Abstract
Although waterborne virus removal may be achieved using separation membrane technologies, such technologies remain largely inefficient at generating virus-free effluents due to the lack of anti-viral reactivity of conventional membrane materials required to deactivating viruses. Here, a stepwise approach towards simultaneous filtration and disinfection of Human Coronavirus 229E (HCoV-229E) in water effluents, is proposed by engineering dry-spun ultrafiltration carbon nanotube (CNT) membranes, coated with anti-viral SnO2 thin films via atomic layer deposition. The thickness and pore size of the engineered CNT membranes were fine-tuned by varying spinnable CNT sheets and their relative orientations on carbon nanofibre (CNF) porous supports to reach thicknesses less than 1 µm and pore size around 28 nm. The nanoscale SnO2 coatings were found to further reduce the pore size down to ∼21 nm and provide more functional groups on the membrane surface to capture the viruses via size exclusion and electrostatic attractions. The synthesized CNT and SnO2 coated CNT membranes were shown to attain a viral removal efficiency above 6.7 log10 against HCoV-229E virus with fast water permeance up to ∼4 × 103 and 3.5 × 103 L.m−2.h−1.bar−1, respectively. Such high performance was achieved by increasing the dry-spun CNT sheets up to 60 layers, orienting successive 30 CNT layers at 45°, and coating 40 nm SnO2 on the synthesized membranes. The current study provides an efficient scalable fabrication scheme to engineer flexible ultrafiltration CNT-based membranes for cost-effective filtration and inactivation of waterborne viruses to outperform the state-of-the-art ultrafiltration membranes.
Keywords: Carbon nanotube membrane, Virus ultrafiltration, Human Coronavirus 229E, Antiviral metal oxide coatings, Fast water permeation
Graphical Abstract
1. Introduction
The affordable access to safe clean water resources is one of the most global challenges to meet nowadays [1], [2], [3]. According to World Health Organization (WHO), about 785 million people suffer from the lack of even a simple drinking water resource, while around 2 billion people consume drinking water contaminated with pathogens [4]. Among waterborne pathogens, viruses are highly widespread microorganisms that have smaller sizes (20–120 nm) and lower infectious doses, making them more challenging to be disinfected [5], [6], [7]. Recent studies have reported the detection of human Coronavirus 2019 (COVID-19) in wastewater through the discharge from households and hospitals [8], [9], [10], [11]. Therefore, the demand for efficient waterborne virus remediation strategies is increased to limit the spread of such virus outbreaks [12], [13], [14]. Conventional remediation methods of waterborne viruses include chlorination [15], [16], UV irradiation [17], [18], ozonation [17], and membrane filtration [13], [19]. However, the production of disinfection by-products is usually associated with chlorination, especially at higher doses of chlorine [20], [21], while the UV irradiation was found to exhibit limited viral removal efficiency with LRV up to 2 log10 [22], [23]. In contrast, ozonation technology was found to achieve LRV of 4.5 log10 against MS2 virus due to the presence of strong reactive oxidizing species [24]. However, this technology was hindered by the high energy demands to generate ozone oxidizing species [25], [26].
Among the different virus remediation methods, membrane filtration technologies are considered as promising routes for cost-effective removal of viruses based on size exclusion and physicochemical interactions between viruses and membrane surfaces [27], [28], [29]. Microfiltration (MF) and ultrafiltration (UF) techniques are also widely used for waterborne virus removal [30], [31]. MF and UF membranes were found to reject various waterborne viruses with LRV of 2–4 log10 [32], [33]. However, complex virus structures with small sizes from 20 to 100 nm may yet diffuse across MF or UF membranes, leading to reduced viral removal efficiency [34]. In comparison to MF and UF, reverse osmosis (RO) and nanofiltration (NF) were emphasized as the most effective membrane filtration methods supporting the remediation of multiple waterborne viruses with LRV up to 6 log10 due to their tight pore size that can reach below 1 nm [35]. Nonetheless, RO and NF technologies require high applied pressures to allow fast water permeation across the tight pores of RO and NF membranes [36], [37]. Membrane fouling is another issue, which deteriorates the RO and NF membrane performance [38] due to the accumulation of several organic contaminants on the membrane surface during wastewater treatment [39], [40], [41]. The key challenge in membrane-based virus remediation is to design highly porous robust membrane structures with narrow pore size distribution and high reactivity towards virus separation and inactivation at fast water permeation [42], [43]. A promising approach to overcome the latter challenge include assembling one-dimensional (1D) nanomaterials, such as polymeric nanofibres [44], inorganic nanowires [45], and carbon nanotubes (CNTs) [46] into resilient macro-scale membrane materials with high porosity and anti-viral reactivity.
In particular, CNTs have attracted a considerable interest to manufacture iso-porous membranes with high adsorption capacity for the removal and inactivation of multiple pathogens, including viruses [47], [48], [49]. The CNT membranes were classified based on their structure into three main types: vertically aligned, bucky-paper (BP), and mixed-matrix CNT membranes [50], [51], [52]. In the vertically aligned CNT membranes, the CNTs were aligned perpendicularly on a substrate to enable the water transport through the CNT interior [53] while in BP and mixed-matrix CNT membranes, the CNTs were randomly oriented within large porous membrane structures [54], [55]. The vertical alignment of CNTs into resilient membrane structures is quite complicated process to achieve and scale-up, while gaps between the nanotubes require to be filled by polymeric or inorganic materials to support the membrane structure, leading to permeability decline [56], [57], [58].
On the other hand, BP and mixed matrix CNT membranes were commonly reported for the removal of waterborne viruses due to their feasible fabrication methodology [19], [59]. BP CNT membranes were fabricated by vacuum filtration of CNT dispersed solutions on microporous supports, resulting in highly porous CNT membranes with relative porosity up to 90%. Single-walled (SW) BP CNT membranes were found to achieve LRV of 3.2 log10 against MS2 bacteriophage [49]. Conversely, Multi-walled (MW) BP CNT membranes were found to exhibit LRV of 5.4 log10 against MS2 bacteriophage [60]. The viral removal efficiency of BP CNT membranes was further improved by applying an electric field (2–3 V) to reach LRV of 6.2 log10 against MS2 bacteriophage [48]. In another fabrication strategy, mixed matrix CNT-based membranes were synthesized by incorporating CNTs with hydrophilic inorganic or polymeric materials to improve removal efficiency of water borne viruses and provide additional functionality on CNT surface. For example, dispersions of CNTs and anti-viral silver (Ag) nanoparticles were coated on microporous supports to generate Ag-CNT nanocomposites with a tight pore size (∼30 nm), and thus LRV above 4 log10 was attained against CoxA9 virus at a water permeance of ∼992 L.m−2.h−1.bar−1 [61]. In another study, CNT-based membranes grafted with amphiphilic block copolymers were found to achieve LRV above 6 log10 against phage viruses at a fast water permeance around 3300 L.m−2.h−1.bar−1 [19]. The current fabrication strategies of CNT membranes may be promising to upscale their production towards virus capture and inactivation from water effluents. However, the fabrication of BP and mixed matrix CNT membranes require complicated wet chemistry methods to disperse and process the CNTs into resilient membrane structures [62]. In addition, CNT-based membranes may still lack proper CNT alignment, pore size and geometry control [59], [63].
Furthermore, hydrophilic metal oxides, such as TiO2, ZnO, and SnO2 nanoparticles and thin film coatings were reported as effective anti-viral and disinfectant nanomaterials through electrostatic interaction, reactive oxygen generation, and virus receptor inactivation mechanisms [64], [65], [66]. Thus, the combination of CNTs with hydrophilic metal oxides via chemical impregnation was found to enhance their wettability and anti-viral properties against waterborne viruses [67], [68]. However, the investigation of anti-viral metal oxide coating on CNT membranes for waterborne virus capture has not been studied yet. Here, a novel method is proposed to synthesize ultrafiltration CNT membranes using CNT dry drawing technology for synergistic separation and inactivation of waterborne viruses. The dry spinning of aligned CNT sheets enables the generation of CNT membranes with fine-tuned nanoscale thicknesses and narrow pore size distributions by controlling the orientation between the dry-spun CNT sheets and inter-CNT contacts. Anti-viral hydrophilic SnO2 coatings were introduced on CNT-based membranes via atomic layer deposition (ALD) technique to fine-tune the pore size and surface charge of the membrane for virus capture via size exclusion and electrostatic interactions at fast water permeance. This technology will support a facile, scalable manufacturing of anti-viral ultrafiltration CNT membranes with controlled pore size and surface charge for cost-effective virus removal and inactivation from water effluents.
2. Materials and methods
2.1. Materials
The precursors of poly(acrylonitrile) (PAN) were purchased from Jilin Tanggu (China). Ethanol and Dimethylformamide (DMF) organic solvents were provided by chem-supply (Australia). The drawable forests of CNTs (diameters: 8–9 nm, density: 40–55 mg.cm−3, height: 280–350 µm) were obtained from Lintec of America, Inc. Tertrakis(dimethylamino)tin(IV) (TDMASn) precursor was obtained from Strem chemicals (USA).
2.2. CNT-based membrane synthesis
First, carbon nanofibre (CNF) support membranes were fabricated via the carbonization of stabilized electrospun 12 wt% PAN membranes as previously reported (Fig. S1) [69]. CNT membranes were then synthesized by the dry spinning of CNT sheets on CNF support membranes fixed on a drum collector with a 15-rpm speed as reported in our recent work (Fig. S2) [70]. Different numbers of CNT layers were collected on the CNF support membranes, ranging from 10 and 30–60 CNT layers to control the thickness of the synthesized CNT membranes. The relative orientation between the dry-spun CNT layers was also controlled by changing the orientation angle between the CNT layers of 30-layer CNT membrane from 0 and 45–90°. The multi-layers of CNT sheets were then densified by ethanol solvent spraying, followed by subsequent evaporation of the solvent [71], [72]. The densified CNT membranes were then kept for drying at room temperature for 24 h until resilient and stable CNT-based membranes were obtained. A graphical presentation of CNT-based membrane fabrication process is described in Fig. S3.
2.3. Synthesis of SnO2-CNT membranes
SnO2-CNT membranes were developed by SnO2 coating of CNT membranes utilizing FIJI F200 ALD facility (Cambridge Nanotech) at 100 °C as reported in our recent work [73]. TDMASn was used as Sn precursor, while oxygen plasma was utilized as an oxygen precursor with a 100-sccm oxygen flow and a 300-W plasma power [74], [75]. Four sequential steps were performed to complete a typical ALD cycle: (i) oxygen plasma exposure (3 s), (ii) Ar gas purging (8 s), (iii) Sn precursor pulsing (0.15 s), and (iv) Ar gas purging (8 s). The thickness of SnO2 coatings on CNT membranes was fine-tuned by changing the SnO2 deposition cycles from 50 and 250–400.
2.4. Material characterization
Scanning electron microscopy (SEM) was utilized to analyze the morphology of CNF, CNT, and SnO2 coated CNT membranes utilizing Zeiss Supra 55VP at an accelerating voltage of 5 kV. Transmission electron microscopy (TEM) was used to evaluate the microstructure of the synthesized membranes using JEM-2100 F at an accelerating voltage of 200 kV.
The thicknesses of pristine CNT membranes were determined by profilometry technique using Olympus LEXT OLS4000 confocal microscope. The thicknesses of SnO2 coatings on reference silicon wafers were measured using ellipsometry technique by M-2000 Ellipsometer (J.A. Woollam, USA). The modelling of SnO2 thickness was then conducted by Complete EASE software using B-spline formula.
The mean pore sizes of the different synthesized membranes were identified using a Quantachrome porometer instrument (3GZH, U.S.A.). A Porofil liquid was utilized to wet the membranes. A pressurized gas of N2 was then applied to push the wetting Porofil liquid through the analyzed membranes at an applied pressure of 6.4–34.5 bar.
X-ray diffraction (XRD) patterns of the synthesized membranes were collected using PANalytical, Xpert Powder. The surface chemistry of the synthesized membranes was also analyzed using X-ray photoelectron spectroscopy (XPS) at RMIT University using Kratos Axis Supra with Al Kα X-ray Source. The analyzed elements were calibrated relative to the carbon standard peak at 284.6 eV [76].
The surface charge of the synthesized membranes was analyzed by a SurPASS 3 streaming potential analyzer (Anton Paar, Australia). KCl electrolyte (0.1 M), HCl (0.1 M) and KOH (0.1 M) titrants were utilized to determine the streaming potentials of the analyzed membranes at a pH range from 2 to 11. The membrane wettability was evaluated utilizing water contact angle measurements by (Biolin Scientific) goniometer. OneAttension Theta Lite software was used to record the measured water contact angles.
The water permeance of the membranes was calculated using cross-flow cell filtration module with a membrane area of 25 × 10−4 m2 at an applied pressure of 0.5 bar. The water permeance was then determined based on Eq. 1 [77]:
| (1) |
2.5. Virus removal performance testing
The Human Coronavirus 229E (HCoV-229E) was utilized as a model virus to evaluate the membrane filtration performance in the department of virology at the University of Melbourne as reported previously [13]. HCoV-229E is a mild respiratory virus, causing flu-like symptoms in humans [78], [79]. In this work, the quantification of HCoV-229E titres was conducted using Median Tissue Culture Infectious Dose (TCID50) on lung fibroblasts (MRC-5). TCID50 is a commonly used protocol to quantify the infectious viruses in the evaluated samples [80]. Briefly, 30 μl filtered solutions were collected, followed by ten-fold serial dilutions. A 50 μl volume of each dilution was then inoculated to 6 wells of a 98-well plate for five days at 37 °C. A light microscope was then used to examine the cytopathology and determine the suspension dilution, which initiates cytopathology in half of the cultured wells. An empty holder was used during virus filtration experiment as a control to assess possible losses of the virus adsorbed on the membrane holder. The initial HCoV-229E virus titre in the influent feed was 5 × 106 TCID50 U/mL, while the viral removal efficiency was evaluated based on the log reduction value (LRV) of HCoV-229E virus calculated by Eq. 2 [48], [81]:
| (2) |
where Cf and Cp represent the virus titre (TCID50 U/mL) of HCoV-229E in the influent feed and effluent permeate, respectively.
3. Results and discussion
Here, the impacts of CNT layers, CNT orientation angle and deposited SnO2 thickness on the morphology and physicochemical properties of CNT-based membranes, are explained and linked to the membrane water permeation performance and viral removal efficiency. The suggested mechanisms for the virus removal are then elucidated based on the physicochemical properties of the synthesized CNT-based membranes.
3.1. CNT-based membrane morphology
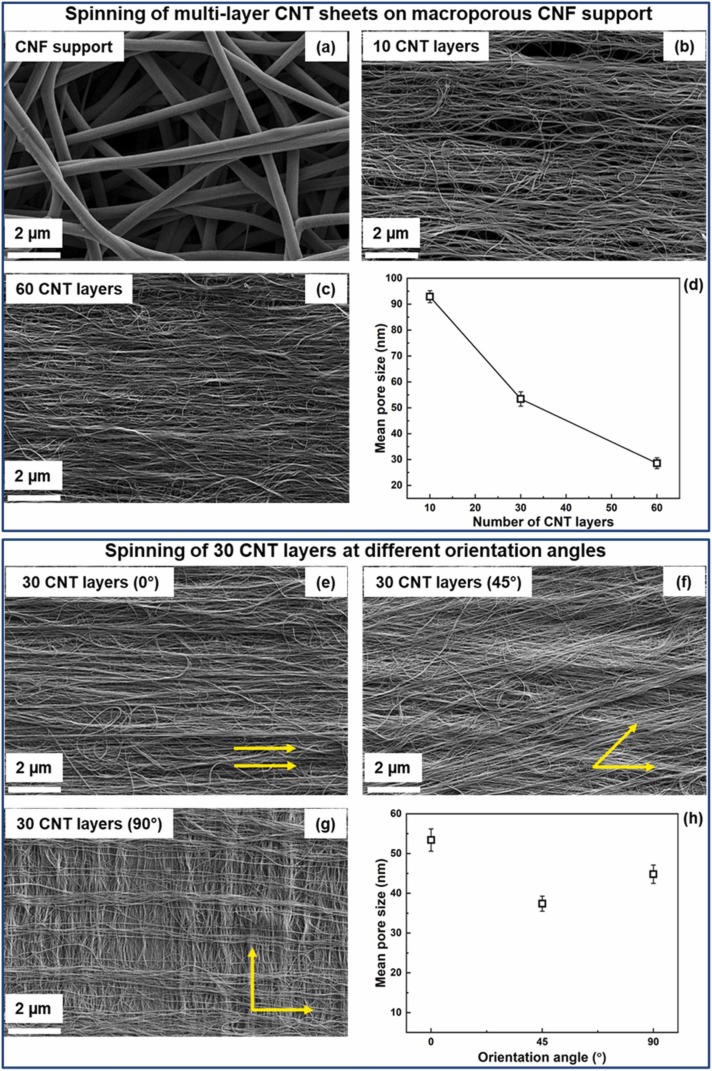
The CNT membranes were synthesized on CNF porous supports via solid state CNT dry spinning technology, followed by ethanol solvent densification to generate resilient CNT-based membranes as described in our recent work [73]. The thickness and orientation of CNT membranes was optimized by changing the number of dry-spun CNT layers and their relative orientations. The SEM morphology of CNF supports and CNT membranes with different CNT layers is shown in Fig. 1a-c. The large pore size of CNF support (∼ 3.6 µm) (Fig. 1a) was significantly reduced with increasing the number of aligned dry-spun CNT layers from 10 to 60 on CNF support to reach down to 92.9 and 28.6 nm, respectively, indicating the full coverage of CNF surface with compact and tightly-stacked CNT membranes (Fig. 1b-d). The reduction of pore size upon assembling multi-layers of CNTs can be explained by the increased CNT membrane thickness from 0.82 to 3.15 µm, corresponding to 10–60 CNT layers as depicted in Fig. S4. The corresponding pore size distributions of CNF and multi-layer CNT membranes are presented in Fig. S6a-c. Another route to fine-tune the membrane morphology and pore size was proposed by changing the orientation angle between the dry-spun CNT layers. As shown in Fig. 1e-h and S6d-f, CNT junctions were generated after orienting the overlapped layers of 30-layer CNT membranes at 45 and 90°, while their mean pore size of 53.4 nm was reduced to 37.4 and 44.8 nm, respectively. The pore size reduction after orienting overlapped CNT layers at varied angles can be attributed to the production of tightly-stacked CNT membranes with CNT junctions. Thus, the generation of CNT junctions between overlapped CNTs at different angles may affect the pore size and geometry of CNT membranes, resulting in improved membrane selectivity [82]. This can be also considered as a cost-effective approach to fine-tune the CNT membrane pore size without using larger number of the dry-spun CNT layers.
Fig. 1.
. (a-c) The SEM morphology of CNF support and multi-layer CNT membranes. (d) The mean pore size variation of CNT membranes with the number of dry-spun CNT layers. (e-g) SEM images of 30-layer CNT membranes oriented at different angles (0,45, and 90°). (h) The change in the CNT membrane pore size with the orientation angle between the dry-spun CNT layers.
3.2. SnO2-CNT membrane morphology and crystallinity
The ALD of anti-viral hydrophilic SnO2 coatings on 30-layer CNT membranes was conducted to induce the surface reactivity and control the pore size without deteriorating the water permeation across hydrophilic SnO2-CNT membranes. As presented across the SEM and TEM images in Fig. 2a-f, the external diameter of CNTs was consistently increased with thicker SnO2 coatings from 5 to 40 nm (Fig. 2g), leading to a reduction in the interspaces between the CNTs. The reduction in the interspaces between the CNTs was found to decrease the pore size of SnO2 coated CNT membranes down to 21.2 nm with 40 nm SnO2 (Fig. 2h and S7). The TGA curves of SnO2 coated CNT membranes were used to calculate the residual SnO2 weight percent after the combustion of CNTs in air above 565 °C (Fig. S8) [83], [84]. The SnO2 weight percent was found to increase from 23.82 wt% (5 nm SnO2-CNT) to 77.22 wt% (40 nm SnO2-CNT) as presented in Fig. 3a, which was in line with the SEM and TEM morphology of larger external diameter of CNTs upon SnO2 deposition. Furthermore, XRD patterns of SnO2 coated CNT membranes revealed the presence of distinctive diffraction peaks at 2θ around 26°, 33°, and 53°, corresponding to (110), (101), and (211) crystal lattice planes of rutile SnO2 (Fig. 3b) [85], [86], [87]. The intensities of SnO2 diffraction peaks were improved at thicker SnO2 coatings due to the increased weight percent of SnO2 [86]. Thus, the conformal deposition of anti-viral SnO2 thin films with controllable thickness and weight percent on CNT membranes was verified to produce SnO2-CNT membranes with fine-tuned pore size and surface reactivity.
Fig. 2.
SEM and corresponding TEM images of SnO2-CNT membranes with varied SnO2 thicknesses: (a-b) 5 nm, (c-d) 25 nm, and (e-f) 40 nm. (g) The variation of SnO2 thickness with ALD cycles. (h) The variation of SnO2-CNT membrane pore size with the SnO2 thickness.
Fig. 3.
(a) The variation of SnO2 weight percent with SnO2 thickness. (b) XRD spectra of the synthesized membranes at different SnO2 thicknesses. (c) The deconvoluted O 1 s spectra of 25 nm SnO2 coated CNT membrane. (d) The deconvoluted Sn 3d spectra of 25 nm SnO2 coated CNT membrane. (e) The variation of streaming zeta potential at different pH values from 2 to 11. (f) The change of membrane wettability with SnO2 thicknesses.
3.3. Chemical composition and physical properties of SnO2-CNT membranes
XPS analysis was used to analyze the chemical composition of SnO2-CNT membranes as presented in Fig. 3c-d and S8–10. In Fig. 3c, the O 1 s main peak of 25 nm SnO2-CNT was deconvoluted into 3 peaks: O–C/O–H (∼531.70 eV), C O (∼532.96 eV), and O-Sn (∼530.46), demonstrating the covalent Sn–O–C bonding between SnO2 and CNTs [88], [89]. In Fig. 3d, the Sn 3d main peak of 25 nm SnO2-CNT was fitted into 2 peaks: Sn 3d3/2 (∼495.06 eV) and Sn 3d5/2 (∼486.60 eV) [83], [90]. The binding energy difference between Sn 3d5/2 and Sn 3d3/2 was measured to be around 8.46 eV with an area ratio of 1.46, which validated the development of tetragonal SnO2 on CNT membranes [76], [89]. The chemical interaction between the deposited SnO2 and CNTs via covalent bonding may alter the surface charge and wettability of CNT-based membranes [88], [89], [91].
The streaming zeta potential of the synthesized membranes was investigated to identify their surface charges at 2–11 pH range (Fig. 3e). The introduction of 5 nm SnO2 coating was found to negatively shift the streaming zeta potential of CNT membranes by 75% to exhibit − 28 mV at pH 6. The membrane surface charge has become more negative with further increase in the deposited SnO2 thickness to reach − 37 mV with 40 nm SnO2 deposition at pH 6. The isoelectric point (IEP) of CNT membranes was also shifted from a pH of 5.1–3.6 upon the deposition of 40 nm SnO2, leading to the generation of more negative charges at broader pH range. The IEP shift exhibited by SnO2-CNT membranes can be attributed to the presence of oxygen containing groups (O–C/O–H) on CNT surface upon SnO2 deposition via oxygen plasma ALD process as presented in the XPS analysis [92], [93], [94]. The presence of oxygen-containing groups on the membrane surface may govern the interactions between waterborne viruses and the active membrane surface [95], [96]. The streaming zeta potential may also affect the wettability of the membrane surface [97].
Thus, the membrane wettability was evaluated by measuring the water contact angle of SnO2-CNT membranes to investigate the impact of SnO2 deposition on the membrane wettability. The water contact angle of pristine CNT membrane was significantly decreased upon the deposition of SnO2 thin films due to their intrinsic hydrophilicity (Fig. 3f) [98], [99], [100]. The water contact angle of pristine CNT membrane was decreased from ∼105–84° upon the deposition of 5 nm SnO2, followed by a significant reduction down to ∼ 25° with thicker SnO2 layers (25–40 nm). The significant reduction in water contact angle at thicker SnO2 layers was due to the presence of hydrophilic SnO2 and the improved charge density on the membrane surface. The improved surface charge and wettability of SnO2-CNT membranes may enable efficient virus removal from aquatic environments.
3.4. Water permeation and viral removal performance of the synthesized membranes
The performance of the synthesized membranes was investigated in terms of water permeance and viral removal efficiency to understand the impact of CNT layering, orientation, and SnO2 coating on the membrane performance. The impact of CNT layering was analyzed by varying the number of dry-spun CNT layers from 10 and 30–60 layers. The 30-layer CNT membrane was selected to analyze the impact of CNT orientation angle from 0 and 45–90° at fixed number of 30 dry-spun CNT layers. The 30-layer CNT membrane aligned at 0° was then selected to analyze the impact of SnO2 coating at fixed CNT orientation angle.
The water permeance of the synthesized membranes was analyzed at an applied pressure of 0.5 bar using crossflow filtration system to evaluate the water permeation performance of the synthesized CNT-based membranes. As shown in Fig. 4a, the CNF support membrane was found to achieve high water permeance of 10.35 × 103 L.m−2.h−1.bar−1 due to their large pore size (∼ 3.6 µm), while the assembly of multi-layer CNTs resulted in a reduction of the water permeance down to 3.83 × 103 L.m−2.h−1.bar−1, corresponding to 60 CNT layers. The achieved water permeance of CNT membranes was still higher than that of the conventional ultrafiltration membranes with only 20–135 L.m−2.h−1.bar−1 [19], [101]. The reduced water permeance of CNT membranes upon increasing the CNT layers was associated with an increased thickness of the selective CNT membranes assembled on the CNF supports from 0.82 µm (10 CNT layers) to 3.15 µm (60 CNT layers), respectively. Interestingly, the orientation of 30-layer CNT membranes at different angles resulted in a reduced water permeance from 4.68 × 103 L.m−2.h−1.bar−1 at 0° to 3.95 × 103 and 4.27 × 103 L.m−2.h−1.bar−1 at 45° and 90°, respectively (Fig. 4b). Such reduced water permeance upon changing the orientation angle between the dry-spun CNT layers can be attributed to the narrowed pore size since CNT junctions were generated between the CNT layers. The introduction SnO2 coatings on CNT membranes was also found to affect their water permeance as depicted in Fig. 4c. The water permeance was slightly enhanced with 5 nm SnO2 coating on the 30-layer CNT membranes to reach 4.92 × 103 L.m−2.h−1.bar−1 owing to the enhanced wettability of membrane surface as explained earlier [100]. However, the water permeance was declined to 4.24 × 103 and 3.51 × 103 L.m−2.h−1.bar−1 with 25 and 40 nm SnO2 coatings, respectively since the impact of pore size reduction became more dominant. Furthermore, the achieved fast water permeance of the developed CNT-based membranes was kept quite stable over the operation time as shown in Fig. S12.
Fig. 4.
Water permeance performance of: (a) CNF support and multi-layer CNT membranes, (b) CNT membranes with different orientation angles between 30 CNT layers, and (c) 30-layer CNT membranes deposited with different SnO2 thicknesses. LRV of HCoV-229E of: (d) CNT-CNF membranes with varied CNT layers, (e) 30-layer CNT membranes with different orientation angles, and (f) 30-layer CNT membranes deposited with different SnO2 thicknesses.
The virus removal efficiency was evaluated using HCoV-229E as a model virus in water. HCoV-229E belongs to the same family of the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) [102]. However, HCoV-229E is classified under the genus Alphacoronaviruses, while SARS-CoV-2 is classified under Betacoronaviruses [103]. Both HCoV-229E and SARS-CoV-2 exhibit similar spike morphology and virion size from 60 to 100 nm [13], [104], [105]. Therefore, HCoV-229E is a typical model virus in this work. When no viruses were detected in the permeate effluent, the virus titre was assumed to be less than 1 TCID50 U/mL for feasible calculation of the virus removal efficiency, and LRV can be reported as higher than log10 (initial virus titre in the influent). This LRV calculation protocol was undertaken and verified in relevant studies in the literature [27], [47], [48], [106].
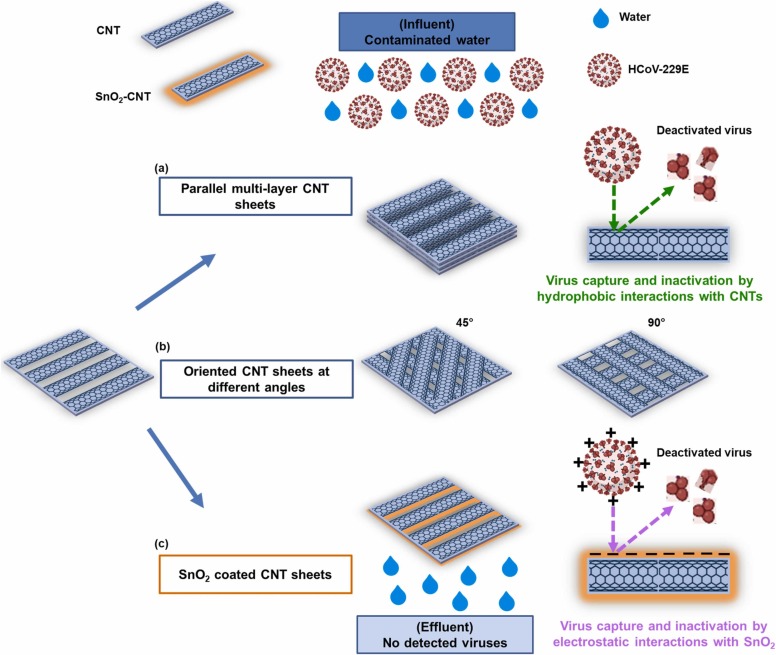
The HCoV-229E virus titre was consistently decreased upon introducing multi-layers of CNTs on the CNF supports (Fig. S13a), while the corresponding log reduction value (LRV) of HCoV-229E was increased accordingly (Fig. 4d). It was also observed that 100% of the virus titre was rejected using the 60-layer CNT membrane with viral removal efficiency above 6.7 log10 due to the produced thicker and tighter CNT membranes upon introducing 60 CNT layers. The influence of CNT orientation at varied angles on the virus removal efficiency of 30-layer CNT membrane is presented in Fig. 4e and S13b. The viral removal efficiency was sharply increased from 5.1 log10 to above 6.7 log10 after orienting the CNTs at 45° due to the tighter pore size and varied pore shapes of the CNT junctions generated between the dry-spun CNT layers at varied orientation angles. The virus rejection mechanism exhibited by the synthesized CNT-based membranes can be explained by the size exclusion and hydrophobic interactions between the virus and the overlapped CNT layers as described in Fig. 5a and b [60], [96], [107]. As depicted in Fig. 4f and S13c, the viral removal efficiency of 30-layer CNT membrane was further enhanced with SnO2 coatings due to the enhanced surface reactivity and reduced pore size. The coating of CNT membrane with 25 nm SnO2 was found to improve the viral removal efficiency from 5.1 to 6.3 log10, while the coating of 40 nm SnO2 was found to boost the viral removal efficiency above 6.7 log10. The virus rejection mechanism by SnO2-CNT membranes can be explained by the size exclusion and electrostatic interactions between the negatively charged membrane surface and the positively charged amine groups present in the virus spike protein as explained in Fig. 5c [64], [65], [67], [108]. In addition, metal oxides, such as SnO2 was reported to exhibit antiviral properties by binding with the viral particles [64], [65], and thus, the viral removal efficiency was improved upon SnO2 coating.
Fig. 5.
A schematic presentation of the virus rejection mechanisms by the different CNT-based membranes with: (a) Parallel multi-layer CNT sheets, (b) Oriented CNT sheets at different angles (0, 45, and 90°), and (c) SnO2 coated CNT sheets.
The above demonstrated performance of the synthesized CNT and SnO2-CNT membranes validates their excellent water permeation and sieving properties towards waterborne virus removal and inactivation compared with relevant CNT membranes reported in the literature ( Table 1). Most of the reported studies have used complicated wet chemistry, followed by vacuum filtration or spray coating methods to deposit tens of micrometres CNT membranes on microporous supports with LRV from 2 to 7 log10 against MS2 bacteriophage [19], [60], [106]. However, the water transport resistance was increased with the larger CNT membrane thickness of above 10 µm [19]. The orientation of CNTs within the membranes could not be controlled with the current fabrication methods, which include randomly aligned CNT-membrane structures. In addition, the functionalization of CNT membranes with anti-viral metal or metal oxides was conducted via several steps of chemical impregnation and hydrothermal techniques, which might affect the intrinsic properties of CNTs. Thus, the uniform coating of CNT membranes with anti-viral metal or metal oxides could not be achieved with the current functionalization strategies. The chemical impregnation of anti-viral metal or metal oxide with CNTs in previous studies was found to display LRV up to 4 log10 against Norovirus and MS2 bacteriophage, respectively [61], [67].
Table 1.
A comparison of the membrane performance with other relevant reported studies.
| Membrane | Fabrication method | Pore size (nm) |
Pressure (bar) |
Virus type | Virus rejection (LRV) | Water permeance (L.m−2.h−1.bar−1) |
Reference |
|---|---|---|---|---|---|---|---|
| MWCNT | Spray pyrolysis | - | 8–11 | MS2 virus | 6–7 log10 | 11.1 | [47] |
| MWCNT | Wet chemistry and Vacuum filtration | ∼100 | - | MS2 virus | 3.7–7.2 log10 | 60–260 | [60] |
| MWCNT | Wet chemistry and Vacuum filtration | ∼93 | - | MS2 virus | 4 log10 | 250 | [106] |
| MWCNT | Wet chemistry and drop casting | ∼280 | 1 | MS2 virus | 0.33 log10 | ∼62.2 | [109] |
| MWCNT-phenol | Wet chemistry and Vacuum filtration | - | - | MS2 virus | 1.54 log10 | - | [110] |
| Ag/MWCNT | Wet chemistry and Vacuum filtration | ∼38 | 5.9 | Norovirus and Poliovirus | > 4 log10 | 988.50 | [61] |
| Cu2O/MWCNT | Chemical impregnation | - | - | MS2 virus | ∼ 6.8 log10 | - | [68] |
| Cu2O/MWCNT | Chemical impregnation | - | - | MS2 virus | 4 log10 | 150 | [67] |
| TiO2/MWCNT | Chemical impregnation | - | - | MS2 virus | 2 log10 | 150 | [67] |
| Metal organic framework-MWCNT | Wet chemistry and drop casting | ∼70 | 1 | MS2 virus | 2.4 log10 | ∼57.4 | [109] |
| Amphiphilic block copolymer/MWCNT | Wet chemistry and Spray coating | ∼44 | 1 | Phage virus | > 6 log10 | 3.33 × 103 | [19] |
| 60-layer MWCNT-CNF aligned at 0° | CNT dry spinning | ∼28 | 0.5 | HCoV-229E | > 6.7 log10 | 3.83 × 103 | This work |
| 30-layer MWCNT-CNF aligned at 45° | CNT dry spinning | ∼37 | 0.5 | HCoV-229E | > 6.7 log10 | 3.95 × 103 | This work |
| 40 nm SnO2 coated 30-layer MWCNT | CNT dry spinning and ALD of SnO2 | ∼21 | 0.5 | HCoV-229E | > 6.7 log10 | 3.51 × 103 | This work |
On the other hand, in the current study, the thickness and orientation of the synthesized CNT membranes were fine-tuned via dry spinning technique of multi-layer CNT sheets, while the uniform coating of anti-viral metal oxide (SnO2) was attained via atomic layer deposition. The synthesized CNT-based membranes exhibited smaller thicknesses down to 1 µm and narrow pore sizes around 21 nm with LRV above 6.7 log10, while the achieved water permeance was 1.5–15 times faster than that attained by previously reported CNT membranes (Table 1). In addition, the atomic layer deposition of anti-viral hydrophilic SnO2 on CNT membranes was found to exhibit LRV above 6.7 log10 against HCoV-229E without compromising the high water permeance. Hence, the synthesized CNT and SnO2 coated CNT membranes with controllable pore size and surface chemistry may enable facile routes for cost-effective virus removal and inactivation at fast water permeation.
4. Conclusions and prospects
In this study, an advanced approach was established to fabricate flexible UF CNT-based membranes for waterborne virus remediation. The proposed dry spinning technology of multi-layer CNTs at different orientation angles on CNF supports enabled the engineering CNT membranes with controllable thickness and pore size down to ∼1 µm and ∼28 nm, respectively. The reactivity of CNT membrane surface was then induced by the coating of hydrophilic antiviral SnO2 thin films via atomic layer deposition technique, while the pore size was further reduced to ∼21 nm. The production of thicker CNT membranes via increased number of CNT layers was found to significantly improve the viral removal efficiency by more than 100% compared with the bare CNF supports. Interestingly, the orientation of successive CNT layers at an orientation angle of 45° was found improve the viral removal efficiency through the developed CNT junctions at different angles. The anti-viral SnO2 coatings were also found to enhance the viral removal efficiency by ∼35% benchmarked with the pristine CNT membranes. The synthesized CNT and SnO2-CNT membranes exhibited 100% HCoV-229E virus removal efficiency above 6.7 log10 with a fast water permeance across the membranes around 4 × 103 L.m−2.h−1.bar−1 and 3.5 × 103 L.m−2.h−1.bar−1, respectively. Overall, this study provides a scaled-up fabrication route of resilient ultrafiltration CNT-based membranes for efficient virus removal from water at an exceptional rate.
CRediT authorship contribution statement
Ahmed O. Rashed: Conceptualization, Methodology, Data acquisition, Writing – original draft preparation; Chi Huynh: Supervision, Writing – reviewing and editing; Andrea Merenda: Data acquisition, Writing – original draft preparation; Julio Rodriguez-Andres: Data acquisition, Writing – original draft preparation; Lingxu e Kong: Supervision, Writing – reviewing and editing; Takeshi Kondo: Supervision, Writing – reviewing and editing; Joselito M. Razal: Supervision, Writing – reviewing and editing; Ludovic F. Dumée: Supervision, Conceptualization, Methodology, Data acquisition, Writing – original draft preparation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
A great part of this study was conducted at Melbourne Centre for Nanofabrication (MCN). The authors acknowledge the Advanced Electron Microscopy instruments at Deakin University and the Microscopy and Microanalysis instruments at RMIT University. The operational team at Carbon Nexus is also acknowledged for the help with the fabrication of CNF support membranes. LINTEC OF AMERICA, INC is greatly acknowledged for providing the utilized CNT forest materials. LFD acknowledges Khalifa University for the support through Project: RC2–2019-007.
Editor: Luigi Rizzo
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jece.2023.110176.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- 1.Qian J., Gao X., Pan B. Nanoconfinement-mediated water treatment: from fundamental to application. Environ. Sci. Technol. 2020;54(14):8509–8526. doi: 10.1021/acs.est.0c01065. [DOI] [PubMed] [Google Scholar]
- 2.Kummu M., Guillaume J.H., de Moel H., Eisner S., Florke M., Porkka M., Siebert S., Veldkamp T.I., Ward P.J. The world's road to water scarcity: shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016;6:38495. doi: 10.1038/srep38495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove W.J., Loucks D.P. Water management: current and future challenges and research directions. Water Resour. Res. 2015;51(6):4823–4839. doi: 10.1002/2014wr016869. [DOI] [Google Scholar]
- 4.WHO., World Health Organization (WHO) Fact sheets/Drinking-water, (2022) Fact sheets.
- 5.Shannon M.A., Bohn P.W., Elimelech M., Georgiadis J.G., Marinas B.J., Mayes A.M. Science and technology for water purification in the coming decades. Nature. 2008;452(7185):301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 6.Pendergast M.M., Hoek E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011;4(6) doi: 10.1039/c0ee00541j. [DOI] [Google Scholar]
- 7.Chen C., Guo L., Yang Y., Oguma K., Hou L.A. Comparative effectiveness of membrane technologies and disinfection methods for virus elimination in water: A review. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran H.N., Le G.T., Nguyen D.T., Juang R.S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.P. SARS-CoV-2 coronavirus in water and wastewater: A critical review about presence and concern. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8(5) doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen D.A., Wigginton K.R. Tracking COVID-19 with wastewater. Nat. Biotechnol. 2020;38(10):1151–1153. doi: 10.1038/s41587-020-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala-Comorera L., Reynolds L.J., Martin N.A., O'Sullivan J.J., Meijer W.G., Fletcher N.F. Decay of infectious SARS-CoV-2 and surrogates in aquatic environments. Water Res. 2021;201 doi: 10.1016/j.watres.2021.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020;8(5) doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merenda A., Bortolassi A.C.C., Rodriguez-Andres J., Al-Attabi R., Schütz J.A., Kujawski W., Shon H.K., Dumée L.F. Hybrid polymer/ionic liquid electrospun membranes with tunable surface charge for virus capture in aqueous environments. J. Water Process Eng. 2021;43 doi: 10.1016/j.jwpe.2021.102278. [DOI] [Google Scholar]
- 14.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - A scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachmadi A.T., Kitajima M., Kato T., Kato H., Okabe S., Sano D. Required chlorination doses to fulfill the credit value for disinfection of enteric viruses in water: a critical review. Environ. Sci. Technol. 2020;54(4):2068–2077. doi: 10.1021/acs.est.9b01685. [DOI] [PubMed] [Google Scholar]
- 16.Ge Y., Zhang X., Shu L., Yang X. Kinetics and mechanisms of virus inactivation by chlorine dioxide in water treatment: a review. Bull. Environ. Contam. Toxicol. 2021;106(4):560–567. doi: 10.1007/s00128-021-03137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong J., Lu Y., Ren Y., Chen Z., Chen M. The virus removal in UV irradiation, ozonation and chlorination. Water Cycle. 2021;2:23–31. doi: 10.1016/j.watcyc.2021.05.001. [DOI] [Google Scholar]
- 18.Storm N., McKay L.G.A., Downs S.N., Johnson R.I., Birru D., de Samber M., Willaert W., Cennini G., Griffiths A. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation. Sci. Rep. 2020;10(1):22421. doi: 10.1038/s41598-020-79600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma D., Li H., Meng Z., Zhang C., Zhou J., Xia J., Wang Y. Absolute and fast removal of viruses and bacteria from water by spraying-assembled carbon-nanotube membranes. Environ. Sci. Technol. 2021;55(22):15206–15214. doi: 10.1021/acs.est.1c04644. [DOI] [PubMed] [Google Scholar]
- 20.Srivastav A.L., Patel N., Chaudhary V.K. Disinfection by-products in drinking water: occurrence, toxicity and abatement. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115474. [DOI] [PubMed] [Google Scholar]
- 21.Du Y., Lv X.T., Wu Q.Y., Zhang D.Y., Zhou Y.T., Peng L., Hu H.Y. Formation and control of disinfection byproducts and toxicity during reclaimed water chlorination: A review. J. Environ. Sci. (China) 2017;58:51–63. doi: 10.1016/j.jes.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Tondera K., Klaer K., Gebhardt J., Wingender J., Koch C., Horstkott M., Strathmann M., Jurzik L., Hamza I.A., Pinnekamp J. Reducing pathogens in combined sewer overflows using ozonation or UV irradiation. Int. J. Hyg. Environ. Health. 2015;218(8):731–741. doi: 10.1016/j.ijheh.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo L., Gernjak W., Krzeminski P., Malato S., McArdell C.S., Perez J.A.S., Schaar H., Fatta-Kassinos D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.136312. [DOI] [PubMed] [Google Scholar]
- 24.Im D., Nakada N., Kato Y., Aoki M., Tanaka H. Pretreatment of ceramic membrane microfiltration in wastewater reuse: a comparison between ozonation and coagulation. J. Environ. Manag. 2019;251 doi: 10.1016/j.jenvman.2019.109555. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Sikora P., Rutgersson C., Lindh M., Brodin T., Bjorlenius B., Larsson D.G.J., Norder H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Environ. Health. 2018;221(3):479–488. doi: 10.1016/j.ijheh.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q., He C., Shan Y., Zhang Z., Li J. Catalytic ozonation of atenolol by Mn-Ce@Al2O3 catalysts: efficiency, mechanism and degradation pathways. J. Environ. Chem. Eng. 2023;11(2) doi: 10.1016/j.jece.2023.109444. [DOI] [Google Scholar]
- 27.Kosiol P., Kahrs C., Thom V., Ulbricht M., Hansmann B. Investigation of virus retention by size exclusion membranes under different flow regimes. Biotechnol. Prog. 2019;35(2) doi: 10.1002/btpr.2747. [DOI] [PubMed] [Google Scholar]
- 28.Dey P., Haldar D., Rangarajan V., Suggala V.S., Saji G., Dilip K.J. Paradigm shift from conventional processes to advanced membrane adsorption-mediated inactivation processes towards holistic management of virus − A critical review. J. Environ. Chem. Eng. 2022;10(6) doi: 10.1016/j.jece.2022.108568. [DOI] [Google Scholar]
- 29.Zhu Y., Chen R., Li Y.Y., Sano D. Virus removal by membrane bioreactors: a review of mechanism investigation and modeling efforts. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116522. [DOI] [PubMed] [Google Scholar]
- 30.Ma H., Hsiao B.S., Chu B. Functionalized electrospun nanofibrous microfiltration membranes for removal of bacteria and viruses. J. Membr. Sci. 2014;452:446–452. doi: 10.1016/j.memsci.2013.10.047. [DOI] [Google Scholar]
- 31.Lu R., Zhang C., Piatkovsky M., Ulbricht M., Herzberg M., Nguyen T.H. Improvement of virus removal using ultrafiltration membranes modified with grafted zwitterionic polymer hydrogels. Water Res. 2017;116:86–94. doi: 10.1016/j.watres.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Gentile G.J., Cruz M.C., Rajal V.B., Fidalgo de Cortalezzi M.M. Electrostatic interactions in virus removal by ultrafiltration membranes. J. Environ. Chem. Eng. 2018;6(1):1314–1321. doi: 10.1016/j.jece.2017.11.041. [DOI] [Google Scholar]
- 33.Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process Eng. 2020;38 doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merenda A., Dumée L.F. Virus remediation in water engineering: are our current technologies up to the challenge? J. Water Process Eng. 2021;44 doi: 10.1016/j.jwpe.2021.102370. [DOI] [Google Scholar]
- 35.Marets N., Kuo D., Torrey J.R., Sakamoto T., Henmi M., Katayama H., Kato T. Highly efficient virus rejection with self-organized membranes based on a crosslinked bicontinuous cubic liquid crystal. Adv. Healthc. Mater. 2017;6(14) doi: 10.1002/adhm.201700252. [DOI] [PubMed] [Google Scholar]
- 36.R. Wlodarczyk, A. Kwarciak-Kozlowska, Treatment of waterborne pathogens by reverse osmosis, in: H. Bridle (Ed.), Waterborne Pathogens2020, pp. 57–80. https://doi.org/10.1016/b978–0-12–818783-8.00004–9.
- 37.Pype M.L., Donose B.C., Marti L., Patureau D., Wery N., Gernjak W. Virus removal and integrity in aged RO membranes. Water Res. 2016;90:167–175. doi: 10.1016/j.watres.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Hachemi C., Enfrin M., Rashed A.O., Jegatheesan V., Hodgson P.D., Callahan D.L., Lee J., Dumee L.F. The impact of PET microplastic fibres on PVDF ultrafiltration performance - A short-term assessment of MP fouling in simple and complex matrices. Chemosphere. 2023;310 doi: 10.1016/j.chemosphere.2022.136891. [DOI] [PubMed] [Google Scholar]
- 39.Virga E., Žvab K., de Vos W.M. Fouling of nanofiltration membranes based on polyelectrolyte multilayers: the effect of a zwitterionic final layer. J. Membr. Sci. 2021;620 doi: 10.1016/j.memsci.2020.118793. [DOI] [Google Scholar]
- 40.Luo Y., Guo W., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., Liang S., Wang X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 473- 2014;474:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 41.Pronk W., Ding A., Morgenroth E., Derlon N., Desmond P., Burkhardt M., Wu B., Fane A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019;149:553–565. doi: 10.1016/j.watres.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 42.Pype M.-L., Lawrence M.G., Keller J., Gernjak W. Reverse osmosis integrity monitoring in water reuse: the challenge to verify virus removal – A review. Water Res. 2016;98:384–395. doi: 10.1016/j.watres.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 43.Zodrow K., Brunet L., Mahendra S., Li D., Zhang A., Li Q., Alvarez P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009;43(3):715–723. doi: 10.1016/j.watres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Fahimirad S., Fahimirad Z., Sillanpaa M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao G., Brastad K.S., Zhang Q., Robinson R., He Z., Li Y. Enhanced disinfection of Escherichia coli and bacteriophage MS2 in water using a copper and silver loaded titanium dioxide nanowire membrane. Front. Environ. Sci. Eng. 2016;10(4) doi: 10.1007/s11783-016-0854-x. [DOI] [Google Scholar]
- 46.Brady-Estevez A.S., Nguyen T.H., Gutierrez L., Elimelech M. Impact of solution chemistry on viral removal by a single-walled carbon nanotube filter. Water Res. 2010;44(13):3773–3780. doi: 10.1016/j.watres.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Mostafavi S.T., Mehrnia M.R., Rashidi A.M. Preparation of nanofilter from carbon nanotubes for application in virus removal from water. Desalination. 2009;238(1):271–280. doi: 10.1016/j.desal.2008.02.018. [DOI] [Google Scholar]
- 48.Rahaman M.S., Vecitis C.D., Elimelech M. Electrochemical carbon-nanotube filter performance toward virus removal and inactivation in the presence of natural organic matter. Environ. Sci. Technol. 2012;46(3):1556–1564. doi: 10.1021/es203607d. [DOI] [PubMed] [Google Scholar]
- 49.Brady-Estevez A.S., Kang S., Elimelech M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small. 2008;4(4):481–484. doi: 10.1002/smll.200700863. [DOI] [PubMed] [Google Scholar]
- 50.Barrejón M., Prato M. Carbon nanotube membranes in water treatment applications. Adv. Mater. Interfaces. 2021;9(1) doi: 10.1002/admi.202101260. [DOI] [Google Scholar]
- 51.Yang H.Y., Han Z.J., Yu S.F., Pey K.L., Ostrikov K., Karnik R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013;4:2220. doi: 10.1038/ncomms3220. [DOI] [PubMed] [Google Scholar]
- 52.Lee B., Baek Y., Lee M., Jeong D.H., Lee H.H., Yoon J., Kim Y.H. A carbon nanotube wall membrane for water treatment. Nat. Commun. 2015;6:7109. doi: 10.1038/ncomms8109. [DOI] [PubMed] [Google Scholar]
- 53.Li S., Liao G., Liu Z., Pan Y., Wu Q., Weng Y., Zhang X., Yang Z., Tsui O.K.C. Enhanced water flux in vertically aligned carbon nanotube arrays and polyethersulfone composite membranes. J. Mater. Chem. A. 2014;2(31):12171–12176. doi: 10.1039/c4ta02119c. [DOI] [Google Scholar]
- 54.Kar S., Bindal R.C., Tewari P.K. Carbon nanotube membranes for desalination and water purification: challenges and opportunities. Nano Today. 2012;7(5):385–389. doi: 10.1016/j.nantod.2012.09.002. [DOI] [Google Scholar]
- 55.Roy S., Jain V., Bajpai R., Ghosh P., Pente A.S., Singh B.P., Misra D.S. Formation of carbon nanotube bucky paper and feasibility study for filtration at the nano and molecular scale. J. Phys. Chem. C. 2012;116(35):19025–19031. doi: 10.1021/jp305677h. [DOI] [Google Scholar]
- 56.Trivedi S., Alameh K. Effect of vertically aligned carbon nanotube density on the water flux and salt rejection in desalination membranes. Springerplus. 2016;5(1):1158. doi: 10.1186/s40064-016-2783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vahdatifar S., Ali Khodadadi A., Mortazavi Y., Greenlee L.F. Functionalized open-ended vertically aligned carbon nanotube composite membranes with high salt rejection and enhanced slip flow for desalination. Sep. Purif. Technol. 2021;279 doi: 10.1016/j.seppur.2021.119773. [DOI] [Google Scholar]
- 58.Yu M., Funke H.H., Falconer J.L., Noble R.D. High density, vertically-aligned carbon nanotube membranes. Nano Lett. 2009;9(1):225–229. doi: 10.1021/nl802816h. [DOI] [PubMed] [Google Scholar]
- 59.Rashed A.O., Merenda A., Kondo T., Lima M., Razal J., Kong L., Huynh C., Dumée L.F. Carbon nanotube membranes – Strategies and challenges towards scalable manufacturing and practical separation applications. Sep. Purif. Technol. 2021;257 doi: 10.1016/j.seppur.2020.117929. [DOI] [Google Scholar]
- 60.Brady-Estevez A.S., Schnoor M.H., Vecitis C.D., Saleh N.B., Elimelech M. Multiwalled carbon nanotube filter: improving viral removal at low pressure. Langmuir. 2010;26(18):14975–14982. doi: 10.1021/la102783v. [DOI] [PubMed] [Google Scholar]
- 61.Kim J.P., Kim J.H., Kim J., Lee S.N., Park H.O. A nanofilter composed of carbon nanotube-silver composites for virus removal and antibacterial activity improvement. J. Environ. Sci. (China) 2016;42:275–283. doi: 10.1016/j.jes.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Ihsanullah Carbon nanotube membranes for water purification: developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019;209:307–337. doi: 10.1016/j.seppur.2018.07.043. [DOI] [Google Scholar]
- 63.Rashed A.O., Esawi A.M.K., Ramadan A.R. Novel polysulfone/carbon nanotube-polyamide thin film nanocomposite membranes with improved water flux for forward osmosis desalination. ACS Omega. 2020;5(24):14427–14436. doi: 10.1021/acsomega.0c00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J., Hu Z., Zabihi F., Chen Z., Zhu M. Progress and perspective of antiviral protective material. Adv. Fiber Mater. 2020;2(3):123–139. doi: 10.1007/s42765-020-00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delumeau L.V., Asgarimoghaddam H., Alkie T., Jones A.J.B., Lum S., Mistry K., Aucoin M.G., DeWitte-Orr S., Musselman K.P. Effectiveness of antiviral metal and metal oxide thin-film coatings against human coronavirus 229E. APL Mater. 2021;9(11) doi: 10.1063/5.0056138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soni V., Khosla A., Singh P., Nguyen V.H., Le Q.V., Selvasembian R., Hussain C.M., Thakur S., Raizada P. Current perspective in metal oxide based photocatalysts for virus disinfection: A review. J. Environ. Manag. 2022;308 doi: 10.1016/j.jenvman.2022.114617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nemeth Z., Szekeres G.P., Schabikowski M., Schrantz K., Traber J., Pronk W., Hernadi K., Graule T. Enhanced virus filtration in hybrid membranes with MWCNT nanocomposite. R. Soc. Open Sci. 2019;6(1) doi: 10.1098/rsos.181294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Domagala K., Jacquin C., Borlaf M., Sinnet B., Julian T., Kata D., Graule T. Efficiency and stability evaluation of Cu(2)O/MWCNTs filters for virus removal from water. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115879. [DOI] [PubMed] [Google Scholar]
- 69.Rashed A.O., Huynh C., Merenda A., Qin S., Maghe M., Kong L., Kondo T., Dumée L.F., Razal J.M. Carbon nanofibre microfiltration membranes tailored by oxygen plasma for electrocatalytic wastewater treatment in cross-flow reactors. J. Membr. Sci. 2023;673 doi: 10.1016/j.memsci.2023.121475. [DOI] [Google Scholar]
- 70.Rashed A.O., Huynh C., Merenda A., Qin S., Maghe M., Kong L., Kondo T., Razal J.M., Dumée L.F. Electrocatalytic ultrafiltration membrane reactors designed from dry-spun self-standing carbon nanotube sheets. Chem. Eng. J. 2023;458 doi: 10.1016/j.cej.2023.141517. [DOI] [Google Scholar]
- 71.Gbordzoe S., Yarmolenko S., Hsieh Y.-Y., Adusei P.K., Alvarez N.T., Fialkova S., Shanov V. Three-dimensional texture analysis of aligned carbon nanotube structures. Carbon. 2017;121:591–601. doi: 10.1016/j.carbon.2017.06.028. [DOI] [Google Scholar]
- 72.Yu X., Zhang X., Zou J., Lan Z., Jiang C., Zhao J., Zhang D., Miao M., Li Q. Solvent-tunable microstructures of aligned carbon nanotube films. Adv. Mater. Interfaces. 2016;3(17):1600352. doi: 10.1002/admi.201600352. [DOI] [Google Scholar]
- 73.Rashed A.O., Huynh C., Merenda A., Qin S., Usman K.A.S., Sadek A., Kong L., Kondo T., Dumée L.F., Razal J.M. Schottky-like photo/electro-catalytic carbon nanotube composite ultrafiltration membrane reactors. Carbon. 2023;204:238–253. doi: 10.1016/j.carbon.2022.12.073. [DOI] [Google Scholar]
- 74.Roy A.K., Dendooven J., Deduytsche D., Devloo-Casier K., Ragaert K., Cardon L., Detavernier C. Plasma-enhanced atomic layer deposition: a gas-phase route to hydrophilic, glueable polytetrafluoroethylene. Chem. Commun. (Camb. ) 2015;51(17):3556–3558. doi: 10.1039/c4cc09474c. [DOI] [PubMed] [Google Scholar]
- 75.Wang C., Zhao D., Grice C.R., Liao W., Yu Y., Cimaroli A., Shrestha N., Roland P.J., Chen J., Yu Z., Liu P., Cheng N., Ellingson R.J., Zhao X., Yan Y. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. A. 2016;4(31):12080–12087. doi: 10.1039/c6ta04503k. [DOI] [Google Scholar]
- 76.Zhu S., Liu J., Sun J. Growth of ultrathin SnO2 on carbon nanotubes by atomic layer deposition and their application in lithium ion battery anodes. Appl. Surf. Sci. 2019;484:600–609. doi: 10.1016/j.apsusc.2019.04.163. [DOI] [Google Scholar]
- 77.He L., Dumée L.F., Feng C., Velleman L., Reis R., She F., Gao W., Kong L. Promoted water transport across graphene oxide–poly(amide) thin film composite membranes and their antibacterial activity. Desalination. 2015;365:126–135. doi: 10.1016/j.desal.2015.02.032. [DOI] [Google Scholar]
- 78.Vassilara F., Spyridaki A., Pothitos G., Deliveliotou A., Papadopoulos A. A rare case of human coronavirus 229e associated with acute respiratory distress syndrome in a healthy adult. Case Rep. Infect. Dis. 2018;2018:6796839. doi: 10.1155/2018/6796839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee F.E., Treanor H., Broaddus J.J.V.C., Mason R.J., Ernst J.D., King T.E., Lazarus S.C. Viral infections. Murray Nadel'S. Textb. Respir. Med. 2016:527–556. [Google Scholar]
- 80.Al-Attabi R., Rodriguez-Andres J., Schütz J.A., Bechelany M., des Ligneris E., Chen X., Kong L., Morsi Y.S., Dumée L.F. Catalytic electrospun nano-composite membranes for virus capture and remediation. Sep. Purif. Technol. 2019;229 doi: 10.1016/j.seppur.2019.115806. [DOI] [Google Scholar]
- 81.Wen J., Tan X., Hu Y., Guo Q., Hong X. Filtration and electrochemical disinfection performance of PAN/PANI/AgNWs-CC composite nanofiber membrane. Environ. Sci. Technol. 2017;51(11):6395–6403. doi: 10.1021/acs.est.6b06290. [DOI] [PubMed] [Google Scholar]
- 82.Beigmoradi R., Samimi A., Mohebbi-Kalhori D. Engineering of oriented carbon nanotubes in composite materials. Beilstein J. Nanotechnol. 2018;9:415–435. doi: 10.3762/bjnano.9.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng Y., Huang J., Qi H., Cao L., Luo X., Li J., Xu Z., Yang J. Controlling the Sn-C bonds content in SnO2@CNTs composite to form in situ pulverized structure for enhanced electrochemical kinetics. Nanoscale. 2017;9(47):18681–18689. doi: 10.1039/c7nr05556k. [DOI] [PubMed] [Google Scholar]
- 84.Zhu S., Liu J., Sun J. Precise growth of Al2O3/SnO2/CNTs composites by a two-step atomic layer deposition and their application as an improved anode for lithium ion batteries. Electrochim. Acta. 2019;319:490–498. doi: 10.1016/j.electacta.2019.07.027. [DOI] [Google Scholar]
- 85.Kumar M., Kumar A., Abhyankar A.C. Occurrence of non-equilibrium orthorhombic SnO2 phase and its effect in preferentially grown SnO2 nanowires for CO detection. RSC Adv. 2015;5(45):35704–35708. doi: 10.1039/c4ra15539d. [DOI] [Google Scholar]
- 86.Wang Y., Su D., Wang C., Wang G. SnO2@MWCNT nanocomposite as a high capacity anode material for sodium-ion batteries. Electrochem. Commun. 2013;29:8–11. doi: 10.1016/j.elecom.2013.01.001. [DOI] [Google Scholar]
- 87.Bashir S., Hossain S.S., Rahman Su, Ahmed S., Amir A.-A., Hossain M.M. Electrocatalytic reduction of carbon dioxide on SnO2/MWCNT in aqueous electrolyte solution. J. CO2 Util. 2016;16:346–353. doi: 10.1016/j.jcou.2016.09.002. [DOI] [Google Scholar]
- 88.Rani N., Khurana K., Jaggi N. Spectroscopic analysis of SnO2 nanoparticles attached functionalized multiwalled carbon nanotubes. Surf. Interfaces. 2021;27 doi: 10.1016/j.surfin.2021.101492. [DOI] [Google Scholar]
- 89.Feng X., Xue J., Zhang T., Zhang Z., Han C., Dai L., Wang L., He Z. Synergistic Catalysis of SnO2-CNTs Composite for VO 2 + /VO(2+) and V(2+)/V(3+) Redox Reactions. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.671575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavithra K., Kumar S.M.S. Embedding oxygen vacancies at SnO2–CNT surfaces via a microwave polyol strategy towards effective electrocatalytic reduction of carbon-dioxide to formate. Catal. Sci. Technol. 2020;10(5):1311–1322. doi: 10.1039/c9cy01960j. [DOI] [Google Scholar]
- 91.Liu M., Zhang S., Dong H., Chen X., Gao S., Sun Y., Li W., Xu J., Chen L., Yuan A., Lu W. Nano-SnO2/carbon nanotube hairball composite as a high-capacity anode material for lithium ion batteries. ACS Sustain. Chem. Eng. 2019;7(4):4195–4203. doi: 10.1021/acssuschemeng.8b05869. [DOI] [Google Scholar]
- 92.Bogdanova N.F., Klebanov A.V., Ermakova L.E., Sidorova M.P., Aleksandrov D.A. Adsorption of ions on the surface of tin dioxide and its electrokinetic characteristics in 1: 1 electrolyte solutions. Colloid J. 2004;66(4):409–417. doi: 10.1023/B:COLL.0000037445.08721.85. [DOI] [Google Scholar]
- 93.Drzymala E., Gruzel G., Pajor-Swierzy A., Depciuch J., Socha R., Kowal A., Warszynski P., Parlinska-Wojtan M. Design and assembly of ternary Pt/Re/SnO2 NPs by controlling the zeta potential of individual Pt, Re, and SnO2 NPs. J. Nanopart. Res. 2018;20(5):144. doi: 10.1007/s11051-018-4244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skwarek E., Bolbukh Y., Tertykh V., Janusz W. Electrokinetic properties of the pristine and oxidized MWCNT depending on the electrolyte type and concentration. Nanoscale Res. Lett. 2016;11(1):166. doi: 10.1186/s11671-016-1367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Innocenzi P., Stagi L. Carbon-based antiviral nanomaterials: graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020;11(26):6606–6622. doi: 10.1039/d0sc02658a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith S.C., Rodrigues D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon. 2015;91:122–143. doi: 10.1016/j.carbon.2015.04.043. [DOI] [Google Scholar]
- 97.Refaat Alawady A., Ali Alshahrani A., Ali Aouak T., Mohamed Alandis N. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020;388 doi: 10.1016/j.cej.2020.124267. [DOI] [Google Scholar]
- 98.Hong X., Wang R., Li S., Fu J., Chen L., Wang X. Hydrophilic macroporous SnO2/rGO composite prepared by melamine template for high efficient photocatalyst. J. Alloy. Compd. 2020;816 doi: 10.1016/j.jallcom.2019.152550. [DOI] [Google Scholar]
- 99.Talinungsang, Upadhaya D., Kumar P., Purkayastha D.D. Superhydrophilicity of photocatalytic ZnO/SnO2 heterostructure for self-cleaning applications. J. Sol. -Gel Sci. Technol. 2019;92(3):575–584. doi: 10.1007/s10971-019-05127-8. [DOI] [Google Scholar]
- 100.Talinungsang, Dhar Purkayastha D., Krishna M.G. Dopant controlled photoinduced hydrophilicity and photocatalytic activity of SnO2 thin films. Appl. Surf. Sci. 2018;447:724–731. doi: 10.1016/j.apsusc.2018.04.028. [DOI] [Google Scholar]
- 101.Shah V., Wang B., Li K. Blending modification to porous polyvinylidene fluoride (PVDF) membranes prepared via combined crystallisation and diffusion (CCD) technique. J. Membr. Sci. 2021;618 doi: 10.1016/j.memsci.2020.118708. [DOI] [Google Scholar]
- 102.D.X. Liu, J.Q. Liang, T.S. Fung, Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae), in: M.A.Z. Dennis Bamford (Ed.), Encyclopedia of Virology2021, pp. 428–440. https://doi.org/10.1016/b978–0-12–809633-8.21501-x.
- 103.Monchatre-Leroy E., Boue F., Boucher J.M., Renault C., Moutou F., Ar Gouilh M., Umhang G. Identification of Alpha and Beta Coronavirus in Wildlife Species in France: Bats, Rodents, Rabbits, and Hedgehogs. Viruses. 2017;9(12) doi: 10.3390/v9120364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.S. Kumar, R. Nyodu, V.K. Maurya, S.K. Saxena, Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Coronavirus Disease 2019 (COVID-19)2020, pp. 23–31. https://doi.org/10.1007/978–981-15–4814-7_3.
- 106.Vecitis C.D., Schnoor M.H., Rahaman M.S., Schiffman J.D., Elimelech M. Electrochemical multiwalled carbon nanotube filter for viral and bacterial removal and inactivation. Environ. Sci. Technol. 2011;45(8):3672–3679. doi: 10.1021/es2000062. [DOI] [PubMed] [Google Scholar]
- 107.Lee S., Nam J.-S., Han J., Zhang Q., Kauppinen E.I., Jeon I. Carbon nanotube mask filters and their hydrophobic barrier and hyperthermic antiviral effects on SARS-CoV-2. ACS Appl. Nano Mater. 2021;4(8):8135–8144. doi: 10.1021/acsanm.1c01386. [DOI] [PubMed] [Google Scholar]
- 108.Alayande A.B., Kang Y., Jang J., Jee H., Lee Y.G., Kim I.S., Yang E. Antiviral nanomaterials for designing mixed matrix membranes. Membr. (Basel) 2021;11(7) doi: 10.3390/membranes11070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta I., Farinas E.T., Mitra S. Development of carbon nanotube-metal organic framework (MOF) hybrid antiviral microfiltration membrane. Sep. Purif. Technol. 2023;315 doi: 10.1016/j.seppur.2023.123766. [DOI] [Google Scholar]
- 110.Gupta I., Azizighannad S., Farinas E.T., Mitra S. Antiviral properties of select carbon nanostructures and their functionalized analogs. Mater. Today Commun. 2021;29 doi: 10.1016/j.mtcomm.2021.102743. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.