Abstract
The mesolimbic dopamine system is the primary neural circuit mediating motivation, reinforcement, and reward-related behavior. The activity of this system and multiple behaviors controlled by it are affected by changes in feeding and body weight, such as fasting, food restriction, or the development of obesity. Multiple different peptides and hormones that have been implicated in the control of feeding and body weight interact with the mesolimbic dopamine system to regulate many different dopamine-dependent, reward-related behaviors. In this review, we summarize the effects of a selected set of feeding-related peptides and hormones acting within the ventral tegmental area and nucleus accumbens to alter feeding, as well as food, drug, and social reward.
Keywords: Dopamine, VTA, Feeding, Peptide, Reward, Drugs
Introduction
The mesolimbic dopamine (DA) system is the primary neural circuit mediating motivation, reinforcement, and reward-related behavior. This system consists of DA neurons of the ventral tegmental area (VTA) that project to multiple forebrain areas, including the nucleus accumbens (NAcc) and several other limbic structures. At rest, DA neurons fire tonically in a steady, low-frequency mode setting a stable background dopaminergic tone, but when exposed to unexpected salient stimuli of positive or negative valence DA neurons fire in a high-frequency phasic mode which produces high extracellular DA concentrations at efferent targets [1–3]. Activation of the mesolimbic pathway is highly rewarding and reinforcing. For example, rats readily press a lever to electrically stimulate regions along the mesolimbic pathway [4] and some rats even forgo food and starve to continue self-stimulating [5]. This behavior was later shown to be dependent on DA neurotransmission [6] and decades of investigation have revealed the breadth of behaviors regulated by the mesolimbic DA system including natural and drug rewards, reinforcement, motivation, and aversion, among others [7–11].
The mesolimbic DA system also plays an important role in the control of feeding and is highly sensitive to changes in feeding status and body weight. For example, fasting and prolonged food restriction increase DA system function and increase the reinforcing qualities of, and motivation to obtain, nearly all rewarding substances [12]. In addition, increases in body weight, such as those seen in obesity, alter DA circuit function and multiple DA-dependent behaviors [13–16]. Some of these effects may be due to the actions of feeding-related neuropeptides and hormones on the mesolimbic DA system. Multiple central and peripheral peptides and hormones that are intricately involved in the control of feeding and body weight to either promote (orexigenic) or inhibit feeding (anorexigenic) have been shown to interact with the mesolimbic DA system to regulate multiple behaviors. This includes feeding, the motivational and rewarding aspects of food, as well as drug-reward associated and social behaviors. In this review, we summarize the effects of a selected set of feeding-related peptides and hormones acting within the VTA and NAcc to alter feeding as well as food, drug, and social reward Table 1. provides an overview of the studies showing the effects of these peptides and hormones on food, alcohol, drug, and social behaviors, which are described in detail in the following sections.
Table 1.
Overview of studies showing effects of discussed peptides and hormones on food, alcohol, drug, and social behavior.
| Peptide | Brain area | Manipulation | Behavioral effect | Citation | |
|---|---|---|---|---|---|
| α-MSH | VTA | Intraparenchymal MCR agonist injection | Reduced the intake of chow and sucrose | (Roseberry, 2013; Yen and Roseberry, 2014) | |
| Decreased sucrose self-administration | (Shanmugarajah et al., 2017) | ||||
| Intraparenchymal MC3R agonist injection | Increased sucrose self-administration but not free feeding on sucrose. | (Pandit et al., 2016) | |||
| Chemogenetic activation of MC3R-expressing neurons | Decreased feeding in female mice | (Dunigan et al., 2021) | |||
| NAcc | Intraparenchymal MC4-R agonist injection | Decreased feeding | (Lerma-Cabrera et al., 2012) | ||
| Intraparenchymal α-MSH injection | Decreased motivation for sucrose | (Pandit et al., 2015) | |||
| AgRP | VTA | Intraparenchymal MCR antagonist injection | Increased chow intake | (Roseberry, 2013) | |
| Increased sucrose self-administration | (Shanmugarajah et al., 2017) | ||||
| Chemogenetic inhibition of MC3R-expressing neurons | Decreased feeding in male mice | (Dunigan et al., 2021) | |||
| NAcc | Intraparenchymal MC4-R antagonist injection | Increased feeding | (Lerma-Cabrera et al., 2012) | ||
| Intraparenchymal AgRP or MCR antagonist injection | Increased motivation for sucrose | (Pandit et al., 2015) | |||
| Blocked reinforcing, motivational and sensitizing effects of cocaine | (Hsu et al., 2005) | ||||
| Global | MC3R knockout and re-expression | Sex-dependently altered sucrose preference and DA turnover. | (Lippert et al., 2014). | ||
| Altered food self-administration under FR1 and PR conditions in food-restricted mice with a reversal in PR responding following MC3R re-expression in DA neurons. | (Mavrikaki et al., 2016) | ||||
| NPY | NAcc | Constitutive activation of NAcc NPY2R | Reduced expression of ethanol-induced behavioral sensitization | (Hayes et al., 2012) | |
| Intraparenchymal NPY or NPY1R agonist injection | Increased ethanol self-administration directly into the posterior VTA. | (Borkar et al., 2016) | |||
| (Desai et al., 2013) | |||||
| Potentiated the rewarding effect of morphine. | (Wang et al., 2020) | ||||
| Prolonged the extinction period following chronic morphine exposure | |||||
| Intraparenchymal NPY5R antagonist injection | Reduced morphine extinction period | (Wang et al., 2020) | |||
| CRF | VTA | Intraparenchymal CRF injection | Reduced motivation for food reward | (Wanat et al., 2013) | |
| Reinstated lever pressing for cocaine | (Wang et al., 2005; Wang et al., 2007) | ||||
| Intraparenchymal CRF antagonist injection | Blocked foot shock-induced reinstatement of cocaine seeking. | (Wang et al., 2005) | |||
| (Boyson et al., 2014). | |||||
| Prevented dopaminergic cross-sensitizations and escalated cocaine self-administration. | |||||
| Intraparenchymal CRFR1 antagonist or CRFR2 agonist injection | Decreased binge-like ethanol drinking (drinking in the dark) | (Rinker et al., 2017; Sparta et al., 2013) | |||
| Intraparenchymal CRFR1 antagonist | Reduce alcohol consumption in an intermittent access two-bottle choice paradigm. | (Hwa et al., 2013) | |||
| (Hwa et al., 2016) | |||||
| (Grieder et al., 2014). | |||||
| Reduced social defeat stress-enhanced ethanol drinking. | |||||
| Prevented anxiety-like behavior during nicotine withdrawal. | |||||
| ShRNA-mediated knockdown of CRFR1 | Reduced cue-induced cocaine seeking but not cue-induced sucrose seeking | (Chen et al., 2014) | |||
| NAcc | Intraparenchymal CRF injection | Enhanced the ability of Pavlovian reward cues to trigger instrumental performance for sucrose reward | (Peciña et al., 2006) | ||
| CRF overexpression | Increased operant responding to nicotine and increased food intake (females> males) | (Uribe et al., 2020). | |||
| MCH | NAcc | Intraparenchymal MCH injection | Increased feeding (only in males) Restored feeding in Pmch null mice to wild-type levels | (Georgescu et al., 2005; Terrill et al., 2020) | |
| (Mul et al., 2011). | |||||
| Intraparenchymal MCHR1 antagonist injection | Decreased feeding | (Georgescu et al., 2005) | |||
| Orexin | VTA | Intraparenchymal OX-A injection | Increased chow, HFD and sucrose intake. | (Terrill et al., 2016) | |
| Attenuated intragastric nutrient-induced hypophagia. | (España et al., 2011) | ||||
| (Harris et al., 2005; Wang et al., 2009) | |||||
| Promoted cocaine self-administration. Reinstated previously extinguished morphine and cocaine preference. | |||||
| Intraparenchymal OX-1R antagonist injection | Attenuated orexigenic effects of ICV ghrelin. | (Cone et al., 2014) | |||
| (Olney et al., 2017) | |||||
| Blunted binge-like ethanol intake. | (Borgland et al., 2009; España et al., 2010) | ||||
| Reduced cocaine self-administration. | |||||
| Prevented acquisition of locomotor sensitization to cocaine. | (Borgland et al., 2006) | ||||
| (James et al., 2011) | |||||
| Attenuated cue-induced cocaine reinstatement. | |||||
| Intraparenchymal OX-1R/OX-2R antagonist injection | Attenuated alcohol self-administration | (Srinivasan et al., 2012) | |||
| NAcc | Intraparenchymal OX-A injection | Increased feeding | (Thorpe and Kotz, 2005) | ||
| Increased the hedonic impact of sucrose taste and increased palatable food intake | (Castro et al., 2016) | ||||
| Intraparenchymal OX-1R antagonist injection | Decreased alcohol intake in excessive but not moderate drinkers | (Lei et al., 2019; Lei et al., 2016) | |||
| (Qi et al., 2013) | |||||
| Attenuated stress-induced morphine reinstatement | |||||
| Oxytocin | VTA | Intraparenchymal OT injection | Decreased chow intake at acute time points. | (Wald et al., 2020) | |
| (Mullis et al., 2013) | |||||
| Decreased sucrose intake. | (Wald et al., 2020) | ||||
| Reduced food motivation and food seeking. (Mullis et al., 2013; Wald et al., 2020) | (Song et al., 2016). | ||||
| (Borland et al., 2018) | |||||
| Reduced place avoidance for the social interaction chamber. | |||||
| Decreased the frequency of seeking social interaction in Operant Social Preference task. | |||||
| Optogenetic stimulation of PVN OT axon terminals | Promoted sociability. | (Hung et al., 2017) | |||
| Intraparenchymal OTR antagonist injection | Increased sucrose intake. | (Mullis et al., 2013) | |||
| Increased the frequency of entering social interaction chambers in Operant Social Preference task. | (Borland et al., 2018) | ||||
| NAcc | Intraparenchymal OT injection | Decreased chow intake in deprived conditions and the consumption of palatable nutritive and non-nutritive sweet solutions. | (Herisson et al., 2016) | ||
| (Baracz et al., 2012; Cox et al., 2017) | |||||
| (Ibragimov et al., 1987; Weber et al., 2018) | |||||
| Attenuated METH-induced CPP, drug seeking and demand. | |||||
| Inhibited cocaine seeking and heroin self-administration. | |||||
| OTR overexpression | Reduced ethanol preference, ethanol intake, and reinstatement of ethanol conditioned place preference. | (Bahi, 2015; Bahi et al., 2016) | |||
| Intraparenchymal OTR antagonist injection | Prevented social CPP. | (Dolen et al., 2013) | |||
| Amylin | VTA | Intraparenchymal AmyR agonist injection | Decreased the intake of chow, sucrose, and HFD primarily through a reduction in meal size. | (Mietlicki-Baase et al., 2015; Mietlicki-Baase et al., 2013b) | |
| (Mietlicki-Baase et al., 2017) | |||||
| Decreased the intake of palatable, non-nutritive sweetener. | (Mietlicki-Baase et al., 2013b) | ||||
| (Kalafateli et al., 2021b) | |||||
| Decreased the motivation to work for sucrose reward. | (Kalafateli et al., 2021a) | ||||
| Blocked alcohol-induced locomotor stimulation and decreased alcohol-induced DA release in the NAcc shell in mice and decreased alcohol intake in rats. | |||||
| Decreased cocaine-evoked locomotor stimulation. | |||||
| Intraparenchymal AmyR antagonist injection | Increased food intake. | (Mietlicki-Baase et al., 2013b) | |||
| VTA CTR knockdown | Produced hyperphagia in HFD-fed animals. | (Mietlicki-Baase et al., 2015) | |||
| NAcc | Intraparenchymal AmyR agonist injection | Blocked alcohol-induced locomotor stimulation. | (Kalafateli et al., 2021b) | ||
| (Kalafateli et al., 2021a) | |||||
| Decreased cocaine-induced locomotor stimulation. | |||||
| Neurotensin | VTA | Intraparenchymal NT injection | Increased latency to eat and reduced food intake in fasted animals. | (Cador et al., 1986; Hawkins, 1986) | |
| (Kelley et al., 1989) | |||||
| Reduced operant responding for food. | |||||
| NTS1R-expressing neuron ablation | Increased the intake of chow, sucrose, and HFD. | (Woodworth et al., 2017). | |||
| GLP-1 | VTA | Intraparenchymal GLP-1R agonist injection | Reduced the intake of palatable food. | (Alhadeff et al., 2012) | |
| Reduced chow intake in fasted animals or when chow was the only caloric source. | (Dickson et al., 2012). | ||||
| (Alhadeff et al., 2012; Mietlicki-Baase et al., 2014; Mietlicki-Baase et al., 2013a) | |||||
| Decreased HFD intake while increasing chow intake in animals fed both diets simultaneously. | (Alhadeff et al., 2012). | ||||
| (Dickson et al., 2012). | |||||
| Decreased 1hr sucrose intake. | (Shirazi et al., 2013) | ||||
| Decreased the motivation to obtain sucrose reward. | (Vallöf et al., 2019). | ||||
| (Schmidt et al., 2016) | |||||
| Decreased alcohol intake. | (Hernandez et al., 2018) | ||||
| Decreased alcohol-induced locomotor behavior. | |||||
| Reduced cocaine self-administration. | |||||
| Reduced cocaine-primed reinstatement. | |||||
| Chemogenetic induction of GLP-1 release from NTS terminals | Reduced HFD intake. | (Wang et al., 2015) | |||
| NAcc | Intraparenchymal GLP-1R agonist injection | Decreased HFD intake while increasing chow intake in animals fed both diets simultaneously. | (Alhadeff et al., 2012; Mietlicki-Baase et al., 2014; Mietlicki-Baase et al., 2013a) | ||
| (Alhadeff et al., 2012). | |||||
| Decreased 1hr sucrose intake. | (Dickson et al., 2012). | ||||
| Decreased the motivation to obtain sucrose reward. | (Vallöf et al., 2019) | ||||
| (Hernandez et al., 2019). | |||||
| Decreased alcohol consumption in alcohol-preferring animals only. | |||||
| Decreased alcohol-induced locomotor response and alcohol CPP. | |||||
| Reduced cocaine-primed reinstatement. | |||||
| Intraparenchymal GLP-1R antagonist injection | Increased sucrose meal size and sucrose palatability. | (Dossat et al., 2013) | |||
Melanocortins
The melanocortin peptides, α-melanocyte-stimulating hormone (α-MSH) and agouti-related protein (AgRP), are well known for their role in the regulation of feeding but they also participate in the control of many other behaviors and physiological functions [17–19]. α-MSH is produced by proteolytic cleavage of proopiomelanocortin (POMC) propeptide produced by POMC neurons of the arcuate nucleus of the hypothalamus (Arc) as well as the commissural nucleus of the solitary tract (NTS). In contrast, Arc AgRP neurons are the sole site of AgRP production and release [17,18,20]. In the central nervous system, melanocortin peptides bind to two receptors, the melanocortin-3 and −4 receptors (MC3R and MC4R) with α-MSH acting as an agonist and AgRP acting as is an inverse agonist/competitive antagonist. AgRP neurons also release neuropeptide Y (NPY) which binds to a family of multiple NPY receptors (NPYR) and is discussed in the next section. The initial evidence showing that melanocortins can interact with mesolimbic DA circuits was the demonstration that either intracerebroventricular (ICV) or direct intra-VTA injection of α-MSH stimulated DA release in efferent projection areas such as the NAcc and the prefrontal cortex, and stimulated grooming and rearing behavior [21–24]. Analysis of the anatomy of the melanocortin circuits and their receptors also provided evidence for their interactions with the mesolimbic DA system. MC3Rs and MC4Rs are expressed in both VTA and NAcc [25–28]. Moreover, VTA is one of the regions with the highest expression of MC3Rs, which are expressed in DA and non-DA neurons [27–29]. Both POMC and AgRP neurons project to the VTA [30–32] and prolyl carboxypeptidase, an enzyme responsible for inactivation of α-MSH, is expressed in the VTA [33] suggesting that mechanisms regulating α-MSH dynamics are present within this region. Surprisingly, with the use of monosynaptic cell-type-specific rabies virus, we recently showed a low degree of synaptic connectivity between Arc neurons, including AgRP/NPY and POMC populations, and multiple VTA neuron subtypes [31]. These results were further validated by another group that showed low amount of AgRP/NPY synaptic terminals in the vicinity of VTA DA neurons compared to the robust labeling in the paraventricular nucleus (PVN) [34]. The same group also reported a lack of inhibitory postsynaptic currents produced by ChR2-mediated activation of AgRP/NPY terminals in the VTA and unchanged basal activity of VTA DAT neurons in response to chemogenetic activation of Arc AgRP/NPY neurons [34]. This lack of synaptic connectivity, coupled with the robust labeling of POMC and AgRP neurons by generic retrograde tracers injected into the VTA [31,35] and the previously reported presence of AgRP terminals in close proximity to putative dopamine neurons of the VTA [30], suggest that Arc feeding peptides act on the VTA circuitry via short distance diffusion. It is currently not known whether α-MSH and AgRP are released from axons of neurons traveling through the VTA on their way to more caudal regions, however. This possibility is less likely than extra-synaptic transmission from direct AgRP and POMC neuronal projections to the VTA as AgRP neurons have been reported to show little collateralization [36]. In contrast to our findings, another group has reported Arc POMC+ synaptic terminals and boutons in the proximity of VTA DA and non-DA neurons [32] and showed that Arc POMC neurons inhibit VTA DA neurons via direct and indirect mechanisms [37]. These findings conflict with the data showing that α-MSH increased extracellular DA in NAcc and increased DA-dependent behaviors however [21,22], as well as the data from our laboratory showing that α-MSH increased the firing rate of MC3R-expressing VTA neurons in acute slices [38]. In this section, we will focus on the effects of melanocortin action within mesolimbic circuits in the regulation of feeding and drug reward whereas the effects on alcohol intake and reward have been discussed in detail in a recent review [39].
Food and food reward:
Melanocortin peptides are well known for their regulation of feeding. Stimulation of POMC neurons and injection of melanocortin receptor (MCR) agonists have been widely shown to decrease food intake and increase energy expenditure and metabolism [40–42], whereas central administration or overexpression of AgRP and activation of AgRP neurons promotes feeding and weight gain [40,43–46]. Some of these effects appear to be mediated through actions in the mesolimbic DA system. For example, our laboratory has shown that administration of non-specific MCR agonists into the VTA decreased the intake of both standard chow and palatable sucrose solutions, and decreased sucrose self-administration, whereas injection of MCR antagonists into the VTA increased chow intake, body weight, and sucrose self-administration [47–49]. Contrary to our findings with the non-selective MCR agonist MTII, another group showed that intra-VTA infusion with D-trp8-γMSH, an MC3R-selective agonist, increased sucrose self-administration but not free feeding on sucrose, illustrating the difficulty in resolving the exact role of MCRs in the VTA [50]. Injection of α-MSH and AgRP into the NAcc shell also produced a decrease and an increase in motivation for sucrose, respectively [51]. Although MCR knockout results in increased weight and fat mass, diabetes, and altered activity [52–55], suggesting that both receptor subtypes are important for energy homeostasis, it isn’t clear whether the MC3R or MC4R a larger role in the effects of α-MSH and AgRP within the mesolimbic DA system circuitry. Global deletion of MC3R altered food self-administration under fixed ratio-1 and progressive ratio conditions in food-restricted mice but not under normal ad libitum feeding, and re-expression of MC3Rs exclusively in DA neurons reversed the changes in progressive but not fixed ratio, responding [56]. MC3R knockout also sex-dependently altered sucrose preference and DA turnover [28]. Taken together, these data suggest that MC3Rs may be the point of interaction between the homeostatic melanocortin peptides and the mesolimbic circuitry, likely through their interactions in the VTA because of the abundance of MC3R in that region [27]. Furthermore, our laboratory recently showed that MC3R-expressing neurons of the VTA control feeding in an activity- and sex-dependent manner such that acute activation of these neurons decreased feeding, but only in females, and acute inhibition of them decreased feeding, but only in males [29] further supporting the contextual and sex-dependent roles of MC3R-mediated signaling in the VTA. There have also been several reports of VTA MC4R-dependent regulation of DA dynamics and feeding [57],58. However, these studies used MC4R-selective ligands that still show significant affinity for MC3R [59]. Because of this, and the abundance of MC3R receptors and low amount of MC4R receptors in the VTA [28], studies utilizing MC4R-selective ligands should be interpreted cautiously. In contrast to the VTA, the NAcc is enriched in MC4R and lacks MC3R [27,60]. Similar to what was observed in the VTA, NAcc injection of MC4-R agonists decreased and antagonists increased feeding [57]. Future studies enabled by the use of recently developed genetic tools and new ligands showing higher specificity for MC3Rs vs MC4Rs will aid in furthering our understanding of mechanisms associated with melanocortin action in the mesolimbic DA system.
Effects on drugs of abuse:
Activation of MCRs appears to have a potentiating effect on drug reward and reinforcement for multiple drugs. For example, ICV injection of non-specific MCR agonists enhanced the reward-potentiating effects of amphetamine on lateral hypothalamus (LH) self-stimulation [61]. Likewise, ICV administration of α-MSH or an MC4R agonist potentiated nicotine reward in an intracranial self-stimulation (ICSS) paradigm in an ovariectomized rat menopausal female model, whereas MC4R antagonists suppressed the ICSS promoting effects of nicotine [62]. MC4R null mice show blunted cocaine locomotor sensitization which is normalized by restoration of MC4R expression in Dopamine receptor D1 (D1R)-expressing neurons [63]. Furthermore, NAcc injection of MCR antagonists blocked multiple aspects of cocaine reward including reinforcing, motivational and sensitizing effects of the drug [60] illustrating the direct involvement of NAcc MCRs in the effects on cocaine reward. The roles of the melanocortin peptides or their receptors within the VTA in the regulation of drug reward have not been investigated to date, however.
NPY
NPY is a potent orexigenic peptide that is expressed widely throughout the CNS including the Arc and structures of the mesolimbic system. For example, NPY is produced locally by NAcc neurons and the NAcc also receives input from Arc NPY neurons [64,65]. As discussed in the previous section, Arc NPY neurons also project to the VTA, and the area receives additional NPY input from the ventrolateral medulla of the brainstem [30,31,66]. Unlike the NAcc, the VTA lacks NPY-expressing cell bodies, and blockade of neuronal peptide transport by colchicine eliminated VTA NPY signal [66] suggesting that in the VTA, NPY immunoreactive fibers are exclusively external. NPY mediates its effects through five known receptors, NPY1R-NPY6R [67] although NPY6R is not expressed in the rat brain. NPY1R and NPY5R are expressed in the VTA and NAcc and NPY5R binding has been confirmed in the latter [68–71]. In ex vivo experiments, NPY has been shown to affect VTA DA neuron activity via multiple pre and postsynaptic mechanisms including direct inhibition of DA neurons, and a decrease in presynaptic glutamatergic and GABAergic transmission [68,72]. The net effect of NPY action in the VTA is currently unknown, however. In the NAcc, NPY infusions increased DA concentrations [70,73] suggestive of NPY-mediated stimulation of DA release.
Food and food reward:
NPY is an important regulator of feeding as shown by the robust increase in feeding produced by central administration of the peptide [74,75] or optogenetic stimulation of Arc NPY neurons [40]. Furthermore, ablation of NPY neurons has been shown to reduce feeding and body weight [76,77]. Additionally, central administration of NPY increased motivation to obtain food pellets, indicating that the mesolimbic system may be one of the targets of NPY [78]. In fact, injection of NPY into the VTA or the NAcc increased motivation to respond for sucrose [79]. The mesolimbic system-mediated effects of NPY in the regulation of feeding appear to be macronutrient selective. For example, intra-VTA or -NAcc injection of NPY did not affect intake of sucrose or regular chow [65,79,80] but increased intake of fat when administered into the NAcc via NPY1R -dependent mechanism [65].
Alcohol intake and reward:
Central administration of NPY or NPY1R agonists have been shown to decrease ethanol intake in genetically vulnerable rodent models [81–83]. Furthermore, NPY also contributes to the regulation of alcohol-directed reinstatement and relapse behaviors. For example, ICV infusion of NPY has been shown to block stress-induced reinstatement of ethanol seeking [84] and, when administered during the withdrawal period, reduced increases in ethanol self-administration in subsequent withdrawal trials [85]. Within the mesolimbic circuitry, activation of NAcc NPY2R reduced expression of ethanol-induced behavioral sensitization [86]. On the other hand, intra-NAcc shell administration of NPY or NPY1R agonists dose-dependently increased ethanol self-administration directly into the posterior VTA, whereas NPY1R antagonist produced the opposite effect [87] potentially suggestive of NPY-mediated enhancement of DA release in the NAcc shell. Little is known about the role of VTA-targeted NPY action in the regulation of ethanol-directed behaviors, however.
Effects on drugs of abuse:
Unlike the mechanisms involved in the regulation of ethanol-directed behaviors which appear to be mediated through NPY1R and NPY2R but not NPY5R [88–90], NPY5R seems to play an important role in NPY effects on drugs of abuse. For example, global knockout of NPY5R attenuated cocaine self-administration and cocaine-induced hyperlocomotion [91]. Furthermore, ICV administration of NPY during abstinence attenuated morphine withdrawal symptoms in an effect mediated via receptors with a NPY5R-like pharmacological profile [92]. NPY administration into NAcc shell also prolonged the extinction period following chronic morphine exposure whereas blockade of NPY5R receptor signaling reduced it [93]. The same group also showed that chronic morphine exposure reversibly decreases NPY expression in the NAcc shell [93]. Despite this evidence implicating NPY5R s, in the NAcc shell, NPY also acts via NPY1R to regulate drug of abuse-related reward behaviors. For example, injections of NPY or NPY1R agonists potentiated and a NPY1R antagonist attenuated the rewarding effect of morphine as measured by ICSS of medial forebrain bundle [94]. The intra-VTA effects of NPY in the regulation of drug-directed behaviors are currently not known and require attention.
Corticotropin-releasing factor
Corticotropin-releasing factor (CRF) is a key neuropeptide in the stress response. CRF is produced by the neurons of multiple hypothalamic and limbic regions including PVN, bed nucleus of the stria terminal is (BNST), and the central amygdala and CRF-containing fibers populate the NAcc core, NAcc medial shell, and the VTA [95–97]. Additionally, a subset of VTA DA neurons also co-express CRF [98]. Within the VTA, DA neurons receive mostly asymmetric (excitatory) input from CRF+ terminals, whereas non-DA neurons receive both symmetric (inhibitory) and asymmetric inputs [99,100]. This anatomical diversity is further supported by electrophysiological studies which showed both inhibitory [101] and excitatory [102,103] effects of CRF on the VTA DA neurons. CRF binds to two receptors, CRFR1 and CRFR2, although the peptide has higher affinity for the former [104]. Both of these receptors are expressed throughout the brain, including the mesolimbic structures [97,105]. In the VTA, CRFR1 is located both presynaptically and postsynaptically [106] and colocalizes with TH and D1R [107]. The expression of CRFR2 in VTA has been difficult to resolve [108] but, as discussed below, has been implicated in behavior. In NAcc neurons, CRFR1s do not colocalize with tyrosine hydroxylase (TH) or D1R [107], whereas dopaminergic terminals populating NAcc express both receptor subtypes suggestive of DA-release regulatory function of CRF in that area [95].. In support of this, direct administration of CRF into NAcc has been shown to elevate DA, but only in animals that were not stressed [95]. The urocortin peptides are CRF analogs that act via CRFR1 and CRFR2. Although the urocortins have been implicated in both feeding and social behavior, their actions directly within the mesolimbic system have not been highly studied, so they will not be discussed here.
Food and food reward:
Stress-facilitated eating, especially of highly palatable and calorically dense foods, is a well-known phenomenon. Yet, little attention has been focused on the interaction between CRF and the mesolimbic system in the regulation of feeding. One study showed that CRF acts in the VTA to reduce motivation for food reward [109]. Conversely, through its action in the NAcc, CRF has been shown to amplify incentive salience of sucrose reward. For example, CRF injected into NAcc shell enhanced the ability of Pavlovian reward cues to trigger instrumental performance for sucrose reward [110]. It is possible that CRF may contribute to the changes in mesolimbic DA system function that occur in response to fasting and food restriction, both of which elevate CRF levels, but this has not been directly tested to date. Overall, more studies are necessary to understand the role of CRF and stress in the regulation of feeding and food reward.
Alcohol intake and reward:
As postulated by the tension reduction hypothesis, stress augments anxiety, and alcohol is then consumed to reduce this anxiety [111]. This hypothesis establishes a clear link between stress and alcohol consumption but the real picture and the mechanisms involved in alcohol dependence are much more complicated as discussed in another review [112]. The physiological effects of the hormonal mediator of stress, CRF, have been strongly linked to alcohol-taking behaviors. For example, CRFR1 antagonists have been shown to decrease alcohol drinking in binge and intermittent access paradigms [113–115], to decrease ethanol self-administration in dependent animals [116], to decrease anxiety-like behavior during ethanol withdrawal [116], and to decrease stress-induced reinstatement of ethanol seeking [117]. The VTA has been established to be an important site of CRF action in the regulation of binge-like ethanol consumption as shown by a decrease in binge-like drinking in response to intra-VTA CRFR1 antagonist administration [118,119]. Interestingly, VTA activation of CRFR2 also decreased binge-like drinking and it has been suggested that the reduction in binge-like ethanol intake produced by CRFR1 antagonism requires intact CRFR2 signaling [118]. Furthermore, BNST CRF input to the VTA, rather than local VTA CRF-producing neurons, has been identified as the driver of binge-like ethanol drinking [118]. VTA CRFR1 antagonism was also shown to reduce alcohol consumption in an intermittent access two-bottle choice paradigm regardless of intrinsic alcohol preference of the animals [115]. VTA CRF signaling via CRFR1 was also shown to be important for social defeat stress-induced escalation of ethanol drinking while signaling via CRFR2 produced non-alcohol specific reductions in intake [120]. Thus, CRF clearly interacts with the mesolimbic DA system to regulate ethanol responses.
Effects on drugs of abuse:
The existence of a positive correlation between stress and drug use is well known. For example, CRF administration facilitated reinstatement of cocaine seeking following extinction [121–123]. CRFR1 has also been consistently shown to be involved in stress-mediated cocaine behaviors including reinforcement and relapse. For example, peripherally administered CRFR1 antagonists reduced cocaine self-administration in multiple paradigms [124,125], blocked stress-induced increase in cocaine conditioned place preference (CPP) [126], attenuated stress-induced reinstatement of cocaine CPP [127,128], and stress-induced reinstatement of cocaine seeking [129,130]. The VTA is one of the key neural substrates of CRF-mediated effects on cocaine behaviors. For example, VTA infusion of CRF reinstated lever pressing for cocaine in cocaine-exposed animals [100,130]. Furthermore, stress-induced reinstatement of cocaine seeking has been linked to the physiological action of CRF in the VTA as shown by blockade of foot shock-induced reinstatement by intra-VTA infusion of general CRF antagonist [100]. The mechanism of CRF action in the VTA is complex and not well understood, however. Both CRFR1 [127,129,131,132] and CRFR2 [100,130] have been causally, and in some studies mutually exclusively, implicated in the VTA-directed CRF effects. However, another study showed that the same behavior can be regulated by both receptors as antagonists for either CRF receptor subtype administered into the VTA during social defeat stress prevented dopaminergic cross-sensitizations and escalated cocaine self-administration in a 24-hour binge protocol [133]. Interestingly, short hairpin RNA-mediated knockdown of VTA CRFR1 reduced cue-induced cocaine seeking but not cue-induced sucrose seeking [132] suggesting differential regulation of drug and natural rewards by CRF.
There is no surprise that stress, and by extension CRF, also participate in the regulation of nicotine-directed behaviors. Blockade of CRF action [134], and specifically the effects mediated by CRFR1 [135], decreased stress-induced reinstatement of nicotine seeking. Additionally, CRF is involved in nicotine withdrawal effects as shown by a decrease in measures of precipitated nicotine withdrawal in response to CRFR1 antagonist treatment [135]. VTA appears to be one of the CRF targets in the modulation of withdrawal-associated behaviors as blockade of VTA CRFR1 prevented anxiety-like behavior during nicotine withdrawal [98]. Overexpression of CRF in NAcc increased operant responding to nicotine in sexually dimorphic manner with females exhibiting higher levels of responding. Furthermore, this effect was shown to be dependent on ovarian hormones as nicotine self-administration was increased to a larger extent in intact compared to ovariectomized females [136]. Interestingly, NAcc CRF overexpression also increased food intake to a larger extent in females than males but this effect was not dependent on ovarian hormones [136].
Melanin-concentrating hormone
Melanin-concentrating hormone (MCH) is a cyclic peptide predominately synthesized by neurons of the LH and zona incerta from a precursor encoded by Pmch gene [137]. LH MCH neurons send widespread projections throughout the brain including dense innervation of the VTA and NAcc [137,138]. MCH binds to two receptors, MCHR1 and MCHR2, but all preclinical research on MCH has focused on the MCHR1-mediated actions of the peptide as MCHR2 is not expressed in rodents [139]. MCHR1 is highly enriched in NAcc shell [140–142] and is coexpressed with DA receptors [143,144]. Low to moderate levels of MCHR1 mRNA have been previously detected in the VTA [140–142] but no detectable VTA MCHR1 expression was found in BAC knock-in mice [145]. Optogenetic stimulation of MCH neurons has been shown to increase NAcc DA [146], yet, in VTA slices, MCH application did not affect firing rate [147] and intra-VTA MCH injections failed to produce changes in DA-dependent behaviors [24]. Together with anatomical data, these results, as well as those discussed below, suggest that VTA is a minor target of MCH whereas NAcc is the primary mesolimbic substrate of MCH action in the regulation of feeding and reward behaviors. The role of MCH in the regulation of feeding and food reward has received the most attention whereas much less is known about the effect of this peptide on the behaviors associated with intake of alcohol and drugs of abuse. This review will discuss food and food reward-related findings whereas the role of MCH in drug abuse disorders has been discussed in greater detail in a recent review [148].
Food and food reward:
The role of MCH as a critical regulator of feeding is supported by both pharmacological and genetic data. For example, loss of Pmch resulted in hypophagia and a lean phenotype [149] whereas overexpression of MCH caused hyperphagia and excess weight gain [150]. Similarly, MCH administered centrally increased feeding and body weight [151,152] whereas systemic treatment with MCHR1 antagonist reduced food intake and body weight in animals with diet-induced obesity [153,154]. In the mesolimbic circuit, MCH exerts its feeding effects by acting primarily in the NAcc shell as shown by an orexigenic response to intra-NAcc shell MCH injection and a reduction of feeding produced by MCHR1 antagonist injected into this area [155]. Additionally, MCH administration to the NAcc shell of Pmch null mice restored feeding to wild-type levels [156]. Recently, Terrill et al. showed that MCH action in the NAcc in the regulation of feeding is sexually dimorphic as NAcc shell treatment with MCH or chemogenetic activation of LH-MCH projections to the region promoted feeding, but only in males. This effect was shown to be estrogen-sensitive as ovariectomy restored the ability of MCH to promote feeding in females [157].
MCH also regulates rewarding and motivational aspects of feeding. For example, MCHR1 antagonists reduced consumption of palatable sweetened condensed milk in sated rats [154]. The NAcc appears to be involved in this effect as shown by MCH-induced increase in sucrose intake when the peptide is administered directly into this region [157]. Peripheral administration of MCHR-1 antagonists or loss of Pmch reduced food self-administration in food-restricted animals as well as high-fat food-reinforced operant responding and sucrose but not saccharin self-administration [156,158,159] suggesting that MCH may be important for the motivational aspects of feeding, especially of nutritive substances. Surprisingly, MCH administration into NAcc shell or chemogenetic activation of NAcc-projecting MCH neurons did not affect operant responding for sucrose, however [157]. In fact, MCH appears to play a complex role in the regulation of intake of sweet substances such that it conveys rewarding signals from the caloric content rather than the taste of the ingested sugars [146,158]. The mechanism or the neural substrates responsible for this effect are currently not known, however. Additionally, conflicting data exist regarding MCH’s effects on reinstatement of food seeking. For example, MCHR1 antagonists reduced MCH-induced reinstatement of food seeking but had no effect on high-fat food, cue, or stress-primed reinstatement [159]. Conversely, cue-induced reinstatement of sucrose seeking was decreased by MCHR1 antagonism [158]. The mechanisms underlying these differences need to be investigated in future experiments.
Orexin
The two orexin (OX) peptides, orexin A and orexin B (OX-A, OX-B), also known as hypocretins, are neuropeptides alternatively spliced from a common precursor and are produced by the neurons of the lateral, dorsomedial, and perifornical areas of the hypothalamus. Interestingly, OX neurons show a similar distribution to MCH neurons and are interspersed with them. The OX peptides bind to two distinct receptors, orexin 1 (OX1R) and orexin 2 (OX2R), to regulate many behaviors including arousal, feeding, and appetitive behaviors [160,161]. Like MCH neurons, OX neurons project widely throughout the brain and OX receptors are expressed in many brain regions including the components of the mesolimbic system [162,163]. Both VTA and NAcc receive input from OX-A- and OX-B-containing neurons of LH and OX-containing terminals are found in close opposition to VTA DA neurons [164]. Moreover, both OXRs are expressed in the VTA and NAcc [162,165,166]. A large body of literature supports the idea that OX regulates DA neurotransmission and DA-dependent behaviors. For example, ICV OX produced classic DA-dependent behaviors such as hyperlocomotion and stereotypy that were blocked by a DA receptor antagonist [167]. Furthermore, OX has been shown to directly activate VTA DA neurons, including those projecting to NAcc shell, and to produce increased DA outflow in the NAcc [147,167–171].
Food and food reward:
Peripheral or ICV injections of OX peptides increased food intake [161,172–178] and central administration of anti-OX antibody or an OX1R-selective antagonist reduced food intake [173,179]. Furthermore, blockage of OX1R also reduced intake of highly palatable food and binge-like consumption of sucrose [180–183]. OX1R-mediated signaling also appears to play an important role in the regulation of food reinforcement and motivation as was illustrated by decreased performance under variable and progressive ratio of reinforcement produced by peripheral OX1R blockade and LH OX knockdown [184]. Moreover, ICV OX-A has also been shown to reinstate previously extinguished food-seeking behavior [185].
Unlike the peripheral effects, intra-VTA administration of OX did not stimulate feeding on chow diet [178]. Conversely, OX-A administered into the VTA increased the intake of high-fat diet (HFD) and sucrose solution in ad-libitum fed rats, and this increase in sucrose feeding was mediated by OX1R as the receptor blockade attenuated sucrose intake [186]. Furthermore, in a hedonic model of feeding in which food-restricted animals are exposed to HFD following chow refeeding, OX-A acted in the VTA to increase intake of both chow and HFD [186]. Together, these experiments suggest that OX acts in the VTA to promote palatable food intake rather than to modulate normal baseline chow feeding. These results are also in line with the idea that VTA OX may play a role in feeding under high-motivation conditions as VTA blockade of OX-1R blunted orexigenic effects of ICV ghrelin [187]. Furthermore, intra-VTA OX-A attenuated intragastric nutrient-induced hypophagia [186] suggesting that OX action in the VTA counteracts postingestive negative feedback.
In the NAcc, infusion of OX-A into the shell of the nucleus increased feeding and locomotor activity, and the effect on feeding but not locomotor activity was attenuated by pretreatment with OX1R antagonist [188]. These results suggest that in the NAcc feeding and locomotion may be modulated by different molecular mechanisms. Injection of OX-A into the previously identified “hedonic hotspot” within the rostral region of the NAcc medial shell, a region that was previously shown to be the target of opioid enhancement of sucrose liking as measured by taste reactivity [189], also increased the hedonic impact of sucrose taste. Meanwhile, the intake of palatable food was enhanced by OX-A injections throughout different regions of NAcc shell [190]. Not much is known about the specific interactions of VTA/NAcc with OX in the regulation of food-motivated behavior, however. Future experiments are necessary to fill this knowledge gap.
Alcohol intake and reward:
Multiple excellent reviews have focused on discussing the role of OX in the regulation of alcohol intake and reward (please refer to [191,192]). In this review, we will specifically focus on literature investigating the direct action of OX on the mesolimbic structures. Like the effects on sucrose and saccharin, peripheral administration of OXR antagonists disrupts binge-like consumption of ethanol [193,194]. Within the VTA, selective blockade of OX1R but not OX2R significantly blunted binge-like ethanol intake [195] suggesting that VTA is one of the neural substrates of OX action in the regulation of ethanol binge drinking. OX action in the VTA is also involved in the regulation of operant behavior directed towards alcohol as shown by the attenuated alcohol self-administration following intra-VTA treatment with dual OX1R/OX2R antagonist [196]. OX has been strongly implicated in regulation of alcohol-directed behaviors in high alcohol consumers and the mesolimbic circuitry appears to be involved in this. For example, systemically administered OX1R antagonist decreased alcohol consumption, preference, self-administration, and reinstatement selectively in high ethanol preferring/responding rats [197]. Similarly, blockade of NAcc shell OX1Rs decreased alcohol intake in a 2 bottle choice drinking in the dark and intermittent access paradigms [198,199] and this effect was only observed in excessive drinkers with no effect in moderate drinkers [199].
Effects on drugs of abuse:
The effects of OX action on the expression of behaviors moderated by the drugs of abuse and involved in addiction have received the most attention and have been reviewed in detail previously [200–202]. OX action in the VTA is strongly implemented in the regulation of reinforcement properties of cocaine. For example, intra-VTA infusion of OX-A promoted cocaine self-administration under multiple models of reinforcement schedule [203] and both systemic and intra-VTA administration of OX1R antagonist reduced cocaine self-administration [204,205]. OX is also involved in the development and expression of drug-induced locomotor sensitization but the effects differ for different drugs. Similar to its systemic effect, OX1R antagonist delivered into the VTA prevented acquisition but not the expression of locomotor sensitization to cocaine [206]. However, systemically administered OX1R or dual OX1R/ OX2R antagonist blocked the expression of sensitization and blocked the expression of amphetamine-induced markers of synaptic plasticity in the VTA [207,208]. OX also acts on different modalities of the mesolimbic system to regulate drug seeking. Intra-VTA administration of OX peptides reinstated previously extinguished morphine and cocaine preference [209,210] and blockade of OX1R in the VTA attenuated cue-induced cocaine reinstatement [211] but not stress-induced cocaine reinstatement [210]. Conversely, blockade of NAcc shell OX1R or OX2R attenuated stress-induced morphine reinstatement but not morphine primed reinstatement [212]. Taken together, it appears that OX acts on the VTA neurons to regulate cue-induced drug-seeking behavior and it acts in the NAcc shell to mediate stress-induced drug-seeking responses.
Oxytocin
Oxytocin (OT) is a neuropeptide produced by the PVN and supraoptic nucleus neurons that is well known for its role in bonding and social behavior but this peptide is also an important regulator of feeding and reward. This review will focus on the actions of OT in the mesolimbic DA system whereas its roles in the regulation of other behaviors are discussed in greater detail in the following reviews [213,214]. OT influences behavior primarily through its action on the oxytocin receptor (OTR), although it can also bind to vasopressin 1A receptors with lower affinity [215]. Both the VTA and NAcc express OTRs [216–218] and receive input from OT-expressing PVH neurons [217,219–222] although OT can also signal via volume transmission [223]. OT has been shown to, directly and indirectly, impact VTA neuron activity [219,224,225], DA release [226,227], and to facilitate DA-dependent behaviors [228,229]. Interestingly, almost 50% of OTR-expressing neurons co-express vGlut, whereas only 10% colocalize with TH [217] suggesting that glutamatergic signaling by VTA neurons plays an important role in OT-mediated effects.
Food and food reward:
Peripheral or central administration of OT decreased feeding in ad libitum and food-deprived conditions and treatment with OTR antagonist increased feeding [230–234]. Likewise, peripheral OT injection attenuated sucrose intake, and treatment with an OTR antagonist increased sucrose consumption [235,236]. OT has also been shown to participate in the regulation of fat intake [229,237–239]. However, systemically administered OT antagonist shifted the preference toward sucrose consumption without affecting total energy intake when animals were given a choice between fat and sucrose diets [235] suggesting that OT preferentially regulates carbohydrate intake. This is consistent with observations from OT null mice which overconsume palatable sucrose solution but not fat emulsion solution [240]. In addition to intake, OT also attenuated food reward-related behaviors such as sucrose self-administration and sucrose seeking [229,241–243], and disrupted the expression but not acquisition of sucrose place preference [244].
Intra-VTA injection of OT has been reported to reduce chow intake when measured at a short-term time point [234] without an overall effect on overnight chow consumption [229]. OT delivery to the VTA also decreased the intake of sucrose but not water in deprived animals [229] suggesting that OT action in the VTA is directed towards sucrose consumption rather than general modulation of reward. The same research group also showed that endogenous OT signaling in the VTA is physiologically relevant to attenuation of sucrose intake as shown by intra-VTA OTR antagonist-mediated increase in sucrose intake [229]. Furthermore, OT delivered to the VTA has been shown to reduce food motivation and food seeking [234] supporting the idea that OT-mediated reduction in food reward contributes to the central anorexigenic effect of this peptide. OT signaling in the NAcc core but not shell also decreased reward-related feeding behaviors such as chow intake in deprived conditions, and the consumption of palatable nutritive and non-nutritive sweet solutions [245]. In obese humans, OT administration has been shown to reduce functional connectivity between the VTA and brain areas involved in the regulation of food-motivated behaviors in response to viewing high-calorie food imagery [246] potentially providing a translational mechanistic insight into OT-mediated anorexigenic effects. In fact, an OT derivative is currently being reviewed by the FDA for the treatment of hyperphagia in individuals with Prader-Willi Syndrome [247], a genetic disorder characterized by insatiable hunger and life-threatening obesity.
Alcohol intake and reward:
In human trials, intranasal OT has been shown to block alcohol withdrawal in dependent individuals [248] illustrating the clinical utility of OT in treatment of alcohol dependency. Animal work conducted by several groups also supports the role of OT in the regulation of alcohol consumption and reward but there is a large knowledge gap regarding the mechanisms or the neural substrates of OT’s action in the regulation of these behaviors. Multiple studies had shown that peripheral and/or central treatment with OT reduced alcohol consumption [249,250], including binge-like intake of alcohol [251], reduced operant responding for ethanol [251,252], and decreased stress- and cue-induced reinstatement of alcohol seeking [253,254], although the latter was only decreased in alcohol-dependent animals. The mesolimbic system is known for regulating all of the afore-mentioned behaviors but very little is known about the action of OT on the mesolimbic circuits in regulation of alcohol-related behaviors. It has been previously shown that ICV OT blocked ethanol-induced DA release in NAcc [252] and overexpression of OTR in NAcc reduced ethanol preference, ethanol intake, and reinstatement of ethanol conditioned place preference [255,256]. The effects of OT in the VTA in the regulation of alcohol-related behaviors remain largely unknown, however.
Effects on drugs of abuse:
The systemic and central effects of OT in the regulation of behaviors associated with drug reward are well documented for multiple substances including cocaine, opioids, methamphetamine (METH), and nicotine, and have been discussed in detail in a recent review [257]. Although the mesolimbic system is known for its involvement in the regulation of addictive behaviors, there is a knowledge gap in understanding how OT interacts with the mesolimbic structures to regulate drug reward. Several studies have examined the actions of OT in the NAcc in regulation of drug-associated behaviors. For example, intra-NAcc OT has been shown to attenuate METH-induced CPP [258], drug seeking and demand [259], as well as METH-primed reinstatement [260]. This latter effect was OTR-independent, however, as shown by the lack of reversal in response to co-administration of OTR antagonist [260]. Similarly, direct infusion of OT into NAcc inhibited cocaine-seeking behavior [261,262] and heroin self-administration [261]. The role of VTA action of OT in modulation of behaviors directed towards drugs of abuse remains largely unexplored and requires attention, however.
Effects on social reward:
OT is well known for its role in social interactions, including social reward, and the mesolimbic system is a critical target of OT’s action in regulation of these behaviors. Intra-VTA administration of OT or OTR agonist showed that activation of VTA OTRs is necessary for the expression of rewarding properties of social interactions [263]. Furthermore, optogenetic stimulation of PVN OT neuron axons in the VTA promoted sociability whereas silencing of PVN OT neuron projections in the VTA reduced social interactions [264] suggesting that endogenous OT signaling in the VTA is necessary for social reward. Interestingly, when the animals were tested in a novel Operant Social Preference task which, unlike CPP, does not contain a memory confound [265], intra-VTA OT decreased the frequency of seeking social interaction and OTR antagonist increased the frequency of entering social interaction chambers [266] showing that, in the VTA, OT plays a complex role in the regulation of reinforcing properties of social interaction. OT action within the NAcc has also been shown to be important for social reward. For example, intra-NAcc injection of OTR antagonist prevented social CPP [267]. This effect was mediated by presynaptic action of OT as OTR deletion from the cells of NAcc did not affect social CPP whereas deletion of presynaptic OTRs, specifically those projecting from dorsal raphe, completely blocked CPP [267].
Amylin
Amylin (Amy) is an anorexigenic peptide co-secreted with insulin by pancreatic β-cells. This peptide signals through the Amy receptor (AmyR) which is a dimerized complex of a calcitonin receptor (CTR) and a receptor activity-modifying protein. Amy inhibits pancreatic glucagon release, delays gastric emptying, reduces gastric acid production, and acts as a satiety agent (the diverse physiological effects of Amy are more thoroughly discussed in a previous review [268]). In 2005, the Amy analog pramlintide acetate received FDA approval for treatment of type 1 and type 2 diabetes [269,270] and, in addition to being a potent glycemic regulator [271], Amy has been shown to reduce food intake in humans [272–274]. The area postrema and NTS have been regarded as the primary central nervous system targets of Amy because lesions of these brain regions completely abolish anorexigenic effect of Amy [275,276]. There is evidence that Amy also works on the mesolimbic DA circuits, however. mRNA for all components of AmyR complex and CTR immunoreactivity have been identified in VTA and NAcc [277,278], and ~63% of CTR-expressing VTA neurons co-express TH while ~12% of TH neurons co-express CTR [279]. Amy binding has been localized to the mesolimbic structures including the NAcc and VTA in autoradiography studies [280,281], and peripherally injected fluorescent-labeled Amy analog salmon calcitonin (sCT) was detected in the VTA and NAcc [282]. sCT also prevented the VTA-stimulated or drug-induced DA release in NAcc shell [283–285] and when administered directly into the VTA reduced food evoked phasic increase in NAcc core DA release [279]. Together with the anatomical findings, these results suggest that Amy may directly influence DA neurotransmission to regulate different behaviors. The involvement of Amy in the regulation of energy homeostasis and natural reward has been well characterized but the involvement of this peptide in the modulation of drug reward received little attention until recently.
Food and food reward:
Peripheral or ICV administration of AmyR agonists reduced food intake and body weight [286–289] whereas peripheral treatment with AmyR antagonist increased feeding [290]. Furthermore, both of these effects were primarily caused by a change in meal size [290,291] suggesting that Amy may facilitate meal-ending satiety. It was recently shown that the anorexigenic and body weight suppressing effects of Amy are true for both genders but are subject to some sex differences as the weight loss was only sustained in males after the discontinuation of long-term AmyR agonist treatment [292].
Consistent with its effects following peripheral administration, intra-VTA AmyR activation by sCT produced hypophagia that was mediated mainly by a reduction in meal size [277,279]. Blockade of AmyR increased food intake and attenuated intake-suppressive effects of peripherally administered sCT [277] suggesting physiological relevance of intra-VTA Amy signaling. sCT action in the VTA also decreased intake of palatable sucrose solution and HFD, again through a reduction in meal size, and decreased 24-hour body weight gain [277,279]. Furthermore, VTA AmyR activation reduced the intake of a palatable, non-nutritive sweetener [293] and reduced the motivation to work for sucrose reward [277] illustrating the peptide’s involvement in regulation of food reward.
Amy action in the VTA may be preferentially targeted to regulation of fat intake as VTA CTR knockdown resulted in hyperphagia and increased body weight gain in rats fed HFD but not chow-fed animals [279] and VTA sCT produced a greater and more rapid suppressive effect on fat intake compared to sucrose [293]. Very little data is available on the action of Amy in NAcc, however. One study showed that Amy acts in the NAcc shell but not core to reduce food intake in food-deprived rats but this effect might have been due to an off-target action of Amy because changes in feeding were absent in a different experiment in which NAcc was targeted to avoid penetration of the lateral ventricle [294].
Mechanistically, Amy appears to modulate DA neurons to regulate feeding. For example, intra-VTA sCT administration attenuated food-induced DA release in the NAcc core [279] and NAcc core D1/D2R agonist blocked intake and body weight-suppressive effects of VTA AmyR activation in chow and HFD fed rats [279]. Further experiments will be necessary to determine how Amy regulates DA and other VTA neurons, however.
Alcohol intake and reward:
Amy signaling is involved in the expression of alcohol responses as shown by the attenuation of both acute and chronic alcohol-directed behaviors in rodents by systemic AmyR activation. For example, systemically administered sCT reduced alcohol-induced locomotor stimulation, alcohol-induced CPP, self-administration, and relapse drinking [284,295] with concurrent attenuation of alcohol-induced DA release in NAcc [284]. Similarly, sCT blocked alcohol-induced locomotor stimulation when administered into the VTA or NAcc shell and intra-VTA sCT decreased alcohol-induced DA release in the NAcc shell in mice and decreased alcohol intake in rats [282].
Effects on drugs of abuse:
Similar to the effects on food and alcohol-dependent NAcc DA release, peripheral treatment with sCT attenuated cocaine and nicotine-induced elevation in accumbal DA [285,296] and inhibited the expression of amphetamine, cocaine, and nicotine-induced hyperlocomotion [285,296–298]. Interestingly, unlike the effects on alcohol, peripheral sCT injection did not affect the rewarding properties or reward-dependent memory of cocaine or nicotine in the CPP paradigm [285,296] suggesting that Amy may regulate alcohol and stimulant drug reward differently. Local activation of VTA or NAcc shell AmyR reduced but did not fully inhibit cocaine-evoked locomotor stimulation, however [285], suggesting possible involvement of other brain areas in Amy-mediated effects. Further experiments will be necessary to fully dissect the mechanism by which Amy influences drug reward.
Neurotensin
Neurotensin (NT) is an anorexigenic peptide released peripherally by intestinal enteroendocrine cells and the adrenal gland as well as centrally by neurons of several regions including the LH. This peptide signals through NT receptors (NTR1, NTR2, NTR3) and has been implicated in the regulation of many different physiological processes and behaviors including analgesia, blood pressure, body temperature, locomotion, and fluid and energy homeostasis. In this review, we will briefly go over the actions of NT in the mesolimbic system in the regulation of energy homeostasis and natural and drug reward but these topics are more thoroughly discussed in other prior reviews [299–301]).
Food and food reward:
Central and peripheral treatment with NT decreased food intake in fasted and sated rodents [302–304] and the anorectic effect of leptin was attenuated in NTR1 knockouts [305]. Substantial evidence suggests that NT interacts with the mesolimbic system to regulate behavior. Neuroanatomical studies support this interaction as NT-expressing neurons, primarily from the LH and medial and lateral preoptic areas, have been shown to project to the VTA [306–308]. Additionally, NT-immunoreactive axon terminals are found in direct contact with dendrites and perikarya of VTA TH+ neurons [309], and NT receptors, primarily NTR1, are expressed in the VTA neurons [310–312] where they are co-expressed with TH [313]. Intra-VTA injection of NT increased latency to eat and reduced food intake in fasted but not sated animals [314,315] and reduced operant responding for food [316]. Conversely, intra-NAcc injection of NT did not alter food intake [317]. Interestingly, ablation of VTA NTR1-expressing neurons in adult mice reduced body weight in chow-fed animals and protected the mice from HFD-induced obesity while increasing their intake of chow or HFD. This lean-promoting phenotype appeared to be caused by increased physical activity and energy expenditure [313]. Similar to chow and HFD, VTA NTR1 neuron ablated mice overconsumed sucrose but appeared to have unaltered motivation for sucrose. However, these mice failed to adjust their sucrose self-administration to energy sufficiency cues such as sucrose pre-feeding or leptin [313] suggesting that VTA NTR1 neurons may serve as a point of interaction between signals conveying metabolic status and motivated behavior.
Effects on drugs of abuse:
Central administration of NT or its analogs blocked amphetamine, cocaine, and nicotine-induced hyperactivity [318,319], decreased self-administration of METH and nicotine [320,321], prevented initiation and expression of sensitization to nicotine [322], and decreased alcohol preference and intake [323,324]. The effect on alcohol appears to be modulated by NTR1 as NTR1 null mice have increased ethanol intake, decreased sensitivity to ethanol-induced ataxia and these mice fail to respond to peripherally injected NT analog [323]. Much is unknown about the neural substrates of the NT action in regulation of drug reward and future experiments are needed to determine the mechanisms underlying NT involvement in these behaviors.
Glucagon-like peptide-1
Glucagon-like peptide-1 (GLP-1) is an anorexigenic incretin secreted from L cells of the small and large intestine and centrally from the preproglucagon neurons of the NTS. Several GLP-1 analogs, including Exendin-4 (Ex-4) and Liraglutide, are FDA-approved for treatment of type 2 diabetes and weight management because they increased insulin production, suppressed food intake, and produced weight loss (for review see [325–327]). In rodents, peripheral injections of Ex-4 decreased food intake and food and drug reward-associated behaviors [328–332], and a large body of data suggests that the mesolimbic circuitry is one of the neuroanatomical substrates of GLP-1’s action in regulation of these behaviors. This is supported anatomically as both VTA and NAcc have been shown to contain GLP1-R mRNA [333], to house GLP-1 containing fibers [334,335], and to receive projections from GLP-1-expressing NTS neurons as identified by anterograde tracing experiments [334–336].
Food and food reward
The mesolimbic system is one of the targets of GLP-1 analogs in regulation of feeding as the reduction in feeding produced by systemic Ex-4 was attenuated by VTA pretreatment with a GLP-1R antagonist [337]. Injections of Ex-4 directly into the VTA also reduced intake of both palatable food and regular chow [329,335,337], and the effects on the palatable HFD intake were shown to be regulated in part by glutamatergic AMPA/kainate receptor signaling [337]. The intake of chow was only reduced in a fasted state or in ad libitum fed rodents when chow is the only caloric source, however [329]. Conversely, activation of VTA and NAcc core and shell GLP-1Rs in animals fed chow and HFD simultaneously decreased HFD intake while increasing chow intake [335,337,338] suggesting that GLP-1 plays a role in regulation of preference for high-energy food. Chemogenetic induction of GLP-1 release from NTS neurons in the VTA has also been shown to reduce HFD intake and to modulate this effect through a reduction in excitatory synaptic strength of VTA DA neurons that project to NAcc medial shell [339]. Ex-4 also decreased 1-hour sucrose intake when administered to the VTA and NAcc core but not NAcc shell suggestive of site-specific macronutrient intake regulation [335]. NAcc core administration of GLP-1R antagonist increased sucrose meal size and sucrose palatability but had no effect on licking for sweet saccharin solution suggesting that GLP-1 acts in the NAcc core to regulate the hedonic value of nutritive foods [340]. Peripheral and central administration of Ex-4 also strongly inhibits food reward-associated behaviors and the mesolimbic structures have been implicated in these responses [329] as shown by a reduction in the motivation to obtain sucrose reward in a progressive ratio paradigm by intra-VTA or intra-NAcc injections of Ex-4 [329]. Taken together, GLP-1 appears to act in the mesolimbic structures to decrease intake as well as the rewarding properties of hedonic foods with a smaller contribution to context-dependent regulation of homeostatic intake.
Alcohol intake and reward
Similar to its effects on food, peripheral injections of GLP-1 or Ex-4 have been shown to supress alcohol intake and to decrease multiple measures of alcohol-induced reward and seeking behaviors [331,341,342]. The effect on alcohol intake is dependent on the baseline consumption of alcohol as alcohol consumption was reduced by GLP-1R agonist only in alcohol-preferring rats while it remained unaffected in natural ‘nondrinkers’ [341,342]. The NAcc is one of the neuroanatomical substrates of GLP-1 mediated effects, as the same behavioral pattern was observed upon intra-NAcc Ex-4 treatment [343]. Unlike its effects on food, however, GLP-1 seems to modulate alcohol-related behaviors through NAcc shell and not core because NAcc shell but not core Ex-4 injections decreased alcohol intake [343,344] as well as alcohol-induced locomotor response and alcohol CPP while leaving chow intake and body weight unaffected [343]. These results suggest that anatomically distinct portions of the mesolimbic circuitry may modulate homeostatic feeding and alcohol-directed behaviors. The effects of GLP-1 in the VTA on alcohol-related behaviors are somewhat conflicting. Intra-VTA Ex-4 has been shown to decrease alcohol intake in rats [342] but this effect on intake could not be replicated by another group, although they did report a decrease in alcohol-induced locomotor behavior [343]. Interestingly, VTA effects were only produced by posterior and not anterior VTA treatment with Ex-4 [343] suggesting that GLP-1 acts on a specific VTA sub-circuit to regulate alcohol-directed behaviors. Taken together, GLP-1 acts on the structures of the mesolimbic system to attenuate alcohol intake and reward and this action and circuitry involved may be distinct from that used to regulate food-associated behaviors.
Effects on drugs of abuse
Systemic or central administration of GLP-1R agonists attenuate addiction-related effects of cocaine, amphetamine, and nicotine including intake, operant behavior, and CPP [330,332,345–349]. In the VTA, Ex-4 reduced cocaine self-administration [350] and primed reinstatement of cocaine seeking [351] while VTA GLP-1R knockdown increased cocaine self-administration [350]. Similarly, Ex-4 has been shown to act in both NAcc shell and core to reduce cocaine-primed reinstatement of drug seeking [352]. Interestingly, unlike GLP-1’s effects on the regulation of intake and rewarding aspects of food, alcohol, cocaine, amphetamine, and nicotine, peripherally administered GLP-1R agonist failed to decrease the addiction-related behavioral effects of opioids including CPP, withdrawal, hyperlocomotion, or self-administration [353] suggestive of differences in the mechanisms regulating effects of opioids and. other natural and drug rewarding substances.
Summary/Conclusions
Multiple peptides and hormones that play important roles in the control of feeding and energy homeostasis can interact with the mesolimbic DA system to regulate an array of DA-dependent behaviors, including food, drug, and social reward (Fig. 1). In some cases, the effects of these peptides are similar across different rewards, but for others there appear to be distinct effects of the peptides on different types of reward (Fig. 1). Furthering our understanding of how these feeding-related peptides interact with mesolimbic circuits to regulate behavior may help identify how changes in feeding and weight alter mesolimbic DA system-dependent behaviors and may allow for new approaches to treat disorders associated with these systems.
Fig. 1.
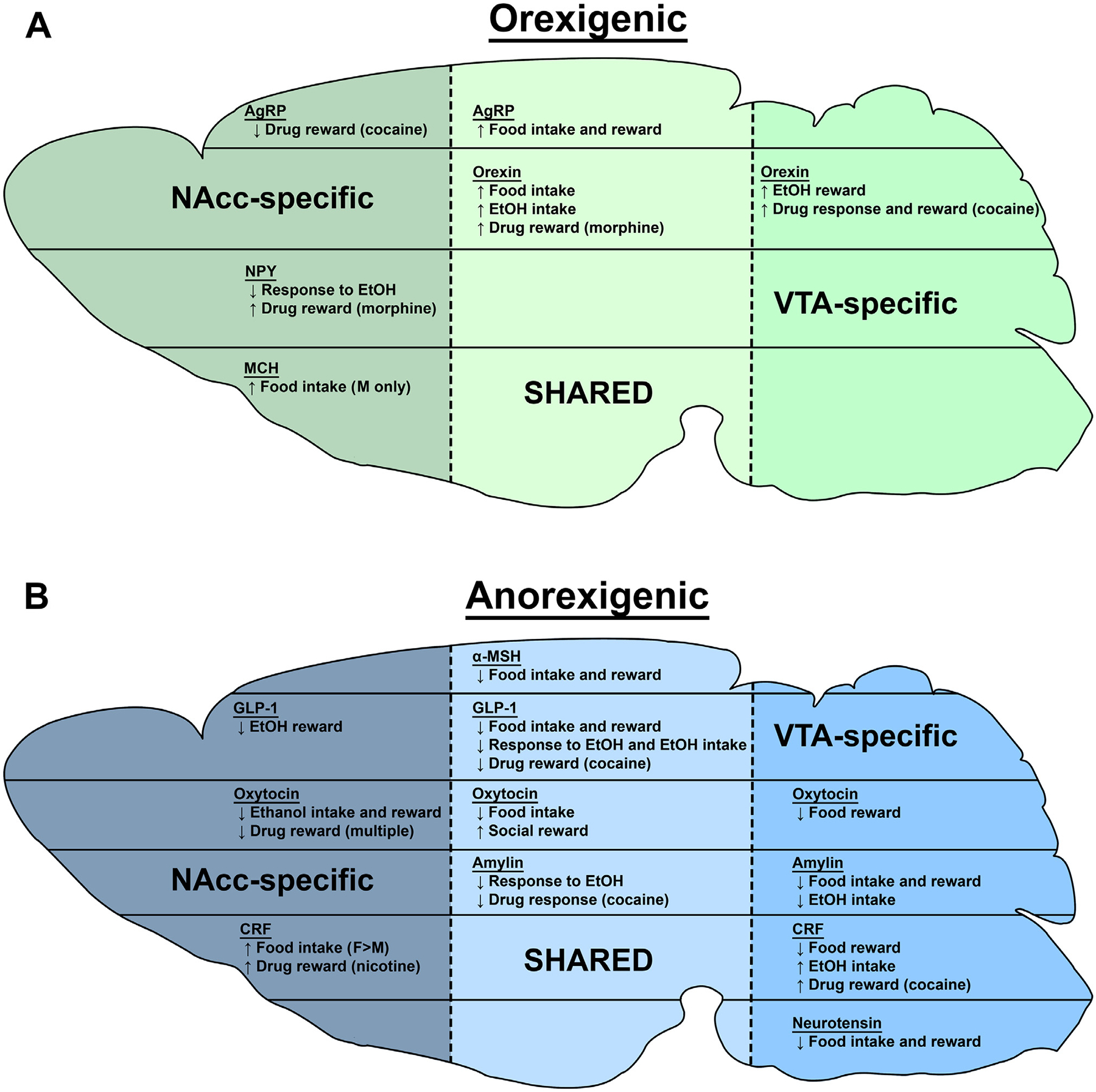
Summary of the behavioral effects of the mesolimbic system action of peptides and hormones reviewed in this manuscript. Sagittal outline of the brain in green summarizes the effects of intra-NAcc and intra-VTA actions of orexigenic peptides (A) while the section in blue summarizes the effects of anorexigenic peptides (B) and hormones on feeding as well as food, drug, and social reward. In general, “intake” refers to free consumption, “reward” refers to behaviors measured by paradigms such as self-administration and CPP, and “response” refers to behaviors such as substance-induced locomotion. “NAcc-“and VTA-specific sections describe effects produced exclusively in that area (which sometimes were due to the lack of response and in other cases due to lack of testing) whereas the SHARED section contains common effects produced by peptide/hormone action in the two brain regions. Abbreviations used: EtOH-alcohol; M-male; F-female.
Funding
Funding for these studies was provided by NIH grant 1R01DK115503 (to AGR).
Footnotes
Declaration of Competing Intrest
The authors declare no conflicts of interest.
Data Availability Statement
No data was generated in this manuscript.
References
- [1].Grace AA, Floresco SB, Goto Y, Lodge DJ, Regulation of firing of dopaminergic neurons and control of goal-directed behaviors, Trends Neurosci 30 (2007) 220–227, doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [2].Paladini CA, Roeper J, Generating bursts (and pauses) in the dopamine midbrain neurons, Neuroscience 282 (2014) 109–121, doi: 10.1016/j.neuroscience.2014.07.032. [DOI] [PubMed] [Google Scholar]
- [3].Morikawa H, Paladini CA, Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms, Neuroscience 198 (2011) 95–111, doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olds J, Milner P, Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain, J Comp Physiol Psychol 47 (1954) 419–427, doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- [5].Stutz RM, Rossi RR, Bowring AM, Competition between food and rewarding brain shock, Physiol Behav 7 (1971) 753–757, doi: 10.1016/0031-9384(71)90144-2. [DOI] [PubMed] [Google Scholar]
- [6].Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM, Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation, Nature 398 (1999) 67–69, doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- [7].Lammel S, Lim BK, Malenka RC, Reward and aversion in a heterogeneous midbrain dopamine system, Neuropharmacology 76 (2014) 351–359, doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bromberg-Martin ES, Matsumoto M, Hikosaka O, Dopamine in Motivational Control: Rewarding, Aversive, and Alerting, Neuron 68 (2010) 815–834, doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schultz W, Dopamine reward prediction-error signalling: a two-component response, Nat Rev Neurosci 17 (2016) 183–195, doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schultz W, Multiple dopamine functions at different time courses, Annu Rev Neurosci 30 (2007) 259–288, doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- [11].Schultz W, Dayan P, Montague PR, A neural substrate of prediction and reward, Science 275 (1997) 1593–1599, doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- [12].Carr KD, Modulatory Effects of Food Restriction on Brain and Behavioral Effects of Abused Drugs, Curr Pharm Des 26 (2020) 2363–2371, doi: 10.2174/1381612826666200204141057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kenny PJ, Reward mechanisms in obesity: new insights and future directions, Neuron 69 (2011) 664–679, doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kenny PJ, Common cellular and molecular mechanisms in obesity and drug addiction, Nat Rev Neurosci 12 (2011) 638–651, doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- [15].Volkow ND, Wang GJ, Baler RD, Reward, dopamine and the control of food intake: implications for obesity, Trends Cogn Sci 15 (2011) 37–46, doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DiLeone RJ, Taylor JR, Picciotto MR, The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction, Nat Neurosci 15 (2012) 1330–1335, doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cone RD, Studies on the physiological functions of the melanocortin system, Endocr Rev 27 (2006) 736–749, doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- [18].Butler AA, The melanocortin system and energy balance, Peptides 27 (2006) 281–290, doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hill JW, Faulkner LD, The Role of the Melanocortin System in Metabolic Disease: New Developments and Advances, Neuroendocrinology 104 (2017) 330–346, doi: 10.1159/000450649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cone RD, Anatomy and regulation of the central melanocortin system, Nat Neurosci 8 (2005) 571–578, doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- [21].Torre E, Celis ME, Cholinergic mediation in the ventral tegmental area of alpha melanotropin induced excessive grooming changes of the dopamine activity in the nucleus accumbens and caudate putamen, Life Sciences 42 (1988) 1651–1658, doi: 10.1016/0024-3205(88)90444-4. [DOI] [PubMed] [Google Scholar]
- [22].Torre E, Celis ME, Alpha msh injected into the substantia nigra or intraventricularly alters behavior and the striatal dopaminergic activity, Neurochemistry International 9 (1986) 85–90, doi: 10.1016/0197-0186(86)90035-5. [DOI] [PubMed] [Google Scholar]
- [23].Klusa V, Svirskis S, Opmane B, Muceniece R, Wikberg JES, Behavioural responses of gamma-MSH peptides administered into the rat ventral tegmental area, Acta Physiologica Scandinavica 167 (1999) 99–104. [DOI] [PubMed] [Google Scholar]
- [24].Sanchez MS, Barontini M, Armando I, Celis ME, Correlation of increased grooming behavior and motor activity with alterations in nigrostriatal and mesolimbic catecholamines after alpha-melanotropin and neuropeptide glutamine-isoleucine injection in the rat ventral tegmental area, Cellular and Molecular Neurobiology 21 (2001) 523–533, doi: 10.1023/a:1013871407464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK, Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat, J Comp Neurol 457 (2003) 213–235, doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- [26].Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK, Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter, J Neurosci 23 (2003) 7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD, Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system, Proc Natl Acad Sci U S A 90 (1993) 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lippert RN, Ellacott KL, Cone RD, Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice, Endocrinology 155 (2014) 1718–1727, doi: 10.1210/en.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dunigan AI, Olson DP, Roseberry AG, VTA MC3R neurons control feeding in an activity- and sex-dependent manner in mice, Neuropharmacology (2021) 108746, doi: 10.1016/j.neuropharm.2021.108746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araújo I, Liu ZW, Horvath TL, AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors, Nat Neurosci 15 (2012) 1108–1110, doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dunigan AI, Swanson AM, Olson DP, Roseberry AG, Whole-brain efferent and afferent connectivity of mouse ventral tegmental area melanocortin-3 receptor neurons, J Comp Neurol (2020), doi: 10.1002/cne.25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qu N, He Y, Wang C, Xu P, Yang Y, Cai X, Liu H, Yu K, Pei Z, Hyseni I, Sun Z, Fukuda M, Li Y, Tian Q, Xu Y, A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia, Mol Psychiatry 25 (2020) 1006–1021, doi: 10.1038/s41380-019-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jeong JK, Diano S, Prolyl carboxypeptidase mRNA expression in the mouse brain, Brain Res 1542 (2014) 85–92, doi: 10.1016/j.brainres.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, Kobzar NP, Salgado IA, Reddy DM, Sun F, Zhang Y, Li Y, Cui G, Krashes MJ, High-fat food biases hypothalamic and mesolimbic expression of consummatory drives, Nat Neurosci 23 (2020) 1253–1266, doi: 10.1038/s41593-020-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].King CM, Hentges ST, Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites, PLoS One 6 (2011) e25864, doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Betley JN, Cao ZF, Ritola KD, Sternson SM, Parallel, redundant circuit organization for homeostatic control of feeding behavior, Cell 155 (2013) 1337–1350, doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Barber TR, Griffanti L, Bradley KM, McGowan DR, Lo C, Mackay CE, Hu MT, Klein JC, Nigrosome 1 imaging in REM sleep behavior disorder and its association with dopaminergic decline, Ann Clin Transl Neurol 7 (2020) 26–35, doi: 10.1002/acn3.50962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].West KS, Lu C, Olson DP, Roseberry AG, α-MSH increases the activity of MC3R-expressing neurons in the ventral tegmental area, J Physiol (2019), doi: 10.1113/JP277193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lerma-Cabrera JM, Carvajal F, Garbutt JC, Navarro M, Thiele TE, The melanocortin system as a potential target for treating alcohol use disorders: A review of pre-clinical data, Brain Res 1730 (2020) 146628, doi: 10.1016/j.brainres.2019.146628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aponte Y, Atasoy D, Sternson SM, AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training, Nat Neurosci 14 (2011) 351–355, doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD, Role of melanocortinergic neurons in feeding and the agouti obesity syndrome, Nature 385 (1997) 165–168, doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- [42].Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS, Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity, Diabetes 51 (2002) 1337–1345, doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- [43].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB, Rapid, reversible activation of AgRP neurons drives feeding behavior in mice, J Clin Invest 121 (2011) 1424–1428, doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL, Overexpression of Agrt leads to obesity in transgenic mice, Nat Genet 17 (1997) 273–274, doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- [45].Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR, A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo, Endocrinology 139 (1998) 4428–4431, doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- [46].Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS, Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein, Science 278 (1997) 135–138, doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- [47].Roseberry AG, Altered feeding and body weight following melanocortin administration to the ventral tegmental area in adult rats, Psychopharmacology (Berl) 226 (2013) 25–34, doi: 10.1007/s00213-012-2879-6. [DOI] [PubMed] [Google Scholar]
- [48].Shanmugarajah L, Dunigan AI, Frantz KJ, Roseberry AG, Altered sucrose self-administration following injection of melanocortin receptor agonists and antagonists into the ventral tegmental area, Psychopharmacology (Berl) (2017), doi: 10.1007/s00213-017-4570-4. [DOI] [PubMed] [Google Scholar]
- [49].Yen HH, Roseberry AG, Decreased consumption of rewarding sucrose solutions after injection of melanocortins into the ventral tegmental area of rats, Psychopharmacology (Berl) (2014), doi: 10.1007/s00213-014-3663-6. [DOI] [PubMed] [Google Scholar]
- [50].Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ, Garner K, Kallo I, Ghanem A, Liposits Z, Conzelmann KK, Vanderschuren LJ, la Fleur SE, Adan RA, Melanocortin 3 Receptor Signaling in Midbrain Dopamine Neurons Increases the Motivation for Food Reward, Neuropsychopharmacology (2016), doi: 10.1038/npp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pandit R, van der Zwaal EM, Luijendijk MC, Brans MA, van Rozen AJ, Oude Ophuis RJ, Vanderschuren LJ, Adan RA, la Fleur SE, Central Melanocortins Regulate the Motivation for Sucrose Reward, PLoS One 10 (2015) e0121768, doi: 10.1371/journal.pone.0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD, A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse, Endocrinology 141 (2000) 3518–3521, doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- [53].Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH, Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass, Nat Genet 26 (2000) 97–102, doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- [54].Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH, Role of the melanocortin-4 receptor in metabolic rate and food intake in mice, Transgenic Res 9 (2000) 145–154, doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- [55].Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F, Targeted disruption of the melanocortin-4 receptor results in obesity in mice, Cell 88 (1997) 131–141, doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- [56].Mavrikaki M, Girardet C, Kern A, Faruzzi Brantley A, Miller CA, Macarthur H, Marks DL, Butler AA, Melanocortin-3 receptors in the limbic system mediate feeding-related motivational responses during weight loss, Mol Metab 5 (2016) 566–579, doi: 10.1016/j.molmet.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lerma-Cabrera JM, Carvajal F, de la Torre L, de la Fuente L, Navarro M, Thiele TE, Cubero I, Control of food intake by MC4-R signaling in the lateral hypothalamus, nucleus accumbens shell and ventral tegmental area: interactions with ethanol, Behav Brain Res 234 (2012) 51–60, doi: 10.1016/j.bbr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergström L, Wikberg JE, The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine, Neuroreport 12 (2001) 2155–2158. [DOI] [PubMed] [Google Scholar]
- [59].Wikberg JE, Melanocortin receptors: perspectives for novel drugs, Eur J Pharmacol 375 (1999) 295–310, doi: 10.1016/s0014-2999(99)00298-8. [DOI] [PubMed] [Google Scholar]
- [60].Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS, Blockade of melanocortin transmission inhibits cocaine reward, Eur J Neurosci 21 (2005) 2233–2242, doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cabeza de Vaca S, Kim GY, Carr KD, The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in ad-libitumfed and food-restricted rats, Psychopharmacology (Berl) 161 (2002) 77–85, doi: 10.1007/s00213-002-0998-1. [DOI] [PubMed] [Google Scholar]
- [62].Upadhya MA, Upadhya HM, Borkar CD, Choudhary AG, Singh U, Chavan P, Sakharkar A, Singru P, Subhedar NK, Kokare DM, Nicotine-induced Brain Stimulation Reward is Modulated by Melanocortin-4 Receptors in Ovariectomized Rats, Neuroscience 431 (2020) 205–221, doi: 10.1016/j.neuroscience.2020.01.035. [DOI] [PubMed] [Google Scholar]
- [63].Cui H, Lutter M, The expression of MC4Rs in D1R neurons regulates food intake and locomotor sensitization to cocaine, Genes Brain Behav 12 (2013) 658–665, doi: 10.1111/gbb.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL, The anatomy of neuropeptide-Y-containing neurons in rat brain, Neuroscience 15 (1985) 1159–1181, doi: 10.1016/0306-4522. [DOI] [PubMed] [Google Scholar]
- [65].van den Heuvel JK, Furman K, Gumbs MC, Eggels L, Opland DM, Land BB, Kolk SM, Narayanan NS, Fliers E, Kalsbeek A, DiLeone RJ, la Fleur SE, Neuropeptide Y activity in the nucleus accumbens modulates feeding behavior and neuronal activity, Biol Psychiatry 77 (2015) 633–641, doi: 10.1016/j.biopsych.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gumbs MCR, Vuuregge AH, Eggels L, Unmehopa UA, Lamuadni K, Mul JD, la Fleur SE, Afferent neuropeptide Y projections to the ventral tegmental area in normal-weight male Wistar rats, J Comp Neurol (2019), doi: 10.1002/cne.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Blomqvist AG, Herzog H, Y-receptor subtypes–how many more? Trends Neurosci 20 (1997) 294–298, doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- [68].Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL, Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat, Eur J Neurosci 23 (2006) 2677–2685, doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- [69].Parker RM, Herzog H, Regional distribution of Y-receptor subtype mRNAs in rat brain, Eur J Neurosci 11 (1999) 1431–1448, doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- [70].Quarta D, Leslie CP, Carletti R, Valerio E, Caberlotto L, Central administration of NPY or an NPY-Y5 selective agonist increase in vivo extracellular monoamine levels in mesocorticolimbic projecting areas, Neuropharmacology 60 (2011) 328–335, doi: 10.1016/j.neuropharm.2010.09.016. [DOI] [PubMed] [Google Scholar]
- [71].Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK, Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor, J Comp Neurol 482 (2005) 217–243, doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- [72].West KS, Roseberry AG, Neuropeptide-Y alters VTA dopamine neuron activity through both pre- and postsynaptic mechanisms, J Neurophysiol 118 (2017) 625–633, doi: 10.1152/jn.00879.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sørensen G, Wegener G, Hasselstrøm J, Hansen TV, Wörtwein G, Fink-Jensen A, Woldbye DP, Neuropeptide Y infusion into the shell region of the rat nucleus accumbens increases extracellular levels of dopamine, Neuroreport 20 (2009) 1023–1026, doi: 10.1097/wnr.0b013e32832d4848. [DOI] [PubMed] [Google Scholar]
- [74].Clark JT, Kalra PS, Crowley WR, Kalra SP, Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats, Endocrinology 115 (1984) 427–429, doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- [75].Vettor R, Zarjevski N, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B, Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y administration to normal rats, Diabetologia 37 (1994) 1202–1208, doi: 10.1007/BF00399793. [DOI] [PubMed] [Google Scholar]
- [76].Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC, Agouti-related peptide-expressing neurons are mandatory for feeding, Nat Neurosci 8 (2005) 1289–1291, doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- [77].Luquet S, Perez FA, Hnasko TS, Palmiter RD, NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates, Science 310 (2005) 683–685, doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- [78].Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T, Effects of neuropeptide Y, insulin, 2-deoxyglucose, and food deprivation on food-motivated behavior, Psychopharmacology (Berl) 120 (1995) 267–271, doi: 10.1007/BF02311173. [DOI] [PubMed] [Google Scholar]
- [79].Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA, Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food, Obesity (Silver Spring) 22 (2014) 1216–1219, doi: 10.1002/oby.20718. [DOI] [PubMed] [Google Scholar]
- [80].Brown CM, Coscina DV, Fletcher PJ, The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens, Peptides 21 (2000) 1279–1287, doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- [81].Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK, Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats, Alcohol Clin Exp Res 25 (2001) 386–390. [PubMed] [Google Scholar]
- [82].Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK, Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats, Alcohol Clin Exp Res 27 (2003) 894–899, doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- [83].Sparta DR, Fee JR, Hayes DM, Knapp DJ, MacNeil DJ, Thiele TE, Peripheral and central administration of a selective neuropeptide Y Y1 receptor antagonist suppresses ethanol intake by C57BL/6J mice, Alcohol Clin Exp Res 28 (2004) 1324–1330, doi: 10.1097/01.alc.0000139829.67958.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M, Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking, Psychopharmacology (Berl) 208 (2010) 417–426, doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- [85].Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M, Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence, Biol Psychiatry 69 (2011) 1091–1099, doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hayes DM, Fee JR, McCown TJ, Knapp DJ, Breese GR, Cubero I, Carvajal F, Lerma-Cabrera JM, Navarro M, Thiele TE, Neuropeptide Y signaling modulates the expression of ethanol-induced behavioral sensitization in mice, Addict Biol 17 (2012) 338–350, doi: 10.1111/j.1369-1600.2011.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Borkar CD, Upadhya MA, Shelkar GP, Subhedar NK, Kokare DM, Neuropeptide Y system in accumbens shell mediates ethanol self-administration in posterior ventral tegmental area, Addict Biol 21 (2016) 766–775, doi: 10.1111/adb.12254. [DOI] [PubMed] [Google Scholar]
- [88].Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD, Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor, Pharmacol Biochem Behav 67 (2000) 683–691, doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- [89].Thiele TE, Koh MT, Pedrazzini T, Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor, J Neurosci 22 (2002) RC208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Thiele TE, Naveilhan P, Ernfors P, Assessment of ethanol consumption and water drinking by NPY Y(2) receptor knockout mice, Peptides 25 (2004) 975–983, doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [91].Sørensen G, Jensen M, Weikop P, Dencker D, Christiansen SH, Loland CJ, Bengtsen CH, Petersen JH, Fink-Jensen A, Wörtwein G, Woldbye DP, Neuropeptide Y Y5 receptor antagonism attenuates cocaine-induced effects in mice, Psychopharmacology (Berl) 222 (2012) 565–577, doi: 10.1007/s00213-012-2651-y. [DOI] [PubMed] [Google Scholar]
- [92].Woldbye DP, Klemp K, Madsen TM, Neuropeptide Y attenuates naloxone-precipitated morphine withdrawal via Y5-like receptors, J Pharmacol Exp Ther 284 (1998) 633–636. [PubMed] [Google Scholar]
- [93].Wang X, Tian Z, Ma J, Feng Z, Ou Y, Zhou M, Peng J, Lv Y, Gao G, Qi S, NPY alterations induced by chronic morphine exposure affect the maintenance and reinstatement of morphine conditioned place preference, Neuropharmacology 181 (2020) 108350, doi: 10.1016/j.neuropharm.2020.108350. [DOI] [PubMed] [Google Scholar]
- [94].Desai SJ, Upadhya MA, Subhedar NK, Kokare DM, NPY mediates reward activity of morphine, via NPY Y1 receptors, in the nucleus accumbens shell, Behav Brain Res 247 (2013) 79–91, doi: 10.1016/j.bbr.2013.03.018. [DOI] [PubMed] [Google Scholar]
- [95].Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE, Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive, Nature 490 (2012) 402–406, doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rodaros D, Caruana DA, Amir S, Stewart J, Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area, Neuroscience 150 (2007) 8–13, doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- [97].Swanson LW, Sawchenko PE, Rivier J, Vale WW, Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study, Neuroendocrinology 36 (1983) 165–186, doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- [98].Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, Chwalek M, Maal-Bared G, Freiling J, Schlosburg JE, Clarke L, Crawford E, Koebel P, Repunte-Canonigo V, Sanna PP, Tapper AR, Roberto M, Kieffer BL, Sawchenko PE, Koob GF, van der Kooy D, George O, VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation, Nat Neurosci 17 (2014) 1751–1758, doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tagliaferro P, Morales M, Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic, J Comp Neurol 506 (2008) 616–626, doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB, Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking, J Neurosci 25 (2005) 5389–5396, doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT, CRF enhancement of GIRK channel-mediated transmission in dopamine neurons, Neuropsychopharmacology 34 (2009) 1926–1935, doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A, Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons, Neuron 39 (2003) 401–407, doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- [103].Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A, Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih, J Physiol 586 (2008) 2157–2170, doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T, Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain, Proc Natl Acad Sci U S A 92 (1995) 836–840, doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA, Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice, Alcohol Clin Exp Res 39 (2015) 1609–1618, doi: 10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hahn J, Hopf FW, Bonci A, Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons, J Neurosci 29 (2009) 6535–6544, doi: 10.1523/JNEU-ROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bernardi RE, Broccoli L, Hirth N, Justice NJ, Deussing JM, Hansson AC, Spanagel R, Dissociable Role of Corticotropin Releasing Hormone Receptor Subtype 1 on Dopaminergic and D1 Dopaminoceptive Neurons in Cocaine Seeking Behavior, Front Behav Neurosci 11 (2017) 221, doi: 10.3389/fnbeh.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE, Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse, J Comp Neurol 428 (2000) 191–212, doi:. [DOI] [PubMed] [Google Scholar]
- [109].Wanat MJ, Bonci A, Phillips PE, CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors, Nat Neurosci 16 (2013) 383–385, doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Peciña S, Schulkin J, Berridge KC, Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol 4 (2006) 8, doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cappell H, Herman CP, Alcohol and tension reduction. A review, Q J Stud Alcohol 33 (1972) 33–64. [PubMed] [Google Scholar]
- [112].Anthenelli R, Grandison L, Effects of stress on alcohol consumption, Alcohol Res 34 (2012) 381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE, Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures, Alcohol Clin Exp Res 32 (2008) 259–265, doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Simms JA, Nielsen CK, Li R, Bartlett SE, Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395, Addict Biol 19 (2014) 606–611, doi: 10.1111/adb.12024. [DOI] [PubMed] [Google Scholar]
- [115].Hwa LS, Debold JF, Miczek KA, Alcohol in excess: CRF receptors in the rat and mouse VTA and DRN, Psychopharmacology (Berl) 225 (2013) 313–327, doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF, Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor, Alcohol Clin Exp Res 26 (2002) 1494–1501, doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- [117].Liu X, Weiss F, Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms, J Neurosci 22 (2002) 7856–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE, Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake, Biol Psychiatry 81 (2017) 930–940, doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, Ron D, Bonci A, Binge ethanol-drinking potentiates corticotropin releasing factor R1 receptor activity in the ventral tegmental area, Alcohol Clin Exp Res 37 (2013) 1680–1687, doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hwa LS, Holly EN, DeBold JF, Miczek KA, Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice, Psychopharmacology (Berl) 233 (2016) 681–690, doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Erb S, Petrovic A, Yi D, Kayyali H, Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context, Psychopharmacology (Berl) 187 (2006) 112–120, doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- [122].Brown ZJ, Tribe E, D’souza NA, Erb S, Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat, Psychopharmacology (Berl) 203 (2009) 121–130, doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- [123].Kupferschmidt DA, Klas PG, Erb S, Cannabinoid CB1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine-induced behavioural sensitization, Br J Pharmacol 167 (2012) 196–206, doi: 10.1111/j.1476-5381.2012.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Goeders NE, Guerin GF, Effects of the CRH receptor antagonist CP-154, 526 on intravenous cocaine self-administration in rats, Neuropsychopharmacology 23 (2000) 577–586, doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- [125].Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF, CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats, Psychopharmacology (Berl) 196 (2008) 473–482, doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA, Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB, Neuropsychopharmacology 34 (2009) 2609–2617, doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lu L, Liu D, Ceng X, Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats, Eur J Pharmacol 415 (2001) 203–208, doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- [128].McReynolds JR, Vranjkovic O, Thao M, Baker DA, Makky K, Lim Y, Mantsch JR, Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice, Psychopharmacology (Berl) 231 (2014) 3953–3963, doi: 10.1007/s00213-014-3535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shaham Y, Erb S, Leung S, Buczek Y, Stewart J, CP-154, 526, a selective, nonpeptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats, Psychopharmacology (Berl) 137 (1998) 184–190, doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- [130].Wang B, You ZB, Rice KC, Wise RA, Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat, Psychopharmacology (Berl) 193 (2007) 283–294, doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- [131].Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR, Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2, J Neurosci 31 (2011) 11396–11403, doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, Chen A, Lawrence AJ, Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice, J Neurosci 34 (2014) 11560–11570, doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA, Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration, J Neurosci 34 (2014) 6659–6667, doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW, Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats, Neuropharmacology 53 (2007) 958–966, doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bruijnzeel AW, Prado M, Isaac S, Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse, Biol Psychiatry 66 (2009) 110–117, doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Uribe KP, Correa VL, Pinales BE, Flores RJ, Cruz B, Shan Z, Bruijnzeel AW, Khan AM, O’Dell LE, Overexpression of corticotropin-releasing factor in the nucleus accumbens enhances the reinforcing effects of nicotine in intact female versus male and ovariectomized female rats, Neuropsychopharmacology 45 (2020) 394–403, doi: 10.1038/s41386-019-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE, The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization, J Comp Neurol 319 (1992) 218–245, doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- [138].Haemmerle CA, Campos AM, Bittencourt JC, Melanin-concentrating hormone inputs to the nucleus accumbens originate from distinct hypothalamic sources and are apposed to GABAergic and cholinergic cells in the Long-Evans rat brain, Neuroscience 289 (2015) 392–405, doi: 10.1016/j.neuroscience.2015.01.014. [DOI] [PubMed] [Google Scholar]
- [139].Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD, Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression, Genomics 79 (2002) 785–792, doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- [140].Saito Y, Cheng M, Leslie FM, Civelli O, Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain, J Comp Neurol 435 (2001) 26–40, doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- [141].Pissios P, Frank L, Kennedy AR, Porter DR, Marino FE, Liu FF, Pothos EN, Maratos-Flier E, Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice, Biol Psychiatry 64 (2008) 184–191, doi: 10.1016/j.biopsych.2007.12.011. [DOI] [PubMed] [Google Scholar]
- [142].Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA, The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat, Eur J Neurosci 12 (2000) 1194–1216, doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- [143].Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ, Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal, J Neurosci 23 (2003) 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O, The melanin-concentrating hormone system modulates cocaine reward, Proc Natl Acad Sci U S A 106 (2009) 6772–6777, doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chee MJ, Pissios P, Maratos-Flier E, Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus, J Comp Neurol 521 (2013) 2208–2234, doi: 10.1002/cne.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM, Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar, Elife 2 (2013) e01462, doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE, Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins, J Neurosci 23 (2003) 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Morganstern I, Gulati G, Leibowitz SF, Role of melanin-concentrating hormone in drug use disorders, Brain Res 1741 (2020) 146872, doi: 10.1016/j.brainres.2020.146872. [DOI] [PubMed] [Google Scholar]
- [149].Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E, Mice lacking melanin-concentrating hormone are hypophagic and lean, Nature 396 (1998) 670–674, doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- [150].Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E, Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance, J Clin Invest 107 (2001) 379–386, doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N, Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats, Int J Obes Relat Metab Disord 26 (2002) 1289–1295, doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- [152].Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Tanaka T, Tokita S, Moriya M, Iwaasa H, Kanatani A, Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone, Am J Physiol Endocrinol Metab 284 (2003) E583–E588, doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- [153].Tavares FX, Al-Barazanji KA, Bishop MJ, Britt CS, Carlton DL, Cooper JP, Feldman PL, Garrido DM, Goetz AS, Grizzle MK, Hertzog DL, Ignar DM, Lang DG, McIntyre MS, Ott RJ, Peat AJ, Zhou HQ, 6-(4-chlorophenyl)-3-substituted-thieno[3, 2-d]pyrimidin-4(3H)-one-based melanin-concentrating hormone receptor 1 antagonist, J Med Chem 49 (2006) 7108–7118, doi: 10.1021/jm060814b. [DOI] [PubMed] [Google Scholar]
- [154].Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C, Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist, Nat Med 8 (2002) 825–830, doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- [155].Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ, The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance, J Neurosci 25 (2005) 2933–2940, doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MM, Sears RM, Homberg JR, Schoffelmeer AN, Adan RA, DiLeone RJ, De Vries TJ, Cuppen E, Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat, PLoS One 6 (2011) e19600, doi: 10.1371/journal.pone.0019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Terrill SJ, Subramanian KS, Lan R, Liu CM, Cortella AM, Noble EE, Kanoski SE, Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner, Neuropharmacology 178 (2020) 108270, doi: 10.1016/j.neuropharm.2020.108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M, Cippitelli A, Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: reduced appetite for calories and suppression of addictive-like behaviors, Pharmacol Biochem Behav 102 (2012) 400–406, doi: 10.1016/j.pbb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- [159].Nair SG, Adams-Deutsch T, Pickens CL, Smith DG, Shaham Y, Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats, Psychopharmacology (Berl) 205 (2009) 129–140, doi: 10.1007/s00213-009-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity, Proc Natl Acad Sci U S A 95 (1998) 322–327, doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior, Cell 92 (1998) 696, doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- [162].Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, Neurons containing hypocretin (orexin) project to multiple neuronal systems, J Neurosci 18 (1998) 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Baldo BA, Daniel RA, Berridge CW, Kelley AE, Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress, J Comp Neurol 464 (2003) 220–237, doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- [164].Fadel J, Deutch AY, Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area, Neuroscience 111 (2002) 379–387, doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- [165].Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, Differential expression of orexin receptors 1 and 2 in the rat brain, J Comp Neurol 435 (2001) 6–25, doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- [166].Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, Distribution of orexin receptor mRNA in the rat brain, FEBS Lett 438 (1998) 71–75, doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- [167].Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T, Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system, Brain Res 873 (2000) 181–187, doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- [168].Borgland SL, Storm E, Bonci A, Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons, Eur J Neurosci 28 (2008) 1545–1556, doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- [169].Baimel C, Lau BK, Qiao M, Borgland SL, Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons, Cell Rep 18 (2017) 1346–1355, doi: 10.1016/j.celrep.2017.01.030. [DOI] [PubMed] [Google Scholar]
- [170].Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T, Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine, J Neurosci 26 (2006) 398–405, doi: 10.1523/JNEU-ROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Vittoz NM, Berridge CW, Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area, Neuropsychopharmacology 31 (2006) 384–395, doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- [172].Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR, The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin, J Endocrinol 160 (1999) R7–12, doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- [173].Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR, A selective orexin-1 receptor antagonist reduces food consumption in male and female rats, Regul Pept 96 (2000) 45–51, doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- [174].Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR, Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice, Regul Pept 104 (2002) 153–159, doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- [175].Lubkin M, Stricker-Krongrad A, Independent feeding and metabolic actions of orexins in mice, Biochem Biophys Res Commun 253 (1998) 241–245, doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- [176].Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T, Orexins/hypocretins regulate drinking behaviour, Brain Res 842 (1999) 256–261, doi: 10.1016/s0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- [177].Morello G, Imperatore R, Palomba L, Finelli C, Labruna G, Pasanisi F, Sacchetti L, Buono L, Piscitelli F, Orlando P, Di Marzo V, Cristino L, Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling, Proc Natl Acad Sci U S A 113 (2016) 4759–4764, doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sweet DC, Levine AS, Billington CJ, Kotz CM, Feeding response to central orexins, Brain Res 821 (1999) 535–538, doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- [179].Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y, Inhibition of food intake by central injection of anti-orexin antibody in fasted rats, Biochem Biophys Res Commun 267 (2000) 527–531, doi: 10.1006/bbrc.1999.1998. [DOI] [PubMed] [Google Scholar]
- [180].White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, York DA, Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity, Peptides 26 (2005) 2331–2338, doi: 10.1016/j.peptides.2005.03.042. [DOI] [PubMed] [Google Scholar]
- [181].Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I, Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: pharmacological and molecular evidence of orexin involvement, Behav Brain Res 272 (2014) 93–99, doi: 10.1016/j.bbr.2014.06.049. [DOI] [PubMed] [Google Scholar]
- [182].Valdivia S, Patrone A, Reynaldo M, Perello M, Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model, PLoS One 9 (2014) e87478, doi: 10.1371/journal.pone.0087478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Choi DL, Davis JF, Fitzgerald ME, Benoit SC, The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats, Neuroscience 167 (2010) 11–20, doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [184].Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ, Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement, Biol Psychiatry 67 (2010) 753–760, doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L, Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior, Proc Natl Acad Sci U S A 102 (2005) 19168–19173, doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Terrill SJ, Hyde KM, Kay KE, Greene HE, Maske CB, Knierim AE, Davis JF, Williams DL, Ventral tegmental area orexin 1 receptors promote palatable food intake and oppose postingestive negative feedback, Am J Physiol Regul Integr Comp Physiol 311 (2016) R592–R599, doi: 10.1152/ajpregu.00097.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Cone JJ, McCutcheon JE, Roitman MF, Ghrelin acts as an interface between physiological state and phasic dopamine signaling, J Neurosci 34 (2014) 4905–4913, doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Thorpe AJ, Kotz CM, Orexin A in the nucleus accumbens stimulates feeding and locomotor activity, Brain Res 1050 (2005) 156–162, doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- [189].Castro DC, Berridge KC, Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”, J Neurosci 34 (2014) 4239–4250, doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Castro DC, Terry RA, Berridge KC, Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’, Neuropsychopharmacology 41 (2016) 2101–2111, doi: 10.1038/npp.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Walker LC, Lawrence AJ, The Role of Orexins/Hypocretins in Alcohol Use and Abuse, Curr Top Behav Neurosci 33 (2017) 221–246, doi: 10.1007/7854_2016_55. [DOI] [PubMed] [Google Scholar]
- [192].Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G, Multiple roles for orexin/hypocretin in addiction, Prog Brain Res 198 (2012) 79–121, doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM, Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models, Front Neurosci 8 (2014) 33, doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Olney JJ, Navarro M, Thiele TE, Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity, Alcohol Clin Exp Res 39 (2015) 21–29, doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Olney JJ, Navarro M, Thiele TE, The Role of Orexin Signaling in the Ventral Tegmental Area and Central Amygdala in Modulating Binge-Like Ethanol Drinking Behavior, Alcohol Clin Exp Res 41 (2017) 551–561, doi: 10.1111/acer.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE, The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration, PLoS One 7 (2012) e44726, doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Moorman DE, Aston-Jones G, Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats, Alcohol 43 (2009) 379–386, doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW, Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking, Front Neurosci 10 (2016) 400, doi: 10.3389/fnins.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, Pedrozo V, Anderson L, Ghotra S, Fouad M, Hopf FW, Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals, Front Neurosci 13 (2019) 88, doi: 10.3389/fnins.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].James MH, Mahler SV, Moorman DE, Aston-Jones G, A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33 (2017) 247–281, doi: 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Baimel C, Borgland SL, Hypocretin/Orexin and Plastic Adaptations Associated with Drug Abuse, Curr Top Behav Neurosci 33 (2017) 283–304, doi: 10.1007/7854_2016_44. [DOI] [PubMed] [Google Scholar]
- [202].Plaza-Zabala A, Maldonado R, Berrendero F, The hypocretin/orexin system: implications for drug reward and relapse, Mol Neurobiol 45 (2012) 424–439, doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- [203].España RA, Melchior JR, Roberts DC, Jones SR, Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration, Psychopharmacology (Berl) 214 (2011) 415–426, doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers, J Neurosci 29 (2009) 11215–11225, doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR, The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system, Eur J Neurosci 31 (2010) 336–348, doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A, Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine, Neuron 49 (2006) 589–601, doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [207].Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ, Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure, Neuropharmacology 58 (2010) 185–194, doi: 10.1016/j.neuropharm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- [208].Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C, The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization, Neurochem Int 56 (2010) 11–15, doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- [209].Harris GC, Wimmer M, Aston-Jones G, A role for lateral hypothalamic orexin neurons in reward seeking, Nature 437 (2005) 556–559, doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- [210].Wang B, You ZB, Wise RA, Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network, Biol Psychiatry 65 (2009) 857–862, doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV, Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking, Int J Neuropsychopharmacol 14 (2011) 684–690, doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- [212].Qi K, Wei C, Li Y, Sui N, Orexin receptors within the nucleus accumbens shell mediate the stress but not drug priming-induced reinstatement of morphine conditioned place preference, Front Behav Neurosci 7 (2013) 144, doi: 10.3389/fnbeh.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Gimpl G, Fahrenholz F, The oxytocin receptor system: structure, function, and regulation, Physiol Rev 81 (2001) 629–683, doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- [214].Lee HJ, Macbeth AH, Pagani JH, Young WS, Oxytocin: the great facilitator of life, Prog Neurobiol 88 (2009) 127–151, doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mustoe A, Schulte NA, Taylor JH, French JA, Toews ML, Leu8 and Pro8 oxytocin agonism differs across human, macaque, and marmoset vasopressin 1a receptors, Sci Rep 9 (2019) 15480, doi: 10.1038/s41598-019-52024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Gould BR, Zingg HH, Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse, Neuroscience 122 (2003) 155–167, doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- [217].Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG, Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets, J Comp Neurol 525 (2017) 1094–1108, doi: 10.1002/cne.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF, Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice, Naunyn Schmiedebergs Arch Pharmacol 376 (2008) 441–448, doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- [219].Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y, Biased Oxytocinergic Modulation of Midbrain Dopamine Systems, Neuron 95 (2017) 368–384 e365, doi: 10.1016/j.neuron.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L, Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping, Cell 162 (2015) 622–634, doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Otero-García M, Agustín-Pavón C, Lanuza E, Martínez-García F, Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice, Brain Struct Funct 221 (2016) 3445–3473, doi: 10.1007/s00429-015-1111-y. [DOI] [PubMed] [Google Scholar]
- [222].Sofroniew MV, Morphology of vasopressin and oxytocin neurones and their central and vascular projections, Prog Brain Res 60 (1983) 101–114, doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- [223].Ludwig M, Leng G, Dendritic peptide release and peptide-dependent behaviours, Nat Rev Neurosci 7 (2006) 126–136, doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- [224].Tang Y, Chen Z, Tao H, Li C, Zhang X, Tang A, Liu Y, Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain, Neuropharmacology 77 (2014) 277–284, doi: 10.1016/j.neuropharm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- [225].Xiao L, Priest MF, Kozorovitskiy Y, Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons, Elife 7 (2018), doi: 10.7554/eLife.33892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A, Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats, Eur J Neurosci 26 (2007) 1026–1035, doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- [227].Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ, Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat, Endocrinology 151 (2010) 2276–2286, doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Stivers JA, Kaltwasser MT, Hill PS, Hruby VJ, Crawley JN, Ventral tegmental oxytocin induces grooming, Peptides 9 Suppl 1 (1988) 223–231, doi: 10.1016/0196-9781(88)90248-3. [DOI] [PubMed] [Google Scholar]
- [229].Mullis K, Kay K, Williams DL, Oxytocin action in the ventral tegmental area affects sucrose intake, Brain Res 1513 (2013) 85–91, doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Arletti R, Benelli A, Bertolini A, Oxytocin inhibits food and fluid intake in rats, Physiol Behav 48 (1990) 825–830, doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- [231].Arletti R, Benelli A, Bertolini A, Influence of oxytocin on feeding behavior in the rat, Peptides 10 (1989) 89–93, doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- [232].Benelli A, Bertolini A, Arletti R, Oxytocin-induced inhibition of feeding and drinking: no sexual dimorphism in rats, Neuropeptides 20 (1991) 57–62, doi: 10.1016/0143-4179(91)90040-p. [DOI] [PubMed] [Google Scholar]
- [233].Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG, Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats, Peptides 12 (1991) 113–118, doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- [234].Wald HS, Chandra A, Kalluri A, Ong ZY, Hayes MR, Grill HJ, NTS and VTA oxytocin reduces food motivation and food seeking, Am J Physiol Regul Integr Comp Physiol 319 (2020) R673–R683, doi: 10.1152/ajpregu.00201.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schiöth HB, Levine AS, Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake, Endocrinology 151 (2010) 4736–4744, doi: 10.1210/en.2010-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Olszewski PK, Shaw TJ, Grace MK, Höglund CE, Fredriksson R, Schiöth HB, Levine AS, Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY, Peptides 30 (2009) 226–233, doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D, Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance, Neuron 69 (2011) 523–535, doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Zhang G, Cai D, Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment, Am J Physiol Endocrinol Metab 301 (2011) E1004–E1012, doi: 10.1152/ajpendo.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE, Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats, Am J Physiol Endocrinol Metab 302 (2012) E134–E144, doi: 10.1152/ajpendo.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Miedlar JA, Rinaman L, Vollmer RR, Amico JA, Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions, Am J Physiol Regul Integr Comp Physiol 293 (2007) R1063–R1068, doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- [241].Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-Hanson T, Nelson J, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG, Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization, Am J Physiol Regul Integr Comp Physiol 310 (2016) R640–R658, doi: 10.1152/ajpregu.00220.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Klockars OA, Klockars A, Levine AS, Olszewski PK, Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake, Neuroreport 29 (2018) 504–510, doi: 10.1097/WNR.0000000000001005. [DOI] [PubMed] [Google Scholar]
- [243].Zhou L, Ghee SM, See RE, Reichel CM, Oxytocin differentially affects sucrose taking and seeking in male and female rats, Behav Brain Res 283 (2015) 184–190, doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Patel D, Sundar M, Lorenz E, Leong KC, Oxytocin Attenuates Expression, but Not Acquisition, of Sucrose Conditioned Place Preference in Rats, Front Behav Neurosci 14 (2020) 603232, doi: 10.3389/fnbeh.2020.603232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Herisson FM, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK, Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake, J Neuroendocrinol 28 (2016), doi: 10.1111/jne.12381. [DOI] [PubMed] [Google Scholar]
- [246].Kerem L, Hadjikhani N, Holsen L, Lawson EA, Plessow F, Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity, Int J Obes (Lond) 44 (2020) 980–989, doi: 10.1038/s41366-019-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Dykens EM, Miller J, Angulo M, Roof E, Reidy M, Hatoum HT, Willey R, Bolton G, Korner P, Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome, JCI Insight 3 (2018), doi: 10.1172/jci.insight.98333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC, Intranasal oxytocin blocks alcohol withdrawal in human subjects, Alcohol Clin Exp Res 37 (2013) 484–489, doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS, Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats, PLoS One 6 (2011) e27237, doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J, Peripheral oxytocin administration reduces ethanol consumption in rats, Pharmacol Biochem Behav 140 (2016) 27–32, doi: 10.1016/j.pbb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC, Oxytocin Reduces Ethanol Self-Administration in Mice, Alcohol Clin Exp Res 41 (2017) 955–964, doi: 10.1111/acer.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID, Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens, Addict Biol 22 (2017) 702–711, doi: 10.1111/adb.12362. [DOI] [PubMed] [Google Scholar]
- [253].King CE, Becker HC, Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice, Psychopharmacology (Berl) 236 (2019) 2613–2622, doi: 10.1007/s00213-019-05233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Hansson AC, Koopmann A, Uhrig S, Bühler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstädt-Klein S, Spanagel R, Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans, Neuropsychopharmacology 43 (2018) 1235–1246, doi: 10.1038/npp.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Bahi A, The oxytocin receptor impairs ethanol reward in mice, Physiol Behav 139 (2015) 321–327, doi: 10.1016/j.physbeh.2014.11.046. [DOI] [PubMed] [Google Scholar]
- [256].Bahi A, Al Mansouri S, Al Maamari E, Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice, Physiol Behav 164 (2016) 249–258, doi: 10.1016/j.physbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- [257].King CE, Gano A, Becker HC, The role of oxytocin in alcohol and drug abuse, Brain Res 1736 (2020) 146761, doi: 10.1016/j.brainres.2020.146761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL, Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference, Behav Brain Res 228 (2012) 185–193, doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- [259].Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G, Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand, Biol Psychiatry 81 (2017) 949–958, doi: 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Baracz SJ, Everett NA, McGregor IS, Cornish JL, Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats, Addict Biol 21 (2016) 316–325, doi: 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- [261].Ibragimov R, Kovács GL, Szabó G, Telegdy G, Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sci 41 (1987) 1265–1271, doi: 10.1016/0024-3205(87)90205-0. [DOI] [PubMed] [Google Scholar]
- [262].Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM, Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats, Int J Neuropsychopharmacol 21 (2018) 677–686, doi: 10.1093/ijnp/pyy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE, Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions, Psychoneuroendocrinology 74 (2016) 164–172, doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, Malenka RC, Gating of social reward by oxytocin in the ventral tegmental area, Science 357 (2017) 1406–1411, doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Borland JM, Frantz KJ, Aiani LM, Grantham KN, Song Z, Albers HE, A novel operant task to assess social reward and motivation in rodents, J Neurosci Methods 287 (2017) 80–88, doi: 10.1016/j.jneumeth.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Borland JM, Grantham KN, Aiani LM, Frantz KJ, Albers HE, Role of oxytocin in the ventral tegmental area in social reinforcement, Psychoneuroendocrinology 95 (2018) 128–137, doi: 10.1016/j.psyneuen.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Dölen G, Darvishzadeh A, Huang KW, Malenka RC, Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin, Nature 501 (2013) 179–184, doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD, Amylin: Pharmacology, Physiology, and Clinical Potential, Pharmacol Rev 67 (2015) 564–600, doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- [269].Singh-Franco D, Robles G, Gazze D, Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus, Clin Ther 29 (2007) 535–562, doi: 10.1016/j.clinthera.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [270].Ryan GJ, Jobe LJ, Martin R, Pramlintide in the treatment of type 1 and type 2 diabetes mellitus, Clin Ther 27 (2005) 1500–1512, doi: 10.1016/j.clinthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- [271].Scherbaum WA, The role of amylin in the physiology of glycemic control, Exp Clin Endocrinol Diabetes 106 (1998) 97–102, doi: 10.1055/s-0029-1211958. [DOI] [PubMed] [Google Scholar]
- [272].Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Lush CW, Chen K, Lacerte C, Burns C, McKay R, Weyer C, Horowitz M, Low-dose pramlintide reduced food intake and meal duration in healthy, normal-weight subjects, Obesity (Silver Spring) 15 (2007) 1179–1186, doi: 10.1038/oby.2007.626. [DOI] [PubMed] [Google Scholar]
- [273].Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S, Wang Y, Burns C, Lush C, Weyer C, Horowitz M, Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes, Diabetologia 48 (2005) 838–848, doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- [274].Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC, Chen KS, Halseth AE, Lush CW, Weyer C, Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study, Am J Physiol Endocrinol Metab 293 (2007) E620–E627, doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- [275].Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E, Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats, Peptides 19 (1998) 309–317, doi: 10.1016/s0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- [276].Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E, The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats, Int J Obes Relat Metab Disord 25 (2001) 1005–1011, doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- [277].Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, Schmidt HD, Hayes MR, Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake, Neuropsychopharmacology 38 (2013) 1685–1697, doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Becskei C, Riediger T, Zünd D, Wookey P, Lutz TA, Immunohistochemical mapping of calcitonin receptors in the adult rat brain, Brain Res 1030 (2004) 221–233, doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [279].Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, Roitman MF, Hayes MR, Amylin modulates the mesolimbic dopamine system to control energy balance, Neuropsychopharmacology 40 (2015) 372–385, doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Beaumont K, Kenney MA, Young AA, Rink TJ, High affinity amylin binding sites in rat brain, Mol Pharmacol 44 (1993) 493–497. [PubMed] [Google Scholar]
- [281].Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K, In vitro autoradiographic localization of amylin binding sites in rat brain, Neuroscience 62 (1994) 553–567, doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- [282].Kalafateli AL, Satir TM, Vallöf D, Zetterberg H, Jerlhag E, An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain, Prog Neurobiol 200 (2021) 101969, doi: 10.1016/j.pneurobio.2020.101969. [DOI] [PubMed] [Google Scholar]
- [283].Whiting L, McCutcheon JE, Boyle CN, Roitman MF, Lutz TA, The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc), Physiol Behav 176 (2017) 9–16, doi: 10.1016/j.physbeh.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Kalafateli AL, Vallöf D, Jerlhag E, Activation of amylin receptors attenuates alcohol-mediated behaviours in rodents, Addict Biol 24 (2019) 388–402, doi: 10.1111/adb.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Kalafateli AL, Aranäs C, Jerlhag E, Activation of the amylin pathway modulates cocaine-induced activation of the mesolimbic dopamine system in male mice, Horm Behav 127 (2021) 104885, doi: 10.1016/j.yhbeh.2020.104885. [DOI] [PubMed] [Google Scholar]
- [286].Lutz TA, Tschudy S, Rushing PA, Scharrer E, Amylin receptors mediate the anorectic action of salmon calcitonin (sCT), Peptides 21 (2000) 233–238, doi: 10.1016/s0196-9781(99)00208-9. [DOI] [PubMed] [Google Scholar]
- [287].Reidelberger RD, Kelsey L, Heimann D, Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats, Am J Physiol Regul Integr Comp Physiol 282 (2002) R1395–R1404, doi: 10.1152/ajpregu.00597.2001. [DOI] [PubMed] [Google Scholar]
- [288].Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM, Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression, Endocrinology 147 (2006) 5855–5864, doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- [289].Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D’Alessio DA, Air EL, Woods SC, Inhibition of central amylin signaling increases food intake and body adiposity in rats, Endocrinology 142 (2001) 5035, doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- [290].Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J, Amylin receptor blockade stimulates food intake in rats, Am J Physiol Regul Integr Comp Physiol 287 (2004) R568–R574, doi: 10.1152/ajpregu.00213.2004. [DOI] [PubMed] [Google Scholar]
- [291].Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E, Amylin decreases meal size in rats, Physiol Behav 58 (1995) 1197–1202, doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- [292].Kalafateli AL, Vestlund J, Raun K, Egecioglu E, Jerlhag E, Effects of a selective long-acting amylin receptor agonist on alcohol consumption, food intake and body weight in male and female rats, Addict Biol 26 (2021) e12910, doi: 10.1111/adb.12910. [DOI] [PubMed] [Google Scholar]
- [293].Mietlicki-Baase EG, McGrath LE, Koch-Laskowski K, Krawczyk J, Reiner DJ, Pham T, Nguyen CTN, Turner CA, Olivos DR, Wimmer ME, Schmidt HD, Hayes MR, Amylin receptor activation in the ventral tegmental area reduces motivated ingestive behavior, Neuropharmacology (2017), doi: 10.1016/j.neuropharm.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Baldo BA, Kelley AE, Amylin infusion into rat nucleus accumbens potently depresses motor activity and ingestive behavior, Am J Physiol Regul Integr Comp Physiol 281 (2001) R1232–R1242, doi: 10.1152/ajpregu.2001.281.4.R1232. [DOI] [PubMed] [Google Scholar]
- [295].Kalafateli AL, Vallöf D, Colombo G, Lorrai I, Maccioni P, Jerlhag E, An amylin analogue attenuates alcohol-related behaviours in various animal models of alcohol use disorder, Neuropsychopharmacology 44 (2019) 1093–1102, doi: 10.1038/s41386-019-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Aranäs C, Vestlund J, Witley S, Edvardsson CE, Kalafateli AL, Jerlhag E, Salmon Calcitonin Attenuates Some Behavioural Responses to Nicotine in Male Mice, Front Pharmacol 12 (2021) 685631, doi: 10.3389/fphar.2021.685631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Clementi G, Valerio C, Emmi I, Prato A, Drago F, Behavioral effects of amylin injected intracerebroventricularly in the rat, Peptides 17 (1996) 589–591, doi: 10.1016/0196-9781(96)00062-9. [DOI] [PubMed] [Google Scholar]
- [298].Twery MJ, Kirkpatrick B, Lewis MH, Mailman RB, Cooper CW, Antagonistic behavioral effects of calcitonin and amphetamine in the rat, Pharmacol Biochem Behav 24 (1986) 1203–1207, doi: 10.1016/0091-3057(86)90171-1. [DOI] [PubMed] [Google Scholar]
- [299].St-Gelais F, Jomphe C, Trudeau LE, The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci 31 (2006) 229–245. [PMC free article] [PubMed] [Google Scholar]
- [300].Schroeder LE, Leinninger GM, Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders, Biochim Biophys Acta Mol Basis Dis 1864 (2018) 900–916, doi: 10.1016/j.bbadis.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Ramirez-Virella J, Leinninger GM, The Role of Central Neurotensin in Regulating Feeding and Body Weight, Endocrinology 162 (2021), doi: 10.1210/endocr/bqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG, Peripheral and central administration of xenin and neurotensin suppress food intake in rodents, Obesity (Silver Spring) 17 (2009) 1135–1143, doi: 10.1038/oby.2008.652. [DOI] [PubMed] [Google Scholar]
- [303].Levine AS, Kneip J, Grace M, Morley JE, Effect of centrally administered neurotensin on multiple feeding paradigms, Pharmacol Biochem Behav 18 (1983) 19–23, doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- [304].Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ, The effect of neurotensin on food consumption in the rat, Eur J Pharmacol 81 (1982) 499–503, doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- [305].Kim ER, Leckstrom A, Mizuno TM, Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice, Behav Brain Res 194 (2008) 66–71, doi: 10.1016/j.bbr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- [306].Zahm DS, Grosu S, Williams EA, Qin S, Bérod A, Neurons of origin of the neurotensinergic plexus enmeshing the ventral tegmental area in rat: retrograde labeling and in situ hybridization combined, Neuroscience 104 (2001) 841–851, doi: 10.1016/s0306-4522(01)00118-x. [DOI] [PubMed] [Google Scholar]
- [307].Geisler S, Zahm DS, Neurotensin afferents of the ventral tegmental area in the rat: [1]re-examination of their origins and [2]responses to acute psychostimulant and antipsychotic drug administration, Eur J Neurosci 24 (2006) 116–134, doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- [308].Woodworth HL, Brown JA, Batchelor HM, Bugescu R, Leinninger GM, Determination of neurotensin projections to the ventral tegmental area in mice, Neuropeptides 68 (2018) 57–74, doi: 10.1016/j.npep.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Woulfe J, Beaudet A, Immunocytochemical evidence for direct connections between neurotensin-containing axons and dopaminergic neurons in the rat ventral midbrain tegmentum, Brain Res 479 (1989) 402–406, doi: 10.1016/0006-8993(89)91649-1. [DOI] [PubMed] [Google Scholar]
- [310].Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC, Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody, Neuropharmacology 39 (2000) 1430–1442, doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- [311].Nicot A, Rostène W, Bérod A, Differential expression of neurotensin receptor mRNA in the dopaminergic cell groups of the rat diencephalon and mesencephalon, J Neurosci Res 40 (1995) 667–674, doi: 10.1002/jnr.490400512. [DOI] [PubMed] [Google Scholar]
- [312].Lépée-Lorgeoux I, Betancur C, Rostène W, Pélaprat D, Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization, Brain Res Dev Brain Res 113 (1999) 115–131, doi: 10.1016/s0165-3806(99)00009-7. [DOI] [PubMed] [Google Scholar]
- [313].Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, Fuller PM, Leinninger GM, Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance, Cell Rep 20 (2017) 1881–1892, doi: 10.1016/j.celrep.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Hawkins MF, Aphagia in the rat following microinjection of neurotensin into the ventral tegmental area, Life Sci 38 (1986) 2383–2388, doi: 10.1016/0024-3205(86)90606-5. [DOI] [PubMed] [Google Scholar]
- [315].Cador M, Kelley AE, Le Moal M, Stinus L, Ventral tegmental area infusion of substance P, neurotensin and enkephalin: differential effects on feeding behavior, Neuroscience 18 (1986) 659–669, doi: 10.1016/0306-4522(86)90061-8. [DOI] [PubMed] [Google Scholar]
- [316].Kelley AE, Cador M, Stinus L, Le Moal M, Neurotensin, substance P, neurokininalpha, and enkephalin: injection into ventral tegmental area in the rat produces differential effects on operant responding, Psychopharmacology (Berl) 97 (1989) 243–252, doi: 10.1007/BF00442258. [DOI] [PubMed] [Google Scholar]
- [317].Hawkins MF, Central nervous system neurotensin and feeding, Physiol Behav 36 (1986) 1–8, doi: 10.1016/0031-9384(86)90064-8. [DOI] [PubMed] [Google Scholar]
- [318].Boules M, Warrington L, Fauq A, McCormick D, Richelson E, A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity, Eur J Pharmacol 426 (2001) 73–76, doi: 10.1016/s0014-2999(01)01197-9. [DOI] [PubMed] [Google Scholar]
- [319].Fredrickson P, Boules M, Yerbury S, Richelson E, Blockade of nicotine-induced locomotor sensitization by a novel neurotensin analog in rats, Eur J Pharmacol 458 (2003) 111–118, doi: 10.1016/s0014-2999(02)02689-4. [DOI] [PubMed] [Google Scholar]
- [320].Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR, Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia, J Pharmacol Exp Ther 336 (2011) 809–815, doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Boules M, Oliveros A, Liang Y, Williams K, Shaw A, Robinson J, Fredrickson P, Richelson E, A neurotensin analog, NT69L, attenuates intravenous nicotine self-administration in rats, Neuropeptides 45 (2011) 9–16, doi: 10.1016/j.npep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- [322].Fredrickson P, Boules M, Yerbury S, Richelson E, Novel neurotensin analog blocks the initiation and expression of nicotine-induced locomotor sensitization, Brain Res 979 (2003) 245–248, doi: 10.1016/s0006-8993(03)02895-6. [DOI] [PubMed] [Google Scholar]
- [323].Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi DS, Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice, Pharmacol Biochem Behav 95 (2010) 235–241, doi: 10.1016/j.pbb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Lee MR, Hinton DJ, Unal SS, Richelson E, Choi DS, Increased ethanol consumption and preference in mice lacking neurotensin receptor type 2, Alcohol Clin Exp Res 35 (2011) 99–107, doi: 10.1111/j.1530-0277.2010.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Lovshin JA, Glucagon-like Peptide-1 Receptor Agonists: A Class Update for Treating Type 2 Diabetes, Can J Diabetes 41 (2017) 524–535, doi: 10.1016/j.jcjd.2017.08.242. [DOI] [PubMed] [Google Scholar]
- [326].Tella SH, Rendell MS, Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance, Ther Adv Endocrinol Metab 6 (2015) 109–134, doi: 10.1177/2042018815580257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Drucker DJ, Habener JF, Holst JJ, Discovery, characterization, and clinical development of the glucagon-like peptides, J Clin Invest 127 (2017) 4217–4227, doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Hayes MR, Skibicka KP, Grill HJ, Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation, Endocrinology 149 (2008) 4059–4068, doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP, The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors, J Neurosci 32 (2012) 4812–4820, doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Egecioglu E, Engel JA, Jerlhag E, The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice, PLoS One 8 (2013) e69010, doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E, The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents, Psychoneuroendocrinology 38 (2013) 1259–1270, doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- [332].Egecioglu E, Engel JA, Jerlhag E, The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice, PLoS One 8 (2013) e77284, doi: 10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Merchenthaler I, Lane M, Shughrue P, Distribution of pre-proglucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system, J Comp Neurol 403 (1999) 261–280, doi:. [DOI] [PubMed] [Google Scholar]
- [334].Dossat AM, Lilly N, Kay K, Williams DL, Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake, J Neurosci 31 (2011) 14453–14457, doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Alhadeff AL, Rupprecht LE, Hayes MR, GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake, Endocrinology 153 (2012) 647–658, doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Rinaman L, Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure, Brain Res 1350 (2010) 18–34, doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR, The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors, Am J Physiol Endocrinol Metab 305 (2013) E1367–E1374, doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR, Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling, J Neurosci 34 (2014) 6985–6992, doi: 10.1523/JNEU-ROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Wang XF, Liu JJ, Xia J, Liu J, Mirabella V, Pang ZP, Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons, Cell Rep 12 (2015) 726–733, doi: 10.1016/j.celrep.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL, Nucleus accumbens GLP-1 receptors influence meal size and palatability, Am J Physiol Endocrinol Metab 304 (2013) E1314–E1320, doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, Tschöp MH, Seeley RJ, Benoit SC, Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats, Biol Psychiatry 72 (2012) 354–360, doi: 10.1016/j.biopsych.2012.01.035. [DOI] [PubMed] [Google Scholar]
- [342].Shirazi RH, Dickson SL, Skibicka KP, Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward, PLoS One 8 (2013) e61965, doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Vallöf D, Kalafateli AL, Jerlhag E, Brain region specific glucagon-like peptide-1 receptors regulate alcohol-induced behaviors in rodents, Psychoneuroendocrinology 103 (2019) 284–295, doi: 10.1016/j.psyneuen.2019.02.006. [DOI] [PubMed] [Google Scholar]
- [344].Abtahi S, Howell E, Currie PJ, Accumbal ghrelin and glucagon-like peptide 1 signaling in alcohol reward in female rats, Neuroreport 29 (2018) 1046–1053, doi: 10.1097/WNR.0000000000001071. [DOI] [PubMed] [Google Scholar]
- [345].Graham DL, Erreger K, Galli A, Stanwood GD, GLP-1 analog attenuates cocaine reward, Mol Psychiatry 18 (2013) 961–962, doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A, The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice, Physiol Behav 149 (2015) 262–268, doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A, Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels, Transl Psychiatry 6 (2016) e809, doi: 10.1038/tp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu XA, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ, GLP-1 acts on habenular avoidance circuits to control nicotine intake, Nat Neurosci 20 (2017) 708–716, doi: 10.1038/nn.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, Davis JF, Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors, Physiol Behav 161 (2016) 140–144, doi: 10.1016/j.physbeh.2016.04.013. [DOI] [PubMed] [Google Scholar]
- [350].Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine, Neuropsychopharmacology 41 (2016) 1917–1928, doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD, Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats, Neuropsychopharmacology 43 (2018) 2000–2008, doi: 10.1038/s41386-018-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD, Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats, Addict Biol 24 (2019) 170–181, doi: 10.1111/adb.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Bornebusch AB, Fink-Jensen A, Wörtwein G, Seeley RJ, Thomsen M, Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs, eNeuro 6 (2019), doi: 10.1523/ENEURO.0443-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was generated in this manuscript.


