Abstract
We present an exploratory post hoc analysis from the phase 3 CheckMate 214 trial of first-line nivolumab plus ipilimumab (NIVO + IPI) versus sunitinib in a subgroup of 108 patients with advanced renal cell carcinoma (aRCC) without prior nephrectomy and with an evaluable primary tumor, a population under-represented in clinical trials. Patients with clear cell aRCC were randomized to NIVO + IPI every 3 week for four doses followed by NIVO monotherapy, or sunitinib every day for 4 wk (6-wk cycle). Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and primary tumor shrinkage were assessed. PFS and ORR were assessed per independent radiology review committee using RECIST version 1.1. With minimum study follow-up of 4 yr for intent-to-treat patients, OS favored NIVO + IPI (n = 53) over sunitinib (n = 55; hazard ratio 0.63, 95% confidence interval 0.40–1.0) among patients without prior nephrectomy. ORR was higher (34% vs 15%; p = 0.0041) and median duration of response was longer with NIVO + IPI versus sunitinib (20.5 vs 14.1 mo); the best overall response was partial response in each arm. A ≥30% reduction in the diameter of intact target renal tumors was achieved in 35% of patients with NIVO + IPI versus 20% with sunitinib. This trial is registered at ClinicalTrials.gov as NCT02231749. Safety was consistent with the global study population. In conclusion, in patients with aRCC without prior nephrectomy and with an evaluable primary tumor, NIVO + IPI showed survival benefits and renal tumor reduction versus sunitinib.
Keywords: Advanced renal cell carcinoma, CheckMate 214, Cytoreductive nephrectomy, Ipilimumab, Nivolumab
Patient summary:
In an exploratory analysis of a large global trial (CheckMate 214), we observed positive outcomes (both survival and tumor response to treatment) with nivolumab plus ipilimumab over sunitinib in a subgroup of patients with advanced kidney cancer who did not undergo removal of their primary kidney tumor. This subset of patients represents a population that has not been studied in clinical trials and for whom outcomes with new immunotherapy combination regimens are not yet known. We conclude that treatment with nivolumab plus ipilimumab offers these patients a survival benefit versus sunitinib, consistent with that observed in the overall study, as well as a notable kidney tumor reduction.
Patients with advanced renal cell carcinoma (aRCC) who do not undergo upfront cytoreductive nephrectomy usually have poor prognosis and clinical trial data are limited for this population [1–3]. However, evidence from the prospective CARMENA trial showed that sunitinib (SUN) alone was noninferior to initial nephrectomy followed by treatment with SUN in patients with aRCC and Memorial Sloan Kettering Cancer Center intermediate-risk or poor-risk disease [4]. In addition, a notable renal tumor reduction was observed with cabozantinib compared with everolimus in pretreated patients without prior nephrectomy in the METEOR trial [5]. Nivolumab plus ipilimumab (NIVO + IPI) is approved for first-line treatment of patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate-/poor-risk aRCC on the basis of results from the randomized phase 3 CheckMate 214 trial [6,7]. In this exploratory post hoc analysis, we assessed the efficacy and safety outcomes with NIVO + IPI versus SUN in a subgroup of patients in CheckMate 214 without prior nephrectomy and with an evaluable primary tumor that is based on minimum study follow-up of 4 yr for intent-to-treat (ITT) patients. The study design details for CheckMate 214 have been published previously [6]. In brief, adults with treatment-naïve aRCC with a clear cell component were stratified by geographic region and IMDC risk (favorable, 0; intermediate, 1–2; poor, 3–6). Patients were randomized 1:1 to intravenous NIVO 3 mg/kg and IPI 1 mg/kg every 3 wk (four doses) followed by NIVO 3 mg/kg every 2 wk or to oral SUN 50 mg every day (4 wk on and 2 wk off; 6-wk cycle). The trial was conducted in accordance with the required institutional review boards and independent ethics committees in accordance with Good Clinical Practice guidelines and is registered at ClinicalTrials.gov as NCT02231749. All patients provided written informed consent [6].
The date of randomization of the last patient in the ITT population was February 23, 2016, and the current database lock was February 25, 2020, which provided an extended minimum study follow-up of 4 yr (the primary analysis reported minimum study follow-up of 17.5 mo; database lock August 7, 2017) [6]. In this post hoc analysis, overall survival (OS), progression-free survival (PFS), and the objective response rate (ORR) were evaluated for ITT patients without prior nephrectomy and one or more evaluable intact renal tumors at baseline. This subgroup is referred to hereafter as “patients without prior nephrectomy and with an evaluable primary tumor.” PFS and ORR were assessed by the independent radiology review committee. Statistical analysis for this exploratory subgroup follows the overall trial methodology, as previously described [6].
In total, 1096 patients were randomized in CheckMate 214 (550 to NIVO + IPI; 546 to SUN) [6]. Of the 108 patients without prior nephrectomy and with an evaluable primary tumor, 53 were randomized to NIVO + IPI and 55 to SUN (Supplementary Fig. 1). Baseline characteristics were largely balanced between the treatment arms (Table 1) [6]. In the subgroup without prior nephrectomy, 58 patients were classified as having IMDC intermediate-risk (NIVO + IPI, n = 26; SUN, n = 32), 48 had poor-risk (NIVO + IPI, n = 26; SUN, n = 22), and two had favorable-risk (NIVO + IPI, n = 1; SUN, n = 1) disease. At baseline the median tumor size by sum of the diameters of target renal tumors was 78.9 mm (range 21–190) for NIVO + IPI patients and 89.3 mm (range 21–246) for SUN patients. The severity of disease burden, as measured by the proportion of patients with at least two sites of target/nontarget tumors, was higher in the subgroup of patients without prior nephrectomy (NIVO + IPI, 96%; SUN, 98%) in comparison to the ITT population (NIVO + IPI, 78%; SUN, 78%), but was balanced between the treatment arms. Of note, the distribution of patients with tumor PD-L1 expression ≥1% across treatment arms was similar between the subgroup and the ITT population (24% vs 22%), although the proportion of patients with IMDC poor-risk disease was greater in the subgroup (44% vs 16%; Table 1).
Table 1 –
Select baseline characteristics of patients without prior nephrectomy and with an evaluable primary tumor and the intent-to-treat population
| Characteristic a | Patients without prior nephrectomy and with an evaluable primary tumor | Intent-to-treat patients [6] | ||
|---|---|---|---|---|
| NIVO + IPI (N = 53) | SUN (N = 55) | NIVO + IPI (N = 550) | SUN (N = 546) | |
| Median age, yr (range) | 64 (40–84) | 64 (34–85) | 62 (26–85) | 62 (21–85) |
|
| ||||
| Sex, n (%) | ||||
| Male | 39 (74) | 43 (78) | 413 (75) | 395 (72) |
| Female | 14 (26) | 12 (22) | 137 (25) | 151 (28) |
|
| ||||
| IMDC risk factors, n (%) b | 1 (2.0) | 1 (2.0) | 125 (23) | 124 (23) |
| Favorable (0) c | 26 (49) | 32 (58) | 334 (61) | 333 (61) |
| Intermediate (1–2) | 26 (49) | 22 (40) | 91 (17) | 89 (16) |
| Poor (3–6) | ||||
|
| ||||
| Region, n (%) | ||||
| United States | 17 (32) | 11 (20) | 154 (28) | 153 (28) |
| Canada/Europe | 16 (30) | 22 (40) | 201 (37) | 199 (36) |
| Rest of the world | 20 (38) | 22 (40) | 195 (35) | 194 (36) |
|
| ||||
| Prior nephrectomy, n (%) | 0 | P0 | 453 (82) | 437 (80) |
|
| ||||
| Sites with target/nontarget tumors, n (%) d | 2 (4.0) | 1 (2.0) | 123 (22) | 118 (22) |
| 1 | 51 (96) | 54 (98) | 427 (78) | 427 (78) |
| ≥2 | ||||
|
| ||||
| Sites of metastasis, n (%) e | ||||
| Lung | 35 (66) | 33 (60) | 381 (69) | 373 (68) |
| Lymph node | 17 (32) | 32 (58) | 246 (45) | 268 (49) |
| Bone f | 14 (26) | 18 (33) | 112 (20) | 119 (22) |
| Liver | 14 (26) | 10 (18) | 99 (18) | 107 (20) |
|
| ||||
| Quantifiable tumor PD-L1 expression, n (%) | N = 48 | N = 54 | N = 499 | N = 503 |
| <1% | 36 (75) | 40 (74) | 386 (77) | 376 (75) |
| ≥1% | 12 (25) | 14 (26) | 113 (23) | 127 (25) |
IMDC = International Metastatic Renal Cell Carcinoma Database Consortium; NIVO + IPI = nivolumab plus ipilimumab; SUN = sunitinib.
Information shown is based on data collected using an interactive voice-response system.
The case report form was the source for the baseline IMDC score.
Two patients in the subgroup without prior nephrectomy and with an evaluable primary tumor were classified as favorable risk per the case report form. Both patients were determined to have one IMDC risk factor present, which was <1 yr from the time of diagnosis to first dose, consistent with intermediate risk categorization. Hence, these patients were not excluded from the current analysis.
The number of target or nontarget tumors at baseline was not reported for one intent-to-treat patient in the SUN arm.
Includes both target and nontarget tumors.
Bone with and without soft-tissue component.
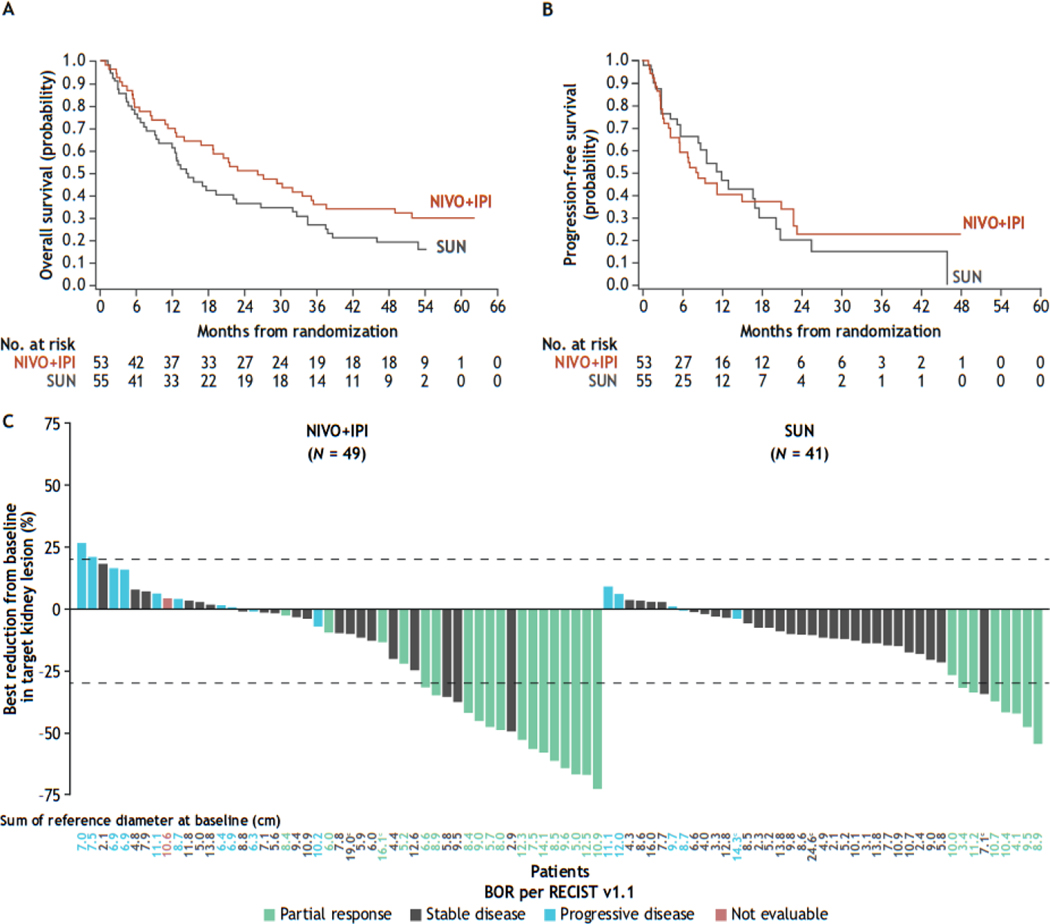
Among patients without prior nephrectomy and with an evaluable primary tumor, the hazard ratio (HR) for OS for NIVO + IPI versus SUN was 0.63 (95% confidence interval [CI] 0.40–1.0; Fig. 1A). Median OS was 26.1 mo (95% CI 14–35) versus 14.3 mo (95% CI 9.7–23), respectively. The HR for PFS was 0.99 (95% CI 0.59–1.7; Fig. 1B). The ORR (95% CI) was higher with NIVO + IPI (34%, 95% CI 22–48%) versus SUN (15%, 95% CI 6.5–27%; Supplementary Table 1), with a best overall response of partial response observed in each arm. The median time to response was 2.8 versus 5.4 mo and the median duration of response (DOR) was 20.5 versus 14.1 mo with NIVO + IPI versus SUN, respectively (Supplementary Fig. 2); the HR for DOR was 0.69 (95% CI 0.17–2.9). Approximately two-thirds of patients in each treatment arm received subsequent therapy (Supplementary Table 2). Eight patients (15%) in the NIVO + IPI arm and four (7.3%) in the SUN arm underwent delayed nephrectomy after their last dose of treatment.
Fig. 1 –
Kaplan-Meier curves for (A) OS a and (B) PFS per IRRC using RECIST version 1.1 b in patients without prior nephrectomy and with an evaluable primary tumor, and (C) maximum reduction in target renal tumors in all response-evaluable patients without prior nephrectomy and with an evaluable primary tumor. c–g BOR = best overall response; CI = confidence interval; HR = hazard ratio; IRRC = independent radiology review committee; NIVO + IPI = nivolumab plus ipilimumab; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumors; SUN = sunitinib.
a Median (95% CI) OS was 26.1 (14–35) mo with NIVO + IPI versus 14.3 (9.7–23) mo with SUN (HR 0.63, 95% CI 0.40–1.0); there were 37 events/53 patients versus 44 events/55 patients, respectively.
b Median (95% CI) PFS was 8.1 (5.5–21) mo with NIVO + IPI versus 11.9 (8.4–18) mo with SUN (HR 0.99, 95% CI 0.59–1.7); there were 33 events/53 patients versus 29 events/55 patients, respectively.
c Patients with a primary tumor at baseline and one or more on-treatment tumor assessments were included.
d Of the 108 patients without nephrectomy, 49/53 patients in the NIVO + IPI arm and 41/55 patients in the SUN arm had a primary tumor at baseline and one or more on-treatment tumor assessments.
e Two patients (3.8%) patients in the NIVO + IPI arm versus three (5.5%) in the SUN arm had more than one evaluable target renal tumor.
f Best reduction shown is the maximum reduction in the sum of the diameters of target renal tumors (a negative value indicates a true reduction; a positive value indicates an increase only observed over time). The horizontal reference line at +20% indicates a 20% increase and the horizontal reference line at −30% indicates a 30% reduction, both consistent with a RECIST version 1.1 response.
g Different colored bars represent overall systemic responses (including but not limited to responses in the primary tumor) according to RECIST version 1.1.
Among patients with an evaluable primary tumor at baseline and one or more on-treatment tumor assessments, a reduction of ≥30% in target renal tumors was achieved in 35% of patients with NIVO + IPI versus 20% with SUN, while an increase in target renal tumors of 20% from baseline occurred in 4.1% of patients with NIVO + IPI and zero patients with SUN (Fig. 1C). The effect of NIVO + IPI on the primary tumor is illustrated in radiographic scans of a patient with aRCC in Supplementary Figure 3.
Any-grade treatment-related adverse events (AEs) occurred in 51/53 patients (96%) treated with NIVO + IPI versus 50/54 (93%) treated with SUN, with grade 3–4 treatment-related AEs in 22/53 (42%) versus 32/54 (59%) patients, respectively. The incidence of treatment-related select AEs (potentially immune-mediated) was consistent with the overall study population (Supplementary Table 3).
In this exploratory subgroup of aRCC patients without prior nephrectomy and with an evaluable primary tumor, a population with poor prognosis and high unmet medical need [1,2], first-line NIVO + IPI showed survival benefits over SUN similar to those for the ITT and intermediate-/poor-risk patients in the overall study [8]. ORR, DOR, and primary tumor shrinkage were also notably improved with NIVO + IPI versus SUN in this subgroup, with an anticipated best overall response of partial response achieved in each arm, consistent with previous findings of lower complete response rates [9,10]. Safety outcomes were generally consistent with the overall study population. Limitations of this analysis include its non-prespecified, post hoc nature and small patient subgroup numbers, although the distribution of patients with unresected primary tumors was well balanced between the treatment arms. In addition, while SUN monotherapy was used as the comparator arm in CheckMate 214, newer tyrosine kinase inhibitor–based therapies are now available [11].
Recent prospective studies are exploring the role and sequencing of nephrectomy for patients with aRCC who receive systemic therapy [4,12,13]. Although underpowered, the SURTIME trial results suggest that pretreatment with SUN improves outcomes in some patients with primary metastatic RCC, and these results help to inform optimal treatment sequencing strategies [12,13]. In addition, the NORDIC-SUN trial (NCT03977571) is investigating deferred nephrectomy in aRCC patients with three or more IMDC risk factors who respond to or have stable disease after upfront NIVO + IPI systemic therapy. Although upfront nephrectomy is not currently recommended for patients with aRCC with poor performance status and intermediate-/poor-risk disease [4,13], prospective studies investigating the role of nephrectomy in the context of contemporary checkpoint inhibitors will continue to inform optimal treatment strategies.
In conclusion, the results presented here show improved efficacy outcomes with NIVO + IPI versus SUN in patients without prior nephrectomy, and further support the established long-term benefits of first-line NIVO + IPI in patients with aRCC.
Supplementary Material
Acknowledgments:
The authors acknowledge the patients and families who made this study possible; the clinical study teams who participated; Dako, an Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28–8 pharmDx assay; and Bristol-Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd. (Osaka, Japan). Editorial assistance was provided by Rachel Maddente of Parexel, funded by Bristol-Myers Squibb. These data were previously presented at the European Society for Medical Oncology (ESMO) Annual Congress in 2020.
Funding/Support and role of the sponsor:
This work was supported by Bristol-Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company (Osaka, Japan). The sponsors played a role in the design and conduct of the study, collection and management of the data, and preparation and review of the manuscript. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672). Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748).
Financial disclosures:
Laurence Albiges certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Laurence Albiges reports consulting fees from Pfizer, Novartis, Bristol-Myers Squibb (BMS), Ipsen, Roche, MSD, AstraZeneca, Merck, Amgen, Astellas, and Exelixis; and other fees from Corvus Pharmaceuticals and Peloton Therapeutics. Nizar M. Tannir reports research funding from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Arrowhead Pharmaceuticals, Takeda, and Epizyme, Inc.; and personal fees from BMS, Exelixis, Pfizer, Novartis, Eisai, Lilly Oncology, Neolukin Therapeutics, Ipsen, Nektar Therapeutics, Surface Oncology, Ono Pharmaceutical, and Oncorena. Mauricio Burotto reports consulting/advisory fees from Roche/Genentech, BMS, MSD Oncology, Novartis, and AstraZeneca; and speaker bureau fees from Roche/Genentech, MSD Oncology, BMS, and AstraZeneca. David McDermott reports research funding from BMS, Merck, Genentech/Roche, Novartis, Peloton Therapeutics, Alkermes, and Prometheus Laboratories; consulting/advisory fees from BMS, Merck, Genentech/Roche, Pfizer, Exelixis, Novartis, X4 Pharma, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, and Lilly; and other fees from Beth Israel Deaconess Medical Center. Elizabeth R. Plimack reports institutional research funding from BMS, AstraZeneca, Merck, Peloton Therapeutics, Pfizer, and Astellas; consulting fees from AstraZeneca, BMS, Genentech, Merck, Pfizer, Exelixis, Incyte, Seattle Genetics, Janssen, Flatiron Health, Infinity Pharma, and MEI Pharma; fees for development of educational presentations from BMS and Merck; and US patent application no. 14/588,503. Philippe Barthélémy reports consulting/advisory fees from BMS, Pfizer, MSD Oncology, Novartis, Ipsen, Roche, AstraZeneca, Merck, and Janssen Cilag; travel and accommodation expenses from Amgen; and honoraria from Astellas Pharma. Camillo Porta reports consulting/advisory fees from Angelini Farma, BMS, MSD Oncology, AstraZeneca, Pfizer, Ipsen, Novartis, EUSA Pharma, Eisai, and Merck Serono; speaker fees from Angelini Farma, BMS, MSD Oncology, Pfizer, Ipsen, EUSA Pharma, Eisai, General Electric, Merck Serono. and AstraZeneca; expert testimony fees from Pfizer and EUSA Pharma; and travel and accommodation expenses from Roche. Thomas Powles reports consulting/advisory fees from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck, MSD, Pfizer, Seattle Genetics, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai, and Roche; honoraria from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck, MSD, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai, and Roche; institutional research funding from AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas Pharma, Johnson & Johnson, and Eisai; and travel and accommodation expenses from Roche, Pfizer, MSD, AstraZeneca, and Ipsen. Frede Donskov reports institutional research funding from BMS, Pfizer, and Ipsen. Saby George reports consulting/advisory fees from Bayer, BMS, Exelixis, Corvus Pharmaceuticals, Genentech, Sanofi/Genzyme, Pfizer, EMD Serono, Seattle Genetics, Eisai, Merck, QED Therapeutics, and Aveo; and research funding from Pfizer, Merck, Agensys, Novartis, BMS, Bayer, Eisai, Seattle Genetics/Astellas, Calithera Biosciences, Immunomedics, Corvus Pharmaceuticals, and Surface Oncology. Christian K. Kollmannsberger reports advisory board fees from Pfizer, Ipsen, Eisai, Roche, AstraZeneca, and BMS; and honoraria from Pfizer, Ipsen, Eisai, and BMS. Howard Gurney reports advisory board fees from Pfizer, Astellas, Ipsen, Roche, and BMS. Marc-Oliver Grimm reports research funding from BMS and Intuitive Surgical; consulting/advisory fees from AstraZeneca, BMS, Ipsen, MSD, Ono Pharmaceutical, Pfizer, Astellas Pharma, EUSA Pharma, and Merck Serono; honoraria from Astellas Pharma, AstraZeneca, BMS, Medac, MSD, Ono Pharmaceutical, Novartis, Pfizer, Ipsen, Merck Serono, and EUSA Pharma; and travel and accommodation expenses from BMS and Merck Serono. Yoshihiko Tomita reports research funding from Ono Pharmaceutical, Takeda, Astellas, and Chugai; consulting/advisory fees from Ono Pharmaceutical; and honoraria from Ono Pharmaceutical, BMS, and Pfizer. Daniel Castellano reports institutional research funding from Janssen Oncology; consulting/advisory fees from Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, BMS, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, and Boehringher Ingelheim; and travel and accommodation expenses from Pfizer, Roche, BMS, and AstraZeneca Spain. Brian I. Rini reports research funding from Pfizer, Merck, GNE/Roche, Aveo, Novartis, Synthorx, Peloton, Compugen, Merck, Arravive, Surface Oncology, and 3D Medicines; and stock ownership in PTC Therapeutics. Toni K. Choueiri reports consulting/advisory fees from Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, BMS, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, LPath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, and ESMO; travel and accommodation expenses from Pfizer, Bayer, Novartis, GlaxoSmithKilne, Merck, BMS, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, LPath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, and ESMO; international patent application numbers PCT/US2018/058430 and PCT/US2018/12209; honoraria from NCCN, UpToDate, Michael J, Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, BMS, Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Health, Kidney Cancer Association, Exelixis, Prometheus, LPath, New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Lilly, Janssen Oncology, and IQvia; and institutional research funding from Pfizer, Novartis, Merck, Exelixis, TRACON Pharma, GlaxoSmithKline, BMS, AstraZeneca, Peloton Therapeutics, Roche/Genentech, Celldex, Agensys, Eisai, Takeda, Prometheus, Ipsen, Corvus Pharmaceuticals, Cerulean Pharma, Seattle Genetics/Astellas, Bayer, Foundation Medicine, Roche, Calithera Biosciences, Analysis Group, and NCI. David Leung is an employee of, owns stock in, has received travel and accommodation expenses from BMS; and reports institutional interest in BMS patents. Shruti Shally Saggi, Chung-Wei Lee, and M. Brent McHenry are employees of and own stock in BMS. Robert J. Motzer reports institutional research funding from Pfizer, Novartis, Eisai, Genentech/Roche, Exelixis, Merck, and BMS; consulting/advisory fees from Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Lilly, AstraZeneca, and EMD Serono; and travel and accommodation expenses from BMS.
Footnotes
Data sharing statement:
The Bristol-Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Renner A, Samtani S, Marin A, et al. Is cytoreductive nephrectomy still a standard of care in metastatic renal cell carcinoma? J Kidney Cancer VHL 2019;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donskov F, Xie W, Overby A, et al. Synchronous versus metachronous metastatic disease: Impact of time to metastasis on patient outcome-results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol Oncol 2020;3:530–9. [DOI] [PubMed] [Google Scholar]
- 3.Courcier J, Dalban C, Laguerre B, et al. Primary renal tumour response in patients treated with nivolumab for metastatic renal cell carcinoma: results from the GETUG-AFU 26 NIVOREN trial. Eur Urol 2021;80:325–9. [DOI] [PubMed] [Google Scholar]
- 4.Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med 2018;379:417–27. [DOI] [PubMed] [Google Scholar]
- 5.Tannir NM, Powles T, Escudier B, et al. Clinical outcomes by nephrectomy status in METEOR, a randomized phase 3 trial of cabozantinib versus everolimus in patients with advanced renal cell carcinoma. Kidney Cancer 2020;4:29–39. [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squibb Bristol-Myers. OPDIVO (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb; 2021. [Google Scholar]
- 8.Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5:e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albiges L, Rini BI, Haanen J, et al. Primary renal tumor shrinkage in patients who did not undergo upfront cytoreductive nephrectomy: subgroup analysis from the phase 3 JAVELIN Renal 101 trial of first-line avelumab + axitinib vs sunitinib for advanced renal cell carcinoma. Presented at the European Society for Medical Oncology (ESMO) annual meeting, September 27–October 1, 2019, Barcelona, Spain. [Google Scholar]
- 10.Soulieres D, Rini BI, Plimack ER, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma: subgroup analysis from KEYNOTE-426 by prior nephrectomy. Presented at the International Kidney Cancer Association (IKCS) 2020 virtual annual meeting, November 6–7, 2020. [Google Scholar]
- 11.Roberto M, Botticelli A, Panebianco M, et al. Metastatic renal cell carcinoma management: From molecular mechanism to clinical practice. Front Oncol 2021;11:657639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: the SURTIME randomized clinical trial. JAMA Oncol 2019;5:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:706–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.