function with an increase in adiposity [15,16]. This affects a remarkably large group of people, with prevalence rates of up to 33.5% observed, e.g., in the older US population [17], which suggests that many obese people simulateously suffer from sarcopenia. Because loss of muscle mass is often accompanied by an increase in fat mass, body weight may remain stable [18, 19], meaning that a stable or even decreasing body weight can mask increasing adiposity [19].
The retirement age is generally between 48 and 67 y in the Organization for Economic Co-operation and Development countries [20]; this is exactly in the age range during which the previously described major changes in body composition occur. Therefore, the retirement phase represents a window of opportunity to decelerate the associated deterioration in body composition. This phase is a period of change. In most cases, this change is not gradual, but occurs abruptly one day when a person no longer needs to go to work and needs to start redesigning their everyday life [12]. A recent longitudinal study showed that 61% of people in this age group changed their lifestyle during the retirement phase [21]. People that changed their lifestyle by reducing risk factors for obesity, such as poor diet or inactivity, showed smaller physical declines over time in later life. This finding underlines the great potential for implementing healthy nutrition and exercise habits and thus increasing the disability-free life expectancy in the retirement phase [21].
Nutrition and exercise interventions are considered as first-line therapies for treating individuals with overweight and obesity [4,11,22]. These should not only be effective in reduction of BF but also in preserving muscle mass. This is even more important in older adults to prevent the occurence of disability [11]. Several systematic reviews have summarized different nutrition and exercise interventions in older persons with overweight and obesity [[23], [24], [25], [26], [27], [28], [29], [30]], but we identified only one that focused on people near retirement age [23]. This review, however, examined the effectiveness of dietary interventions on healthy eating habits and not on obesity parameters such as body composition or anthropometric parameters.
Thus, prior to the current review, a comprehensive systematic review and network meta-analysis (NMA) of the effects of nutrition and exercise interventions in persons near retirement age was lacking. Such an analysis is highly beneficial for the scientific community and clinical practice, because the results can provide recommendations for effective interventions for people who are overweight or obese in this target group. The specific aim of conducting this systematic review using NMA methodology was to assess which nutrition and exercise interventions are most effective for improving the body composition (fat mass and muscle mass), BMI, and waist circumference (WC) in persons with overweight or obesity near retirement age (55 to 70 y of age).
Methods
This systematic review and NMA of randomized controlled trials (RCTs) has been registered in PROSPERO (International Prospective Register of Systematic Reviews, https://www.crd.york.ac.uk/prospero/, identifier CRD42021276465). The principles of the Preferred Reporting Items for Systematic Review and Meta-analysis 2021 were applied for reporting NMAs [31].
Search strategy
We conducted a comprehensive literature search to identify RCTs that had been published in PubMed (MEDLINE), EMBASE via OVID, CINAHL via EBSCO Host, and the Cochrane Central Register of Controlled Trails (CENTRAL) via the Cochrane Library from the inception of these databases and up to July 12, 2022. No language or calendar date restrictions were set. In addition, we conducted a manual search of reference lists from eligible studies and Google Scholar. We also searched for gray literature in the online platforms available via https://clinicaltrials.gov/ and the WHO International Clinical Trials Registry (ICTRP). The literature review was conducted by 2 authors (MT, SB) independently.
We used the following search terms: “obesity,” “diet,” “exercise,” “train∗,” and “physical activity.” Additionally, we used the following MeSH terms: “obesity,” “diet,” and “exercise.” As part of the search strategy, the search terms were combined using the Boolean operators AND and OR. We applied filters for RCTs and the respective age group (middle-aged and aged) and made small adaptations for each database searched (see Supplementary Table 1).
Study selection
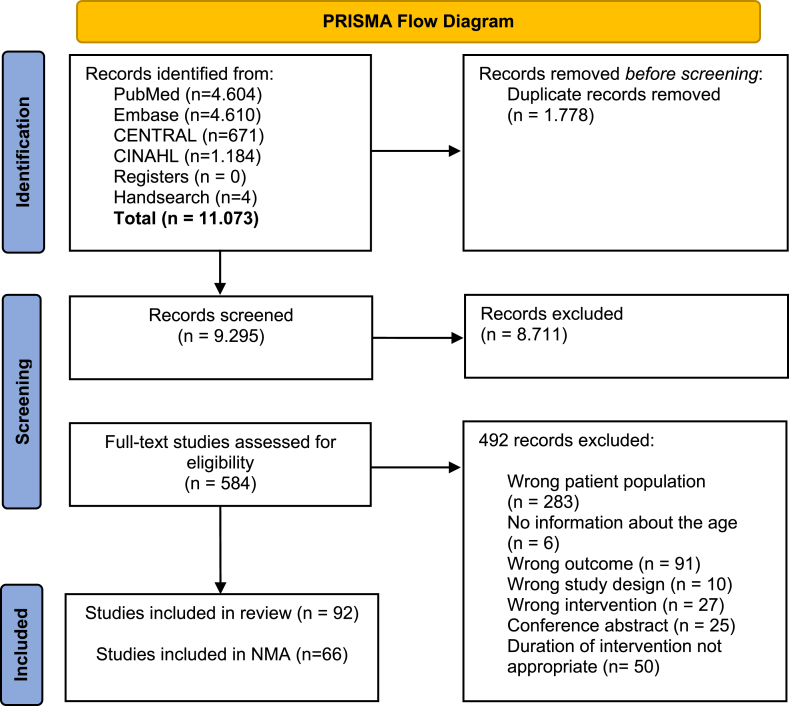
The study selection process was conducted with the systematic review software COVIDENCE (Veritas Health Innovation). We included RCTs using a parallel or crossover design, based on our predefined Population, Intervention, Control, Outcome (PICO) question (Table 1). We excluded studies focusing on persons with specific health conditions, such as cancer, type 2 diabetes, heart failure, or pulmonary diseases, as well as studies with specific target groups, such as soccer players or truck drivers. We further excluded RCTs with pharmaceutical or behavioral interventions other than nutrition or activity interventions for obesity and studies that lacked a clear description of the intervention and weight maintenance studies. Title and abstract screening as well as full-text screening were performed based on inclusion and exclusion criteria by 2 authors independently of one another (MT, SB, DE). The numbers and reasons for the exclusion of studies are listed in the flow chart (see Figure 1). Any disagreements were resolved by a discussion involving a third person (DE).
TABLE 1.
Inclusion criteria for RCTs based on the PICO question
| Parameters | Search strategy |
|---|---|
| Participants | Community dwelling, persons with overweight or obesity (BMI > 25 to 40 kg/m2) near the retirement age (mean age in the single studies between 55 and 70 y, independent of the CI) |
| Intervention | Any nutrition or exercise intervention for a duration between 8 wk and 6 mo |
| Control | No intervention, any other intervention |
| Outcomes | BF in kg, %BF, LBM/FFM, BMI, WC |
| Study design | Randomized controlled trials |
Abreviations: BF, body fat; WC, waist circumference.
FIGURE 1.
PRISMA flow diagram of the literature review and study selection process
Data extraction and quality assessment
Two reviewers independently extracted data from the final included full-text articles, and disagreements were resolved by a discussion involving a third person. We generated a standardized data extraction template, including the study characteristics, patient characteristics, intervention(s), adherence to the intervention(s), and patient outcomes. Prior to the data extraction, we piloted the template of 2 studies to identify and edit possible shortcomings in the template. The methodological quality of the RCTs was assessed by 2 independent reviewers using the Cochrane Risk of Bias tool, referring to the Cochrane Handbook for Systematic Reviews of Interventions [32].
Data synthesis: interventions
Based on the different interventions used in the identified studies, we created 12 pragmatic intervention/control categories: 1) no intervention, 2) energy restriction (i.e., caloric restriction of 500 to 1000 kcal), 3) energy restriction plus high protein intake (1.1–1.7 g/kg body weight/d), 4) intermittent fasting (5:2 diet), 5) mixed exercise (aerobic and resistance training), 6) resistance training, 7) aerobic training, 8) high-protein intake plus resistance training, 9) energy restriction plus high-protein intake plus exercise, 10) energy restriction plus resistance training, 11) energy restriction plus aerobic training, and 12) energy restriction plus mixed exercises (aerobic and resistance training combined). Studies that could not be assigned to any of these categories and studies that included study arms comparing similar interventions that would have been in the same category were described narratively.
Statistical analysis
The change in the outcome measures was used for the statistical analysis. This change was calculated as the mean difference (MD) between the baseline and follow-up for each treatment group. Most included studies provided means and standard deviations at baseline and for specific follow-up dates. We then calculated the MD and the standard deviation of change referring to the Cochrane Handbook for Systematic Reviews of Interventions and assuming a correlation of 0.85 [33]. This value was chosen because we observed a high correlation between the baseline and follow-up measures in studies where baseline, follow-up, and change measures were reported. If not directly provided, further steps were taken to calculate the corresponding values. This included the use of P values and confidence intervals; where ranges were reported, we applied the same methodology as Hozo et al. [34]. The NMAwas based on a random effects model, and correlations in multi-arm studies were considered [35]. The common heterogeneity variance used in the random effects model was estimated with a generalized DerSimonian-Laird estimator [36]. To assess inconsistency, the between-designs Q-value was calculated based on a full design-by-treatment interaction model for random effects [37]. If studies had more than one arm belonging to the same combined treatment group, we combined the corresponding means, standard deviations, and sample sizes [38]. We used Egger’s test to appraise the data for potential publication bias, i.e., to identify asymmetry in the funnel plot [39]. For models where we compared effects within the same outcome measure, we used the MD. In models where we compared more than one outcome measure, we used the standardized mean difference (SMD) to assure comparability. For one model, we prioritized data, choosing the first available outcome measures in the following order: BF in %, BF in kg, muscle mass (LBM/ FFM), WC, then BMI. Here, we considered the different direction of positive effects for the fat mass and muscle mass.
Additionally, we performed several subgroup and sensitivity analyses. First, we analyzed data stratified by sex, from studies with only females, only males, or reporting on both sexes separately. Second, we distinguished between the duration of the intervention(s) being less or equal to 14 wk or being more than 14 wk. Third, we repeated analyses excluding all studies identified as having a high risk of bias. Fourth, we grouped the interventions even further into the following 3 categories: nutrition, exercise, or nutrition plus exercise. In all subgroup analyses, we used one fat mass as an outcome measure (body fat or BF in %) and muscle mass as an outcome measure (LBM/FFM), deciding on a case-by-case basis and using the ones that were reported more frequently in order to include the highest number of studies in the analysis. A 2-sided P value of less than 0.05 was considered to be statistically significant. All analyses were performed in R (Version 4.1.3).
Results
We identified and screened 11,073 records, from which 92 RCTs met the selection criteria (Figure 1). Of these, 6 RCTs included interventions that could not be assigned to one of the 12 intervention/control categories (i.e., different types of oil supplementation, vegan diet, and Mediterranean diet), 2 RCTs included less than 8 participants, and 18 RCTs compared interventions within the same intervention category (e.g., comparison of energy restriction with target 500 kcal and 1000 kcal). For these reasons, we included 66 studies [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105]] that involved nutrition and exercise interventions in persons with overweight/obesity near retirement age and included a total of 4957 participants in the final NMA. Figure 1 illustrates the literature review process.
Characteristics of the included RCTs
Of all 92 identified studies, we identified 21 RCTs that focused on nutrition interventions alone, 25 RCTs that focused on exercise interventions alone, and 46 RCTs that combined nutrition and exercise interventions. Overall, we identified 82 different interventions described in the studies, which we assigned to 12 categories created based on the interventions used in the primary studies.
Most studies included both male and female participants (n = 51), followed by studies that only included female participants (n = 36). The included studies were conducted in 19 different countries, and mostly in the United States (n = 49), see Table 2. The sample sizes ranged from 11 to 543 participants. The intervention period ranged from 8 wk to 6 mo, with the most common intervention periods being 12 wk (n = 33 or 36%), 26 wk (n = 21 or 23%), and 16 wk (n = 11 or 12%). Most of the exercise studies (n = 19 or 76%) performed training sessions 3 times a week for 20 to 60 min. The most common nutrition intervention was energy restriction (with or without meal replacement, mostly with a reduction in fat content and/or carbohydrates, including low- and very low-calorie diets).
TABLE 2.
Study characteristics of included studies and their assigned intervention groups
| Author (y) | Country | Sample Size | Sex | No. of Arms | Duration of Intervention | BC measurement | Intervention [Intervention Group] | Adherence | Incl. in NMA |
|---|---|---|---|---|---|---|---|---|---|
| Combined Nutrition and Exercise Interventions | |||||||||
| Amamou et al. [41] 2017 | Canada | 26 | Male + Female | 2 | 16 wk | DXA | Not mentioned | Yes | |
| Amati et al. [42] 2008 | United States | 64 | Male + Female | 3 | 16 wk | DXA | Not mentioned | Yes | |
| Avila et al. [43] 2010 | United States | 27 | Male + Female | 2 | 10 wk | ADP | Intervention 1 dietary interv.: 85% Intervention 2 dietary interv: 98%, RT: 96% |
Yes | |
| Beebe et al. [44] 2013 | United States | 26 | Female | 2 | 16 wk | No BC measured | Not mentioned | Yes | |
| Bopp et al. [45] 2008 | United States | 70 | Female | 3 | 20 wk | DXA | Intervention 1 Not mentioned Intervention 2 Dietary compliance: 100.1% +/- 0.4 Intervention 3 Not mentioned |
Yes | |
| Bouchard et al.[46] 2009 | United States | 46 | Female | 4 | 12 wk | DXA | Not mentioned | Yes | |
| Brennan et al. [107] 2020 | United States | 61 | Male + Female | 3 | 26 wk | DXA |
|
Not mentioned | No |
| Brochu et al. [47] 2009 | Canada | 107 | Female | 2 | 16 wk | DXA | Intervention 1 80-90% Intervention 2 80-90% |
Yes | |
| Deibert et al. [48] 2011 | Germany | 35 | Male | 3 | 12 wk | Skinfold measurement |
|
Intervention 1 90% Intervention 2 90% Intervention 3 90% |
Yes |
| Dubé et al. [49] 2011 | United States | 16 | Male + Female | 2 | 16 wk | DXA | Not mentioned | Yes | |
| Evans et al. [50] 2021 | United States | 61 | Female | 3 | 26 wk | DXA |
|
Intervention 1 dietary adherence not mentioned Intervention 2 75% exercise Intervention 3 75% exercise |
Yes |
| Felix-Soriano et al. [108] 2021 | Spain | 85 | Female | 4 | 16 wk | DXA |
|
Intervention 1 95% Intervention 2 95% Intervention 3 95% Intervention 4 95% |
No |
| Galbreath et al. [51] 2018 | United States | 54 | Female | 3 | 14 wk | BIA, DXA | Intervention 1 70% Intervention 2 70% Intervention 3 70% |
Yes | |
| Grossman et al. [109] 2018 | United States | 11 | Female | 2 | 16 wk | DXA |
|
Intervention 1 100% Intervention 2 60% |
No |
| Hays et al. [110] 2004 | United States | 34 | Male + Female | 3 | 14 wk | BOD POD |
|
Not mentioned | No |
| Haywood et al. [53] 2018 | Australia | 117 | Male + Female | 3 | 12 wk | DXA | Not mentioned | Yes | |
| Hsu et al. [106] 2021 | Taiwan | 69 | Male + Female | 3 | 12 wk | DXA | No Intervention Not mentioned Intervention 2 90% Intervention 3 94% |
Yes | |
| Jefferson et al. [52] 2015 | United States | 32 | Male + Female | 2 | 20 wk | DXA | Intervention 1 84% Intervention 2 86% |
Yes | |
| Jo et al. [111] 2019 | United States | 11 | Male + Female | 2 | 14 wk | DXA |
|
Not mentioned | No |
| Kelly et al. [112] 2014 | United States | 24 | Male + Female | 2 | 12 wk | DXA, CT |
|
Intervention1 diet + exercise: 97% Intervention2 diet + exercise: 97% |
No |
| McNeil et al. [54] 2015 | Canada | 93 | Female | 2 | 26 wk | DXA | Not mentioned | Yes | |
| Messier et al. [55] 2010 | Canada | 107 | Female | 2 | 26 wk | DXA | Not mentioned | Yes | |
| Mulya et al. [113] 2017 | United States | 20 | Male + Female | 2 | 12 wk | DXA |
|
Intervention 1 83.3% Intervention 2 84.3% |
No |
| Muollo et al. [114] 2019 | Italy | 38 | Male + Female | 2 | 24 wk | DXA |
|
Not mentioned | No |
| Muollo et al. [115] 2021 | Italy | 27 | Male + Female | 2 | 24 wk | DXA |
|
Intervention 1 81.4% Intervention 2 80.2% |
No |
| Nicklas et al. [58] 2019 | United States | 155 | Male + Female | 3 | 20 wk | DXA | Intervention 1 85.8% Intervention 2 Exercise: 89.9%; 99.2% Intervention 3 Exercise 91.2%; 100% |
Yes | |
| Nicklas et al. [57] 2015 | United States | 126 | Male + Female | 2 | 20 wk | DXA | Intervention 1 attendance: 86% Intervention2 attendance: 89% |
Yes | |
| Nicklas et al. [56] 2009 | United States | 112 | Female | 3 | 20 wk | DXA, CT | Intervention 1 dietary compliance: 99.8% (SD 1.4%) Intervention2 dietary compl.: 100.3% (SD 1.8); exercise: 92.6% (SD 5.5) Intervention 3 dietary compl.: 100.4% (SD 1,7); exercise: 90% (SD 8.7) |
Yes | |
| Sakurai et al. [59] 2013 | Japan | 66 | Male + Female | 4 | 12 wk | BC analyzer | Not mentioned | Yes | |
| Santanasto et al. [60] 2011 | United States | 36 | Male + Female | 2 | 26 wk | DXA, CT | Not mentioned | Yes | |
| Shah et al. [61] 2009 | United States | 18 | Male + Female | 2 | 26 wk | DXA | Not mentioned | Yes | |
| Solomon et al. [116] 2013 | United States | 20 | Male + Female | 2 | 12 wk | DXA |
|
Not mentioned | No |
| St-Onge et al. [74] 2013 | Canada | 89 | Male + Female | 2 | 26 wk | DXA | Not mentioned | Yes | |
| Valente et al. [73] 2011 | United States | 27 | Male + Female | 2 | 10 wk | ADP | Intervention 1 85% Intervention 2 98% |
Yes | |
| van Gemert et al. [117] 2015 | Netherlands | 243 | Male + Female | 3 | 16 wk | DXA |
|
Intervention 1 80% Intervention 2 80% Intervention 3 80% |
No |
| Verreijen et al. [71] 2017 | Netherlands | 100 | Male + Female | 4 | 10 wk | ADP (BOD POD) | Intervention 1 Not mentioned Intervention 2 mean adherence to exercise program: 2.9 +- 0.3 times/wk Intervention 3 mean adherence to exercise program: 2.9 +- 0.3 times/wk Intervention 4 Not mentioned |
Yes | |
| Verreijen et al. [72] 2015 | Netherlands | 60 | Male + Female | 2 | 13 wk | DXA | Intervention 1 food consupmtion: 91%; exercise program: 72% Intervention 2 food consumption: 97%; exercise program: 88% |
Yes | |
| Villareal et al. [70] 2017 | United States | 160 | Male + Female | 4 | 26 wk | DXA | Not mentioned | Yes | |
| Wang et al. [69] 2015 | United States | 70 | Female | 2 | 20 wk | DXA | Not mentioned | Yes | |
| Wasserfurth et al. [67] 2020 | Germany | 134 | Male + Female | 4 | 12 wk | BIA | Not mentioned | Yes | |
| Waters et al. [67] 2021 | United States | 160 | Male + Female | 4 | 26 wk | DXA | Not mentioned | Yes | |
| Weiss et al. [66] 2021 | United States | 52 | Male + Female | 3 | 12 wk | DXA | Not mentioned | Yes | |
| Yassine et al. [65] 2009 | United States | 24 | Male + Female | 2 | 12 wk | Hydrostatic weighing, CT | Intervention 1 94% Intervention 2 94% |
Yes | |
| Yoshimura et al. [64] 2014 | Japan | 75 | Male + Female | 2 | 12 wk | Underwater weighing | Intervention 1 Diet only: 97% Intervention 2 Exercise: 81%, diet: 93% |
Yes | |
| You et al. [63] 2004 | United States | 34 | Female | 2 | 26 wk | DXA, CT | Intervention 1 diet class: 80% Intervention 2 diet + ex: 78%; exercise sessions: 78% |
Yes | |
| You et al. [62] 2006 | United States | 45 | Female | 3 | 20 wk | DXA | Intervention 1 Not mentioned Intervention 2 exercise compl: 92.3 ± 1.7% Intervention 3 exercise compl: 87.9 ± 2.3% |
Yes | |
| Exercise Interventions | |||||||||
| Ballor et al. [75] 1996 | United States | 18 | Male + Female | 2 | 12 wk | Underwater weighing | Not mentioned | Yes | |
| Bocalini et al. [76] 2012 | Brazil | 44 | Female | 4 | 12 wk | Skinfold measurement | Not mentioned | Yes | |
| Boukabous et al. [120] 2019 | Canada | 18 | Female | 2 | 8 wk | DXA |
|
Intervention 1 92.7% Intervention 2 92.7% |
No |
| Carneiro et al. [121] 2021 | Brazil | 40 | Female | 2 | 15 wk | DXA |
|
Not mentioned | No |
| Cavalcante et al. [77] 2018 | Brazil | 57 | Female | 3 | 12 wk | DXA | Intervention 1 session attending ≥ 85% Intervention 2 session attending ≥ 85% No intervention Not mentioned |
Yes | |
| Conley et al. [79] 2018 | Australia | 23 | Male | 2 | 26 wk | No BC measured | Intervention 1 82% Intervention 2 83% |
Yes | |
| Faramarzi et al. [80] 2018 | Iran | 40 | Female | 4 | 8 wk | Skinfold measurement | Not mentioned | Yes | |
| Fritz et al. [81] 2018 | Spain | 63 | Female | 3 | 8 wk | BIA, DXA | Intervention 1 91.6 ± 3.3% Intervention 2 93.3 ± 3.1% No intervention Not mentioned |
Yes | |
| Izzicupo et al. [122] 2017 | Italy | 30 | Female | 2 | 12 wk | Skinfold |
|
Not mentioned | No |
| Kallings et al. [82] 2009 | Sweden | 101 | Male + Female | 2 | 26 wk | BIA | Not mentioned | Yes | |
| Kim et al. [83] 2019 | Korea | 20 | Male | 2 | 12 wk | BIA | Not mentioned | Yes | |
| Li et al. [84] 2021 | China | 29 | Male + Female | 3 | 12 wk | DXA | Not mentioned | Yes | |
| Nunes et al. [85] 2019 | Brazil | 24 | Female | 2 | 12 wk | DXA | Not mentioned | Yes | |
| Park et al. [86] 2015 | Korea | 20 | Female | 2 | 12 wk | BC analyzer | Not mentioned | Yes | |
| Park et al. [87] 2020 | Korea | 20 | Male | 2 | 12 wk | BIA | Not mentioned | Yes | |
| Phillips et al. [88] 2012 | United States | 23 | Female | 2 | 12 wk | Skinfold measurement | Intervention 1 attendance exercise: 100% No intervention attendance: 90% |
Yes | |
| Puengsuwan et al. 2020 [89] | Thailand | 55 | Female | 2 | 15 wk | Skinfold measurement | No intervention Not mentioned Intervention 2 exercise training: 90% |
Yes | |
| Ribeiro et al. [90] 2020 | Brazil | 33 | Female | 2 | 8 wk | DXA | No intervention Not mentioned Intervention 1 participating in sessions ≥ 85% |
Yes | |
| Rossi et al. [91] 2016 | Brazil | 70 | Female | 3 | 16 wk | DXA | Not mentioned | Yes | |
| Siu et al. [95] 2021 | Hong Kong | 543 | Male + Female | 3 | 12 wk | No BC measured | No intervention Not mentioned Intervention 1 class attendance: 67% Intervention 2 class attendance: 70% |
Yes | |
| Sjögren et al. [92] 2012 | Sweden | 73 | Male + Female | 2 | 26 wk | BIA | Not mentioned | Yes | |
| Stewart et al. [93] 2005 | United States | 104 | Male + Female | 4 | 26 wk | DXA | No intervention 90% Intervention 1 90% |
Yes | |
| Tomeleri et al. [94] 2016 | Brazil | 38 | Female | 2 | 8 wk | DXA | No intervention Not mentioned Intervention 1 session participating ≥ 85% |
Yes | |
| Church et al. [78] 2009 | United States | 411 | Female | 4 | 26 wk | Skinfold measurement | No intervention Not mentioned Intervention 1 exercise: 99.5% Intervention 2 exercise: 99.3 Intervention 3 exercise: 99.2% |
Yes | |
| Irwin et al. [104] 2003 | United States | 173 | Female | 2 | 12 wk | DXA | Not mentioned | Yes | |
| Nutritional Interventions | |||||||||
| Backx et al. [103] 2016 | Netherlands | 61 | Male + Female | 2 | 12 wk | DXA | Not mentioned | Yes | |
| Barbour et al. [123] 2015 | Australia | 63 | Male + Female | 2 | 12 wk | DXA |
|
Intervention 1 80% No intervention Not mentioned |
No |
| Barnard et al. [118] 2022 | United States | 62 | Male + Female | 2 | 16 wk | DXA |
|
Intervention 1 84% Intervention 2 84% |
No |
| Barnard et al. [119] 2005 | United States | 59 | Female | 2 | 14 wk | BOD POD |
|
Not mentioned | No |
| Beaver et al. [102] 2015 | United States | 24 | Male + Female | 2 | 12 wk | DXA, CT | Intervention 1 Self-reported compliance to dietary interv: 97.5 ± 3.3% Intervention 2 Self-reported compliance to dietary interv: 92.2 ± 9.3% |
Yes | |
| Beaver et al. [100] 2019 | United States | 96 | Male + Female | 2 | 26 wk | DXA | Intervention 1 attendance at educational sessions: 88%; self-reported meal replacement: 92.7% No intervention attendance at educational sessions: 84% |
Yes | |
| Dengo et al. [101] 2010 | United States | 36 | Male + Female | 2 | 12 wk | DXA, CT | Not mentioned | Yes | |
| Dennis et al. [133] 2010 | United States | 48 | Male + Female | 2 | 12 wk | DXA |
|
Intervention 1 Water intake compliance: 90 ± 2% |
No |
| Englert et al. [99] 2021 | Germany | 54 | Female | 2 | 12 wk | BIA | Not mentioned | Yes | |
| Goss et al. [125] 2020 | United States | 34 | Male + Female | 2 | 8 wk | DXA |
|
Not mentioned | No |
| Ilich et al. [126] 2019 | United States | 135 | Female | 3 | 26 wk | DXA |
|
No intervention compliance with suppl: 73% Intervention 2 compliance with suppl: 73% Intervention 3 compliance with placebo: 82.7% |
No |
| Katz et al. [127] 2012 | United States | 46 | Male + Female | 2 | 8 wk | No BC measured |
|
Not mentioned | No |
| Kristensen et al. [128] 2012 | Denmark | 72 | Female | 2 | 12 wk | DXA |
|
Intervention 1 compl. with provided food: 91.5% Intervention 2 compl. with provided food: 94.2% |
No |
| Njike et al. [129] 2017 | United States | 32 | Male + Female | 2 | 12 wk | BIA |
|
Not mentioned | No |
| Ogilvie et al. [98] 2021 | United States | 34 | Female | 2 | 26 wk | DXA | Not mentioned | Yes | |
| Porter Starr et al. [97] 2019 | United States | 39 | Male + Female | 2 | 26 wk | BOD POD | Not mentioned | Yes | |
| Serra et al. [96] 2019 | United States | 82 | Male + Female | 2 | 26 wk | DXA | Intervention 1 attendance to education: 88% self-reported compl: 93% No intervention attendance to education. session: 84% |
Yes | |
| Shapses et al. [130] 2004 |
United States | 58 | Female | 4 | 25 wk | DXA |
|
Intervention 1 90% Intervention 2 90% Intervention 3 85% Intervention 4 85% |
No |
| Teng et al. [105] 2013 | Malaysia | 56 | Male | 2 | 12 wk | Body composition analyzer | Not mentioned | Yes | |
| Wien et al. [131] 2003 |
United States | 65 | Male + Female | 2 | 24 wk | BIA |
|
Not mentioned | No |
| Christensen et al. [124] 2011 | Denmark | 192 | Male + Female | 2 | 16 wk | BIA |
|
Intervention 1 after 8 wks: 91%/after 16 wks: 90% Intervention 2 8 wks: 94%; 16 wks: 93% |
No |
Abbreviations: ADP, air-displacement plethysmograph; AT, aerobic training; BC, body composition; LCD, low-calorie diet; RT, resistance training.
Intervention groups: [1] No intervention, [2] energy restriction (300-1000 kcal + meal replacement), [3] energy restriction + high protein, [4] 5:2 diet, [5] mixed exercise (AT+RT), [6] resistance training, [7] aerobic training, [8] resistance training + high protein, [9] energy restriction + high protein + exercise (mainly RT), [10] energy restriction + resistance training, [11] energy restriction + aerobic training, [12] energy restriction + mixed exercise.
Risk of bias
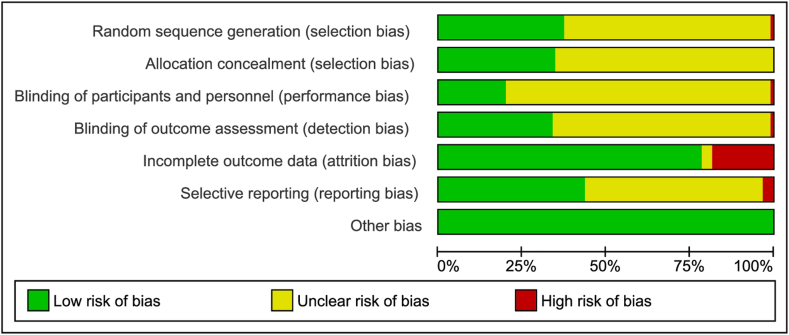
Overall, 19 RCTs (21 %) were rated as having a high risk of bias for at least one domain. Thirty-four studies (37%) were judged as having a low risk of bias in the domain random sequence generation, whereas 32 studies (35%) had a low risk of bias in the domain allocation concealment. The assessment of participant and personnel blinding revealed that 18 studies (20%) had a low risk of bias, and 31 studies (34%) had a low risk of bias for blinding the outcome assessor. Seventy-two (78%) of the studies had a low risk for presenting incomplete outcome data, and 40 (43%) had a low risk for selective reporting. The Egger’s test results for publication bias were not significant for all outcomes (P < 0.05). A summary of risk of bias assessment is provided in Figure 2.
FIGURE 2.
Risk of bias summary.
NMA
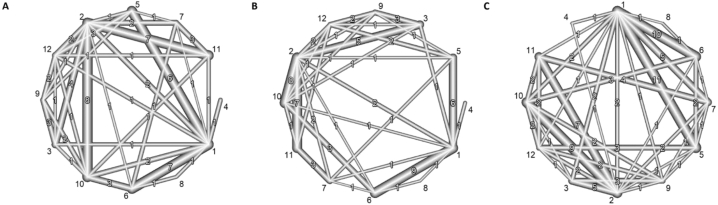
We conducted the NMA model separately for the 5 outcomes (%BF, BF in kg, LBM/FFM, WC, BMI) and one model using all outcomes. The network geometry for the outcomes BF mass in kg and LBM/FFM, as well as the network geometry using all studies according to the predefined prioritization of outcomes is presented in Figure 3. All other network geometries can be found in Supplementary Figure 1. By grouping interventions into specific treatment groups, we obtained a dense network that enabled us to make many direct comparisons. The network where we used the prioritization shows the highest number of direct comparisons. Figure 4 illustrates the results of the NMA for all outcomes separately as well as for the single model using all studies with a prioritization of outcomes.
FIGURE 3.
Network graphs comparing the structure of the network regarding (A) body fat in kg, (B) LBM/FFM, and (C) ALL according to the outcome prioritization. The numbers within the graphs represent the numbers of direct comparisons, while the thickness of the lines is proportional to the inverse standard error of the estimates. The numbers outside the graphs represent the intervention numbers as follows: 1) no intervention, 2) energy restriction, 3) energy restriction plus high-protein intake, 4) 5:2 diet, 5) mixed exercise (aerobic and resistance training), 6) resistance training, 7) aerobic training, 8) resistance training plus high-protein intake, 9) energy restriction plus high protein and exercise, 10) energy restriction plus resistance training, 11) energy restriction plus aerobic training, and 12) energy restriction plus mixed exercises.
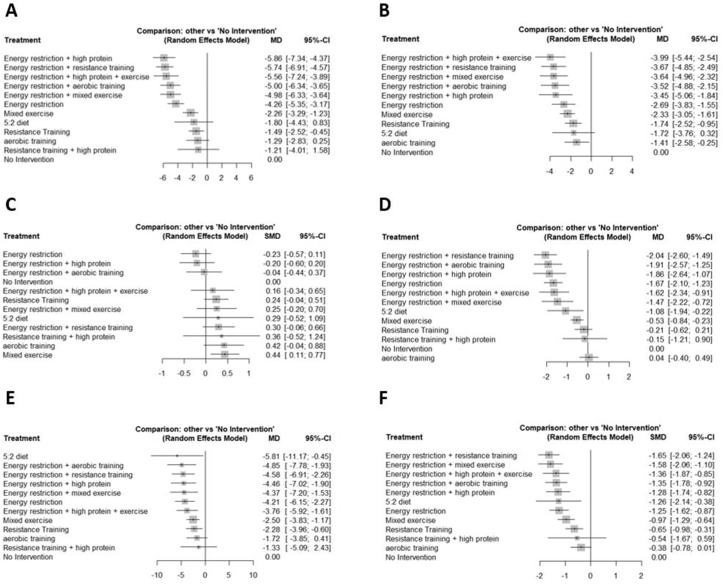
FIGURE 4.
Summary effect estimates of the different nutrition and exercise interventions on (A) BF in kg, (B) %BF, (C) LBM/FFM, (D) BMI, (E) WC and (F) ALL according to the outcome prioritization. BF, body fat; WC, waist circumference.
Fat mass
Absolute BF mass was the most common outcome, and this was included in 47 studies (74 pairwise comparisons, 12 treatment groups), whereas relative BF mass was included in 37 studies (50 pairwise comparisons, 11 treatment groups). In general, energy restriction combined with any kind of exercise and combined with high-protein content were effective strategies for losing fat mass. The most effective intervention for losing BF in % was energy restriction combined with a high-protein content and exercise (P < 0.001, MD: −3.99, 95%: CI −5.44, −2.54). For losing BF in kg, the most effective intervention was energy restriction combined with a high-protein intake (P < 0.001, MD: −5.86, 95% CI: −7.34, −4.37). Energy restriction alone also led to significant loss of fat mass, but to a lesser extent (BF in kg: P < 0.001, MD: −4.26, 95% CI: −5.35, −3.17, BF in %: P < 0.001, MD: −2.69, 95% CI: −3.83, −1.55). Resistance training or mixed exercise alone also enabled the significant loss of BF, but to a considerably lesser extent than energy restriction alone. No significant effect in terms of reducing BF was observed for the 5:2 diet and resistance training combined with a high-protein diet. Aerobic training was only significant in terms of losing BF in % (P = 0.02, MD: −1.41, 95% CI: −2.58, −0.25).
Muscle mass (LBM/FFM)
LBM or FFM was determined as an outcome in 48 studies (72 pairwise comparisons, 12 treatment groups). Only mixed exercise significantly increased LBM or FFM (P = 0.0089, SMD: 0.44, 95% CI: 0.11, 0.77). All other interventions managed to preserve muscle mass. However, we observed a nonsignificant trend that energy restriction alone seemed to result in a loss of muscle mass (P = 0.189, SMD: −0.23, 95% CI: −0.57, 0.11).
BMI
For the outcome BMI, 38 studies were available (60 pairwise comparisons, 12 treatment groups). The most effective intervention for BMI reduction was energy restriction combined with resistance training (P < 0.001, MD: −2.04, 95% CI: −2.60, −1.49), followed by energy restriction with aerobic training (P < 0.001, MD: −1.91, 95% CI: −2.57, −1.25), energy restriction with high-protein content (P < 0.001, MD: −1.86, 95% CI: −2.64, −1.07), and energy restriction alone (P < 0.001, MD: −1.67, 95% CI: −2.10, −1.23). We found no significant effects on BMI for the interventions of resistance training, resistance training with a high-protein diet, or aerobic training.
WC
WC was measured in 25 studies (40 pairwise comparisons, 12 treatment groups). All interventions except aerobic training and resistance training with a high-protein diet significantly decreased WC. Highly effective treatments for reducing WC were either energy restriction alone or combined with any kind of exercise or high-protein diet. The most effective treatments were the 5:2 diet (P = 0.03, MD: −5.81, 95% CI: −11.17, −0.45) and energy restiction with aerobic training (P = 0.001, MD: −4.85, 95% CI: −7.78, −1.93), energy restriction combined with resistance training (P < 0.001, MD: −4.58, 95% CI: −6.91, −2.26), energy restriction with high-protein content (P < 0.001, MD: −4.46, 95% CI: −7.02, −1.90), energy restriction with mixed exercise (P = 0.003, MD: −4.37, 95% CI: −7.20, −1.53), or energy restriction alone (P < 0.001, MD: −4.21, 95% CI: −6.15; −2.27).
All outcomes combined
In the model combining all outcomes according to our prioritization, 65 studies were available (98 pairwise comparisons). The most effective interventions were energy restriction with resistance training (P < 0.001, SMD: −1.65, 95% CI: −2.06, −1.24), energy restriction with mixed exercise (P < 0.001, SMD: −1.58, 95% CI: −2.06, −1.10), and energy restriction with high-protein content and exercise (P < 0.001, SMD: −1.36, 95% CI: −1.87, −0.85). However, BF mass was considered in 58 pairwise comparisons, LBM/FFM only in 3 pairwise comparisons, and WC in 5 pairwise comparisons in this model, meaning that the model mainly emphasized fat mass reduction.
Inconsistency
We observed no signs of inconsistency in the networks when comparing changes in BF in kg (P = 0.859), BF in % (P = 0.986), LBM/FFM (P = 0.232), and prioritization (P = 0.461). However, we observed inconsistencies in the network when comparing changes in BMI (P < 0.001) and WC (P < 0.001). In the network comparing the change in BMI, it was necessary to remove 3 studies to obtain a P value above the level of significance [66,95,106]. In the network comparing the change in WC, we found 3 studies [52,66,101] that contributed to inconsistency; when removing all 3 of them, the network no longer showed signs of inconsistency (P = 0.219), but the same overall results were obtained as for the main analysis.
Subgroup and sensitivity analyses
No considerable differences were observed when only analyzing studies with women or men. An intervention duration of more than 14 wk was associated with more pronounced weight loss, but the order of effectiveness did not change between categories of interventions. Results did not change substantially when excluding studies with a high risk of bias. The subgroup analysis results supported the hypothesis that interventions combining nutrition and exercise most effectively improve body composition and anthropometric parameters.
Summary of studies not included in the network meta-analysis
In our literature review, we identified 26 studies [[107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132]] that could not be included in the network meta-analysis. Eleven of these studies were with mixed nutrition and exercise interventions, 3 were with only exercise interventions, and 12 were with only nutritional interventions. All 26 studies were highly heterogeneous in terms of the interventions used; therefore, they are difficult to compare. A detailed overview about the used interventions as well as the main results of the single studies can be found in Supplementary Table 2.
Discussion
The aim of conducting this systematic review and NMA was to evaluate which nutrition and exercise interventions most effectively improve body composition (fat mass and muscle mass) and anthropometric measures (BMI and WC) in persons with overweight or obesity near or around retirement age. In the NMA models, we identified several effective nutrition and exercise interventions for this target group. A reduction in BF could be best achieved by applying the measures of energy restriction combined with any kind of exercise or with high-protein intake. Energy restriction alone also reduced BF, but to a lesser extent, and on the contrary, energy restriction alone tended to decrease muscle mass. Muscle mass could only be significantly increased with mixed exercise (resistance and aerobic) interventions, but all other interventions that included exercise effectively preserved muscle mass. A decrease in BMI and/or WC could be achieved with nearly every intervention except aerobic training alone, resistance training alone, or resistance training combined with a high-protein diet. Overall, the most effective strategy for loss of fat mass while maintaining or increasing muscle mass was the combination of energy restriction and exercise (resistance or mixed) and/or a high-protein diet.
Energy restriction to achieve a negative energy balance is still a key therapeutic weight loss strategy recommended in evidence-based guidelines [22,133]. However, according to our results, energy restriction alone does not seem to be an appropriate approach for persons in retirement age. Although it results in weight and fat loss, it tends to result in a loss of muscle mass, which may be considered as an adverse effect as it increases risk of disability, metabolic impairments, mortality, or a low quality of life in this group [134]. In addition, LBM loss is a major factor in weight regain, as LBM is the main driver of energy expenditure [135].
Because aging is associated with muscle loss, great efforts should be made to preserve or even increase muscle mass as well as muscle function and quality in aging or aged persons. Our results show that muscle preservation can be effectively achieved by combining resistance training, mixed exercise, or mixed exercise with high-protein content foods with an energy-restricted diet. This result is similar to those of other systematic reviews that concluded that resistance training added to energy-restricted diets prevents muscle loss in other target groups, e.g., in generally older individuals [25,136]. According to our results, resistance training or the combination of resistance and aerobic training yielded much better results than aerobic training alone regarding muscle mass preservation. This result also agrees with those of other studies. A recent systematic review in older adults, for example, concluded that only resistance training could effectively improve muscle strength, whereas aerobic training could not [137].
The term energy-restricted diet is referred to in several different approaches, including low-carbohydrate or low-fat diets, low- and very low-calorie diets, diets using formula products, time-restricted eating, and many more [138]. Our results do not allow us to determine which approach is best because it was not possible to examine all different approaches to reach energy restriction due to the limited number of similar studies that included people near retirement age. However, the specific approach might not be important as long as the energy intake is below the energy requirements (currently 500 to 1000 kcal). For example, a systematic review comparing intermittent and continous energy restrictions in adults did not find different effects, but both forms of energy restriction resulted in similar amounts of weight loss [139]. Whether weight loss is achieved with a moderate, low- or very low-calorie diet also seems to be of secondary importance, but guidelines address concerns that a low- or very low-calorie diet may be less likely to be nutritionally complete [133], also because very low-calorie intake may induce more intense LBM loss [140]. Another systematic review found that low-fat diets are not more successful than higher-fat, low-carbohydrate diets with regard to long-term weight change in adults [141]. The protein amount, source, and quality are also important components of an energy-restricted diet for maintaining muscle [142]. The amino acid composition (and notably the essential amino acid content) and the timing of protein intake (and especially regarding exercise training) [143] but also of other nutrients should be considered, such as vitamin D [144], as well as the fat quality, either alone or combined with other interventions [145].
This evidence implies that several potentially effective ways to restrict energy intake exist. It is likely to be much more important that persons with overweight or obesity find an approach that suits their lifestyle [133]. The time near retirement is a period that offers a unique opportunity for changing lifestyle habits; this time should be used to change diet and behavior in ways that are comfortable over longer periods of time and ensure a healthy diet over the long term to maintain weight loss. Women and men may also react differently to nutritional and exercise interventions [146], but this was not supported by our NMA results because the subgroup analyses separating men and women did not reveal different results from those of the full analysis.
Risk of bias assessment results indicate that risk of bias of the included studies was mostly unclear, i.e., the reporting was very poor in most of the included studies, and risk of reporting bias was mostly unclear or high. Furthermore, only 48% of all identified studies investigating nutritional interventions could be included in the NMA. This was due to the heterogeneity of the nutritional intervention studies (i.e., highly heterogeneous interventions) and the (frequent) lack of a control group with no intervention or usual care. These studies compared similar interventions but yielded few results in terms of observed differences between the study arms. The quality of the studies including the exercise interventions was much higher. Of these, 88% of the exercise studies and 83% of the studies including nutritional interventions combined with exercise interventions could be included in the NMA, demonstrating a much higher homogeneity. This may also be due to the fact that there are many more different food and diet options available to improve body composition than exercise options.
This systematic review and meta-analysis included a considerably high number of studies with the predefined target group and was conducted systematically based on the recommendations in the Cochrane Collaboration Handbook. This NMA allowed an indirect comparison to be included in the statistical model, enhancing the significance and coverage of the whole model [[147], [148], [149]]. However, this study also had some limitations. The method used to group treatments actually treated different treatments as the same, which might have biased our results.
The included studies of this review are heterogeneous, varying in terms of the study duration, the participants’ sex, age and weight, or the country in which the studies were conducted. We found that some of our networks showed evidence of inconsistency. We were able to partly explain the cause of this inconsistency. Although we could not reject the null hypothesis of no inconsistency, this does not imply that the network is consistent. Nevertheless, this study contributes important evidence that should be considered when developing recommendations for improving body composition aimed at persons with overweight and obesity near retirement age.
Conclusion
The overall results of this NMA indicate that the most effective strategy to improve body composition, i.e., losing fat without increasing risk of sarcopenia in persons with obesity around retirement age, was combining energy restriction with resistance training or with mixed exercise (resistance combined with aerobic exercise) and/or high-protein intake. Without training, an energy-restricted diet with or without added protein helped individuals lose fat mass but also tended to result in losses of muscle mass. To lose fat while preserving muscle, interventions involving aerobic training, intermittent fasting, resistance training combined with a high-protein diet, and energy restriction alone or in combination with a high-protein diet were not suitable, because they either tend to decrease muscle mass or do not reduce BF.
The important life period near retirement provides individuals with an opportunity to start establishing new healthy nutrition and exercise habits and to incorporate evidence-based nutrition and exercise interventions into their daily routines. The main aim is to prevent dependency and disability in older age. Healthcare professionals involved in the management of persons with obesity must be aware that an energy-restricted diet alone probably contributes to the development of sarcopenic obesity in persons of retirement age. To simultaneously lose weight and maintain muscle mass, the combination of energy restriction and resistance training is probably the best way forward.
Acknowledgments
The authors’ responsibilities were as follows – DE, SB: designed research; MT, SB, DE, LR: conducted research; SE: performed statsistical analysis; DE, SB, LR, MT: wrote the manuscript; JDS, PJW, TV, YB, ACJ: reviewed and edited the manuscript; DE: had primary responibility for the final content; and all authors: read and approved the final manuscript.
Funding
The SO-NUTS project is funded by JPI HDHL, the funding agencies supporting this work are: the Netherlands Organisation for Health Research and Development (ZonMw), French National Research Agency (ANR), Federal Ministry of Education, Science and Research represented by the Austrian Research Promotion Agency (BMBWF represented by FFG), Spanish State Research Agency (AEI: PCI2020-120683-2) and the Ministry of Education, Youth and Sports Department of Research and Development (MSMT). This project has received funding from the European Union’s Horizon 2020 research and innovation program under the ERA-NET Cofund action No. 727565.
Author disclosures
The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.04.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Peralta M., Ramos M., Lipert A., Martins J., Marques A. Prevalence and trends of overweight and obesity in older adults from 10 European countries from 2005 to 2013. Scand. J. Public Health. 2018;46(5):522–529. doi: 10.1177/1403494818764810. [DOI] [PubMed] [Google Scholar]
- 2.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Vol. 360. NCHS Data Brief; 2020. pp. 1–8. (Prevalence of obesity and severe obesity among adults: United States, 2017-2018). [PubMed] [Google Scholar]
- 3.NCD-Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/s0140-6736(16)30054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yannakoulia M., Poulimeneas D., Mamalaki E., Anastasiou C.A. Dietary modifications for weight loss and weight loss maintenance. Metabolism. 2019;92:153–162. doi: 10.1016/j.metabol.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Ogden C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age. JAMA. 2018;319(16):1723–1725. doi: 10.1001/jama.2018.3060. 2007-2008 to 2015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global BMI Mortality Collaboration. Di Angelantonio E., Bhupathiraju S., Wormser D., Gao P., Kaptoge S., et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi: 10.1016/s0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alwan A. World Health Organization; 2011. Global status report on noncommunicable diseases 2010.https://apps.who.int/iris/handle/10665/44579 [Internet] Available from: [Google Scholar]
- 8.Villareal D.T., Apovian C.M., Kushner R.F., Klein S. American Society for Nutrition, NAASO, The Obesity Society, Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr. 2005;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 9.Peto R., Whitlock G., Jha P. Effects of obesity and smoking on U.S. life expectancy. N. Engl. J. Med. 2010;362(9):855–856. doi: 10.1056/NEJMc1000079. [DOI] [PubMed] [Google Scholar]
- 10.Ogliari G., Ryg J., Andersen-Ranberg K., Scheel-Hincke L.L., Masud T. Association between body mass index and falls in community-dwelling men and women: a prospective, multinational study in the Survey of Health, Ageing and Retirement in Europe (SHARE) Eur. Geriatr. Med. 2021;12(4):837–849. doi: 10.1007/s41999-021-00485-5. [DOI] [PubMed] [Google Scholar]
- 11.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., et al. European guidelines for obesity management in adults. Obes. Facts. 2015;8(6):402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoufour J.D., Tieland M., Barazzoni R., Ben Allouch S., van der Bie J., Boirie Y., et al. The relevance of diet, physical activity, exercise, and persuasive technology in the prevention and treatment of sarcopenic obesity in older adults. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.661449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canning K.L., Brown R.E., Jamnik V.K., Kuk J.L. Relationship between obesity and obesity-related morbidities weakens with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(1):87–92. doi: 10.1093/gerona/glt026. [DOI] [PubMed] [Google Scholar]
- 14.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunan E., Wright C.L., Semola O.A., Subramanian M., Balasubramanian P., Lovern P.C., et al. Obesity as a premature aging phenotype - implications for sarcopenic obesity. GeroScience. 2022;44(3):1393–1405. doi: 10.1007/s11357-022-00567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahat G. Sarcopenic obesity: a hot yet under considered evolving concept. Eur. Geriat. Med. 2022;13(5):1023–1024. doi: 10.1007/s41999-022-00674-w. [DOI] [PubMed] [Google Scholar]
- 17.Batsis J.A., Mackenzie T.A., Emeny R.T., Lopez-Jimenez F., Bartels S.J. Low lean mass with and without obesity, and mortality: results from the 1999-2004 national health and nutrition examination survey. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72(10):1445–1451. doi: 10.1093/gerona/glx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raguso C.A., Kyle U., Kossovsky M.P., Roynette C., Paoloni-Giacobino A., Hans D., et al. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin. Nutr. 2006;25(4):573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Kuk J.L., Saunders T.J., Davidson L.E., Ross R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Organization for Economic Co-operation and Development . 2019. OECD Pensions at a Glance. [Google Scholar]
- 21.Robinson S.M., Westbury L.D., Ward K., Syddall H., Cooper R., Cooper C., et al. Is lifestyle change around retirement associated with better physical performance in older age?: insights from a longitudinal cohort. Eur. J. Ageing. 2021;18(4):513–521. doi: 10.1007/s10433-021-00607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharton S., Lau D.C.W., Vallis M., Sharma A.M., Biertho L., Campbell-Scherer D., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lara J., Hobbs N., Moynihan P.J., Meyer T.D., Adamson A.J., Errington L., et al. Effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2014;12:60. doi: 10.1186/1741-7015-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witham M.D., Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39(2):176–184. doi: 10.1093/ageing/afp251. [DOI] [PubMed] [Google Scholar]
- 25.Sardeli A.V., Komatsu T.R., Mori M.A., Gáspari A.F., Chacon-Mikahil M.P.T. Resistance training prevents muscle loss induced by caloric restriction in obese elderly individuals: a systematic review and meta-analysis. Nutrients. 2018;10(4):423. doi: 10.3390/nu10040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostás I., Pótó L., Mátrai P., Hegyi P., Tenk J., Garami A., et al. In middle-aged and old obese patients, training intervention reduces leptin level: a meta-analysis. PLOS ONE. 2017;12(8) doi: 10.1371/journal.pone.0182801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poscia A., Milovanovic S., La Milia D.I., Duplaga M., Grysztar M., Landi F., et al. Effectiveness of nutritional interventions addressed to elderly persons: umbrella systematic review with meta-analysis. Eur. J. Public Health. 2018;28(2):275–283. doi: 10.1093/eurpub/ckx199. [DOI] [PubMed] [Google Scholar]
- 28.McTigue K.M., Hess R., Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity (Silver Spring) 2006;14(9):1485–1497. doi: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 29.Liao C.D., Tsauo J.Y., Wu Y.T., Cheng C.P., Chen H.C., Huang Y.C., et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2017;106(4):1078–1091. doi: 10.3945/ajcn.116.143594. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.E., O’Connor L.E., Sands L.P., Slebodnik M.B., Campbell W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr. Rev. 2016;74(3):210–224. doi: 10.1093/nutrit/nuv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. John Wiley & Sons; Chichester, UK: 2019. [Google Scholar]
- 33.Higgins J.P., Li T., Deeks J.J. In: Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. John Wiley & Sons; Chichester, UK: 2019. Choosing effect measures and computing estimates of effect; pp. 143–176. [Google Scholar]
- 34.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarzer G., Carpenter J.R., Rücker G. Springer; New York City: 2015. Network meta-analysis, Meta-analysis with R; pp. 187–216. [Google Scholar]
- 36.Rücker G., Krahn U., König J., Efthimiou O., Schwarzer G. 2021. Netmeta: Network Meta-Analysis using Frequentist Methods.https://github.com/guido-s/netmeta [Internet]. R package version 2.0-0. Available from: [Google Scholar]
- 37.Higgins J.P., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rücker G., Cates C.J., Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res. Synth. Methods. 2017;8(4):392–403. doi: 10.1002/jrsm.1259. [DOI] [PubMed] [Google Scholar]
- 39.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu K.J., Liao C.D., Tsai M.W., Chen C.N. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta-analysis. Nutrients. 2019;11(9):2163. doi: 10.3390/nu11092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amamou T., Normandin E., Pouliot J., Dionne I.J., Brochu M., Riesco E. Effect of a high-protein energy-restricted diet combined with resistance training on metabolic profile in older individuals with metabolic impairments. J. Nutr. Health Aging. 2017;21(1):67–74. doi: 10.1007/s12603-016-0760-8. [DOI] [PubMed] [Google Scholar]
- 42.Amati F., Dubé J.J., Shay C., Goodpaster B.H. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J. Appl. Physiol. 2008;105(3):825–831. doi: 10.1152/japplphysiol.90384.2008. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avila J.J., Gutierres J.A., Sheehy M.E., Lofgren I.E., Delmonico M.J. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur. J. Appl. Physiol. 2010;109(3):517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- 44.Beebe N., Magnanti S., Katkowski L., Benson M., Xu F., Delmonico M.J., et al. Effects of the addition of t’ai chi to a dietary weight loss program on lipoprotein atherogenicity in obese older women. J. Altern. Complement. Med. 2013;19(9):759–766. doi: 10.1089/acm.2012.0531. [DOI] [PubMed] [Google Scholar]
- 45.Bopp M.J., Houston D.K., Lenchik L., Easter L., Kritchevsky S.B., Nicklas B.J. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet. Assoc. 2008;108(7):1216–1220. doi: 10.1016/j.jada.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard D.R., Soucy L., Sénéchal M., Dionne I.J., Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- 47.Brochu M., Malita M.F., Messier V., Doucet E., Strychar I., Lavoie J.M., et al. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J. Clin. Endocrinol. Metab. 2009;94(9):3226–3233. doi: 10.1210/jc.2008-2706. [DOI] [PubMed] [Google Scholar]
- 48.Deibert P., Solleder F., König D., Vitolins M.Z., Dickhuth H.H., Gollhofer A., et al. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male. 2011;14(4):273–279. doi: 10.3109/13685538.2011.565091. [DOI] [PubMed] [Google Scholar]
- 49.Dubé J.J., Amati F., Toledo F.G., Stefanovic-Racic M., Rossi A., Coen P., et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147–1156. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans E.M., Straight C.R., Reed R.A., Berg A.C., Rowe D.A., Johnson M.A. Exercise and protein effects on strength and function with weight loss in older women. Med. Sci. Sports Exerc. 2021;53(1):183–191. doi: 10.1249/mss.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 51.Galbreath M., Campbell B., LaBounty P., Bunn J., Dove J., Harvey T., et al. Effects of adherence to a higher protein diet on weight loss, markers of health, and functional capacity in older women participating in a resistance-based exercise program. Nutrients. 2018;10(8):1070. doi: 10.3390/nu10081070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jefferson M.E., Nicklas B.J., Chmelo E.A., Crotts C.I., Shaltout H.A., Diz D.I., et al. Effects of resistance training with and without caloric restriction on arterial stiffness in overweight and obese older adults. Am. J. Hypertens. 2016;29(4):494–500. doi: 10.1093/ajh/hpv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haywood C.J., Prendergast L.A., Purcell K., Le Fevre L., Lim W.K., Galea M., et al. Very low calorie diets for weight loss in obese older adults-a randomized trial. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73(1):59–65. doi: 10.1093/gerona/glx012. [DOI] [PubMed] [Google Scholar]
- 54.McNeil J., Schwartz A., Rabasa-Lhoret R., Lavoie J.M., Brochu M., Doucet É. Changes in leptin and peptide YY do not explain the greater-than-predicted decreases in resting energy expenditure after weight loss. J. Clin. Endocrinol. Metab. 2015;100(3):E443–E452. doi: 10.1210/jc.2014-2210. [DOI] [PubMed] [Google Scholar]
- 55.Messier V., Rabasa-Lhoret R., Doucet E., Brochu M., Lavoie J.M., Karelis A., et al. Effects of the addition of a resistance training programme to a caloric restriction weight loss intervention on psychosocial factors in overweight and obese post-menopausal women: a Montreal Ottawa New Emerging Team study. J. Sports Sci. 2010;28(1):83–92. doi: 10.1080/02640410903390105. [DOI] [PubMed] [Google Scholar]
- 56.Nicklas B.J., Wang X., You T., Lyles M.F., Demons J., Easter L., et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am. J. Clin. Nutr. 2009;89(4):1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicklas B.J., Chmelo E., Delbono O., Carr J.J., Lyles M.F., Marsh A.P. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am. J. Clin. Nutr. 2015;101(5):991–999. doi: 10.3945/ajcn.114.105270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicklas B.J., Brinkley T.E., Houston D.K., Lyles M.F., Hugenschmidt C.E., Beavers K.M., et al. Effects of caloric restriction on cardiorespiratory fitness, fatigue, and disability responses to aerobic exercise in older adults with obesity: a randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(7):1084–1090. doi: 10.1093/gerona/gly159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakurai R., Fujiwara Y., Saito K., Fukaya T., Kim M.J., Yasunaga M., et al. Effects of a comprehensive intervention program, including hot bathing, on overweight adults: a randomized controlled trial. Geriatr. Gerontol. Int. 2013;13(3):638–645. doi: 10.1111/j.1447-0594.2012.00955.x. [DOI] [PubMed] [Google Scholar]
- 60.Santanasto A.J., Glynn N.W., Newman M.A., Taylor C.A., Brooks M.M., Goodpaster B.H., et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J. Obes. 2011;2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah K., Stufflebam A., Hilton T.N., Sinacore D.R., Klein S., Villareal D.T. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17(12):2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You T., Murphy K.M., Lyles M.F., Demons J.L., Lenchik L., Nicklas B.J. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int. J. Obes. (Lond) 2006;30(8):1211–1216. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]
- 63.You T., Berman D.M., Ryan A.S., Nicklas B.J. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J. Clin. Endocrinol. Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 64.Yoshimura E., Kumahara H., Tobina T., Matsuda T., Watabe K., Matono S., et al. Aerobic exercise attenuates the loss of skeletal muscle during energy restriction in adults with visceral adiposity. Obes. Facts. 2014;7(1):26–35. doi: 10.1159/000358576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yassine H.N., Marchetti C.M., Krishnan R.K., Vrobel T.R., Gonzalez F., Kirwan J.P. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss E.P., Albert S.G., Reeds D.N., Kress K.S., McDaniel J.L., Klein S., et al. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am. J. Clin. Nutr. 2016;104(3):576–586. doi: 10.3945/ajcn.116.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waters D.L., Aguirre L., Gurney B., Sinacore D.R., Fowler K., Gregori G., et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J. Gerontol. A Biol. Sci. Med. Sci. 2022;77(1):131–139. doi: 10.1093/gerona/glab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wasserfurth P., Nebl J., Schuchardt J.P., Müller M., Boßlau T.K., Krüger K., et al. Effects of exercise combined with a healthy diet or Calanus finmarchicus oil supplementation on body composition and metabolic markers-a pilot study. Nutrients. 2020;12(7):2139. doi: 10.3390/nu12072139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X., You T., Murphy K., Lyles M.F., Nicklas B.J. Addition of exercise increases plasma adiponectin and release from adipose tissue. Med Sci Sports Exerc. 2015;47(11):2450–2455. doi: 10.1249/mss.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villareal D.T., Aguirre L., Gurney A.B., Waters D.L., Sinacore D.R., Colombo E., et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verreijen A.M., Engberink M.F., Memelink R.G., van der Plas S.E., Visser M., Weijs P.J. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr. J. 2017;16(1):10. doi: 10.1186/s12937-017-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verreijen A.M., Verlaan S., Engberink M.F., Swinkels S., de Vogel-van den Bosch J., Weijs P.J. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015;101(2):279–286. doi: 10.3945/ajcn.114.090290. [DOI] [PubMed] [Google Scholar]
- 73.Valente E.A., Sheehy M.E., Avila J.J., Gutierres J.A., Delmonico M.J., Lofgren I.E. The effect of the addition of resistance training to a dietary education intervention on apolipoproteins and diet quality in overweight and obese older adults. Clin. Interv. Aging. 2011;6:235–241. doi: 10.2147/cia.S23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.St-Onge M., Rabasa-Lhoret R., Strychar I., Faraj M., Doucet É., Lavoie J.M. Impact of energy restriction with or without resistance training on energy metabolism in overweight and obese postmenopausal women: a Montreal Ottawa New Emerging Team group study. Menopause. 2013;20(2):194–201. doi: 10.1097/gme.0b013e318261f22a. [DOI] [PubMed] [Google Scholar]
- 75.Ballor D.L., Harvey-Berino J.R., Ades P.A., Cryan J., Calles-Escandon J. Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metabolism. 1996;45(2):179–183. doi: 10.1016/s0026-0495(96)90050-5. [DOI] [PubMed] [Google Scholar]
- 76.Bocalini D.S., Lima L.S., de Andrade S., Madureira A., Rica R.L., Dos Santos R.N., et al. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin. Interv. Aging. 2012;7:551–556. doi: 10.2147/cia.S33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavalcante E.F., Ribeiro A.S., do Nascimento M.A., Silva A.M., Tomeleri C.M., Nabuco H.C.G., et al. Effects of different resistance training frequencies on fat in overweight/obese older women. Int. J. Sports Med. 2018;39(7):527–534. doi: 10.1055/a-0599-6555. [DOI] [PubMed] [Google Scholar]
- 78.Church T.S., Martin C.K., Thompson A.M., Earnest C.P., Mikus C.R., Blair S.N. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLOS ONE. 2009;4(2) doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conley M., Le Fevre L., Haywood C., Proietto J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. 2018;75(1):65–72. doi: 10.1111/1747-0080.12372. [DOI] [PubMed] [Google Scholar]
- 80.Faramarzi M., Bagheri L., Banitalebi E. Effect of sequence order of combined strength and endurance training on new adiposity indices in overweight elderly women. Isokinet. Exer. Sci. 2018;26(2):105–113. doi: 10.3233/IES-172195. [DOI] [Google Scholar]
- 81.Fritz N.B., Juesas Á., Gargallo P., Calatayud J., Fernández-Garrido J., Rogers M.E., et al. Positive effects of a short-term intense elastic resistance training program on body composition and physical functioning in overweight older women. Biol. Res. Nurs. 2018;20(3):321–334. doi: 10.1177/1099800418757676. [DOI] [PubMed] [Google Scholar]
- 82.Kallings L.V., Sierra Johnson J., Fisher R.M., Faire U., Ståhle A., Hemmingsson E., et al. Beneficial effects of individualized physical activity on prescription on body composition and cardiometabolic risk factors: results from a randomized controlled trial. Eur. J. Cardiovasc. Prev. Rehabil. 2009;16(1):80–84. doi: 10.1097/HJR.0b013e32831e953a. [DOI] [PubMed] [Google Scholar]
- 83.Kim S.W., Jung W.S., Park W., Park H.Y. Twelve weeks of combined resistance and aerobic exercise improves cardiometabolic biomarkers and enhances red blood cell hemorheological function in obese older men: a randomized controlled trial. Int. J. Environ. Res. Public Health. 2019;16(24):5020. doi: 10.3390/ijerph16245020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X., Han T., Zou X., Zhang H., Feng W., Wang H., et al. Long-term high-intensity interval training increases serum neurotrophic factors in elderly overweight and obese Chinese adults. Eur. J. Appl. Physiol. 2021;121(10):2773–2785. doi: 10.1007/s00421-021-04746-w. [DOI] [PubMed] [Google Scholar]
- 85.Nunes P.R.P., Martins F.M., Souza A.P., Carneiro M.A.S., Nomelini R.S., Michelin M.A., et al. Comparative effects of high-intensity interval training with combined training on physical function markers in obese postmenopausal women: a randomized controlled trial. Menopause. 2019;26(11):1242–1249. doi: 10.1097/gme.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 86.Park S.M., Kwak Y.S., Ji J.G. The effects of combined exercise on health-related fitness, endotoxin, and immune function of postmenopausal women with abdominal obesity. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/830567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park W., Jung W.S., Hong K., Kim Y.Y., Kim S.W., Park H.Y. Effects of moderate combined resistance- and aerobic-exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int. J. Environ. Res. Public Health. 2020;17(19):7233. doi: 10.3390/ijerph17197233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillips M.D., Patrizi R.M., Cheek D.J., Wooten J.S., Barbee J.J., Mitchell J.B. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med. Sci. Sports Exerc. 2012;44(11):2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- 89.Puengsuwan P., Kuo C.H., Chaunchaiyakul R., Nanagara R., Leelayuwat N. Wand stretching exercise decreases abdominal obesity among adults with high body mass index without altering fat oxidation. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.565573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ribeiro A.S., Schoenfeld B.J., Dos Santos L., Nunes J.P., Tomeleri C.M., Cunha P.M., et al. Resistance training improves a cellular health parameter in obese older women: a randomized controlled trial. J. Strength Cond. Res. 2020;34(10):2996–3002. doi: 10.1519/jsc.0000000000002773. [DOI] [PubMed] [Google Scholar]
- 91.Rossi F.E., Fortaleza A.C., Neves L.M., Buonani C., Picolo M.R., Diniz T.A., et al. Combined training (aerobic plus strength) potentiates a reduction in body fat but demonstrates no difference on the lipid profile in postmenopausal women when compared with aerobic training with a similar training load. J. Strength Cond. Res. 2016;30(1):226–234. doi: 10.1519/jsc.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 92.Sjögren P., Sierra-Johnson J., Kallings L.V., Cederholm T., Kolak M., Halldin M., et al. Functional changes in adipose tissue in a randomised controlled trial of physical activity. Lipids Health Dis. 2012;11:80. doi: 10.1186/1476-511x-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stewart K.J., Bacher A.C., Hees P.S., Tayback M., Ouyang P., Jan de Beur S. Exercise effects on bone mineral density relationships to changes in fitness and fatness. Am. J. Prev. Med. 2005;28(5):453–460. doi: 10.1016/j.amepre.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 94.Tomeleri C.M., Ribeiro A.S., Souza M.F., Schiavoni D., Schoenfeld B.J., Venturini D., et al. Resistance training improves inflammatory level, lipid and glycemic profiles in obese older women: a randomized controlled trial. Exp. Gerontol. 2016;84:80–87. doi: 10.1016/j.exger.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Siu P.M., Yu A.P., Chin E.C., Yu D.S., Hui S.S., Woo J., et al. Effects of tai chi or conventional exercise on central obesity in middle-aged and older adults: a three-group randomized controlled trial. Ann. Intern. Med. 2021;174(8):1050–1057. doi: 10.7326/m20-7014. [DOI] [PubMed] [Google Scholar]
- 96.Serra M.C., Beavers D.P., Henderson R.M., Kelleher J.L., Kiel J.R., Beavers K.M. Effects of a hypocaloric, nutritionally complete, higher protein meal plan on regional body fat and cardiometabolic biomarkers in older adults with obesity. Ann. Nutr. Metab. 2019;74(2):149–155. doi: 10.1159/000497066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porter Starr K.N., Orenduff M., McDonald S.R., Mulder H., Sloane R., Pieper C.F., et al. Influence of weight reduction and enhanced protein intake on biomarkers of inflammation in older adults with obesity. J. Nutr. Gerontol. Geriatr. 2019;38(1):33–49. doi: 10.1080/21551197.2018.1564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogilvie A.R., Watford M., Wu G., Sukumar D., Kwon J., Shapses S.A. Decreased fasting serum glucogenic amino acids with a higher compared to normal protein diet during energy restriction in women: a randomized controlled trial. Amino Acids. 2021;53(9):1467–1472. doi: 10.1007/s00726-021-03053-0. [DOI] [PubMed] [Google Scholar]
- 99.Englert I., Bosy-Westphal A., Bischoff S.C., Kohlenberg-Müller K. Impact of protein intake during weight loss on preservation of fat-free mass, resting energy expenditure, and physical function in overweight postmenopausal women: a randomized controlled trial. Obes. Facts. 2021;14(3):259–270. doi: 10.1159/000514427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beavers K.M., Nesbit B.A., Kiel J.R., Sheedy J.L., Arterburn L.M., Collins A.E., et al. Effect of an energy-restricted, nutritionally complete, higher protein meal plan on body composition and mobility in older adults with obesity: a randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(6):929–935. doi: 10.1093/gerona/gly146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dengo A.L., Dennis E.A., Orr J.S., Marinik E.L., Ehrlich E., Davy B.M., et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55(4):855–861. doi: 10.1161/hypertensionaha.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beavers K.M., Gordon M.M., Easter L., Beavers D.P., Hairston K.G., Nicklas B.J., et al. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study. J. Nutr. Health Aging. 2015;19(1):87–95. doi: 10.1007/s12603-015-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Backx E.M., Tieland M., Borgonjen-van den Berg K.J., Claessen P.R., van Loon L.J., de Groot L.C. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int. J. Obes. (Lond) 2016;40(2):299–304. doi: 10.1038/ijo.2015.182. [DOI] [PubMed] [Google Scholar]
- 104.Irwin M.L., Yasui Y., Ulrich C.M., Bowen D., Rudolph R.E., Schwartz R.S., et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289(3):323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 105.Teng N.I., Shahar S., Rajab N.F., Manaf Z.A., Johari M.H., Ngah W.Z. Improvement of metabolic parameters in healthy older adult men following a fasting calorie restriction intervention. Aging Male. 2013;16(4):177–183. doi: 10.3109/13685538.2013.832191. [DOI] [PubMed] [Google Scholar]
- 106.Hsu K.J., Chien K.Y., Tsai S.C., Tsai Y.S., Liao Y.H., Chen J.J., et al. Effects of exercise alone or in combination with high-protein diet on muscle function, aerobic capacity, and physical function in middle-aged obese adults: a randomized controlled trial. J. Nutr. Health Aging. 2021;25(6):727–734. doi: 10.1007/s12603-021-1599-1. [DOI] [PubMed] [Google Scholar]
- 107.Brennan A.M., Standley R.A., Yi F., Carnero E.A., Sparks L.M., Goodpaster B.H. Individual response variation in the effects of weight loss and exercise on insulin sensitivity and cardiometabolic risk in older adults. Front. Endocrinol. (Lausanne) 2020;11:632. doi: 10.3389/fendo.2020.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Félix-Soriano E., Martínez-Gayo A., Cobo M.J., Pérez-Chávez A., Ibáñez-Santos J., Palacios Samper N., et al. Effects of DHA-rich n-3 fatty acid supplementation and/or resistance training on body composition and cardiometabolic biomarkers in overweight and obese post-menopausal women. Nutrients. 2021;13(7):2465. doi: 10.3390/nu13072465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grossman J.A., Arigo D., Bachman J.L. Meaningful weight loss in obese postmenopausal women: a pilot study of high-intensity interval training and wearable technology. Menopause. 2018;25(4):465–470. doi: 10.1097/gme.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 110.Hays N.P., Starling R.D., Liu X., Sullivan D.H., Trappe T.A., Fluckey J.D., et al. Effects of an ad libitum low-fat, high-carbohydrate diet on body weight, body composition, and fat distribution in older men and women: a randomized controlled trial. Arch. Intern. Med. 2004;164(2):210–217. doi: 10.1001/archinte.164.2.210. [DOI] [PubMed] [Google Scholar]
- 111.Jo E., Worts P.R., Elam M.L., Brown A.F., Khamoui A.V., Kim D.H., et al. Resistance training during a 12-week protein supplemented VLCD treatment enhances weight-loss outcomes in obese patients. Clin. Nutr. 2019;38(1):372–382. doi: 10.1016/j.clnu.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 112.Kelly K.R., Navaneethan S.D., Solomon T.P., Haus J.M., Cook M., Barkoukis H., et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med. Sci. Sports Exerc. 2014;46(5):920–926. doi: 10.1249/mss.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mulya A., Haus J.M., Solomon T.P., Kelly K.R., Malin S.K., Rocco M., et al. Exercise training-induced improvement in skeletal muscle PGC-1α-mediated fat metabolism is independent of dietary glycemic index. Obesity (Silver Spring) 2017;25(4):721–729. doi: 10.1002/oby.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muollo V., Rossi A.P., Milanese C., Masciocchi E., Taylor M., Zamboni M., et al. The effects of exercise and diet program in overweight people - Nordic walking versus walking. Clin. Interv. Aging. 2019;14:1555–1565. doi: 10.2147/cia.S217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muollo V., Rossi A.P., Milanese C., Zamboni M., Rosa R., Schena F., et al. Prolonged unsupervised Nordic walking and walking exercise following six months of supervision in adults with overweight and obesity: a randomised clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021;31(4):1247–1256. doi: 10.1016/j.numecd.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 116.Solomon T.P., Haus J.M., Cook M.A., Flask C.A., Kirwan J.P. A low-glycemic diet lifestyle intervention improves fat utilization during exercise in older obese humans. Obesity (Silver Spring) 2013;21(11):2272–2278. doi: 10.1002/oby.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Gemert W.A., Schuit A.J., van der Palen J., May A.M., Iestra J.A., Wittink H., et al. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: the SHAPE-2 trial. Breast Cancer Res. 2015;17(1):120. doi: 10.1186/s13058-015-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnard N.D., Alwarith J., Rembert E., Brandon L., Nguyen M., Goergen A., et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. J. Am. Nutr. Assoc. 2022;41(2):127–139. doi: 10.1080/07315724.2020.1869625. [DOI] [PubMed] [Google Scholar]
- 119.Barnard N.D., Scialli A.R., Turner-McGrievy G., Lanou A.J., Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005;118(9):991–997. doi: 10.1016/j.amjmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 120.Boukabous I., Marcotte-Chénard A., Amamou T., Boulay P., Brochu M., Tessier D., et al. Low-volume high-intensity interval training (HIIT) versus moderate-intensity continuous training on body composition, cardiometabolic profile and physical capacity in older women. J. Aging Phys. Act. 2019;27(4):879–889. doi: 10.1123/japa.2018-0309. [DOI] [PubMed] [Google Scholar]
- 121.Carneiro M.A.S., Oliveira Júnior G.N., de Sousa J.F.R., Orsatti C.L., Murta E.F.C., Michelin M.A., et al. Effect of whole-body resistance training at different load intensities on circulating inflammatory biomarkers, body fat, muscular strength, and physical performance in postmenopausal women. Appl. Physiol. Nutr. Metab. 2021;46(8):925–933. doi: 10.1139/apnm-2020-0746. [DOI] [PubMed] [Google Scholar]
- 122.Izzicupo P., D’Amico M.A., Di Blasio A., Napolitano G., Di Baldassarre A., Ghinassi B. Nordic walking increases circulating VEGF more than traditional walking training in postmenopause. Climacteric. 2017;20(6):533–539. doi: 10.1080/13697137.2017.1366979. [DOI] [PubMed] [Google Scholar]
- 123.Barbour J.A., Howe P.R., Buckley J.D., Bryan J., Coates A.M. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7(9):7381–7398. doi: 10.3390/nu7095343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Christensen P., Bliddal H., Riecke B.F., Leeds A.R., Astrup A., Christensen R. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clin. Obes. 2011;1(1):31–40. doi: 10.1111/j.1758-8111.2011.00006.x. [DOI] [PubMed] [Google Scholar]
- 125.Goss A.M., Gower B., Soleymani T., Stewart M., Pendergrass M., Lockhart M., et al. Effects of weight loss during a very low carbohydrate diet on specific adipose tissue depots and insulin sensitivity in older adults with obesity: a randomized clinical trial. Nutr. Metab. (Lond) 2020;17:64. doi: 10.1186/s12986-020-00481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ilich J.Z., Kelly O.J., Liu P.Y., Shin H., Kim Y., Chi Y., et al. Role of calcium and low-fat dairy foods in weight-loss outcomes revisited: results from the randomized trial of effects on bone and body composition in overweight/obese postmenopausal women. Nutrients. 2019;11(5):1157. doi: 10.3390/nu11051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katz D.L., Davidhi A., Ma Y., Kavak Y., Bifulco L., Njike V.Y. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J. Am. Coll. Nutr. 2012;31(6):415–423. doi: 10.1080/07315724.2012.10720468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kristensen M., Toubro S., Jensen M.G., Ross A.B., Riboldi G., Petronio M., et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J. Nutr. 2012;142(4):710–716. doi: 10.3945/jn.111.142315. [DOI] [PubMed] [Google Scholar]
- 129.Njike V.Y., Kavak Y., Treu J.A., Doughty K., Katz D.L. Snacking, satiety, and weight: a randomized, controlled trial. Am. J. Health Promot. 2017;31(4):296–301. doi: 10.4278/ajhp.150120-QUAN-676. [DOI] [PubMed] [Google Scholar]
- 130.Shapses S.A., Heshka S., Heymsfield S.B. Effect of calcium supplementation on weight and fat loss in women. J. Clin. Endocrinol. Metab. 2004;89(2):632–637. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wien M.A., Sabaté J.M., Iklé D.N., Cole S.E., Kandeel F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. 2003;27(11):1365–1372. doi: 10.1038/sj.ijo.0802411. [DOI] [PubMed] [Google Scholar]
- 132.Dennis E.A., Dengo A.L., Comber D.L., Flack K.D., Savla J., Davy K.P., et al. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 2010;18(2):300–307. doi: 10.1038/oby.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.National Institute for Health Care and Excellence . 2014. Obesity: identification, assessment and management.https://www.nice.org.uk/guidance/cg189 [Internet] Available from: [PubMed] [Google Scholar]
- 134.Batsis J.A., Villareal D.T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ravussin E., Smith S.R., Ferrante A.W., Jr. Physiology of energy expenditure in the weight-reduced state. Obesity (Silver Spring) 2021;29(Suppl 1):S31–S38. doi: 10.1002/oby.23095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weinheimer E.M., Sands L.P., Campbell W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68(7):375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 137.Wang H., Huang W.Y., Zhao Y. Efficacy of exercise on muscle function and physical performance in older adults with sarcopenia: an updated systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2022;19(13):8212. doi: 10.3390/ijerph19138212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCarthy D., Berg A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients. 2021;13(7):2473. doi: 10.3390/nu13072473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cioffi I., Evangelista A., Ponzo V., Ciccone G., Soldati L., Santarpia L., et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018;16(1):371. doi: 10.1186/s12967-018-1748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaston T.B., Dixon J.B., O’Brien P.E. Changes in fat-free mass during significant weight loss: a systematic review. Int. J. Obes. (Lond) 2007;31(5):743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 141.Tobias D.K., Chen M., Manson J.E., Ludwig D.S., Willett W., Hu F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968–979. doi: 10.1016/S2213-8587(15)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boirie Y., Guillet C. Fast digestive proteins and sarcopenia of aging. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21(1):37–41. doi: 10.1097/mco.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 143.Gryson C., Walrand S., Giraudet C., Rousset P., Migné C., Bonhomme C., et al. Fast proteins” with a unique essential amino acid content as an optimal nutrition in the elderly: growing evidence. Clin. Nutr. 2014;33(4):642–648. doi: 10.1016/j.clnu.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Domingues-Faria C., Boirie Y., Walrand S. Vitamin D and muscle trophicity. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20(3):169–174. doi: 10.1097/mco.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 145.Tessier A.J., Chevalier S. An update on protein, leucine, omega-3 fatty acids, and vitamin D in the prevention and treatment of sarcopenia and functional decline. Nutrients. 2018;10(8):1099. doi: 10.3390/nu10081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Christensen P., Meinert Larsen T., Westerterp-Plantenga M., Macdonald I., Martinez J.A., Handjiev S., et al. Men and women respond differently to rapid weight loss: metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW) Diabetes Obes. Metab. 2018;20(12):2840–2851. doi: 10.1111/dom.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chaimani A., Caldwell D.M., Li T., Higgins J.P., Salanti G. In: Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. John Wiley & Sons; Chichester, UK: 2019. Undertaking network meta-analyses; pp. 285–320. [Google Scholar]
- 148.Schwarzer G., Carpenter J.R., Rücker G. Springer; New York City: 2015. Small-study effects in meta-analysis, Meta-analysis with R; pp. 107–141. [Google Scholar]
- 149.Schwingshackl L., Buyken A., Chaimani A. Network meta-analysis reaches nutrition research. Eur. J. Nutr. 2019;58(1):1–3. doi: 10.1007/s00394-018-1849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.