Abstract
Treatment of carotid body tumors (CBTs) can be complicated by the presence of hormonal activity. This case describes the treatment of a 65-year-old woman that presented with an abnormally high blood pressure and was found to have a neck mass. Diagnostic imaging along with urine metanephrines revealed this mass to be a hormonally active CBT. Careful resection along with preoperative treatment with an alpha blockade allowed for a successful complete removal of the tumor without any complications. Although CBTs tend to be benign, and hormonally active tumors are rare, one must always maintain a level of suspicion of hormonal activity to prevent catastrophic operative events.
Keywords: Carotid body tumor, Metanephrine, Paraganglioma, Neuroendocrine tumors
Background
Carotid body tumors (CBTs) are rare neoplasms that typically present as slow-growing hypervascular masses located at the carotid bifurcation.1,2 While most CBTs are benign, they can grow and compress adjacent structures including nerves and vessels. Sporadic tumors are typically asymptomatic and found incidentally on imaging studies, via palpation during routine physical exams or incidentally by the patient. However, larger CBTs may present with pain, vertigo, dysphagia, hoarseness, or nervous dysfunction (including the vagus nerve and cranial nerves IX, XI, and XII).3,4 Most CBTs are inactive, but approximately 3–4% of CBTs are hormonally active. Patients that have active tumors will typically present with an initial complaint of new-onset or worsening hypertension.5 This report entails the presentation, treatment, and outcome of a patient with a hormonally active CBT.
The patient consented to publication of her case details and images.
Case presentation
A 65-year-old woman presented to the emergency room after an episode of malaise, dizziness, sense of impending doom, and an abnormally elevated blood pressure. At presentation, her blood pressure was 195/42 with a heart rate of 84. Later in the same hospitalization, she was found to have a new diagnosis of atrial fibrillation with rates from 120 to 150. She was also noted to have a mass on her left neck which had been growing in size along with hoarseness (likely secondary to pressure on the recurrent laryngeal nerve). This was evaluated with MRI and CT imaging, revealing a 2 × 3 × 4.5 cm Shamblin 2 CBT (Figs. 1a, 1b, and 1c). She denied any personal or family history of paragangliomas.
Fig. 1.

a, b, and c. CT imaging of carotid body tumor.
Due to her new onset atrial fibrillation and hypertension, there was concern for a hormonally active tumor. A hormonal workup revealed elevated urine metanephrines, plasma norepinephrine and plasma dopamine levels. An abdominal CT scan was negative for adrenal tumors. Along with managing her atrial fibrillation with a calcium channel blocker and anticoagulation, she was started on alpha-blockade for 6 weeks with planned surgical resection. Prior to resection, her blood pressure was monitored at a pre-operative appointment to clinically assess success of alpha blockade. Additionally, the patient performed frequent home blood pressure checks and reported her findings to her primary care provider.
Pre-operative embolization was not completed as it was felt in this case that the risks were greater than the benefits. Intraoperatively, the tumor was found to be partially encasing the ICA and ECA, consistent with a Shamblin 2 classification (Fig. 2). It was resected in the peri-adventitial plane and the feeding vessels were ligated (Figs. 3a and 3b). The vagus and hypoglossal nerves were identified and preserved. Post-operatively, the patient had some difficulty swallowing but passed a formal swallow evaluation. The tumor was sent for pathology and found to have features consistent with paraganglioma. It was decided to not send for genetics due to unilaterality, lack of family history, and lack of other tumors. She was discharged on post-operative day one. On outpatient follow up, her swallowing returned to normal. Her hormone levels were checked and had also returned to normal, so her alpha blockade was discontinued. An ultrasound one month after surgery showed normal appearing carotid arteries without evidence of tumor recurrence. Post-operatively, hormonal lab checks were done at one month, six month, and then one year intervals.
Fig. 2.
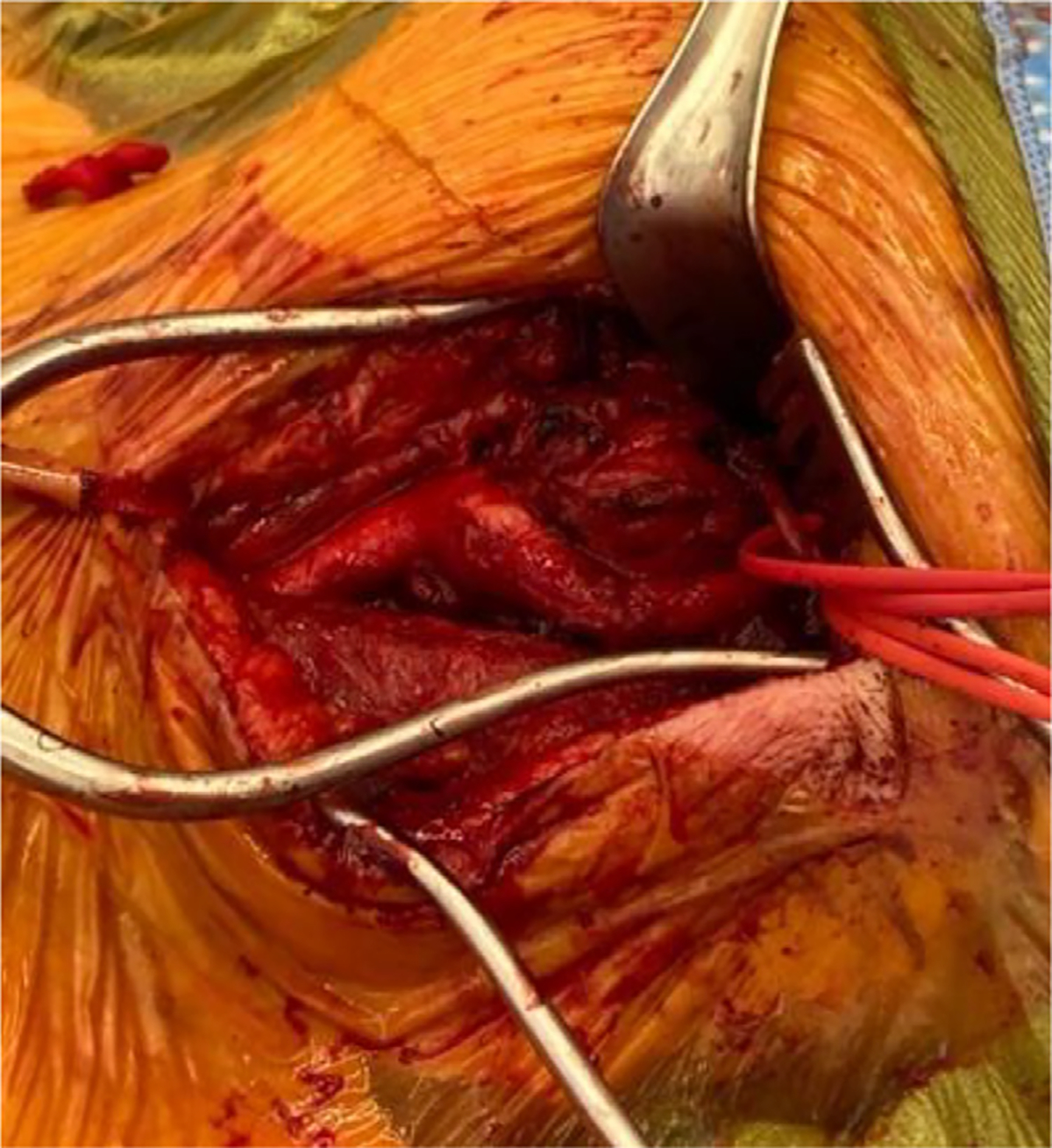
Intraoperative view of carotid body tumor prior to resection.
Fig. 3.

a and b. Intraoperative view of carotid body tumor after resection with identification and isolation of external carotid artery (white umbilical tape), internal carotid artery (red vessel loop), vagus nerve (black silk tie), and hypoglossal nerve (yellow vessel loop).
Discussion
This case reports a rare presentation of a hormonally active CBT, with new onset atrial fibrillation, hypertensive urgency, and associated neck mass. The carotid body is the largest collection of paraganglia in the head and neck. It acts as a chemoreceptor that activates the sympathetic nervous system in response to arterial hypoxemia. The carotid body typically measures approximately 3–5 mm but can become larger than 8 cm with repeated hypoxic stimulations.1,6 Paragangliomas are estimated to represent approximately 0.012% of all tumors found in humans and 0.6% of tumors found specifically in the head and neck region. CBTs make up about 65% of all head and neck paragangliomas — meaning they account for less than 1% of all tumors found in this area.7 These tumors also have a much higher incidence in women than men (approximately 30:1), and typically present around age 50.8
CBTs are usually sporadic, with about 25% being hereditary1. Genetic mutations are the only known risk factor for development of CBTs beyond hypoxic stimulation. Hereditary CBTs are often caused by mutations of the succinate dehydrogenase genes, including SDHD, SDHB, and SDHC. Compared to sporadic, hereditary CBTs often present bilaterally, and often present with other paragangliomas, such as vagal, temporal, tympanic, or jugular. Additionally, patients with hereditary tumors will often present earlier in life and typically have at least one affected parent due to the observed autosomal dominant inheritance pattern.3
Identification of CBTs early is highly beneficial. Palpation of the head and neck during routine physicals is extremely important for screening purposes. This is because finding CBTs when they are smaller in size allows for earlier surgical intervention with less risks. Radiologic examination of these tumors typically shows them splaying the internal carotid artery (ICA) and external carotid artery (ECA). Computed tomography (CT) and magnetic resonance imaging (MRI) are the most used methods of identification and categorization. Categorization of CBTs occurs with the Shamblin system, which measures the involvement of the carotid vessels.9
Treatment of CBTs most typically involves surgical excision; radiation therapy (RT) is reserved for rare cases in which the patient is unfit for surgery, or the tumor is nonresectable. Surgical resection of CBTs has been associated with a morbidity rate of 35% and a mortality rate of 1%. Recurrent tumor growth and other serious complications have been reported following RT.8,10–12 Preoperative embolization has become increasingly popular in efforts to make resection a safer option. The benefit of embolization is reduction of blood loss intra-operatively. However, there is no significant difference in the number of surgical complications and the decreased blood loss may not have a clinically significant value. Additionally, if embolization is used, there is a risk of embolic particle reflux into nontargeted vasculature.13–15
Hormonal activity of CBTs should always be assessed as up to 4% of CBTs can be hormonally active. Active CBTs often present with hypertension. This is because most often, CBTs will secrete norepinephrine (with a minority secreting epinephrine or dopamine). To assess hormonal activity, catecholamine levels should be obtained. If these levels are increased, additional imaging should be performed to rule out intraabdominal tumors such as pheochromocytomas. Prior to operating on a hormonally active CBT, patients should be preoperatively treated with an alpha-blockade for at least two weeks to normalize blood pressure. This is of paramount importance to prevent severe fluctuations both during tumor resection and post-operatively.5 This blockade can be discontinued following resection, though monitoring of blood pressure should be maintained for several months post-surgically. Beyond hormonal activity, some CBTs have malignant potential. Up to 10% of all CBTs are believed to be malignant. Primary tumor recurrence is the most common form of malignancy.16
Conclusion
This case highlights the importance of considering the possibility of hormonal activity when managing a patient with a CBT. If catecholamines are not assessed in a hormonal active patient, catastrophic operative events can occur. If hormonal activity is discovered, patients should be treated with an alpha blockade prior to the operation. Post-operatively, CBTs should be monitored for recurrence or other signs of malignancy.
Acknowledgments
This work was funded in part by the National Institute of General Medical Sciences (5U54GM104942) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK128569).
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests
Samantha Minc reports financial support was provided by National Institute of General Medical Sciences. Samantha Minc reports financial support was provided by National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Hoang VT, Trinh CT, Lai TAK, Doan DT, Tran TTT. Carotid body tumor: a case report and literature review. J Radiol Case Rep. 2019;13(8):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head Neck Pathol. 2009;3(4):303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offergeld C, Brase C, Yaremchuk S, et al. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics. 2012;67(Suppl 1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Guercio L, Narese D, Ferrara D, Butrico L, Padricelli A, Porcellini M. Carotid and vagal body paragangliomas. Transl Med UniSa. 2013;6:11–15. [PMC free article] [PubMed] [Google Scholar]
- 5.Colen TY, Mihm FG, Mason TP, Roberson JB. Catecholamine-secreting paragangliomas: recent progress in diagnosis and perioperative management. Skull Base. 2009;19(6):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Cuevas S, López-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 m above sea level. Head Neck. 1998;20(5):374–378. [DOI] [PubMed] [Google Scholar]
- 7.Lozano-Sánchez FS, Muñoz A, las Heras JA, González-Sarmiento R, García-Cenador MB. Carotid paragangliomas related to form involving multiple systems (syndromes and diseases): a systematic literature review. Int J Rare Dis Disord. 2020;3:24. [Google Scholar]
- 8.Sajid MS, Hamilton G, Baker DMJoint Vascular Research Group. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34(2):127–130. [DOI] [PubMed] [Google Scholar]
- 9.Arya S, Rao V, Juvekar S, Dcruz AK. Carotid body tumors: objective criteria to predict the Shamblin group on MR imaging. AJNR Am J Neuroradiol. 2008;29(7):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdagni R, Amichetti M. Radiation therapy of carotid body tumors. Am J Clin Oncol. 1990;13(1):45–48. [DOI] [PubMed] [Google Scholar]
- 11.Cleere EF, Martin-Grace J, Gendre A, Sherlock M, O’Neill JP. Contemporary management of paragangliomas of the head and neck. Laryngoscope Investig Otolaryngol. 2021;7(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhm M, Polterauer P, Gstöttner W, et al. Diagnostic and therapeutic approaches to carotid body tumors: review of 24 patients. Arch Surg. 1997;132(3):279–284. [DOI] [PubMed] [Google Scholar]
- 13.LaMuraglia GM, Fabian RL, Brewster DC, Pile-Spellman J, Darling RC, Cambria RP, et al. The current surgical management of carotid body paragangliomas. J Vasc Surg. 1992;15(6):1038–1044 discussion 1044–5. [DOI] [PubMed] [Google Scholar]
- 14.Gözen ED, Teveto ğlu F, Kara S, K ı z ı lk ı l ı ç O, Yener HM. Is preoperative embolization necessary for carotid paraganglioma resection: experience of a tertiary center. Ear Nose Throat J. 2022;101(4):NP180–NP185. [DOI] [PubMed] [Google Scholar]
- 15.Texakalidis P, Charisis N, Giannopoulos S, Xenos D, Rangel-Castilla L, Tassiopoulos AK, et al. Role of preoperative embolization in carotid body tumor surgery: a systematic review and meta-analysis. World Neurosurg. 2019;129:503–513 e2. [DOI] [PubMed] [Google Scholar]
- 16.Xing J, Cheng Y, Ying H, Guan M, Jia N, Bai C. Systemic treatment of a metastatic carotid body tumor. Medicine. 2020;99(47):e22811. [DOI] [PMC free article] [PubMed] [Google Scholar]


