Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is a complex retrovirus encoding regulatory and accessory genes in four open reading frames (ORF I to IV) of the pX region. Emerging evidence indicates an important role for the pX ORF I-encoded accessory protein p12I in viral replication, but its contribution to viral pathogenesis remains to be defined. p12I is a conserved, membrane-associated protein containing four SH3-binding motifs (PXXP). Its interaction with the interleukin-2 (IL-2) receptor β- and γ-chains implies an involvement of p12I in intracellular signaling pathways. In addition, we have demonstrated that expression of pX ORF I p12I is essential for persistent infection in rabbits. In contrast, standard in vitro systems have thus far failed to demonstrate a contribution of p12I to viral infectivity and ultimately cellular transformation. In this study we developed multiple in vitro coculture assays to evaluate the role of p12I in viral infectivity in quiescent peripheral blood mononuclear cells to more accurately reflect the virus-cell interactions as they occur in vivo. Using these assays, we demonstrate a dramatic reduction in viral infectivity in quiescent T lymphocytes for a p12 mutant viral clone (ACH.p12) in comparison to the wild-type clone ACH. Moreover, addition of IL-2 and phytohemagglutinin during the infection completely rescued the ability of ACH.p12 to infect primary lymphocytes. When newly infected primary lymphocytes are used to passage virus, ACH.p12 also exhibited a reduced ability to productively infect activated lymphocytes. Our data are the first to demonstrate a functional role for pX ORF I in the infection of primary lymphocytes and suggest a role for p12I in activation of host cells during early stages of infection.
Human T-lymphotropic virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia. The viral infection is also associated with tropical spastic paraparesis/HTLV-1-associated myelopathy and a variety of other immune-mediated disorders (42). In addition to the common retroviral genes gag, pol, and env, HTLV-1 also contains several regulatory and accessory genes encoded in four open reading frames (ORF) in the pX region of the viral genome (pX ORF I to IV) (4, 5, 9, 10). Two of these open reading frames, pX ORF III and pX ORF IV, encode the regulatory proteins Rex and Tax, respectively, which have been extensively characterized. Rex is a 27-kDa nucleolus localizing phosphoprotein that increases the cytoplasmic accumulation of unspliced and singly spliced RNA (24). Tax is a 40-kDa nucleus-localizing protein which increases viral transcription from the HTLV-1 long terminal repeat (LTR), as well as many cellular genes involved in host cell proliferation (23).
Much less is known regarding the role of accessory proteins p12I, p27I, p13II and p30II encoded by pX ORF I and II in the replication and pathogenesis of HTLV-1. The mRNA of these proteins has been detected in cells derived from adult T-cell leukemia and tropical spastic paraparesis/HTLV-1-associated myelopathy patients as well as asymptomatic carriers (4, 5, 30). Moreover, humoral immune responses have been reported against p13II and p30II as well as p12I in infected patients (6, G. A. Dekaban, unpublished data). Furthermore, cytotoxic T lymphocytes isolated from a variety of infected subjects with and without disease recognize peptides representing regions in all four of the accessory proteins, indicating the chronic production of these proteins during HTLV-1 infections (37).
The accessory protein p12I, encoded in pX ORF I, is a 99-amino-acid hydrophobic protein which localizes to cellular endomembranes (29). The protein has two putative transmembrane domains and contains four minimal proline-rich SH3 binding motifs (PXXP), which are commonly found in proteins involved in intracellular signaling pathways (18). PXXP motifs 1 and 3 (amino acids 8 to 11 and 70 to 73) are highly conserved among viral strains (18). Taken together, these two findings imply a function for p12I in modulating intracellular signaling pathways. Moreover, p12I associates with the β- and γ-chains of the interleukin-2 (IL-2) receptor (34) as well as the 16-kDa subunit of the vacuolar H+-ATPase (19).
Recent studies designed to analyze the role of p12I in viral replication failed to demonstrate a contribution of p12I to viral replication and immortalization of primary lymphocytes in vitro (15, 39). These studies, however, were performed with target cells activated by the presence of IL-2 and phytohemagglutinin (PHA). In contrast, we recently demonstrated that selective ablation of p12I dramatically decreases the infectivity of an infectious molecular clone of HTLV-1, ACH, in a rabbit model of infection (13). If p12I increased viral infectivity by activation of quiescent primary cells, conditions involving a highly activated target cell population would override the requirement for p12I expression. Support for such a hypothesis comes from biochemical and functional similarities of HTLV-1 p12I with human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) Nef. Nef is also a hydrophobic, membrane-associated protein that contains an SH3 binding motif (20). This motif facilitates interactions of Nef with cell signaling pathways, in particular those involving the Src kinases Hck, Lck, and Lyn (38). Functionally, Nef is required for optimal in vivo viral infectivity in a manner similar to HTLV-1 p12I (26, 27). Importantly, Spina et al. (41) and Miller et al. (32) demonstrated that Nef is required for induction of HIV replication in quiescent primary T lymphocytes in vitro (32, 41). This effect was further observed in CD4+ cell lines infected with low virus inputs (8).
In this study we used multiple in vitro coculture assays to test the biological function of p12I in viral infectivity and replication. These assays are based on the coculture of a variety of HTLV-1-producing cells with naive, quiescent peripheral blood mononuclear cells (PBMC) in the absence of exogenous stimuli to more accurately reflect the virus-cell interactions in vivo. Using these assays, we demonstrate a dramatic reduction in the viral infectivity of a p12 mutant molecular clone of HTLV-1 (ACH.p12) in primary lymphocytes. Furthermore, upon addition of mitogens to the coculture, we observed restoration of the mutant's ability to infect quiescent target cells. These data provide the first evidence that HTLV-1 p12I is required for optimal viral infectivity in quiescent primary cells and suggest a role for p12I in T-lymphocyte activation.
MATERIALS AND METHODS
Plasmids and cells.
The ACH plasmid is an infectious molecular clone of HTLV-1 (14) and has been described previously (28). ACH.p12 contains a deletion in the splice acceptor of the third exon of the pX ORF I DNA, resulting in complete ablation of p12I expression without affecting the expression of any other viral genes encoded in this region (13). Normal uninfected human PBMC were obtained by leukophoresis and maintained as previously described (35). All HTLV-1-transformed or -immortalized cell lines were maintained in RPMI 1640 supplemented with 15% fetal bovine serum (FBS), 1% streptomycin-penicillin, and 1% glutamine (complete RPMI [cRPMI]). Human IL-2 (hIL-2; Roche Molecular Biochemicals, Indianapolis, Ind.) was added at 10 U/ml where indicated. HUT-102 (21) and MT-2 (33) are HTLV-1-transformed cell lines. The immortalized, IL-2-dependent cell lines ACH.1, ACH.2, ACH.p12.2, and ACH.p12.4 were generated by transfection of PBMC with ACH or ACH.p12, as described (13). Jurkat is an HTLV-1-negative human T-cell line (American Type Culture Collection catalog no. TIB-152). The 293T cell line is a human epithelial cell line, 293, which stably expresses the simian virus 40 T antigen (kind gift of G. Franchini, National Institutes of Health [NIH]). 293T were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 1% streptomycin-penicillin, and 1% glutamine (complete DMEM [cDMEM]).
Flow cytometry and PI staining.
Flow cytometric analysis of expression of cell surface markers CD3, CD4, CD8, CD25, and CD69 was performed as described (12). All above antibodies were from BD PharMingen (San Diego, Calif.) and were used according to the manufacturer's recommendations. Propidium iodide (PI) (Sigma, St. Louis, Mo.) staining for DNA content of cells to measure proliferation was performed essentially as described (25). In brief, 107 cells were fixed in 70% ethanol for 1 h at −20°C. Cells were then pelleted, resuspended in low-molecular-weight DNA extraction buffer (0.05 M Na2HPO4, 25 mM citric acid, 0.1% Triton X-100 [pH 7.8]), and stained with PI (20 μg/ml) plus RNase A (50 μg/ml) in phosphate-buffered saline. Data were collected using a Coulter Epics Elite flow cytometer and analyzed using Epics Elite software, version 4.1 (Coulter Corp., Miami, Fla.). Samples stained with fluorescein isothiocyanate-conjugated anti-CD3 or PE-conjugated anti-CD8 antibodies, respectively, were included for color compensation.
Quantification of proviral copy number.
HTLV-1 proviral copy number per cell was determined using real-time PCR in a Roche Light Cycler (Roche Molecular Biochemicals). Genomic DNA was obtained by affinity column separation (QiAmp; Qiagen; Santa Clarita, Calif.). Genomic DNA (50 ng, equivalent to ≈6,730 cells) was amplified in the presence of 4 mM MgCl2 using HTLV-1 gag-specific primers SG166 and SG296 (17) at 0.5 μM. For quantification purposes, a dilution series of a vector containing the gag target region (StyIΔ28) was amplified in parallel as previously described (1). Experiments were done in duplicate. Proviral copy number per cell was derived by dividing the total number of copies per reaction with the cell equivalent of 50 ng of DNA (6,730 cells). The sensitivity of the assay was estimated to be 0.005 proviral copies/cell.
Evaluation of HTLV-1 expression.
Viral p19 antigen production of the ACH.1, ACH.2, ACH.p12.2, and ACH.p12.4 cell lines was measured in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) in quadruplicate samples (Zeptomatrix, Buffalo, N.Y.) according to the manufacturer's protocol. Western blot analysis was used to evaluate the expression of cell-associated viral proteins as described (11). Briefly, 5 × 106 cells immortalized with either ACH or ACH.p12 were lysed, and 20 μg of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer to nitrocellulose, viral proteins were detected by an anti-HTLV-1 human antiserum (Scripps Laboratories, San Diego, Calif.). Blots were then stripped and reprobed with monoclonal antibodies against the surface envelope protein gp46 (IC-11) (36) and the matrix protein p19 (Zeptomatrix), respectively, and a polyclonal antibody against the viral transactivator Tax (no. 6505, NIH AIDS Research and Reference Reagent Program). Bands were visualized with appropriate secondary antibodies conjugated to horseradish peroxidase using chemiluminescence.
Electron microscopy.
Viral particle morphology was analyzed by electron microscopy. 293T cells transfected with ACH and ACH.p12 or HTLV-1-transformed cell lines were fixed in 3% glutaraldehyde, treated with 1.33% osmium tetroxide, and dehydrated. Embedded samples (Sponate 12 Resin; Ted Pella Inc., Redding, Calif.) were sectioned with an LKB ultratome and stained with uranyl acetate and lead citrate. Viral particles were examined from stained sections using a Phillips 300 electron microscope.
In vitro infection of primary cells.
Infection of PBMC was performed by coculture with HTLV-1-producing cell lines. Target PBMC were either taken directly from cryopreservation (quiescent) or prestimulated for 4 days with hIL-2 (10 U/ml) and PHA (2 μg/ml) (activated) in cRPMI.
For infection of PBMC by transfected human 293T kidney epithelial cells, 293T were transiently transfected with ACH and ACH.p12 as previously described (39) to generate 293T-ACH and 293T-ACH.p12 effector cell populations, respectively. Viral antigen production was monitored in cell culture supernatants every 24 h posttransfection, followed by complete medium changes. At 48 h posttransfection, 106 293T cells were seeded per well in a six-well plate. To provide optimal conditions for the coculture with target PBMC, medium was changed from cDMEM to cRPMI at 72 h posttransfection. After another 24 h, viral p19 antigen production in the ACH- and ACH.p12-transfected 293T cells was tested and consistently found to be equal. Then 293T cells were lethally γ-irradiated (10,000 rads), provided with fresh cRPMI, and overlaid with 106 naive quiescent or activated PBMC. Wells containing irradiated 293T-ACH and 293T-ACH.p12 cells alone were treated identically to control for residual p19 production by irradiated effector cells. After 7 days of coculture, PBMC were separated from 293T cells by gentle resuspension and subsequent aspiration, washed, and reseeded in fresh cRPMI supplemented with hIL-2 (10 U/ml). Postcoculture supernatants were taken at 1, 3, 7, 14, 21, and 28 days postwash and analyzed for the presence of viral antigen by p19 ELISA in comparison to a standard curve and a negative control well containing medium alone.
Viral p19 detected in wells containing either irradiated 293T-ACH or 293T-ACH.p12 alone was subtracted as background antigen production. Data points of the mean of triplicate samples ± standard error of the mean (SEM) representing two independent experiments were analyzed for statistical significance using Student's t test.
For coculture of PBMC with ACH and ACH.p12 cell lines, the effector cells were equilibrated for viral p19 antigen production as determined by ELISA, lethally irradiated (10,000 rads), washed, and added to naive quiescent or activated PBMC at a 1:10 ratio. Wells containing irradiated effector cells alone were cultured in parallel as above to control for background p19 production. Coculture was performed in 24-well plates for 96 h in cRPMI without IL-2 or PHA unless otherwise indicated. After coculture, cells were washed and reseeded into fresh cRPMI supplemented with hIL-2 (10 U/ml). Supernatants were tested for viral p19 antigen as above. Results were summarized for pools of triplicate samples representing four independent experiments.
Infectivity assays using newly infected PBMC as effector cells were carried out as follows. Naive PBMC (106) stimulated with hIL-2 and PHA were cocultured with lethally irradiated ACH and ACH.p12 cell lines in the presence of hIL-2 and PHA for 7 days to produce newly infected PBMC effector cells (passage 2 [P2] cells). At the end of coculture, these effector cells were washed, reseeded in cRPMI containing hIL-2 (10 U/ml), and cultured alone for an additional 7 days. Then, effector cells were equilibrated for p19 production, lethally irradiated (10,000 rads), and added to naive quiescent or activated PBMC at a 1:10 ratio. Coculture of P2-ACH and P2-ACH.p12 with either quiescent or activated PBMC or without target cells as a background control was performed for 7 days. At the end of coculture, PBMC were washed and reseeded in cRPMI supplemented with hIL-2 (10 U/ml). Supernatants were tested for HTLV-1 p19 as described above. Data points of the mean of triplicate samples ± SEM representing two independent experiments were analyzed for statistical significance using Student's t test.
RESULTS
Characterization of PBMC target cells.
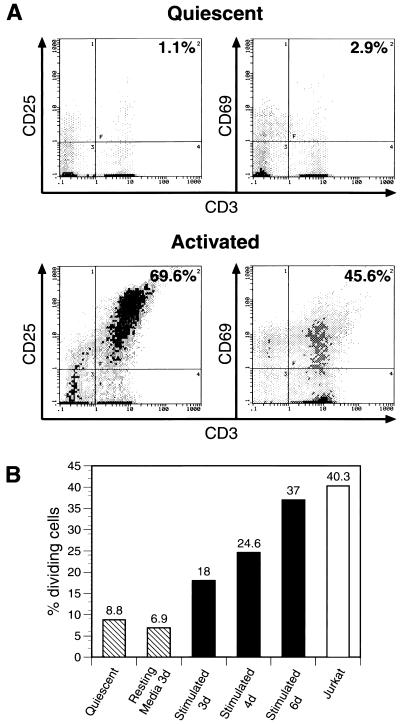
To evaluate the activation status of the PBMC used as target cells in all in vitro infectivity assays, we performed flow cytometric analysis of T-cell activation markers (CD 25/IL-2 receptor α and CD69) on quiescent or activated PBMC. As expected, stimulation of PBMC with IL-2 and PHA caused an increase in the expression of activation markers CD25 and CD69 (Fig. 1A) within the examined CD3+ T-cell population. To confirm that upregulation of CD69 and CD25 correlated with increased proliferation, the DNA content of quiescent and activated PBMC populations was determined by PI staining. As predicted, IL-2-PHA treatment of PBMC caused an increase in cellular proliferation in a time-dependent fashion from 3 to 6 days compared to an actively dividing T-cell line (Jurkat) (Fig. 1B). No significant modification of apoptotic cell (sub-G1) peak was observed (data not shown).
FIG. 1.
Characterization of PBMC used as target cells in infectivity assays. (A) Flow cytometry specific for activation markers CD25 and CD69 was performed on 106 quiescent (directly out of cryopreservation) or activated (4 days in culture with hIL-2 [10 U/ml] and PHA [2 μg/ml]) PBMC. Activated PBMC show a marked increase in surface marker expression. (B) PI staining for DNA content in quiescent (hatched) and activated (solid) for 3, 4, or 6 days as indicated PBMC. Percentage of dividing cells reflects cells in S and G2/M phases of the cell cycle. “Resting media 3d” (hatched) indicates a 3-day culture of PBMC in RPMI supplemented with 15% FBS, which represents the medium conditions used for coculture experiments. Jurkat (open bar) is a transformed human T-cell line.
Transmission of HTLV-1 from transfected 293T cells.
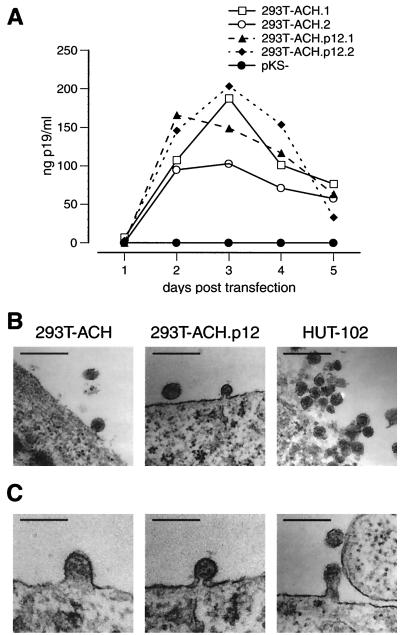
Previous studies have demonstrated successful virus production by 293T kidney epithelial cells transiently transfected with the HTLV-1 infectious clone ACH (39). To examine whether selective ablation of pX ORF I expression would directly influence the infectivity of ACH in primary cells, we used transfected, lethally irradiated 293T cells to infect either activated or quiescent PBMC. 293T cells transfected with ACH (293T-ACH) or ACH.p12 (293T-ACH.p12) produced high and comparable levels of viral p19 antigen as early as 48 h posttransfection (Fig. 2A) and released viral particles similar in shape to those of the HTLV-1-transformed cell line HUT-102 (Fig. 2B). Viral particles in all stages of maturation were detected in transfected 293T cells (Fig. 2C). As expected, these were lower in number than in HUT-102 cells, which we have previously reported to contain high proviral copy numbers (1). To further evaluate whether cell-to-cell contact between 293T and quiescent PBMC would activate the PBMC population, quiescent PBMC were cocultured with nontransfected 293T cells in cRPMI for 7 days and then subjected to flow cytometric analysis for the expression of cellular activation markers CD25 and CD69. Cell-to-cell contact between 293T cells and quiescent PBMC did not activate the PBMC, as no significant increase in expression of CD25 or CD69 surface markers could be detected (data not shown).
FIG. 2.
Viral antigen and particle production by transfected 293T cells. (A) Viral p19 antigen production in 293T cells transiently transfected in duplicate with ACH or ACH.p12. Culture supernatant samples were taken every 24 h, followed by complete medium changes. 293T cells produced high amounts of viral p19 antigen, with peak production at 3 days posttransfection. Levels between ACH- and ACH.p12-transfected cells are equal. (B) Electron microscopy showing HTLV-1 particle release from transfected 293T cells in comparison to the HTLV-1-transformed cell line HUT-102. Magnification, ×53,000. Bar, 200 nm. (C) Viral particles at different stages during the maturation process in transfected 293T cells. Particles are shown in the transition from early and late budding to release and are representative samples from 293T-ACH as well as 293T-ACH.p12 cells. Micrographs were taken at a magnification of ×53,000 and further magnified electronically. Bar, 90 nm.
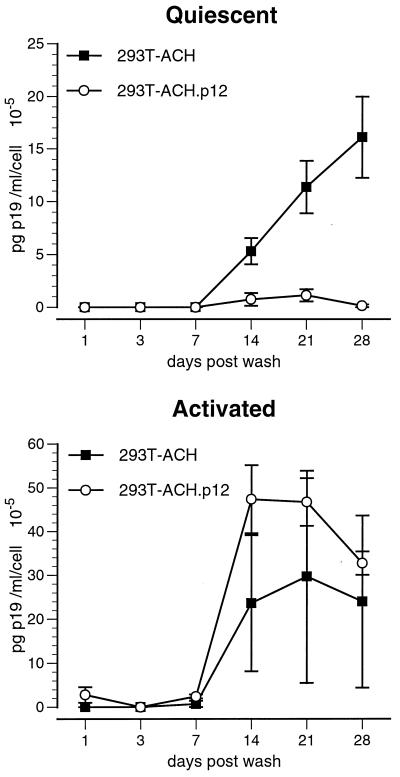
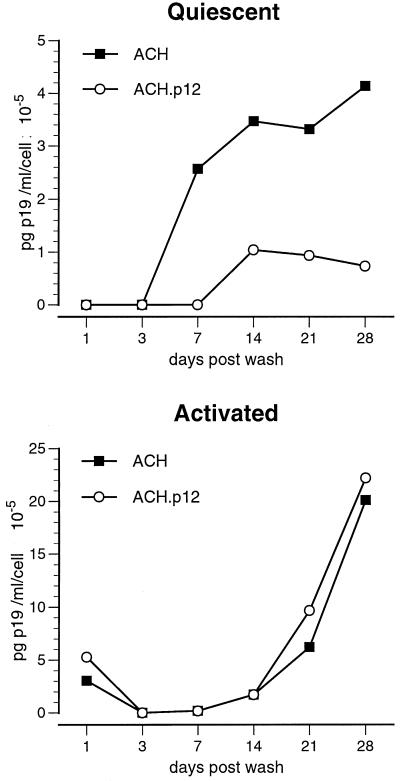
To test the cell-to-cell transmission of wild-type and p12I-deleted HTLV-1, we cocultured lethally irradiated 293T cells transfected with ACH (293T-ACH) or ACH.p12 (293T-ACH.p12) with either quiescent or activated PBMC. Virus produced by ACH.p12-transfected 293T cells had a significantly reduced infectivity in quiescent primary cells (Student's t test), while no significant differences were observed in activated PBMC (Fig. 3). To determine whether these results are due to an ability of the 293T-ACH cells but not the 293T-ACH.p12 cells to activate the PBMC target population, we performed flow cytometric analysis of CD25 and CD69 expression on PBMC target cells 4 weeks after coculture. We did not observe a difference in CD25 and CD69 expression on either initially quiescent or activated PBMC cocultured with 293T-ACH or 293T-ACH.p12 (data not shown).
FIG. 3.
Decreased viral infectivity of ACH.p12 produced in transfected 293T cells. Values represent de novo viral p19 antigen detected in cell culture supernatants of PBMC infected by coculture with 293T-ACH or 293-ACH.p12 cells. Data points are the means of triplicate samples ± SEM and represent two independent experiments. ACH.p12 produced in 293T cells has a significantly lower infectivity in quiescent PBMC than the wild type, while no significant difference can be observed in activated PBMC. Statistical analysis was performed using Student's t test (P < 0.01).
Viral antigen production in ACH and ACH.p12 cell lines.
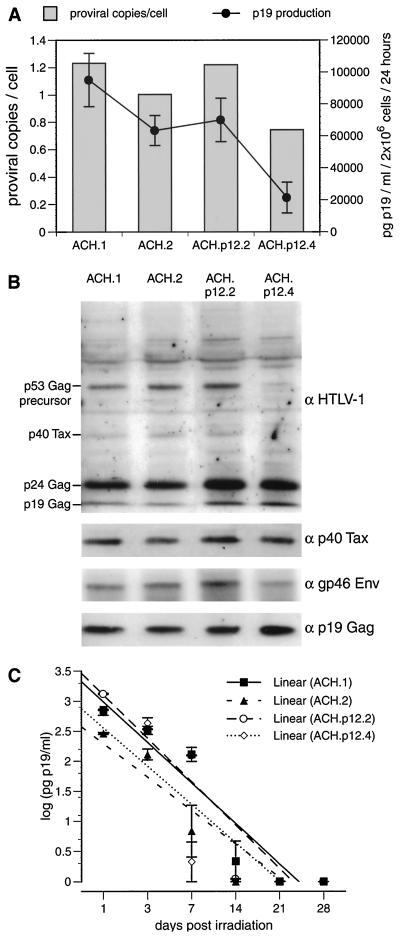
Next, we evaluated the effects of selective ablation of HTLV-1 p12I on the lymphocyte-to-lymphocyte transmission of the virus. CD4+ or CD8+ PBMC cell lines immortalized with either ACH or ACH.p12 (12) were used to infect activated or quiescent PBMC. The mutation fidelity and lack of p12I expression in the ACH.p12 cell lines was confirmed by PCR-based methods as described (12) (data not shown). To further characterize the ACH- and ACH.p12-immortalized cell lines, we measured the p19 antigen production of four of these lines and determined their proviral load per cell (Fig. 4A). As expected, individual differences between the four cell lines were observed in regard to viral p19 antigen production and proviral copy number per cell. However, these differences were not dependent upon the expression status of p12I. The variations in viral antigen production by the two ACH and the two ACH.p12 cell lines correlated with the number of proviral copies per cell, as determined by gag-specific quantitative real-time PCR. To test the expression of various other cell-associated viral proteins in the ACH and ACH.p12 cell lines, we performed Western blot analysis. The major cell-associated viral proteins p19Gag, p24Gag, p40Tax, and the p53Gag precursor detected by a polyclonal HTLV-1 antiserum in the four cell lines were similar except for decreased levels of the p53Gag precursor in ACH.p12.4 (Fig. 4B). Further analysis with specific polyclonal antibodies against Tax and monoclonal antibodies against gp46Env and p19Gag also demonstrated similar expression levels of these proteins among the four cell lines.
FIG. 4.
Characterization of PBMC immortalized with ACH and ACH.p12. (A) Comparison of viral p19 antigen production and proviral copy number in two ACH- and ACH.p12-immortalized PBMC cell lines. p19 values are represented as averages of quadruplicate samples ± SEM; proviral copy number is shown as an average of two independent experiments. (B) Immunoblot of cellular lysates from ACH- and ACH.p12-immortalized cell lines using a human anti-HTLV-1 antiserum. The membrane was subsequently stripped and reprobed with a polyclonal antibody against Tax and monoclonal antibodies against gp46 and p19. (C) Decay of viral p19 antigen production in ACH and ACH.p12 cell lines after lethal γ-irradiation. Cell culture supernatants were taken 24 h after complete medium changes. Values plotted on a logarithmic scale for regression analysis are the mean of triplicate samples ± SEM and represent two independent experiments. The slopes of the four curves do not differ statistically (P > 0.05) by χ2 test, indicating a similar decay of p19 production in these cell lines after irradiation.
To ascertain whether the ACH and ACH.p12 cell lines would be affected equally by lethal γ-irradiation (10,000 rads), we measured the decay of their p19 antigen production over 28 days postirradiation. Decay of virus production correlated with decay of overall cell number (data not shown) and was not significantly different between the ACH and ACH.p12 lines, as confirmed by regression analysis and the χ2 test (Fig. 4C).
Transmission of HTLV-1 from immortalized PBMC cell lines.
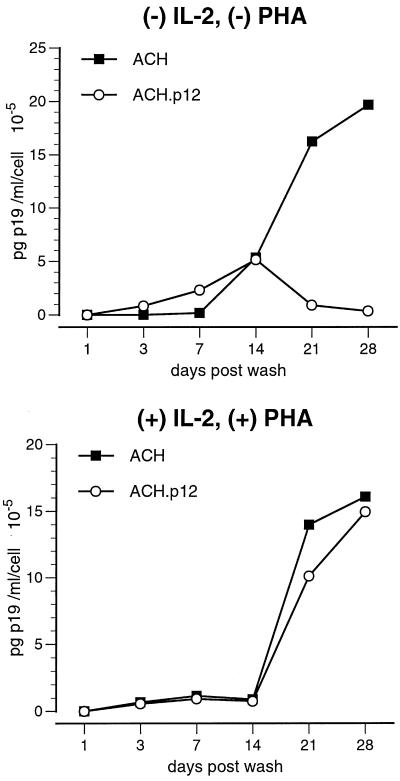
We next examined whether the ACH- and ACH.p12-immortalized PBMC lines had the same ability to transmit HTLV-1 to quiescent and activated PBMC by coculture in the absence of IL-2 and PHA. While ACH- and ACH.p12-immortalized PBMC lines were equally efficient in transmitting virus to activated PBMC, an approximately 10-fold-reduced infectivity was detected for ACH.p12-expressing cell lines cocultured with quiescent PBMC regardless of the combination of ACH and ACH.p12 cell lines used (Fig. 5).
FIG. 5.
Decreased viral infectivity for ACH.p12 in quiescent primary lymphocytes. Quiescent and activated PBMC were cocultured with ACH or ACH.p12 cell lines for 96 h in 15% cRPMI in the absence of exogenous stimuli, such as hIL-2 or PHA, and washed and reseeded in fresh cRPMI containing hIL-2 (10 U/ml). Supernatants were assayed for viral p19 antigen production by ELISA for 28 days postwash. Data points are the averages for a pool of triplicate samples and represent four independent experiments. While ACH and ACH.p12 equally infect activated PBMC, ACH.p12 has an approximately 10-fold-reduced infectivity in quiescent PBMC.
In a second set of experiments, we asked whether addition of exogenous stimuli to the coculture medium could rescue the ability of the ACH.p12 “knockout” lines to infect quiescent PBMC. For this purpose, ACH and ACH.p12 lines were cocultured with initially quiescent PBMC in either the absence or presence of IL-2 and PHA. As expected, the addition of both IL-2 and PHA to the coculture medium completely restored the ability of ACH.p12 to infect primary cells (Fig. 6).
FIG. 6.
Rescue of ACH.p12 infectivity by the addition of exogenous mitogens. Quiescent PBMC were infected by coculture with ACH and ACH.p12 cell lines in the presence or absence of hIL-2 (10 U/ml) and PHA (2 μg/ml) for 3 days, washed, and reseeded into fresh cRPMI containing hIL-2 (10 U/ml). De novo p19 production by newly infected PBMC was monitored for 28 days postwash by ELISA; all other conditions were as described. While ACH.p12 has a decreased infectivity in the absence of IL-2 and PHA, it infects quiescent PBMC as efficiently as ACH in the presence of these stimuli. Data points shown are the averages for a pool of triplicate samples and represent four independent experiments.
Reduced infectivity of ACH.p12 in quiescent and activated PBMC during serial viral passage.
To establish an environment for the cell-to-cell transmission of HTLV-1 that resembles most closely the cellular events during the natural infection, we infected PBMC by coculture with ACH- and ACH.p12-immortalized cell lines. These newly infected PBMC (P2 cells) were then used as effector cells in an infectivity assay as described above. To confirm that these PBMC produced comparable amounts of virus, we analyzed their p19 production by ELISA. P2-ACH and P2-ACH.p12 cells produced equal amounts of viral p19 antigen per cell (approximately 15 × 10−5 pg/ml per cell; data not shown). To determine whether the newly infected PBMC had similar phenotypes, flow cytometry was performed on cell surface markers CD3, CD4, CD8, CD25, and CD69. As expected for PBMC, both cell groups were mixed populations of T cells and expressed similar levels of the activation marker CD69. However, the P2-ACH effector population had an up to twofold increase in the expression of the IL-2Rα chain (CD25). This overall increase was caused by the presence of an approximately 4- to 10-fold-greater number of CD3+ CD25high lymphocytes compared to the P2-ACH.p12 cells (data not shown).
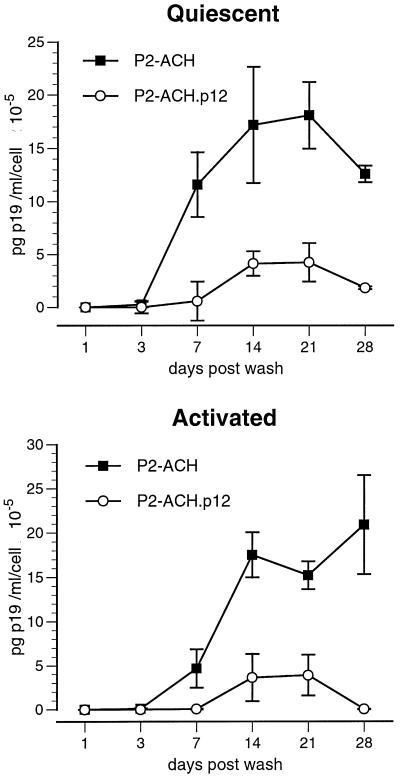
We then tested the ability of PBMC newly infected with ACH and ACH.p12 (P2 cells) to transmit virus to activated or quiescent PBMC. Two weeks after infecting PBMC with ACH and ACH.p12 to create P2-ACH and P2-ACH.p12, these P2 cells were used to pass on virus to quiescent or activated PBMC. Interestingly, ACH.p12 had a significantly reduced infectivity in quiescent as well as activated PBMC, as determined by Student's t test (Fig. 7). Last, we investigated, whether p12I induced changes in the phenotype of the activated or quiescent target cells which could help explain the increased infectivity of the wild-type virus ACH. Infected PBMC were analyzed by flow cytometry 4 weeks after coculture for the expression of CD4, CD8, and the lymphocyte activation markers CD25 and CD69. No significant alteration of the T-cell phenotype in the ACH-infected PBMC was observed in comparison to the ACH.p12-infected PBMC for both activated and quiescent target cells (data not shown).
FIG. 7.
Decreased viral infectivity for newly infected P2-ACH.p12 cells in primary lymphocytes. Quiescent and activated PBMC were cocultured with PBMC populations newly infected with ACH or ACH.p12 (P2-ACH and P2-ACH.p12) for 7 days in 15% cRPMI in the absence of stimuli, such as IL-2 or PHA. After coculture, cells were washed and reseeded in fresh medium including hIL-2 (10 U/ml). Supernatants were assayed for viral p19 antigen production by ELISA for 28 days postwash. Data points are the means of triplicate samples ± SEM and represent two independent experiments. A significantly reduced infectivity was observed for P2-ACH.p12 in quiescent and activated PBMC target cells (Student's t test, P < 0.01).
DISCUSSION
In this study we have investigated whether selective ablation of HTLV-1 p12I reduces viral infectivity in primary lymphocytes. Using three independent approaches to evaluate the infectivity of a wild-type infectious clone of HTLV-1 (ACH) and an ACH mutant with selective ablation of pX ORF I p12I (ACH.p12), we demonstrate a dramatically reduced infectivity for ACH.p12 in quiescent PBMC. If ACH.p12 is transmitted from newly infected cells, a reduction in viral infectivity can also be observed in activated target PBMC. Our data are the first to describe a phenotype in cell culture for a mutant of HTLV-1 lacking expression of pX ORF I p12I. Furthermore, our results suggest an involvement of HTLV-1 p12I in activation of host cells during early stages of infection. These findings are consistent with our previous studies that demonstrated a requirement for HTLV-1 p12I in viral infectivity in a rabbit model of infection (13).
We first infected PBMC by coculture with 293T cells transfected with ACH and ACH.p12 in order to test whether the mutation in p12I would directly affect the ability of the proviral clone to produce infectious virus. While particles produced in both 293T-ACH and 293T-ACH.p12 were equally infectious in activated PBMC, we observed a significantly reduced ability of 293T-ACH.p12 cells to infect quiescent PBMC. These differences are most likely directly linked to viral infectivity rather than to cell-mediated effects, since coculture of uninfected 293T cells with quiescent PBMC neither changed the PBMC's overall CD3 CD4 CD8 phenotype nor caused an increase in the expression of the lymphocyte activation markers CD25 and CD69. Flow cytometric analysis at 28 days after coculture of PBMC infected by 293T-ACH and 293T-ACH.p12 revealed no difference in activation as measured by CD25 and CD69 expression. This was regardless of the initial activation status of the PBMC before coculture.
To determine whether these differences in viral infectivity were reproducible in a biologically more relevant system, we used PBMC cell lines immortalized with ACH and ACH.p12 as effector cells. These cells provide a useful system to examine the lymphocyte-to-lymphocyte transmission of HTLV-1 because they have been extensively characterized and phenotypically resemble the natural effector cell (12). Further analysis of the ACH and ACH.p12 lines revealed that they produced similar levels of viral p19 antigen, which roughly correlated with the number of integrated proviruses. Importantly, besides reduced expression levels of the p53Gag precursor in ACH.p12.4, both ACH and ACH.p12 cells produced similar amounts of all other cell-associated viral proteins, especially the transactivator protein Tax. In addition, previous studies by Robek et al. (39) showed equal Tax activity in ACH and ACH.p12 cell lines using an LTR-luciferase reporter plasmid. These studies also demonstrated no effect of the mutation in pX ORF I p12I on the expression and function of Rex (39). In addition to similar viral parameters, all ACH and ACH.p12 cell lines exhibited a similar sensitivity to γ-irradiation. Thus, we concluded that any differences in the ability of these cell lines to transmit virus to naive PBMC would be related to the expression of pX ORF I. When using these cells to infect PBMC by coculture, we observed a dramatic reduction in the ability of the ACH.p12 lines to infect quiescent PBMC. Consistent with our findings using transfected 293T cells, no reduction in infectivity was demonstrated in activated PBMC. Moreover, rescue experiments showing that addition of mitogens to the coculture restored the ability of ACH.p12 to infect quiescent PBMC suggest that HTLV-1 p12I is involved in activation of host cells during early stages of infection.
Since we could not completely rule out that differences in viral infectivity are caused by effects due to long-term culture of ACH and ACH.p12 cells, we performed a third set of experiments to compare ACH and ACH.p12. Using PBMC newly infected with ACH and ACH.p12 (P2-ACH and P2-ACH.p12) to passage virus to naive PBMC, we observed significant decreases in viral infectivity in quiescent as well as activated PBMC for HTLV-1 lacking pX ORF I expression (P2-ACH.p12). One hypothesis consistent with our results is that the naive PBMC in the second passage are exposed to lower levels of infectious virus than in a coculture with ACH- and ACH.p12-immortalized cell lines. This is supported by reduced p19 output of the P2 effector cells and by previous studies that showed decreased infectivity for a nef mutant of HIV in CD4+ cell lines infected with low virus inputs (8). In addition, we believe that not only the number of viral particles shed by the effector cells but also the quality of the cellular transmission of these particles by optimal effector-target cell contact influence the overall infectivity of this highly cell-associated virus. In support of this hypothesis, we observed an increase in the number of CD3+ CD25high lymphocytes in the P2-ACH cells compared to the P2-ACH.p12 cells.
Ours is the first study to show the requirement for HTLV-1 p12I expression for efficient viral infectivity in primary lymphocytes. In contrast to previous studies that did not find an involvement of p12I in viral infectivity in vitro (13, 15, 39), we have used quiescent primary cells as target cells and have examined viral infectivity during early stages of infection. The effect mediated by HTLV-1 p12I is not unprecedented, as a variety of viral proteins are required only for efficient viral replication or pathogenesis in vivo, most prominently the Nef protein of HIV and SIV. Nef has marked biochemical and functional similarities with HTLV-1 p12I. Like p12I, Nef is hydrophobic (20) and highly conserved (3, 7, 43) and contains an SH3 binding motif that facilitates the functional interaction of Nef with many cellular signaling proteins (38) and that is required for Nef-mediated increases in viral infectivity and activation of infected host cells (7, 40). More importantly, Nef is critical for efficient viral infectivity in vivo (16, 27). Moreover, two reports showed that selective ablation of HIV nef dramatically decreases viral infectivity in quiescent CD4+ lymphocytes in vitro, while no difference from the wild type was observed in fully activated target cells (32, 41). Findings of these studies were later supported by numerous other reports using a variety of cellular systems (2, 22, 31), providing further parallels to this study.
In summary, this report demonstrates a positive contribution of p12I to the HTLV-1 life cycle in primary cells that is consistent with our findings in the animal model of HTLV-1 infection (13). We propose that HTLV-1 p12I is involved in the activation of host cells during early stages of infection. This would provide maximal virus production, resulting in an increased rate of infection of other naive target cells. The exact intracellular pathways that p12I might interact with remain to be elucidated. Due to the conserved nature of the four SH3 binding motifs (PXXP) in p12I, it is likely that it interacts specifically with certain cellular SH3 domain-containing proteins that are involved in stimulatory signaling pathways. Biochemical delineation of the specific interactions of p12I with cellular signaling pathways will further strengthen the knowledge about the molecular mechanisms of HTLV-1-induced pathogenesis and the feasibility of a p12I mutant virus as an attenuated live vaccine.
ACKNOWLEDGMENTS
This work was supported by grants CA-55185 (M.L.), RR-14324 (M.D.L.), and CA-63417 (L.R.) from the NIH and CA-70259 from the Ohio State University, Comprehensive Cancer Center. B. Albrecht is supported by a fellowship from Boehringer Ingelheim Fonds. M. Lairmore is supported by an Independent Scientist Career Award from the National Institutes of Health.
We thank Richard Meister in the Center for Retrovirus Research Cytometry Laboratory for assistance with flow cytometry, Evelyn Handley for performing electron microscopic analyses, and Tim Voijt for preparation of figures. Furthermore, we are indebted to James DeWille for providing PCR facilities. We also thank David Derse, Maureen Shuh, Michael Robek, and Andrew Phipps for valuable technical advice and Weiqing Zhang and Celine D'Souza for helpful discussions.
REFERENCES
- 1.Albrecht B, Collins N D, Newbound G C, Ratner L, Lairmore M D. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. doi: 10.1016/s0166-0934(98)00087-1. [DOI] [PubMed] [Google Scholar]
- 2.Alexander L, Du Z J, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 Nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asamitsu K, Morishima T, Tsuchie H, Kurimura T, Okamoto T. Conservation of the central proline-rich (PxxP) motifs of human immunodeficiency virus type 1 Nef protein during the disease progression in two hemophiliac patients. FEBS Lett. 1999;459:399–404. doi: 10.1016/s0014-5793(99)01288-0. [DOI] [PubMed] [Google Scholar]
- 4.Berneman Z N, Gartenhaus R B, Reitz M S, Blattner W A, Manns A, Hanchard B, Ikehara O, Gallo R C, Klotman M E. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman M E. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y M, Chen S H, Fu C Y, Chen J Y, Osame M. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I Tof, Rex and Tax in HTLV-I-infected people with differing clinical status. Int J Cancer. 1997;71:196–202. doi: 10.1002/(sici)1097-0215(19970410)71:2<196::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Hoxie J P, Parks W P. The conserved core of human immunodeficiency virus type 1 Nef is essential for association with Lck and for enhanced viral replication in T-lymphocytes. Virology. 1999;264:5–15. doi: 10.1006/viro.1999.9937. [DOI] [PubMed] [Google Scholar]
- 8.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciminale V, D'Agostino D, Zotti L, Franchini G, Felber B K, Chieco-Bianchi L. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology. 1996;209:445–456. doi: 10.1006/viro.1995.1277. [DOI] [PubMed] [Google Scholar]
- 10.Ciminale V, Pavlakis G N, Derse D, Cunningham C P, Felber B K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cockerell G L, Lairmore M D, De B, Rovnak J, Hartley T, Miyoshi I. Persistent infection of rabbits with HTLV-I: patterns of anti-viral reactivity and detection of virus by gene amplification. Int J Cancer. 1990;45:127–130. doi: 10.1002/ijc.2910450123. [DOI] [PubMed] [Google Scholar]
- 12.Collins N D, D'Souza C, Albrecht B, Robek M D, Ratner L, Ding W, Green P L, Lairmore M D. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins N D, Newbound G C, Albrecht B, Beard J L, Ratner L, Lairmore M D. Selective ablation of human T-cell lymphotropic virus type 1 p12(I) reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 14.Collins N D, Newbound G C, Ratner L, Lairmore M D. In vitro CD4(+) lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell lymphotropic virus type 1. J Virol. 1996;70:7241–7246. doi: 10.1128/jvi.70.10.7241-7246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 16.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich G D, Greenberg S, Abbott M A. Detection of human T-cell lymphoma/leukemia viruses. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Harcourt Brace Jovanovich; 1990. pp. 325–336. [Google Scholar]
- 18.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 19.Franchini G, Mulloy J C, Koralnik I J, Lo Monico A, Sparkowski J J, Andresson T, Goldstein D J, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel A D, Young J A. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Gazdar A F, Carney D N, Bunn P A, Russell E K, Jaffe E S, Schechter G P, Guccion J G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980;55:409–417. [PubMed] [Google Scholar]
- 22.Glushakova S, Grivel J C, Suryanarayana K, Meylan P, Lifson J D, Desrosiers R, Margolis L. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J Virol. 1999;73:3968–3974. doi: 10.1128/jvi.73.5.3968-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The retroviridae. New York, N.Y: Plenum Press; 1994. pp. 277–311. [Google Scholar]
- 24.Hollsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotz M A, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994;15:237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- 26.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 27.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high viral loads and for the development of AIDS. Cell. 1991;65:651. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 28.Kimata J T, Wong F, Wang J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 29.Koralnik I J, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koralnik I J, Gessain A, Klotman M E, Lo Monico A, Berneman Z N, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc Natl Acad Sci USA. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messmer D, Ignatius R, Santisteban C, Steinman R M, Pope M. The decreased replicative capacity of simian immunodeficiency virus SIVmac239deltanef is manifest in cultures of immature dendritic cells and T cells. J Virol. 2000;74:2406–2413. doi: 10.1128/jvi.74.5.2406-2413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 34.Mulloy J C, Crowley R W, Fullen J, Leonard W J, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12(I) protein binds the interleukin-2 receptor beta and gamma(c) chains and affects their expression on the cell surface. J Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newbound G C, Andrews J M, Orourke J, Brady J N, Lairmore M D. Human T-cell lymphotropic virus type 1 tax mediates enhanced transcription in CD4+ T lymphocytes. J Virol. 1996;70:2101–2106. doi: 10.1128/jvi.70.4.2101-2106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palker T, Tanner M, Scearce R, Streilen R, Clark M, Haynes B. Mapping of immunogenic regions of human T-cell leukemia virus type 1 (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989;142:971–978. [PubMed] [Google Scholar]
- 37.Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar M C. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J Exp Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renkema H G, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–D283. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- 39.Robek M D, Wong F H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchiyama T. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 43.Zanotto P M, Kallas E G, de Souza R F, Holmes E C. Genealogical evidence for positive selection in the nef gene of HIV-1. Genetics. 1999;153:1077–1089. doi: 10.1093/genetics/153.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]