Abstract
Introduction
Pregnancy is a risk factor for severe coronavirus disease 2019 (COVID‐19) and adverse pregnancy outcomes. We aimed to explore maternal characteristics, pregnancy outcomes, vaccination status, and virus variants among pregnant women admitted to intensive care units (ICU) with severe COVID‐19.
Material and methods
We identified pregnant women admitted to ICU in Sweden (n = 96), Norway (n = 31), and Denmark (n = 16) because of severe COVID‐19, from national registers and clinical databases between March 2020 and February 2022 (Denmark), August 2022 (Sweden), or December 2022 (Norway). Their background characteristics, pregnancy outcome, and vaccination status were compared with all birthing women and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) test‐positive pregnant women during the same time period. We calculated the number admitted to ICU per 10 000 birthing and per 1000 SARS‐CoV‐2 test‐positive women during the Index, Alpha, Delta, and Omicron periods.
Results
Women admitted to ICU had a higher mean body mass index, were more often of non‐Scandinavian origin, had on average lower education and income levels, had a higher proportion of chronic and pregnancy‐related conditions, delivered preterm, had neonates with low Apgar scores, and had more infants admitted to neonatal care, compared with all birthing and test‐positive pregnant women. Of those admitted to ICU, only 7% had been vaccinated before admission. Overall, the highest proportion of women admitted to ICU per birthing was during the Delta period (4.1 per 10 000 birthing women). In Norway, the highest proportion admitted to ICU per test‐positive pregnant women was during the Delta period (17.8 per 1000 test‐positive), whereas the highest proportion of admitted per test‐positive in Sweden and Denmark was seen during the Index period (15.4 and 8.9 per 1000 test‐positive, respectively).
Conclusions
Admission to ICU because of COVID‐19 in pregnancy was a rare event in the Scandinavian countries, but women who were unvaccinated, of non‐Scandinavian origin, and with lower socio‐economic status were at higher risk of admission to ICU. In addition, women admitted to ICU for COVID‐19 had higher risk of adverse pregnancy outcomes.
Keywords: intensive care unit, register, vaccination, virus variant
Socio‐economic factors, non‐Scandinavian origin, and vaccination status were associated with admission to ICU for COVID‐19 in pregnancy in the Scandinavian countries, which highlights the importance of targeted preventive measures towards risk groups.
Abbreviations
- COVID‐19
coronavirus disease 2019
- DCOD
Danish COVID‐19 in pregnancy database
- DNPR
Danish National Patient Register
- ICU
intensive care unit
- MBRN
Medical Birth Registry of Norway
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SPR
Swedish Pregnancy Register
Key message.
Socio‐economic factors, non‐Scandinavian origin, and vaccination status were associated with admission to ICU for COVID‐19 in pregnancy in the Scandinavian countries, which highlights the importance of targeted preventive measures towards risk groups.
1. INTRODUCTION
Pregnant women seem to be at similar risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as non‐pregnant women. 1 However, pregnancy is a risk factor for developing severe coronavirus disease 2019 (COVID‐19), admittance to intensive care unit (ICU), invasive ventilation, and of dying because of COVID‐19. 1 , 2 , 3 Severe COVID‐19 is further associated with adverse pregnancy outcomes such as preterm delivery, neonatal intensive care admission, and SARS‐CoV‐2 positivity in offspring. 2 , 4 Different SARS‐CoV‐2 variants might be associated with varying degrees of disease severity and adverse pregnancy outcomes. 5 , 6 COVID‐19 vaccines are safe in pregnancy and protective against severe disease. 7 , 8 , 9 Yet, a lower vaccine uptake is seen in pregnant women compared with non‐pregnant women of fertile age. 10 , 11 Surveillance data from the International Network of Obstetrics Survey System show that 80%–100% of pregnant women admitted to critical care as a result of severe COVID‐19 were non‐vaccinated. 9
There are currently limited data from population‐based studies of contemporary cohorts of pregnant women on pregnancy outcomes in pregnant women admitted to ICU with severe COVID‐19. Using prospectively collected national quality‐ and population‐based register data from Sweden, Norway and Denmark, we explored maternal characteristics, pregnancy outcomes, vaccination status, and virus variants among pregnant women admitted to ICU because of severe COVID‐19.
2. MATERIAL AND METHODS
2.1. Study participants and design
This study included all women giving birth after 22 weeks of gestation (live or stillbirth) identified from the Swedish Pregnancy Register (SPR), the Medical Birth Registry in Norway (MBRN), and the Danish National Patient Register (DNPR). We included births from March 1, 2020, with end of follow up at the latest available date in each country; August 18, 2022 in Sweden, December 1, 2022 in Norway, and December 31, 2021 in Denmark. To avoid oversampling of short pregnancies towards end of follow up (preterm deliveries would be registered as delivered while ongoing pregnancies would not yet be registered if delivery were after end of follow up), only pregnancies with the opportunity to reach 42 completed gestational weeks by the end of follow up in the registries were included. The SPR includes 94% of all births (21 of 24 regions) in Sweden, the DNPR includes 97% of all births in Denmark, while MBRN includes all births in Norway. Unique personal identification numbers, assigned to all citizens at birth or immigration, enabled linkage between national registers. The study populations are shown in Figure 1. The registers and data sources are described in more detail in Appendix S1.
FIGURE 1.
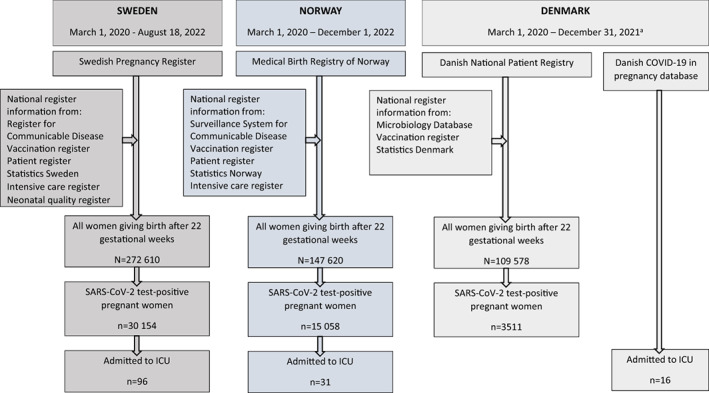
Study cohorts in Sweden, Norway, and Denmark. aThe intensive care unit cases in the Danish COVID‐19 in pregnancy database (DCOD) were identified up to February 28, 2022.
2.2. Severe COVID‐19 in pregnancy
Severe COVID‐19 in pregnancy was defined as admission to ICU because of COVID‐19 during pregnancy and up to 14 days postpartum in Sweden and Norway and up to delivery in Denmark, in women giving birth (live or stillbirth) after 22 weeks of gestation. In Sweden, information on pregnant women admitted to ICU, with COVID‐19 as the primary ICU diagnosis, was retrieved through linkage between the national Swedish Intensive Care Register and the SPR. In Norway, ICU admissions were identified based on the Norwegian Intensive Care Register and the Norwegian Patient Registry. The Norwegian Intensive Care Registry was used to identify all women who had been admitted to the ICU during pregnancy. The Norwegian Patient Registry was subsequently used to identify the women that had been specifically admitted to ICU for COVID‐19 as indicated by having a registration of either International Classification of Diseases 10th revision (ICD‐10) codes U07.1, U07.2, U09, or U10 as the primary diagnoses. In Denmark, pregnant women admitted to ICU because of COVID‐19 were identified through the Danish COVID‐19 in pregnancy database (DCOD). 12 Women admitted to ICU were included during the same study period as for all birthing women in each country, except for Denmark, where end of follow up for those admitted to ICU was February 28, 2022.
2.3. Maternal characteristics and pregnancy outcomes
Information on maternal characteristics and pregnancy outcomes were collected from the Swedish Register for Communicable Diseases, the Norwegian Surveillance System for Communicable Diseases, the Danish Microbiology Database, SPR, MBRN, DNPR, the Swedish National Patient Register, the Swedish Neonatal Quality Register, registers at Statistics Sweden, Norway, and Denmark, national vaccination registries, and DCOD.
Individuals were defined as infected by SARS‐CoV‐2 during pregnancy if they had a positive polymerase chain reaction or antigen test (Denmark only, from December 2020) for SARS‐CoV‐2 between conception and date of delivery. Date of conception was estimated from routine ultrasound measurements (>95%), or from last menstrual period, embryo transfer date in assisted reproductive treatment pregnancies, or clinical assessment if ultrasound measurements were missing. We identified four calendar time periods by the circulating SARS‐CoV‐2 virus variant that dominated: Index (March 1, 2020 to January 31, 2021), Alpha (February 1, 2021 to June 30, 2021), Delta (July 1, 2021 to December 31, 2021) and Omicron (January 1, 2022 to onwards). 13 , 14 No data from the Omicron period were available from Denmark, as end of follow up for the population‐based data was December 2021.
Maternal characteristics included maternal age (continuous), pre‐pregnancy body mass index (BMI, kg/m2) (continuous), smoking status at start of pregnancy (yes/no), parity (nulliparous/multiparous), multiple birth (yes/no), region of birth (Scandinavia, other European countries, Middle East/Africa, other), cohabitation with partner (yes/no), educational level (≤9 years, 10–12 years, >12 years), household income (in tertiles), underlying chronic medical conditions (diabetes, essential hypertension, cardiovascular, renal or lung disease, or previous thrombosis; yes/no), and pregnancy‐related conditions (gestational diabetes, gestational hypertension, pre‐eclampsia, HELLP [Hemolysis, Elevated Liver enzymes, Low Platelets counts], eclampsia or thrombosis; yes/no). Education and income level were not available for the Danish ICU cohort.
Pregnancy outcomes included stillbirth, preterm delivery (<37 completed gestational weeks), spontaneous preterm delivery (ICD‐10 code O60.1 or a live birth/stillbirth <259 days of gestation with spontaneous onset reported in the registries), iatrogenic preterm delivery (ICD‐10 code O60.3 or live birth/stillbirth <259 days with induced labor or cesarean delivery without labor), very preterm delivery (<32 completed gestational weeks), mode of delivery (vaginal, emergency and elective cesarean section), small‐for‐gestational age by the 10th centile according to Marsál et al, 15 Apgar score 7 or less at 5 minutes, and admittance to neonatal care.
COVID‐19 vaccination of pregnant women was recommended from May 2021 in Sweden, August 2021 in Norway, and July 2021 in Denmark. We identified three separate groups based on timing of first vaccine dose: (1) before pregnancy, (2) during pregnancy, and (3) unvaccinated during pregnancy. Timing of subsequent doses was not considered. For those admitted to ICU, vaccination status in relation to date of admission was also investigated.
2.4. Statistical analyses
Data were analyzed separately for each country as they were hosted at different institutions, and the results were subsequently aggregated. We compared maternal characteristics, pregnancy outcomes, and vaccination status for women admitted to ICU with all birthing women (independent of SARS‐CoV‐2 test status, as Sweden lacked national information on negative test results of those tested for SARS‐CoV‐2), and further to the subgroup of SARS‐CoV‐2 test‐positive pregnant women. Cells with fewer than five events were not allowed to be displayed due to privacy regulations.
To explore potential temporal patterns related to the dominating SARS‐CoV‐2 variants, the number of pregnant women admitted to ICU per 10 000 birthing women and per 1000 test‐positive pregnant women during the specified calendar period for the dominating SARS‐CoV‐2 variant, was calculated. The reference group of all birthing women and test‐positive women were from the same calendar period as those admitted to ICU.
Analyses were performed with STATA version 15.
2.5. Ethics statement
This study was approved by the Swedish Ethical Review Authority on April 23, 2020 (approval numbers: 2020‐01499), on June 18, 2020 (2020–02468) and on March 3, 2021 (2021‐00274), the Regional Committee for Medical and Health Research Ethics of South/East Norway on May 14, 2020 (#141135), the Danish Patient Safety Authority on April 24, 2020 (reg. no. 31‐1521‐252) and the regional Data Protection Agency in Region Zealand on March 23, 2020 (reg. no. REG‐022‐2020) and with the Danish Data Protection Agency via Statistics Denmark. Each committee provided a waiver of consent for participants.
3. RESULTS
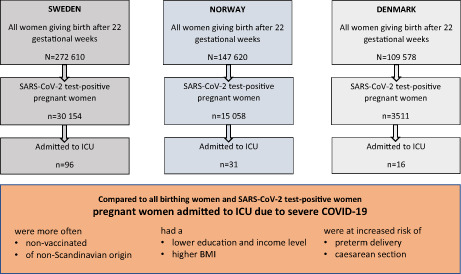
A total of 529 808 women gave birth after 22 weeks of gestation in the three Scandinavian countries during the defined study periods. Among all birthing women, 48 723 (9%) tested positive for SARS‐CoV‐2 during pregnancy—30 154 (11.1%) in Sweden, 15 058 (10.2%) in Norway, and 3511 (3.2%) in Denmark. Concurrently, 143 pregnant women were admitted to ICU because of severe COVID‐19, of which 96 (3.8 per 10 000 birthing women and 3.7 per 1000 test‐positive pregnant women) were admitted in Sweden, 31 (2.1 per 10 000 birthing women and 2.1 per 1000 test‐positive pregnant women) in Norway and 16 (1.5 per 10 000 birthing women and 4.6 per 1000 test‐positive pregnant women) in Denmark.
Table 1 displays maternal characteristics among those admitted to ICU compared with all birthing women, and SARS‐CoV‐2 test‐positive pregnant women. Women admitted to ICU because of severe COVID‐19 had a higher mean BMI, were more frequently of non‐Scandinavian origin, had lower education and income levels, were more likely to be multiparous, and had a higher proportion of chronic and pregnancy‐related conditions, compared with all birthing women and test‐positive pregnant women. In addition, women admitted to ICU were more likely to live without a partner, compared with test‐positive pregnant women. Country‐specific characteristics of the study population are displayed in Table S1.
TABLE 1.
Characteristics of pregnant women admitted to intensive care unit (ICU) because of severe COVID‐19, all birthing women, and SARS‐CoV‐2 test‐positive pregnant women in Sweden, Norway, and Denmark.
| Admitted to ICU a n = 143 | All birthing women b N = 529 808 | Test‐positive pregnant women c n = 48 723 | ||||
|---|---|---|---|---|---|---|
| n | % | N | % | n | % | |
| Age (years), mean ± SD | 31.3 ± 5.1 | 30.8 ± 4.7 | 31.0 ± 4.7 | |||
| BMI (kg/m2), mean ± SD | 29.9 ± 6.9 | 25.0 ± 5.2 | 25.0 ± 5.1 | |||
| Missing | 8 | 5.6 | 22 421 | 4.2 | 1737 | 4.0 |
| Smoking | ||||||
| Yes | 7 | 4.9 | 19 159 | 3.6 | 1128 | 2.3 |
| Missing | 7 | 4.9 | 33 716 | 6.6 | 3222 | 7.5 |
| Parity | ||||||
| Nulliparous | 43 | 30.1 | 232 608 | 43.9 | 18 823 | 38.6 |
| Multiparous | 100 | 69.9 | 297 920 | 56.2 | 29 900 | 61.4 |
| Multiple birth | ||||||
| Yes | <5 | N/A | 9025 | 1.7 | 767 | 1.6 |
| Region of birth | ||||||
| Scandinavia | 56 | 39.2 | 391 290 | 73.9 | 34 392 | 70.6 |
| Other European countries | 13 | 9.1 | 42 561 | 8.0 | 4148 | 8.5 |
| Middle East/Africa | 52 | 36.4 | 57 541 | 10.9 | 6316 | 13.0 |
| Other/missing | 22 | 15.4 | 38 416 | 7.3 | 3867 | 6.9 |
| Living with a partner | ||||||
| Yes | 123 | 86.0 | 478 546 | 90.3 | 44 727 | 91.8 |
| Missing | <5 | N/A | 9053 | 1.7 | 827 | 1.7 |
| Educational level d | ||||||
| ≤9 years | 28 | 19.6 | 46 151 | 8.7 | 4141 | 8.5 |
| 10–12 years | 51 | 35.7 | 137 869 | 26.0 | 13 540 | 27.8 |
| >12 years | 42 | 29.4 | 290 086 | 54.8 | 26 341 | 54.1 |
| Missing | 22 | 15.4 | 55 429 | 10.5 | 4701 | 9.6 |
| Household income d | ||||||
| 1st tertile | 53 | 37.1 | 143 570 | 27.1 | 10 148 | 20.8 |
| 2nd tertile | 37 | 25.9 | 143 610 | 27.1 | 9606 | 19.7 |
| 3rd tertile | 19 | 13.3 | 143 506 | 27.1 | 8815 | 18.1 |
| Missing | 34 | 23.8 | 99 122 | 18.7 | 20 154 | 41.4 |
| Chronic conditions e | ||||||
| Yes | 24 | 16.8 | 58 039 | 11.0 | 5318 | 10.9 |
| Pregnancy conditions f | ||||||
| Yes | 38 | 26.6 | 63 962 | 12.1 | 6047 | 12.4 |
Total number of pregnant women admitted to ICU because of severe COVID‐19, identified during the same time period as for all birthing women for each country, except from Denmark where cases from DCOD were included up till February 28, 2022. Due to privacy reasons cells with fewer than five events are not displayed.
Total number of birthing women between March 1, 2020 and August 18, 2022 in Sweden, December 1, 2022 in Norway and December 31, 2021 in Denmark.
Total number of SARS‐CoV‐2 test‐positive pregnant women, identified during the same time period as for all birthing women for each country.
Education level and household income was not available for the Danish ICU data identified in DCOD.
According to ICD‐10: Diabetes (E10, E11, O24.0–O24.3), essential hypertension (O10, I10–I15), cardiovascular (I20–I25, I30–I52, I61, I62, I64, I67–I79, Q20–Q28), renal (N03, N11, N18) or lung disease (J40–J47), and/or previous thrombosis (I26, O22.3, O22.5, O87.1, O87.3, I63, I65, I66, I74, I81, I82).
According to ICD‐10: Gestational diabetes (O24.4), gestational hypertension (O13.9), pre‐eclampsia/HELLP (Hemolysis, Elevated Liver enzymes, Low Platelets counts)/eclampsia (O14.0, O14.1, O14.2, O14.9, O15), and/or thrombosis during pregnancy (O22.3, O22.5).
Table 2 shows pregnancy outcomes among women admitted to ICU, all birthing women, and SARS‐CoV‐2 test‐positive pregnant women. Two out of three (66%) women admitted to ICU delivered by cesarean section (18% emergency and 48% elective) compared with approximately 18% of all birthing women and SARS‐CoV‐2 test‐positive pregnant women. Additionally, larger proportions of stillbirth, preterm, and very preterm delivery, small‐for‐gestational age, low Apgar score, and newborn admitted to neonatal care were observed among those admitted to ICU compared with all birthing women and SARS‐CoV‐2 test‐positive pregnant women.
TABLE 2.
Pregnancy outcomes among pregnant women admitted to intensive care unit (ICU) because of severe COVID‐19, all birthing women, and SARS‐CoV‐2 test‐positive pregnant women in Sweden, Norway, and Denmark.
| Admitted to ICU n = 143 | All birthing women N = 529 808 | Test‐positive pregnant women n = 48 723 | ||||
|---|---|---|---|---|---|---|
| n | % | N | % | N | % | |
| Stillbirth | 6 | 4.2 | 1511 | 0.3 | 136 | 0.3 |
| Preterm delivery <37 weeks | 77 | 53.8 | 28 564 | 5.4 | 2471 | 5.1 |
| Spontaneous a | 5 | 3.5 | 19 203 | 3.6 | 1498 | 3.1 |
| Iatrogenic b | 72 | 50.3 | 9361 | 1.8 | 973 | 2.0 |
| Very preterm delivery <32 weeks | 36 | 25.2 | 4339 | 0.8 | 359 | 0.8 |
| Mode of delivery | ||||||
| Vaginal | 49 | 31.5 | 435 012 | 82.1 | 40 206 | 82.5 |
| Emergency cesarean section | 25 | 17.5 | 52 064 | 9.8 | 4475 | 9.2 |
| Elective cesarean section | 69 | 48.3 | 42 732 | 8.1 | 4042 | 8.3 |
| Small‐for‐gestational age | 21 | 14.7 | 48 047 | 9.1 | 4233 | 8.7 |
| Apgar score <7 at 5 min | 19 | 13.3 | 8224 | 1.6 | 785 | 1.6 |
| Admittance to neonatal care c | 73 | 51.0 | 49 090 | 9.3 | 4078 | 8.4 |
ICD‐10 O60.1—Spontaneous preterm labor with preterm delivery (live birth or stillbirth) <259 gestational days.
ICD‐10 O60.3—Preterm delivery without spontaneous start of labor, or live birth or stillbirth <259 gestational days with induced labor or cesarean delivery without labor.
At least one admission to a neonatal unit, independent of cause or severity.
Vaccination status in the three countries is displayed in Table 3. Only 10 of 143 women (7%) were vaccinated with at least one dose at the time of admission to ICU. Among the unvaccinated women admitted to ICU, 67% were vaccinated after pregnancy within the follow‐up period, whereas 26% remained unvaccinated until the end of follow up (data not tabulated).
TABLE 3.
Vaccination status among pregnant women admitted to intensive care unit (ICU) because of severe COVID‐19, all birthing women, and SARS‐CoV‐2 test‐positive pregnant women in Sweden, Norway, and Denmark, and combined.
| Admitted to ICU | All birthing women | Test‐positive pregnant women | |||||
|---|---|---|---|---|---|---|---|
| Vaccination status a | n | % | N | % | n | % | |
| Sweden | Total | 96 | 100.0 | 272 610 | 100.0 | 30 154 | 100.0 |
| First dose before pregnancy | <5 | N/A | 39 348 | 14.4 | 9487 | 31.5 | |
| First dose during pregnancy | <5 | N/A | 40 739 | 14.9 | 4918 | 16.3 | |
| Not vaccinated during pregnancy | 90 | 93.8 | 192 523 | 70.6 | 15 749 | 52.2 | |
| Norway | Total | 31 | 100.0 | 147 620 | 100.0 | 15 058 | 100.0 |
| First dose before pregnancy | <5 | N/A | 27 903 | 18.9 | 8250 | 54.8 | |
| First dose during pregnancy | <5 | N/A | 18 161 | 12.3 | 2728 | 18.1 | |
| Not vaccinated during pregnancy | 28 | 90.3 | 101 556 | 68.8 | 4080 | 27.1 | |
| Denmark | Total | 16 | 100.0 | 109 578 | 100.0 | 3511 | 100.0 |
| First dose before pregnancy | 0 | 0 | 583 | 0.5 | 22 | 0.6 | |
| First dose during pregnancy | 0 | 0 | 8502 | 7.8 | 196 | 5.6 | |
| Not vaccinated during pregnancy | 16 | 100.0 | 100 493 | 91.7 | 3293 | 93.8 | |
| Combined | Total | 143 | 100.0 | 529 808 | 100.0 | 48 723 | 100.0 |
| First dose before/during pregnancy | 10 | 7.0 | 135 236 | 25.5 | 25601 | 52.5 | |
| Not vaccinated during pregnancy | 133 | 93.0 | 394 572 | 74.5 | 23 122 | 47.5 | |
In pregnant women admitted to ICU, the vaccination date for those vaccinated during pregnancy, was before the admission date.
Figure 2 presents the number of pregnant women admitted to ICU per 10 000 birthing women and 1000 SARS‐CoV‐2 test‐positive pregnant women by calendar periods corresponding to the circulating dominating virus variants. For the three countries overall, the highest proportion of women admitted to ICU per 10 000 birthing women was during the Delta period (4.1 per 10 000 birthing women). In Norway, the highest number of women admitted to ICU per test‐positive pregnant women was during the Delta period (17.8 per 1000 test‐positive), whereas the highest number admitted per test‐positive in Sweden and Denmark was seen during the Index period (15.4 and 8.9 per 1000 test‐positive, respectively).
FIGURE 2.

Number of pregnant women admitted to intensive care units per 10 000 birthing women and 1000 SARS‐CoV‐2 test‐positive pregnant women, during different periods of dominating virus variants in Sweden, Norway, Denmark, and combined.
4. DISCUSSION
In this population‐based study including nearly all delivering women in Scandinavia, we explored maternal characteristics, pregnancy outcomes, and vaccination status in pregnant women admitted to ICU because of severe COVID‐19. We found that women who were unvaccinated, multiparous, with lower education and income levels, with higher BMI and co‐morbidities, and of non‐Scandinavian origin, were more frequently admitted to ICU because of severe COVID‐19. Admission to ICU was further associated with adverse pregnancy outcomes.
In line with previous studies from other countries, 2 , 16 , 17 our data from Scandinavia confirm that admission to ICU because of severe COVID‐19 in pregnancy is rare. Still, it is important to explore potential maternal characteristics related to admission to ICU and subsequent outcomes of those admitted, as this may identify risk groups among pregnant women. As in other studies, 3 , 5 , 16 , 18 , 19 we also saw that those admitted to ICU had a higher mean BMI and higher proportion of chronic and pregnancy conditions than test‐positive pregnant women not admitted to ICU and all birthing women.
We have previously shown that women of non‐Scandinavian origin have an increased risk of testing positive to SARS‐CoV‐2 in pregnancy, 20 and are less likely to be vaccinated against COVID‐19 in pregnancy. 21 In this study we further show that non‐Scandinavian origin is associated with admission to ICU because of severe COVID‐19. In an Italian study, SARS‐CoV‐2 test‐positive pregnant women from high migration pressure countries were significantly more likely to be hospitalized because of COVID‐19 pneumonia. 16 In a study from Canada, non‐white pregnant women were disproportionately represented among SARS‐CoV‐2‐related hospital admissions. 2 Overall, these results highlight the needs for more efficient approaches to secure equal access to health care and targeted measures and information towards risk groups.
In a systematic review and meta‐analysis by Allotey et al, the overall risk of mother‐to‐child transmission of SARS‐CoV‐2 was shown to be low, although the risk seem to be increased with severe maternal disease. 4 Severe COVID‐19 in pregnancy has further been suggested to increase the risk of cesarean section, 16 preterm delivery, 2 and admission to neonatal care. 22 We saw an increased proportion of cesarean section, preterm, and very preterm deliveries among women admitted to ICU with COVID‐19. We also observed that infants born to mothers admitted to ICU more often had lower Apgar scores at 5 minutes. Gestational age and low Apgar scores are major determining factors of neonatal death, 23 as well as for the child's developmental and neurological health later in life. 24 , 25
Vaccination rates in the Scandinavian countries is high among birthing women. 9 , 21 However, we saw that up to 10% of the women admitted to ICU were vaccinated, which is in line with previous studies. 9 , 17 , 26 Interestingly, one in four women admitted to ICU remained unvaccinated after pregnancy until the end of follow up. A Danish study showed that SARS‐CoV‐2 infection initially offered a high level of sustained protection against re‐infection, comparable with that by vaccines; however, with the introduction of the Omicron variant, the protection dramatically decreased. 13 Our findings emphasize the need for continued efforts to increase vaccination uptake in pregnant women, especially in subgroups known to have lower vaccination uptake. 21
Several studies have suggested that the Alpha and Delta variants of SARS‐CoV‐2 were associated with more severe maternal infection and adverse pregnancy outcomes compared with the Index variant. 5 , 16 As in these previous studies, we lacked variant sequencing data. Nevertheless, when using time periods corresponding to the dominating virus variants to explore admission patterns to ICU, we saw that the highest proportion of admissions was during the Delta period (combined data from the three countries), whereas the highest number of admissions per test‐positive pregnant women was seen in the Index period. Importantly, in the first few months of the pandemic, only those with severe symptoms of infection were tested, 27 which may explain the higher number of admitted per test‐positive. In addition, clinicians may have been more prone to admit pregnant women with COVID‐19 to ICU early in the pandemic when knowledge about the disease was limited. Likewise, it is not clear from the results of this study whether the lowest number of admissions per birthing and test‐positive women during the Omicron period was the result of a less severe infection and/or because of a higher vaccination rate among pregnant women. 21
The difference in proportion of test‐positive individuals between Sweden (11.1%), Norway (10.2%), and Denmark (3.2%) may be multifactorial, but was primarily because we lacked population data from Denmark from the first months of 2022, when test‐positive rates increased profoundly. 28 Compared with Sweden and Norway, where ICU cases were identified from register‐based information, the ICU cases in Denmark were identified from a separate clinical database (DCOD), which included pregnant women testing positive for SARS‐CoV‐2 up to the date of delivery. In the Swedish and Norwegian data, 12 of 127 ICU cases were admitted postpartum. However, all but one of these 12 tested positive for SARS‐CoV‐2, 0–8 days before delivery. Hence, despite the differences in identifying severe COVID‐19 in pregnancy between Denmark and Sweden/Norway, the results will not be particularly affected, and including data from three countries increases the strength of the study on a rare event.
The strengths of this study include its population‐based design with complete register‐ and database information prospectively collected for maternal characteristics and pregnancy outcomes on all pregnant women admitted to ICU within three Scandinavian countries during the Index to Omicron periods of the pandemic. Compared with other studies we were able to differentiate between ICU admissions because of severe COVID‐19 and admissions for obstetric care with coincidental SARS‐CoV‐2 infection, 17 and include national vaccination data. 2 Further, we compared those admitted to ICU with test‐positive pregnant women tested throughout pregnancy, and all birthing women during the same time period, in contrast to using non‐pregnant hospitalized test‐positive women, 16 or historical comparison cohorts. 19
This study has several limitations. We were not able to perform multivariable analyses because of the small numbers of ICU admissions, which also prohibited country‐specific analyses, and results should therefore be interpreted as exploratory. In addition, we lacked information on all pregnancies ending before 22 weeks of gestation, so pregnant women admitted to ICU with a miscarriage were not included in this study. Further, we had no information on sequencing data of virus variants, nor information on symptomatology and interventions of those admitted to ICU.
5. CONCLUSION
In this population‐based study in the Scandinavian countries, we found that admission to ICU because of severe COVID‐19 in pregnancy was rare. However, pregnant women who were unvaccinated, of non‐Scandinavian background, with a lower socio‐economic status, and with a higher BMI, were at increased risk of admission to ICU. Women admitted to ICU for COVID‐19 also had a higher risk of adverse pregnancy outcomes.
AUTHOR CONTRIBUTIONS
The study was initiated by AKÖ and OS, and designed by AKÖ, MCM, AJMA, SEH, and OS. Statistical analyses were performed by AKÖ, MCM, AJMA, AVH, and SKU. The initial draft was written by AKÖ, with support of interpretation of findings and critical revision from all authors. All authors had full access to data, and reviewed and approved the final version of the article submitted for publication.
FUNDING INFORMATION
This work was funded by NordForsk (project number 105545 and 135876), the Research Council of Norway (project number 324312) and through its Centers of Excellence funding scheme (project number 262700), the European Research Council under the European Union's Horizon 2020 Research and Innovation Program (grant agreement number 947684). The Danish COVID‐19 in pregnancy database (DCOD) was funded by The Region of Southern Denmark and Region Zealand's shared fund for joint health research projects (Reg. no. A767).
CONFLICT OF INTEREST STATEMENT
OS reports shares in Evidensa AB, a Pregnancy App “OneMillionBabies”. The rest of the authors report no conflicts of interest.
Supporting information
Appendix S1:
Table S1:
ACKNOWLEDGMENTS
We would like to thank Jonas Söderling at the Swedish Pregnancy Register, and the clinicians at the Danish units reporting to the DCOD (in alphabetical order): Eva K. Andersen, Charlotte Sander Andersen, Line Strand Andersen, Lise Lotte Torvin Andersen, Charlotte Brix Andersson, Christine T. Bæk, Anne‐Line Brülle, Lars Burmester, Tine Clausen, Richard Farlie, Arense Gulbech, Lea Hansen, Gitte Hedermann, Birgitte Henriksen, Lone Hvidman, Mette Holm Ibsen, Fjola Jonsdottir, Lisbeth Jønsson, Kamilla K. Karlsen, Mohammed Khalil, Åse Klemmensen, Birgitte Lindved, Julie Milbak, Ditte Møller Monica Lund Pedersen, Sidsel L. Rathcke, Elisabeth Rønneberg, Anne Nødgaard Sørensen, Manrinda Kaur Tatla, Dorthe Thisted, Annette Thorsen‐Meyer, Karen Wøjdeman, and Marianne Vestgaard.
Örtqvist AK, Magnus MC, Aabakke AJM, et al. Severe COVID‐19 during pregnancy in Sweden, Norway, and Denmark. Acta Obstet Gynecol Scand. 2023;102:681‐689. doi: 10.1111/aogs.14552
REFERENCES
- 1. Magnus MC, Oakley L, Gjessing HK, et al. Pregnancy and risk of COVID‐19: a Norwegian registry‐linkage study. BJOG. 2022;129:101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McClymont E, Albert AY, Alton GD, et al. Association of SARS‐CoV‐2 infection during pregnancy with maternal and perinatal outcomes. JAMA. 2022;327:1983‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allotey J, Chatterjee S, Kew T, et al. SARS‐CoV‐2 positivity in offspring and timing of mother‐to‐child transmission: living systematic review and meta‐analysis. BMJ. 2022;376:e067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vousden N, Ramakrishnan R, Bunch K, et al. Severity of maternal infection and perinatal outcomes during periods of SARS‐CoV‐2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Med. 2022;1:e000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS‐CoV‐2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327:1469‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Haberg SE. Covid‐19 vaccination during pregnancy and first‐trimester miscarriage. N Engl J Med. 2021;385:2008‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engjom H, van den Akker T, Aabakke A, et al. Severe COVID‐19 in pregnancy is almost exclusively limited to unvaccinated women ‐ time for policies to change. Lancet Reg Health Eur. 2022;13:100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blakeway H, Prasad S, Kalafat E, et al. COVID‐19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(236):e1‐e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skjefte M, Ngirbabul M, Akeju O, et al. COVID‐19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aabakke AJM, Petersen TG, Wojdemann K, et al. Risk factors for and pregnancy outcomes after SARS‐CoV‐2 in pregnancy according to disease severity: a nationwide cohort study with validation of the SARS‐CoV‐2 diagnosis. Acta Obstet Gynecol Scand. 2023;00:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michlmayr D, Hansen CH, Gubbels SM, et al. Observed protection against SARS‐CoV‐2 reinfection following a primary infection: a Danish cohort study among unvaccinated using two years of nationwide PCR‐test data. Lancet Reg Health Eur. 2022;20:100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Public Health Agency of Sweden . Statistics on SARS‐CoV‐2 variants of special interest. 2022. Available from: https://www.folkhalsomyndigheten.se/smittskydd‐beredskap/utbrott/aktuella‐utbrott/covid‐19/statistik‐och‐analyser/sars‐cov‐2‐virusvarianter‐av‐sarskild‐betydelse/
- 15. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843‐848. [DOI] [PubMed] [Google Scholar]
- 16. Donati S, Corsi E, Maraschini A, Salvatore MA; ItOSS‐COVID‐19 Working Group . SARS‐CoV‐2 infection among hospitalised pregnant women and impact of different viral strains on COVID‐19 severity in Italy: a national prospective population‐based cohort study. BJOG. 2022;129:221‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stock SJ, Carruthers J, Calvert C, et al. SARS‐CoV‐2 infection and COVID‐19 vaccination rates in pregnant women in Scotland. Nature. 2022;28:504‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vousden N, Bunch K, Morris E, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS‐CoV‐2 infection in the UK from march to September 2020: a national cohort study using the UK obstetric surveillance system (UKOSS). PloS One. 2021;16:e0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engjom H, Aabakke AJM, Klungsoyr K, et al. COVID‐19 in pregnancy‐characteristics and outcomes of pregnant women admitted to hospital because of SARS‐CoV‐2 infection in the Nordic countries. Acta Obstet Gynecol Scand. 2021;100:1611‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Örtqvist AK, Magnus MC, Soderling J, et al. The association between maternal characteristics and SARS‐CoV‐2 in pregnancy: a population‐based registry study in Sweden and Norway. Sci Rep. 2022;12:8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Örtqvist AK, Dahlqwist E, Magnus MC, et al. COVID‐19 vaccination in pregnant women in Sweden and Norway. Vaccine. 2022;40:4686‐4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norman M, Naver L, Soderling J, et al. Association of Maternal SARS‐CoV‐2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325:2076‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med. 2020;383:49‐57. [DOI] [PubMed] [Google Scholar]
- 24. Razaz N, Cnattingius S, Persson M, Tedroff K, Lisonkova S, Joseph KS. One‐minute and five‐minute Apgar scores and child developmental health at 5 years of age: a population‐based cohort study in British Columbia, Canada. BMJ Open. 2019;9:e027655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Persson M, Razaz N, Tedroff K, Joseph KS, Cnattingius S. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: population based cohort study in Sweden. BMJ. 2018;360:k207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engjom HM, Ramakrishnan R, Vousden N, et al. Severity of maternal SARS‐CoV‐2 infection and perinatal outcomes of women admitted to hospital during the omicron variant dominant period using UK obstetric surveillance system data: prospective, national cohort study. BMJ Med. 2022;1:e000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ludvigsson JF. The first eight months of Sweden's COVID‐19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109:2459‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Statens Serum Institut–covid‐19 ‐ Danmark (web page in Danish) . Available from: https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1:
Table S1:


