Abstract
Indoleamine-2,3 dioxygenase is a rate-limiting enzyme in the tryptophan catabolism in kynurenine pathways that has an immunosuppressive effect and supports cancer cells to evade the immune system in different cancer types. Diverse cytokines and pathways upregulate the production of indoleamine-2,3 dioxygenase enzymes in the tumor microenvironment and cause more production and activity of this enzyme. Ultimately, this situation results in anti-tumor immune suppression which is in favor of tumor growth. Several inhibitors such as 1-methyl-tryptophan have been introduced for indoleamine-2,3 dioxygenase enzyme and some of them are widely utilized in pre-clinical and clinical trials. Importantly at the molecular level, indoleamine-2,3 dioxygenase is positioned in a series of intricate signaling and molecular networks. Here, the main objective is to provide a focused view of indoleamine-2,3 dioxygenase enhancer pathways and propose further studies to cover the gap in available information on the function of indoleamine-2,3 dioxygenase enzyme in the tumor microenvironment.
Keywords: IDO, Kynurenine, IDO inhibitor, Immunosuppression, Cancer, Tumor microenvironment
Introduction
Cancer is one of the highest causes of death and an important obstacle to improving life expectancy. In 2020, the coronavirus disease (COVID-19) pandemic caused a reduction in diagnosis and treatment services for cancer because of fear of COVID-19 exposure and diminished in-person health services [1]. Consequently, merely in the united states, new cases and deaths number to cancer are estimated at 2,370,000 and 640,000, respectively, in 2022 [2]. Estimating the consequences of the COVID-19 pandemic on cancer diagnosis and treatment at the world population level may demand some decades due to the delay in releasing the population big data in healthcare.
The classic definition suggests that mutations in different genes, such as LEP, TP53, and NeuroD1, are the key role players in chronic diseases such as diabetes and cancer [3–5]. However, nowadays it is demonstrated that most cancer types are not only genetic disorders but also metabolic disorders. Among many metabolic pathways, tryptophan (TRP) metabolism is one of the most vital and fundamental biological processes for all cell types including cancer cells [6]. Indeed, TRP is an aromatic and essential amino acid that mammals are not able to synthesize it, therefore, dietary sources of TRP is required [7]. Indeed, TRP undergoes complex metabolic routes, resulting in the production of many types of signaling molecules [8]. Due to this importance, during the last two decades, TRP metabolism received significant attention in pre-clinical and clinical studies.
Indoleamine-2,3 dioxygenase (IDO) is the first rate-limiting step during the catabolism of TRP [9]. It is coded by the IDO gene which in the human genome, is located on chromosome 8p12. The IDO gene family contains IDO1 and IDO2, but IDO2 in comparison to IDO1 has a weaker performance. Thus, IDO2 is considered with a less efficiency on the TRP metabolism [10]. The IDO contains 407 amino acids heme-containing and is a cytoplasmic protein [9]. IDO initiates its biological influences by metabolizing TRP into kynurenine (Kyn). Kyn causes to decrease in anti-tumor immunity of T cells via aryl hydrocarbon receptor (AhR) signaling. Altogether, the IDO is well known for its immunosuppressive function and indirect action in favor of tumor microenvironment (TME) growth [11].
The tumor has a complex and dynamic microenvironment that usually consists of different cell types, such as cancer cells, stromal cells, endothelial cells, and immune cells. Tumor-infiltrating immune cells can be classified into two main groups. One group has the tumor-antagonizing characteristic that contains effector T cells, natural killer (NK) cells, dendritic cells (DCs), M1-polarized macrophages, and N1-polarized neutrophils. And the other group is tumor-promoting cells namely, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) [12]. In addition to immune cells, cytokines such as interleukin (IL) 6, IL-10, and Transforming growth factor-beta (TGF-β) are essential role players in the TME that cause chronic inflammatory state and immunosuppression [13].
In this review, we focus on the characteristics of the IDO and the pathways that induce the production of IDO in the TME. In addition, the gap in information regarding single-cell RNA-seq analysis and IDO function in the TME is addressed.
Characteristics of IDO enzyme
In 1936, Kotake and Masayama discovered an enzyme in the liver of mammals and named it tryptophan-oxygenase, which changed later to tryptophan-dioxygenase [14]. During evolution, the main features of the IDO enzyme have been conserved in vertebrates. Pioneering investigations unveiled that IDO was highly expressed in placenta tissue. The function of this enzyme is vital for protecting embryos from the maternal immune system [15]. In the human placenta, IDO is express in the glandular epithelium in the decidua [16]. One of the main function of the IDO is to maintain the tolerance in placenta [17]. In placenta, a specific cell type which is called extravillous trophoblasts (EVT) invade the uterine implantation site in order to remodeling and adapting the blood flow for feed fetus. Thereby, EVT come across with a direct contact with maternal cells [18]. EVT significantly express IDO and the activity of IDO is an important role player in order to suppress the proliferation of the T cells. Hence, the function of IDO protects the fetal tissue against the rejection by the maternal immune system and consequently, reduces the chance of abortion [17]. Since, the function of the IDO results in supressing the appropriate function of T cells, tumor cells use IDO as an advantage. Indeed, tumor cells express IDO and support IDO expression in other cell types in the TME in order to reduce the capacity of anti-tumor immunity [19].
Fundamentally, the characteristics of IDO1, IDO2, and tryptophan 2,3 dioxygenase (TDO) enzymes are different from each other. From an enzymatic perspective, IDO1 is a monomeric enzyme that is located in the majority of the tissues and is responsible for the regulatory role of immune responses. Indeed, the low Michaelis–Menten kinetics (Km) rate of IDO1 allows it to efficiently deplete TRP in the local microenvironment [20]. Whereas IDO2 is not as effective as IDO1, and the capacity of IDO2 for TRP degradation is not considered high. Indeed, the Km of the IDO2 enzyme is almost 100-fold higher than IDO1 and TDO in the physiological concentration of TRP. Specifically, in humans and mice, the Km of IDO2 is approximately 6.8 and 12 mM, respectively [20]. In comparison to IDO, less is known about the characteristics of TDO. Similar to IDO, TDO is a heme-containing enzyme and its Km is about 0.135 mM [21, 22]. In addition, IDO1, IDO2, and TDO enzymes have different expression levels in various tissues. The majority of TDO is localized exclusively in the liver, whereas IDO1 is usually detected in other organs such as the placenta, peripheral nervous system, and central nervous systems [23]. Although much less is known about IDO2, it is suggested that it may be expressed at a lower level in the liver, testis, and thyroid [20]. Finally, these three enzymes have different expression levels in cancer types. In various human cancers, IDO1 and, less frequently, TDO are expressed and cause tumor immune resistance features. Interestingly, IDO2 is not expressed so often in human tumors [20].
IDO in kynurenine pathway and its inhibitors
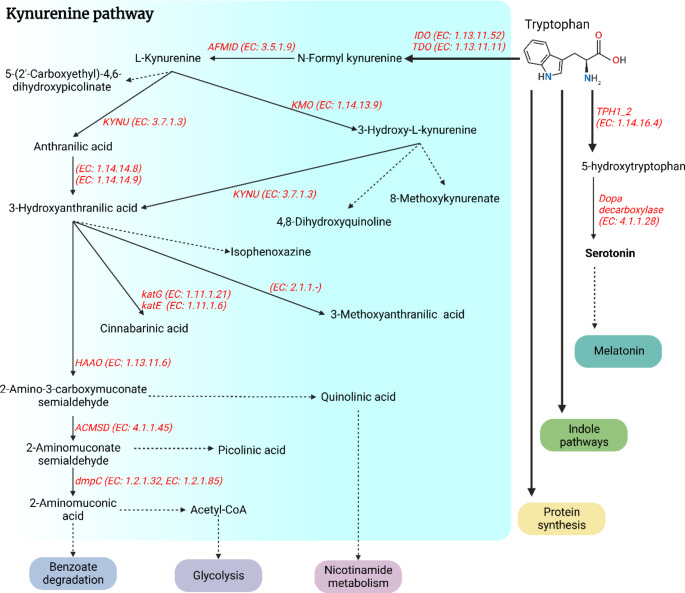
TRP is an essential amino acid for humans and in different physiological conditions it is able to enter into four different biochemical pathways namely, the melatonin synthesis pathway, indole pathway, protein synthesis, and Kyn pathway [24]. The indole pathway appears merely in the intestinal flora of mammals. It is the source of indole products such as indole-3-acetic acid and indole-3-carboxaldehyde which are involved in intestinal immunity regulation via AhR [25]. The Kyn pathway absorbs the majority of available TRP. In most organs, around 95% of TRP is converted into N-Formylkynurenine by IDO1, IDO2, or TDO [26]. Subsequently, N-Formylkynurenine is converted to Kyn by the formamidase enzyme [27]. As an endogenous system, the Kyn pathway contains immunosuppressive characteristics that participate in inflammation and long-term immune tolerance control [28]. Also, this pathway produces important metabolites such as quinolinic acid which ultimately end up in nicotinamide adenine dinucleotide (NAD+) production via the Preiss-Handler pathway and 2-Aminomuconic acid which ends up in glycolysis and benzoate degradation processes [29, 30]. Interestingly, the metabolism of TRP in the brain is different than in other organs. Approximately 1% of dietary TRP consumption is related to the production of Serotonin, N-acetylserotonin, and melatonin in the brain [31]. In this complex organ, during the first step of serotonin synthesis, which is the rate-limiting step of serotonin synthesis, TRP is converted to 5-hydroxytryptophan by the function of the tryptophan hydroxylase 1 (TPH1) and 2 (TPH2) enzymes. In the second step, 5-hydroxytryptophan is converted to serotonin by reacting with the aromatic L-amino acid decarboxylase enzyme (DOPA decarboxylase). Importantly, in the brain, the available concentration of TRP is the regulator for the activity of the TPH1 and TPH2 enzymes (Fig. 1) [31, 32].
Fig. 1.
Summary of the kynurenine pathway of tryptophan metabolism. The solid arrows represent one enzymatic step process and the dash arrows represent more than one enzymatic or non-enzymatic step. The Enzyme Commission number (EC) and symbols of enzymes are shown in red and italics
Among different IDO inhibitors such as BMS-986,205, INCB024360 (Epacadostat), NLG919 (Navoximod), and Norharmane; 1-methyl-tryptophan (1-MT) has been utilized in many studies [33]. 1-MT induces the rejection of fetuses capable of beginning the maternal immune response. Historically, utilizing 1-MT indicated the importance and extraordinary capacity of the IDO1 enzyme in the immune system’s tolerance [15, 34]. The importance of IDO is not limited only to preclinical studies, several clinical trials have started to investigate the role of IDO inhibitors in different cancer types (Table 1). 1-methyl-D-tryptophan (D-1MT) and 1-methyl-L-tryptophan (L-1MT) are two stereoisomers of 1-MT that provide different effects on blocking IDO depending on the cell type [35]. It was noted that L-1MT abolishes IDO1 activity, whereas D-1MT nearly exclusively inhibits IDO2 [36]. Interestingly, the L isomer has higher biochemical activity than the D isomer [37].
Table 1.
IDO inhibitors in clinical trials
| R | IDO Inhibitor(s) | Phase | Cancer types | Drug testing in combination with | Status | clinicaltrials.gov Identifier |
|---|---|---|---|---|---|---|
| 1 | Indoximod | 2 | Metastatic breast cancer |
Docetaxel Paclitaxel |
Completed | NCT01792050 |
| 2 | Epacadostat | 2 |
Ovarian cancer/ Genitourinary (GU) tumors |
Tamoxifen | Terminated | NCT01685255 |
| 3 | Indoximod | 1/2 | Metastatic pancreatic adenocarcinoma; Metastatic pancreatic cancer | NabPaclitaxel; Gemcitabine | Completed | NCT02077881 |
| 4 | SHR9146 | 1 | Solid tumor; Metastatic cancer; Neoplasm malignant |
SHR-1210 Apatinib |
Unknown | NCT03491631 |
| 5 | BMS- 986,205 | 2 | Endometrial adenocarcinoma; Endometrial carcinosarcoma |
BMS- 986,205 Nivolumab |
Active, not recruiting | NCT04106414 |
| 6 | Indoximod | 1/2 | Glioblastoma multiforme; Glioma; Gliosarcoma; Malignant brain tumor |
Temozolomide Bevacizumab |
Completed | NCT02052648 |
| 7 | Indoximod | 1 | Non-small cell lung cancer; Progression of non-small cell lung cancer; Non-small cell lung cancer recurrent |
Docetaxel Tergenpumatucel-L |
Terminated | NCT02460367 |
| 8 | 1-methyl-Dtryptophan | 1 | Breast cancer; Lung cancer; Melanoma; Pancreatic cancer; Solid tumors | - | Terminated | NCT00739609 |
| 9 | INCB024360 | 1 |
Ovarian cancer; Fallopian tube carcinoma; Primary peritoneal carcinoma |
Fludarabine; Cyclophosphamide | Completed | NCT02118285 |
| 10 | Epacadostat | 1/2 | Breast cancer female; Breast neoplasm female |
INCMGA00012; Low dose Cyclophosphamide; Interferon inoculation |
Recruiting | NCT03328026 |
| 11 | Epacadostat | 2 | Gastrointestinal stromal tumors | Pembrolizumab | Completed | NCT03291054 |
| 12 | KHK2455 | 1 | Urothelial carcinoma | Avelumab | Active, not recruiting | NCT03915405 |
| 13 | LY3381916 | 1 | Solid tumor; Non small cell lung cancer; Renal cell carcinoma; Triple negative breast cancer | LY3300054 | Terminated | NCT03343613 |
| 14 | Indoximod | 1 | Ependymoma; Medulloblastoma; Glioblastoma; Primitive; Neuroectoderma | Cyclophosphamide; Etoposide; Ibrutinib | Recruiting | NCT05106296 |
| 15 | Epacadostat | 1/2 | Solid Tumor |
Oxaliplatin; Leucovorin; 5-Fluorouracil; Gemcitabine; nab-Paclitaxel; Carboplatin; Paclitaxel; Pemetrexed; Cyclophosphamide; Cisplatin; platinum agents |
Completed | NCT03085914 |
| 16 | Epacadostat | 1/2 | Solid Tumors | Nivolumab; Ipilimumab; Lirilumab | Terminated | NCT03347123 |
| 17 | Epacadostat | 1/2 | Recurrent fallopian tube cancer; Recurrent ovarian epithelial cancer; Recurrent primary peritoneal cavity cancer; Stage IA fallopian tube cancer; Stage IA ovarian epithelial cancer; Stage IA primary peritoneal cavity cancer; Stage IB fallopian tube cancer; Stage IB ovarian epithelial cancer; Stage IB primary peritoneal cavity cancer; Stage IC fallopian tube cancer; and 23 more | ALVAC(2)-NYESO-1 (M)/TRICOM vaccine | Withdrawn | NCT01982487 |
| 18 | NLG802 | 1 | Solid Tumor | - | Completed | NCT03164603 |
| 19 | Epacadostat | 1/2 | Fallopian tube carcinoma; Ovarian carcinoma; Primary peritoneal carcinoma | Poly ICLC | Completed | NCT02166905 |
| 20 | Indoximod | 1/2 | Metastatic Melanoma; Stage III Melanoma; Stage IV Melanoma | Pembrolizumab; Nivolumab; Ipilimumab | Completed | NCT02073123 |
| 21 | - | 2 | Endometrium cancer | Celecoxib 200 mg capsule | Unknown | NCT03896113 |
| 22 | GDC-0919 | 1 | Solid Tumor | - | Completed | NCT02048709 |
| 23 | Indoximod | 1 | Glioblastoma multiforme; Glioma; Gliosarcoma; Malignant brain tumor; Ependymoma; Medulloblastoma; Diffuse intrinsic pontine glioma; Primary CNS tumor | Etoposide; Cyclophosphamide; Temozolomide | Completed | NCT02502708 |
| 24 | HTI-1090 | 1 | Advanced Solid Tumors | - | Completed | NCT03208959 |
| 25 | Epacadostat | 1 |
Stage III fallopian tube cancer AJCC v7; Stage III ovarian cancer AJCC v6 and v7; Stage III primary peritoneal cancer AJCC v7; Stage IIIA fallopian tube cancer AJCC v7; Stage IIIA ovarian cancer AJCC v6 and v7; Stage IIIA primary peritoneal cancer AJCC v7; Stage IIIB fallopian tube cancer AJCC v7; Stage IIIB ovarian cancer AJCC v6 and v7; Stage IIIB primary peritoneal cancer AJCC v7; Stage IIIC fallopian tube cancer AJCC v7; and 5 more |
- | Active, not recruiting | NCT02042430 |
| 26 | BMS-986,205 | 1/2 |
Metastatic hepatocellular carcinoma; Stage III hepatocellular carcinoma AJCC v8; Stage IIIA hepatocellular carcinoma AJCC v8; Stage IIIB hepatocellular carcinoma AJCC v8, Stage IV hepatocellular carcinoma AJCC v8; Stage IVA hepatocellular carcinoma AJCC v8; Stage IVB hepatocellular carcinoma AJCC v8; Unresectable hepatocellular carcinoma |
Nivolumab | Active, not recruiting | NCT03695250 |
| 27 | DN1406131 | 1 | Advanced solid tumors | - | Unknown status | NCT03641794 |
| 28 | BMS-986,205 | 1/2 | Advanced cancer | - | Recruiting | NCT03459222 |
| 29 | Epacadostat | 2 |
ATM gene mutation; Deleterious BRCA1 gene Mutation; Deleterious BRCA2 gene Mutation; Homologous recombination Deficiency; Pancreatic ductal Adenocarcinoma; Stage II pancreatic cancer AJCC v8; Stage IIA pancreatic cancer AJCC v8; Stage IIB pancreatic cancer AJCC v8; Stage III pancreatic cancer AJCC v8; Stage IV pancreatic cancer AJCC v8 |
Pembrolizumab | Withdrawn | NCT03432676 |
| 30 | BMS-986,205 | 2 | Lip; Oral Cavity Squamous Cell Carcinoma; Pharynx; Larynx; Squamous cell carcinoma | Nivolumab | Recruiting | NCT03854032 |
| 31 | Epacadostat | 2 |
Mucosal melanoma; Recurrent melanoma; Recurrent uveal melanoma; Stage IIIA skin melanoma; Stage IIIA uveal melanoma; Stage IIIB skin melanoma; Stage IIIB uveal melanoma; Stage IIIC skin melanoma; Stage IIIC uveal melanoma; Stage IV skin melanoma; Stage IV uve |
MELITAC 12.1 Peptide Vaccine | Completed | NCT01961115 |
| 32 | Epacadostat | 2 | Sarcoma | Pembrolizumab | Active, not recruiting | NCT03414229 |
| 33 | INCB024360 | 1 | Glioblastoma; Glioblastoma multiforme | Ipilimumab; Anti-GITR monoclonal antibody MK-4166; Nivolumab | Terminated | NCT03707457 |
| 34 | BMS-986,205 | 1 | Glioblastoma | Nivolumab; Temozolomide | Recruiting | NCT04047706 |
| 35 | Epacadostat | 2 | Myelodysplastic syndromes | - | Completed | NCT01822691 |
Last but not least, salinomycin (an antibacterial and coccidiostat ionophore therapeutic drug) decreases the expression levels of IDO1 and IDO2. It represses the Janus kinase/Signal transducer and activator of transcription (JAK/STAT) and nuclear factor-κB (NF-κB) pathways with the collaboration of interferon-gamma (IFNγ). It is suggested that salinomycin diminishes Kyn production and consequently acts against tumor favor [38].
IDO in immune cells
The TME contains a wide range of immune cells generally including macrophages, DCs, T cells, MDSCs, mast cells, and NK cells [39]. Macrophages contain two main subtypes namely, M1 and M2 macrophages. While M1 macrophage has tumor-resistant characteristics, M2 macrophage has tumor-promoting capabilities. M2 macrophages, generally assumed tumor-associated macrophages (TAMs). TAMs are considered as the major inflammatory cells in TME [40]. TAMs have tumor supporting characteristics by modifying angiogenesis, extracellular matrix and chronic inflammation. Also, they support immune suppression processes via various signaling pathways such as NF-κB and Jak‐STAT3 [39]. It is shown that IDO plays an important role in macrophage differentiation and induces macrophages to M2 sub type [41].
DCs are vital role players in TME which contain different subtypes with anti-tumor or tumorigenesis characteristics. It is illustrated that plasmacytoid dendritic cells (pDCs) are involved in tumorigenesis process, while, inflammatory DC (inf-DC) and conventional DC (cDC) have a controversial role in TME [42]. IDO by consuming the available TRP in TME cause to activation of DCs [43]. DCs themselves also are able to produce IDO during reaction to different immunogenic and tolerogenic molecules. Overall, IDO production in DCs can prevent a potent anti-tumor response [44].
Despite anti-tumor immune function by T cells, they can also cause immune tolerance [45]. IDO via different signalings such as Fas-mediated and Vav1 signalings can suppress T cells. Indeed, the Vav1 signaling pathway is one of the adjusters of T cell homeostasis [46]. Interestingly, it is revealed that IDO specific CD8+ T cells can determine IDO+ suppressive cells such as IDO-expressing DCs and destroy them. Thereby, IDO specific CD8+ T cells are able to enhance T cell immunity versus tumor-associated antigen [47]. Tregs increase IDO production in antigen presenting cells (APCs) through cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4)/B7 signaling in the lymph nodes that are draining tumor sites [48].
Gradually by growing the TME immunosuppressive cells such as MDSCs and Treg cells begin to prohibit the proliferation and activity of cytotoxic T cells. MDSCs are a heterogeneous population that repress the function of T and NK cells to assist development of tumor, pre-metastatic niche, and immunotherapy resistance [49]. By the activity of IDO, MDSCs cause and preserve of immune tolerance and tumor immune escape by boosting the function of Treg cells [50].
Mostly, it is still unknown whether mast cells and NK cells are able to produce IDO in human tumor microenvironments. Canine mast cell tumour cells express IDO in order to control the levels of TRP and KYN [51]. Also, it is reported that cell to cell contact of mast cells and monocyte derived DCs significantly expand the expression of IDO in monocyte derived DCs. Some studies showed that IDO expression in cancer associated fibroblasts suppresses NK cell activity [48]. Recently, it is demonstrated that the activity of IDO can interrupt NK cells function by reducing the NKG2D ligand in non-small cell lung cancer [52]. Although many studies dedicated to understand the function of IDO in the tumor microenvironment, many effects of the function IDO on immune cells and their subtypes have yet to be well understood.
Finally, IDO via toll-like receptor-4-myeloid differentiation primary response 88 (TLR4/MyD88) pathways controls the activation of NF-κB and the presence of proinflammatory cytokines, such as IL-1β, IL-6, TNFα (Tumour Necrosis Factor α), IL‑23, and IL‑17 A [53].
IDO expresses in various cancer types
IDO1 in cancer types
The IDO1 expression is significantly high in some cancer types, such as triple-negative breast cancer, and prostate cancer [54–56]. In bladder cancer, microRNA-153 via inhibiting IL-6/STAT3/VEGF (Vascular endothelial growth factor) signaling targets the IDO1 expression and, consequently, reduces the cancer progression [57]. In addition, short hairpin RNA blocks the IDO1 function and leads to reducing the progression of lung cancer. It reduces the IL‑2 and TNFα but raises the expression levels of inhibitory receptors Programmed death‑1 (PD‑1) as well as B-and-T-lymphocyte attenuator (BTLA) in T lymphocyte cells [58]. In addition, interferon-induced guanylate-binding protein 1 (GBP1) assists IDO1 in migrating to extracellular space and facilitates proliferation and metastasis. Intercepting the extracellular secretion of IDO1 reduces T cell exhaustion and consequently improves the anti-tumor impact of PD‑1 inhibitors [59].
In prostate cancer, it is shown that high levels of IDO1 expression are related to low Gleason score and prostate-specific antigen (PSA) levels [60]. Also, in the E.G7-OVA tumor model, silencing the IDO1 gene in DC is illustrated as an effective therapeutic strategy [61]. In colorectal carcinoma and esophageal cancers, the higher expression levels of IDO1 and PD-L1 significantly correlated with a high mitotic index and poor survival rate [62, 63].
In cancer types such as breast and colorectal, IDO1 co-expressed with cyclooxygenase-2 (COX2) is a weak independent predictor for overall survival [64]. Also, in triple-negative breast cancer, the expression of IDO1 has an association with forkhead box P3 (FoxP3) positive cells [65]. Interestingly, it is suggested that IDO1 blocking might interrupt CTLA-4 signaling in breast cancer cells [66]. Specifically, in colorectal cancer IDO1 activates the phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) pathway that promotes tumor progression [67]. CD8A+/IDO1+ is introduced as a prognostic characteristic of overall survival and biomarker for colon cancer. Despite an elevated level of infiltrated CD8, a subtype of colon cancer with a high ratio of CD8A+/IDO1+ cells causes robust poor survival [68]. In addition, in pancreatic ductal adenocarcinoma, IDO inhibitors boost the cytotoxicity effect of γδ T cells on some of the pancreatic ductal adenocarcinoma cells but not all of them [69].
IDO2 and TDO in cancer types
By controlling IDO2 and producing more Kyn concentration, thyroid cancer cells suppress NK cell cytotoxicity. Indeed, the expression of NK receptors such as NKG2D and NKp46 are inhibited by STAT1 and STAT3 pathways, which regulate the promoter regions of these receptors [70]. In the squamous cell carcinomas subgroup, IDO2 and PD-L1 have a high co-expression. This expression level of IDO2 is a potential prognostic biomarker in non-small cell lung cancer [71]. IDO2 depletion in-vivo model with Lewis lung carcinoma declined tumor growth and modified tryptophan accumulation and Kyn reductions in TME; as a result, induced aggression of immune cells [72].
In comparison to IDO1 and IDO2, TDO is less known in TME. It is illustrated that the expression TDO is high in the SK-Mel-28 melanoma cell line, infiltrating polymorphonuclear leukocytes and endothelium in cervical TMEs [73, 74]. Combination therapy of PEG-KYNase and immune-checkpoint inhibitors remarkably decreased the level of Kyn by downregulating the IDO1 and TDO expression in the TMEs such as melanoma and breast cancer [75].
Enhancer pathways of IDO enzyme production
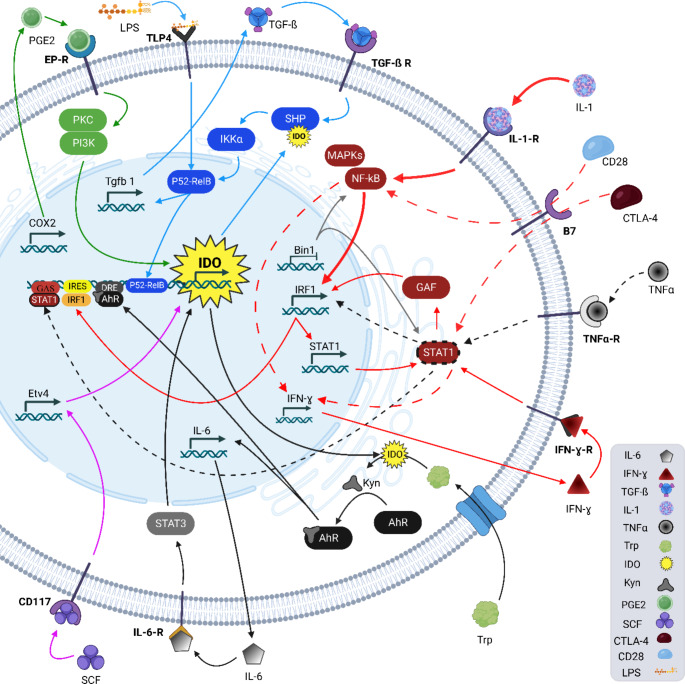
Different TMEs might express various levels of IDO and alterations in the expression of IDO in the immune cells cause modifications in the immune responses. Different pathways with positive feedback such as the AhR pathway can increase IDO production. Illustrated pathways in Fig. 2 are inductor mechanisms that support the expression of IDO.
Fig. 2.
Summary of IDO enzyme enhancer pathways. Each color or style of the arrow shows a group of related events
Activated AhR increases the expression level of IDO and the IL-6 gene. Consequently, IL-6, via an autocrine manner by mediating SATA-3, maintains the expression level of the IDO (Fig. 2 – black arrows) [76]. Also, RelB (v-rel reticuloendotheliosis viral oncogene homolog B) is associated with AhR in the modulation of IDO concentration [77].
IDO expression is stimulated by proinflammatory cytokines such as TNFα, IFNγ, IL-1, lipopolysaccharide (LPS), and prostaglandin E2 (PGE2) [77–79]. IL-1 and TNFα together raise IFN-induced IDO activity [80]. IL-1 initiates a signaling cascade that causes the activation of NF-κB and mitogen activated protein kinases (MAPKs) and induces expression of interferon regulatory factor 1 (IRF1) [81, 82]. TNFα synergistically provokes IDO gene expression by STAT-1 and NFκB-dependent IRF1 signaling [83]. In addition, IFNγ phosphorylates STAT1 and induces the expression of IRF1 by mediating GAF (IFN-gamma activated factor), and consequently, the expression of IRF1 induces the expression of STAT1 and then IDO [84]. In DCs, the B7 receptor signaling causes production of IFN-γ by STAT1, p38 MAPK, and NF-κB (Fig. 2 – red, black-dashed and red dash arrows) [85].
Bridging integrator 1 (Bin1) was identified as an MYC-interacting protein with the ability to the tumor suppression function. In various cancers like primary breast cancer and metastatic prostate cancers, functional deletions in the BIN1 gene have been reported [86, 87]. The loss of BIN1 gene expression is correlated with raised NF-κB and STAT1-dependent expression of IDO [88]. Furthermore, studies on cancer cell lines revealed that IDO expression is connected to COX2 and prostaglandin E2 that act as autocrine signaling by the PGE2 receptor. This cascade stimulates IDO transcription via the PKC and PI3K signaling [21]. In cancer cells, activation of Etv4 (ETS Variant Transcription Factor 4) by CD117 (KIT - tyrosine-protein kinase) driven signaling induces IDO expression (Fig. 2 – green, gray, and purple arrows) [89].
TGF-β stimulates the expression of SHPs via Smad-dependent and PI3K-dependent pathways. Additionally, IDO recruits SHP1/SHP2 to stimulate the noncanonical NF-kB pathway by provoking phosphorylation of the kinase IKKα and p52-RelB. Subsequently, it stimulates the genes encoding IDO and TGF-β [90, 91]. Also, in DCs, LPS is an inductor of RelB which increases the IDO level (Fig. 2 – blue arrows) [77]. Importantly, T cells are considered as one of the protagonists of the first line against the cancer battle [92]. Many studies showed that IDO via different mechanisms manipulates T cells’ function and causes anti-cancer immunosuppression, ultimately supporting TME. Furthermore, via increasing TGF-β production, IDO imposes modifications to the T cell populations [90].
Overall, IDO is a potential tool for supporting tumor cells to alter the TME in favor of tumor growth and progression. The expression ratio of IDO in the TME is adjusted by different mechanisms within an intricate network. The IDO network is a potential area to investigations for new cancer therapy targets.
Conclusions and perspectives
The function of IDO is vital for immune tolerance in the placenta and other normal tissues, however, cancer cells also utilize the characteristics of the IDO to support their growth and survival. Indeed, IDO is located at the core of complicated signaling cascades, which can change the fate of tumor progression by manipulating different cell populations, such as T cells in the TME. In addition, IDO is becoming a hot topic in cancer therapy, hence, several IDO blockers such as Epacadostat, BMS986205, PF-06840003, Navoximod, Indoximod, NLG802, and LY3381916 entered to the clinical stage [93].
Immune checkpoints functionally are enormously important in both physiological and disease conditions, however in cancer types immune checkpoints might be associated with the immune escape phenomenon. For example, it is proposed that CTLA-4 signaling might support the immune escape of cancer cells [94]. In tumors with a high IDO expression, the AhR pathway is more active that causing high resistance to immune checkpoint blockers [95]. The elevated expression and activity of IDO are connected to primary resistance to immunotherapy in patients with non-small cell lung cancer [96]. Also, IDO expression is linked to the progression of breast cancer and unsatisfactory response to neoadjuvant chemotherapy [97]. It is suggested that the co-expression of IDO and PD-L1 could be used to indicate a poor pathologic response after neoadjuvant chemoradiotherapy [98]. Despite efforts, many questions related to the function of IDO in the TME have yet to be well understood.
Basically, one of the challenging obstacles in cancer therapy is that while some patients respond to the treatments very well, others have relatively poor responses. The single-cell RNA sequencing technique in the recent decade has been remarkably advantageous in clarifying the reasons for this phenomenon. Although many studies have used single-cell RNA sequencing to study various aspects of cancer, there is a profound lack of single-cell RNA sequencing analysis on the role of IDO in TME. Using single-cell RNA sequencing analysis on different in-vivo and in-vitro models such as IDO knockout mouse models, IDO knockout cancer cells, or IDO overexpressed cancer cells will provide a wide overview of numerous gene expression alterations expressions. This information has a high potential to shed light many unknown aspects of the role of IDO in the TME as well as other immunological disorders.
Last but not least, the future perspective of immuno-oncology is dependent on the combination of immunotherapies. Since, most cell types in the TME are under the impact of signaling cascades of IDO, combining IDO inhibitors or IDO-linked cytokines such as TGF-β is a new approach in immuno-oncology. Specifically, it is not well understood whether inhibiting TGF-β potentialized the immune toxicities of other treatments [99]. Also, the concentration and timing of TGF-β blocking agents combined with other therapies are critical [100]. During the treatment of cancer with IDO and TGF-β inhibitors, the homeostatic functions of IDO and TGF-β should be considered precisely and not be compromised. Therefore, yet multifold doubts need to be clarified to utilize the therapeutic potential of IDO and TGF-β in clinical approaches.
Overall, IDO is a high-potential spot for conducting many new researches related to understanding cancers’ cellular behavior, identifying novel therapeutic targets, and designing novel treatment strategies.
Acknowledgements
The authors would like to appreciate BioRender.com for its services. Figure 2 is created with Biorender.com.
Author contributions
All authors contributed to the study conception and design. The original idea for the article was designed by Parviz Azimnasab-sorkhabi, Maryam Soltani-asl, and Jose´ Roberto Kfoury Junior. Literature searches were performed by Parviz Azimnasab-sorkhabi, Maryam Soltani-asl, and Túlio Teruo Yoshinaga. The first draft of the manuscript was written by Parviz Azimnasab-sorkhabi, and Maryam Soltani-asl. Critically revised was performed by Parviz Azimnasab-sorkhabi, Maryam Soltani-asl, Maria Lucia Zaidan Dagli, Cristina de Oliveira Massoco Salles Gomes, and Jose´ Roberto Kfoury Junior. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Declarations
The author thanks the National Council for the Improvement of Higher Education (CAPES) and the National Council for Scientific and Technological Development (CNPq) for the scholarship’s support.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yabroff KR, Wu XC, Negoita S, Stevens J, Coyle L, Zhao J, et al. Association of the COVID-19 pandemic with patterns of Statewide Cancer Services. J Natl Cancer Inst. 2022;114(6):907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–590. doi: 10.1097/cm9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham D-V, Park P-H. Tumor metabolic reprogramming by Adipokines as a critical driver of Obesity-Associated Cancer Progression. Int J Mol Sci. 2021;22(3):1444. doi: 10.3390/ijms22031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.asl MS, Azimnasab-sorkhabi P, Abolfathi A-A, aghdam YH. Identification of nucleotide polymorphism within the NeuroD1 candidate gene and its association with type 1 diabetes susceptibility in iranian people by polymerase chain reaction-restriction fragment length polymorphism. J Pediatr Endocrinol Metab. 2020;33(10):1293–1297. doi: 10.1515/jpem-2019-0441. [DOI] [PubMed] [Google Scholar]
- 5.Azimnasab-sorkhabi P, Soltani-asl M, Kfoury Junior J, Algenstaedt P, Moammadzadeh Ghosi F, Hashemi aghdam Y The impact of leptin and its receptor polymorphisms on type 1 diabetes in a population of northwest Iran. Annals of Human Biology. 10.1080/03014460.2022.2134453 [DOI] [PubMed]
- 6.Gyamfi J, Kim J, Choi J (2022) Cancer as a metabolic disorder. Int J Mol Sci 23. 10.3390/ijms23031155 [DOI] [PMC free article] [PubMed]
- 7.Murthy GG, Prideaux MA, Armstrong M, Kenney HM, Latchney SE, Susiarjo M, et al. Characterization of the temporal, cell-specific and interferon-inducible patterns of indoleamine 2,3 dioxygenase 1 (IDO1) expression in the human placenta across gestation. Placenta. 2021;115:129–138. doi: 10.1016/j.placenta.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Fiore A, Murray PJ. Tryptophan and indole metabolism in immune regulation. Curr Opin Immunol. 2021;70:7–14. doi: 10.1016/j.coi.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16(4):354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46(9):2155–2163. doi: 10.1007/s00726-014-1766-3. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei X, Lei Y, Li J-K, Du W-X, Li R-G, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88:106939. doi: 10.1016/j.intimp.2020.106939. [DOI] [PubMed] [Google Scholar]
- 14.Booth ES, Basran J, Lee M, Handa S, Raven EL. Substrate oxidation by Indoleamine 2,3-Dioxygenase: EVIDENCE FOR a COMMON REACTION MECHANISM. J Biol Chem. 2015;290(52):30924–30930. doi: 10.1074/jbc.M115.695684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 16.Kudo Y, Koh I, Sugimoto J. Localization of indoleamine 2,3-Dioxygenase-1 and indoleamine 2,3-Dioxygenase-2 at the human maternal-fetal interface. Int J Tryptophan Res. 2020;13:1178646920984163. doi: 10.1177/1178646920984163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hönig A, Rieger L, Kapp M, Sütterlin M, Dietl J, Kämmerer U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2004;61(2):79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018;9:2597. doi: 10.3389/fimmu.2018.02597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asghar K, Farooq A, Zulfiqar B, Loya A. Review of 10 years of research on breast cancer patients: focus on indoleamine 2,3-dioxygenase. World J Clin Oncol. 2021;12(6):429–436. doi: 10.5306/wjco.v12.i6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Baren N, Van den Eynde BJ (2016) Tryptophan-degrading enzymes in Tumoral Immune Resistance. Front Immunol 6(34). 10.3389/fimmu.2015.00034 [DOI] [PMC free article] [PubMed]
- 21.Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen ED, et al. Constitutive IDO1 expression in human tumors is driven by Cyclooxygenase-2 and mediates intrinsic Immune Resistance. Cancer Immunol Res. 2017;5(8):695. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 22.Meng B, Wu D, Gu J, Ouyang S, Ding W, Liu ZJ. Structural and functional analyses of human tryptophan 2,3-dioxygenase. Proteins. 2014;82(11):3210–3216. doi: 10.1002/prot.24653. [DOI] [PubMed] [Google Scholar]
- 23.Widner B, Werner ER, Schennach H, Fuchs D. An HPLC method to determine tryptophan and kynurenine in serum simultaneously. Adv Exp Med Biol. 1999;467:827–832. doi: 10.1007/978-1-4615-4709-9_105. [DOI] [PubMed] [Google Scholar]
- 24.Dolivo DM, Larson SA, Dominko T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell Mol Life Sci. 2018;75(20):3663–3681. doi: 10.1007/s00018-018-2880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 26.Song X, Si Q, Qi R, Liu W, Li M, Guo M et al (2021) Indoleamine 2,3-Dioxygenase 1: a promising therapeutic target in malignant tumor. Front Immunol 12. 10.3389/fimmu.2021.800630 [DOI] [PMC free article] [PubMed]
- 27.Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncological Sci. 2017;3(2):52–56. doi: 10.1016/j.jons.2017.04.001. [DOI] [Google Scholar]
- 28.Krupa A, Kowalska I (2021) The Kynurenine pathway-new linkage between Innate and adaptive immunity in Autoimmune Endocrinopathies. Int J Mol Sci 22(18). 10.3390/ijms22189879 [DOI] [PMC free article] [PubMed]
- 29.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD + metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa STPd, Cabral L, Lacerda-Júnior GV, Noronha MF, Ottoni JR, Sartoratto A, et al. Exploring the genetic potential of a fosmid metagenomic library from an oil-impacted mangrove sediment for metabolism of aromatic compounds. Ecotoxicol Environ Saf. 2020;189:109974. doi: 10.1016/j.ecoenv.2019.109974. [DOI] [PubMed] [Google Scholar]
- 31.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musajo L, Benassi CA (1964) Aspects of Disorders of the Kynurenine pathway of Tryptophan Metabolism in Man. In: Sobotka H, Stewart CP (eds) Advances in Clinical Chemistry. Elsevier, pp 63–135. DOI:10.1016/S0065-2423(08)60373-X [DOI] [PubMed]
- 33.Günther J, Däbritz J, Wirthgen E (2019) Limitations and Off-Target Effects of Tryptophan-Related IDO inhibitors in Cancer Treatment. Front Immunol 10. 10.3389/fimmu.2019.01801 [DOI] [PMC free article] [PubMed]
- 34.Nelp MT, Kates PA, Hunt JT, Newitt JA, Balog A, Maley D et al (2018) ;115(13):3249. 10.1073/pnas.1719190115 [DOI] [PMC free article] [PubMed]
- 35.Wirthgen E, Kanitz E, Tuchscherer M, Tuchscherer A, Domanska G, Weitschies W, et al. Pharmacokinetics of 1-methyl-L-tryptophan after single and repeated subcutaneous application in a porcine model. Exp Anim. 2016;65(2):147–155. doi: 10.1538/expanim.15-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the Preferred biochemical target of the Antitumor Indoleamine 2,3-Dioxygenase inhibitory compound d-1-Methyl-Tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.Can-07-1872. [DOI] [PubMed] [Google Scholar]
- 37.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.Can-06-2925. [DOI] [PubMed] [Google Scholar]
- 38.Ebokaiwe AP, Njoya EM, Sheng Y, Zhang Z, Li S, Zhou Z, et al. Salinomycin promotes T-cell proliferation by inhibiting the expression and enzymatic activity of immunosuppressive indoleamine-2,3-dioxygenase in human breast cancer cells. Toxicol Appl Pharmcol. 2020;404:115203. doi: 10.1016/j.taap.2020.115203. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8(10):4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutilier AJ, Elsawa SF (2021) Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci 22(13). 10.3390/ijms22136995 [DOI] [PMC free article] [PubMed]
- 41.Wang XF, Wang HS, Wang H, Zhang F, Wang KF, Guo Q, et al. The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell Immunol. 2014;289(1–2):42–48. doi: 10.1016/j.cellimm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Wylie B, Macri C, Mintern JD, Waithman J (2019) Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers (Basel) 11(4). 10.3390/cancers11040521 [DOI] [PMC free article] [PubMed]
- 43.Hwang SL, Chung NP, Chan JK, Lin CL, Indoleamine 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;2(3):167–175. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- 44.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41(6–7):738–764. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh DY, Fong L, Newell EW, Turk MJ, Chi H, Chang HY, et al. Toward a better understanding of T cells in cancer. Cancer Cell. 2021;39(12):1549–1552. doi: 10.1016/j.ccell.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Li R, Wei F, Yu J, Li H, Ren X, Hao X. IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther. 2009;8(14):1402–1408. doi: 10.4161/cbt.8.14.8882. [DOI] [PubMed] [Google Scholar]
- 47.Sørensen RB, Hadrup SR, Svane IM, Hjortsø MC, thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117(7):2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meireson A, Devos M, Brochez L IDO Expression in Cancer: Different Compartment, Different Functionality? Frontiers in Immunology 2020; 11. 10.3389/fimmu.2020.531491 [DOI] [PMC free article] [PubMed]
- 49.Law AMK, Valdes-Mora F, Gallego-Ortega D (2020) Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 9(3). 10.3390/cells9030561 [DOI] [PMC free article] [PubMed]
- 50.Li F, Zhao Y, Wei L, Li S, Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 2018;19(8):695–705. doi: 10.1080/15384047.2018.1450116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuda A, Hata A, Tanaka A, Matsuda H. Canine mast cell tumour cells regulate tryptophan catabolism via the expression of indoleamine 2,3-dioxygenase. Res Vet Sci. 2021;137:159–162. doi: 10.1016/j.rvsc.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Fang X, Guo L, Xing Z, Shi L, Liang H, Li A, et al. IDO1 can impair NK cells function against non-small cell lung cancer by downregulation of NKG2D Ligand via ADAM10. Pharmacol Res. 2022;177:106132. doi: 10.1016/j.phrs.2022.106132. [DOI] [PubMed] [Google Scholar]
- 53.Kang Y, Su G, Sun J, Zhang Y. Activation of the TLR4/MyD88 signaling pathway contributes to the development of human hepatocellular carcinoma via upregulation of IL-23 and IL-17A. Oncol Lett. 2018;15(6):9647–9654. doi: 10.3892/ol.2018.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, et al. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag Res. 2019;11:475–481. doi: 10.2147/cmar.S184221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahm CD, Johnson LE, McNeel DG. Increased indoleamine 2,3-dioxygenase activity and expression in prostate cancer following targeted immunotherapy. Cancer Immunol Immunother. 2019;68(10):1661–1669. doi: 10.1007/s00262-019-02394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tierney JF, Vogle A, Finnerty B, Zarnegar R, Ghai R, Gattuso P, et al. Indoleamine 2,3-Dioxygenase-1 Expression in Adrenocortical Carcinoma. J Surg Res. 2020;256:90–95. doi: 10.1016/j.jss.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Mao S, Shi D, Zhang J, Zhang Z, Guo Y, et al. MicroRNA-153 Decreases Tryptophan Catabolism and Inhibits Angiogenesis in Bladder Cancer by Targeting Indoleamine 2,3-Dioxygenase 1. Front Oncol. 2019;9:619. doi: 10.3389/fonc.2019.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang K, Wang Z, Hu Y, Huang Y, Yuan K, Yu Y. Gene silencing of indoleamine 2,3–dioxygenase 1 inhibits lung cancer growth by suppressing T–cell exhaustion. Oncol Lett. 2020;19(6):3827–3838. doi: 10.3892/ol.2020.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng Y, Wang W, Chen M, Chen K, Xia X, Zhou S, et al. GBP1 Facilitates Indoleamine 2,3-Dioxygenase Extracellular Secretion to Promote the Malignant Progression of Lung Cancer. Front Immunol. 2020;11:622467. doi: 10.3389/fimmu.2020.622467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira JM, Dellê H, Camacho CP, Almeida RJ, Reis ST, Matos YST, et al. Indoleamine 2,3-dioxygenase expression in the prognosis of the localized prostate cancer. Int Urol Nephrol. 2020;52(8):1477–1482. doi: 10.1007/s11255-020-02414-0. [DOI] [PubMed] [Google Scholar]
- 61.Endo R, Nakamura T, Kawakami K, Sato Y, Harashima H. The silencing of indoleamine 2,3-dioxygenase 1 (IDO1) in dendritic cells by siRNA-loaded lipid nanoparticles enhances cell-based cancer immunotherapy. Sci Rep. 2019;9(1):11335. doi: 10.1038/s41598-019-47799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hacking S, Vitkovski T, Jain S, Jin C, Chavarria H, Wu D, et al. Clinical Significance of Program Death Ligand-1 and Indoleamine-2,3-Dioxygenase Expression in Colorectal Carcinoma. Appl Immunohistochem Mol Morphol. 2021;29(3):201–208. doi: 10.1097/pai.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg AJ, Wainwright DA, Rademaker A, Galvez C, Genet M, Zhai L, et al. Indoleamine 2,3-dioxygenase 1 and overall survival of patients diagnosed with esophageal cancer. Oncotarget. 2018;9(34):23482–23493. doi: 10.18632/oncotarget.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asghar K, Loya A, Rana IA, Abu Bakar M, Farooq A, Tahseen M, et al. Association between Cyclooxygenase-2 and Indoleamine 2,3-Dioxygenase Expression in Breast Cancer Patients from Pakistan. Asian Pac J Cancer Prev. 2019;20(11):3521–3525. doi: 10.31557/apjcp.2019.20.11.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asghar K, Loya A, Rana IA, Bakar MA, Farooq A, Tahseen M, et al. Forkhead box P3 and indoleamine 2,3-dioxygenase co-expression in Pakistani triple negative breast cancer patients. World J Clin Oncol. 2020;11(12):1018–1028. doi: 10.5306/wjco.v11.i12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azimnasab-Sorkhabi P, Soltani-Asl M, Yoshinaga TT, Massoco CO, Kfoury Junior JR. IDO blockade negatively regulates the CTLA-4 signaling in breast cancer cells. Immunol Res. 2023 doi: 10.1007/s12026-023-09378-0. [DOI] [PubMed] [Google Scholar]
- 67.Bishnupuri KS, Alvarado DM, Khouri AN, Shabsovich M, Chen B, Dieckgraefe BK, et al. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019;79(6):1138–1150. doi: 10.1158/0008-5472.Can-18-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang R, Li T, Wang W, Gan W, Lv S, Zeng Z, et al. Indoleamine 2, 3-Dioxygenase 1 and CD8 Expression Profiling Revealed an Immunological Subtype of Colon Cancer With a Poor Prognosis. Front Oncol. 2020;10:594098. doi: 10.3389/fonc.2020.594098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonescheit H, Oberg H-H, Gonnermann D, Hermes M, Sulaj V, Peters C et al (2020) Influence of Indoleamine-2,3-Dioxygenase and Its Metabolite Kynurenine on γδ T Cell Cytotoxicity against Ductal Pancreatic Adenocarcinoma Cells. Cells 9(5). 10.3390/cells9051140 [DOI] [PMC free article] [PubMed]
- 70.Park A, Yang Y, Lee Y, Kim MS, Park Y-J, Jung H et al (2019) Indoleamine-2,3-Dioxygenase in Thyroid Cancer Cells Suppresses Natural Killer Cell Function by Inhibiting NKG2D and NKp46 Expression via STAT Signaling Pathways. J Clin Med 8(6). 10.3390/jcm8060842 [DOI] [PMC free article] [PubMed]
- 71.Mandarano M, Bellezza G, Belladonna ML, Vannucci J, Gili A, Ferri I, et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool. Front Immunol. 2020;11:839. doi: 10.3389/fimmu.2020.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamasuge W, Yamamoto Y, Fujigaki H, Hoshi M, Nakamoto K, Kunisawa K, et al. Indoleamine 2,3-dioxygenase 2 depletion suppresses tumor growth in a mouse model of Lewis lung carcinoma. Cancer Sci. 2019;110(10):3061–3067. doi: 10.1111/cas.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Venancio PA, Consolaro MEL, Derchain SF, Boccardo E, Villa LL, Maria-Engler SS, et al. Indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase expression in HPV infection, SILs, and cervical cancer. Cancer Cytopathol. 2019;127(9):586–597. doi: 10.1002/cncy.22172. [DOI] [PubMed] [Google Scholar]
- 74.Cecchi M, Paccosi S, Silvano A, Eid AH, Parenti A (2021) Dexamethasone Induces the Expression and Function of Tryptophan-2-3-Dioxygenase in SK-MEL-28 Melanoma Cells. Pharmaceuticals (Basel) 14(3). 10.3390/ph14030211 [DOI] [PMC free article] [PubMed]
- 75.Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol. 2018;36(8):758–764. doi: 10.1038/nbt.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M et al (2014) Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR, vol 5. Oncotarget, p 4 [DOI] [PMC free article] [PubMed]
- 77.Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM. The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep. 2017;7(1):43337. doi: 10.1038/srep43337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Q, Wang P-p, Huang Z-l, Peng L, Lin C, Gao Z et al (2016) Tumoral indoleamine 2, 3-dioxygenase 1 is regulated by monocytes and T lymphocytes collaboration in hepatocellular carcinoma.Oncotarget; 7(12) [DOI] [PMC free article] [PubMed]
- 79.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106(7):2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirey KA, Jung J-Y, Maeder GS, Carlin JM. Upregulation of IFN-γ Receptor Expression by Proinflammatory Cytokines Influences IDO Activation in Epithelial Cells. J Interferon Cytokine Res. 2006;26(1):53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 82.Wang T, Wang J. K63-linked polyubiquitination of IRF1: an essential step in the IL-1 signaling cascade. Cell Mol Immunol. 2014;11(5):407–409. doi: 10.1038/cmi.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson CM, Hale PT, Carlin JM. The Role of IFN-γ and TNF-α-Responsive Regulatory Elements in the Synergistic Induction of Indoleamine Dioxygenase. J Interferon Cytokine Res. 2005;25(1):20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuasa K, Hijikata T. Distal regulatory element of the STAT1 gene potentially mediates positive feedback control of STAT1 expression. Genes Cells. 2016;21(1):25–40. doi: 10.1111/gtc.12316. [DOI] [PubMed] [Google Scholar]
- 85.Logue EC, Sha WC. CD28-B7 bidirectional signaling: a two-way street to activation. Nat Immunol. 2004;5(11):1103–1105. doi: 10.1038/ni1104-1103. [DOI] [PubMed] [Google Scholar]
- 86.Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC–interacting protein with features of a tumour suppressor. Nat Genet. 1996;14(1):69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- 87.Cher ML, Bova GS, Moore DH, Small EJ, Carroll PR, Pin SS, et al. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 1996;56(13):3091–3102. [PubMed] [Google Scholar]
- 88.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 89.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen W. IDO: more than an enzyme. Nat Immunol. 2011;12(9):809–811. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- 91.Iacono A, Pompa A, De Marchis F, Panfili E, Greco FA, Coletti A, et al. Class IA PI3Ks regulate subcellular and functional dynamics of IDO1. EMBO Rep. 2020;21(12):e49756. doi: 10.15252/embr.201949756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathewson ND, Ashenberg O, Tirosh I, Gritsch S, Perez EM, Marx S, et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell. 2021;184(5):1281–1298. doi: 10.1016/j.cell.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang K, Wu Y-H, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14(1):68. doi: 10.1186/s13045-021-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Azimnasab-sorkhabi P, Soltani-asl M, Kfoury Junior JR Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) as an undetermined tool in tumor cells.Human Cell 2023DOI: 10.1007/s13577-023-00893-8 [DOI] [PubMed]
- 95.Campesato LF, Budhu S, Tchaicha J, Weng C-H, Gigoux M, Cohen IJ, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun. 2020;11(1):4011. doi: 10.1038/s41467-020-17750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kocher F, Amann A, Zimmer K, Geisler S, Fuchs D, Pichler R, et al. High indoleamine-2,3-dioxygenase 1 (IDO) activity is linked to primary resistance to immunotherapy in non-small cell lung cancer (NSCLC) Transl Lung Cancer Res. 2021;10(1):304–313. doi: 10.21037/tlcr-20-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol. 2020;85(1):77–93. doi: 10.1007/s00280-019-04009-8. [DOI] [PubMed] [Google Scholar]
- 98.Zhou S, Zhao L, Liang Z, Liu S, Li Y, Liu S et al (2019) Indoleamine 2,3-dioxygenase 1 and Programmed Cell Death-ligand 1 Co-expression Predicts Poor Pathologic Response and Recurrence in Esophageal Squamous Cell Carcinoma after Neoadjuvant Chemoradiotherapy. Cancers (Basel) 11(2). 10.3390/cancers11020169 [DOI] [PMC free article] [PubMed]
- 99.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun. 2017;8:15221. doi: 10.1038/ncomms15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.