Abstract
Objective:
To examine the association between pregnancy and medication for opioid use disorder (MOUD) initiation and discontinuation among reproductive-age women receiving treatment for OUD in the United States.
Methods:
We conducted a retrospective cohort study of people, gender recorded as female, ages 18–45 years, in the Merative™ MarketScan® Commercial and Multi-State Medicaid Databases (2006–2016). OUD and pregnancy status were identified based on inpatient or outpatient claims for established ICD-9/10 diagnosis and procedure codes. The main outcomes were buprenorphine and methadone initiation and discontinuation, determined using pharmacy and outpatient procedure claims. Analyses were conducted at the treatment episode level. Adjusting for insurance status, age, and co-occurring psychiatric and substance use disorders, we used logistic regression to estimate MOUD initiation, and Cox regression to estimate MOUD discontinuation.
Results:
Our sample included 101,772 reproductive-age people with OUD encompassing 155,771 treatment episodes (mean age 30.8 years, 64.4% Medicaid, 84.1% White), of whom 2,687 (3.2%, encompassing 3,325 episodes) were pregnant. In the pregnant group, 51.2% of treatment episodes (1,703/3,325) involved psychosocial treatment without MOUD, in comparison to 61.1% (93,156/152,446) in the non-pregnant comparator group. In adjusted analyses assessing likelihood of initiation for individual MOUDs, pregnancy status was associated with an increase in buprenorphine (adjusted odds ratio [aOR]=1.57 [95% CI=1.44, 1.70]) and methadone initiation (aOR=2.04 [1.82, 2.27]). MOUD discontinuation rates at 270 days were high for both buprenorphine (72.4% for non-pregnant vs 59.9% for pregnant episodes) and methadone (65.7% for non-pregnant vs 54.1% for pregnant episodes). Pregnancy was associated with a decreased likelihood of discontinuation at 270 days for both buprenorphine (adjusted hazard ratio [aHR]=0.71 [0.67, 0.76]) and methadone (aHR=0.68 [0.61, 0.75]), in comparison to non-pregnancy status.
Conclusions:
Although a minority of reproductive-age people with OUD in the United States are initiated on MOUD, pregnancy is associated with significant increase in treatment initiation and reduced risk of medication discontinuation.
Keywords: pregnancy, opioid use disorder, perinatal, reproductive age, buprenorphine, methadone, retention
Précis:
This study uses administrative data to show that pregnancy is a time period of increased opioid use disorder treatment utilization, although treatment discontinuation remains very high.
INTRODUCTION
The number of pregnant people with opioid use disorder (OUD) has increased in the United States in recent years.(1–3) Medications to treat opioid use disorder (MOUD), which includes methadone and buprenorphine, are considered the “standard of care” for OUD treatment in pregnancy. Even though the use of MOUD is associated with large improvements in pregnancy outcomes, studies from state-wide databases report that up to 50% of pregnant people with OUD do not receive such medications.(4, 5)
Pregnancy status is posited to represent a period of increased health care access, utilization, and OUD treatment engagement.(6–10) However, no known studies have used national data to evaluate rates of MOUD receipt in pregnant and non-pregnant people with OUD in our review of peer-reviewed articles in PubMed (using keywords “pregnancy”, “opioid use disorder”, “buprenorphine”, “MOUD”, and “methadone”, all publications until September 1, 2022). Despite treatment engagement being considered a clinically meaningful outcome that is associated with lower overdose risk,(11) there is a paucity of research specifically comparing initiation and discontinuation rates for buprenorphine and methadone treatment in a national sample of reproductive-age people with OUD. Because large-scale national insurance claims provide a powerful tool for studying OUD outcomes during pregnancy, we used such data to calculate the initiation rates for buprenorphine and methadone (relative to psychosocial treatment without MOUD) and evaluated the association between pregnancy status and treatment initiation among treatment-receiving reproductive-age people in the United States. Among people who initiated buprenorphine or methadone, we computed the percentage of people who discontinued treatment and examined the association between pregnancy status and MOUD discontinuation.
METHODS
We used the Merative™ MarketScan® Commercial and Multi-State Medicaid Databases (2006–2016), which includes all inpatient, outpatient, and prescription drug claims from over 200 million unique people in the United States with Medicaid or employee-sponsored commercial health insurance, spanning insured active employees, non-Medicare retirees, and dependents who are insured by employer-sponsored plans. Information regarding enrollment, clinical data (such as pregnancy status), and pharmacy claims, were extracted. Because no identifiable private data was used, this study was exempt from IRB review by Washington University.
We included treatment-receiving reproductive-age (18–45 years) people, with a diagnosis of OUD (defined by International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes 305.5, 304.0, 304.7, F11) and at least one claim for MOUD or psychosocial treatment involving an OUD diagnosis (Appendix 1, available online at http://links.lww.com/xxx). The database includes two gender categories: male and female. The people included in our study were listed as “female.” Based on previous methods,(12) we excluded people who initiated both buprenorphine and methadone in a single episode (n=1,659), as the goal of the study was to examine the association between a particular medication and initiation. Among people whose gender was recorded as female, we further excluded non-reproductive age people >45 years (n=28,559) and people initiated on naltrexone (n=15,549). As pregnancy was our exposure variable, we sought to decrease heterogeneity in the amount of exposure and thus excluded people who initiated MOUD very late in pregnancy and at delivery time (more than beyond 210 days after fertilization; n=1,303), or people who became pregnant within six months of initiating treatment (n=1,870). This culminated in a final analytic sample of 101,772 people, encompassing 155,771 treatment episodes (Figure 1), of whom 2,687 people experienced at least one pregnancy. We excluded people initiated on naltrexone, as naltrexone’s safety profile is not well understood in pregnancy(13) and is not FDA approved for the treatment of OUD in the perinatal period.
Figure 1:
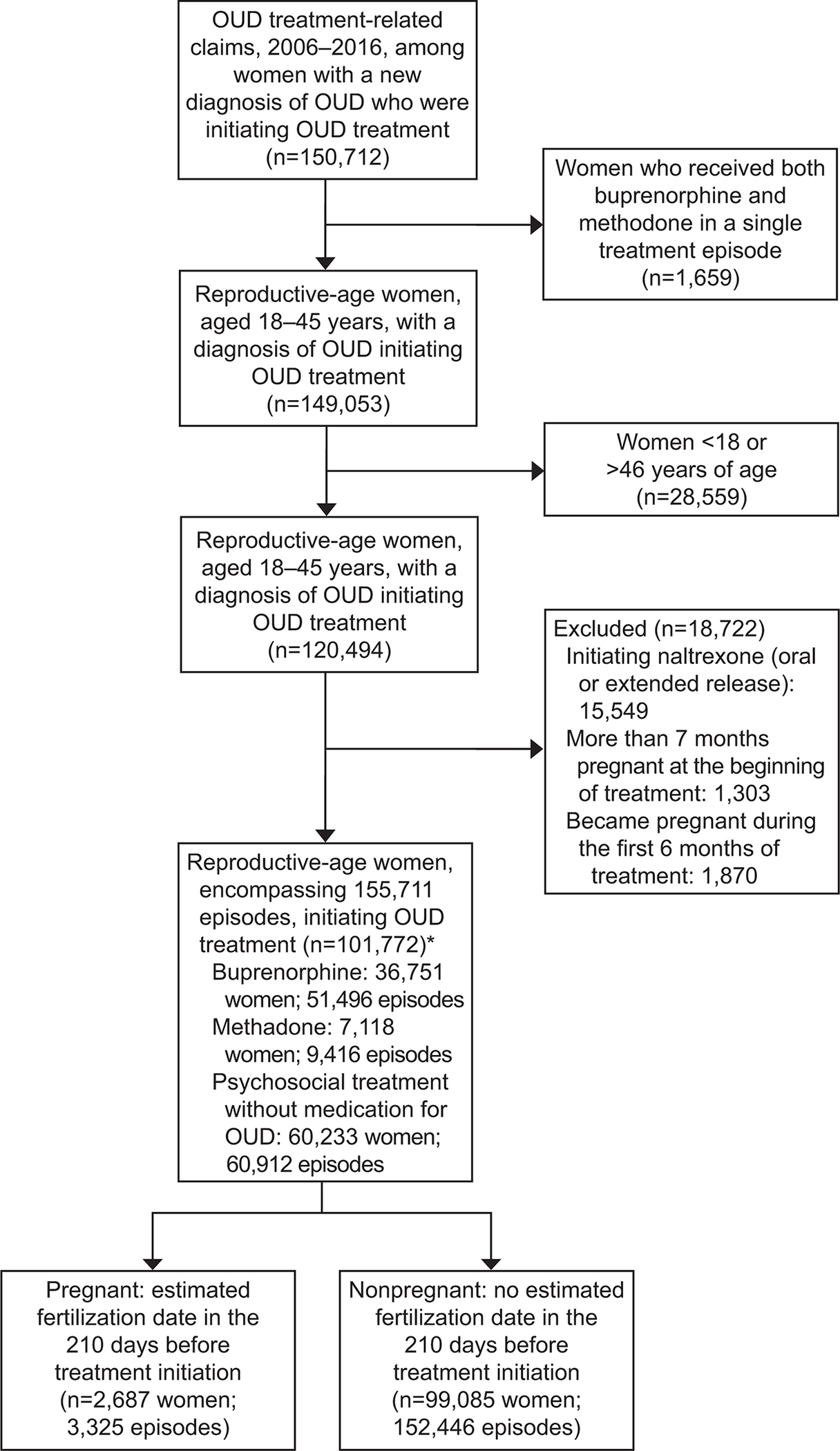
Derivation of analytic sample. *Individuals can have multiple episodes and types. Thus, the number of total individuals is less than the sum of individuals in each group. OUD, opioid use disorder.
Our unit of analysis was the treatment episode. We defined buprenorphine and methadone treatment episodes by continuous receipt, with no lapse in fills and dispensing, respectively, exceeding more than 45 days. In the case of psychosocial treatment episodes, each episode was defined by the presence of healthcare claims for counseling and/or psychosocial treatment, without a gap exceeding 45 days. The length of an episode of care was computed as the number of days between the date of the first treatment claim and the date on which the last claim occurred. Individuals were permitted to undergo multiple treatment episodes, but were required to have a minimum of 6 months of pharmacy and medical coverage prior to the first treatment episode, which constitutes a baseline look-back period for covariate assessment.(14) OUD treatment services were characterized using Current Procedural Terminology (CPT) codes, the Healthcare Common Procedure Coding System (HCPCS) system, National Drug Code numbers, and ICD 9/10 codes (Appendix 1, http://links.lww.com/xxx).
The primary predictor variable was pregnancy status (derived from delivery codes) of <7 months (210 days) gestation at the time of OUD treatment initiation. Using established methods by Palmstein and Huybrechts et al.,(15) we identified fertilization dates by first finding insurance claims for deliveries using CPT-4 and ICD-9 procedure codes and included people with multiple pregnancies (Appendix 2, available online at http://links.lww.com/xxx). We identified the date of fertilization using the delivery date as a reference point, with the calculated fertilization date defined as 270 days prior to delivery (or 245 days if a preterm birth occurred).(16)
The two primary outcome variables of interest were: (1) the binary outcome of MOUD initiation among people in OUD treatment [yes vs. no (meaning psychosocial treatment without MOUD)]; and, (2) the survival-time outcome of treatment discontinuation among the sub-sample of people receiving MOUD treatment. We defined MOUD initiation to include a new treatment episode for the FDA-approved treatments for OUD that are accepted as the standard of care to be used during pregnancy: buprenorphine and methadone. Individuals were considered to have initiated buprenorphine or methadone if they did not have a preexisting treatment claim for either in the 45 days preceding their first MOUD script. Episodes involving more than one kind of MOUD were not included in the analyses, whereas episodes that included both MOUD and psychosocial treatment were classified as an MOUD episode. Buprenorphine scripts were identified via national drug codes, whereas methadone was identified using HCPCS procedure code H0020, in order to exclude people prescribed methadone for chronic pain. We assumed an active prescription for buprenorphine or methadone procedure code for dispensing to connote medication consumption;(17) in other words, a 30-day script meant that an individual was assumed to have taken medication for each of the 30 days covered.
Covariates included factors demonstrating associations with OUD treatment outcomes in other samples(11, 18): insurance status (commercial vs. Medicaid), self-reported race (only available among Medicaid enrollees), age at start of OUD treatment (in years), and other comorbidities measured in the prepregnancy period by the presence of ICD-9/10 diagnostic codes for: mood disorder (depression or bipolar disorder), anxiety disorders (composite of posttraumatic stress disorder, generalized anxiety disorder, panic disorder, obsessive compulsive disorder, social anxiety, anxiety disorder unspecified), or co-occurring SUDs (alcohol, cannabis, cocaine, methamphetamine, sedative, tobacco use disorder or dependence). Race was included as a covariate due to previous work showing racial/ethnic disparities in MOUD utilization.(5)
All analyses were conducted using SAS version 9.4. First, we used chi-square tests to compute descriptive statistics for Medicaid status, race, and co-occurring SUDs, and comorbid psychiatric disorders by pregnancy status. The Wilcoxon-sum rank test was used to compute univariate statistics for age by pregnancy status. Next, to determine the relationship between pregnancy status and medication initiation, we used logistic regression to estimate the association between pregnancy status and initiation of MOUD treatment, first with unadjusted analyses and subsequently with adjusted analyses controlling for insurance status, race (among Medicaid enrollees), co-occurring SUDs, and comorbid psychiatric disorders. We computed variance inflation factors (VIF) to evaluate for multicollinearity, finding no significant collinearity among covariates using a highly conservative threshold of <2.0. Cluster robust standard errors, via Taylor series linearization(19), accounted for the possibility of multiple treatment episodes per individual. Next, among people receiving MOUD, we used the Kaplan-Meier procedure to estimate the unadjusted time to medication discontinuation, stratifying by pregnancy status. Finally, we employed a Cox proportional hazards regression model to the estimate the time to medication discontinuation by pregnancy status, first with unadjusted analyses and subsequently with adjusted hazard ratios obtained after controlling for covariates and accounting for multiple episodes per person. In addition to multivariate models adjusting for insurance, age, and comorbidities, we estimated separate models among Medicaid enrollees adjusting for race, age, and comorbidities (as racial data was only available for Medicaid enrollees).
Because it was possible for a person to be included in both the pregnant and non-pregnant groups (as the treatment episode is the unit of analysis), we conducted sensitivity analyses: 1) limiting observations to one treatment episode per person (each person’s first episode), and 2) limiting observations to one pregnancy per person.
RESULTS
As shown in Table 1, of all persons meeting study criteria with OUD in the cohort in the overall cohort, 2,687 (2.6%) were pregnant at the time of OUD treatment initiation. The median age was 30.8 (SD ±7.1) years (27.8 [SD±5.0] pregnant vs 30.9 [SD±7.2] non-pregnant, p<.001). Overall,36,216 (35.6%) had private insurance (444 [16.5%] pregnant vs 35,772 [36.1%] non-pregnant)) and 65,556 (64.4%) were Medicaid recipients (2,243 [83.5%] pregnant vs 63,313 [63.9%] non-pregnant, p<.001). Among Medicaid enrollees, 3,842 (5.9%) self-reported their race as Black (144 [6.4%] pregnant vs 3,698 [5.8%] non-pregnant), 629 (1%) as Hispanic (23 [1.0%] pregnant vs 606 [1.0%] non-pregnant), 3,438 (5.2%) as “none of the above race/ethnicity groups” (152 [6.8%] pregnant vs 3,286 [5.2%] non-pregnant) 55,119 (84.1%) as White (1,875 [83.6%] pregnant v 53,244 [84.1%] non-pregnant), while 2,528 (3.9%) had missing race/ethnicity information (49 [2.2%] pregnant vs 2,479 (3.9%) non-pregnant). Co-occurring substance use disorders in the 6 months preceding treatment initiation were common; 11,750 (11.6%) had alcohol use disorder (220 [8.2%] pregnant vs 11,530 [11.6%] non-pregnant, p<.001), 13,446 (13.2%) had cannabis use disorder (351 [13.1%] pregnant vs 13,095 [13.2%] non-pregnant, p=.81), 14,218 (14.0%) had a stimulant use disorder (cocaine or amphetamine; 368 [13.7%] pregnant vs 13,850 [14.0%] non-pregnant, p=0.68), and 46,294 (45.5%) had a tobacco use disorder or dependence (1,248 [46.5%] pregnant vs 45,046 [45.5%] non-pregnant, p=0.31). Mood and anxiety disorders were particularly common at 43.7% (n=44,434) (911 [33.9%] pregnant vs 43,523 [43.9%] non-pregnant, p<.001) and 36.7% (n=37,324) (804 [29.9%] pregnant vs 36,520 [36.9%] non-pregnant, p<.001), respectively. 2,406 (2.4%) overall had psychotic disorders (34 [1.3%] pregnant vs 2,372 [2.4%] non-pregnant, p<.001).
Table 1:
Characteristics of Reproductive-Age Women Initiating OUD Treatment
| All, n=101,772 | Pregnant, n=2,687 | Not-Pregnant, n=99,085 | P-value | ||
|---|---|---|---|---|---|
| Age Median (SD) | 30.8 ±7.1 years | 27.8 ±5.0 years | 30.9 ±7.2 years | ||
| Insurance | Medicaid Enrollees | 65,556 (64.4) | 2,243 (83.5) | 63,313 (63.9) | <.001 |
| Commercial (Private) Insurance Enrollees | 36,216 (35.6) | 444 (16.5) | 35,772 (36.1) | ||
| Race (Among Medicaid Enrollees) | Black | 3,842 (5.9) | 144 (6.4) | 3,698 (5.8) | 0.01 |
| Hispanic | 629 (1.0) | 23 (1.0) | 606 (1.0) | ||
| White | 55,119 (84.1) | 1,875 (83.6) | 53,244 (84.1) | ||
| None of the above | 3,438 (5.2) | 152 (6.8) | 3,286 (5.2) | ||
| Missing | 2,528 (3.9) | 49 (2.2) | 2,479 (3.9) | ||
| Commercial (Private) Insurance Enrollees /Race Unavailable | 36,216 (35.6) | 444 (16.5) | 35,772 (36.1) | ||
| Alcohol Use Disorder | 11,750 (11.6) | 220 (8.2) | 11,530 (11.6) | <.001 | |
| Cannabis Use Disorder | 13,446 (13.2) | 351 (13.1) | 13,095 (13.2) | 0.81 | |
| Sedative Use Disorder | 8,181 (8.0) | 157 (5.8) | 8,024 (8.1) | <.001 | |
| Stimulant (Cocaine or Amphetamine) Use Disorder | 14,218 (14.0) | 368 (13.7) | 13,850 (14.0) | 0.68 | |
| Tobacco Use Disorder | 46,294 (45.5) | 1,248 (46.5) | 45,046 (45.5) | 0.31 | |
| Mood Disorder (Major Depression or Bipolar Disorder) | 44,434 (43.7) | 911 (33.9) | 43,523 (43.9) | <.001 | |
| Anxiety Disorder | 37,324 (36.7) | 804 (29.9) | 36,520 (36.9) | <.001 | |
| Psychotic Disorder | 2,406 (2.4) | 34 (1.3) | 2,372 (2.4) | <.001 | |
Overall, 36,751 (36.1%) were initiated on buprenorphine, in comparison to 7,118 (7.0%) initiated on methadone, and 60,223 (59.1%) initiated psychosocial treatment without MOUD (reference group). For people with OUD who were pregnant, 51.2% of treatment episodes (1,703/3,325) involved psychosocial treatment without MOUD, in comparison to 61.1% (93,156/152,446) in the non-pregnant comparator group. Among pregnant people, 35.5% (1,180/3,325) of episodes involved any buprenorphine, in comparison to 33.0% (50,316/152,446) of episodes among non-pregnant people. Among pregnant people, 13.3% of episodes (442/3,325) involved any methadone, as opposed to 5.9% (8,974/152,446) among non-pregnant people. Among pregnant people who were initiated on any buprenorphine, 60.4% (692/1,145) received buprenorphine alone, 4,141 out of the 36,129 non-pregnant people (11.5%) initiated on any buprenorphine received buprenorphine alone (Appendix 2, http://links.lww.com/xxx).
In adjusted logistic regression models, treatment episodes in which an individual was pregnant at the time of OUD treatment initiation was associated with an increase in both any buprenorphine (unadjusted odds ratio [uaOR]=1.12 [1.04, 1.20 ], adjusted odds ratio [aOR]=1.57 [95% CI=1.44, 1.70]) and methadone initiation (uOR=2.45 [2.21, 2.73], aOR=2.04 [1.82, 2.27]). We observed similar findings in models adjusting for race among Medicaid enrollees (Figure 2), as well as sensitivity analyses that limited observations to one treatment episode per person and one pregnancy per person (Appendix 3, available online at http://links.lww.com/xxx).
Figure 2:
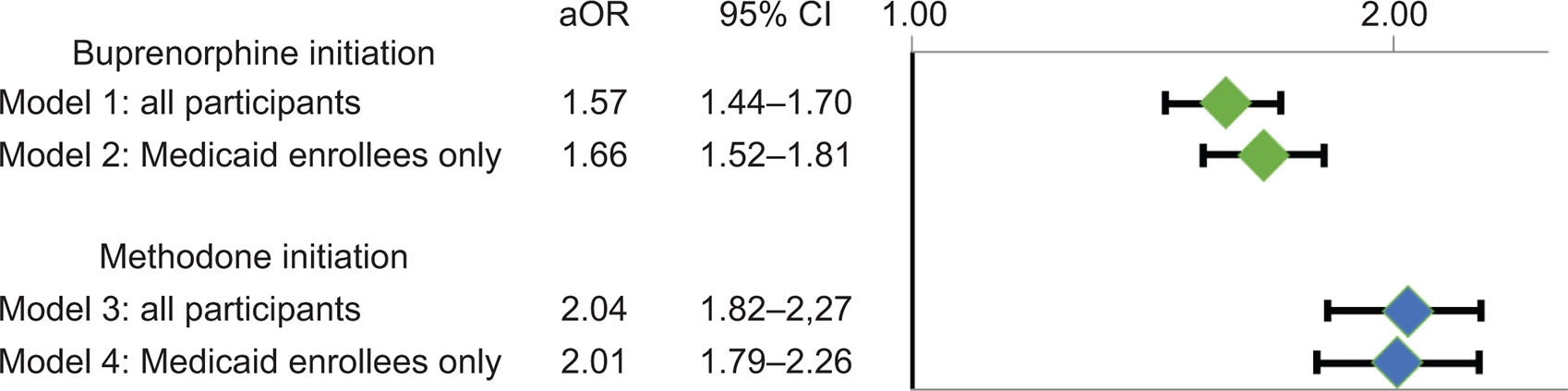
Adjusted odds ratios for medication for opioid use disorder initiation among pregnant versus nonpregnant reproductive-aged women with opioid use disorder. Model 1: Logistic regression models showing the odds of buprenorphine initiation associated with pregnant (n=2,687) compared with nonpregnant people (n=99,084), controlling for age, insurance status, co-occurring substance use disorders (alcohol, cannabis, sedative, stimulant, tobacco), co-occurring anxiety disorders, co-occurring psychotic disorders, and co-occurring mood (bipolar, major depression) disorders. Model 2: Logistic regression models showing the odds of methadone initiation associated with pregnant (n=2,243) compared with nonpregnant people (n=63,313), controlling for age, race (data only available for Medicaid enrollees), co-occurring substance use disorders (alcohol, cannabis, sedative, stimulant, tobacco), co-occurring anxiety disorders, co-occurring psychotic disorders, and co-occurring mood (bipolar, major depression) disorders. Model 3: Logistic regression models showing the odds of methadone initiation associated with pregnant (n=2,687) compared with nonpregnant people (n=99,084), controlling for age, insurance status, co-occurring substance use disorders (alcohol, cannabis, sedative, stimulant, tobacco), co-occurring anxiety disorders, co-occurring psychotic disorders, and co-occurring mood (bipolar, major depression) disorders. Model 4: Logistic regression models showing the odds of methadone initiation associated with pregnant (n=2,243) compared with nonpregnant people (n=63,313), controlling for age, race (data only available for Medicaid enrollees), co-occurring substance use disorders (alcohol, cannabis, sedative, stimulant, tobacco), co-occurring anxiety disorders, co-occurring psychotic disorders, and co-occurring mood (bipolar, major depression) disorders.
The median time to buprenorphine discontinuation or censoring was 119 days (IQR=262 days for non-pregnant vs IQR=355.5 days pregnant people). The median time to methadone discontinuation or censoring was 175 days (IQR=273 days for non-pregnant vs IQR=451 days pregnant people). At 180 days, 61.5% (31,660/51,496) of buprenorphine episodes were discontinued (47.5% [561/1,180] for pregnant vs 61.8% [31,099/50,316] for non-pregnant), increasing to 72.1% (37,116/51,496) at 270 days (59.9% [707/1,180] for pregnant vs 72.4% [36,409/50,316] non-pregnant). In the case of methadone, 50.7% (4,776/9,416) of episodes were discontinued at 180 days (34.2% [151/442] for pregnant vs 51.5% [4,625/8,974] for non-pregnant), increasing to 65.2% (6,136/9,416) at 270 days (54.1% [239/442] for pregnant vs 65.7% [5,897/8,974] for non-pregnant).
Figure 3 illustrates unadjusted Kaplan-Meier curves for buprenorphine discontinuation during the first 270 days of treatment, for which unadjusted log-rank tests showed that discontinuation was significantly lower among episodes associated with pregnancy in comparison with episodes with not associated with pregnancy χ2 = 59.89; DF=1; P< .001, Figure 3A). Similarly, Kaplan-Meier curves depict decreased methadone treatment discontinuation for episodes with pregnancy status in comparison to episodes with non-pregnancy status (χ2 = 46.59; DF=1; P< .001, Figure 3B).
Figure 3:
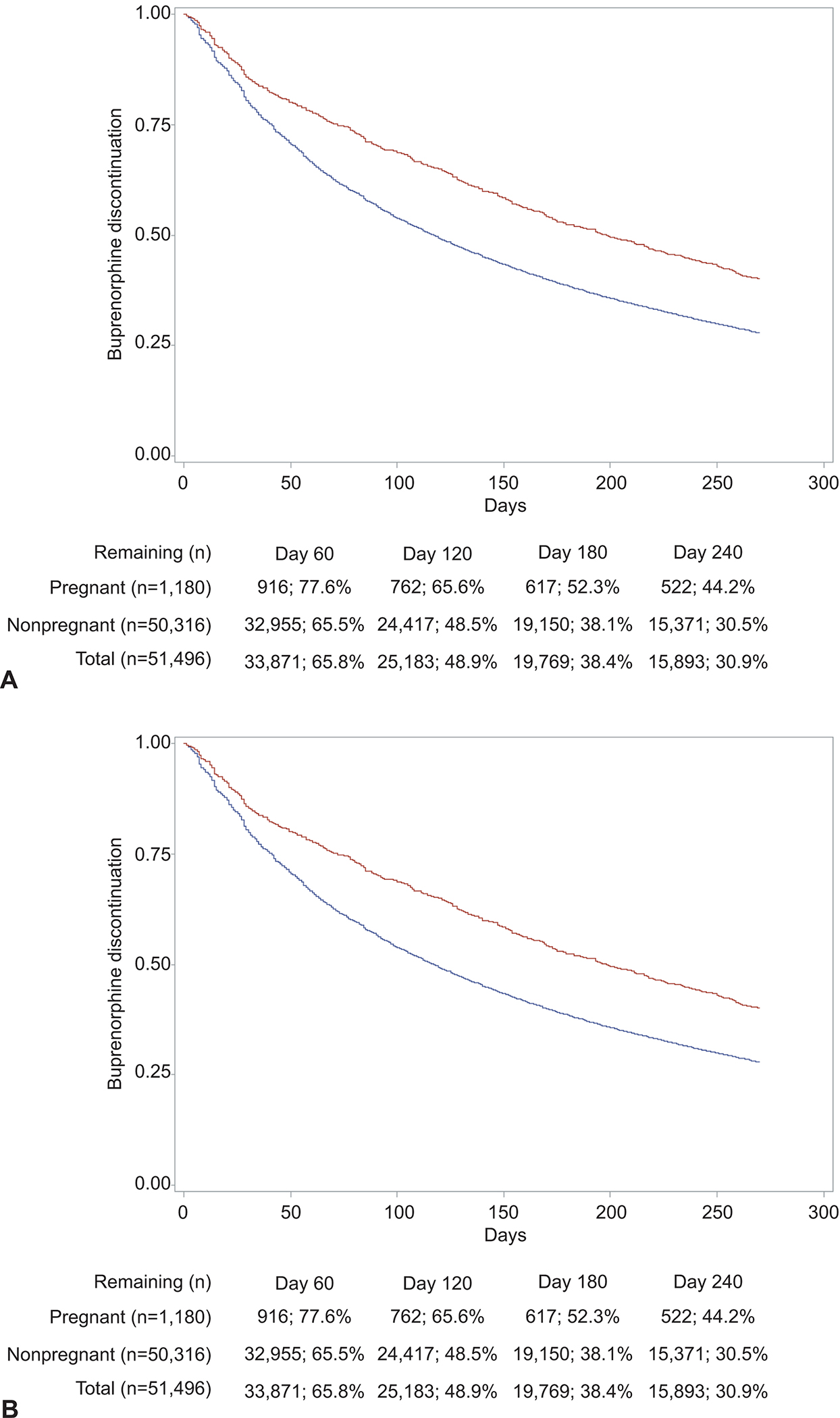
Comparison of medication for opioid use disorder discontinuation among pregnant (red) versus nonpregnant (blue) reproductive-aged women with opioid use disorder. A. Buprenorphine. Length of treatment (days) (χ2=59.89; degrees of freedom=1; P<.001). B. Methadone. Length of treatment (days) (χ2=46.59; degrees of freedom=1; P<.001).
In adjusted Cox proportional hazards regression models (Model 1, Appendix 4 [Appendix 4 is available online at http://links.lww.com/xxx]), episodes with pregnancy status were associated with decreased rates of treatment discontinuation relative to episodes with non-pregnancy status, for both buprenorphine (unadjusted HR [uaHR]= 0.79 [0.75, 0.84]; adjusted hazard ratio [aHR]= 0.71 [0.67, 0.76]) and methadone (uaHR=0.71 [0.64, 0.78]; aHR=0.68 [0.61, 0.75]). We observed similar findings in for models controlling for race/ethnicity among Medicaid enrollees (Model 2, Appendix 4, http://links.lww.com/xxx), as well as sensitivity analyses that limited observations to one treatment episode per person and one pregnancy per person (Models 3–6, Appendix 4, http://links.lww.com/xxx).
DISCUSSION
Using multi-state insurance claims across the United States, we identified that pregnancy status was significantly associated with increased insurance claims for MOUD initiation and improved retention in MOUD. These findings support previous literature showing that pregnancy status is an important window of opportunity for intervention in the treatment of OUD. Childbirth can bring increased capability for behavioral change as well as increased system resources to support individuals needing treatment for chronic health conditions.(20, 21) Despite this hopeful finding, our study also shows that in both pregnant and non-pregnant people with OUD, the majority of individuals do not receive the standard of care, MOUD, which supports studies illustrating the myriad logistical barriers experienced by patients attempting to access MOUD such as case load caps, X-waiver logistical difficulties, prohibitive costs, geographic inaccessibility, and long wait times.(22)
Unfortunately, among individuals who do receive MOUD, MOUD treatment discontinuation remains high, with more than one-half discontinuing methadone and approximately one-half discontinuing buprenorphine 180 days into treatment, higher rates in this observational dataset than in the Maternal Opioid Treatment: Human Experimental Research ( MOTHER trial) sample (33% buprenorphine, 18% methadone).(23) It is important to frame these findings in the greater context of structural barriers to health care in reproductive-aged people, as the high rate of medication discontinuation during pregnancy is not a phenomenon limited to MOUD. Treatment discontinuation has been problematic for other chronic conditions in pregnancy like depression,(24) hypertension, (25) and diabetes, with notably up to one-third of people who used insulin before pregnancy discontinuing insulin after the start of pregnancy.(26) The specific reasons for MOUD treatment discontinuation—ranging from self-discontinuation to discontinuation by formal medical directive—cannot be inferred from this analysis and warrant further investigation.
There are a number of limitations that need to be emphasized. First, pharmacy claims do not always reflect actual consumption of medication, and misclassification bias cannot be ruled out. However, for MOUDs in the MarketScan Databases, our group has found that proportion of days covered in a treatment episode was high, suggesting few gaps between refills and providing some evidence of likely consumption.(27) Second, the MarketScan data is limited to services billed through insurance claims, which will not capture persons receiving methadone or buprenorphine via out-of-pocket payment; as many OUD treatment programs do not accept insurance, our study may be underestimating the number of people receiving MOUD, especially methadone dispensing.(3) Thirdly, the generalizability of our study is limited by its age (extending to 2016), preceding recent increases in fentanyl use in the U.S, and we are unable to address state-by-state heterogeneity in MOUD initiation, an important factor to consider given differential prioritization of treatment access for pregnancy (and criminalization of substance use during pregnancy) tends to vary by state (28). Fourth, we are limited by an absence of race/ethnicity data for commercial insurance claims and limited detail for race/ethnicity for Medicaid, which is an important limitation given sharply increasing rates of death among non-Hispanic Black people with OUD(29) and racial disparities in the type of MOUD received by patients.(30)
Finally, our study uses a “best case scenario” cohort for study, as many vulnerable patients-- such as people who are uninsured, incarcerated, and/or postpartum – are not included. Our analysis was conducted over time periods in which many pregnant women were incarcerated for OUD; this is important to consider given the punitive legal landscape for pregnant people who use substances in the U.S.(31, 32), such that OUD is criminalized (33, 34). Given the association of OUD with low SES and poor insurance coverage,(35) it is a significant limitation that we only include people with 6 months of insurance coverage prior to MOUD initiation, excluding populations at especially high risk of poor OUD outcomes. Our analysis used a “best case scenario” cohort for study, encompassing reproductive-age people with OUD with at least 6 months of insurance coverage before being treated for OUD who, if pregnant, started treatment before the third trimester. Future studies will need to evaluate postpartum MOUD use, as most OUD deaths occur after delivery, especially in the latter postpartum months.(1, 36, 37)
In summary, results find that pregnancy status represents a time period of increased MOUD treatment utilization, spanning medication initiation and retention. However, treatment discontinuation rates are high for buprenorphine and methadone across both pregnant and non-pregnant people with OUD. As mental health-related causes, spanning overdose and suicide, are now the leading cause of pregnancy associated death in the United States(38), intricate systems of power and socioeconomic structures that pose barriers to health care utilization in pregnancy warrant further investigation.
Supplementary Material
Acknowledgments
The authors thank Nuri Farber, MD for providing funding via the Washington University Psychiatry Residency Research Education R25 Program (PRREP) of Washington University. We also than k Jeremy Goldbach, PhD, and Kathleen Bucholz, PhD, of the Transdisciplinary Training in Addictions Research (TranSTAR) T32 Program of Washington University for providing funding and assistance with data interpretation. We thank Matthew Keller, MS, and John Sahrmann, MA, from the Center for Administrative Data Research (CADR) of Washington University as they assisted with data acquisition, management, and storage. Dr. Farber, Dr. Goldbach, Dr. Bucholz, Mr. Keller and Mr. Sahrmann were not compensated for this work.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
Caitlin E. Martin reported money paid to her institution for her efforts on this study from NIDA. Ebony B. Carter reported money paid to her institution for her efforts on this study from the NIH, American Diabetes Association, Robert Wood Johnson Foundation. She also reported money paid to her for speaking engagements. Laura J. Bierut reported money paid to her institutions for her efforts on this study from NIH. She reported money paid to her from the NHGRI Advisory Council, the Research Triangle Institute, and the FDA. She is listed as an inventor on U.S. Patent 8080371,”Markers for Addiction,” covering use of SNPs in determining the diagnosis, prognosis and treatment of addiction. The other authors did not report any potential conflicts of interest.
PEER REVIEW HISTORY
Received October 31, 2022. Received in revised form December 26, 2022. Accepted January 12, 2023. Peer reviews and author correspondence are available at http://links.lww.com/xxx.
REFERENCES
- 1.Schiff DM, Nielsen T, Terplan M, Hood M, Bernson D, Diop H, et al. Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts. Obstet Gynecol 2018. Aug;132(2):466–74. DOI: 10.1097/AOG.0000000000002734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayatbakhsh MR, Kingsbury AM, Flenady V, Gilshenan KS, Hutchinson DM, Najman JM. Illicit drug use before and during pregnancy at a tertiary maternity hospital 2000–2006. Drug Alcohol Rev 2011. Mar;30(2):181–7. DOI: 10.1111/j.1465-3362.2010.00214.x [DOI] [PubMed] [Google Scholar]
- 3.Schiff DM, Work EC.; Foley B.; Applewhite R; Diop H; Goulland; Gupta; Hoeppner BB; Peacock-Chambers E; Vilsaint CL; Bernstein JA; Bryant AS. Perinatal Opioid Use Disorder Research, Race, and Racism: A Scoping Review Pediatrics 2022;149(3). DOI: 10.1542/peds.2021-052368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saia KA, Schiff D, Wachman EM, Mehta P, Vilkins A, Sia M, et al. Caring for Pregnant Women with Opioid Use Disorder in the USA: Expanding and Improving Treatment (vol 5, pg 257, 2016). Curr Obstet Gynecol 2016. Dec;5(4):257–263-. DOI: 10.1007/s13669-016-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiff DM, Nielsen T, Hoeppner BB, Terplan M, Hansen H, Bernson D, et al. Assessment of Racial and Ethnic Disparities in the Use of Medication to Treat Opioid Use Disorder Among Pregnant Women in Massachusetts. Jama Netw Open 2020. May 26;3(5). doi: 10.1001/jamanetworkopen.2020.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiff DM, Nielsen TC, Hoeppner BB, Terplan M, Hadland SE, Bernson D, et al. Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder. Am J Obstet Gynecol 2021. Oct;225(4). doi: 10.1016/j.ajog.2021.04.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forray A, Foster D. Substance Use in the Perinatal Period. Curr Psychiat Rep 2015. Nov;17(11). DOI: DOI: 10.1007/s11920-015-0626-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forray A, Merry B, Lin HQ, Ruger JP, Yonkers KA. Perinatal substance use: A prospective evaluation of abstinence and relapse. Drug Alcohol Depen 2015. May 1;150:147–55. DOI: 10.1016/j.drugalcdep.2015.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadland SE, Bagley SM, Rodean J, Silverstein M, Levy S, Larochelle MR, et al. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. Jama Pediatr 2018. Nov;172(11):1029–37. DOI: DOI: 10.1001/jamapediatrics.2018.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo-Ciganic WH, Donohue JM, Kim JY, Krans EE, Jones BL, Kelley D, et al. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidem Dr S 2019. Jan;28(1):80–9. DOI: 10.1002/pds.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. Jama Netw Open 2020. Feb;3(2). DOI: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mintz CM, Presnall NJ, Sahrmann JM, Borodovsky JT, Glaser PEA, Bierut LJ, et al. Age disparities in six-month treatment retention for opioid use disorder. Drug Alcohol Depen 2020. Aug 1;213. DOI: 10.1016/j.drugalcdep.2020.108130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HE, Chisolm MS, Jansson LM, Terplan M. Naltrexone in the treatment of opioid-dependent pregnant women: common ground. Addiction 2013. Feb;108(2):255–6. DOI: 10.1111/j.1360-0443.2012.03811.x [DOI] [PubMed] [Google Scholar]
- 14.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, et al. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidem Dr S 2013. May;22(5):542–50. DOI: 10.1002/pds.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PLoS One 2013;8(6):e67405. DOI: 10.1371/journal.pone.0067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margulis AV, Palmsten K, Andrade SE, Charlton RA, Hardy JR, Cooper WO, et al. Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results. Pharmacoepidem Dr S 2015. Apr;24(4):335–42. DOI: 10.1002/pds.3743 [DOI] [PubMed] [Google Scholar]
- 17.Xu KY, Borodovsky JT, Presnall N, Mintz CM, Hartz SM, Bierut LJ, et al. Association Between Benzodiazepine or Z-Drug Prescriptions and Drug-Related Poisonings Among Patients Receiving Buprenorphine Maintenance: A Case-Crossover Analysis. Am J Psychiat 2021. Jul;178(7):651–9. DOI: 10.1176/appi.ajp.2020.20081174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin CE, Scialli A, Terplan M. Unmet substance use disorder treatment need among reproductive age women. Drug Alcohol Depen 2020. Jan 1;206. DOI: 10.1016/j.drugalcdep.2019.107679 [DOI] [PubMed] [Google Scholar]
- 19.Huang F Analyzing group level effects with clustered data using Taylor series linearization. Practical Assessment, Research, and Evaluation 2014;19(13):1–9. DOI: 10.7275/ntnk-d929 [DOI] [Google Scholar]
- 20.Kuo C, Schonbrun YC, Zlotnick C, Bates N, Todorova R, Kao JCW, et al. A Qualitative Study of Treatment Needs Among Pregnant and Postpartum Women with Substance Use and Depression. Subst Use Misuse 2013;48(14):1498–508. doi: 10.3109/10826084.2013.800116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JC, Roman-Urrestarazu A, Brayne C. Differences in receipt of opioid agonist treatment and time to enter treatment for opioid use disorder among specialty addiction programs in the United States, 2014–17. Plos One 2019. Dec 12;14(12). DOI: 10.1371/journal.pone.0226349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedrick BS, O’Donnell C, Marx CM, Friedman H, Carter EB, Stout MJ, et al. Barriers to accessing opioid agonist therapy in pregnancy. Am J Obst Gynec Mfm 2020. Nov;2(4). doi: 10.1016/j.ajogmf.2020.100225. [DOI] [PubMed] [Google Scholar]
- 23.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal Abstinence Syndrome after Methadone or Buprenorphine Exposure. New Engl J Med 2010. Dec 9;363(24):2320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikman A, Skalkidou A, Wikstrom AK, Lampa E, Kramer MS, Yong EL, et al. Factors associated with re-initiation of antidepressant treatment following discontinuation during pregnancy: a register-based cohort study. Arch Women Ment Hlth 2020. Oct;23(5):709–17. DOI: 10.1056/NEJMoa1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano LC, Bateman BT, Rodriguez LAG, Hernandez-Diaz S. Prescription of antihypertensive medications during pregnancy in the UK. Pharmacoepidem Dr S 2014. Oct;23(10):1051–8. doi: 10.1002/pds.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao LW, Cesta CE, Pazzagli L. The role of socioeconomic factors on discontinuation of insulin during pregnancy-methodological challenges from a Swedish register-based study. J Public Health-Heid 2022. Feb;30(2):487–94. DOI: 10.1007/s10389-020-01307-x [DOI] [Google Scholar]
- 27.Mintz CM, Xu KY, Presnall NJ, Hartz SM, Levin FR, Scherrer JF, et al. Analysis of Stimulant Prescriptions and Drug-Related Poisoning Risk Among Persons Receiving Buprenorphine Treatment for Opioid Use Disorder. Jama Netw Open 2022. May 11;5(5). DOI: 10.1001/jamanetworkopen.2022.11634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White SA, McCourt A, Bandara S, Goodman DJ, Patel E, McGinty EE. Implementation of State Laws Giving Pregnant People Priority Access to Drug Treatment Programs in the Context of Coexisting Punitive Laws. Women Health Iss 2022. Oct 19. DOI: 10.1016/j.whi.2022.09.001 [DOI] [PubMed] [Google Scholar]
- 29.CDC. Overdose death rates increased significantly for Black, American Indian/Alaska Native people in 2020. 2022.
- 30.Hansen HB, Siegel CE, Case BG, Bertollo DN, DiRocco D, Galanter M. Variation in Use of Buprenorphine and Methadone Treatment by Racial, Ethnic, and Income Characteristics of Residential Social Areas in New York City. J Behav Health Ser R 2013. Jul;40(3):367–77. DOI: 10.1007/s11414-013-9341-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sue K Getting Wrecked: Women, Incarceration, and the American Opioid Crisis: UC Press; 2019. [Google Scholar]
- 32.Sufrin C Jailcare: Finding the Safety Net for Women behind Bars: UC Press; 2017. [Google Scholar]
- 33.Premkumar A, Kerns J, Huchko MJ. “A Resume for the Baby”: Biosocial Precarity and Care of Substance-Using, Pregnant Women in San Francisco. Cult Med Psychiatry 2020. Mar;44(1):35–55. DOI: 10.1007/s11013-019-09634-9 [DOI] [PubMed] [Google Scholar]
- 34.Meinhofer A, Witman A, Maclean JC, Bao Y. Prenatal substance use policies and newborn health. Health Econ 2022. Jul;31(7):1452–67. DOI: 10.1002/hec.4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick S, Faherty LJ, Dick AW, Scott TA, Dudley J, Stein BD. Association Among County-Level Economic Factors, Clinician Supply, Metropolitan or Rural Location, and Neonatal Abstinence Syndrome. Jama-J Am Med Assoc 2019. Jan 29;321(4):385–93. DOI: 10.1001/jama.2018.20851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin CE, Parlier-Ahmad AB. Addiction treatment in the postpartum period: an opportunity for evidence-based personalized medicine. Int Rev Psychiatr 2021. Aug 18;33(6):579–90. DOI: 10.1080/09540261.2021.1898349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen T, Bernson D, Terplan M, Wakeman SE, Yule AM, Mehta PK, et al. Maternal and infant characteristics associated with maternal opioid overdose in the year following delivery. Addiction 2020. Feb;115(2):291–301. DOI: 10.1111/add.14825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trost S, Beauregard J, Njie F, Berry J, Harvey A, Goodman D. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. In: Centers for Disease Control and Prevention UDoHaHS, editor.; 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


