Abstract
Cell-based therapies are being developed for various neurodegenerative diseases that affect the central nervous system (CNS). Concomitantly, the roles of individual cell types in neurodegenerative pathology are being uncovered by genetic and single-cell studies. With a greater understanding of cellular contributions to health and disease and the arrival of promising approaches to modulate them, effective therapeutic cell products are now emerging. This review examines how the ability to generate diverse CNS cell types from stem cells, along with a deeper understanding of cell-type-specific functions and pathology, are advancing preclinical development of cell products for the treatment of neurodegenerative diseases.
eTOC summary
This broad review highlights stem cell-derived cell products that are in development for treating neurodegenerative disease. The review describes how in-depth characterization of healthy and pathological central nervous system tissues are guiding preclinical studies of cell replacement therapies.
Introduction
“Stem cell research is the key to developing cures for degenerative conditions like Parkinson’s and motor neuron disease from which I and many others suffer.”
Stephen Hawking
Neurodegenerative diseases of the CNS (the brain, retina, and spinal cord) affect all ages. They can range from congenital leukodystrophies that impair the white matter in childhood, to those with increased prevalence during aging, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and age-related macular degeneration (AMD) (Figure 1). Mental illnesses may have a degenerative component, such as schizophrenia, which is characterized by cortical gray matter loss and signs of accelerated aging in patients.1 Some neurodegenerative diseases are prevalent, including AD, AMD and PD, while others such as Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), corticobasal syndrome (CBS), multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) are rare. These diseases may have genetic, environmental, or complex etiology. Many neurodegenerative conditions progress to dementia, which is predicted to affect approximately 150 million people globally in 2050 with an economic burden of $10 trillion,2,3 and untold cost to patients and their families. Although neurodegenerative diseases are currently incurable, for some, treatments are available to alleviate symptoms. A good example is PD, where L-DOPA administration and deep brain stimulation can improve quality of life, although these treatments do not address the underlying disease and its inexorable progression.
Figure 1: Primary functional impact of neurodegenerative diseases and affected brain regions.
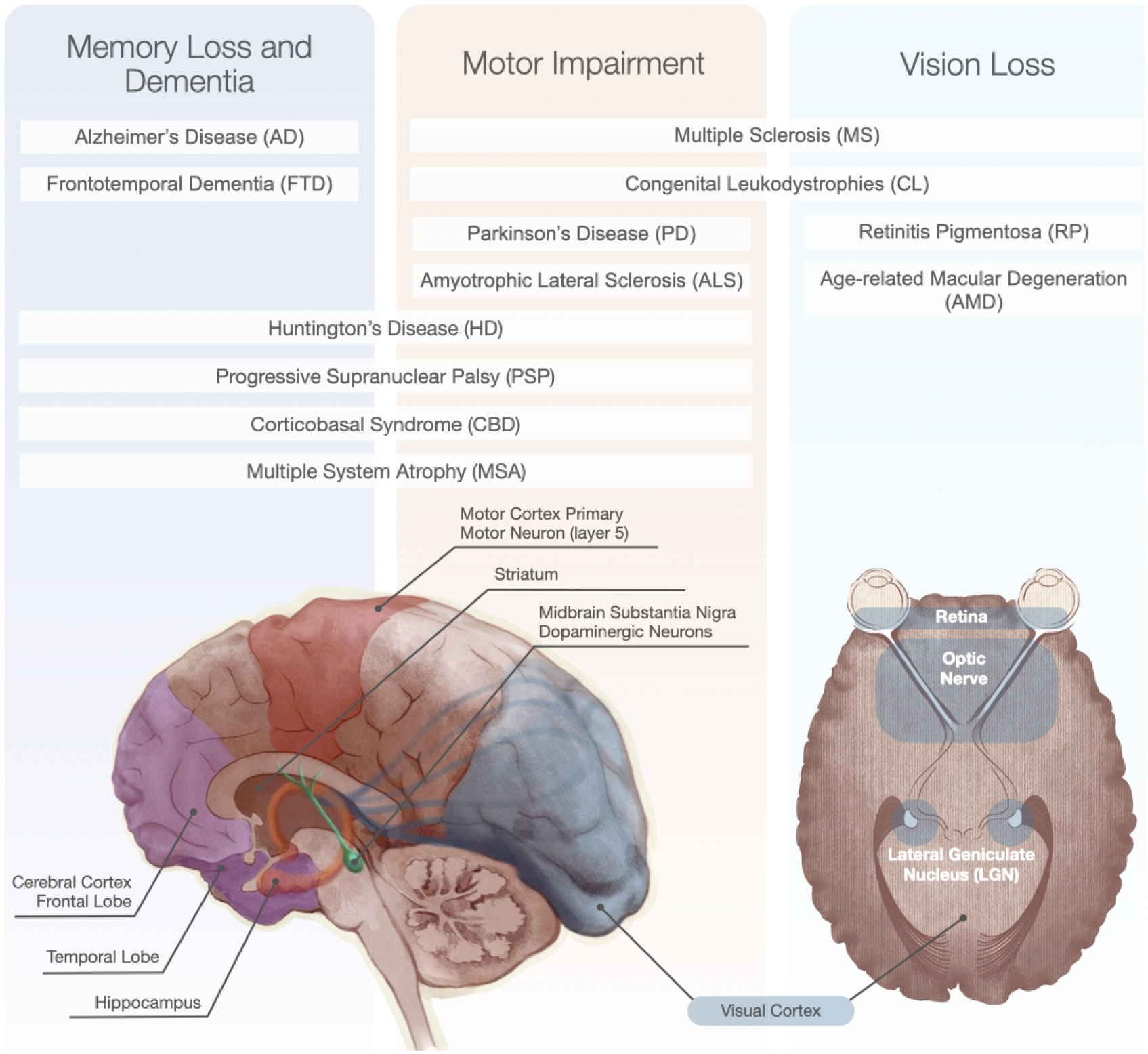
Patients experience symptoms that are primarily related to the particular neuronal and glial cell degeneration that occurs in different CNS areas in different diseases.
Neurodegeneration is characterized by the loss of neurons, but typically involves multiple interdependent cell types. A landmark discovery showed that ALS resulted from motor neuron defects and non-cell autonomous ‘killing’ by astrocytes,4 which could be exacerbated by microglial activation.5 It is now generally understood that macroglia (astrocytes and oligodendrocytes) and microglia play key roles in disease processes. The multicellular and multisystem involvement that characterizes neurodegenerative diseases has been well-documented by neuropathologists. However, through more recent genetic and functional studies, we now appreciate the causal contributions of each cellular player in unprecedented detail. Several excellent reviews have covered the cell therapies for specific neurodegenerative diseases, including PD,6–8 ALS,9,10 retinal degeneration,11 and multiple sclerosis (MS).12 This review instead takes a cell type-centric approach. It focuses on some of the major cellular products in development and highlights how greater knowledge of cell subtypes and states can guide preclinical work to assess their feasibility in the treatment of neurodegenerative diseases.
Development of cell products for clinical use
We are now able to generate numerous CNS cell types from human pluripotent stem cells (hPSCs) (Figure 2). Successful cell production is confirmed by comparing the product with human CNS tissue from fetal to adult stages and establishing the degree of similarity. In particular, single-cell and single-nucleus RNA sequencing (sc/nucRNA-seq) approaches have revolutionized our ability to test whether differentiation protocols generate authentic cell products. Small molecules and growth factors guide hPSCs to produce regionally-patterned neural progenitor cells (NPCs), then specific neural progeny, including major cell types affected by neurodegenerative disease in the forebrain,13–18 retina,19–22 midbrain,23 spinal cord,24 and throughout the nervous system25–30 (Figure 2). Vascular cells are derived from hPSCs by first differentiating into mesoderm and then adding factors to guide production of endothelial cells (ECs) and mural cells, such as pericytes and smooth muscle cells.26,31 Some brain pericytes arise from the neural crest,32 which can be achieved in vitro through initial neural patterning of hPSCs.28 Microglia are produced by guiding hPSCs to differentiate into yolk sac and then mesodermal hematopoietic progenitors, which subsequently produce monocytes and microglia.33 Although these approaches aim to recapitulate the signals that cells experience throughout development in a condensed timeframe, it may still take months to generate desired cell types with reasonable purity. Expression of genetic inducers can rapidly convert hPSCs into target cells. Examples are the overexpression of NGN2 to make neurons,34 or NFIA to make astrocytes,35,36 albeit with recognized differences from their in vivo counterparts.
Figure 2: Examples of hPSC protocols to generate the major CNS cell types affected in neurodegenerative diseases.
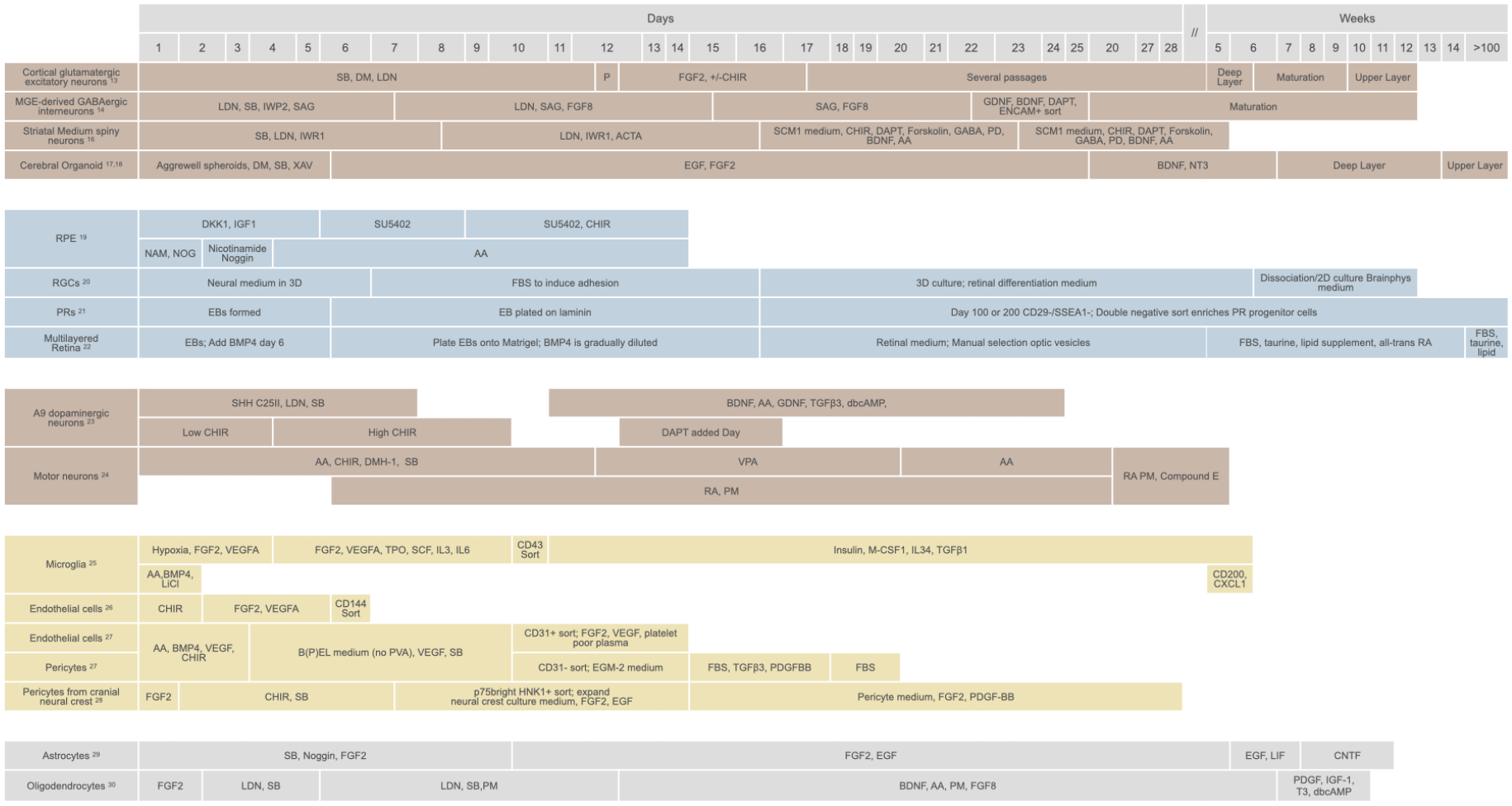
An overview showing the key molecules applied and the approximate time taken to generate the cell products. AA=Ascorbic Acid; ACTA=Activin A; BDNF=brain derived neurotrophic factor; CHIR=CHIR99021, GSK3 Inhibitor, WNT activator; DAPT= gamma secretase inhibitor, Notch pathway inhibitor; dbcAMP=dibutyryl cyclic AMP; DKK1=dickkopf 1, WNT inhibitor; DM=Dorsomorphin, ALK2, ALK3, and ALK6 inhibitor; DMH-1= ALK1, ALK2, and ALK3 inhibitor; EB=Embryoid body; EGF=epidermal growth factor; FGF2=fibroblast growth factor 2; FGF8=fibroblast growth factor 8; FBS=fetal bovine serum; GDNF=glial cell derived growth factor; IGF1=insulin like growth factor 1; IL3=interleukin 3; IL6=interleukin 6; IL34=interleukin 34; IWP=WNT production inhibitor; IWR=WNT response inhibitor; LDN=LDN193189, ALK2 and ALK3 inhibitor; LiCl=Lithium chloride; LIF=Leukemia inhibitory Factor; M-CSF1= macrophage colony stimulating factor; NAM=Nicotinamide; NOG=Noggin; NT3=Neurotrophin 3; P=Passage; PD=PD0332991, CDK4/6 inhibitor; PDGFBB=platelet derived growth factor BB; PM=Purmorphamine; PVA=polyvinyl alcohol; RA=retinoic acid; SAG=sonic hedgehog signaling agonist; SB=SB431542, ALK4, 5 and 7 inhibitor; SCF= Stem Cell Factor; SSEA-1= stage-specific embryonic antigen 1; SHH=sonic hedgehog; T3=Triiodothyronine; TGFβ3=Transforming growth factor β-3; TPO=Thrombopoietin; VEGFA=vascular endothelial growth factor A; VPA=Valproic acid; XAV=XAV-939, Tankyrase blocker, inhibits WNT signaling.
Generally, less mature cells integrate and connect better with the host tissue compared to highly differentiated cells. This applies, for instance, to several neural cell types, such as dopaminergic neurons,37,38 oligodendrocytes,39 astrocytes,40 and retinal pigment epithelial (RPE) cells.41 Hence, it is important to consider the cell stage when designing the optimal cell therapy for a particular neurodegenerative indication.
Along with improved protocols to generate 2D defined cell products, substantial advances have been made in creating 3D organoids that recapitulate the developmental order and cell composition of brain regions, such as the cerebral cortex.42 In some cases, these structures reproduce the cytoarchitectural organization, for instance, the multi-layered retina.22,43 The addition of vascular cells and microglia to neural organoids enables the creation of more complex in vitro constructs.44–47 These 3D structures may be part of the manufacturing process to create a desired cell type, such as retinal photoreceptors, or serve directly as multicell transplant products.
Cell transplantation strategies for neurodegenerative diseases typically aim for long-term cell survival. Therefore, immune rejection is an important consideration. The immune privilege of the CNS affords some protection. Allogeneic transplants have been demonstrated in patients several years post-transplantation, as seen for RPE cells and dopaminergic neurons.48,49 This immune privilege can be compromised during surgical delivery of the cell product. Hence, a typical clinical regimen will include a period of immunosuppression after transplantation. Another option is to deliver autologous cells, for example, using patient-derived iPSCs, when cell reprogramming, differentiation, and product release testing can occur within a viable therapeutic time-window.50 The autologous approach demands a robust manufacturing process that results in a safe and effective product, reproducibly across different donors.51,52 Autologous use of iPSCs presents considerable cost challenges, although these can be alleviated by implementing automation and closed, controlled systems during manufacturing.52,53
Although many preclinical studies indicate that autologous iPSC products will likely be effective, some have reported an immune response after injection back into the donor, for instance, due to neoepitope generation following mutations in mitochondrial DNA.50,54 iPSCs designed for protection from the immune system are being developed.55 Approximately 150 different iPSC lines would be sufficient to provide HLA-matched cells for about 90% of the population in the UK or Japan, although this approach is less effective for highly diverse populations.55 Alternatively, iPSCs can be engineered to evade immune detection. For example, iPSCs that lack MHC class I and class II genes and overexpress CD47 (an ‘immune cloaking’ anti-phagocytosis molecule) produce hypoimmune cells that are not detected by an MHC mis-matched host.56 Several approaches to generate hypoimmune cells are being pursued.55 One challenge is a potential tumorigenic conversion of cells in the hypoimmune transplant, which might then evade immune detection. In such an event, inclusion of a safety switch, such as a suicide gene to kill the transplanted cells, is being contemplated.57 However, one must consider the negative effects on the CNS of the patient caused by precipitously eliminating integrated neural cells by this mechanism.
The pathway and standards (https://www.isscr.org/standards) for developing stem-cell-based products are being refined, including for autologous therapies.51,58 It is essential to follow rigorous regulatory guidance, such as issued by the FDA in the U.S. (https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances). In developing a cell product for transplantation, a robust GMP-compliant cell manufacturing process is needed. Tests of the final cell product should demonstrate identity, purity, and potency prior to release for functional studies in animals or humans. Cell products contain diverse subpopulations and their mechanism of action is typically multifactorial. Hence, the critical quality attributes (CQA), those characteristics that make a cell product therapeutically beneficial for a specific indication, are often difficult to elucidate. To address this challenge, sc/nucRNA-seq is proving highly informative to characterize a cell product’s identity and purity pre- and post-grafting. This information can be correlated with clinical outcomes to identify predictive biomarkers of CQAs for use in the manufacture and release of cell products. Recognizing the need for an in-depth characterization of cell products, the NIH in the U.S. supports a regenerative-medicine-innovation-project-linked effort, Catalyst http://rmidatahub.org/.
At the FDA, an Investigational New Drug (IND) application to commence a clinical trial includes safety and efficacy testing. Testing is typically done in animal models, although increasingly in vitro methods and other alternatives are being pursued, supported by a recent U.S. law rescinding animal testing requirements.59 Extensive testing is recommended to assure cell products are not tumorigenic or demonstrate undesirable biodistribution after transplantation. In animal testing, the cells should be administered into the same region intended in patients and over a suitable dose range to establish safety. To achieve long-term engraftment of human cells for assessing efficacy and safety in animals, immunosuppression, or the use of immunodeficient animals such as NSG or rag2−/− mice, is often employed.
Generation of clinical products at sufficient scale is an important consideration.60 Although some CNS products have been tested at low doses (for example 50,000 cells per retina for RPE cells61), others require millions of cells per patient to achieve substantial engraftment. Moreover, sufficient product must be made to enable animal testing in the preclinical stage, and to supply cells for the clinical trial. Most cell products are cryopreserved during the manufacturing process, and such banks are subject to on-going stability testing, requiring additional samples. Some CNS cell products are designed for a ‘thaw and inject’ approach, which has substantial manufacturing efficiency and distribution advantages, for example Lineage Cell Therapeutic’s hPSC-derived RPE cell product (NCT02286089). Other cryopreserved cells require some manipulation post-thawing, such as washing to remove freezing medium or a period of cell culture, prior to final formulation and release. As cell manufacturing technologies are advancing rapidly, opportunities to develop more robust, scaled, and efficient processes are growing. Furthermore, regulators expect more rigorous manufacturing, storage, and release processes, ‘chemistry, manufacturing, and controls’ (CMC), to be developed as clinical trials advance. This consideration is important for products eligible for the FDA’s RMAT (Regenerative Medicine Advanced Therapy) designation, which requires a relatively mature manufacturing process. CMC can be improved by employing quality-by-design principles that optimize product manufacture, holding CQA uppermost.62 Improvements include, for example, stage appropriate scale-up, removing animal components, such as fetal bovine serum, from the manufacturing process or developing a cryopreserved product as the trial progresses.
Cell products for the treatment of neurodegeneration
Midbrain Dopaminergic neurons
In PD, ventral midbrain (VM) substantia nigra A9 dopaminergic neurons that project to the striatum degenerate, leading to motor impairments (Figure 1). Transplantation of human fetal VM neurons into the striatum showed long-term benefit in a subset of PD patients. These pioneering studies also highlighted the importance of defining the cell types in the graft, as contaminating serotonergic neurons could precipitate dyskinesia, debilitating involuntary movements.63 Using hPSCs solves procurement issues associated with fetal sources and enables manufacture of a more consistent cell product at scale. hPSCs differentiated into VM cells produced A9-enriched dopaminergic neurons that were stable and functional upon transplantation in animals.64,65 Moreover, optogenetic control of dopamine release from the grafted cells tuned the motor improvement in a PD mouse model, establishing transplant functionality.66 This preclinical work led to clinical-grade manufacturing and an IND application23,67 to support a clinical trial of human embryonic stem cell (hESC)-derived dopaminergic cells as treatment option in PD (NCT04802733).
Several protocols have been established that produce dopaminergic-neuron-based transplants effective in reversing signs of PD in animal models, including non-human primates.6,68,69 In general, progenitors produce a more effective graft than mature VM neurons, but the type of progenitor can matter. By correlating the pre-transplantation transcriptome of one cell product with graft outcome, it was shown that enrichment for caudal VM was beneficial.70 Timed exposure to FGF8b enhanced caudal VM production, enabling development of a clinical grade process.70 Globally, a number of cell products for PD are in, or advancing toward, early stage trials,6–8 including autologous approaches.71,72 Recognizing the benefit of working together, the international PD cell transplant community shares information and findings: http://www.gforce-pd.com/.
As PD products become more advanced, there are opportunities for product optimization. Sc/nucRNA-seq studies cataloguing human dopaminergic neurons in vivo have identified around 10 distinct populations, including subtypes that are vulnerable or resilient to PD.73–75 In-depth in vivo characterization also benefits analysis of hPSC-derived VM lineages in culture.76,77 This analysis revealed surface markers, CLSTN2 and PTPRO, that enriched for dopaminergic progenitors, producing a more stable and efficacious graft with fewer unwanted cell types.77 Studies of a different PD product identified some unexpected perivascular cells in the graft.78,79 These findings further illustrate the value of iterative product manufacture informed by deep analysis of the pre-and post-transplant cells, the developing and mature human tissue, and functional outcomes. Even though this approach relies on animal studies in the pre-clinical phase, ultimately, it should be done in patients. Notably, brain tissue collected from deceased patients several years after fetal VM transplantation showed evidence of Lewy bodies in the graft, aggregates of α-synuclein that are a pathological hallmark of PD.80 While this occurred rarely and only after more than 10 years post-grafting, it suggests product protection could be improved, perhaps by harnessing knowledge about resilient dopaminergic neuron phenotypes.
Forebrain GABAergic interneurons
A multitude of interneurons, defined by CNS location, morphology, peptide expression and circuitry, regulate neural activity. Lineage mapping studies in animals revealed that most forebrain interneurons are born in the embryonic ventral forebrain progenitor zones, the medial, lateral, and caudal ganglionic eminences (MGE, LGE and CGE), then migrate widely. Recent scRNA-seq profiling of the developing human fetal forebrain documents the phenotypes of MGE, LGE and CGE progenitors and of cortical progenitors that produce some cortical interneurons.81,82
Forebrain GABAergic interneurons dampen excitatory neuron activity and fine-tune their output. hPSCs patterned to MGE-type progenitors produced GABAergic interneurons83,84 that after transplantation into the hippocampus of a mouse epilepsy model migrated extensively, formed synaptic connections with host excitatory neurons, dampened seizures and improved cognition.14 A chemically matured GABAergic interneuron transplant shows similar benefit in a mouse epilepsy model, with optogenetic stimulation of graft neurons controlling seizure activity.85 With this promising therapeutic strategy, an hPSC-derived MGE-type GABAergic interneuron product (NRTX-1001) recently entered Phase I/II trial for patients with intractable temporal lobe epilepsy (NCT05135091). Several other diseases with neurodegenerative elements show impaired GABAergic interneuron functions, including schizophrenia and neuropathic pain.86,87,88 Moreover, degenerative diseases affecting the cortex, such as AD and FTD, are associated with epileptiform phenomena and excitotoxicity, in which glutamatergic neurons become overactive and die.89,90 Hence, in these diverse disorders, increasing GABAergic interneuron function could be therapeutic, and the progress of clinical trial NCT05135091 and similar upcoming trials that seek to increase inhibitory neuron activity will be eagerly followed.
As we understand better how interneuron subtypes contribute to brain function and disease, we can envision many more therapeutic applications.91 It has, however, proven difficult to generate specific subtypes such as fast-spiking, parvalbumin-expressing GABAergic interneurons in a reasonable number and time-frame, which remains an active area of research. Overexpression of LHX6 in human induced pluripotent stem cells (hiPSCs) accelerated production of somatostatin and parvalbumin interneurons in vitro and enhanced their engraftment in vivo.92 Another hurdle is understanding how interneurons survive and integrate into different brain regions. In mice, survival of transplanted interneurons depends on their gamma protocadherin expression.93 Transplantation of hPSC-derived MGE-type progenitors into the neonatal rat striatum produced predominantly striatal-type rather than cortical-type interneurons.94 This result shows the importance of considering the recipient as a selective or instructive environment affecting outcomes. With further advances in hPSC-interneuron manufacture and attaining successful engraftment, the considerable therapeutic potential of this cell type in different neurodegenerative disease settings may be realized.
Retinal neurons and RPE
The retina consists of the neural retina and RPE cells, a crucial support cell that maintains retinal homeostasis, photoreceptor function and vision. RPE cell dysfunction and death in the central retina, the macula, underlies AMD, the leading cause of blindness in the elderly. Other neurodegenerative diseases primarily affect the neural retina directly, including retinitis pigmentosa that leads to death of photoreceptor cells, and glaucoma that kills retinal ganglion cells (RGCs) the sole neural connection between the retina and brain. Several stem-cell-derived retinal cell products are being developed to combat retinal degenerations (Figure 3). Additional approaches aim to augment the environment with other neural cell types or trophic factors to improve retinal cell survival. A human fetal brain-derived neural progenitor product CNS10-NPC is being tested as a subretinal injection for patients with retinitis pigmentosa (NCT04284293). JCyte has successfully completed a Phase II study of human fetal retinal progenitor cells injected into the vitreous, releasing trophic factors to prevent photoreceptor degeneration in patients with retinitis pigmentosa (NCT03073733). Subretinal injection of a gene therapy expressing a neurotrophic factor to prevent cone photoreceptor cell death in retinitis pigmentosa patients is in clinical trial (NCT05748873).
Figure 3: Retinal cell replacement.
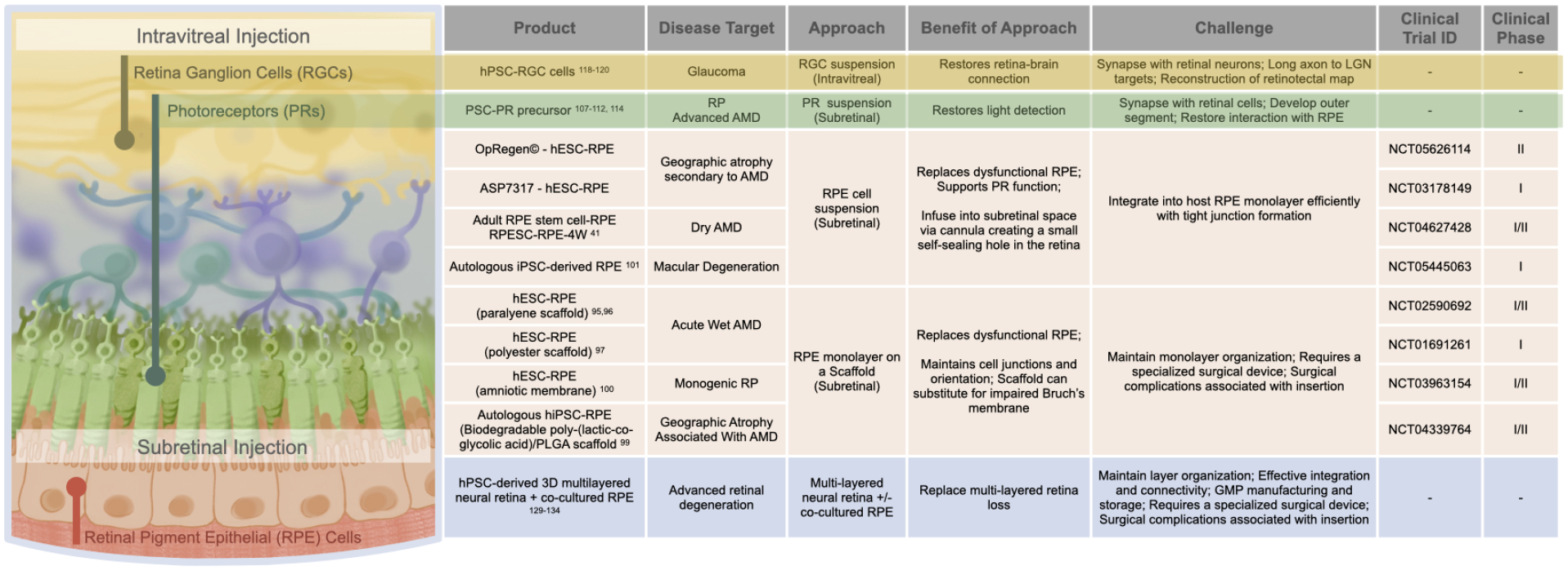
Examples of on-going studies and some of the challenges of using stem cell-derived retinal cell products, both single cell type and multi-cell grafts, to replace dysfunctional retinal cells. PR=photoreceptor.
Ongoing clinical trials for RPE replacement use hESCs, hiPSCs,11 and adult cadaver sourced RPE stem cells41 (NCT04627428) as the starting cell source. Two main sub-retinal transplant product types are being pursued: an RPE cell suspension or an RPE monolayer on an engineered scaffold, which may be permanent95–98 or biodegradable.99,100 The first U.S. clinical trial for autologous hiPSCs uses patient-derived RPE cells on a biodegradable scaffold99 (NCT04339764). The immune privilege of the subretinal space can be compromised by disease and trauma, so it is important to compare autologous and allogeneic approaches.99,101 Overall, early stage RPE transplantation trials are demonstrating that the approach is safe with promising signs of efficacy for patients with AMD.102
Although traditionally viewed as a homogeneous monolayer, scRNA-seq analyses of RPE cells acutely isolated from the adult human eye revealed multiple subclusters representing different subtypes and states.103 Newly identified markers for peripheral and macular RPE provide insight into how these specialized RPE subtypes develop.103 Artificial-intelligence-based image analysis has revealed unique characteristics of RPE cells in macular versus peripheral regions with different disease vulnerabilities.104 Comparison of healthy and AMD RPE using scRNA-seq and proteomics confirmed an involvement of the complement system in AMD and highlighted relevant signaling pathways, such as WNT and prostaglandin signaling.105 Clearly, the RPE has complex composition and contributions to degenerative retinal diseases, which will inform future therapy development. For example, biasing hPSC-RPE cells towards macular phenotypes may generate a more effective AMD product.
This new understanding of RPE cell diversity is guiding a deeper analysis of existing RPE transplant products. Adult RPE stem cells produce a range of RPE subtypes and states. Tracking their differentiation over time has revealed molecular markers for the progenitor cells that are most effective at visual rescue in animals, including a long non-coding RNA TREX whose expression is positively correlated with RPE cell integration into a pre-formed human RPE monolayer in vitro.41,106 Hence, by defining RPE cell products more completely and correlating with functional outcomes, we can find CQA biomarkers defining identity, purity, and potency, beyond the canonical RPE markers.
Transplantation of retinal neurons is more challenging than transplantation of RPE cells, primarily due to the need for functional synaptic connectivity. Nevertheless, preclinical studies have documented the benefit of photoreceptor transplantation for vision rescue.107–109 Intriguingly, mouse photoreceptor cells injected into the mouse retina formed cytoplasmic bridges with existing host photoreceptors,109–111 providing an unexpected mechanism for transferring cell contents and improving photoreceptor health. However, such fusion events occur less often when using human donor cells in a xenotransplant.112 Human photoreceptors include rods and cones, each with functional sub-categories. ScRNA-seq analysis has revealed distinct subtypes of cones spatially organized in the human macula.113 Human photoreceptor progenitors transplanted into rcd1/PDE6B mutant dogs with advanced inherited retinal degeneration survived with immunosuppression, showed no tumor formation, and differentiated largely into cone photoreceptors with evidence of synaptic contacts.114 These results represent an encouraging step towards using these progenitors in a clinical trial.
Successful transplantation of RGCs is particularly challenging given their long and precise trajectories,115,116 primarily to the lateral geniculate nucleus of the thalamus in humans (Figure 1). About 40 subtypes of mouse RGCs have been identified by scRNA-seq,117 and categorization of human RGCs in healthy and glaucomatous eyes is on-going. Multiple types of RGCs can be generated from hPSCs, enhancing studies of their specialized functions.20 hESC-derived RGCs integrated and formed presumptive synaptic contacts in ex vivo retinal explants,118 while cells injected intravitreally in adult rats survived and migrated into the RGC layer.119 NGN2 induction generates human iRGCs that protected resident retinal cells from neurodegeneration after optic nerve crush injury.120 Still, efficient integration and connectivity remain substantial hurdles.121 It will be valuable to determine if different RGC subtypes, such as those resistant to glaucoma or those with more regenerative ability,122,123 can improve outcomes. Optic nerve repair may also benefit from stimulating a glial progenitor present in the human lamina where RGC axons enter the nerve prior to becoming myelinated.124
For advanced retinal degeneration, in which much of the tissue has been lost, multicell type transplants are being contemplated. Proof of concept has come from pioneering surgeries in which the central retina is translocated over healthier, more peripheral RPE in AMD patients.125–128 Despite the high degree of operative complications, some patients showed vision benefit. Our ability to produce multilayered retina from hPSCs provides new opportunities for retinal transplantation (Figure 3).129,130 Several studies have examined the transplantation of hPSC-derived 3D retinal sheets into mice, nude rats, and monkeys. These grafts survived well, although they were frequently disorganized with inclusion of rosette structures rather than well-integrated layers. Younger stage grafts showed evidence of photoreceptor formation, and some studies provided evidence for light detection originating from the graft.130–134 Incorporating RPE with the neural retinal sheet is challenging and the tissues frequently separate after transplantation in vivo.135 Nevertheless, these pioneering studies map a path towards a graft that retains effective retinal cell organization and integrates sufficiently to benefit patients with severe vision loss. Concomitantly with this transplantation research, considerable hurdles are being addressed to establish clinical grade manufacturing of the envisioned 3D retinal products.136
Astrocytes
Astrocytes are distributed throughout the CNS and play multiple key roles. They support neurons, synaptic function, regulate brain homeostasis, innate immunity, metabolism, and blood brain barrier (BBB) integrity.137,138 In disease or injury, astrocytes become ‘reactive’, exhibiting complex morphological and gene expression changes with altered secretome and cell-cell interactions that may have both positive and negative effects.137–139
We have long recognized the heterogeneity of astrocytes, which is related to their specific location, morphology, function, age, and state of disease, and enhanced by recent sc/nucRNA-seq analyses.140–142 In neurodegenerative disease they may have beneficial roles, such as clearance of pathological tau and α-synuclein, but also contribute to pathology, for example, by spreading toxic molecules.139,141,143,144 Specific astrocyte phenotypes can be disease diagnostic, for example, tufted astrocytes with tau pathology in PSP.145,146 The understanding that astrocytes can be beneficial players is driving development of astrocyte products for several neurodegenerative diseases, including ALS, PD, HD, and AD.147,148
During CNS development, astrocytes originate from multipotent NPCs and neural stem cells (NSCs), such as radial glia in the cerebral cortex.149 NSCs first generate neurons, then later glia progenitor cells (GCPs) that make both astrocytes and oligodendrocytes.150 GCPs derived from fetal human brain and injected into neonatal and adult mice showed widespread astrocyte integration and improved outcomes in several models of neurodegenerative diseases, such as ALS and MS.150 Interestingly, human GPCs and their derived astrocytes supported enhanced learning and plasticity compared to analogous mouse cells, suggesting the human astrocytes conferred greater functionality.151
GCPs can be generated from hPSCs and biased toward astrocyte differentiation.29,152 When produced from disease-derived hPSCs, they model glial contributions to disease, such as neurotoxicity.144,153,154 For instance, when CD44+ astrocyte progenitors produced from hESCs carrying the mutant huntingtin gene of HD, were transplanted into the neonatal mouse brain, they impaired motor learning, indicating that glial pathology alone was sufficient to yield aspects of an HD phenotype. Conversely, transplanted wildtype astrocytes improved cognitive and motor outcomes in an HD mouse model.155 A number of neuropsychiatric disorders, most notably schizophrenia, are accompanied by marked astrocytic pathology, and GPCs and astrocytes produced from patient-derived hiPSCs disrupt normal behavior, cognition and sleep patterns when transplanted into neonatal mice.156
Astrocytes can be generated from hPSCs by first creating NPCs and then treating with CNTF and LIF.29,157 By guiding NPCs to different rostro-caudal domains, astrocytes were produced that retained regional identity after transplantation.152 hESC-derived astrocytes transplanted into an ALS hSOD1G93A mouse model slowed disease onset, although intrathecal administration in this experiment led largely to meningeal engraftment.158 hiPSC-derived astrocytes directly injected into the adult mouse brain acquired morphologies similar to those in human brain, including the primate-enriched intralaminar and varicose forms. Importantly, the implanted astrocytes responded to AD pathology similarly to endogenous astrocytes in patients with early-stage AD.159 However, the engrafted cells did not show the degree of migration and integration seen with GCP injection, perhaps indicating that transplantation of more mature astrocytes is not as effective as transplanting progenitor stages.
Astrocyte states are being increasingly explored to promote recovery from neurodegenerative diseases. For example, inactivation of SIRT1 can switch reactive mouse astrocytes to an anti-inflammatory phenotype that reduces pathology in an animal model of inflammatory demyelinating disease.160 Studies have defined how interactions with disease-activated microglia can alter astrocytes to exhibit a pathologic ‘killer’ state.139 In this state, they release neurotoxic saturated lipids, which can be compensated by strategies such as lowering the lipid synthesis enzyme ELOVL1, improving recovery in an axonal injury model.161 In the future, we anticipate the translation of these findings into improved astrocyte-targeting products that may benefit multiple neurodegenerative diseases.
Oligodendrocytes
Oligodendrocytes wrap axons in an insulating, lipid-rich myelin sheath that results in rapid, saltatory conduction of action potentials. Demyelinating diseases cause a failure of neural function due to slowed and interrupted axonal conduction. Leukodystrophies are heritable diseases that primarily affect oligodendrocytes, leading to dysmyelination and often neurodegeneration. They frequently have a childhood onset and are first noted when developmental delays in motor coordination and speech are observed. Examples include Krabbe, Tay-Sachs, and Canavan diseases, among others.162 Leukodystrophies have diverse underlying genetic causes, many converging on lipid metabolism impairment, which particularly affects oligodendrocytes given their role in myelin production.163 MS is a more common demyelinating disease that has an immunological component. Several neurodegenerative and psychiatric diseases include prominent oligodendrocyte pathology and white matter loss, such as FTD, AD, PD, HD, MSA and schizophrenia. In MSA, α-synuclein builds up primarily in oligodendrocytes, leading to widespread degeneration.164 Some white matter diseases can be treated by blood cell infusion, such as the recently approved therapy for cerebral adrenoleukodystrophy in which a patient’s hematopoietic stem cells (HSCs) are engineered to express a normal ABCD1 gene before reinfusion.165 Several related strategies are in preclinical development for other leukodystrophies.166 Similarly, patients with MS can benefit from HSC transplantation to ‘reset’ their immune system.167 However, when there is substantial loss of myelin that is not endogenously repaired, an oligodendrocyte replacement therapy is required.168,169
Proof of concept for successful oligodendrocyte replacement has been demonstrated by engrafting human fetal GCPs into animal models of myelin deficiency, such as the immunocompromised shiverer (shi/shi)×rag2−/− mouse model. Direct injection into the neonatal brain targeting the main white matter tracts led to a remarkable result: widespread replacement of mouse with human oligodendrocytes, and significantly extended survival in some recipients.170 Importantly, human fetal GCPs can also remyelinate in adult mice after chemically induced demyelination and in adult shiverer mice.171 Similar promising results have been observed using hPSCs as the oligodendrocyte source, with widespread migration, maturation and remyelination in nude rats after radiation treatment,30 neonatal (shi/shi)×rag2−/− mice,172 adult nude rats after traumatic injury,173 and adult mice after spinal cord injury.174 One of the challenges of manufacturing hPSC-derived oligodendrocytes is their protracted development over months.30 Direct reprograming, for example, by expressing SOX10, OLIG2 and NKX6.2 in hPSCs, rapidly produces induced oligodendrocyte-like cells that are effective in animal models of demyelinating disease.175
During normal development, oligodendrocytes originate in different regions of the CNS and then migrate widely, occupying different domains depending on their origin.176 Investigations of oligodendrocyte populations in the white matter of human brains using sc/nucRNA-seq analysis has revealed six different subclasses and altered proportions between healthy individuals and MS patients177. They also confirmed transcriptional heterogeneity in several neurodegenerative diseases, such as HD, PD, and AD.178 It will be interesting to learn whether some oligodendrocytes subtypes and states are better at engrafting, self-renewing, and recovering myelin in specific disease settings.
Microglia
Microglia are the resident macrophages in the CNS. They migrate into the early developing CNS, where they are long-lived and sustained by self-renewal.179 Microglia tile through the CNS, forming an expansive network that maintains homeostasis, surveilles for cellular debris and infectious agents, and participates in response and repair processes.180 Rather than simplifying microglial behavior into a dichotomous switch between ramified/resting versus amoeboid/reactive states, multiple microglial states are being recognized through single-cell analyses, providing the level of detail needed to better understand their biology.181
In neurodegenerative diseases, microglia benefit processes such as debris clearance, but also contribute to pathology, for example by spreading abnormal tau or α-synuclein.182,183 Impaired microglia can underlie neurodegenerative diseases, such as adult leukodystrophy caused by mutations in the CSF1 receptor (CSF1R), which is important for microglial survival.184,185 Their critical roles have been brought further into focus through genetic association studies. GWAS analysis has uncovered over 70 loci associated with AD.186 Surprisingly, most candidate AD risk modifying genes are strongly expressed by microglia and monocytes, converging on core functions such as efferocytosis.187,188 Functional genomic and sc/nucRNA-seq analyses across multiple tauopathies in human brain has revealed stage- and disease-specific microglial responses.189 This understanding and further study of AD-risk genes enriched in microglia, such as TREM2 and MS4A,190,191 or genes important for microglial regulation such as progranulin (GRN)192,193 are spurring approaches to treat neurodegenerative diseases by manipulating microglia (Figure 4). For example, TREM2-triggering antibodies that promote the transition from a homeostatic to a disease-associated microglial state194,195 are advancing through clinical trial for AD.196 Given the association of TREM2 with other neurodegenerative conditions such as ALS, PD, and FTD,197 it may prove to be a valuable multi-disease approach. Tempering this is the understanding that increased TREM2 could be pathological,196,198,199 necessitating carefully developed treatment regimens.
Figure 4: Approaches to target microglia to combat neurodegenerative diseases.
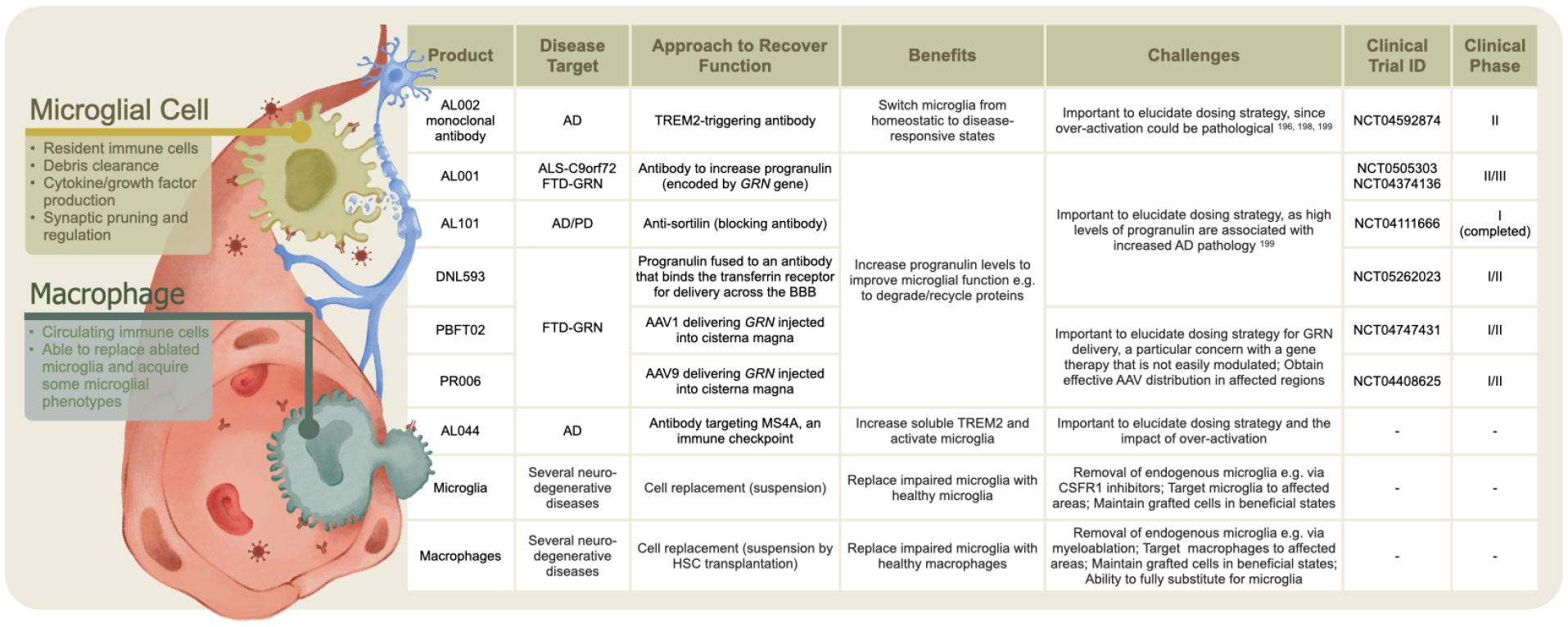
Greater understanding of microglial contribution to neurodegenerative pathologies has led to biologics targeting microglial function and to cell replacement strategies using microglia or macrophages.
Microglia can be generated from hPSCs by mimicking their mesodermal and hemopoietic developmental trajectory25,200,201 or by direct induction. For example hiPSCs with dox-inducible cassettes for PU.1, MAFB, CEBPα, CEBPβ, IRF5 and IRF8, produced iTF microglia that resembled other hPSC-derived microglia, albeit with some distinctions from microglia derived from human brain.202 Subsequent addition of CRISPRi and CRISPRa machinery enabled a large-scale genetic screen for molecular drivers that alter iTF microglial states. For example, microglia expressing SPP1, associated with aging and exposure to amyloid or tau pathology, are increased or decreased in abundance by knocking down MAPK14 or CSF1R respectively.202 CRISPR-based functional testing has the exciting potential to reveal ways microglial states can be manipulated to promote their therapeutic potential.
Because of their central roles in surveillance, microglia respond chameleon-like to altered environments. One challenge in studying human brain microglia is that they change significantly soon after isolation.203 Moreover, hiPSC-derived microglia in culture are highly variable between individuals,204 as seen in primary microglia.205 Transplanting human microglia into the murine brain circumvents some of these issues. hPSC-derived microglia injected into the perinatal mouse forebrain integrated widely and adopted phenotypes similar to human microglia in vivo. Their survival depends on hCSF1 that can be incorporated into the model, e.g. in Rag2−/− Il2rγ−/− hCSF1KI or MITRG mice.206,207 Microglia injected into a MITRGx5xFAD model showed acquisition of human disease-associated microglial signatures, including upregulation of TREM2.207 Fascinatingly, in an AD mouse model that lacks microglia, pathology was shifted from cerebral plaques to cerebral amyloid angiopathy, with brain calcification and cerebral hemorrhage.208 This pathology was reversed by a single injection of wild-type microglia.208
Hence, xenotransplantation can shed light on microglial contributions to pathology and provide preclinical evidence supporting safety and efficacy. For a transplantation to be successful, endogenous microglia may need to be reduced (for instance, using the CSF1R inhibitors PLX3397 or PLX647) to provide open niches for new cells to occupy.209 Preclinical studies of PLX3397 and PLX647, which cross the BBB, demonstrate that microglial removal is safe in adult mice and non-human primates without significant changes in inflammation or cognition and shows benefits in AD, PD, ALS and prion disease models.209,210 PLX3397 is an FDA-approved drug for patients with brain cancer.211 Excitingly, human microglia engineered to resist CSF1R inhibitors show more widespread integration in the adult murine brain than wildtype microglia after PLX3397 treatment, potentially revealing an effective way to achieve replacement in patients.212 In a different approach, mice treated with the myeloablation agent busulfan followed by HSC transplantation showed extensive microglial clearing and replacement with peripheral macrophages.213 A similar macrophage replacement strategy slowed progression of neurodegeneration caused by prosaposin deficiency.214 Future studies will determine the extent of human macrophage replacement of microglial functions, as transplanted cells exhibit persisting differences compared to brain microglia.214,215 These studies encourage further assessment of the potential benefits of microglial replacement in multiple neurodegenerative indications, including genetic deficiencies, such as lysosomal storage diseases. They also hint at a potential to prevent diseases, such as AD, from progressing to a state of wide-spread, multi-cell loss.
Vascular cells
The brain vasculature delivers essential nutrients and helps to recycle interstitial and cerebrospinal fluids. ECs in the brain vasculature are firmly connected by tight junctions, forming the BBB that regulates the exchange of circulating molecules. Vascular involvement is common across neurodegenerative diseases.216 The majority of AD patients have brain blood vessel defects, sometimes predating symptom onset by decades.216,217 Vascular abnormalities that contribute to neurodegeneration include reduced cerebral blood flow, BBB breakdown, vascular stiffening, endothelial dysfunction, and pericyte loss. For this reason, vascular repair is being examined as a potential therapeutic option for several neurodegenerative conditions, including traumatic brain injury,218 ischemia,219 and ALS.220
hPSCs can be differentiated efficiently into mesoderm-derived ECs, pericytes and smooth muscle cells, albeit with lower trans-endothelial electrical resistance than in the brain.26,31 Methods to generate brain ECs with high trans-endothelial electrical resistance from hPSCs require additional research to determine the factors regulating this aspect of brain vessel function. Studies demonstrate that some approaches to produce brain ECs from hPSCs actually produce epithelial cells with high trans-endothelial electrical resistance that lack canonical EC markers.221,222 However, these markers can be gained by enforcing expression of ETV2, ERG and FLI1.222 hiPSC-derived vascular cells transplanted into a rat model of white matter infarct improved hind limb movement and remyelination in the infarct region.219 hPSCs differentiated into cranial neural crest generate pericyte-like cells that promote BBB repair and reduce neuronal loss after transplantation into a mouse model of stroke.28
Hence, both ECs and mural cells are important in neurodegenerative pathogenesis. Sc/nucRNA-seq analyses provide a deeper understanding of the heterogeneity of different vascular beds,223 and the responses of large and small vessel cells to neurodegenerative diseases, such as AD224–226 PD227 and HD.228 Sc/nucRNA-seq analysis of white matter in patients with vascular dementia has revealed a subset of disease-associated ECs expressing genes implicated in cell death and protein folding and a subset expressing genes associated with angiogenesis and oligodendrocyte maturation.229 For AD, sc/nucRNA-seq analysis of the prefrontal cortex highlighted ECs from patient samples with increased expression of angiogenic growth factors and receptors (such as EGFL7, FLT1, VWF) and genes associated with antigen-presentation (such as B2M and HLA-E), implying functions in vessel regrowth and immune responses.225 A method to improve snucRNA-seq analysis of vascular cells (VINE-seq) has revealed selective vulnerability of a subset of pericytes specialized to maintain the extracellular matrix which may contribute to the loss of BBB integrity seen in AD.224 Notably, this study also showed that 30 of the top 45 genes linked to AD by GWAS are vascular-cell-associated. SnucRNA-seq analysis of APOE4 carriers shows APOE and NFAT dysregulation in pericytes and that targeting of calcineurin/NFAT signaling reduces APOE-associated cerebral amyloid angiopathy pathology in model systems.226 Hence, these exciting studies are highlighting promising targets for vascular-cell-focused therapies.
Neural stem cells
NSCs build the CNS, create multiple types of neurons and glia, and seem perfectly suited for replacement therapies for disorders, in which a variety of cells are lost.230 Pioneering clinical studies tested human fetal-derived NSCs for neurological conditions, such as Batten disease, Pelizaeus-Merzbacher disease, spinal cord injury, AMD, and AD.231 NSC transplantation proved safe with signs of positive efficacy. A human fetal-derived NSC line transduced to express GDNF improved motor neuron survival and animal lifespan in an SOD1 rat model of ALS232,233 and demonstrated safety in an early-phase clinical trial (NCT05306457).234 Cell delivery is a challenge for any neurodegenerative disease. Typically, direct cell injection is the envisioned initial path. Several studies indicate that intravascular administration can be effective using NSCs with high α4β1 integrin expression, with brain penetration and improved outcomes demonstrated in an ALS mouse model.235
NSCs are highly diverse regionally and temporally, which biases their ability to produce specific types of progeny.236 Mouse NSCs patterned to the visual cortex can survive and extend appropriate connections when transplanted into the visual but not the motor cortex of adult mice.237 Hence, matching the NSC product may be an important factor to accomplish effective multi-cell replacement in different CNS regions. In HD, the loss of striatal medium spiny neurons progresses to a devastating degeneration of the basal forebrain and cerebral cortex. Transplanting hESC-derived NSCs into the brains of a P6 mouse model of HD showed improved histology, motor and cognitive function.238 In a similar approach, transplanting hiPSC-derived NPCs produced neurons, astrocytes and oligodendrocytes and improved cognitive and motor outcomes in an HD mouse model, with overall benefits greater than those obtained with human GCPs.239 These studies demonstrate the value of using multipotent progenitors to replace lost CNS cell types. Moreover, incorporating developing vascular cells along with NPCs improved engraftment and vessel structure in a mouse model of stroke, supporting the feasibility of yet more complex, mixed cell products for extensive CNS repair.240 hPSC-derived cortical organoids engrafted into the neonatal rat brain received thalamocortical and corticocortical inputs, extended axons, and responded to optogenetic stimulation driving reward-seeking behaviour.241 In adult mouse cortex, human cerebral organoids also engraft, become vascularized, show microglial colonization, rhythmic electrical activity and optogenetic-stimulated graft-host connectivity.242 Excitingly, cortical organoids transplanted into adult mice equipped with electrodes to monitor brain activity integrate and show electrophysiological cortical responses to visual stimuli.243 Successful transplantation of organoids into the monkey cerebral cortex has also been demonstrated.244 These studies suggest that in the future, hPSC-derived 3D neural grafts could potentially treat important aspects of the multicellular, regional neural cell loss associated with complex neurodegenerative diseases.
Summary of Progress and Future Perspectives
Active clinical evaluation is ongoing for stem-cell-derived dopaminergic neurons for PD, RPE for AMD and GABAergic interneurons for epilepsy. Progress in these areas includes a deeper characterization of the cell products and graft outcomes. The goals are to better define CQA biomarkers and produce improved or refined products for specific indications. Technologies such as sc/nucRNA-seq benefit these preclinical studies and may, in the future, be employed widely in manufacturing and release criteria, aided by the implementation of cross-platform benchmarking.245 Several years of preclinical research indicate that astrocyte and oligodendrocyte transplantation might be beneficial for a variety of demyelinating and neurodegenerative diseases, and we anticipate clinical evaluation shortly. Exciting progress in achieving effective microglial replacement in animal models opens new opportunities for cross-disease therapies, given their broad immunomodulatory role. However, maintaining microglia in a beneficial state could be challenging. In cases where the goal is wide-spread cell replacement after microglial or macroglial transplantation, the effects of such a drastic change on human CNS function are yet to be determined, although animal studies are encouraging. Restoring neuronal connectivity, both local and long-range, remain substantial hurdles. However, there are positive signs from retinal and cerebral transplants that despite disrupted organization, connections with host cells can improve functionality. Finally, the impact of the human host environment, and how it changes with disease stage, is an important consideration for successful grafting. Looking forward, we anticipate that the preclinical pipeline for neurodegenerative diseases will include cell products that are genetically manipulated to be resilient, slow disease progression, enhance cell function or control cell states for specific disease indications.
Conclusion
Increased knowledge of individual brain cell phenotypes and states, through in-depth characterization of tissue and stem-cell-derived cells, is guiding development of neural cell therapeutics. With brisk translation, there is a good reason to think that human stem-cell-based products will produce disease-altering therapies to improve the lives of patients with currently incurable neurodegenerative diseases. Although there are challenges, we are encouraged by progress to date, and take inspiration from Steven Hawking’s last message to spur us to take care of our world and future: “Be brave, be determined, overcome the odds. It can be done”.
Acknowledgements
I’m indebted to Jeffrey H. Stern, NSCI; Jason S. Meyer, U. Indiana; Lorenz Studer, Memorial Sloan Kettering Cancer Center; Steven A. Goldman, U. Rochester; Mathew Blurton-Jones, U.C. Irvine; and Arnold Kriegstein, UCSF for their invaluable input on the manuscript. The illustrations are the original work of Yangzi Isabel Tian.
Funding support from NIH: R35NS097277, R01EY032138, U01AG072464 and RF1NS123568.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
ST is co-founder of Luxa Biotech developing an RPE therapy for AMD and has patents related to RPE cell therapy: Retinal pigment epithelial stem cells, Patent number: 8481313; Methods of treating a retinal disease by Retinal pigment epithelial stem cells, Patent number: 10034916; ST has advised BlueRock therapeutics, Vita Therapeutics and SANA Biotechnology.
References
- 1.Stone WS et al. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophr Res 243, 154–162, doi: 10.1016/j.schres.2022.03.004 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators, G. B. D. D. F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125, doi: 10.1016/S2468-2667(21)00249-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nandi A et al. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine 51, 101580, doi: 10.1016/j.eclinm.2022.101580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagai M et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10, 615–622, doi: 10.1038/nn1876 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahsen BF et al. Non-neuronal cells in amyotrophic lateral sclerosis - from pathogenesis to biomarkers. Nat Rev Neurol 17, 333–348, doi: 10.1038/s41582-021-00487-8 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Barbuti PA et al. Recent Advances in the Development of Stem-Cell-Derived Dopaminergic Neuronal Transplant Therapies for Parkinson’s Disease. Mov Disord 36, 1772–1780, doi: 10.1002/mds.28628 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Barker RA, Parmar M, Studer L & Takahashi J Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell 21, 569–573, doi: 10.1016/j.stem.2017.09.014 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Cha Y, Park TY, Leblanc P & Kim KS Current Status and Future Perspectives on Stem Cell-Based Therapies for Parkinson’s Disease. J Mov Disord, doi: 10.14802/jmd.22141 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin TJ et al. Potential of Cellular Therapy for ALS: Current Strategies and Future Prospects. Front Cell Dev Biol 10, 851613, doi: 10.3389/fcell.2022.851613 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sironi F, De Marchi F, Mazzini L & Bendotti C Cell therapy in ALS: An update on preclinical and clinical studies. Brain Res Bull 194, 64–81, doi: 10.1016/j.brainresbull.2023.01.008 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Van Gelder RN et al. Regenerative and restorative medicine for eye disease. Nat Med 28, 1149–1156, doi: 10.1038/s41591-022-01862-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JA et al. Stem Cell Therapies for Progressive Multiple Sclerosis. Front Cell Dev Biol 9, 696434, doi: 10.3389/fcell.2021.696434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strano A, Tuck E, Stubbs VE & Livesey FJ Variable Outcomes in Neural Differentiation of Human PSCs Arise from Intrinsic Differences in Developmental Signaling Pathways. Cell Rep 31, 107732, doi: 10.1016/j.celrep.2020.107732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham M et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15, 559–573, doi: 10.1016/j.stem.2014.10.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald M, Sotuyo N, Tischfield DJ & Anderson SA Generation of cerebral cortical GABAergic interneurons from pluripotent stem cells. Stem Cells 38, 1375–1386, doi: 10.1002/stem.3252 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Smith-Geater C et al. Aberrant Development Corrected in Adult-Onset Huntington’s Disease iPSC-Derived Neuronal Cultures via WNT Signaling Modulation. Stem Cell Reports 14, 406–419, doi: 10.1016/j.stemcr.2020.01.015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowles KR et al. ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids. Cell 184, 4547–4563 e4517, doi: 10.1016/j.cell.2021.07.003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SJ et al. Reliability of human cortical organoid generation. Nat Methods 16, 75–78, doi: 10.1038/s41592-018-0255-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leach LL et al. Induced Pluripotent Stem Cell-Derived Retinal Pigmented Epithelium: A Comparative Study Between Cell Lines and Differentiation Methods. J Ocul Pharmacol Ther 32, 317–330, doi: 10.1089/jop.2016.0022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer KB et al. Retinal Ganglion Cell Diversity and Subtype Specification from Human Pluripotent Stem Cells. Stem Cell Reports 10, 1282–1293, doi: 10.1016/j.stemcr.2018.02.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliardi G et al. Characterization and Transplantation of CD73-Positive Photoreceptors Isolated from Human iPSC-Derived Retinal Organoids. Stem Cell Reports 11, 665–680, doi: 10.1016/j.stemcr.2018.07.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capowski EE et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146, doi: 10.1242/dev.171686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TW Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use. Cell Stem Cell 28, 343–355 e345, doi: 10.1016/j.stem.2021.01.005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du ZW et al. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun 6, 6626, doi: 10.1038/ncomms7626 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abud EM et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94, 278–293 e279, doi: 10.1016/j.neuron.2017.03.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertucci T, Kakarla S, Kim D & Dai G Differentiating Human Pluripotent Stem Cells to Vascular Endothelial Cells for Regenerative Medicine, Tissue Engineering, and Disease Modeling. Methods Mol Biol 2375, 1–12, doi: 10.1007/978-1-0716-1708-3_1 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Orlova VV et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc 9, 1514–1531, doi: 10.1038/nprot.2014.102 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Sun J et al. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat Commun 11, 5196, doi: 10.1038/s41467-020-19042-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perriot S, Canales M, Mathias A & Du Pasquier R Differentiation of functional astrocytes from human-induced pluripotent stem cells in chemically defined media. STAR Protoc 2, 100902, doi: 10.1016/j.xpro.2021.100902 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piao J et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 16, 198–210, doi: 10.1016/j.stem.2015.01.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams IM & Wu JC Generation of Endothelial Cells From Human Pluripotent Stem Cells. Arterioscler Thromb Vasc Biol 39, 1317–1329, doi: 10.1161/ATVBAHA.119.312265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etchevers HC, Vincent C, Le Douarin NM & Couly GF The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059–1068, doi: 10.1242/dev.128.7.1059 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Speicher AM, Wiendl H, Meuth SG & Pawlowski M Generating microglia from human pluripotent stem cells: novel in vitro models for the study of neurodegeneration. Mol Neurodegener 14, 46, doi: 10.1186/s13024-019-0347-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulme AJ, Maksour S, St-Clair Glover M, Miellet S & Dottori M Making neurons, made easy: The use of Neurogenin-2 in neuronal differentiation. Stem Cell Reports 17, 14–34, doi: 10.1016/j.stemcr.2021.11.015 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X et al. Fast Generation of Functional Subtype Astrocytes from Human Pluripotent Stem Cells. Stem Cell Reports 11, 998–1008, doi: 10.1016/j.stemcr.2018.08.019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchieu J et al. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol 37, 267–275, doi: 10.1038/s41587-019-0035-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiller BM et al. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. NPJ Regen Med 7, 24, doi: 10.1038/s41536-022-00221-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganat YM et al. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest 122, 2928–2939, doi: 10.1172/JCI58767 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warrington AE, Barbarese E & Pfeiffer SE Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res 34, 1–13, doi: 10.1002/jnr.490340102 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Filous AR et al. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev Neurobiol 70, 826–841, doi: 10.1002/dneu.20820 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis RJ The Developmental Stage of Adult Human Stem Cell-Derived Retinal Pigment Epithelium Cells Influences Transplant Efficacy for Vision Rescue. Stem Cell Reports 9, 42–49, doi: 10.1016/j.stemcr.2017.05.016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arlotta P & Pasca SP Cell diversity in the human cerebral cortex: from the embryo to brain organoids. Curr Opin Neurobiol 56, 194–198, doi: 10.1016/j.conb.2019.03.001 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Fligor CM, Huang KC, Lavekar SS, VanderWall KB & Meyer JS Differentiation of retinal organoids from human pluripotent stem cells. Methods Cell Biol 159, 279–302, doi: 10.1016/bs.mcb.2020.02.005 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Matsui TK, Tsuru Y, Hasegawa K & Kuwako KI Vascularization of human brain organoids. Stem Cells 39, 1017–1024, doi: 10.1002/stem.3368 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Xu R et al. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Reports 16, 1923–1937, doi: 10.1016/j.stemcr.2021.06.011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Epelboim A & Christian KM Modeling neuro-immune interactions using human pluripotent stem cells. Curr Opin Neurobiol 79, 102672, doi: 10.1016/j.conb.2022.102672 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Sun XY et al. Generation of vascularized brain organoids to study neurovascular interactions. Elife 11, doi: 10.7554/eLife.76707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashani AH et al. Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration. Stem Cell Reports 17, 448–458, doi: 10.1016/j.stemcr.2022.01.001 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li JY & Li W Postmortem Studies of Fetal Grafts in Parkinson’s Disease: What Lessons Have We Learned? Front Cell Dev Biol 9, 666675, doi: 10.3389/fcell.2021.666675 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamanaka S Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 27, 523–531, doi: 10.1016/j.stem.2020.09.014 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Jha BS, Farnoodian M & Bharti K Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl Med 10, 198–208, doi: 10.1002/sctm.20-0242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madrid M, Sumen C, Aivio S & Saklayen N Autologous Induced Pluripotent Stem Cell-Based Cell Therapies: Promise, Progress, and Challenges. Curr Protoc 1, e88, doi: 10.1002/cpz1.88 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Bohrer LR et al. Automating iPSC generation to enable autologous photoreceptor cell replacement therapy. J Transl Med 21, 161, doi: 10.1186/s12967-023-03966-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deuse T et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat Biotechnol 37, 1137–1144, doi: 10.1038/s41587-019-0227-7 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Petrus-Reurer S et al. Immunological considerations and challenges for regenerative cellular therapies. Commun Biol 4, 798, doi: 10.1038/s42003-021-02237-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deuse T et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37, 252–258, doi: 10.1038/s41587-019-0016-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wunderlich S et al. Targeted biallelic integration of an inducible Caspase 9 suicide gene in iPSCs for safer therapies. Mol Ther Methods Clin Dev 26, 84–94, doi: 10.1016/j.omtm.2022.05.011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker RA et al. Lessons learnt, and still to learn, in first in human stem cell trials. Stem Cell Reports, doi: 10.1016/j.stemcr.2022.11.019 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.m. W FDA no longer needs to require animal tests before human drug trials. doi: 10.1126/science.adg6264 (2023). [DOI] [Google Scholar]
- 60.Campbell A et al. Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med 4, 1155–1163, doi: 10.5966/sctm.2014-0294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz SD, Tan G, Hosseini H & Nagiel A Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci 57, ORSFc1-9, doi: 10.1167/iovs.15-18681 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Lipsitz YY, Timmins NE & Zandstra PW Quality cell therapy manufacturing by design. Nat Biotechnol 34, 393–400, doi: 10.1038/nbt.3525 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Politis M et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med 2, 38ra46, doi: 10.1126/scitranslmed.3000976 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Kriks S et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551, doi: 10.1038/nature10648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy NS et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 12, 1259–1268, doi: 10.1038/nm1495 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Steinbeck JA et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol 33, 204–209, doi: 10.1038/nbt.3124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piao J et al. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 28, 217–229 e217, doi: 10.1016/j.stem.2021.01.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doi D et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat Commun 11, 3369, doi: 10.1038/s41467-020-17165-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kikuchi T et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548, 592–596, doi: 10.1038/nature23664 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Kirkeby A et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell 20, 135–148, doi: 10.1016/j.stem.2016.09.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schweitzer JS et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N Engl J Med 382, 1926–1932, doi: 10.1056/NEJMoa1915872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loring JF Autologous Induced Pluripotent Stem Cell-Derived Neurons to Treat Parkinson’s Disease. Stem Cells Dev 27, 958–959, doi: 10.1089/scd.2018.0107 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Fiorenzano A, Sozzi E, Parmar M & Storm P Dopamine Neuron Diversity: Recent Advances and Current Challenges in Human Stem Cell Models and Single Cell Sequencing. Cells 10, doi: 10.3390/cells10061366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamath T et al. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat Neurosci 25, 588–595, doi: 10.1038/s41593-022-01061-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aguila J et al. Spatial RNA Sequencing Identifies Robust Markers of Vulnerable and Resistant Human Midbrain Dopamine Neurons and Their Expression in Parkinson’s Disease. Front Mol Neurosci 14, 699562, doi: 10.3389/fnmol.2021.699562 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smits LM et al. Single-cell transcriptomics reveals multiple neuronal cell types in human midbrain-specific organoids. Cell Tissue Res 382, 463–476, doi: 10.1007/s00441-020-03249-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu P et al. Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcomes in a Parkinson’s disease model. J Clin Invest 132, doi: 10.1172/JCI156768 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tiklova K et al. Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat Commun 11, 2434, doi: 10.1038/s41467-020-16225-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiklova K et al. Author Correction: Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat Commun 11, 3630, doi: 10.1038/s41467-020-17421-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kordower JH, Chu Y, Hauser RA, Freeman TB & Olanow CW Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14, 504–506, doi: 10.1038/nm1747 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Yu Y et al. Interneuron origin and molecular diversity in the human fetal brain. Nat Neurosci 24, 1745–1756, doi: 10.1038/s41593-021-00940-3 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Delgado RN et al. Individual human cortical progenitors can produce excitatory and inhibitory neurons. Nature 601, 397–403, doi: 10.1038/s41586-021-04230-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicholas CR et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 12, 573–586, doi: 10.1016/j.stem.2013.04.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maroof AM et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572, doi: 10.1016/j.stem.2013.04.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Q et al. Human cortical interneurons optimized for grafting specifically integrate, abort seizures, and display prolonged efficacy without over-inhibition. Neuron, doi: 10.1016/j.neuron.2022.12.014 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hughes DI & Todd AJ Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurotherapeutics 17, 874–885, doi: 10.1007/s13311-020-00936-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jahangir M, Zhou JS, Lang B & Wang XP GABAergic System Dysfunction and Challenges in Schizophrenia Research. Front Cell Dev Biol 9, 663854, doi: 10.3389/fcell.2021.663854 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kruse AO & Bustillo JR Glutamatergic dysfunction in Schizophrenia. Transl Psychiatry 12, 500, doi: 10.1038/s41398-022-02253-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murley AG et al. GABA and glutamate deficits from frontotemporal lobar degeneration are associated with disinhibition. Brain 143, 3449–3462, doi: 10.1093/brain/awaa305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Y, Zhao M, Han Y & Zhang H GABAergic Inhibitory Interneuron Deficits in Alzheimer’s Disease: Implications for Treatment. Front Neurosci 14, 660, doi: 10.3389/fnins.2020.00660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Southwell DG et al. Interneurons from embryonic development to cell-based therapy. Science 344, 1240622, doi: 10.1126/science.1240622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuan F et al. Induction of human somatostatin and parvalbumin neurons by expressing a single transcription factor LIM homeobox 6. Elife 7, doi: 10.7554/eLife.37382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mancia Leon WR et al. Clustered gamma-protocadherins regulate cortical interneuron programmed cell death. Elife 9, doi: 10.7554/eLife.55374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Noakes Z et al. Human Pluripotent Stem Cell-Derived Striatal Interneurons: Differentiation and Maturation In Vitro and in the Rat Brain. Stem Cell Reports 12, 191–200, doi: 10.1016/j.stemcr.2018.12.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Y et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res 48, 186–191, doi: 10.1159/000338749 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Brant Fernandes RA et al. An Innovative Surgical Technique for Subretinal Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigmented Epithelium in Yucatan Mini Pigs: Preliminary Results. Ophthalmic Surg Lasers Imaging Retina 47, 342–351, doi: 10.3928/23258160-20160324-07 (2016). [DOI] [PubMed] [Google Scholar]
- 97.da Cruz L et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 36, 328–337, doi: 10.1038/nbt.4114 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Liu Z et al. Surgical Transplantation of Human RPE Stem Cell-Derived RPE Monolayers into Non-Human Primates with Immunosuppression. Stem Cell Reports 16, 237–251, doi: 10.1016/j.stemcr.2020.12.007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma R et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med 11, doi: 10.1126/scitranslmed.aat5580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ben M’Barek K et al. Clinical-grade production and safe delivery of human ESC derived RPE sheets in primates and rodents. Biomaterials 230, 119603, doi: 10.1016/j.biomaterials.2019.119603 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Zhang H et al. Transplantation of GMP-grade human iPSC-derived retinal pigment epithelial cells in rodent model: the first pre-clinical study for safety and efficacy in China. Ann Transl Med 9, 245, doi: 10.21037/atm-20-4707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raimondi R et al. Where Are We with RPE Replacement Therapy? A Translational Review from the Ophthalmologist Perspective. Int J Mol Sci 23, doi: 10.3390/ijms23020682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Z et al. A Single-Cell Transcriptome Atlas of the Human Retinal Pigment Epithelium. Front Cell Dev Biol 9, 802457, doi: 10.3389/fcell.2021.802457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ortolan D et al. Single-cell-resolution map of human retinal pigment epithelium helps discover subpopulations with differential disease sensitivity. Proc Natl Acad Sci U S A 119, e2117553119, doi: 10.1073/pnas.2117553119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Senabouth A et al. Transcriptomic and proteomic retinal pigment epithelium signatures of age-related macular degeneration. Nat Commun 13, 4233, doi: 10.1038/s41467-022-31707-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farjood F, M. J, Wang Y, Williams AL, Zhao C, Borden S, Alam N, Prusky G, Temple S, Stern JH, Boles NC. PREPRINT:Identifying Biomarkers of Retinal Pigment Epithelial Cell Stem Cell-derived RPE Cell Heterogeneity and Transplantation Efficacy. BIRXIV (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pearson RA et al. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103, doi: 10.1038/nature10997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh MS et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A 110, 1101–1106, doi: 10.1073/pnas.1119416110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Decembrini S et al. Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation. Mol Ther 25, 634–653, doi: 10.1016/j.ymthe.2016.12.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santos-Ferreira T et al. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun 7, 13028, doi: 10.1038/ncomms13028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pearson RA et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun 7, 13029, doi: 10.1038/ncomms13029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gonzalez-Cordero A et al. Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors. Stem Cell Reports 9, 820–837, doi: 10.1016/j.stemcr.2017.07.022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voigt AP et al. Human photoreceptor cells from different macular subregions have distinct transcriptional profiles. Hum Mol Genet 30, 1543–1558, doi: 10.1093/hmg/ddab140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ripolles-Garcia A et al. Systemic immunosuppression promotes survival and integration of subretinally implanted human ESC-derived photoreceptor precursors in dogs. Stem Cell Reports 17, 1824–1841, doi: 10.1016/j.stemcr.2022.06.009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martersteck EM et al. Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep 18, 2058–2072, doi: 10.1016/j.celrep.2017.01.075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsai NY et al. Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin. Nat Neurosci 25, 659–674, doi: 10.1038/s41593-022-01068-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rheaume BA et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun 9, 2759, doi: 10.1038/s41467-018-05134-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Croteau LP et al. Ex Vivo Integration of Human Stem Retinal Ganglion Cells into the Mouse Retina. Cells 11, doi: 10.3390/cells11203241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang X et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor Neurol Neurosci 38, 131–140, doi: 10.3233/RNN-190941 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Luo Z et al. Directly induced human retinal ganglion cells mimic fetal RGCs and are neuroprotective after transplantation in vivo. Stem Cell Reports 17, 2690–2703, doi: 10.1016/j.stemcr.2022.10.011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams PR, Benowitz LI, Goldberg JL & He Z Axon Regeneration in the Mammalian Optic Nerve. Annu Rev Vis Sci 6, 195–213, doi: 10.1146/annurev-vision-022720-094953 (2020). [DOI] [PubMed] [Google Scholar]
- 122.VanderWall KB et al. Differential susceptibility of retinal ganglion cell subtypes in acute and chronic models of injury and disease. Sci Rep 10, 17359, doi: 10.1038/s41598-020-71460-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tapia ML, Nascimento-Dos-Santos G & Park KK Subtype-specific survival and regeneration of retinal ganglion cells in response to injury. Front Cell Dev Biol 10, 956279, doi: 10.3389/fcell.2022.956279 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernstein SL et al. The optic nerve lamina region is a neural progenitor cell niche. Proc Natl Acad Sci U S A 117, 19287–19298, doi: 10.1073/pnas.2001858117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Machemer R & Steinhorst UH Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol 231, 635–641, doi: 10.1007/BF00921957 (1993). [DOI] [PubMed] [Google Scholar]
- 126.Eckardt C, Eckardt U & Conrad HG Macular rotation with and without counter-rotation of the globe in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 237, 313–325, doi: 10.1007/s004170050239 (1999). [DOI] [PubMed] [Google Scholar]
- 127.Toth CA & Freedman SF Macular translocation with 360-degree peripheral retinectomy impact of technique and surgical experience on visual outcomes. Retina 21, 293–303, doi: 10.1097/00006982-200108000-00001 (2001). [DOI] [PubMed] [Google Scholar]
- 128.Skaf AR & Mahmoud T Surgical treatment of age-related macular degeneration. Semin Ophthalmol 26, 181–191, doi: 10.3109/08820538.2011.577133 (2011). [DOI] [PubMed] [Google Scholar]
- 129.Arthur P et al. Bioengineering Human Pluripotent Stem Cell-Derived Retinal Organoids and Optic Vesicle-Containing Brain Organoids for Ocular Diseases. Cells 11, doi: 10.3390/cells11213429 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xue Y et al. The Prospects for Retinal Organoids in Treatment of Retinal Diseases. Asia Pac J Ophthalmol (Phila) 11, 314–327, doi: 10.1097/APO.0000000000000538 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uyama H et al. Competency of iPSC-derived retinas in MHC-mismatched transplantation in non-human primates. Stem Cell Reports 17, 2392–2408, doi: 10.1016/j.stemcr.2022.09.014 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tu HY et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39, 562–574, doi: 10.1016/j.ebiom.2018.11.028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Assawachananont J et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2, 662–674, doi: 10.1016/j.stemcr.2014.03.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shirai H et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A 113, E81–90, doi: 10.1073/pnas.1512590113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas BB et al. Co-grafts of Human Embryonic Stem Cell Derived Retina Organoids and Retinal Pigment Epithelium for Retinal Reconstruction in Immunodeficient Retinal Degenerate Royal College of Surgeons Rats. Front Neurosci 15, 752958, doi: 10.3389/fnins.2021.752958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cobb H, Aparicio-Domingo S & Canto-Soler MV Transitioning into GMP-Compliance: Alternative Methods for Producing Retinal Organoids for Transplantation. Transl Vis Sci Technol 10, 9, doi: 10.1167/tvst.10.10.9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sofroniew MV Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol 41, 758–770, doi: 10.1016/j.it.2020.07.004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sofroniew MV & Vinters HV Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35, doi: 10.1007/s00401-009-0619-8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487, doi: 10.1038/nature21029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Escartin C et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24, 312–325, doi: 10.1038/s41593-020-00783-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Majo M, Koontz M, Rowitch D & Ullian EM An update on human astrocytes and their role in development and disease. Glia 68, 685–704, doi: 10.1002/glia.23771 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Al-Dalahmah O et al. Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol Commun 8, 19, doi: 10.1186/s40478-020-0880-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Acioglu C, Li L & Elkabes S Contribution of astrocytes to neuropathology of neurodegenerative diseases. Brain Res 1758, 147291, doi: 10.1016/j.brainres.2021.147291 (2021). [DOI] [PubMed] [Google Scholar]
- 144.Gomes C et al. Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells. Stem Cell Reports 17, 1636–1649, doi: 10.1016/j.stemcr.2022.05.006 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dickson DW, Rademakers R & Hutton ML Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17, 74–82, doi: 10.1111/j.1750-3639.2007.00054.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roemer SF et al. Rainwater Charitable Foundation criteria for the neuropathologic diagnosis of progressive supranuclear palsy. Acta Neuropathol 144, 603–614, doi: 10.1007/s00401-022-02479-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hastings N, Kuan WL, Osborne A & Kotter MRN Therapeutic Potential of Astrocyte Transplantation. Cell Transplant 31, 9636897221105499, doi: 10.1177/09636897221105499 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Valori CF, Possenti A, Brambilla L & Rossi D Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders. Cells 10, doi: 10.3390/cells10082019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rash BG et al. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc Natl Acad Sci U S A 116, 7089–7094, doi: 10.1073/pnas.1822169116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martins-Macedo J et al. Glial restricted precursor cells in central nervous system disorders: Current applications and future perspectives. Glia 69, 513–531, doi: 10.1002/glia.23922 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han X et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353, doi: 10.1016/j.stem.2012.12.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krencik R, Weick JP, Liu Y, Zhang ZJ & Zhang SC Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 29, 528–534, doi: 10.1038/nbt.1877 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia VJ et al. Huntington’s Disease Patient-Derived Astrocytes Display Electrophysiological Impairments and Reduced Neuronal Support. Front Neurosci 13, 669, doi: 10.3389/fnins.2019.00669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barbar L et al. CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes. Neuron 107, 436–453 e412, doi: 10.1016/j.neuron.2020.05.014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benraiss A et al. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat Commun 7, 11758, doi: 10.1038/ncomms11758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Windrem MS et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 21, 195–208 e196, doi: 10.1016/j.stem.2017.06.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tcw J et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports 9, 600–614, doi: 10.1016/j.stemcr.2017.06.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Izrael M et al. Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1(G93A) and NSG animal models. Stem Cell Res Ther 9, 152, doi: 10.1186/s13287-018-0890-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Preman P et al. Human iPSC-derived astrocytes transplanted into the mouse brain undergo morphological changes in response to amyloid-beta plaques. Mol Neurodegener 16, 68, doi: 10.1186/s13024-021-00487-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang W et al. SIRT1 inactivation switches reactive astrocytes to an antiinflammatory phenotype in CNS autoimmunity. J Clin Invest 132, doi: 10.1172/JCI151803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guttenplan KA et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107, doi: 10.1038/s41586-021-03960-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bonkowsky JL, Keller S & Aap Section on Neurology, C. o. G. Leukodystrophies in Children: Diagnosis, Care, and Treatment. Pediatrics 148, doi: 10.1542/peds.2021-053126 (2021). [DOI] [PubMed] [Google Scholar]
- 163.Nowacki JC, Fields AM & Fu MM Emerging cellular themes in leukodystrophies. Front Cell Dev Biol 10, 902261, doi: 10.3389/fcell.2022.902261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krismer F & Wenning GK Multiple system atrophy: insights into a rare and debilitating movement disorder. Nat Rev Neurol 13, 232–243, doi: 10.1038/nrneurol.2017.26 (2017). [DOI] [PubMed] [Google Scholar]
- 165.Keam SJ Elivaldogene Autotemcel: First Approval. Mol Diagn Ther 25, 803–809, doi: 10.1007/s40291-021-00555-1 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Rossini L, Durante C, Marzollo A & Biffi A New Indications for Hematopoietic Stem Cell Gene Therapy in Lysosomal Storage Disorders. Front Oncol 12, 885639, doi: 10.3389/fonc.2022.885639 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cencioni MT et al. Immune Reconstitution Following Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis: A Review on Behalf of the EBMT Autoimmune Diseases Working Party. Front Immunol 12, 813957, doi: 10.3389/fimmu.2021.813957 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goldman SA, Mariani JN & Madsen PM Glial progenitor cell-based repair of the dysmyelinated brain: Progression to the clinic. Semin Cell Dev Biol 116, 62–70, doi: 10.1016/j.semcdb.2020.12.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carmichael ST & Llorente IL The Ties That Bind: Glial Transplantation in White Matter Ischemia and Vascular Dementia. Neurotherapeutics, doi: 10.1007/s13311-022-01322-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Windrem MS et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565, doi: 10.1016/j.stem.2008.03.020 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Windrem MS et al. Human Glial Progenitor Cells Effectively Remyelinate the Demyelinated Adult Brain. Cell Rep 31, 107658, doi: 10.1016/j.celrep.2020.107658 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang S et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252–264, doi: 10.1016/j.stem.2012.12.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu L et al. Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Res Ther 6, 93, doi: 10.1186/s13287-015-0087-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kawabata S et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Reports 6, 1–8, doi: 10.1016/j.stemcr.2015.11.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ehrlich M et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci U S A 114, E2243–E2252, doi: 10.1073/pnas.1614412114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Foerster S, Hill MFE & Franklin RJM Diversity in the oligodendrocyte lineage: Plasticity or heterogeneity? Glia 67, 1797–1805, doi: 10.1002/glia.23607 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Jakel S et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547, doi: 10.1038/s41586-019-0903-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seeker LA & Williams A Oligodendroglia heterogeneity in the human central nervous system. Acta Neuropathol 143, 143–157, doi: 10.1007/s00401-021-02390-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Askew K et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep 18, 391–405, doi: 10.1016/j.celrep.2016.12.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Augusto-Oliveira M et al. What Do Microglia Really Do in Healthy Adult Brain? Cells 8, doi: 10.3390/cells8101293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paolicelli RC et al. Microglia states and nomenclature: A field at its crossroads. Neuron 110, 3458–3483, doi: 10.1016/j.neuron.2022.10.020 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.George S et al. Microglia affect alpha-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener 14, 34, doi: 10.1186/s13024-019-0335-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maphis N et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755, doi: 10.1093/brain/awv081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ferrer I The Primary Microglial Leukodystrophies: A Review. Int J Mol Sci 23, doi: 10.3390/ijms23116341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rademakers R et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 44, 200–205, doi: 10.1038/ng.1027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bellenguez C et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 54, 412–436, doi: 10.1038/s41588-022-01024-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Novikova G et al. Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat Commun 12, 1610, doi: 10.1038/s41467-021-21823-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Romero-Molina C, Garretti F, Andrews SJ, Marcora E & Goate AM Microglial efferocytosis: Diving into the Alzheimer’s disease gene pool. Neuron 110, 3513–3533, doi: 10.1016/j.neuron.2022.10.015 (2022). [DOI] [PubMed] [Google Scholar]
- 189.Rexach JE et al. Tau Pathology Drives Dementia Risk-Associated Gene Networks toward Chronic Inflammatory States and Immunosuppression. Cell Rep 33, 108398, doi: 10.1016/j.celrep.2020.108398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Deming Y et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci Transl Med 11, doi: 10.1126/scitranslmed.aau2291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin SC et al. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet 23, 5838–5846, doi: 10.1093/hmg/ddu277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mendsaikhan A, Tooyama I & Walker DG Microglial Progranulin: Involvement in Alzheimer’s Disease and Neurodegenerative Diseases. Cells 8, doi: 10.3390/cells8030230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Logan T et al. Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic. Cell 184, 4651–4668 e4625, doi: 10.1016/j.cell.2021.08.002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McQuade A et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat Commun 11, 5370, doi: 10.1038/s41467-020-19227-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mazaheri F et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep 18, 1186–1198, doi: 10.15252/embr.201743922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carling G, Luo W & Gan L Friend turned foe: TREM2 agonist in battles against tau. J Exp Med 220, doi: 10.1084/jem.20221850 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xie M, Zhao S, Bosco DB, Nguyen A & Wu LJ Microglial TREM2 in amyotrophic lateral sclerosis. Dev Neurobiol 82, 125–137, doi: 10.1002/dneu.22864 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Song W et al. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement 13, 381–387, doi: 10.1016/j.jalz.2016.07.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suarez-Calvet M et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol Med 10, doi: 10.15252/emmm.201809712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McQuade A et al. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener 13, 67, doi: 10.1186/s13024-018-0297-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Muffat J et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med 22, 1358–1367, doi: 10.1038/nm.4189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Drager NM et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat Neurosci 25, 1149–1162, doi: 10.1038/s41593-022-01131-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, doi: 10.1126/science.aal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tcw J et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233 e2225, doi: 10.1016/j.cell.2022.05.017 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lopes KP et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat Genet 54, 4–17, doi: 10.1038/s41588-021-00976-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mancuso R et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci 22, 2111–2116, doi: 10.1038/s41593-019-0525-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hasselmann J et al. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron 103, 1016–1033 e1010, doi: 10.1016/j.neuron.2019.07.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kiani Shabestari S et al. Absence of microglia promotes diverse pathologies and early lethality in Alzheimer’s disease mice. Cell Rep 39, 110961, doi: 10.1016/j.celrep.2022.110961 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Green KN, Crapser JD & Hohsfield LA To Kill a Microglia: A Case for CSF1R Inhibitors. Trends Immunol 41, 771–784, doi: 10.1016/j.it.2020.07.001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hillmer AT et al. Microglial depletion and activation: A [(11)C]PBR28 PET study in nonhuman primates. EJNMMI Res 7, 59, doi: 10.1186/s13550-017-0305-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Butowski N et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 18, 557–564, doi: 10.1093/neuonc/nov245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chadarevian JP et al. Engineering an inhibitor-resistant human CSF1R variant for microglia replacement. J Exp Med 220, doi: 10.1084/jem.20220857 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sailor KA et al. Hematopoietic stem cell transplantation chemotherapy causes microglia senescence and peripheral macrophage engraftment in the brain. Nat Med 28, 517–527, doi: 10.1038/s41591-022-01691-9 (2022). [DOI] [PubMed] [Google Scholar]
- 214.Shibuya Y et al. Treatment of a genetic brain disease by CNS-wide microglia replacement. Sci Transl Med 14, eabl9945, doi: 10.1126/scitranslmed.abl9945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shemer A et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun 9, 5206, doi: 10.1038/s41467-018-07548-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sweeney MD, Kisler K, Montagne A, Toga AW & Zlokovic BV The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21, 1318–1331, doi: 10.1038/s41593-018-0234-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Govindpani K et al. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J Clin Med 8, doi: 10.3390/jcm8050651 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Srivastava AK et al. Human umbilical cord blood cells restore vascular integrity in injured rat brain and modulate inflammation in vitro. Regen Med 14, 295–307, doi: 10.2217/rme-2018-0106 (2019). [DOI] [PubMed] [Google Scholar]
- 219.Xu B, Kurachi M, Shimauchi-Ohtaki H, Yoshimoto Y & Ishizaki Y Transplantation of iPS-derived vascular endothelial cells improves white matter ischemic damage. J Neurochem 153, 759–771, doi: 10.1111/jnc.14949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Monsour M, Garbuzova-Davis S & Borlongan CV Patching Up the Permeability: The Role of Stem Cells in Lessening Neurovascular Damage in Amyotrophic Lateral Sclerosis. Stem Cells Transl Med 11, 1196–1209, doi: 10.1093/stcltm/szac072 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Delsing L et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 36, 1816–1827, doi: 10.1002/stem.2908 (2018). [DOI] [PubMed] [Google Scholar]
- 222.Lu TM et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2016950118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chavkin NW & Hirschi KK Single Cell Analysis in Vascular Biology. Front Cardiovasc Med 7, 42, doi: 10.3389/fcvm.2020.00042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang AC et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892, doi: 10.1038/s41586-021-04369-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lau SF, Cao H, Fu AKY & Ip NY Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc Natl Acad Sci U S A 117, 25800–25809, doi: 10.1073/pnas.2008762117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Blanchard JW et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med 26, 952–963, doi: 10.1038/s41591-020-0886-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Huang J, Liu L, Qin L, Huang H & Li X Single-Cell Transcriptomics Uncovers Cellular Heterogeneity, Mechanisms, and Therapeutic Targets for Parkinson’s Disease. Front Genet 13, 686739, doi: 10.3389/fgene.2022.686739 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garcia FJ et al. Single-cell dissection of the human brain vasculature. Nature 603, 893–899, doi: 10.1038/s41586-022-04521-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mitroi DN, Tian M, Kawaguchi R, Lowry WE & Carmichael ST Single-nucleus transcriptome analysis reveals disease- and regeneration-associated endothelial cells in white matter vascular dementia. J Cell Mol Med 26, 3183–3195, doi: 10.1111/jcmm.17315 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zholudeva LV, Jin Y, Qiang L, Lane MA & Fischer I Preparation of Neural Stem Cells and Progenitors: Neuronal Production and Grafting Applications. Methods Mol Biol 2311, 73–108, doi: 10.1007/978-1-0716-1437-2_7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tsukamoto A, Uchida N, Capela A, Gorba T & Huhn S Clinical translation of human neural stem cells. Stem Cell Res Ther 4, 102, doi: 10.1186/scrt313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klein SM et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther 16, 509–521, doi: 10.1089/hum.2005.16.509 (2005). [DOI] [PubMed] [Google Scholar]
- 233.Thomsen GM et al. Transplantation of Neural Progenitor Cells Expressing Glial Cell Line-Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis. Stem Cells 36, 1122–1131, doi: 10.1002/stem.2825 (2018). [DOI] [PubMed] [Google Scholar]
- 234.Baloh RH et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial. Nat Med 28, 1813–1822, doi: 10.1038/s41591-022-01956-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nizzardo M et al. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 23, 342–354, doi: 10.1093/hmg/ddt425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bonnefont J & Vanderhaeghen P Neuronal fate acquisition and specification: time for a change. Curr Opin Neurobiol 66, 195–204, doi: 10.1016/j.conb.2020.12.006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Michelsen KA et al. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron 85, 982–997, doi: 10.1016/j.neuron.2015.02.001 (2015). [DOI] [PubMed] [Google Scholar]
- 238.Reidling JC et al. Human Neural Stem Cell Transplantation Rescues Functional Deficits in R6/2 and Q140 Huntington’s Disease Mice. Stem Cell Reports 10, 58–72, doi: 10.1016/j.stemcr.2017.11.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Park HJ et al. Human iPSC-derived neural precursor cells differentiate into multiple cell types to delay disease progression following transplantation into YAC128 Huntington’s disease mouse model. Cell Prolif 54, e13082, doi: 10.1111/cpr.13082 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Krzyspiak J et al. Donor-derived vasculature is required to support neocortical cell grafts after stroke. Stem Cell Res 59, 102642, doi: 10.1016/j.scr.2021.102642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Revah O et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 610, 319–326, doi: 10.1038/s41586-022-05277-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mansour AA et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36, 432–441, doi: 10.1038/nbt.4127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wilson MN et al. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat Commun 13, 7945, doi: 10.1038/s41467-022-35536-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kitahara T et al. Axonal Extensions along Corticospinal Tracts from Transplanted Human Cerebral Organoids. Stem Cell Reports 15, 467–481, doi: 10.1016/j.stemcr.2020.06.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen X et al. A multi-center cross-platform single-cell RNA sequencing reference dataset. Sci Data 8, 39, doi: 10.1038/s41597-021-00809-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


