Abstract
Background
Hypertension is a chronic, multifactorial clinical condition characterized by sustained high blood pressure levels. It is often associated with functional-structural alterations of target organs, which include heart, brain, kidneys, and vasculature.
Objective
This study highlights the recent correlation between the immune system and hypertension and its repercussions on target-organ damage.
Methods
The descriptors used for the search of the study were “hypertension”, “immunity”, and “target organs”. The methodology of the study followed the main recommendations of the PRISMA statement.
Results
The damage to the vasculature arises mainly from the migration of T cells and monocytes that become pro-inflammatory in the adventitia, releasing TNF-α, IFN-γ, and IL-17, which induce endothelial damage and hinder vascular relaxation. In the renal context, the inflammatory process associated with hypertension culminates in renal invasion by leukocytes, which contribute to the injury of this organ by mechanisms of intense sympathetic stimulation, activation of the renin-angiotensin system, sodium retention, and aggravation of oxidative stress. In the cardiac context, hypertension increases the expression of pro-inflammatory elements, such as B, T, and NK cells, in addition to the secretion of IFN-γ, IL-17, IL-23, and TNF-α from angiotensin II, reactive oxygen species, and aldosterone. This pro-inflammatory action is also involved in brain damage through SphK1. In view of the above, the participation of the immune system in hypertension-induced injuries seems to be unequivocal.
Conclusion
Therefore, understanding the multifactorial mechanisms related to hypertension will certainly allow for more efficient interventions in this condition, preventing target organ damage.
Keywords: Hypertension, immune system, immunologic factors, target organs, heart, blood vessels, kidney, brain
1. INTRODUCTION
Systemic arterial hypertension (SAH) is a chronic, multifactorial clinical condition characterized by sustained high levels of blood pressure (BP), represented by systolic pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg, with pressure values greater than 130 x 85 mmHg already classified as prehypertension [1]. It is often associated with functional and/or structural alterations of target organs, which include heart, brain, kidneys, and blood vessels, in addition to metabolic alterations, which have been widely associated with the metabolic syndrome [1, 2]. For this reason, SAH constitutes one of the most important known, identifiable, and controllable risk factors for the development of cardiovascular diseases, such as heart attack, stroke, coronary atherosclerosis, heart failure, and atrial fibrillation [3, 4]. The risk of developing coronary atherosclerosis and stroke is directly related to blood pressure; therefore, an increase in systolic and diastolic pressures implies an exponential increase in the risk of involvement, as demonstrated by the fact that a 20 mmHg increase in systolic pressure or 10 mmHg is responsible for doubling the risk for both events [5]. Furthermore, hypertension is responsible for at least 45% of deaths from heart disease and 51% of deaths from stroke worldwide [1].
These cardiovascular diseases have been the leading cause of death in the world, accounting for about 30% of all deaths. An increase in the percentage has been shown when it comes to developing countries, such as Brazil [6]. Due to the high morbidity and mortality related to hypertension, resulting mainly from its chronic condition and for remaining asymptomatic for many years, there is great interest and search for the improvement of its early detection and proper control [1, 7, 8]. Thus, it becomes crucial to understand the mechanisms underlying hypertension, its subsequent damage to the kidneys, heart, brain, and vascular system, and the immunological pathophysiology of this damage. A deep understanding of these factors would be providential to the evaluation of hypertensive condition to classify it according to the risk stratification and propose the best and most adequate therapeutic interventions so that the complications from this condition can be minimized [5].
Considering the impacts caused by hypertension, especially on the cardiovascular system, it is observed that blood pressure control is essential for cardiovascular risk reduction. The treatment for hypertension, which consists of both the use of medications and lifestyle changes, should be carried out continuously and followed for life. However, it is common to find patients that discontinue the treatment at some point, becoming non-adherent to chronic treatment. Non-adherence behavior represents a major impasse in disease control. This can accelerate the appearance of damage to target organs and cause their failure. Patients who are treatment adherent present favorable outcomes that could be almost three times better responses compared to non-adherent patients [9].
Several subsets of immune cells infiltrate the target tissues and release pro-hypertensive molecules, including cytokines, matrix metalloproteinases (MMPs), and reactive oxygen species (ROS), which promote damage to the vessel wall, kidneys, heart, and central nervous system by mediating tissue injury, preventing vascular relaxation, and increasing sodium reabsorption [10]. In this context, the overactivation of the renin-angiotensin-aldosterone system (RASA), which has regulatory functions for blood pressure and body fluid homeostasis, promotes an inflammatory response by activating the immune system. Increased levels of angiotensin II generate vascular and renal damage, sodium retention, and endothelial dysfunction [11].
Another important mechanism in the pathophysiology of hypertension is the organic damage caused by oxidative stress through the oxidation/antioxidation relationship arising from factors released during the response to injury. Activated immune cells generate excess ROS through Nox-dependent mechanisms, which leads to cytokine production. These modifications lead to a disruption of oxidation-reduction signaling. Thus, oxidative stress promotes low-grade or persistent inflammation and angiotensin II activation [12].
The role of MMP expression in the immune mechanism of SAH was also observed. It has been found that their activity is increased during the global inflammatory process since they are secreted by pro-inflammatory cells, and their secretion is stimulated by cytokines. In the inflammatory setting, MMPs are responsible for degrading various proteins in the extracellular matrix (ECM), including collagen, elastin, and gelatin [13, 14]. They are also associated with dysfunction of the vascular endothelium and vascular smooth muscle cells and can stimulate changes in cardiomyocytes. This whole process can, for example, lead to vascular remodeling, being the first step in the development of cardiovascular complications already mentioned. At the same time, researchers have demonstrated that MMP-2 and MMP-9 may be involved in the abnormal tissue remodeling associated with AH in kidney disease by pathogenic remodeling of the ECM, leading to nephrosclerosis and ultimately to chronic kidney disease (CKD). Many researchers have observed a noticeably higher level of MMP-9 in the serum of hypertensive patients compared to normotensive controls [15].
In addition, mononuclear cells (including lymphocytes) and the deposition of immunoglobulins and complement proteins were found to be demonstrated in the renal interstitium adjacent to injured renal tubules and glomeruli in hypertensive humans. More recently, it was demonstrated that hypertensive individuals present glomerulosclerosis and renal fibrosis that accompany the infiltration of macrophages and T lymphocytes in the renal interstitium, affecting the parenchymal injury, which may lead to CKD [16].
In this context, the main objective of this study is to present how the immune system is involved in the body damage observed in SAH, using previous investigations in the literature. This study highlights recent findings on SAH and the immune system, as well as its repercussions on target organs, such as vessels, kidney, heart, and brain.
2. METHODS
This study is a literature-based review, carried out in six steps, as recommended by the anagram “PICOS”: identification and choice of theme to be researched, definition of descriptors and scientific databases, definition of inclusion and exclusion criteria, search for studies in scientific databases, selection of eligible studies, and critical analysis of the selected studies [17]. The entire basis of the application of the “PICOS” anagram followed the main recommendations of the “PRISMA statement” [18], which is geared at dividing this process into four steps: identification, selection, eligibility, and inclusion.
Using the Pubmed, MEDLINE, and SciELO databases, publications relevant to the proposal were identified, with the last search conducted on May 25th, 2021. The following descriptors were used to locate the specific articles for this study: hypertension, immunity, and target organs; the presence of all three descriptors is required. Inclusion criteria included English and Portuguese language studies that investigated the relationship between immunological aspects of hypertension and target organ damage. Only papers published in the last ten years were selected. The authors independently screened the titles and abstracts for eligibility by the paired selection method. Potentially eligible studies were subjected to full-text review to determine whether they met the predetermined inclusion criteria. Any disagreement was resolved by consensus among the reviewers.
3. RESULTS
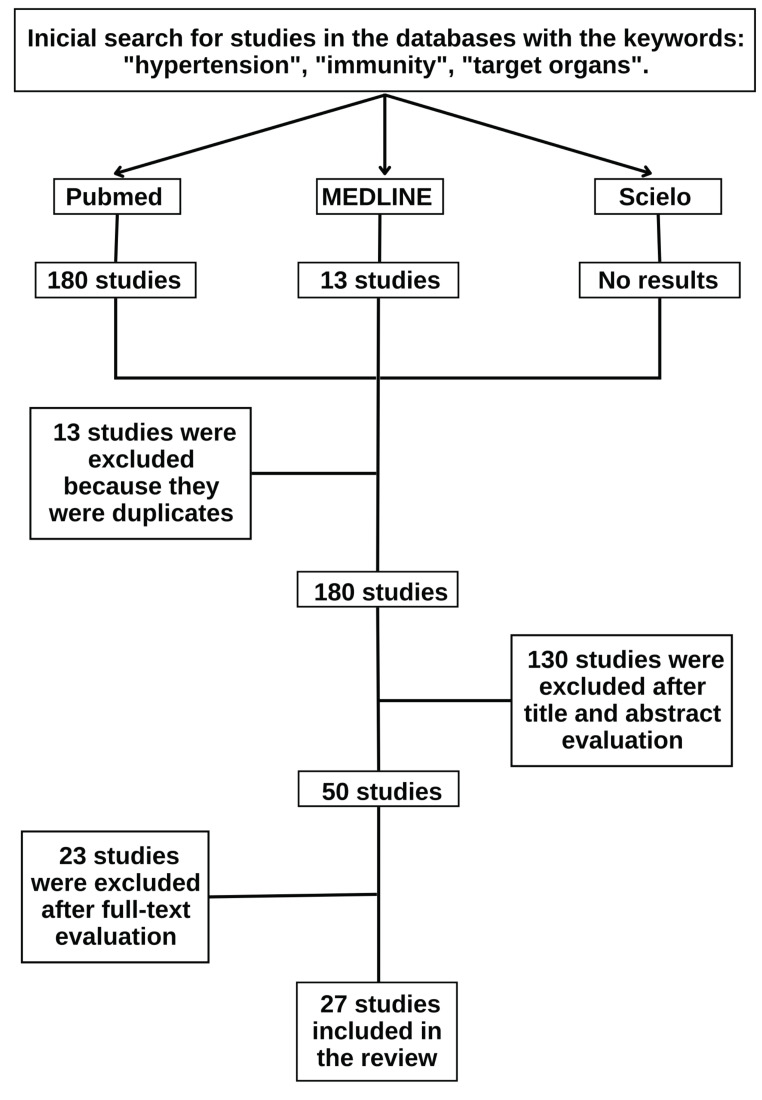
Following the PRISMA recommendations, the authors found 180 studies in the initial search, excluding 13 duplicate studies, representing the first stage, i.e., identification. Then, the selection stage began, divided into two parts. In the first part, the titles and abstracts were read, and 130 studies were excluded. This can be explained because although the studies presented the descriptors mentioned in the title or abstract, they did not establish a direct correlation between possible immunological mechanisms and the occurrence of target organ damage in hypertension, addressing only, in most cases, the existence of a relationship between the progression of hypertension and inflammatory processes. In the second part, the remaining studies after the selection based on reading the titles and abstracts (50) were selected by reading the full text, and 23 studies were excluded. At the end of this selection step, the remaining 27 eligible studies were included in the present study, as shown in Fig. (1).
Fig. (1).
Flow of the search and selection of studies according to PRISMA recommendation.
The 23 included eligible studies served as a theoretical reference basis for the construction of the results of the present study, which were described for the four most relevant target organs in the context of the immunological picture of hypertension: the vasculature, the kidneys, the heart, and the encephalon.
3.1. Damage to the Vasculature
During hypertension, immune cells infiltrate the blood vessels promoting tissue damage and hindering vascular relaxation. Thus, endothelial dysfunction and inflammatory infiltration of the vessel wall are crucial factors in the development of hypertension [19].
Migration of monocytes, neutrophils and macrophages to the vascular wall during hypertension induces the production of chemokines, cytokines, and reactive oxygen species (ROS), contributing to oxidative stress [20]. In addition, neutrophils secrete mediators responsible for the inflammatory response, such as elastase and myeloperoxidase [21].
3.1.1. T-cell Migration
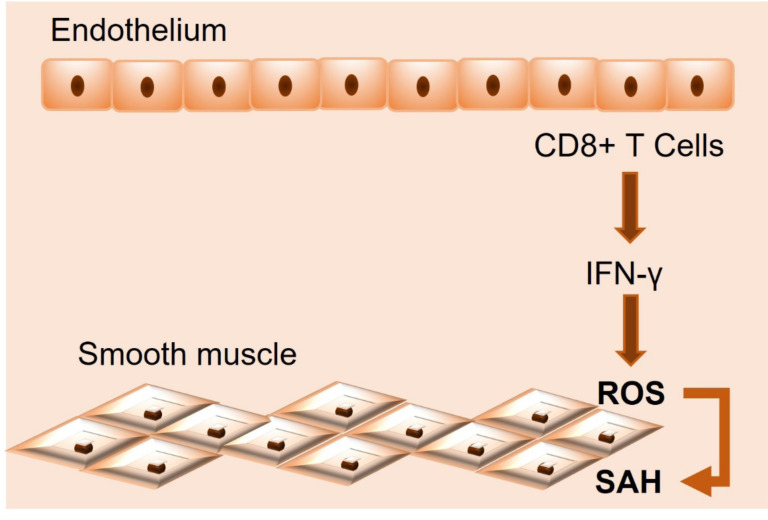
T cells and monocytes infiltrate the vessel wall and intensify local inflammation and tissue damage. In both angiotensin II (Ang II)-induced and high salt-induced hypertension, T cells become pro-inflammatory in the adventitia of vessels and start producing pro-inflammatory cytokines, such as interferon-gamma (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin 17A (IL-17A), which intensify hypertensive responses and induce endothelial dysfunction [22]. Under hypertensive stimuli, activated T cells, particularly CD8+ T cells, migrate into the vessels and produce IFN-γ, which, as observed in mice, induces superoxide generation in vascular smooth muscle cells, indicating that activated T cells can increase superoxide production in both kidneys and blood vessels by secretion of IFN-γ [23] (Fig. 2). T-cell activation and overproduction of these pro-inflammatory cytokines have been observed in both experimental hypertension in mice and hypertension in humans and are associated with significant increases in chemokine receptors that direct these immune cells to blood vessels [24].
Fig. (2).
Systemic arterial hypertension (SAH) development into blood vessels. Abbreviations: IFN-Ɣ = interferon-gamma; ROS = reactive oxygen species.
3.1.2. Oxidative Stress
Studies have shown that hypertension caused by Ang II infusion and vascular dysfunction is associated with increased vascular superoxide production. The isoforms of NADPH oxidase (NOX), NOX1-5, are the major sources of superoxide in vessels, where they play an important role in vascular remodeling and dysfunction [20].
Hypertensive stimuli promote an increase in vascular oxidative stress. Superoxide production is increased due to increased NOX activity, and this effect is associated with reduced nitric oxide (NO) production and impaired endothelium-dependent vasodilation [24]. Hypertension-induced oxidative stress also affects the function of vascular smooth muscle cells, which play an important role in vessel contractility. In the vasculature, these ROS induce vasoconstriction, which impairs endothelium-dependent vascular relaxation [19].
Increased NADPH oxidase activity is strongly involved in regulating the activation of immune cells and their recruitment to the vasculature. Macrophage-derived ROS and inflammatory cytokines provide endothelial dysfunction. In addition, ROS production within immune cells is essential for immune activation and the development of the vascular inflammatory response [25].
The studies showed that inhibition of these ROS caused a reduction in blood pressure and improved endothelial function through increased NO production. These findings prove the damaging role of ROS in the vasculature since excess ROS increase endothelial cell differentiation and apoptosis and control the vascular tone and endothelial function, contributing to endothelial dysfunction [24]. Pro-inflammatory cells are thought to be the main source of superoxide in the vascular wall [20].
3.1.3. IL-17
Interleukin 17 (IL-17) increases blood pressure and decreases NO-dependent vascular relaxation responses by activation of RhoA/Rho kinase. Several experiments showed that vessels from IL-17-deficient mice preserved vascular function and decreased superoxide production. In addition, a significant reduction in vessel T-cell infiltration was observed in response to chronic infusion of Ang II [22].
It has also been observed that IL-17 induces increased expression of pro-inflammatory cytokines, chemokines, adhesion molecules, and matrix metalloproteases. In vitro studies have described that IL-17A activates tubular epithelial cells, vascular smooth muscle cells, endothelial cells, and fibroblasts to release a wide variety of pro-inflammatory mediators, including chemokines, which, in turn, are involved in the recruitment of T cells, macrophages, and monocytes to the injured vascular wall [26].
Other studies have described that IL-17 is associated with increased ROS production, modulation of nitric oxide levels, and activation of protein kinases [26] and that in mice deficient in this interleukin, increased superoxide production did not occur [27]. IL-17 causes damage to vascular smooth muscle cells by directing the local generation of ROS, CCL2 (chemokine ligand 2), interleukin 8 and interleukin 6 [28].
Salleh et al. showed that serum IL-17A levels are significantly increased in humans with hypertension compared to those without hypertension. T-cell production is increased in mice infused with Ang II. IL-17A-deficient mice were found to be protected from endothelial cell dysfunction and hypertension caused by chronic Ang II infusion. Similarly, immunolabeling of IL-17A, but not IL-17F, reduced blood pressure in Ang II-infused mice. In this study, both Th17 and γδT cells produced equal amounts of IL-17A during Ang II hypertension [29]. In a subsequent study, Norlander et al. demonstrated that IL-17A stimulates sodium and water retention by acting on several sodium transporters within the kidney. It was also shown that IL-17A promotes inhibitory phosphorylation of endothelial nitric oxide synthase, reducing endothelial nitric oxide production and, when infused into mice, causes hypertension [30]. Consistent with these findings, infusion of IL-17A has been shown to increase the hypertensive response to Ang II infusion.
Mice deficient in the pro-inflammatory cytokine IL-17A have been shown to exhibit attenuated hypertension, reduced vascular inflammation, and preserved vascular function in response to Ang II-induced hypertension [31].
3.1.4. IL-10
Interleukin 10 (IL-10) has a protective function for vessels in the presence of hypertension. IL-10 was observed to limit Ang II-mediated vascular oxidative stress, and in IL-10-deficient mice, superoxide scavenging improved vascular function. Furthermore, the treatment of hypertensive mice with IL-10 reduced systolic blood pressure and NADPH oxidase activity, limiting oxidative stress [22]. Meanwhile, IL-10-deficient mice exhibit increased NADPH oxidase activity and microvascular endothelial dysfunction with variable effects on hypertensive response [27].
3.1.5. Ang II
Ang II was observed to cause aortic wall thickening and collagen deposition in mice [20]. The chronic infusion of Ang II significantly impaired vasodilation and endothelium-dependent vascular relaxation to acetylcholine (Ach) compared to the control group treated with saline solution [23]. Ang II significantly increased superoxide production in the aortic wall as well as aortic NADPH oxidase activity. The studies demonstrated that the levels of NADPH oxidase NOX1, NOX2, and NOX4 in the aorta artery were significantly elevated by Ang II treatment in mice, which contributed to vascular dysfunction [20].
Ang II has significant pro-inflammatory actions, inducing the recruitment of monocytes and lymphocytes into the subendothelial space, increasing the expression of cell adhesion molecules (CAM), platelet aggregation, and the secretion of cytokines, chemokines, and growth factors, all of which are implicated in the development of atherosclerosis and vascular wall inflammation [19]. By binding to angiotensin 1 receptors (AT1R1), Ang II determines the differentiation of immune cells and the subsequent production of pro-inflammatory cytokines, such as IL-6, IFN-γ, and TNFα. Ang II also contributes to inflammation by stimulating NADH and NADPH oxidase, thus increasing ROS, including superoxide production [24].
Blockade of T-cell co-stimulatory receptors was also found to significantly contribute to the control hypertension arising from the effects of Ang II and deoxycorticosterone acetate (DOCA) [32]. Similarly, deletion of CD70, which is a major factor in memory T-cell formation, has been shown to prevent recurrent episodes of hypertension. It was also found that deletion of gamma delta T cells represented an improvement in hypertensive response and vascular dysfunction caused by Ang II infusion [33]. Studies such as these have highlighted the importance of immune cells in the development of hypertension and its associated target organ damage, particularly with regard to the role of Ang II.
3.2. Kidney Damage
Chronically elevated blood pressure levels are associated with functional rarefaction of the kidney, fibrotic replacement of renal parenchymal tissue, and defects in glomerular function [34]. Hypertension has been found to be an inflammatory process that triggers the accumulation and infiltration of different subsets of cellular immune cells in target organs, including the kidneys.
Studies point out that these cells interact, in positive feedback pathways, with hypertensive agents, such as Ang II and excess salt. This environment stimulates the activation of immune system cells and the release of pro-inflammatory cytokines, which culminate in renal leukocyte invasion, as occurs with AT1 receptor activation [35] and sodium-mediated hypertonicity [36]. Thus, renal injury is sustained by mechanisms independent of blood pressure. This can be seen in a study in which therapy with the angiotensin-converting enzyme inhibitor, quinapril, reduced renal glomerular and tubular damage and the accumulation of inflammatory cells. In research, an experimental model was used in rodents in which inhibition of the renin-angiotensin system (RAS) limited renal inflammation and oxidative stress independently of BP [35]. Moreover, chronic inflammation can reduce the reactivity of the anterior glomerular vessels, increase renin release, activate the afferent renal nerve, and increase vasoconstriction, promoting glomerular dysfunction and proteinuria [37].
3.2.1. T-Lymphocytes
The main hypothesis is that stimulated by hypertensive agents, such as Ang II or high salt, T cells are activated and assume a pro-inflammatory profile, infiltrating the kidney and secreting a series of cytokines of this profile, such as IL-6, IL-1β, IFN-γ, TNF-α, and IL-17A [38]. Some consequences of this expression would be sodium retention in the kidneys, vascular dysfunction (with increased vascular resistance), and intense sympathetic stimulation, which contribute to increased blood pressure and directly to the aggravation of organ damage [38]. A practical example of how this process occurs is in the finding that salt increases IL-17A production from CD4+ T cells via a serum glucocorticoid-regulated kinase 1-dependent (SGK1-dependent) pathway. These hypotheses are reinforced by the fact that mice lacking SGK1 T cells are protected from Ang II-induced endothelial and glomerular dysfunction and renal injury [34]. Excessive salt intake also increased renal infiltration of T lymphocytes associated with albuminuria and glomerular and tubular damage. One of the possible causes of T cell damage is that they act by stimulating renal sodium retention, possibly by suppressing NO synthase-3 and cyclooxygenase-2 in the kidney [27].
Furthermore, another study pointed out that the profile of Treg (anti-inflammatory) cells was reduced in hypertensive rats by deoxycorticosterone acetate salt, which altered the balance of these cells with other T-cell populations of pro-inflammatory profile. These changes were inhibited by spironolactone and anti-IL-17 antibody [39]. Innate immune cells and γδ T cells contribute to inflammation, both directly and by activating adaptive immunity, inducing pro-inflammatory cytokines, such as IL-17 and IFN-γ, and the production of autoantibodies, leading to vascular and renal injury [36].
3.2.2. Cytokines
There is a robust body of evidence that IL-17 plays a decisive role in kidney damage. This cytokine is released by T cells and induces, in addition to oxidative stress in vessels, increased sodium reabsorption both in the proximal tubule through the Na+/H+ exchanger and in the distal contouring tubule through the Na+/Cl- co-transporter [27]. Another finding is that anti-IL-17A antibodies attenuated lymphocyte infiltration into the kidneys of animals infused with Ang II [22] and that IL-17A -/- models abolished activation of the sodium chloride co-transporter and epithelial sodium channel and protected mice from glomerular and tubular injury [22].
Regarding TNF-α, it has been found that its expression is associated with a global activation of the SRA [40]. In kidneys, it is believed to contribute to increased injury to the glomerulus by increasing the risk of ischemia (via vasoconstriction) in a mechanism independent of blood pressure while also impairing sodium excretion [40]. Another study pointed out that this cytokine is directly toxic to glomerular epithelial cells [27]. Moreover, in rats, TNF inhibition attenuates the glomerular and tubular damage accumulated by hypertension from various causes and blunts the chronic hypertensive response to Ang II [27].
Another cytokine that can be directly associated with kidney injury is IL-6. Its levels were also increased by Ang II, with consequent induction of pro-fibrotic and endothelin-1 gene expression. IL-6 promoted a rightward shift in the renal natriuresis-pressure relationship and increased angiotensinogen expression in cultured renal proximal tubular cells, altering renal function [24]. Additionally, IL-6 gene deletion prevented renal dysfunction in mice [24]. Pharmacological inhibition of this interleukin preserved renal function in rats and reduced monocyte infiltration into the kidney [41].
3.2.3. Oxidative Stress
As in vessels in the kidney, macrophage-derived ROS and inflammatory cytokines induce endothelial and epithelial dysfunction, which compromises pressure natriuresis, stimulates renal vasoconstriction, and potentiates tissue damage effects that also contribute to sustained hypertension [25]. The availability of NO in the renal microvasculature is also compromised by the fact that these phenomena increase RAS activation.
In this case, renal injury is associated with two phenomena. ROS stimulate the activation of transcription factors (nuclear factor-kB, signal transducer and activator of activating protein 1 transcription, and hypoxia-inducible factor 1), pro-inflammatory genes, the production of chemokines and cytokines, and the recruitment and activation of inflammatory and immune cells. All these factors promote cardiovascular and renal inflammation and fibrosis, which are important processes underlying vascular injury and target organ damage in hypertension [42]. Furthermore, excessive ROS production in response to oxidative stress can affect all intrinsic renal cell types. Oxidative stress leads to podocyte apoptosis and subsequently to segmental glomerulosclerosis, which results in hypertension-induced kidney damage [43]. Oxidative stress also induces myofibroblast accumulation in the kidney and remodeling of the extracellular matrix of the tubular interstitium [43].
It was also found that mice lacking p47 phox, a subunit of NADPH oxidase, had impaired vascular superoxide production and the development of hypertension in response to Ang II infusion attenuated. At the same time, it was realized that transgenic overexpression of p22 phox leads to age-related hypertension and increases hypertension in response to Ang II [44].
3.2.4. Macrophages
Some involvement of these cells in hypertension pathogenesis or kidney injury may be indicated by the increase in macrophages relative to normotensive controls, as observed in post-mortem kidney analyses [25]. It is believed that, like other immune cells, pro-hypertensive factors polarize macrophages to a pro-inflammatory profile (M1 profile). In several models, expression of the chemokine receptor CCR2 on monocytes facilitates their recruitment to the kidney with consequent exacerbations in hypertensive kidney injury [27]. In turn, M1 would be responsible for contributing to renal injury by promoting sodium retention in the kidney through epithelial cell injury and vascular remodeling, the generation of ROS, and the secretion of pro-inflammatory cytokines, such as TNF-α or IL-1β [25].
For example, Fehrenbach et al. demonstrated that Dahl SS rats, when fed a high salt diet, exhibited a significant increase in macrophages infiltrating the kidney compared to control rats. Most of these macrophages exhibited a phenotype of pro-inflammatory M1-type macrophages. These studies demonstrated that myeloid cells encountering a high sodium microenvironment drive the activation of monocytes, DCs and macrophages to promote hypertension and target organ damage [45].
3.2.5. Other Components Involved
In addition to the immune system entities listed, dendritic cells and Toll-like receptors have also been associated with hypertensive kidney injury. In the former case, it has been indicated that these cells are involved in the renal infiltration of T cells and the presentation of antigens to the same cells in various hypertension models. Once dendritic cells are activated, this may represent susceptibility to hypertension [27]. Nevertheless, hypertension only occurs in the presence of activatable T lymphocytes. In the second case, several subtypes of TLRs (TLR2, TLR3, TLR4, TLR7, TLR8, and TLR9) seem to be involved in target organ injury, regardless of the model used. It is noteworthy that TLR4 and TLR2 seem to play some role in kidney injury [46].
3.3. Damage to the Heart and Brain
3.3.1. Heart
Like vessels and kidneys, the heart can also be a target of immune activity triggered by hypertension. The increase in Ang II plasma levels [34], the high salt content, and the accumulation of ROS propitiate the infiltration of pro-inflammatory elements (T lymphocytes, neutrophils, and macrophages) in those structures [38, 41]. A significant increase in Th17 expression of the retinoid-related orphan receptor γt (RORγt) and cytokines, including IL-17, IL-23, and TNF-α, have been shown in lymphocytes isolated from mouse heart tissue infused with Ang II [39].
On the other hand, the absence of hypertension stimulus causes a reduction in the expression of the transcription factor related to scurf protein (FoxP3), expressed in Treg, and of the cytokine IL-10 [39], as well as depletion of the interleukin 8 beta receptor (CXCR2), which reduces the number of infiltrating neutrophils and protects against ischemia- and reperfusion-induced cardiac injury [20].
Furthermore, the large amount of Ang II obtained experimentally by infusion was found to be associated with collagen deposition in cardiac tissue [22], inflammation, and fibrosis. The latter was reversed with N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP), which could reduce collagen deposition and reverse inflammation and fibrosis in the heart and kidney when infused chronically in rats with hypertension and myocardial infarction [20]. In turn, in hypertensive rats treated with prolyl oligopeptidase (POP) inhibitors, Ac-SDKP was found to be reduced [24].
Another simulated hypertensive condition in DOCA-salt rats was associated with an increased ratio of Th17 to Treg lymphocytes in the heart [39]. Moreover, thymosin beta-4 (Tβ4), capable of controlling cell morphogenesis and motility, preventing inflammation and fibrosis of the myocardium, was found to be increased in hypertensive individuals, causing lesions in the cardiac muscle [24].
Some studies have shown that hypertension causes the accumulation of activated Th17 cells in the cardiac wall. The deposition of these cells leads to remodeling and fibrosis, and thus to cardiac hypertrophy, arrhythmia, or heart failure. Th17 cells also produce Il-17-, a pro-inflammatory cytokine, which increases pressure levels by inhibiting nitric oxide production by the endothelium, increasing the formation of ROS, and generating fibrosis [18].
The changes in the immune system generated from hypertension exert effects mediated by the influence of pro-inflammatory cytokines on endothelial NO synthase (eNOS) expression by components of oxidative stress and modulation of cardiac metabolic function, all of which are essential in cardiac remodeling, hypertrophy, and hypertensive cardiomyopathy. Regarding cardiac fibrosis, intense effects were observed in relation to M1/M2 macrophage balance in the cardiac tissue [25].
Aldosterone is another element involved in the generation of heart damage from elevated blood pressure. This hormone is persistently elevated in hypertensive individuals; it causes cardiac damage and also renal and vascular damage. Aldosterone generates a cascade of activation of the innate immunity (neutrophils, macrophages/monocytes, dendritic cells, and natural killer cells), the innate-like γδ T cells, and the adaptive CD4+ or T helper cells, which become Th1, Th2, Th17, and regulatory T cells. This can result in more damage since aldosterone can remodel and fibrosis those organs. Thus, this hormone is closely related to the cycle of initial damage, inflammatory activation as an attempt to repair, and subsequent additional damage. Besides, it is a factor that predisposes to increased blood pressure and promotes primary hypertension [47].
3.3.2. Brain
The damage to the central nervous system, specifically the brain, also occurs by the action of the immune system in hypertensive patients. This damaging action occurs due to the pro-inflammatory T-cells arising from the effect exerted by the high amount of Ang II and the high salt content on T cells. This same mechanism is responsible for generating damage to several other target organs, as discussed in other sections [22].
In addition, a study using Western blotting, flow cytometry (fluorescence-activated cell sorting - FACS) and mass spectrometry in a mouse model of Ang II-induced hypertension showed that hypertension causes an increase in sphingosine kinase 1 (SphK1) levels and, consequently, in the amount of sphingosine-1-phosphate (S1P). This protein, in turn, was stipulated to be responsible for contributing to the chemotaxis of CD3+ T cells to the brain tissue and an increase in the amount of T cells that would be involved in inflammatory and degenerative processes, even causing cognitive dysfunction [48].
In recent years, extensive evidence has demonstrated the role of cerebral perivascular macrophages (PVMs) in the development of hypertension. Pro-inflammatory mediators, such as IL-1β, increase PGE2 expression within PVMs, resulting in increased sympathetic activation and consequent BP elevation. Furthermore, other neuronal cells, such as pericytes and astrocytes in the blood-brain barrier (BHE), can also be affected by systemic inflammation. As a consequence of the impairment of the cells in the BHE, there is an increased infiltration of immune cells into the brain and, thus, neuronal overexpression of PVMs [12]. Table 1 shows the main immune system factors involved in target organ damage during SAH.
Table 1.
Main immune system factors that promote target organs damage during SAH.
| Organ | Immune Factor Involved |
|---|---|
| Blood vessels | T-cell activation produces pro-inflammatory cytokines (IFN-γ, TNF-α, IL-17A) Monocytes |
| Kidney | T-cell activation secrets IL-6, IL-1β, IFN-γ, TNF-α, IL-17A M1-type macrophages Dendritic cells Toll-like receptors |
| Heart | T-lymphocytes, neutrophils, macrophages IL-17, IL-23, TNF-α |
| Brain | T-cells increase Cerebral perivascular macrophages IL-1β |
IFN-γ = Interferon-gamma; TNF-α = Tumor necrosis factor-α; IL = interleukin
4. DISCUSSION
It was found from the results presented that hypertension promotes inflammatory lesions in target organs mainly through the infiltration of immune cells into tissues, such as vessels, kidney, heart, and brain.
It has been observed that, to a large extent, the vascular damage is due to the infiltration of T cells and monocytes, which start producing pro-inflammatory cytokines in the adventitia of the vessels. Additionally, the increased oxidative stress resulting from hypertension promotes increased ROS, which, in turn, increases endothelial cell differentiation and apoptosis, and impairs endothelium-dependent vasodilation by decreasing NO production. IL-17 and IL-10 have also been found to play an important role in hypertensive vessel damage, as these interleukins are associated with increased expression of pro-inflammatory cytokines and ROS. Angiotensin II has also been related to increased infiltration of inflammatory cells into the vessel wall, as well as increased ROS production. Through experimental studies on mice, it was also found that when there was a deficiency in the aforementioned cells and mediators, the mice showed less hypertensive damage resulting from the expression of pro-inflammatory cytokines and ROS. Therefore, this fact suggests the importance of these components in the pathophysiology of hypertensive damage caused to the vessel wall.
At the same time, it becomes evident that inflammatory mechanisms, the renin-angiotensin system, and salt excess act synergistically in the occurrence of hypertensive damage to the kidneys. According to the literature reviewed, a positive feedback process occurs, in which the increased levels of renin and angiotensin stimulate the infiltration of inflammatory cells in this organ, which contributes to the increased retention and decreased excretion of salt and stimulates the perpetuation of this cycle so that the damage is sustained by mechanisms independent of blood pressure.
In this context, activated T cells, with a pro-inflammatory profile, play a prominent role in several studies, contributing to increased sodium retention, vascular dysfunctions, and increased sympathetic activity, which promote oxidative stress in the kidneys, leading to fibrosis, sclerosis, and apoptosis of podocytes that culminate in albuminuria and tubular damage that occur in hypertension.
A decreased profile of regulatory T cells, which attenuates the inflammatory environment, has also been listed as a contributing factor. Some of the cytokines cited as mediators of this process are IL-17, TNF-alpha, and IL-6, with studies mentioning that the decrease in their activity is associated with a reduction in the mechanisms of injury.
The damage to the heart mediated by arterial hypertension is widely demonstrated from multiple mechanisms that produce pro-inflammatory elements in the organ, similar to the damage suffered by other target organs. In this sense, factors such as high salt content, high ROS levels and high Ang II plasma levels, consequently, also high levels of aldosterone, are responsible for the activation of the innate and adaptive immune system, triggering an intense pro-inflammatory response that, despite having been activated with protective intention by the organism in reaction to an aggressive systemic situation of high blood pressure, will cause cardiac damage mediated specifically by factors, such as IL-17, IL-23, Th17, TNF-α, neutrophil chemotaxis, macrophages with pro-inflammatory action and T lymphocytes. In the brain, the same outcome of lymphocyte activation from Ang II and high salt content could be observed, constituting systemic damage from arterial hypertension.
Although some of the studies that demonstrate such mechanisms have been carried out on animals, restricting the quality of the evidence, specifically those studies that deal with the influence of Th17 on cardiac damage; the works in general point to the result of an imbalance in pro and anti-inflammatory function that leads to the establishment and progression of inflammation generating cycles of damage, thus resulting in tissue fibrosis and loss of organ function. In addition, studies have shown that the inflammatory immune action itself is responsible for promoting the cycle of target organ damage, which occurs mainly in the central nervous system, both from the passage of more immune cells from an increasingly blood-brain barrier damaged by inflammation, as well as by the elevation of blood pressure caused by the action of cerebral perivascular macrophages [49, 50].
CONCLUSION
SAH is still a risk factor for the development of serious chronic diseases that concomitantly affect several physiological systems and reduce life expectancy and quality of life. Among the hypotheses suggested as responsible for the deleterious effects of SAH is the exacerbated activation of the immune system. Apparently, SAH mediates a generalized inflammatory response in the body, impairing the functioning of blood vessels, heart, kidneys, and brain. For example, it has been described that during hypertension, immune cells infiltrate the blood vessels, promoting endothelial dysfunction and hindering vascular relaxation. In the kidneys, infiltration and accumulation of different subsets of immune cells have been observed. However, in the heart, infiltration of pro-inflammatory elements has been observed. Moreover, in the brain, tissue damage may be due to pro-inflammatory T cells. Considering the above, the participation of the immune system in systemic lesions induced by SAH seems to be unequivocal. Understanding the multifactorial mechanisms related to SAH will certainly allow for a more efficient treatment and, perhaps, the recovery of patients, since, to date, there is no cure for this condition.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- SAH
Systemic arterial hypertension
- BP
Blood Pressure
- MMPs
Matrix Metalloproteinases
- ROS
Reactive Oxygen Species
- RASA
Renin-Angiotensin-Aldosterone System
- ECM
Extracellular Matrix
- S1P
Sphingosine-1-Phosphate
- PVMs
Perivascular Macrophages
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
REFERENCES
- 1.Barroso W.K.S., Rodrigues C.I.S., Bortolotto L.A., et al. Diretrizes brasileiras de hipertensão arterial – 2020. Arq. Bras. Cardiol. 2021;116(3):516–658. doi: 10.36660/abc.20201238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Veen P.H., Geerlings M.I., Visseren F.L.J., et al. Hypertensive target organ damage and longitudinal changes in brain structure and function. Hypertension. 2015;66(6):1152–1158. doi: 10.1161/HYPERTENSIONAHA.115.06268. [DOI] [PubMed] [Google Scholar]
- 3.Lionakis N., Mendrinos D., Sanidas E., Favatas G., Georgopoulou M. Hypertension in the elderly. World J. Cardiol. 2012;4(5):135–147. doi: 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. W: A global brief on hypertension: Silent killer, global public health crisis. 2013.
- 5.Fuchs F.D., Whelton P.K. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromfield S., Muntner P. High blood pressure: The leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep. 2013;15(3):134–136. doi: 10.1007/s11906-013-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie P.D., Allison M.A., Avilés-Santa M.L., et al. Prevalence of hypertension, awareness, treatment, and control in the hispanic community health study/study of latinos. Am. J. Hypertens. 2014;27(6):793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiório C.E., Cesar C.L.G., Alves M.C.G.P., Goldbaum M. Prevalência de hipertensão arterial em adultos no município de São Paulo e fatores associados. Rev. Bras. Epidemiol. 2020;23:e200052. doi: 10.1590/1980-549720200052. [DOI] [PubMed] [Google Scholar]
- 9.Correa N.B., Faria A.P., Junior H.M., Modolo R. Não adesão ao tratamento farmacológico anti-hipertensivo como causa de controle inadequado da hipertensão arterial. Rev Bras Hipertens. 2016;23(3):58–65. [Google Scholar]
- 10.Brouwers S., Sudano I., Kokubo Y., Sulaica E.M. Arterial hypertension. Lancet. 2021;398(10296):249–261. doi: 10.1016/S0140-6736(21)00221-X. [DOI] [PubMed] [Google Scholar]
- 11.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Kardiol. Pol. 2019;77(2):71–159. doi: 10.5603/KP.2019.0018. [DOI] [PubMed] [Google Scholar]
- 12.Kućmierz J, Frąk W, Młynarska E, Franczyk B, Rysz J. Molecular interactions of arterial hypertension in its target organs. Int. J. Mol. Sci. 2021;22(18):9669. doi: 10.3390/ijms22189669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond G.R., Vinh A., Guzik T.J., Sobey C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019;19(8):517–532. doi: 10.1038/s41577-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 14.Bisogni V., Cerasari A., Pucci G., Vaudo G. Matrix metalloproteinases and hypertension-mediated organ damage: Current insights. Integr. Blood Press. Control. 2020;13:157–169. doi: 10.2147/IBPC.S223341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of enzyme inhibition and medicinal chemistry. 2016. p. 31(sup1): 177- 83. [DOI] [PubMed]
- 16.Mattson D.L., Dasinger J.H., Abais-Battad J.M. Amplification of salt-sensitive hypertension and kidney damage by immune mechanisms. Am. J. Hypertens. 2021;34(1):3–14. doi: 10.1093/ajh/hpaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvão T.F., Pereira M.G. Revisões sistemáticas da literatura: Passos para sua elaboração. Epidemiol. Serv. Saude. 2014;23(1):183–184. doi: 10.5123/S1679-49742014000100018. [DOI] [Google Scholar]
- 18.Sarkis-Onofre R., Catalá-López F., Aromataris E., Lockwood C. How to properly use the PRISMA statement. Syst. Rev. 2021;10(1):117. doi: 10.1186/s13643-021-01671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piqueras L., Sanz M.J. Angiotensin II and leukocyte trafficking: New insights for an old vascular mediator. Role of redox-signaling pathways. Free Radic. Biol. Med. 2020;157:38–54. doi: 10.1016/j.freeradbiomed.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Zhang Q., Wu H., et al. NIU, K. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am. J. Hypertens. 2015;28(11):1339–1346. doi: 10.1093/ajh/hpv034. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Zhao X.C., Cui W., et al. Genetic and pharmacologic inhibition of the chemokine receptor CXCR2 prevents experimental hypertension and vascular dysfunction. Circulation. 2016;134(18):1353–1368. doi: 10.1161/CIRCULATIONAHA.115.020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikolajczyk T.P., Guzik T.J. Adaptive immunity in hypertension. Curr. Hypertens. Rep. 2019;21(9):68. doi: 10.1007/s11906-019-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X.N., Li C., Liu Y., et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ. Res. 2017;120(10):1584–1597. doi: 10.1161/CIRCRESAHA.116.310480. [DOI] [PubMed] [Google Scholar]
- 24.Tanase D.M., Gosav E.M., Radu S., et al. Arterial hypertension and interleukins: Potential therapeutic target or future diagnostic marker? Int. J. Hypertens. 2019;2019:1–17. doi: 10.1155/2019/3159283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small H.Y., Migliarino S., Czesnikiewicz-Guzik M., Guzik T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018;125:104–115. doi: 10.1016/j.freeradbiomed.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 26.Orejudo M., Rodrigues-Diez R.R., Rodrigues-Diez R., et al. Interleukin 17A participates in renal inflammation associated to experimental and human hypertension. Front. Pharmacol. 2019;10:1015. doi: 10.3389/fphar.2019.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen Y., Crowley S.D. Renal effects of cytokines in hypertension. Curr. Opin. Nephrol. Hypertens. 2018;27(2):70–76. doi: 10.1097/MNH.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Wang J., Yang P., Song X., Li Y. Elevated Th17 cell proportion, related cytokines and mRNA expression level in patients with hypertension-mediated organ damage: A case control study. BMC Cardiovasc. Disord. 2022;22(1):257. doi: 10.1186/s12872-022-02698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh M.A., Norlander A.E., Madhur M.S. Inhibition of interleukin-17A, but not interleukin-17F, signaling lowers blood pressure, and reduces end-organ inflammation in angiotensin II–induced hypertension. JACC Basic Transl. Sci. 2016;1(7):606–616. doi: 10.1016/j.jacbts.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norlander A.E., Saleh M.A., Kamat N.V., et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II–induced hypertension. Hypertension. 2016;68(1):167–174. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norlander A.E., Saleh M.A., Pandey A.K., et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2017;2(13):e92801. doi: 10.1172/jci.insight.92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Beusecum J.P., Moreno H., Harrison D.G. Innate immunity and clinical hypertension. J. Hum. Hypertens. 2022;36(6):503–509. doi: 10.1038/s41371-021-00627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison D.G., Coffman T.M., Wilcox C.S. Pathophysiology of hypertension. Circ. Res. 2021;128(7):847–863. doi: 10.1161/CIRCRESAHA.121.318082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norlander A.E., Madhur M.S., Harrison D.G. The immunology of hypertension. J. Exp. Med. 2018;215(1):21–33. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowley S.D., Rudemiller N.P. Immunologic effects of the renin-angiotensin system. J. Am. Soc. Nephrol. 2017;28(5):1350–1361. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel U.O., Bode M., Kurts C., Ehmke H. Salt, inflammation, IL‐17 and hypertension. Br. J. Pharmacol. 2019;176(12):1853–1863. doi: 10.1111/bph.14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai C.L., Xing J.P., Liu X.H., et al. Relationships of inflammatory factors and risk factors with different target organ damage in essential hypertension patients. Chin. Med. J. (Engl.) 2017;130(11):1296–1302. doi: 10.4103/0366-6999.206343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X., Crowley S.D. Inflammation in salt-sensitive hypertension and renal damage. Curr. Hypertens. Rep. 2018;20(12):103. doi: 10.1007/s11906-018-0903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du Y.N., Tang X.F., Xu L., Chen W.D., Gao P.J., Han W.Q. SGK1-FoxO1 signaling pathway mediates Th17/Treg imbalance and target organ inflammation in angiotensin II-induced hypertension. Front. Physiol. 2018;9:1581. doi: 10.3389/fphys.2018.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justin Rucker A., Crowley S.D. The role of macrophages in hypertension and its complications. Pflugers Arch. 2017;469(3-4):419–430. doi: 10.1007/s00424-017-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenzel P. Monocytes as immune targets in arterial hypertension. Br. J. Pharmacol. 2019;176(12):1966–1977. doi: 10.1111/bph.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Touyz R.M., Rios F.J., Alves-Lopes R., Neves K.B., Camargo L.L., Montezano A.C. Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 2020;36(5):659–670. doi: 10.1016/j.cjca.2020.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai W., Zhang Z., Huang Y., Sun H., Qiu L. Vaccarin alleviates hypertension and nephropathy in renovascular hypertensive rats. Exp. Ther. Med. 2018;15(1):924–932. doi: 10.3892/etm.2017.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J., Saleh M.A., Kirabo A., et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest. 2015;126(1):50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehrenbach D.J., Abais-Battad J.M., Dasinger J.H., Lund H., Mattson D.L. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am. J. Physiol. Renal Physiol. 2019;317(2):F361–F374. doi: 10.1152/ajprenal.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bomfim G.F., Cau S.B.A., Bruno A.S., Fedoce A.G., Carneiro F.S. Hypertension: A new treatment for an old disease? Targeting the immune system. Br. J. Pharmacol. 2019;176(12):2028–2048. doi: 10.1111/bph.14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira N.S., Tostes R.C., Paradis P., Schiffrin E.L. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021;34(1):15–27. doi: 10.1093/ajh/hpaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Don-Doncow N., Vanherle L., Zhang Y., Meissner A. T-cell accumulation in the hypertensive brain: A role for sphingosine-1-phosphate-mediated chemotaxis. Int. J. Mol. Sci. 2019;20(3):537. doi: 10.3390/ijms20030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su R., Huang Y., Zhang D., Xiao G., Wei L. SRDFM: Siamese response deep factorization machine to improve anti-cancer drug recommendation. Brief. Bioinform. 2022;23(2):bbab534. doi: 10.1093/bib/bbab534. [DOI] [PubMed] [Google Scholar]
- 50.Wang X., Song X. New medical image fusion approach with coding based on SCD in wireless sensor network. J. Electr. Eng. Technol. 2015;10(6):2384–2392. doi: 10.5370/JEET.2015.10.6.2384. [J. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.