Abstract
Objectives
Childhood obesity affects multiple organs in the body and is associated with both significant morbidity and ultimately premature mortality. Childhood obesity, especially dyslipidemia, can lead to early atherosclerosis and premature cardiovascular disease (CVD) in adulthood. The detection of exhaled volatile organic compounds (VOCs) in the breath offers the opportunity for the discovery of novel disease-specific biomarkers. This study aimed to identify VOCs that correlate with childhood obesity accompanied by dyslipidemia.
Methods
A total of 82 overweight or obese children between the ages of 8 and 12 years were recruited from the exercise on obesity adolescents in Peking (EXCITING) study (NCT04984005). The breath VOCs of the participants were measured by gas chromatography-mass spectrometry (GC-MS). The classification was performed using principal component analysis (PCA) of the relative abundance of VOCs. The difference between the obese and overweight groups with or without dyslipidemia was analyzed.
Results
Among the 82 children, 25 were overweight, of whom 10 had dyslipidemia. The other 57 children were obese, and 17 of them had dyslipidemia. Obese children with dyslipidemia had higher triglycerides and elevated non–high-density lipoprotein-cholesterol compared to overweight children without dyslipidemia. We confirmed 13 compounds based on database well matches (average score > 80) for mass spectra and refractive index. These 13 VOCs were grouped into three chemical functional groups: saturated hydrocarbons, aromatic hydrocarbons and unsaturated aldehydes. For obese children with dyslipidemia, the PCA scatter plot of the three chemical groups was obviously separated from the other groups. Some of the candidates, including heptadecane, naphthalene, and cis-6-nonnenol, were significantly higher in obese children with dyslipidemia than in overweight groups with or without dyslipidemia.
Conclusion
A suite of VOCs from three chemical function groups, saturated hydrocarbons, aromatic hydrocarbons, and unsaturated aldehydes, were separated in the obese children with dyslipidemia. Heptadecane, naphthalene, and cis-6-nonenol were significantly elevated in obese children with dyslipidemia. Our findings underscore the potential value of the candidate VOCs for future risk categorization.
Key words: childhood, obesity, dyslipidemia, volatile organic compounds
Introduction
Obesity is becoming increasingly prevalent in pediatric populations worldwide. Among children and adolescents aged 5–19 years, the prevalence of obesity has increased even more dramatically: obesity increased from less than 1% in 1975 to 6% in girls and 8% in boys in 2016. This percentage is equivalent to more than 124 million obese children and adolescents worldwide.[1] Childhood obesity affects multiple organs in the body and is associated with both significant morbidity and ultimately premature mortality.[2] Mendelian randomization in adolescents and young adults indicated that increased adiposity with multiple cardiometabolic risk markers adversely influences the metabolite profile, implying that identifying key metabolic markers of adiposity during early life could help to facilitate the early monitoring and prevention of cardiovascular diseases in later life.[3]
The analysis of volatile organic compounds (VOCs) in the breath for noninvasive disease detection and monitoring is an emergent research field that has the potential to reshape current clinical practice.[4,5] The detection of exhaled VOCs in the breath offers the opportunity for the discovery of novel disease-specific biomarkers.[6] Patterns of VOCs, as detected by an electronic nose, can distinguish patients with chronic obstructive pulmonary disease from those with asthma.[7]
Breath testing is a simple and safe alternative to more invasive investigations in children. Breath VOCs could be useful for childhood obesity screening and phenotyping, identifying children who could benefit from personalized therapeutic strategies.[8] One study demonstrated that a panel of four VOCs could identify the presence of overweight/obesity with excellent accuracy. Further analysis revealed that breath isoprene, 1-decene, 1-octene, ammonia, and hydrogen sulfide were significantly higher in the obese group than in the lean group.[9] To date, the significance of VOCs in childhood obesity with dyslipidemia remains elusive.
Therefore, this study aimed to assess (1) the feasibility of breath VOC testing using untargeted gas chromatography-mass spectroscopy (GC-MS) to distinguish obese children with dyslipidemia and (2) the ability to identify VOCs that correlate with childhood obesity accompanied by dyslipidemia. The different categories of VOCs observed could help to provide insights into the pathophysiological processes and pathways leading to the development of childhood obesity with dyslipidemia.
Methods
This study was designed and performed in accordance with the Declaration of Helsinki and after obtaining approval from the institutional review board of Peking University Third Hospital (Approval No. LM2021316). Written informed consent was obtained from participants and parents. A schematic diagram of the study design and procedures is shown in Figure 1.
Figure 1.
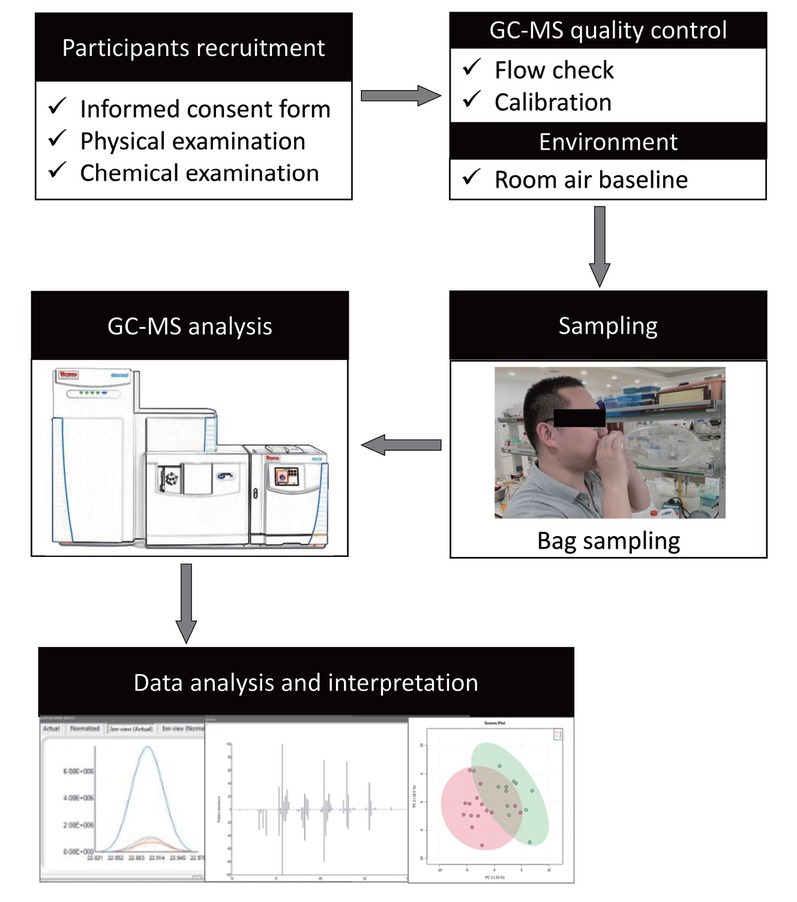
The schematic flowchart of the study design and procedures of the present study. GC-MS: gas chromatography-mass spectroscopy.
Participant recruitment
A total of 87 overweight or obese children between the ages of 8 and 12 years were recruited from the exercise on obesity adolescents in Peking (EXCITING) study (NCT04984005), and five of them were excluded because of abnormal liver function or routine blood examination results. Demographic data were obtained, and clinical variables were recorded, including standard procedures for height and weight. The body mass index (BMI) was also calculated for each child.
Overweight was defined by a BMI ≥ 85th percentile, and obesity was defined by a BMI ≥ 95th percentile adjusted for age and gender.[10] Dyslipidemia in this pilot study was defined as having one or more of the following three criteria for childhood metabolism syndrome: (1) low high-density lipoprotein (HDL)-cholesterol, defined as concentrations < 1.03 mmol/L (40 mg/dL); (2) elevated non-HDL-cholesterol (non-HDL-C), defined as concentrations ≥ 3.76 mmol/L (145 mg/dL); and (3) hypertriglyceridemia, defined as triglyceride (TG) level ≥ 1.47 mmol/L (130 mg/dL).[11]
Children with the following conditions were excluded from the study: (1) subjects with liver, kidney, pulmonary, or heart dysfunction; (2) subjects with any known hereditary diseases or malignant tumors; (3) subjects with allergic diseases or with any recent drug history within 2 weeks before admission; (4) subjects with acute and/or chronic infections; (5) subjects intolerant to exercise training; and (6) subjects with any evidence of a psychiatric disorder.
Exhaled breath collection
All the study subjects fasted overnight without breakfast before the collection of the breath sample to reduce contamination by food. Given that all the participants were children, we simplified the gas collection process to ensure feasibility. After 5 min of tidal breathing, the participants inhaled maximally, and the expiratory port was connected to a 2-L disposable Teflon® FEP bag, which was capped, transferred, and analyzed within 6 h. All the standardized gas collection processes were under the supervision of one of the experimenters. To standardize the conditions, the indoor air was also collected for background elimination.
Untargeted GC–MS and raw data processing
The exhaled breath samples were analyzed using a Q Exactive GC Orbitrap mass spectrometer (Thermo Scientific, USA) at Peking University Analytical Instrumentation Center. All exhaled breath samples were processed with solid-phase microextraction (SPME). A 50/30 μm DVB/ CARBOXEN/PDMS-coated 24 G needle was introduced by first piercing the SPME fiber sheath into the sampling bags, and then the breath sample was exposed and placed in a 37℃ incubator for 30 min before the fiber was retracted and secured in the SPME fiber sheath.
The SPME needle was withdrawn and immediately introduced into the GC injection port, and the fiber was exposed to the 200℃ inlets for 30 s to release the absorbed compounds. The GC oven temperature gradient was programmed as follows: begin at 40℃, hold at 40℃ for 1 min, ramp to 180℃ at a rate of 5℃/min, and hold at 180℃ for 2 min, with a total run time of 31 min. The MS transfer line temperature was 250℃, the ion source temperature was 200℃, the electron energy was 70 eV, and a mass-to-charge ratio (m/z) range of 30–550 was scanned.
TraceFinder software, version 5.0 (Thermo Fisher Scientific, Waltham, MA, USA) was used to process the data automatically. All potential VOCs were cataloged in a “breath matrix” and sorted by retention index, mass spectra, and subsequent deconvolution, peak area, and relative abundance. The original data were the GC-MS peak intensity with the background signal removed. The breath matrix was refined by iterations of validation and data processing to remove VOCs present in environmental air; the identities of coeluting components and aligned data were verified to correct for “between-run” shifts in retention behavior.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software, version 8 (GraphPad Software Inc., La Jolla, CA, USA) or IBM SPSS statistics software (version 23.0; IBM Corp., Armonk, NY, USA). Two-way analysis of variance (ANOVA) and post hoc analysis were performed to compare the obese versus overweight groups with or without dyslipidemia. Pearson’s chi-square test was used for categorical factors. A normal distribution was found for all continuous variables. Data are presented as the mean ± standard error. P < 0.05 was considered statistically significant. The classification was performed using principal component analysis (PCA) of the mass scanning peaks, namely, the relative abundance of VOCs, by the online free tutools platform (http://www.cloudtutu.com).
Results
Participants’ characteristics
A total of 82 overweight or obese adolescents were ultimately included. The demographic features and laboratory examination results are listed in Table 1. Among the subjects, 25 were overweight with BMI ≥ 85th percentile, and 10 of them had dyslipidemia. The other 57 were obese children with BMI ≥ 95th percentile, and 17 of them had dyslipidemia. There was no significant difference in routine blood examinations, liver function (aspartate aminotransferase), or kidney function (creatine) among obese individuals with dyslipidemia, obese individuals without dyslipidemia, overweight individuals with dyslipidemia, and overweight individuals without dyslipidemia. Obese children with dyslipidemia had higher TGs and elevated non-HDL-c than overweight children without dyslipidemia. Interestingly, children with obesity with dyslipidemia had a slightly elevated leukocyte amount compared with those who were overweight without dyslipidemia.
Table 1.
Participants’ characteristics and related parameters
| Characteristics | Control (n = 55) |
Dyslipidemia (n = 27) |
||
|---|---|---|---|---|
| Overweight (n = 15) | Obesity (n = 40) | Overweight (n = 10) | Obesity (n = 17) | |
| Age, yr | 10 ± 1 | 10 ± 1 | 10 ± 1 | 10 ± 1 |
| Male, n | 3 | 20 | 4 | 9 |
| Height, cm | 148.2 ± 7.7 | 148.4 ± 7.6 | 148.7 ± 6.1 | 147.6 ± 6.4 |
| Weight, kg | 46.4 ± 5.6 | 56.9 ± 9.5* | 47.9 ± 5.6 | 56.6 ± 10.0 |
| BMI, kg/m2 | 21.0 ± 0.9 | 25.7 ± 2.7**** | 21.6 ± 0.9 | 25.8 ± 3.1# |
| Glucose, mmol/L | 5.2 ± 0.3 | 5.0 ± 0.3 | 5.0 ± 0.2 | 5.1 ± 0.4 |
| HbA1c, % | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.1 | 5.5 ± 0.3 |
| Non-HDL-C, mmol/L | 2.82 ± 0.59 | 2.74 ± 0.46 | 4.66 ± 0.51**** | 3.57 ± 0.74##$$$$ |
| TG, mmol/L | 0.75 ± 0.23 | 0.83 ± 0.24 | 0.87 ± 0.26 | 1.52 ± 0.65##$$$$ |
| HDL-C, mmol/L | 1.46 ± 0.19 | 1.38 ± 0.19 | 1.40 ± 0.23 | 1.23 ± 0.30 |
| ApoA1, g/L | 1.33 ± 0.19 | 1.35 ± 0.17 | 1.36 ± 0.24 | 1.32 ± 0.24 |
| ApoB, g/L | 0.62 ± 0.10 | 0.64 ± 0.10 | 0.97 ± 0.14**** | 0.81 ± 0.14#$$$$ |
| AIP | 1.97 ± 0.54 | 2.03 ± 0.48 | 3.44 ± 0.79**** | 2.96 ± 0.62$$$$ |
| WBC, ×109/L | 6.93 ± 1.20 | 7.10 ± 1.09 | 7.14 ± 1.88 | 7.72 ± 1.17* |
| HGB, g/L | 134 ± 8 | 138 ± 8 | 136 ± 6 | 137 ± 7 |
| Platelet, ×109/L | 329 ± 59 | 336 ± 54 | 370 ± 65 | 341 ± 57 |
| AST, U/L | 23 ± 5 | 26 ± 8 | 27 ± 4 | 30 ± 20 |
| Cr, μmol/L | 55 ± 5 | 55 ± 7 | 52 ± 5 | 56 ± 4 |
| 25-OH vitamin D, ng/mL | 17 ± 6 | 18 ± 5 | 17 ± 3 | 18 ± 4 |
Data are presented as mean ± SD. *P < 0.05 versus overweight control, ****P < 0.0001 versus overweight control, #P < 0.05 versus overweight dyslipidemia, ##P < 0.01 versus overweight dyslipidemia; $$$$P < 0.0001 versus overweight control. AST: aspartate transaminase; AIP: atherogenic index of plasma; BMI: body mass index; Cr: creatinine; Hb1Ac: glycated hemoglobin; HDL-c: high-density lipoprotein-cholesterol; HGB: hemoglobin; non-HDL-c: non–high-density lipoprotein-cholesterol; TG: triglyceride; WBC: white blood cell.
Categorization of metabolomic breathprint in obesity with dyslipidemia
Stepwise variable selection was performed using the mass scanning ion peak data. A total of 138 VOCs were obtained after data pretreatment, such as deconvolution, background subtraction, and peak alignment. To exclude exogenous compounds, Human Metabolome Database (HMDB) annotations were used to select the putative metabolites, and 56 VOCs met the criteria. We confirmed 13 compounds based on database well matches (average score > 80) for mass spectra and refractive index. For these 13 VOCs, PCA scatter plots showed separation among obese and overweight participants with or without dyslipidemia (Figure 2). According to the chemical characteristics, VOCs were grouped into three chemical functional groups: saturated hydrocarbons, aromatic hydrocarbons, and unsaturated aldehydes. For obesity with dyslipidemia participants, the PCA scatter plot of the three previously mentioned chemical groups was obviously separated from the other groups. The results revealed that this group had some potential characteristics compared with the others.
Figure 2.
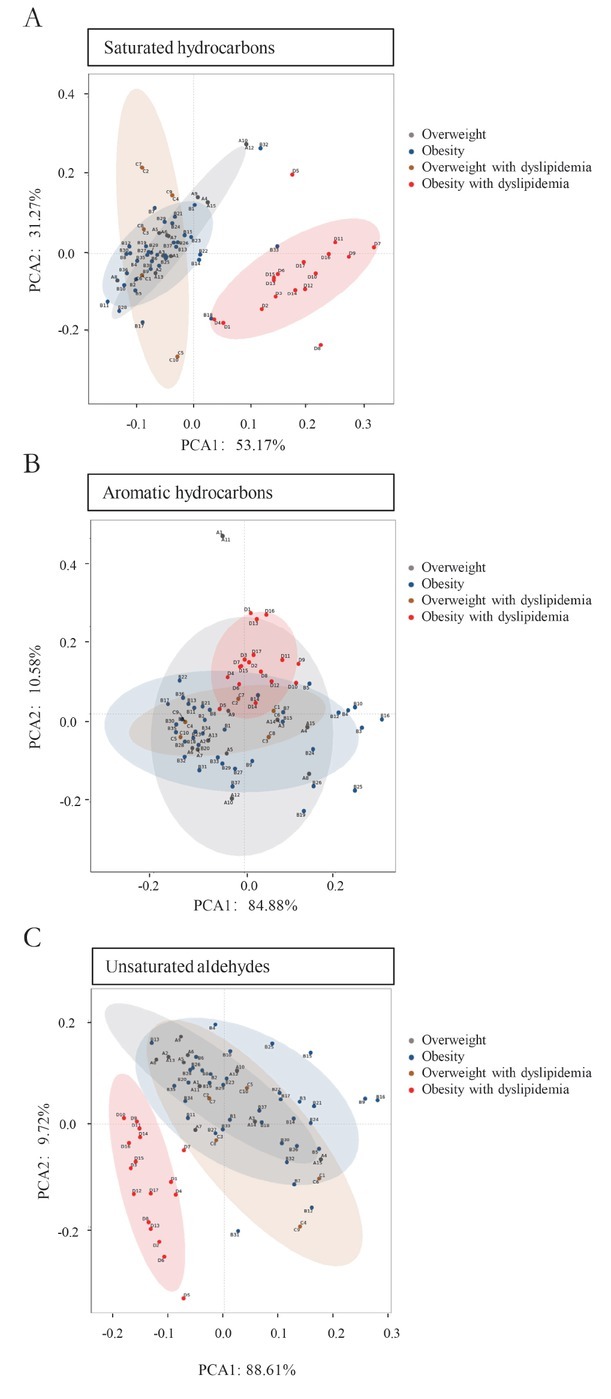
Principal component analysis of certain categories of VOCs and a scatter plot showing separation among obesity and overweight participants with or without dyslipidemia. (A) Saturated hydrocarbons, (B) aromatic hydrocarbons, and (C) unsaturated aldehydes. VOCs: volatile organic compounds; PCA: principal component analysis.
VOC changes in children with obesity with dyslipidemia
The relative abundances of some saturated hydrocarbons, such as heptadecane, undecane, and dodecane, showed increasing trends in obese children with dyslipidemia (Figure 3A). Heptadecane, one of the candidates of the saturated hydrocarbons, was statistically significantly higher in obese children with dyslipidemia than in overweight children with ortholiposis (P < 0.01). At the same time, Figure 3B shows that four aromatic hydrocarbon VOCs—naphthalene, methylnaphthalene, benzene, 1,2,3,4-tetramenthyl, and phenol—were present at higher levels in the obese children with dyslipidemia. Naphthalene, one of the selected aromatic hydrocarbons, was significantly higher in obese children with dyslipidemia than in overweight children with or without dyslipidemia (all P < 0.05). As shown in Figure 3C, a suite of unsaturated aldehydes, such as cis-6-nonenol, 2-ocetenal, and d-limonene, were also elevated in obese children with dyslipidemia. Furthermore, the relative abundance of cis-6-nonenol was significantly higher in obese children with dyslipidemia than in the overweight children with ortholiposis (P < 0.05).
Figure 3.
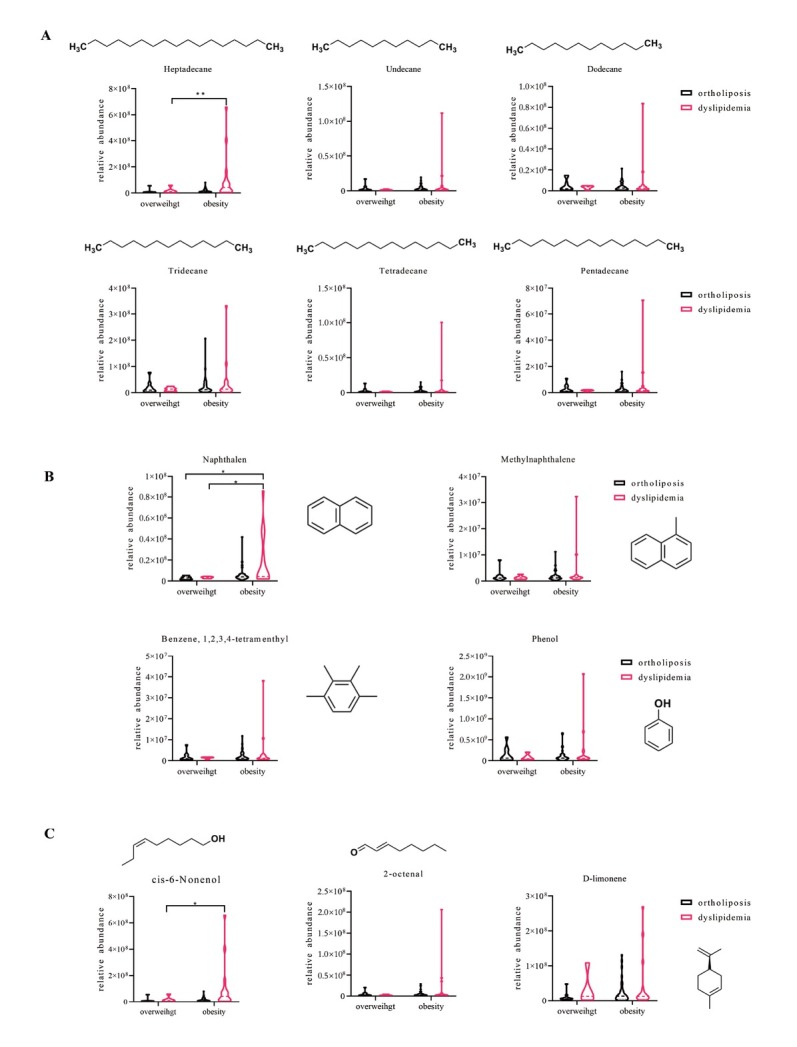
Relative levels of specific VOCs in obese and overweight children with or without dyslipidemia. (A) Saturated hydrocarbons, including heptadecane, undecane, dodecane, tridecane, tetradecane, and pentadecane. (B) Aromatic hydrocarbons, including naphthalene, methylnaphthalene, benzene, 1,2,3,4-tetramenthyl, and phenol. (C) Unsaturated aldehydes, including cis-6-nonenol, 2-octenal, and d-limonene. *P < 0.05; **P < 0.01. VOCs: volatile organic compounds.
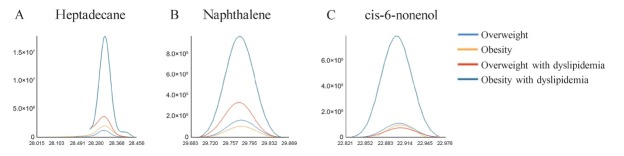
Figure 4 shows a comparison of the selected extraction chromatography of the breath from an obese child and an overweight child with or without dyslipidemia for these three category compounds.
Figure 4.
Selected extraction chromatogram of the representative VOCs that distinguish between obese and overweight participants with or without dyslipidemia. (A) Heptadecane, (B) naphthalene, (C) cis-6-nonenol. VOCs: volatile organic compounds.
Discussion
The principal findings of this study are as follows: (1) obese children with dyslipidemia have a unique pattern of VOCs compared with overweight children without dyslipidemia and (2) a suite of saturated hydrocarbons, aromatic hydrocarbons, and unsaturated aldehydes are elevated in obese children with dyslipidemia. These findings could provide a potential strategy to classify the high risk of childhood obesity with dyslipidemia.
Over the last few decades, obesity has become an increasing public health problem worldwide.[12] Obesity is associated with hypertension, angina, hypertension, diabetes, and arthritis, and it leads to a wide variety of other illnesses.[13, 14, 15] The increasing prevalence of obesity in childhood and adolescence poses an ever-increasing problem since a large proportion of overweight children tend to become obese adults.[16, 17] Obesity, especially dyslipidemia, begins at an early age and significantly increases cardiovascular risk later in life.[18] As previous studies have revealed,[19] our data also showed that obese children with dyslipidemia had both higher TG and non-HDL-c levels. However, our study demonstrated that obese children might not always have dyslipidemia and vice versa. This different phenotype of dyslipidemia or obesity could arise from different internal mechanisms and incur future risks.[20] The method of differentiating various types of obesity with or without dyslipidemia will benefit the understanding and treatment of certain complications. Traditional methods, including laboratory examinations, have shown limitations in diagnostic efficiency. The innovative method of noninvasive detection of breath VOCs could offer the opportunity to dive in greater depth.
Human breath offers several benefits for diagnostic applications in children, including simple access and noninvasive collection. Breath is a rich source of clinically relevant biological information. The assessment of VOCs for disease diagnosis or prognosis is a growing area of research.[4] Some researchers have demonstrated that breath tests could dramatically improve the early detection of conditions such as lung cancer or noncommunicable chronic disease.[21] A panel of VOCs, including ethylbenzene, 2-octenal, and octadecyne, was found to differentiate asthmatic children from healthy children.[22] Moreover, exhaled VOCs from chronic obstructive pulmonary disease patients, such as nitric oxide, have the ability to distinguish unstable patients from stable patients. Exhaled VOCs or their relative concentrations might provide childhood obesity clues or risk classification. A previous study found that a panel of VOCs, including isoprene, 1-decene, 1-octene, ammonia, and hydrogen sulfide, could identify the presence of overweight/obesity with excellent accuracy.[9]
In the present study, we screened three categories of different VOCs, including saturated hydrocarbons, aromatic hydrocarbons, and unsaturated aldehydes, which were discrepant between obese children with and without dyslipidemia. Some of the compounds are components of microorganisms or are metabolized by the microbiome in the human body,[23,24] and they have been found to possess biological functions. Phenol is involved in sulfur metabolism and has been reported to be a uremic toxin that is elevated in chronic kidney disease patients.[25, 26] Some saturated hydrocarbons, such as heptadecane and dodecane, have shown anti-inflammatory and antiallergic effects in rats.[27,28] Many studies have reported that d-limonene effectively plays a valuable role in the prevention of several neoplastic and degenerative diseases.[29, 30] Recently, it has been reported that volatile hydrocarbons and aldehydes in body fluids and breath are attributed to oxidative stress.[31] Some studies have demonstrated that a source of hydrocarbons and aldehydes in exhaled breath is the peroxidation of lipids.[32,33] The process can be mediated by enzymes, such as lipoxygenase, cyclooxygenases, or cytochrome P450 (CYP450),[34] or nonenzymatic peroxidation through oxygen radical oxidative routes.[35] To some extent, exhaled hydrocarbons and aldehydes in this study might represent a state of inflammation in obesity with dyslipidemia. Coincidentally, our study showed that obese children with dyslipidemia had slightly higher leukocytes than overweight children without dyslipidemia. Since inflammation is a key mediator of several metabolic diseases,[36] the elevation of certain VOCs in obese children with dyslipidemia could contribute to the transitional status of diseases such as atherosclerosis, diabetes mellitus, and nonalcoholic fatty liver disease and progression from obesity with dyslipidemia to adult cardiovascular diseases. Exhaled VOCs might play an important role in the dynamic monitoring of obese children with dyslipidemia with the potential ability to predict the prevalence of adult illnesses.
Our study has several limitations, including that only overweight or obese individuals were enrolled in the study. This fact is partially due to the EXCITING study focusing on this group of children, and the primary aim was to evaluate the effects of exercise training on these children This study was cross sectional, and the VOCs were determined at a single time point. Performing serial breath testing on children in both healthy and obese states could help us to better understand the effects of changes in VOCs on different biological processes and their potential impacts on obesity development and progression. Second, the results in the present study will need to be verified in a validation cohort since the preliminary findings were obtained in an 82-subject cohort. In addition, the breath VOCs were collected with bags, which are convenient, inexpensive, and disposable for protection from potentially infectious samples. However, there are also potential drawbacks, including compound degradation and compound interactions within the bag. Therefore, sorbent tubes or electronic noses have been introduced in some other studies. Finally, the VOCs lacked quantitative analysis, and exogenous VOCs were eliminated from the database, which might have affected the interpretation of VOCs from a fuller perspective.
This work provides a strategy to dive into the potential VOCs to classify risk in childhood, revealing promising new clues for future studies to investigate obese children with dyslipidemia.
Conclusion
In the present study, a suite of VOCs from three chemical function groups, saturated hydrocarbons, aromatic hydrocarbons, and unsaturated aldehydes, were separated in obese children with dyslipidemia. Heptadecane, naphthalene, and cis-6-nonenol were significantly elevated in obese children with dyslipidemia. Our findings could reveal the potential value of the candidate VOCs for future risk categories.
Funding Statement
This work was supported by the following funds: National Key Research and Development Program of China (No. 2020YFA0803800, No. 2020YFA0803803), National Natural Science Foundation of China (No. U20A20345, No. 81900315, No. 21976005), and Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases, Chinese Academy of Medical Sciences (No. 2021RU003).
Footnotes
Availability of Data and Materials
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The institutional review board of Peking University Third Hospital approved the protocol (Approval No. LM2021316). Written informed consent was obtained from each participant and his or her parents.
Conflict of Interest
Ming Xu is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this member and his research group.
Contributor Information
Dr. Jiang Zhou, Email: zhoujiang@pku.edu.cn.
Prof. Ming Xu, Email: xuminghi@bjmu.edu.cn.
Reference
- 1.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horesh A, Tsur AM, Bardugo A, Twig G. Adolescent, Childhood Obesity, Excess Morbidity. Mortality in Young Adulthood-a Systematic Review. Curr Obes Rep. 2021;10:301–10. doi: 10.1007/s13679-021-00439-9. [DOI] [PubMed] [Google Scholar]
- 3.P Würtz, Q Wang, AJ Kangas, RC Richmond, J Skarp, M Tiainen. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haworth JJ, Pitcher CK, Ferrandino G, Hobson AR, Pappan KL, Lawson JLD. Breathing new life into clinical testing and diagnostics: perspectives on volatile biomarkers from breath. Crit Rev Clin Lab Sci. 2022 doi: 10.1080/10408363.2022.2038075. [DOI] [PubMed] [Google Scholar]
- 5.Kaloumenou M, Skotadis E, Lagopati N, Efstathopoulos E, Tsoukalas D. Breath Analysis: A Promising Tool for Disease Diagnosis-The Role of Sensors. Sensors (Basel) 2022;22:1238. doi: 10.3390/s22031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim B, Basanta M, Cadden P, Singh D, Douce D, Woodcock A. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax. 2011;66:804–9. doi: 10.1136/thx.2010.156695. [DOI] [PubMed] [Google Scholar]
- 7.Fens N, Zwinderman AH, MP van der Schee, SB de Nijs, E Dijkers, AC Roldaan. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2009;180:1076–82. doi: 10.1164/rccm.200906-0939OC. [DOI] [PubMed] [Google Scholar]
- 8.Rondanelli M, Perdoni F, Infantino V, Faliva MA, Peroni G, Iannello G. Volatile Organic Compounds as Biomarkers of Gastrointestinal Diseases and Nutritional Status. J Anal Methods Chem. 2019;2019:7247802. doi: 10.1155/2019/7247802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhouri N, Eng K, Cikach F, Patel N, Yan C, Brindle A. Breathprints of childhood obesity: changes in volatile organic compounds in obese children compared with lean controls. Pediatr Obes. 2015;10:23–9. doi: 10.1111/j.2047-6310.2014.221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373–92. doi: 10.1016/S2213-8587(21)00045-0. [DOI] [PubMed] [Google Scholar]
- 11.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;5:S213–56. doi: 10.1542/peds.2009-2107C. 128 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefan N, HU Häring, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018;6:249–58. doi: 10.1016/S2213-8587(17)30292-9. [DOI] [PubMed] [Google Scholar]
- 13.Sorop O, Olver TD, J van de Wouw, I Heinonen, RW van Duin, DJ Duncker. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res. 2017;113:1035–45. doi: 10.1093/cvr/cvx093. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Xiong H, Ren T.. Repair of damaged pancreatic β cells: New hope for a type 2 diabetes reversal? J Transl Intern Med. 2021;9:150–51. doi: 10.2478/jtim-2021-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei S, Feng X, Luo J, Guo L, Pan Q.. Obesity and coronavirus disease 2019. J Transl Intern Med. 202210:207–18. doi: 10.2478/jtim-2022-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JD, Fu E, Kobayashi MA. Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu Rev Clin Psychol. 2020;16:351–78. doi: 10.1146/annurev-clinpsy-100219-060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Lu Y, Gokulnath P, Vulugundam G, Li G, Li J. et al. Benefits of physical activity on cardiometabolic diseases in obese children and adolescents. J Transl Intern Med. 2022;10:236–45. doi: 10.2478/jtim-2022-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messiah SE, Vidot DC, Gurnurkar S, Alhezayen R, Natale RA, Arheart KL. Obesity is significantly associated with cardiovascular disease risk factors in 2- to 9-year-olds. Journal of clinical hypertension (Greenwich, Conn) 2014;16:889–94. doi: 10.1111/jch.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calcaterra V, R De Giuseppe, G Biino, M Mantelli, S Marchini, G Bendotti. Relation between circulating oxidized-LDL and metabolic syndrome in children with obesity: the role of hypertriglyceridemic waist phenotype. J Pediatr Endocrinol Metab. 2017;30:1257–63. doi: 10.1515/jpem-2017-0239. [DOI] [PubMed] [Google Scholar]
- 20.Khafagy R, Dash S. Obesity and Cardiovascular Disease: The Emerging Role of Inflammation. Front Cardiovasc Med. 2021;8:768119. doi: 10.3389/fcvm.2021.768119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issitt T, Wiggins L, Veysey M, Sweeney ST, Brackenbury WJ, Redeker K. Volatile compounds in human breath: critical review and meta-analysis. J Breath Res. 2022;16:024001. doi: 10.1088/1752-7163/ac5230. [DOI] [PubMed] [Google Scholar]
- 22.Gahleitner F, Guallar-Hoyas C, Beardsmore CS, Pandya HC, Thomas CP. Metabolomics pilot study to identify volatile organic compound markers of childhood asthma in exhaled breath. Bioanalysis. 2013;5:2239–47. doi: 10.4155/bio.13.184. [DOI] [PubMed] [Google Scholar]
- 23.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–75. doi: 10.1016/j.cgh.2013.02.015. e1–3. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Liu S, Jin G, Yang X, Zhou YJ. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol Adv. 2020;44:107628. doi: 10.1016/j.biotechadv.2020.107628. [DOI] [PubMed] [Google Scholar]
- 25.Duranton F, Cohen G, R De Smet, M Rodriguez, J Jankowski, R Vanholder. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–70. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan S, Li Z, Wang Y, Liang L, Liu F, Qiao Y. et al. A comprehensive weighted gene co-expression network analysis uncovers potential targets in diabetic kidney disease. J Transl Intern Med. 2022;10:359–68. doi: 10.2478/jtim-2022-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi D, Kang W, Park T. Anti-Allergic. Anti-Inflammatory Effects of Undecane on Mast Cells and Keratinocytes. Molecules. 2020;25:1554. doi: 10.3390/molecules25071554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLoS One. 2013;8:e59316. doi: 10.1371/journal.pone.0059316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anandakumar P, Kamaraj S, Vanitha MK. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem. 2021;45:e13566. doi: 10.1111/jfbc.13566. [DOI] [PubMed] [Google Scholar]
- 30.Yuan F, Zhang Q, Dong H, Xiang X, Zhang W, Zhang Y. et al. Effects of des-acyl ghrelin on insulin sensitivity and macrophage polarization in adipose tissue. J Transl Intern Med. 2021;9:84–97. doi: 10.2478/jtim-2021-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratcliffe N, Wieczorek T, Drabińska N, Gould O, Osborne A. De Lacy Costello B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: an aid to understanding the origins of volatile organic compounds from the human body. J Breath Res. 2020;14:034001. doi: 10.1088/1752-7163/ab7f9d. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Pal S, Mitra M. Significance of Exhaled Breath Test in Clinical Diagnosis: A Special Focus on the Detection of Diabetes Mellitus. J Med Biol Eng. 2016;36:605–24. doi: 10.1007/s40846-016-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev. 2012;112:5949–66. doi: 10.1021/cr300174a. [DOI] [PubMed] [Google Scholar]
- 34.Massey KA, Nicolaou A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem Soc Trans. 2011;39:1240–6. doi: 10.1042/BST0391240. [DOI] [PubMed] [Google Scholar]
- 35.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 36.Donath MY, Meier DT, M Böni-Schnetzler. Inflammation in the Pathophysiology and Therapy of Cardiometabolic Disease. Endocr Rev. 2019;40:1080–91. doi: 10.1210/er.2019-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]