Abstract
Chimeric antigen receptor (CAR) T-cell therapy has shown remarkable efficacy in treating highly refractory and relapsing hematological malignancies in pediatric and adult patients. However, this promising therapy is limited by severe and potentially life-threatening toxicities. Cytokine release syndrome (CRS) is the most commonly observed of these toxicities. The cardiovascular manifestations of CRS include tachycardia, hypotension, left ventricular dysfunction, arrhythmias, troponin elevation, cardiogenic shock, and pulmonary edema. Recent data suggest that cardiotoxicities may be transient and reversible in younger patients with few cardiac comorbidities; however, cardiotoxicities may be fatal in older patients with significant cardiac risk factors. The literature remains sparse regarding long-term cardiotoxicities associated with CAR-T cell therapy. Furthermore, consensus guidelines for monitoring and prevention of cardiotoxicities remain ill-defined. Therefore, this review will detail the cardiovascular toxicities of CAR T-cell therapy seen in clinical trials and observational studies, summarize treatment approaches for CRS, outline the currently adopted surveillance protocols for CAR T-cell associated cardiotoxicity, and explore the future directions of research in this rapidly emerging field.
Keywords: CAR T-cell, Cardio-oncology, cytokine release syndrome, cardiomyopathy, cardiotoxicity, chimeric antigen receptor, CD19, BCMA
1. INTRODUCTION
Over the last two decades, targeted immunotherapies, such as monoclonal antibody therapy and checkpoint inhibitors, have been established as the standard of care for many malignancies. However, a tipping point in the clinical development of immunotherapies has been the emergence of adoptive cell transfer (ACT), which involves utilizing patients’ immune cells to treat their cancers. Of the several types of ACTs, chimeric antigen receptor (CAR) T-cell therapy has been the most clinically successful [1]. CAR T-cell therapy rose to prominence after being utilized to treat a pediatric patient with relapsing acute lymphoblastic leukemia in 2012 [2]. As such, there has been a dramatic increase in clinical trials studying various CAR T-cell therapies for hematologic and solid tumors, with more than 180 trials being conducted worldwide utilizing this novel therapy [3].
Although proven to be efficacious against relapsing and remitting hematologic malignancies, with response rates of up to 90%, CAR T-cell therapy is limited by severe life-threatening cardiotoxicities, occurring independently and as sequelae of varying degrees of cytokine release syndrome (CRS) [4-8]. With the continual adoption of this therapy, cardiovascular toxicities are becoming a significant concern since the majority of patients under consideration for this therapy have cardiovascular comorbidities and prior exposure to high doses of cardiotoxic chemotherapies and mediastinal radiation. Therefore, the purpose of this review was to detail the cardiovascular toxicities of CAR T-cell therapy seen in clinical trials and observational studies. Moreover, we sought to identify the mechanisms of these cardiotoxicities and present the currently adopted treatment approaches and future methods currently under investigation. Given the novelty of CAR T-cell therapy, there are no consensus guidelines for the monitoring and prevention of cardiotoxicities; therefore, we present the currently proposed surveillance protocols from cardio-oncology experts.
2. CAR T-CELL DESIGN AND ENGINEERING
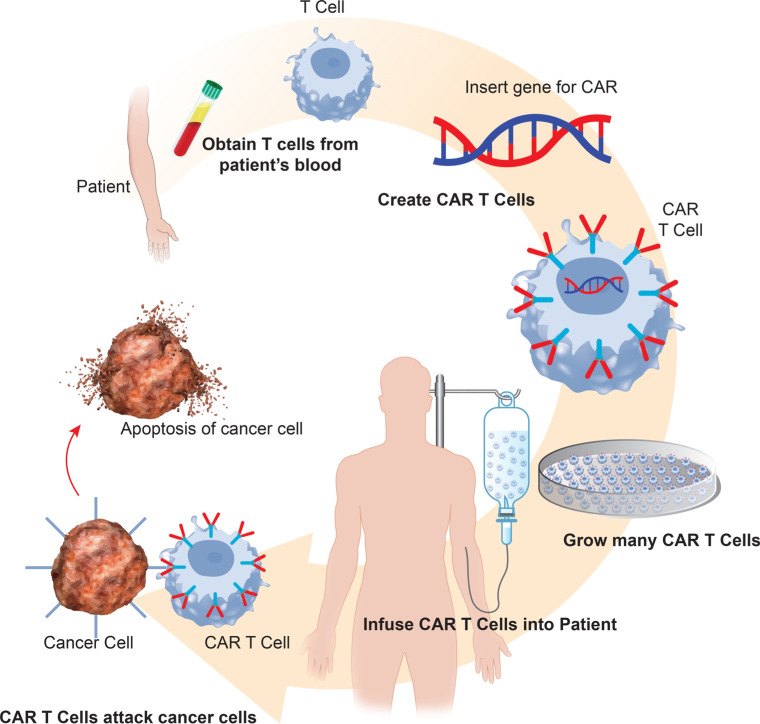
CARs are synthetic immunoreceptors composed of a variable portion from an antibody that redirects the specificity and function of T-cells, augmenting T-cells’ ability to target specific antigens [9]. This technology rapidly generates cancer-targeting T-cells, bypassing the barriers and incremental kinetics of active immunization, thereby generating a supra-physiological anti-cancer response [10]. In addition to their immediate antitumor benefits, CAR T-cells also provide long-term benefits as they promote immune surveillance to prevent tumor recurrence by continually targeting malignant cells and assisting in activating tumor-infiltrating lymphocytes [9]. CAR T-cells are created by extracting autologous T-cells from patients via apheresis (Fig. 1). These T-cells are genetically modified ex vivo with lentiviral or retroviral vectors to transduce the specific tumor-associated antigen into the T-cell, such as CD19. Next, the CAR T-cells undergo rapid proliferation ex vivo to generate therapeutic quantities; the manufacturing process requires 2 to 3 weeks [11]. Before receiving the CAR T-cell infusion, a patient must undergo lympho-depleting chemotherapy with either cyclophosphamide or fludarabine; this suppresses a patient’s endogenous T-cells, promoting selective expansion in vivo of the infused CAR T-cells [12].
Fig. (1).
CAR T-cell therapy manufacturing process.
In clinical trials, CAR T-cell therapy has shown remarkable efficacy for treating a variety of highly refractory and relapsing hematologic malignancies. CAR T-cells targeting the B-cell receptor CD19 have effectively been used to treat acute lymphoblastic leukemia [13-16], chronic lymphocytic leukemia [17-19], and large B-cell lymphoma [20-24]. Moreover, CAR T-cell therapy targeting B-cell maturation antigen (BCMA) has shown effectiveness in treating multiple myeloma [25, 26]. Currently, research is focused on expanding the clinical applicability of CAR T-cell therapy to also include Hodgkin lymphoma [27, 28] and solid tumor malignancies [29-32]. Furthermore, the success with hematologic malignancies has inspired research into the application of CAR T-cell therapy to treat autoimmune diseases, infections such as HIV, and transplant rejection [33]. The currently approved CAR T-cell therapies targeting CD19 are axicabtagene ciloleucel (Yescarta®) [34], brexucabtagene autoleucel (Tecartus™) [35], lisocabtagene maraleucel (Breyanzi®) [36], and tisagenlecleucel (Kyrmriah®) [37]. The sole approved BCMA targeting therapy is idecabtagene vicleucel (Abecma™) [38].
Legend: DNA is extracted from the patient using leukapheresis. T-cells are genetically modified with viral or nonviral vectors allowing for the insertion of the CAR gene into the T-cell. CAR T-cells undergo rapid proliferation ex vivo to generate therapeutic quantities. Patients undergo lymphodepletion therapy, and the CAR T-cells are infused into the patient where they can target and destroy cancer cells.
3. CYTOKINE RELEASE SYNDROME
The most notorious side effect associated with CAR T-cell therapy is CRS, mediated by the release of various inflammation cytokines, as discussed below. CRS presents as a constellation of symptoms and can affect multiple organ systems. However, the most characteristic clinical signs include fever, tachycardia, hypotension, and hypoxia. Symptoms can range from mild to severe, with life-threatening associations, such as cardiac dysfunction, respiratory distress, neurologic toxicities, coagulopathy, liver failure, and renal failure [39]. CRS is characterized by delayed onset of symptoms, occurring days to weeks after treatment, which contrasts with cytokine storm, which typically occurs within minutes or hours of treatment initiation [40]. The consensus approach for clinical grading of CRS severity by the American Society for Transplantation and Cellular Therapy (ASTCT) is shown in Table 1 [41]. The degree of CRS is of substantial importance as higher degrees of CRS have been strongly associated with the development of adverse cardiovascular outcomes [7,42]. Notability, CRS has also been previously associated with monoclonal antibody therapies, specifically, alemtuzumab (anti-CD52), rituximab (anti-CD-20), and muromonab (anti-CD3) [43].
Table 1.
ASTCT Consensus cytokine release syndrome grading scale.
| Clinical Symptoms | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Fever | Tempurature ≥ 38°C | Tempurature ≥ 38°C | Tempurature ≥ 38°C | Tempurature ≥ 38°C |
| - | - | with | with | with |
| Hypotension | None | Hypotension but not requiring vasopressors | Hypotension requiring vasopressors with or without vasopressin | Hypotension requiring multiple vasopressors (excluding vasopressin) |
| and/or | and/or | and/or | ||
| Hypoxia | None | Hypoxia requiring low-flow (≤6L per minute) nasal cannula | Hypoxia requiring high-flow nasal cannula (>6L per minute), nonrebreather, or Venturi mask | Hypoxia requiring positive pressure ventilation (CPAP, BiPAP, or mechanical ventilation) |
Abbreviations: ASTCT; American Society for Transplantation and Cellular Therapy, BiPAP; bilevel positive airway pressure, CPAP; continuous positive airway pressure.
3.1. Immunopathogenesis of Cytokine Release Syndrome
CAR T-cells are activated when their T-cell receptor engages with antigens (CD16 or BCMA) expressed on malignant cells. The activated T-cell releases cytokines and chemokines, including interleukin (IL)-2, soluble IL-2Rα, interferon-gamma (IFNγ), IL-6, soluble IL-6R, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Furthermore, bystander myeloid cells, such as monocytes, macrophages, and dendritic cells, also contribute to the inflammatory state by secreting IL-1RA, IL-10, IL-6, IL-8, tumor necrosis factor-alpha (TNFα) chemokine C-X-C ligand 10 (CXCL10), C-X-C ligand 9 (CXCL9), interferon-alpha (IFNα), C-C motif chemokine ligand 3 (CCL3), C-C motif chemokine ligand 3 (CCL4), and soluble IL-6R [4]. These cytokines mediate the activation of prostaglandins, which precipitate fever, myalgias, fatigue, hypotension, tachycardia, capillary leak, central nervous system toxicity, and cardiovascular toxicities [44]. However, of the involved cytokines, IL-6 is the most critical cytokine mediating the toxicity seen with CRS [42]. This immunological inflammatory cascade is the intended mechanism of CAR T-cell therapy; however, high-grade CRS is only manifested when these immunologic processes are activated to supraphysiological proportions. In actuality, every patient treated with CAR T-cells therapy is expected and desired to have some degree of CRS, as grade 1 disease indicates a response to therapy [5, 45]. Moreover, greater CRS severity has been correlated with large tumor bulk at the time of administration and greater infused dosages of CAR T-cells [13].
3.2. Management of Cytokine Release Syndrome
The cardiotoxicity associated with CAR T-cell therapy is correlated with grade 2 or higher CRS [7]. The management of CRS is in accordance with the grade of toxicity (Table 1). All patients hospitalized with signs of CRS, regardless of grade, should have frequent monitoring of vital signs, neurological assessments, blood counts, electrolytes, coagulation assays, and inflammatory markers [41]. Furthermore, patients should be placed on continuous telemetry and have a 12-lead electrocardiogram and a baseline echocardiogram. The clinical signs and symptoms of CRS should prompt an evaluation of cardiac biomarkers, troponin and brain natriuretic peptide [39]. Currently, evidence endorsing the correlation between adverse cardiovascular events and elevated troponin remains sparse; therefore, routine monitoring is controversial. However, observational data have shown elevated troponin levels after CAR T-cell therapy in the majority of patients who went on to develop major adverse cardiac events (MACE). Furthermore, in patients with elevated troponin levels, the risk for MACE increased 1.7-fold for each 12-hour period from the onset of CRS [6].
The stepwise approach to CRS management is shown in Fig. (2). Grade 1 or mild CRS is managed with supportive care, which includes volume maintenance with intravenous fluids while being cognizant of the patient’s fluid balance to avoid overload [4]. Supportive care at this stage also includes acetaminophen for fever, hypothermia blankets, ibuprofen as a second-line option for fever, empiric broad-spectrum antibiotics, and filgrastim if the patient is neutropenic. Patients should also be assessed for concurrent infection with blood cultures, urine cultures, and chest radiography [4].
Fig. (2).
Management of cytokine release syndrome. Abbreviations: ASTCT; American Society for Transplantation and Cellular Therapy, CRS; Cytokine release syndrome, IL; interleukin.
Grade 2 CRS is considered mild to severe and is hallmarked by the onset of hypotension [41]. This toxicity grade should be promptly treated with boluses of isotonic intravenous fluids. In addition to fluid resuscitation, an IL-6 antagonist, such as tocilizumab dosed at 8 mg/kg or siltuximab dosed at 11 mg/kg, should be considered if hypotension is refractory to fluid boluses. As discussed previously, this approach is beneficial as IL-6 is the most crucial cytokine mediating the toxicities of CRS [42]. Tocilizumab can be repeated after 6 hours if necessary, for a maximum dose is 800 mg. Furthermore, serum CRP levels should be monitored since its production by hepatocytes is mediated by IL-6 [46]. Therefore, elevated CRP levels correlate with elevated IL-6 production, and a decrease in CRP indicates an adequate response to IL-6 antagonists. A return of CRP to baseline is a reassuring sign that CRS has concluded. In numerous cases, tocilizumab administration has resulted in a rapid and complete resolution of hemodynamic instability with CRS [13, 47]. Moreover, the use of IL-6 antagonists has not been associated with a higher rate of cancer relapse [9]. If hypotension persists, the patient should be transferred to an intensive-care unit (ICU), and low-dose vasopressors should be initiated and titrated for a goal systolic blood pressure of greater than 90 mmHg [41]. At this time, bedside echocardiography should be obtained to assess for CRS-associated LV dysfunction [4]. In the setting of LV dysfunction leading to cardiogenic shock, inotropic support should be initiated with dobutamine or milrinone [48]. Hypoxia associated with CRS can present as non-cardiogenic pulmonary edema or pleural effusions and should be managed with supplemental oxygen, noninvasive and invasive ventilation, diuresis, and thoracentesis when indicated [41].
Grade 3 and 4 CRS is considered severe and should be managed in the ICU. At this stage, CRS is deemed to be refractory to IL-6 antagonists. Therefore, in addition to IL-6 antagonists, high-dose corticosteroids should be administered; specifically, methylprednisolone 1g/day IV or dexamethasone 10 mg IV every 6 hours, with an increase to 20 mg every 6 hours if symptoms are persistently refractory [41]. Cautious use of corticosteroids was initially recommended, given that they inhibit T-cell activity, thus hindering the CAR T-cell response; however, recent data suggest that high-dose corticosteroids do not influence the efficacy and kinetics of CAR T-cells [49].
Although preliminary, there is developing evidence that IL-1 antagonists, such as anakinra, and TNFα antagonists, such as infliximab or etanercept, may have a role in CRS management. However, these inflammatory cytokines are inconsistently elevated in patients presenting with CRS, compared to IL-6, which is reliably elevated in CRS [50]. Animal studies accessing the use of anakinra for CRS confirmed that anakinra with or without tocilizumab effectively hindered the immune response from monocytes and abolished lethal neurotoxicity [51]. Notably, tocilizumab has been observed to be less effective in treating CRS-related neurotoxicity and hemophagocytic lymphohistiocytosis since it does not cross the blood-brain barrier [50]. Moreover, these studies suggest that the severity of CAR T-cell-associated CRS may be mediated by IL-6, IL-1, and nitric oxide produced by macrophages. Therefore, in the setting of insufficiently suppressed IL-1, anakinra may aid in reducing CRS mortality [52]. In a case report of 2 patients who had CRS following BCMA CAR T-cell therapy for multiple myeloma, anakinra was used after these patients failed to improve with tocilizumab. The investigators noted significant improvement in CRS symptoms, suggesting a synergistic role of anakinra with tocilizumab [53]. A phase II clinical trial is currently underway exploring the role of anakinra in the CRS management protocol [54]. The use of TNFα antagonists for CRS in the literature has been limited to case reports and smaller series [42, 55]. However, in patients with CRS after BCMA CAR T-cell therapy for multiple myeloma, etanercept showed efficacy in curtailing CRS in patients with elevated TNFα levels [55].
4. CARDIOTOXICITIES WITH CAR T-CELL THERAPY
4.1. Pathophysiology of Cardiomyopathy
Although cardiotoxicity associated with CAR T-cell therapy predominantly occurs in the context of high-grade CRS, the specific mechanisms have not been definitively established [48]. Current evidence suggests a multifactorial origin of CAR T-cell-associated cardiomyopathy. During high-grade CRS, vascular leak has been identified as a significant contributor to cardiomyopathy. Retrospective studies have noted a frequent diagnosis of capillary leak syndrome (CLS) after CAR T-cell therapy, with patients having an average positive fluid balance of 140 ml/kg over the 3 days leading up to the onset of shock [5]. CLS presents as a constellation of hypotension, pulmonary edema, systemic edema, hemoconcentration, hypoproteinemia, and shock, occurring due to an acute increase in vascular permeability and resulting in the loss of protein-rich fluid from the intravascular space. Furthermore, similar to CRS, this syndrome has been associated with increased expression of IL-2, IL-6, IFNγ, IL-12, and CXCL10 [56]. Although, of these inflammatory cytokines, a higher concentration of IL-6 has been noted in patients with CLS versus patients without CLS symptoms, similar to the association of IL-6 with high-grade CRS. Furthermore, the occurrence of CLS has not been associated with age, gender, pre-treatment tumor burden, or type and dosage of CAR T-cells used in contrast to CRS [57].
Another proposed mechanism of LV systolic dysfunction after CAR T-cell therapy is the occurrence of stress-induced or Takotsubo cardiomyopathy, presenting similarly to sepsis-induced cardiomyopathy. In reported cases of stress-induced cardiomyopathy after CAR T-cell therapy, the onset was typically rapid with high severity of dysfunction; however, the LV systolic dysfunction was reversible [42]. The stressor, in this case, would be the supraphysiologic inflammatory response from CRS. Finally, an additional proposed mechanism involves direct T-cell mediated cardiac cytotoxicity. This process has been described during the histopathological analysis of 2 patients who received CAR T-cell therapy directed against melanoma-associated antigen 3 (MAGE-A3) to treat stage III/IV melanoma. Both patients developed cardiogenic shock and died within a few days of treatment initiation. Autopsy revealed gross findings of severe myocardial damage, and histopathological analysis was consistent with myocardial necrosis from immunologically mediated destruction from T-cell infiltration, revealing a histological pattern similar to that for allograft rejection. This variant of autoimmune toxicity is proposed to involve off-target cross-reactivity between T-cells and titin, a sarcomere protein expressed in striated muscle. Interestingly, mutations in titin are known to cause dilated cardiomyopathy. Autopsy samples from both patients confirmed high titers of titin by reverse transcriptase-polymerase chain reaction [58]. Although compelling, a similar histopathological analysis in patients after CD19 CAR T-cell therapy has yet to be conducted; therefore, evidence is speculative.
4.2. Cardiotoxicity Data from Clinical Trials
Data from the landmark clinical trials, which led to the United States Food and Drug Administration (FDA) approval of the current CAR T-cell therapies, are presented in Table 2 [23, 24, 59-62]. In these trials, the highest rate of CRS, regardless of grading, was seen with axicabtagene ciloleucel in the ZUMA-1 (study evaluating the safety and efficacy of KTE-C19 in adult participants with refractory aggressive non-Hodgkin lymphoma) trial at 93% [23]. However, the highest rate of grade 3 or higher CRS was seen with tisagenlecleucel in the ELIANA (study of efficacy and safety of CTL019 in pediatric ALL patients) trial at 46% [59]. Notably, ELIANA was the only trial to include a pediatric population. However, the highest rate of grade 3 or higher CRS in adults was also seen with tisagenlecleucel in the JULIET (study of efficacy and safety of CTL019 in adult DLBCL patients) trial. Conversely, the lowest rate of total CRS and grade 3 or higher CRS was seen with lisocabtagene maraleucel in TRANSCEND NHL 001 (study evaluating the safety and pharmacokinetics of JCAR017 in B-cell non-Hodgkin lymphoma) trial at 42% and 2%, respectively [61].
Table 2.
Pivotal trials leading to FDA approval of CAR T-cell therapies
| CAR T-cell therapy |
Axicabtagene Ciloleucel
(Yescarta®) |
Tisagenlecleucel (Kyrmriah®) | Tisagenlecleucel (Kyrmriah®) |
Brexucabtagene Autoleucel
(Tecartus™) |
Lisocabtagene Maraleucel
(Breyanzi®) |
Idecabtagene Vicleucel (Abecma™) |
|---|---|---|---|---|---|---|
| Trial (year) | ZUMA-1 (2017)[23] | JULIET (2018)[60] | ELIANA (2018)[61] | ZUMA-2 (2020)[62] | TRANSCEND NHL 001 (2020)[63] | KarMMa (2021)[64] |
| Disease (target) | Adult LBCL (CD19) | Adult LBCL (CD19) | Pediatric B-ALL (CD19) | Adult MCL (CD19) | Adult LBCL (CD19) | Multiple Myeloma (BCMA) |
| Population size (n) | 101 | 93 | 75 | 68 | 269 | 128 |
| Objective response | 82% | 50% | 83% | 93% | 73% | 73% |
| Complete response | 54% | 40% | 60% | 67% | 53% | 33% |
| CRS (%) | 94 (93%) | 64 (58%) | 58 (77%) | 61 (91%) | 113 (42%) | 107 (84%) |
| Grade 3 or higher CRS | 13 (13%) | 24 (22%) | 35 (46%) | 15% | 6 (2%) | 7 (5%) |
| Hypotension | 60 (59%) | 29 (26%) | 22 (29%) | 35 (51%) | 68 (25%) | 22 (17%) |
| Requiring vasopressors | 14 (14%) | 8 (9%) | 13 (17%) | 15 (22%) | 7 (3%) | NR |
| Tachycardia | 39 (39%) | 12 (11%) | 3 (4%) | 21 (31%) | 42 (16%) | NR |
| Serious adverse cardiovascular events | NR | NR | 14 (19%) | NR | NR | NR |
| Tocilizumab usage | 49 (49%) | 16 (14%) | 36 (48%) | 42 (62%) | 48 (18%) | 67 (52%) |
CRS; Cytokine release syndrome, ELIANA; Study of efficacy and safety of CTL019 in pediatric ALL patients, FDA; the United States Food and Drug Administration, JULIET; Study of efficacy and safety of CTL019 in adult DLBCL patients, KarMMa; Efficacy and safety study of bb2121 in subjects with relapsed and refractory multiple myeloma, LCB; large B-Cell lymphoma, MCL; mantle-cell lymphoma, NR; not reported, TRANSCEND NHL 001; Study evaluating the safety and pharmacokinetics of JCAR017 in B-cell non-Hodgkin lymphoma, ZUMA-1; Study evaluating the safety and efficacy of KTE-C19 in adult participants with refractory aggressive non-Hodgkin lymphoma, ZUMA-2; Study to evaluate the efficacy of brexucabtagene autoleucel (KTE-X19) in participants with relapsed/refractory mantle cell lymphoma.
Of these clinical trials, MACE data were only reported for tisagenlecleucel in the ELIANA trial. In this trial, the investigators reported instances of pulmonary edema in 5% of patients, left ventricular dysfunction in 4%, cardiac arrest in 4%, and cardiac failure in 4% [59]. In the other clinical trials, MACE was not reported independently; rather, hypotension with and without the need for vasopressors and tachycardia were reported as mutual components of CRS. Severe hypotension requiring vasopressors was most commonly seen with brexucabtagene autoleucel and tisagenlecleucel, occurring in 22% and 17%, respectively. Furthermore, tachycardia occurred in 39% of patients treated with axicabtagene ciloleucel. However, a wide array of MACE occurring as sequelae of CRS were not characterized in clinical trials but were reported in observational studies. These studies included patients in the pediatric and adult population, and the MACE included arrhythmias, hypotension, left ventricular systolic dysfunction, and cardiogenic shock [5-8, 48, 63].
4.3. Cardiotoxicity Data from Observational Studies in Pediatric and Young Adults
Two retrospective studies sought to identify specific MACE with CAR T-cell therapies in the pediatric and young adult population. The first was a retrospective cohort study published in 2017 by Burstein and colleagues, including 98 patients who received tisagenlecleucel (CTL019 or CTL119) for relapsed/refractory ALL. There were no CAR T-cell cardiac-related deaths in the cohort. However, the investigators reported hypotension requiring vasopressor support in 24 (25%) patients with a mean onset of 5 days; 21 of these patients had life-threatening symptoms requiring tocilizumab. Of the patients requiring vasopressor support, 10 had new evidence of left ventricular (LV) systolic dysfunction found on echocardiogram. Of these 10 patients, 6 required milrinone for cardiac support, and 6 had new ST-segment-related changes on the electrocardiogram. 7 out of 10 patients had persistent LV systolic dysfunction at hospital discharge. However, at 6-month follow-up, LV dysfunction had recovered in 6 out of 7 patients. Risk factors for cardiogenic shock were preexisting systolic or diastolic dysfunction; however, cardiomyopathy, previous total body radiation, or high dose anthracycline therapy were not associated with increased adverse events [48].
The second retrospective study from Shalabi and colleagues published in 2020 included 52 patients treated with CD19 CAR T-cells for relapsed/refractory B ALL or non-Hodgkin lymphoma. All patients had prior exposure to anthracycline chemotherapy agents. CRS occurred in 37 patients with a median onset of 5 days. Grade 3 or higher disease occurred in 9 (24%) patients, 9 patients required vasopressor support, and 7 received tocilizumab or steroids. Of the patients who developed CRS, 6 (16%) had cardiac dysfunction, defined by a greater than 10% reduction in LV ejection fraction from baseline or new-onset grade 2 or higher LV dysfunction by global longitudinal strain. In patients with systolic dysfunction, 4 had elevated troponin. A single case of post-infusion cardiac arrest required temporary mechanical circulatory support with an intra-aortic balloon pump. This patient had a peak troponin elevation of 6.23 ng/mL and had a drop in LV ejection fraction from 64% to 20%. None of the patients with cardiac dysfunction developed any clinically significant arrhythmias. In addition, no clinically significant cardiac dysfunction occurred in patients who did not develop CRS. At 28 days post CAR T-cell infusion, the cardiac dysfunction had resolved in 4 out of 6 (67%) patients. The remaining 2 patients had a full recovery of cardiac function at 3-month follow-up; interesting, both these patients had the highest pre-treatment anthracycline exposure [8].
Therefore, clinical data in pediatrics and young adults suggest that cardiotoxicities occurring after CAR T-cell therapy, although catastrophic in some circumstances, is predominantly self-limited. The MACE seen were LV systolic dysfunction occurring in 10% to 16% of patients with one instance of cardiac arrest. Furthermore, the majority of patients with cardiac dysfunction had a recovery to their pre-treatment baseline within 3 to 6 months.
4.4. Cardiotoxicity Data from Observational Studies in Adults
Three observational studies have been conducted evaluating the cardiovascular toxicities with CAR T-cell therapies in the adult population. The first retrospective cohort study in adults, published in 2019 by Alvi and colleagues, included 137 patients who received CD19 CAR T-cell therapy. Approximately 50% of patients received commercial CAR T-cells: axicabtagene ciloleucel and tisagenlecleucel. The remainder received non-commercial, investigational CAR T-cells. In addition, 79% of patients had prior anthracycline exposure, and 28% had mediastinal radiation. CRS occurred in 59% of patients after a median of 5 days, with grade 3 or higher CRS occurring in 6 (4%) patients. All MACE occurred with grade 2 or higher CRS, with higher grades correlating with increased risk. Elevated troponin was seen in 29 (54%) patients; the median time from infusion to troponin elevation was 16 days. The investigators noted that troponin elevations were more likely in older patients with cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, atrial fibrillation/flutter, and coronary artery disease. In addition, higher CRS grades correlated with an increased likelihood of troponin elevations. Baseline echocardiography data pre and post-CAR T-cell therapy were only available in 29 of the 137 patients. Of these, 8 (28%) had a new decrease in LV ejection fraction. All patients with a decreased ejection fraction post-infusion also had troponin elevations. However, no data were included in this study regarding LV systolic function recovery. Moreover, 17 (12%) MACE were reported in this study: 6 cardiovascular deaths, 6 instances of decompensated heart failure, and 5 new-onset arrhythmias. Of the deaths, 3 occurred in patients with new-onset heart failure or tachyarrhythmias, which were complicated by hypotension and shock. The remaining 3 deaths occurred in patients with circulatory shock followed by cardiac arrest. Of the patients with decompensated heart failure, 4 had a new-onset disease while 2 had previous stable disease; all had an NT proBNP level of greater than 3000 pg/ml, and all required intravenous diuretics. Of the new-onset arrhythmias, 2 patients had supraventricular tachycardia, 3 had atrial fibrillation or flutter, and all required intravenous amiodarone or metoprolol. All patients with MACE had an elevated troponin, except 1 patient who had pre-existing heart failure and suffered from an acute decompensation. These results indicate that serial troponin measurements may be valuable in monitoring impending cardiac events in patients after CAR T-cell infusion and determining patients who may benefit from early tocilizumab administration6.
The second retrospective cohort was published in 2020 by Lefebvre and colleagues and included 145 patients who received CD19 CAR T-cell therapy for diffuse large B-cell lymphoma (30%), ALL (25%), or CLL (45%). There was exposure to anthracyclines in 60% of patients in this study. Patients received commercial (axicabtagene ciloleucel and tisagenlecleucel) or non-commercial CAR T-cells. CRS occurred in 104 (72%) patients with a median onset of 6 days. The investigators identified 41 MACE occurring after a median time of 11 days post-infusion in 31 (21%) patients. Moreover, the incidence of cardiac events was 17% in the first 30 days, 19% at 6 months, and 21% at 12 months following infusion [7]. These results suggest short and long-term cardiac risk with this therapy. In 17 of the 31 patients with MACE, there was a decrease in LV ejection fraction when events occurred, from a mean baseline of 62 ± 7% to 49 ± 14%. There were 22 occurrences of symptomatic heart failure, 12 episodes of atrial fibrillation, 1 event of supraventricular tachycardia, 1 event of nonsustained ventricular tachycardia, 2 episodes of acute coronary syndrome, and 2 cardiac deaths. The 2 cardiac deaths resulted from the pulseless electrical activity and massive pulmonary embolism with ST-segment elevation myocardial infarction. Furthermore, MACE were correlated with patients who used aspirin, statins, insulin, and had prior atrial fibrillation and elevated baseline creatinine [7]. Therefore, these results suggest that patients with high-risk cardiovascular profiles were more susceptible to MACE.
Finally, a retrospective review published in 2020 by Ganatra and colleagues examined 187 adult patients who received CD19 CAR T-cell for non-Hodgkin lymphoma to detect the incidence of cardiomyopathy. Axicabtagene ciloleucel was administered to 97% of patients. A total of 155 (83%) patients had CRS with more than 50% having grade 2 or higher CRS. Serial echocardiogram data were available for 116 patients. Of these patients, 12 (10%) developed new or progressive cardiomyopathy with an average decline in LV ejection fraction from 58% to 37%, at a median onset of 12.5 days after CAR T-cell infusion. Of the patients with cardiomyopathy, LV ejection fraction improved in 9 patients over an average of 169 days, 6 patients had a full recovery, and 3 had a partial recovery. Cardiac deaths were reported in the 3 patients without recovery in LV ejection fraction. The majority of cardiomyopathy patients had grade 2 or higher CRS. Furthermore, the investigators noted that cardiomyopathy was more likely in older patients with greater cardiovascular risk factors, such as hyperlipidemia, coronary artery disease, and the use of renin-angiotensin inhibitors or beta-blockers. Anthracycline or mediastinal radiation exposure did not differ between patients with and without cardiomyopathy [63].
Therefore, in contrast to pediatrics and young adults, clinical data in adults suggest that cardiotoxicity in the setting of grade 2 or higher CRS is not always reversible, and patients with increased cardiovascular risk factors retain a greater risk for fatal cardiac events. Furthermore, troponin may be a valuable indicator of cardiotoxicity to guide the administration of tocilizumab. Of the currently available data on the cardiotoxicities with CAR T-cells, the majority of MACE has been noted to occur within 6 months of infusion and predominantly in the context of CRS. However, longitudinal data establishing the occurrence of long-term or late-onset cardiotoxicities have not yet been described. A single retrospective study by Cordeiro and colleagues published in 2020 examined late adverse events after CAR T-cell therapy in 86 patients with an average follow-up of 28 months. The most common late adverse events identified were cytopenia, immune-related events, infections, and neurologic and psychiatric events. Notably, no MACE were reported [64]. Although reassuring, more longitudinal data in patients who suffered from MACE and those who did not will be necessary to establish long-term risks with CAR T-cell therapy.
5. CARDIAC SCREENING AND MONITORING IN PATIENTS UNDERGOING CAR T-CELL THERAPY
It would be anticipated that patients receiving CAR T-cell therapy would be more susceptible to cardiotoxicities given their prior exposure to anthracycline chemotherapy agents and mediastinal radiation; however, observational studies have found no association [6, 7, 48, 63]. However, cardiovascular risk factors have been associated with an increased propensity for MACE in patients receiving CAR T-cell therapy, although evidence is limited to observational studies [6, 7, 63]. Cardiac evaluation before CAR T-cell therapy varies significantly between various centers, given the paucity of data in the literature. As there are no concrete guidelines regarding cardiovascular risk evaluation before CAR T-cell therapy, the American Society of Clinical Oncology (ASCO) clinical practice guidelines for preventing and monitoring cardiac dysfunction for cardiotoxic anti-cancer therapies should be considered [65]. Furthermore, the cardio-oncology service at the University College of London Hospital has established a cardiac screening and monitoring protocol (Fig. 3) [66] to be used in conjunction with the ASCO guidelines. These recommendations include starting with a detailed cardiovascular history and physical examination, particularly screening for cardiovascular risk factors, such as hypertension, diabetes, dyslipidemia, obesity, and smoking, prior to treatment initiation [65].
Fig. (3).
The University College of London Hospital cardiac screening and monitoring protocol for CAR T-cell therapy.
Abbreviations: CRS; Cytokine release syndrome, ECG; electrocardiogram, NT-proBNP; N-terminal-pro hormone brain natriuretic peptide.
All patients planned for CAR T-cell therapy should undergo cardiac risk assessment with troponin, brain natriuretic peptide, 12-lead electrocardiogram, echocardiogram, and cardiac magnetic resonance imaging (MRI) with a fast scan protocol. A cardio-oncology team should evaluate those with abnormalities on diagnostic tests or those identified as having high cardiac risk before CAR T-cell initiation [66]. Furthermore, exercise tolerance should be assessed in patients with coronary artery disease or multiple cardiovascular risk factors. An imaging stress test should be obtained for patients with poor exercise capacity to rule out obstructive coronary artery disease. In patients with obstructive coronary artery disease or other severe cardiac risk factors, a risk versus benefits discussion with the patient and cardio-oncology team should be pursued, focusing on the patient’s functional status and prognosis with the malignancy. Patients with preexisting heart failure are at high risk for decompensation due to large fluid shifts with CAR T-cells; therefore, volume status should be closely monitored in these patients with attention to maintaining euvolemia with diuretics [39]. In addition, initiation of cardioprotective medications, beta-blockers, and angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) should be considered [66]. A single case report has been published utilizing the invasive pulmonary artery pressure monitoring device, CardioMEMS, in a patient with symptomatic heart failure. This patient had an LV ejection fraction of 25%, presumably due to anthracycline toxicity. CardioMEMS was used reliably to determine cardiac hemodynamics and guide diuretic therapy [67]. In the future, CardioMEMS may become a valuable component of CAR T-cell monitoring, allowing for administration to patients with severely reduced LV function, which is common in this patient population who have had exposure to large dosage anthracyclines and mediastinal radiation.
After treatment initiation of CAR T-cell therapy, as part of cardiac surveillance, it is strongly recommended at 7 days post-treatment to obtain repeat biomarker levels, 12-lead electrocardiogram, and echocardiogram. If abnormalities are detected on surveillance diagnostics or signs of CRS, patients should be admitted to the hospital. Cardiac MRI or multigated acquisition (MUGA) scan should be obtained, and the cardio-oncology team should review the case. At this time, cardioprotective medications should be administered in patients with LV dysfunction [66]. Moreover, all patients should have cardio-oncology follow-up at 3 months post-treatment. Cardiac diagnostics, including biomarkers, 12-lead electrocardiogram, and echocardiogram, should be repeated. In addition, the ASCO guidelines recommend a repeat surveillance echocardiogram between 6 to 12 months after completion of therapy in these asymptomatic but high-risk patients [65].
CONCLUSION AND FUTURE DIRECTION
CAR T-cell therapy has shown remarkable efficacy for treating relapsing and remitting hematologic malignancies. The success of CAR T-cells has inspired research into applying this novel therapy to the treatment of autoimmune diseases, infections such as HIV, and transplant rejection. However, therapy is limited by the life-threatening CRS and associated cardiotoxicities, with CRS of varying degrees occurring as often as 93% in clinical trials. CRS management is well established; however, future studies utilizing alternative immunosuppressive agents, such as IL-1 or TNFa antagonists, will be essential to optimize CRS treatment protocols to minimize the toxic effects of CAR T-cells while maintaining their efficacy. Currently, the data regarding specific adverse cardiovascular events, such as post-infusion LV dysfunction, arrhythmia, and cardiogenic shock, is limited to retrospective studies. MACE have been strongly associated with grade 2 or higher CRS, with higher grade correlated with increased risk. Furthermore, data from pediatric and young adult populations indicate that cardiac dysfunction in these patients is self-limited, with the majority of patients with cardiac dysfunction having recovered to their pre-treatment baseline by 6 months. However, retrospective data in adults suggest that cardiotoxicities can lead to irreversible cardiac dysfunction, with increased susceptibility for cardiotoxicity in patients with cardiovascular risk factors.
Currently, the long-term cardiovascular effects of CAR T-cell therapy, such as the risk for metabolic syndrome, hypertension, vascular disease, and cardiomyopathy, are unknown. Therefore, future longitudinal prospective studies are needed to characterize these toxicities further and establish risks in various patient populations. In addition, as CAR T-cell therapy is in its infancy, guidelines for monitoring, treatment, and prevention of cardiotoxicities remain ill-defined. However, as this therapy becomes more widely adopted worldwide, future clinical data will be imperative in establishing screening and treatment recommendations utilizing multimodality cardiac imaging and novel cardiac biomarkers. However, until consensus guidelines are established, clinicians should continue to use the University College of London Hospital cardio-oncology protocol for CAR T-cell cardiac screening and monitoring.
SELECTION CRITERIA
MEDLINE and PubMed were searched for original articles. All identified articles were published in English. The MeSH terms used were “antigens, CD19”, “cardiotoxicity/etiology”, “cardiotoxicity/diagnosis” “cardiovascular diseases/etiology*”, “clinical trials as topic”, “cytokines/metabolism”, “cytokine release syndrome/etiology*”, “humans”, “hematologic neoplasms/therapy”, “immunotherapy, adoptive/adverse effects*”, “lymphoma, large B-cell, diffuse/therapy*”, “lymphoma, mantle-cell/therapy*”, “multiple myeloma/drug therapy”, “neoplasms/ therapy*”, “receptors, antigen, T-cell/therapeutic use*”, “receptors, chimeric antigen/therapeutic use*”, “ventricular dysfunction, left/etiology”. The reference list of identified articles and their citation data were also searched using the Web of Science for related papers.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- CAR
Chimeric antigen receptor
- CRS
Cytokine release syndrome
- ACT
adoptive cell transfer
- BCMA
B-cell maturation antigen
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise
REFERENCES
- 1.Yang J.C., Rosenberg S.A. Adoptive t-cell therapy for cancer. Adv. Immunol. 2016;130:279–294. doi: 10.1016/bs.ai.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum L. Tragedy, perseverance, and chance - The story of CAR-T Therapy. N. Engl. J. Med. 2017;377(14):1313–1315. doi: 10.1056/NEJMp1711886. [DOI] [PubMed] [Google Scholar]
- 3.Tang J., Shalabi A., Hubbard-Lucey V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018;29(1):84–91. doi: 10.1093/annonc/mdx755. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu S.S., Tummala S., Kebriaei P., et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald J.C., Weiss S.L., Maude S.L., et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic Leukemia. Crit. Care Med. 2017;45(2):e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvi R.M., Frigault M.J., Fradley M.G., et al. Cardiovascular events among adults treated with chimeric antigen receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019;74(25):3099–3108. doi: 10.1016/j.jacc.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre B., Kang Y., Smith A.M., Frey N.V., Carver J.R., Scherrer-Crosbie M. Cardiovascular effects of car T cell therapy: A retrospective study. JACC CardioOncology. 2020;2(2):193–203. doi: 10.1016/j.jaccao.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalabi H., Sachdev V., Kulshreshtha A., et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J. Immunother. Cancer. 2020;8(2):8. doi: 10.1136/jitc-2020-001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter A.I., Pont M.J., Riddell S.R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131(24):2621–2629. doi: 10.1182/blood-2018-01-785840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15(1):31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude S.L., Frey N., Shaw P.A., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si Lim S.J., Grupp S.A., DiNofia A.M. Tisagenlecleucel for treatment of children and young adults with relapsed/refractory B-cell acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2021;68(9):e29123. doi: 10.1002/pbc.29123. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Rivière I., Gonen M., et al. Long-Term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter D.L., Hwang W.T., Frey N.V., et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turtle C.J., Hay K.A., Hanafi L.A., et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 2017;35(26):3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraietta J.A., Lacey S.F., Orlando E.J., et al. Determinants of response and resistance to CD19 Chimeric Antigen Receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer J.N., Dudley M.E., Kassim S.H., et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer J.N., Somerville R.P.T., Lu T., et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum Interleukin-15 levels. J. Clin. Oncol. 2017;35(16):1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke F.L., Neelapu S.S., Bartlett N.L., et al. Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol. Ther. 2017;25(1):285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelapu S.S., Locke F.L., Bartlett N.L., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster S.J., Bishop M.R., Tam C.S., et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 25.Ali S.A., Shi V., Maric I., et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brudno J.N., Maric I., Hartman S.D., et al. T cells genetically modified to express an anti-b-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple Myeloma. J. Clin. Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C.M., Wu Z.Q., Wang Y., et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin Lymphoma: An open-label phase I trial. Clin. Cancer Res. 2017;23(5):1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 28.Ramos C.A., Ballard B., Zhang H., et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 2017;127(9):3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown C.E., Alizadeh D., Starr R., et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adusumilli P.S., Cherkassky L., Villena-Vargas J., et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis C.U., Savoldo B., Dotti G., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed N., Brawley V.S., Hegde M., et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-Positive sarcoma. J. Clin. Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldini C.R., Ellis G.I., Riley J.L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 2018;18(10):605–616. doi: 10.1038/s41577-018-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchkouj N., Kasamon Y.L., de Claro R.A., et al. FDA approval summary: Axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin. Cancer Res. 2019;25(6):1702–1708. doi: 10.1158/1078-0432.CCR-18-2743. [DOI] [PubMed] [Google Scholar]
- 35.FDADISCO. Burst Edition: FDA approval of Tecartus (brexucabtagene autoleucel) for adult patients with relapsed or refractory Bcell precursor acute lymphoblastic leukemia. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-tecartus-brexucabtagene-autoleucel-adult-patients-relapsed-or#:~:text=On%20October%201%2C%202021%2C%20the,cell%20precursor%20acute%20lymphoblastic%20leukemia (Accessed on May 25, 2022).
- 36.FDADISCO. Burst Edition: Breyanzi (lisocabtagene maraleucel). Available from: https://www.fda.gov/drugs/resources-information-approveddrugs/fda-disco-burst-edition-breyanzi-lisocabtagene-maraleucel (Accessed on May 25, 2022).
- 37.O’Leary M.C., Lu X., Huang Y., et al. FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res. 2019;25(4):1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 38.FDADISCO. Burst Edition: FDA approval of ABECMA (idecabtagene vicleucel) the first FDA approved cell-based gene therapy for the treatment of adult patients with relapsed or refractory multiple myeloma. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-abecma-idecabtagene-vicleucel-first-fda-approved-cell-based (Accessed on May 25, 2022).
- 39.Ganatra S., Carver J.R., Hayek S.S., et al. Chimeric antigen receptor T-Cell therapy for cancer and heart: JACC council perspectives. J. Am. Coll. Cardiol. 2019;74(25):3153–3163. doi: 10.1016/j.jacc.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter D., Frey N., Wood P.A., Weng Y., Grupp S.A. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 2018;11(1):1–2. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for Cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D.W., Gardner R., Porter D.L., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., et al. Cytokine release syndrome. J. Immunother. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asnani A. Cardiotoxicity of immunotherapy: Incidence, diagnosis, and management. Curr. Oncol. Rep. 2018;20(6):44. doi: 10.1007/s11912-018-0690-1. [DOI] [PubMed] [Google Scholar]
- 45.Leick M.B., Maus M.V. Toxicities associated with immunotherapies for hematologic malignancies. Best Pract. Res. Clin. Haematol. 2018;31(2):158–165. doi: 10.1016/j.beha.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Schuster S.J., Svoboda J., Chong E.A., et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burstein D.S., Maude S., Grupp S., Griffis H., Rossano J., Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: A single-institution experience. Biol. Blood Marrow Transplant. 2018;24(8):1590–1595. doi: 10.1016/j.bbmt.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Liu S., Deng B., Yin Z., et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. doi: 10.1038/s41408-020-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riegler L.L., Jones G.P., Lee D.W. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther. Clin. Risk Manag. 2019;15:323–335. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norelli M., Camisa B., Barbiera G., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 52.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24(6):731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jatiani S.S., Aleman A., Madduri D., et al. Myeloma CAR-T CRS management With IL-1R antagonist anakinra. Clin. Lymphoma Myeloma Leuk. 2020;20(9):632–636.e1. doi: 10.1016/j.clml.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Library of Medicine. Anakinra for the prevention of cytokine release syndrome and neurotoxicity in patients with B-cell lymphoma receiving CD19-Targeted CAR-T cell therapy. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04359784 (Accessed on May 25, 2022).
- 55.Zhang L., Wang S., Xu J., et al. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp. Hematol. Oncol. 2021;10(1):16. doi: 10.1186/s40164-021-00209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddall E., Khatri M., Radhakrishnan J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017;92(1):37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y., Feng J., Shao M., Huang H. Profile of capillary-leak syndrome in patients received chimeric antigen receptor T cell therapy. Blood. 2018;132(Suppl. 1):5204–4. doi: 10.1182/blood-2018-99-117602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linette G.P., Stadtmauer E.A., Maus M.V., et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maude S.L., Laetsch T.W., Buechner J., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M., Munoz J., Goy A., et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abramson J.S., Palomba M.L., Gordon L.I., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 62.Munshi N.C., Anderson L.D., Jr, Shah N., et al. Idecabtagene vicleucel in relapsed and refractory multiple Myeloma. N. Engl. J. Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 63.Ganatra S., Redd R., Hayek S.S., et al. Chimeric antigen receptor T-Cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-Hodgkin lymphoma. Circulation. 2020;142(17):1687–1690. doi: 10.1161/CIRCULATIONAHA.120.048100. [DOI] [PubMed] [Google Scholar]
- 64.Cordeiro A., Bezerra E.D., Hirayama A.V., et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol. Blood Marrow Transplant. 2020;26(1):26–33. doi: 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh A.K., Chen D.H., Guha A., Mackenzie S., Walker J.M., Roddie C. CAR T cell therapy-related cardiovascular outcomes and management: Systemic disease or direct cardiotoxicity? JACC CardioOncology. 2020;2(1):97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanelidis A.J., Raikhelkar J., Kim G., et al. CardioMEMS-Guided CAR T cell therapy for lymphoma in a patient with anthracycline-induced cardiomyopathy. JACC Cardio Oncol. 2020;2(3):515–518. doi: 10.1016/j.jaccao.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]