Abstract
The salience network, including the insula and anterior cingulate cortex (ACC), has been implicated in nicotine addiction. Structural imaging studies have reported diminished insula and ACC gray matter volumes (GMVs) in smokers as compared to nonsmokers. However, it remains unclear how insula and ACC GMVs may relate to years of smoking, addiction severity, or behavioral traits known to dispose individuals to smoking. Here, with a dataset curated from the Human Connectome Project and voxel-based morphometry, we replicated the findings of smaller GMVs of the insula and medial prefrontal cortex, including the dorsal ACC and supplementary motor area (dACC/SMA), in (70 heavy < 209 light < 209 never) smokers matched in age, sex, and average daily num ber of drinks. The GMVs of the insula or dACC/SMA were not significantly correlated with years of smoking or Fagerstrom Test for Nicotine Dependence (FTND) scores. Heavy relative to never smokers demonstrated higher externalizing and internalizing scores, as evaluated by the NIH Emotion. In heavy smokers, the dACC/SMA but not insula GMV was positively correlated with both externalizing and internalizing scores. The findings together confirm volumetric changes in the salience network in heavy smokers and suggest potentially distinct dysfunctional roles of the insula and dACC/SMA in chronic smoking.
Keywords: Nicotine use disorder, Saliency circuit, VBM, Externalizing, Internalizing
1. Introduction
Nicotine addiction is one of the leading causes of preventable morbidity and mortality [1–3]. Individuals with nicotine use disorders have great difficulty in managing craving and controlling their impulse to smoke [4–7]. Approximately 70% of smokers engaged in a treatment program relapse within 6 to 12 months [8, 9, see 10 for a review]. When abstinent, smokers experience anxiety and depression as well as craving, likely as a result of the allostatic changes in interoception elicited by chronic exposure to nicotine [11].
Cigarette smoking has been associated with externalizing [e.g., aggression and impulsivity; 12, 13] and internalizing [e.g. depression and anxiety; see 14 for reviews, 15] traits. For instance, an earlier study associated higher levels of sensation seeking with greater appetitive craving (anticipation of pleasure from smoking) and higher levels of urgency and lack of perseverance with negative affect craving (anticipation of relief from aversive states) in smokers [16]. A more recent study showed that, as compared to individuals without a depressive disorder, those with subsyndromal depression or depressive episodes were more likely to smoke [17]. These findings are in line with prior evidence showing co-occurrence of externalizing and internalizing problems and substance use [18].
A growing body of neuroimaging research has been examining the neural correlates of cigarette smoking as well as externalizing and internalizing behaviors. The insula and anterior cingulate cortex (ACC), two key nodes of the salience network, have been implicated in cigarette smoking and craving [19–21]. Positively correlated with individual variation in nicotine addiction severity, insula and ACC responses to smoking vs. neutral cues distinguished smokers who relapsed from those who maintained abstinence [22]. In addition, greater insula reactivity to negative emotional stimuli was associated with more severe smoking [23]. Studies have also implicated the insula and ACC in impulse control dysfunction and other externalizing and internalizing traits in nicotine addiction [see 24, 25, 26, 27 for reviews]. In a flanker task, smokers vs. non-smokers showed lower insula and ACC activation and weaker insula connectivity with the prefrontal cortex during incongruent versus congruent trials [28]. Resting-state fMRI studies showed weaker insula connectivity with the ACC, in association with more errors during incongruent trials in a Stroop task, in young adult smokers relative to non-smokers [29, 30]. Regional responses to smoking vs. neutral cues in the inferior, middle, and frontal gyri and ACC were positively correlated with depression severity in smokers abstinent overnight [20]. These findings together suggest intricate roles of the salience network in smoking, externalizing and internalizing behaviors.
With structural brain imaging, studies including meta-analyses have identified gray matter volume (GMV) loss in the insula and ACC in smokers as compared to never smokers [31–38]. Insula GMVs were negatively correlated with the Fagerström Test for Nicotine Dependence (FTND) scores in cigarette smokers [39]. However, another study demonstrated insula volumes in negative correlation with pack-years but positive correlation with FTND scores in cigarette smokers [40]. Further, lesions of the insula disrupted the desire to smoke [41] and led to less severe withdrawal symptoms [42] in human smokers, suggesting a potentially causal role of the insula in generating the urge to smoke [see 21, 43 for reviews]. Thus, although smokers demonstrated smaller insula volumes, lesions of the insula obliterated the desire to smoke. These seemingly contrasting findings suggest that the relationship between insula GMV and craving as well as nicotine addiction severity merits further investigation.
Morphometric studies have also implicated the insula and ACC in externalizing and internalizing behaviors that may conduce to smoking or other substance use [31, 44–46, see 47 for a review]. For instance, lower insula thickness was associated with more externalizing behaviors, and thinner ACC predicted less reduction in externalizing behaviors at one-year follow-up in a sample of community adolescents [48]. Smaller insula volume was associated with more lifetime problem conducts in male adolescents with early-onset conduct disorder [49]. As compared to never smokers, current smokers showed smaller ACC GMV and greater depression severity [31]. Relative to controls, patients with heroin dependence showed smaller insula and ACC GMVs as well as higher depression and anxiety scores [46].
In the current study, we aimed to examine how insula and ACC GMVs differed between heavy, light, and never smokers and to relate insula and ACC GMVs to years of smoking, nicotine addiction severity, as well as externalizing and internalizing behaviors. We addressed these aims using the data curated from the Human Connectome Project (HCP). We employed voxel-based morphometry to estimate the insula and ACC GMVs in heavy, light, and never smokers and controlled for age, sex, years of education, alcohol use and total intracranial volume in data analyses.
2. Materials and methods
2.1. Dataset: subjects and assessments
In the S1200 release, the HCP contains clinical, behavioral, and 3T magnetic resonance (MR) imaging data of 1206 young adults (1113 with structural MR scans). The HCP participants were without severe neurodevelopmental, neuropsychiatric or neurologic disorders. A total of 330 participants reported lifetime history of smoking - smoking cigarettes on at least 100 separate occasions. Nicotine addiction severity was assessed with the Fagerström Test for Nicotine Dependence (FTND), with a score ranging from 0 to 10 and a higher score indicating more severe dependence [50]. In the HCP FTND scores > 6 were re-coded as 6 (n = 26). The current study included 83 heavy smokers with an FTND score > 3 (indicative of dependence; [51]). However, 13 of the 83 smokers were excluded from further analyses because of excessive head movement (translation > 2 mm or rotation > 2° in any dimension) during the scans or problematic image quality after normalization (n = 11), or missing measures of alcohol use (n = 2). Therefore, the heavy-smoker group comprised 70 participants (29 women). We also included a group of 209 light smokers (97 women) with an FTND score ≤ 3. In the HCP, years of smoking were recorded as 5 (1–5 years), 10 (6–10 years), 15 (11–15 years), or 18 (16 + years). A total of 635 participants never smoked. We selected a largest possible number of never smokers that matched the smoker groups in mean age, sex composition, and alcohol consumption, as indexed by the number of drinks consumed daily over the prior 12 months, in order to control for the effects of alcohol use [52]. The sample size of never smokers was 209 (80 women).
Table 1 shows age, sex, years of education, average daily number of drinks in the prior 12 months, years of smoking, FTND score, and total intracranial volume (TIV) for heavy, light, and never smokers. The three groups showed significant pair-wise differences in the years of education (p’s < 0.001).
Table 1.
Clinical characteristics of heavy, light, and never smokers.
| Heavy smokers(n = 70) | Light smokers(n = 209) | Never smokers(n = 209) | |
|---|---|---|---|
| Age (years) | 29.79 ± 3.05 | 29.27 ± 3.79 | 29.54 ± 3.41 |
| Sex (M/F) | 41/29 | 112/97 | 129/80 |
| Education (years) | 13.60 ± 1.87 | 14.49 ± 1.84 | 15.11 ± 1.74 |
| Daily # drinks/prior year | 3.26 ± 1.71 | 2.93 ± 1.71 | 2.98 ± 1.41 |
| FTND score | 4.83 ± 0.88 | 1.14 ± 1.11 | / |
| Years of smoking | 15.14 ± 3.34 | 13.00 ± 4.19 | / |
| TIV (ccm) | 1511 ± 209 | 1507 ± 176 | 1540 ± 182 |
| Extn score | 12.23 ± 8.74 | 10.06 ± 7.32 | 8.38 ± 6.36 |
| Intn score | 14.44 ± 12.56 | 10.34 ± 7.36 | 9.24 ± 8.59 |
Note: Values are mean ± SD. M: males; F: females. FTND scores > 6 were re-coded as 6. Years of smoking were re-coded as 5 (1–5 years), 10 (6–10 years), 15 (11–15 years), or 18 (16 + years). FTND scores and years of smoking were not available for never smokers. TIV: total intracranial volume; Extn: externalizing score; Intn: internalizing score. The three groups were matched in age, sex, and average daily number of drinks in the prior 12 months, but not in the years of education. Two-sample t tests showed significantly higher FTND scores (p < 0.001) and longer years of smoking (p < 0.001) in heavy smokers as compared to light smokers. The three groups did not differ in the TIV. With age, sex, years of education, and average daily number of drinks as covariates, univariate ANCOVA showed significant differences in the Extn (F = 5.63, p = 0.004) and Intn scores (F = 7.67, p = 0.001) across groups. Post-hoc multiple comparisons showed significantly higher Extn scores in heavy smokers as compared to never smokers (p = 0.005) and significantly higher Intn scores in heavy smokers than light smokers (p = 0.003) and never smokers (p’s < 0.001).
Participants were assessed with the Achenbach Adult Self Report (ASR), a part of the Achenbach System of Empirically Based Assessment (ASEBA) taxonomy [53], with the ASR_Extn_Raw score (Extn) and ASR_Intn_Raw score (Intn) quantifying the extent of externalizing (i.e., aggressive behavior, rule-breaking behavior, and intrusiveness) and internalizing (i.e., anxious/depressed, withdrawn, and somatic complaints) behaviors/problems, respectively. Higher scores indicate more symptoms. We performed one-way ANOVA to examine whether heavy, light, and never smokers differed in externalizing and internalizing behaviors with age, sex, average daily number of drinks, and years of education as covariates.
2.2. MRI protocol and voxel-based morphometry
A customized 3T Siemens Connectome Skyra with a standard 32-channel Siemens receiver head coil and a body transmission coil was used in the MRI scanning. T1-weighted high-resolution structural images were acquired using a 3D MPRAGE sequence with 0.7 mm isotropic resolution (FOV = 224 × 224 mm, matrix = 320 × 320, 256 sagittal slices, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, FA = 8°).
We used voxel-based morphometry (VBM) to estimate the GMVs of brain regions with the CAT12 (Version 12.7) toolbox and followed the suggested defaults of the CAT12 manual [54; http://dbm.neuro.unijena.de/vbm]: 1) individuals’ structural images were spatially normalized to the same stereotactic space; 2) the normalized images were segmented into gray matter, white matter, and cerebrospinal fluid; and 3) the gray matter (GM) images were smoothed. Specifically, a spatial adaptive non-local means (SANLM) denoising filter was first applied to the initial voxel-based processing [55], followed by internal resampling to accommodate low-resolution images and anisotropic spatial resolutions. Subsequently, data were processed by bias-correction and affine-registration, followed by the unified segmentation [56]. After segmentation, the brain was parcellated into left and right hemisphere, subcortical areas, and cerebellum by skull-stripping. A local intensity transformation of all tissue classes was performed to reduce the effects of higher GM intensities in the motor cortex, basal ganglia, or occipital lobe, followed by adaptive maximum a posteriori segmentation with partial volume estimation [57]. The tissue segments were then spatially normalized to a common reference space using DARTEL registrations [58]. The GM maps were smoothed by convolution with an isotropic Gaussian kernel (FWHM = 8 mm). Data quality was checked by using the modules of display slices and VBM data homogeneity in the CAT12.
2.3. Group statistics of clinical metrics and GMVs
In group statistics on the clinical measures, we first performed a 3 (heavy, light, never smokers) by 2 (men, women) analysis of covariance (ANCOVA) with age, average daily number of drinks, and years of education as covariates. If we did not observe a significant group-by-sex interaction in the ANCOVA, we combined men and women and performed univariate ANCOVA to examine the differences between heavy, light, and never smokers, with age, sex, average daily number of drinks, and years of education as covariates.
In group statistics on the GMVs, we performed a 3 (heavy, light, never smokers) by 2 (men, women) ANCOVA for the whole brain, with age, average daily number of drinks, years of education as well as the total intracranial volume (TIV) as covariates. We evaluated the results at voxel p < 0.001, uncorrected, in combination with cluster p < 0.05, corrected for family-wise error (FWE) of multiple comparisons, on the basis of Gaussian random field theory as implemented in SPM, following the reporting standards [59]. If the ANCOVA did not show clusters with significant group-by-sex interaction, we combined men and women and performed a one-way ANOVA for the whole brain with the same covariates.
For the brain regions that showed significant group differences in GMVs from whole-brain analyses, we computed the regional GMVs for individual subjects and performed linear correlations with clinical measures. For the groups of heavy and light smokers separately, we performed Pearson’s correlations on the regional GMVs with years of smoking and FTND score. For all three groups separately, we performed Pearson’s correlations between the regional GMVs and externalizing/internalizing traits. All these correlations were computed with age, sex, years of education, average daily number of drinks, and TIV as covariates. Slope tests were used to confirm the group differences in the correlations [60].
The insula can be parcellated into functionally distinct subregions: dorsal-anterior (dAI), ventral-anterior (vAI), and mid-posterior (PI) insula [61]. To examine whether the subregions were distinguishable in the differences in GMVs between smoking groups, we computed the GMVs for the insula subregions for regions of interest (ROI) analyses. In addition, we performed ROI analyses on the ACC and insula thickness data, including left/right insula, left/right rostral ACC, and left/right caudal ACC, as provided by the HCP. We used univariate ANCOVA on each volumetric measure to examine group differences, with age, sex, years of education, average daily number of drinks, and TIV as covariates. If the group effect was significant, we further performed post-hoc comparisons and evaluated the results with Bonferroni correction.
3. Results
3.1. Clinical measures
We first examined potential influences of sex on clinical measures. With age, years of education, and average daily number of drinks as covariates, a 3 (heavy, light, never smokers) by 2 (men, women) ANCOVA did not showed a significant group-by-sex interaction for externalizing (F = 1.39, p = 0.249) or internalizing (F = 0.41, p = 0.665) scores. Thus, men and women were combined in covariance analyses. With age, sex, years of education, and average daily number of drinks as covariates, univariate ANCOVA showed significant differences in externalizing (F = 5.63, p = 0.004) and internalizing scores (F = 7.67, p = 0.001) across smoking groups. Post-hoc comparisons showed significantly higher externalizing scores in heavy (12.23 ± 8.74) vs. never (8.38 ± 6.36) smokers (p = 0.005) but no significant differences between light (10.06 ± 7.32) and heavy (p = 0.324) or between light and never (p = 0.089) smokers. Heavy smokers (14.44 ± 12.56) showed significantly higher Intn scores than light (10.34 ± 7.36; p = 0.003) and never (9.24 ± 8.59; p < 0.001) smokers, but the internalizing scores of light and never smokers were not significantly different (p = 1.000). These measures are shown in Table 1.
Furthermore, with age, sex, years of education and average daily number of drinks as covariates, pair-wise Pearson’s regressions did not reveal a significant correlation of externalizing and internalizing scores with years of smoking or FTND score in heavy smokers (p’s ≥ 0.348). Without considering multiple comparisons, light smokers showed greater externalizing scores in link with longer smoking years (r = 0.14, p = 0.043) but no other significant correlations (p’s ≥ 0.095).
3.2. Regional GMVs: whole-brain analyses
With age, years of education, average daily number of drinks, and TIV as covariates, a 3 (heavy, light, never smokers) by 2 (men, women) ANCOVA did not show any clusters with a significant group-by-sex interaction. In men and women combined, voxel-wise covariance analysis with age, sex, years of education, average daily number of drinks, and TIV as covariates showed significant differences in a cluster of insula/inferior frontal gyrus, pars orbitalis (cluster size k = 616, MNI coordinates x = 46, y = 32, z = 9, peak Z = 4.19) at voxel level p < 0.001 uncorrected. We followed with pair-wise group comparisons and evaluated the results at voxel p < 0.001, uncorrected, in combination with a cluster p < 0.05, corrected for FWE of multiple comparisons. It showed smaller GMV in heavy vs. never smokers in a cluster of insula/superior temporal gyrus (insula/STG; cluster size k = 1565, MNI coordinates x = −42, y = −15, z = −14, peak Z = 4.30; Fig. 1 A – left panel) and a cluster of the dorsal anterior cingulate cortex/supplementary motor area (dACC/SMA; cluster size k = 1244, MNI coordinates x = −18, y = 21, z = 28, peak Z = 4.27; Fig. 1 B – left panel). No clusters showed GMV differences in other group contrasts. We computed the insula/STG and dACC/SMA GMVs of individual subjects for post-hoc analyses.
Fig. 1.
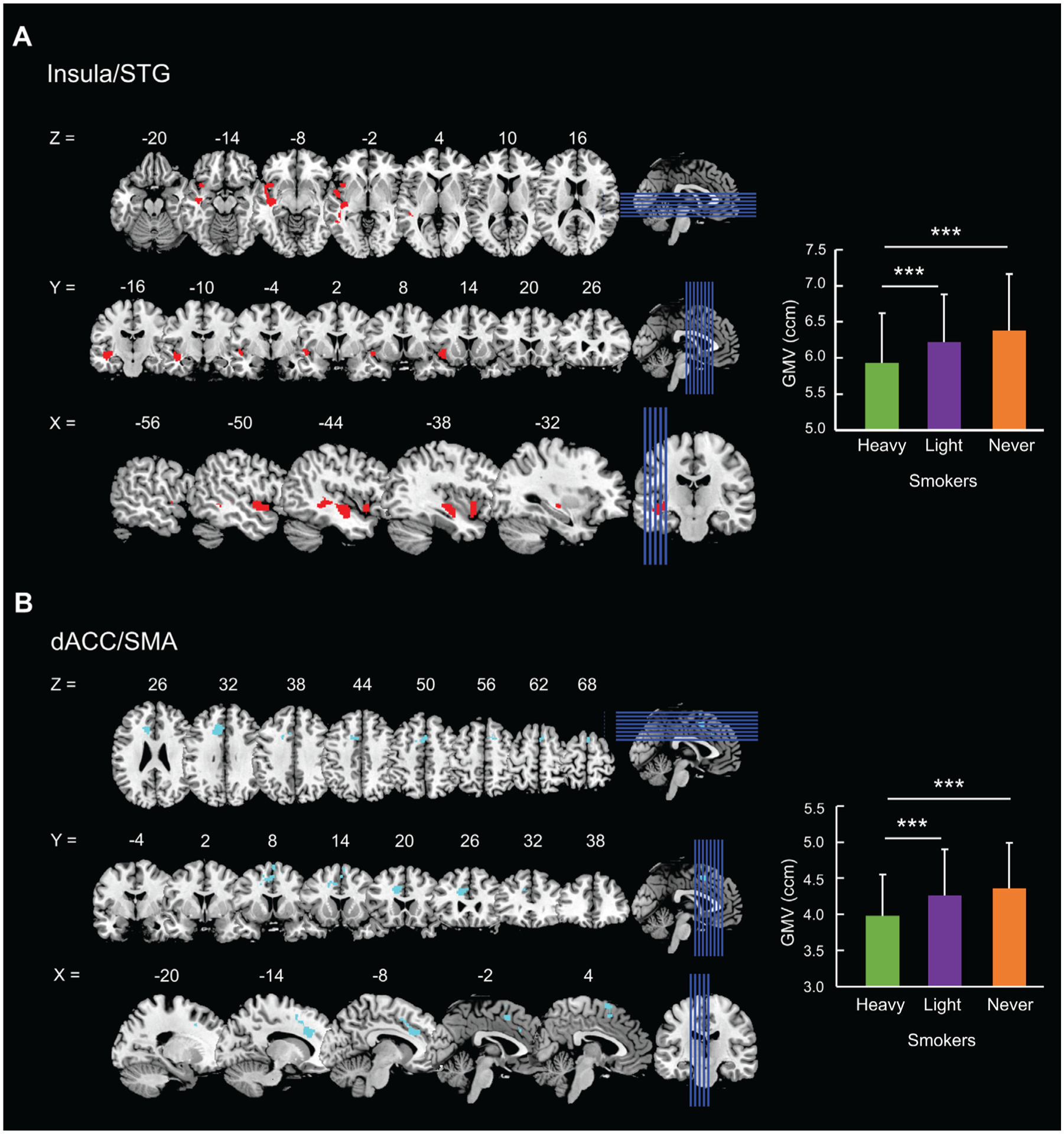
Whole-brain analysis identified the (A) insula/superior temporal gyrus (insula/STG) and (B) dorsal anterior cingulate cortex/supplementary motor area (dACC/SMA) showing smaller GMVs (left) in heavy vs. never smokers. We computed the GMVs and visualized the group differences in bars (right; error bar = SD). * * * p < 0.001 (Bonferroni correction for post-hoc multiple comparisons).
The ANCOVA did not show a significant group-by-sex interaction effect for insula/STG (F = 0.71, p = 0.494) or dACC/SMA (F = 0.73, p = 0.481) GMVs. With age, sex, years of education, average daily number of drinks, and TIV as covariates, univariate ANOVA showed significant differences in the insula/STG GMV across groups (F = 12.68, p < 0.001). As shown in Fig. 1 A (right panel), post-hoc comparisons showed significantly lower insula/STG GMV in heavy (5.93 ± 0.69 ccm) vs. light (6.22 ± 0.66) smokers (p < 0.001) and vs. never (6.38 ± 0.78) smokers (p < 0.001) and showed no significant differences between light and never smokers (p = 0.054). With the same set of covariates, univariate ANOVA showed significant differences in the dACC/SMA GMV across groups (F = 11.87, p < 0.001). As shown in Fig. 1 B (right panel), post-hoc comparisons showed significantly lower dACC/SMA GMV in heavy (3.98 ± 0.57) vs. light (4.26 ± 0.64) smokers (p < 0.001) and vs. never (4.36 ± 0.63) smokers (p < 0.001) but showed no significant differences between light and never smokers (p = 0.617).
3.3. Relationships between regional GMVs and clinical measures
The correlations of insula/STG and dACC/SMA GMVs with clinical measures are summarized in Table 2. As shown in Fig. 2 A and 2 B, regional GMVs were not significantly correlated with either years of smoking or FTND scores in heavy or light smokers (p’s ≥ 0.078). As shown in Fig. 2 C and 2 D, insula GMV was not significantly correlated with externalizing or internalizing scores (p’s ≥ 0.161) in any group. The GMV of the dACC/SMA was positively correlated with both externalizing (r = 0.36, p = 0.003) and internalizing (r = 0.30, p = 0.017) scores in heavy smokers but not in light or never smokers (p’s ≥ 0.220). Slope tests showed that the correlations between the dACC/SMA GMV and externalizing score in heavy smokers was significantly stronger than in never smokers (T = 2.30, p = 0.022).
Table 2.
Correlations of insula/STG and dACC/SMA GMVs with clinical measures.
| Insula/STG GMV | dACC/SMA GMV | ||||||
|---|---|---|---|---|---|---|---|
| Correlation | Slope test | Correlation | Slope test | ||||
| r | P | T value (p) | r | P | T value (p) | ||
| Years of smoking | Heavy | −0.22 | 0.078 | 1.26 (0.209) | −0.01 | 0.918 | 0.52 (0.606) |
| Light | −0.02 | 0.818 | −0.06 | 0.382 | |||
| FTND score | Heavy | 0.19 | 0.137 | 1.54 (0.124) | 0.05 | 0.696 | 0.19 (0.852) |
| Light | −0.02 | 0.740 | 0.06 | 0.383 | |||
| Extn | Heavy | 0.07 | 0.578 | H vs. L: 0.15 (0.883) | 0.36 | 0.003** | H vs. L: 1.94 (0.053) |
| Light | 0.10 | 0.161 | L vs. N:0.17 (0.868) | 0.04 | 0.622 | L vs. N:0.37 (0.709) | |
| Never | 0.07 | 0.306 | N vs. H: 0.003 (0.998) | 0.01 | 0.880 | N vs. H: 2.30 (0.022*) | |
| Intn | Heavy | 0.13 | 0.307 | H vs. L: 0.75 (0.455) | 0.30 | 0.017* | H vs. L: 0.62 (0.533) |
| Light | 0.02 | 0.791 | L vs. N: 0.21 (0.834) | 0.09 | 0.220 | L vs. N: 1.03 (0.302) | |
| Never | −0.01 | 0.844 | N vs. H: 0.90 (0.369) | −0.01 | 0.866 | N vs. H: 1.71 (0.089) | |
Note: dACC/SMA: dorsal anterior cingulate cortex/supplementary motor area; STG: superior temporal gyrus; GMV: gray matter volume; H: heavy smokers; L: light smokers; N: never smokers; Extn: Externalizing score; Intn: Internalizing score.
p < 0.005.
p < 0.05.
Fig. 2.
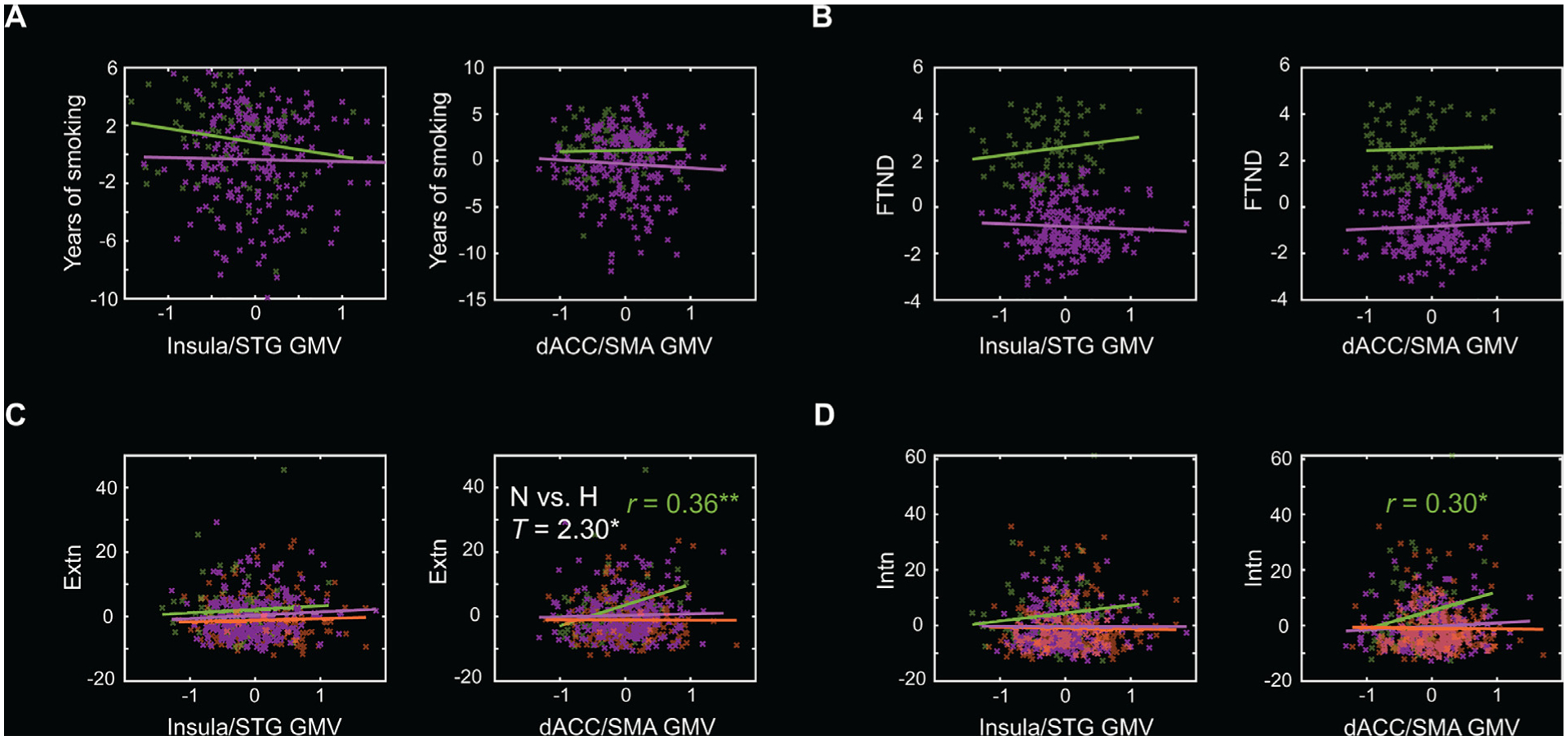
Scatter plots to show the correlations of insula/STG GMV and dACC/SMA GMV with (A) years of smoking, (B) FTND score, (C) externalizing (Extn), and (D) internalizing (Intn) score, with age, sex, average daily number of drinks, years of education, and TIV as covariates for the three groups separately. There were no data of years of smoking or FTND scores for never smokers. Residuals are presented here. Solid lines represent the regressions. Heavy (H) smokers: green; Light smokers: purple; Never (N) smokers: orange. Significant r and slope test T values are marked with * * p < 0.005, or * p < 0.05 (two-tailed).
3.4. Subregional GMVs of insula and thickness of insula and ACC
Subregional GMVs of the insula and the statistics of the ANCOVA are presented in Supplementary Table S1. All group effects for the subregional GMVs were significant (Supplementary Figure S1). For the dorsal-anterior insula, heavy smokers (14.50 ± 1.55 ccm) showed significantly lower GMV than light (14.94 ± 1.53; p = 0.011) and never (15.13 ± 1.70; p = 0.024) smokers, but there was no significant difference between light and never smokers (p = 1.000). For the ventral-anterior insula, heavy smokers (4.82 ± 0.56) showed significantly lower GMV than never (5.06 ± 0.61; p = 0.017) but not light (4.97 ± 0.53; p = 0.077) smokers; there was no significant difference between light and never smokers (p = 1.000). For the mid-posterior insula, heavy smokers (11.16 ± 1.31) showed significantly lower GMV than light (11.52 ± 1.24; p = 0.014) and never (11.61 ± 1.37; p = 0.031) smokers, but there was no significant difference between light and never smokers (p = 1.000).
The dorsal-anterior insula GMV was negatively correlated with years of smoking (r = −0.30, p = 0.017), but positively correlated with the FTND score in heavy smokers (r = 0.30, p = 0.016). The ventral-anterior insula GMV was positively correlated with externalizing scores in light smokers (r = 0.15, p = 0.030). Slope tests showed that none of these correlations differed between groups. None of the other correlations between subregional GMVs and clinical measures was significant. These results are summarized in Supplementary Table S2.
The thickness of the insula and the statistics of the ANCOVA are presented in Supplementary Table S3. The smoker group effect was significant only for the left insula (F = 4.59, p = 0.011). Post-hoc comparisons showed that the left insula thickness was greater in never (3.08 ± 0.13 mm) as compared to heavy (3.02 ± 0.16 mm; p = 0.035) and light (3.04 ± 0.14 mm; p = 0.043) smokers, but there were no significant differences between heavy and light smokers (p = 1.000). With age, sex, years of education, and average daily number of drinks as covariates, the left insula thickness was not significantly correlated with years of smoking or FTND scores in heavy or light smokers or with externalizing or internalizing scores in any smoker group.
4. Discussion
Smokers relative to never smokers showed smaller GMVs of both the insula and dACC/SMA, consistent with prior reports of GMV deficits in the salience network [31–37]. Insula GMV was negatively correlated with years of smoking in heavy smokers, though only at trend-level significance (p = 0.078). The dACC/SMA but not insula GMV was positively correlated with externalizing and internalizing scores in heavy smokers. These findings suggest potentially differentiable roles of the insula and dACC/SMA in nicotine addiction. We discuss the main findings in the below.
4.1. Insula and dACC/SMA GMVs vs. years of smoking and FTND score
Insula volumes were negatively correlated with years of smoking, suggesting that the volumetric deficits may result directly from the effects of cigarette smoking [39, 62]. A previous study also demonstrated loss of insula volume in alcohol dependent individuals, half of whom were smokers, relative to healthy controls [63]. In fact, reduction of insula volumes has been noted in alcohol, cocaine, and methamphetamine addiction, all frequently comorbid with nicotine use disorders [64]. In animal studies, chronic nicotine administration resulted in neuronal loss [65, see 66, 67 for reviews, 68] and diminished ACC-striatal functional connectivity [69], in broad accord with the current findings. It should be noted that studies have also employed other morphometric measures, such as gray matter density (GMD) and cortical thickness, which are not commensurate with GMV [70, 71]. Thus, whereas studies consistently found lower insula and/or ACC GMV in smokers, the findings were at odds for GMD [32, 34, 38].
Although previous studies have implicated the insula in drug craving and addiction severity [64, 72], we did not find insula volumes in significant correlation with FTND score in heavy or light smokers. An earlier VBM study of cigarette smokers demonstrated insula volumes in negative correlation with pack-years but positive correlation with FTND scores across subjects [40]. In methamphetamine users, insula GMV showed a positive correlation with subjectively reported craving [73]. Although the latter study did not include non-drug using controls for contrasts, an earlier work of the ENIGMA Addiction Consortium demonstrated reduction of insula GMV in methamphetamine, cocaine and other substance use disorders [SUD; 74]. Thus, it is possible that insula GMV is positively associated with craving across multiple SUDs. Again, studies employing other morphometric markers, including GMD and cortical thickness, did not yield consistent findings [75].
Considered along with studies showing that lesions of the insula virtually abolished craving to smoke [41, 42], the seemingly discrepant findings of insula GMVs in negative correlation with years of smoking in the current study but positive correlation with FTND score in Peng et al. [40] may suggest two non-exclusive possibilities. First, insula supports craving, and smokers who sustained less neurotoxic effects on the insula would demonstrate higher craving and nicotine addiction severity. Second, as a compensatory process, smokers who exhibited larger volumes are more vulnerable to craving and nicotine dependence. The literature of neuropsychology provides evidence for these compensatory processes. For instance, the ventromedial prefrontal cortex (vmPFC) regulates physiological arousal, with higher vmPFC activity associated with dampened skin conductance responses [76, 77]. On the other hand, lesions of the vmPFC led to diminished arousal elicited by affective stimuli [see 78, 79 for reviews], akin to insula lesions abolishing craving to smoke. More broadly, in a recent study of cocaine users, exposure to drug vs. neutral cues elicited “deactivation” of bilateral parahippocampal gyri (PHG); however, less deactivation of PHG response to drug vs. neutral cues was associated with higher chronic cocaine craving [80]. It was posited that chronic cocaine exposure led to diminished response to saliency and those who engaged functional compensation, though illadaptively, exhibited more intense drug craving. These findings together suggest a complex, likely age- or chronicity-related relationships between nicotine consumption, insula volumetrics, and craving/addiction severity, which can probably only be resolved with longitudinal studies.
Although smokers demonstrated loss of dACC/SMA volumes, as compared to non-smokers, dACC/SMA volumes were not correlated with years of nicotine use or FTND scores. These findings suggest a lesser role of the dACC/SMA in supporting craving and addiction severity. A recent meta-analysis showed diminished dACC/SMA volumes in individuals with behavioral addictions vs. healthy controls, suggesting a broader role of these medial prefrontal cortical structures in the pathophysiology of addiction [81], although the study did not control for cigarette smoking. Likewise, longitudinal research with within-subject analyses would confirm whether smaller dACC/SMA dispose individuals to rather than reflecting the consequences of smoking.
4.2. Insula and dACC/SMA GMVs vs. externalizing/internalizing scores
Heavy smokers showed more externalizing symptoms as compared to never smokers, in line with previous findings of impulsive and sensation seeking behaviors both from questionnaires [82] and laboratory assessments [83–85]. Heavy and light smokers also showed more internalizing symptoms, consistent with comorbidity of nicotine use disorder and depression and anxiety [see 86, 87 for reviews]. In incentive learning theory, negative emotional states promote cigarette smoking over alternative actions [see 88 for a review], although mood symptoms may also reflect consequences of nicotine addiction [89–91].
The dACC/SMA but not insula volumes were positively correlated with both externalizing and internalizing symptom severity in heavy smokers. This finding contrasts with earlier reports of ACC volumetric deficits and more impulsive and risk-seeking behaviors as well as higher rates of depression in drug addicted individuals [31, 92], and of inverse correlations of ACC GMV with impulsivity [93] and of SMA GMV with “worry ” severity [94] in healthy individuals. Moreover, smaller dACC GMV was associated with higher depression and anxiety scores in a group of participants comprising both heroin users and healthy controls [46]. However, other studies showed that impulsivity as measured with Barratt Impulsivity Scale-11 was positively correlated with the ACC GMV in healthy young adults [95] and high-impulsivity smokers with greater ACC volume may be more sensitive to craving [21].
We did not observe a significant relationship between insula GMVs with externalizing or internalizing scores. In an earlier VBM study, patients with severe substance use and conduct problems showed smaller insula GMV as compared to controls; however, smaller insula GMV was associated with lower impulsivity as measured by a composite score of disinhibited behaviors in the patients [96]. In contrast, another study showed that, although not different from healthy controls’, insula volumes were negatively correlated with impulsivity in teenagers with borderline personality disorders [97]. In adolescents, thinner left insular cortex was associated with higher externalizing behaviors, and thinner left ACC predicted less reduction in externalizing behaviors one year later [48]. In animal work, impulsivity was predicted by the thinness of the insular cortex in rats [98]. Here, we also observed thinner left insula in heavy and light smokers but not a correlation of insula thickness with clinical measures. Thus, the findings appear to be mixed in how insula volumes may relate to impulsivity. More research is warranted to investigate how structural brain changes underlie the comorbidity of substance use and externalizing and internalizing behaviors.
4.3. Limitations of the study and conclusions
A few limitations should be considered for the current study. First, smokers in the HCP are young adults (ages 22–35) with only 31% reporting an FTND score ≥ 6, suggesting they are at an early stage of nicotine addiction. Further, it should also be noted that we did not correct for multiple comparisons in evaluating the correlation of GMVs with clinical variables. The current findings thus need to be considered preliminary. Second, prolonged alcohol use has detrimental effects on brain structure and functions [see 99 for a review]. The HCP data set does not provide a measure of life-time alcohol use, and we used average daily number of drinks over the prior 12 months to represent alcohol use severity and as a covariate in data analyses. Nonetheless, we cannot rule out the effects of chronic alcohol exposure on the current findings. Third, as with earlier studies, we reported cross-sectional findings and it remains unclear whether the volumetric deficits precede or reflect the consequences of cigarette smoking. The light smokers showed intermediate levels of insula and ACC GMVs, as compared to heavy and never smokers. This along with the finding of dorsal-anterior insula GMV each in negative and positive correlation with years of smoking and FTND score in heavy smokers seems to suggest volumetric deficits as a consequence of smoking. However, studies with a longitudinal design are needed to evaluate the causal relationships between cerebral structural abnormalities and nicotine use. Fourth, we did not examine sex differences because of the small sample size. Women and men demonstrate differences in neural markers of nicotine addiction [100] and of externalizing and internalizing traits [101–104]. Finally, the insula comprises functionally distinct subregions [61]. Although the insula subregions did not appear distinguishable in terms of GMV differences between heavy and never smokers, more studies are needed to investigate the potentially distinct roles of these subregions in nicotine addiction and its comorbidities.
To conclude, we replicated smaller insula and dACC/SMA GMVs and more severe externalizing and internalizing behaviors in heavy smokers relative to never smokers. Higher insula GMV was potentially associated with nicotine addiction severity and higher dACC/SMA GMVs may contribute to more externalizing and internalizing behaviors in smokers. Our findings support potentially distinct dysfunctional roles of the insula and dACC/SMA in cigarette smoking and suggest insula and dACC/SMA GMVs as important neural markers in the research of the pathophysiology and treatment of nicotine addiction [see 105 for a review].
Supplementary Material
Acknowledgement
The current study is supported by NIH grants DA045189 and DA051922 (C-SRL). The NIH is otherwise not involved in the conceptualization of the study, experimental design, or data analyses, or in the decision to publish the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no competing interests in the current work.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: https://doi.org/10.1016/j.addicn.2021.100003.
References
- [1].Breslau N, et al. , Nicotine dependence in the United States: prevalence, trends, and smoking persistence, Arch. Gen. Psychiatry 58 (9) (2001) 810–816. [DOI] [PubMed] [Google Scholar]
- [2].Grant BF, Shmulewitz D, Compton WM, Nicotine Use and DSM-IV Nicotine Dependence in the United States, 2001–2002 and 2012–2013, Am. J. Psychiatry 177 (11) (2020) 1082–1090. [DOI] [PubMed] [Google Scholar]
- [3].Ng M, et al. , Smoking prevalence and cigarette consumption in 187 countries, 1980–2012, JAMA 311 (2) (2014) 183–192. [DOI] [PubMed] [Google Scholar]
- [4].Turner JR, et al. , Translational research in nicotine dependence, Cold Spring Harb. Perspect. Med 3 (3) (2013) a012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benowitz NL,Nicotine addiction N. Engl. J. Med 362 (24) (2010) 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laviolette SR, van der Kooy D, The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour, Nat. Rev. Neurosci 5 (1) (2004) 55–65. [DOI] [PubMed] [Google Scholar]
- [7].Gray MA, Critchley HD, Interoceptive basis to craving, Neuron 54 (2) (2007) 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ferguson J, et al. , The English smoking treatment services: one-year outcomes, Addiction 100 (Suppl 2) (2005) 59–69. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Rodriguez O, et al. , Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NE-SARC), Drug Alcohol. Depend 132 (3) (2013) 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Piasecki TM, Relapse to smoking, Clin. Psychol. Rev 26 (2) (2006) 196–215. [DOI] [PubMed] [Google Scholar]
- [11].Zhou X, et al. , Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study, Addict. Behav 34 (4) (2009) 365–373. [DOI] [PubMed] [Google Scholar]
- [12].Dakwar E, Popii M, Coccaro EF, Lifetime history of cigarette smoking associated with aggression and impulsivity in both healthy and personality disordered volunteers, J. Pers. Disord 25 (5) (2011) 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fite PJ, et al. , The relation between childhood proactive and reactive aggression and substance use initiation, J. Abnorm. Child Psychol 36 (2) (2008) 261–271. [DOI] [PubMed] [Google Scholar]
- [14].Morrell HER, Cohen LM, Cigarette Smoking, Anxiety, and Depression, J. Psychopathol. Behav. Assess 28 (4) (2006) 281–295. [Google Scholar]
- [15].Acton GS, Measurement of impulsivity in a hierarchical model of personality traits: implications for substance use, Subst. Use Misuse 38 (1) (2003) 67–83. [DOI] [PubMed] [Google Scholar]
- [16].Doran N, et al. , Impulsivity and cigarette craving: differences across subtypes, Psychopharmacology (Berl) 207 (3) (2009) 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stubbs B, et al. , Association between depression and smoking: A global perspective from 48 low- and middle-income countries, J. Psychiatr. Res 103 (2018) 142– 149. [DOI] [PubMed] [Google Scholar]
- [18].Chan Y−F, Dennis ML, Funk RR, Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment, J. Subst. Abuse Treat 34 (1) (2008) 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Claus ED, et al. , Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity, Neuropsychopharmacology 38 (12) (2013) 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kushnir V, et al. , Enhanced smoking cue salience associated with depression severity in nicotine-dependent individuals: a preliminary fMRI study, Int. J. Neuropsychopharmacol 16 (5) (2013) 997–1008. [DOI] [PubMed] [Google Scholar]
- [21].Garavan H, Insula and drug cravings, Brain Struct. Funct 214 (5–6) (2010) 593–601. [DOI] [PubMed] [Google Scholar]
- [22].Janes AC, et al. , Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings, Drug Alcohol. Depend. 179 (2017) 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dias NR, Peechatka AL, Janes AC, Insula reactivity to negative stimuli is associated with daily cigarette use: A preliminary investigation using the Human Connectome Database, Drug Alcohol. Depend 159 (2016) 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bechara A, Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective, Nat. Neurosci 8 (11) (2005) 1458–1463. [DOI] [PubMed] [Google Scholar]
- [25].Namkung H, Kim SH, Sawa A, The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology, Trends Neurosci. 40 (4) (2017) 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Naqvi NH, Bechara A, The hidden island of addiction: the insula, Trends Neurosci. 32 (1) (2009) 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paulus MP, Stein MB, An insular view of anxiety, Biol. Psychiatry 60 (4) (2006) 383–387. [DOI] [PubMed] [Google Scholar]
- [28].Fedota JR, et al. , Insula Demonstrates a Non-Linear Response to Varying Demand for Cognitive Control and Weaker Resting Connectivity With the Executive Control Network in Smokers, Neuropsychopharmacology 41 (10) (2016) 2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Y, et al. , The implication of salience network abnormalities in young male adult smokers, Brain Imaging Behav. 11 (4) (2017) 943–953. [DOI] [PubMed] [Google Scholar]
- [30].Bi Y, et al. , Altered resting state functional connectivity of anterior insula in young smokers, Brain Imaging Behav. 11 (1) (2017) 155–165. [DOI] [PubMed] [Google Scholar]
- [31].Fritz HC, et al. , Current smoking and reduced gray matter volume-a voxel-based morphometry study, Neuropsychopharmacology 39 (11) (2014) 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gallinat J, et al. , Smoking and structural brain deficits: a volumetric MR investigation, Eur. J. Neurosci 24 (6) (2006) 1744–1750. [DOI] [PubMed] [Google Scholar]
- [33].Hanlon CA, et al. , Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers, Addict. Biol 21 (1) (2016) 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brody AL, et al. , Differences between smokers and nonsmokers in regional gray matter volumes and densities, Biol. Psychiatry 55 (1) (2004) 77–84. [DOI] [PubMed] [Google Scholar]
- [35].Liao Y, et al. , Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study, Addict. Biol 17 (6) (2012) 977–980. [DOI] [PubMed] [Google Scholar]
- [36].Pan P, et al. , Chronic smoking and brain gray matter changes: evidence from meta–analysis of voxel-based morphometry studies, Neurol. Sci 34 (6) (2013) 813–817. [DOI] [PubMed] [Google Scholar]
- [37].Yang Z, et al. , Meta-analysis of brain gray matter changes in chronic smokers, Eur. J. Radiol 132 (2020) 109300. [DOI] [PubMed] [Google Scholar]
- [38].Zhang X, et al. , Factors underlying prefrontal and insula structural alterations in smokers, Neuroimage 54 (1) (2011) 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang C, et al. , Gray matter volumes of insular subregions are not correlated with smoking cessation outcomes but negatively correlated with nicotine dependence severity in chronic smokers, Neurosci. Lett 696 (2019) 7–12. [DOI] [PubMed] [Google Scholar]
- [40].Peng P, et al. , Brain Structure Alterations in Respect to Tobacco Consumption and Nicotine Dependence: A Comparative Voxel-Based Morphometry Study, Front. Neuroanat 12 (2018) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Naqvi NH, et al. , Damage to the insula disrupts addiction to cigarette smoking, Science 315 (5811) (2007) 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Abdolahi A, et al. , Damage to the insula leads to decreased nicotine withdrawal during abstinence, Addiction 110 (12) (2015) 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Naqvi NH, Bechara A, The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making, Brain Struct. Funct 214 (5–6) (2010) 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Blondino CT, et al. , The Association between Internalizing and Externalizing Severity with Current Use of Cigarettes, E-cigarettes, and Alcohol in Adults: Wave 1 of the Population Assessment of Tobacco and Health (PATH) Study, Addict. Behav (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pedersen MU, et al. , Externalizing behavior problems are related to substance use in adolescents across six samples from Nordic countries, Eur. Child Adolesc. Psychiatry 27 (12) (2018) 1551–1561. [DOI] [PubMed] [Google Scholar]
- [46].Lin WC, et al. , Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study, Psychiatry Res. 201 (2) (2012) 89–97. [DOI] [PubMed] [Google Scholar]
- [47].Potvin S, et al. , Cigarette Cravings, Impulsivity, and the Brain, Front. Psychiatry 6 (2015) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tanzer M, et al. , Cortical thickness of the insula and prefrontal cortex relates to externalizing behavior: Cross-sectional and prospective findings, Dev. Psychopathol (2020) 1–11. [DOI] [PubMed] [Google Scholar]
- [49].Fairchild G, et al. , Brain structure abnormalities in early-onset and adolescent-onset conduct disorder, Am. J. Psychiatry 168 (6) (2011) 624–633. [DOI] [PubMed] [Google Scholar]
- [50].Heatherton TF, et al. , The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire, Br. J. Addict 86 (9) (1991) 1119– 1127. [DOI] [PubMed] [Google Scholar]
- [51].de Meneses-Gaya C, et al. , Psychometric qualities of the Brazilian versions of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index, Nicotine Tob. Res 11 (10) (2009) 1160–1165. [DOI] [PubMed] [Google Scholar]
- [52].Chou SP, et al. , The epidemiology of DSM-5 nicotine use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III, J. Clin. Psychiatry 77 (10) (2016) 1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Achenbach TM, Rescorla L, Manual For the ASEBA Adult Forms & Profiles, University of Vermont, Burlington, VT, 2003. [Google Scholar]
- [54].Gaser C, Dahnke R, CAT-a computational anatomy toolbox for the analysis of structural MRI data, HBM 2016 (2016) 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Manjón JV, et al. , Adaptive non-local means denoising of MR images with spatially varying noise levels, J. Magn. Reson. Imaging 31 (1) (2010) 192–203. [DOI] [PubMed] [Google Scholar]
- [56].Ashburner J, Friston K, Unified segmentation, Neuroimage 26 (3) (2005) 839–851. [DOI] [PubMed] [Google Scholar]
- [57].Tohka J, Zijdenbos A, Evans A, Fast and robust parameter estimation for statistical partial volume models in brain MRI, Neuroimage 23 (1) (2004) 84–97. [DOI] [PubMed] [Google Scholar]
- [58].Ashburner J, A fast diffeomorphic image registration algorithm, Neuroimage 38 (1) (2007) 95–113. [DOI] [PubMed] [Google Scholar]
- [59].Poldrack RA, et al. , Guidelines for reporting an fMRI study, Neuroimage 40 (2) (2008) 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zar JH, Biostatistical Analysis, Pearson Education; India, 1999. [Google Scholar]
- [61].Kelly C, et al. , A convergent functional architecture of the insula emerges across imaging modalities, Neuroimage 61 (4) (2012) 1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nakamura H, et al. , Activation of fronto-limbic system in the human brain by cigarette smoking: evaluated by a CBF measurement, Keio J. Med 49 (2000) A122–A124. [PubMed] [Google Scholar]
- [63].Grodin EN, et al. , Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence, Drug Alcohol. Depend 179 (2017) 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Naqvi NH, et al. , The insula: a critical neural substrate for craving and drug seeking under conflict and risk, Ann. N. Y. Acad. Sci 1316 (2014) 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Abreu-Villaca Y, Seidler FJ, Slotkin TA, Does prenatal nicotine exposure sensitize the brain to nicotine-induced neurotoxicity in adolescence? Neuropsychopharmacology 29 (8) (2004) 1440–1450. [DOI] [PubMed] [Google Scholar]
- [66].Ferrea S, Winterer G, Neuroprotective and neurotoxic effects of nicotine, Pharmacopsychiatry 42 (6) (2009) 255–265. [DOI] [PubMed] [Google Scholar]
- [67].Slotkin TA, Nicotine and the adolescent brain: insights from an animal model, Neurotoxicol. Teratol 24 (3) (2002) 369–384. [DOI] [PubMed] [Google Scholar]
- [68].Abreu-Villaca Y, et al. , Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal, Neuropsychopharmacology 29 (5) (2004) 879–890. [DOI] [PubMed] [Google Scholar]
- [69].Keeley RJ, et al. , Intrinsic differences in insular circuits moderate the negative association between nicotine dependence and cingulate-striatal connectivity strength, Neuropsychopharmacology 45 (6) (2020) 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gennatas ED, et al. , Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adulthood, J. Neurosci 37 (20) (2017) 5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Winkler AM, et al. , Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies, Neuroimage 53 (3) (2010) 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Due DL, et al. , Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging, Am. J. Psychiatry 159 (6) (2002) 954–960. [DOI] [PubMed] [Google Scholar]
- [73].Qi C, et al. , Structural Imaging-Based Biomarkers for Detecting Craving and Predicting Relapse in Subjects With Methamphetamine Dependence, Front. Psychiatry 11 (2021) 599099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mackey S, et al. , Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects, Am. J. Psychiatry 176 (2) (2019) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Morales AM, et al. , Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers, Neuropsychopharmacology 39 (8) (2014) 1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang S, et al. , Ventromedial prefrontal cortex and the regulation of physiological arousal, Soc. Cogn. Affect. Neurosci 9 (7) (2014) 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhang S, et al. , Barratt Impulsivity and Neural Regulation of Physiological Arousal, PLoS One 10 (6) (2015) e0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schneider B, Koenigs M, Human lesion studies of ventromedial prefrontal cortex, Neuropsychologia 107 (2017) 84–93. [DOI] [PubMed] [Google Scholar]
- [79].McCormick C, et al. , Comparing and Contrasting the Cognitive Effects of Hippocampal and Ventromedial Prefrontal Cortex Damage: A Review of Human Lesion Studies, Neuroscience 374 (2018) 295–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang W, et al. , Noradrenergic correlates of chronic cocaine craving: neuromelanin and functional brain imaging, Neuropsychopharmacology 46 (4) (2021) 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Qin K, et al. , Shared gray matter alterations in individuals with diverse behavioral addictions: A voxel-wise meta-analysis, J. Behav. Addict 9 (1) (2020) 44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Balevich EC, Wein ND, Flory JD, Cigarette smoking and measures of impulsivity in a college sample, Subst. Abus 34 (3) (2013) 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Amlung M, et al. , Steep delay discounting and addictive behavior: a meta-analysis of continuous associations, Addiction 112 (1) (2017) 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Green L, Myerson J, On the Complexity of Discounting, Choice Situations, and People, Perspect. Behav. Sci 42 (3) (2019) 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bickel WK, Odum AL, Madden GJ, Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers, Psychopharmacology (Berl.) 146 (4) (1999) 447–454. [DOI] [PubMed] [Google Scholar]
- [86].Fluharty M, et al. , The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review, Nicotine Tob. Res 19 (1) (2017) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moylan S, et al. , How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways, Brain Behav. 3 (3) (2013) 302–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mathew AR, et al. , Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model, Addiction 112 (3) (2017) 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Steuber TL, Danner F, Adolescent smoking and depression: which comes first? Addict. Behav 31 (1) (2006) 133–136. [DOI] [PubMed] [Google Scholar]
- [90].Fergusson DM, Goodwin RD, Horwood LJ, Major depression and cigarette smoking: results of a 21-year longitudinal study, Psychol. Med 33 (8) (2003) 1357. [DOI] [PubMed] [Google Scholar]
- [91].Audrain-McGovern J, et al. , Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol. Depend 103 (3) (2009) 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Qiu YW, et al. , The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals, Neurosci. Lett. 538 (2013) 43–48. [DOI] [PubMed] [Google Scholar]
- [93].Matsuo K, et al. , A voxel-based morphometry study of frontal gray matter correlates of impulsivity, Hum. Brain Mapp. 30 (4) (2009) 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hilbert K, et al. , Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization, Psychiatry Res 234 (3) (2015) 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cho SS, et al. , Morphometric correlation of impulsivity in medial prefrontal cortex, Brain Topogr. 26 (3) (2013) 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dalwani MS, et al. , Female adolescents with severe substance and conduct problems have substantially less brain gray matter volume, PLoS One 10 (5) (2015) e0126368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Takahashi T, et al. , Insular cortex volume and impulsivity in teenagers with first-presentation borderline personality disorder, Prog. Neuropsychopharmacol. Biol. Psychiatry 33 (8) (2009) 1395–1400. [DOI] [PubMed] [Google Scholar]
- [98].Belin-Rauscent A, et al. , Impulsivity is predicted by the thinness of the insular cortex in rats, Mol. Psychiatry 21 (4) (2016) 445. [DOI] [PubMed] [Google Scholar]
- [99].Zahr NM, Kaufman KL, Harper CG, Clinical and pathological features of alcohol-related brain damage, Nat. Rev. Neurol 7 (5) (2011) 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhang S, et al. , Resting-state functional connectivity of the basal nucleus of meynert in cigarette smokers: dependence level and gender differences, Nicotine Tob. Res. 19 (4) (2017) 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Dhingra I, et al. , Sex differences in neural responses to reward and the influences of individual reward and punishment sensitivity, BMC Neurosci. 22 (1) (2021) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ide JS, et al. , Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel-based morphometry study of the ABCD project data, Neuroimage 220 (2020) 117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Li G, et al. , Sex Differences in Neural Responses to the Perception of Social Interactions, Front. Hum. Neurosci 14 (2020) 565132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li G, et al. , Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits, Neuroimage 221 (2020) 117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Menossi HS, et al. , Neural bases of pharmacological treatment of nicotine dependence - insights from functional brain imaging: a systematic review, CNS Drugs 27 (11) (2013) 921–941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


