Abstract
Ligand-enabled Pd-catalyzed regioselective α,β-dehydrogenation of carbonyl compounds via β-methylene C─H activation has recently emerged as a promising transformation. Herein, we report the realization of β,γ-dehydrogenation and subsequent vinyl C─H olefination reactions of free carboxylic acids, thus providing a unique method for the structural diversification of aliphatic acids containing α-quaternary centers through sequential functionalizations of two β-C─H bonds and one γ-C─H bond. This tandem dehydrogenation-olefination-lactonization reaction offers a one-step preparation of β-alkylidene-γ-lactones, which are often difficult to prepare through conventional methods, from inexpensive and abundant free aliphatic acids. A variety of free aliphatic acids, such as isosteviol and grandiflorolic acid natural products, and olefins are compatible with the reported protocol. The newly-designed bidentate oxime ether-pyridone and morpholine-pyridone ligands are crucial for this tandem reaction to proceed. Notably, these ligands also enable preferential methylene C─H activation over the previously reported, competing process of methyl C─H bond olefination.
Graphical Abstract
1. Introduction
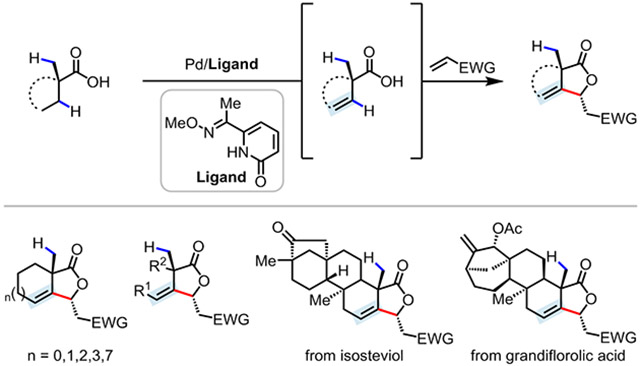
The desaturation of carbonyl compounds is an important transformation in organic chemistry, owing to the versatile reactivity of the double bond in downstream applications.1 Despite significant advances in the desaturation of ketone and aldehyde substrates, aliphatic carboxylic acids, which are ubiquitous and highly versatile motifs, are less amenable to dehydrogenation reactions. In 2017, the Newhouse group reported an elegant method for the α,β-desaturation of free carboxylic acids via zinc enediolates, using an allyl-Pd catalyst and allyl acetate as the stoichiometric oxidant.2 Recently, our group developed a pair of PdII-catalyzed α,β-dehydrogenation reactions of free carboxylic acids through β-methylene C─H activation, delivering α,β-unsaturated carboxylic acids or γ-alkylidene butenolides (Scheme 1A).3 The success of this protocol hinged on the use of bidentate pyridine-pyridone ligands with different bite angles to overcome product inhibition: (1) the five-membered chelating ligand L6 was designed to preferentially activate C(sp3)─H bonds over the vinyl C─H bonds of α,β-unsaturated carboxylic acid intermediates; (2) the six-membered chelating ligand L5 promoted a tandem vinyl C─H activation and coupling with alkynyl bromide. This β-C─H activation-enabled α,β-dehydrogenation process prompted us to investigate whether this strategy could be exploited to promote β,γ-dehydrogenation in the absence of α-C─H bonds, thereby achieving the functionalization of remote γ-methylene C─H bonds, a feat unachievable using enolate chemistry. An early study from our group in 2008 demonstrated that, in the presence of oxazoline directing groups (DGs) and stoichiometric quantities of Pd, a complex exhibiting β,γ-dehydrogenation could be isolated in 63% yield (Scheme 1B).4 Although a catalytic protocol was developed, it suffered from limited scope (a single cyclopentanecarboxylic acid example), low efficiency, and required the use of exogenous oxazoline DGs (Scheme 1B). In addition, auxiliary-enabled visible light-induced distal desaturation of alcohol or amine substrates using Pd catalyst has been reported.5 Therefore, the regioselective β,γ-dehydrogenation of native substrates such as carboxylic acids via C─H activation remains a significant challenge.6
Scheme 1.
Regioselective Dehydrogenation of Carboxylic Acids via β-C─H Activation
Achieving regioselective β,γ-dehydrogenation necessitates the preferential activation of methylene C─H bonds, despite the possible presence of more accessible α-methyl groups.7 Recently, our collaboration with the Sorensen group yielded an efficient synthesis of benzocyclobutenes through the methylene-selective C─H arylation of ketones.8 Computational studies showed that this selectivity for methylene C─H activation was driven by ligand–substrate interactions and the geometry of the Pd during the competing oxidative addition events. Despite the elegance of this intramolecular C─H arylation approach, designing ligands to obtain selectivity for methylene C─H bonds over methyl C─H bonds remains a formidable challenge.
Herein, we report a tandem Pd-catalyzed β,γ-dehydrogenation and vinyl C─H olefination of free carboxylic acids for the synthesis of a wide range of β-alkylidene-γ-lactones (Scheme 1C). The key to success was the design of bidentate oxime ether-pyridone and morpholine-pyridone ligands to achieve double functionalization through sequential methylene and vinyl C─H activation. This protocol has several key advantages over other C─H functionalization reactions: (1) the use of native carboxylic acids as substrates without the installation of exogenous directing groups, (2) exclusive β-methylene selectivity in the presence of primary β-C─H bonds, (3) the functionalization of γ-methylene C─H bonds, and (4) compatibility with a broad range of carboxylic acids such as isosteviol and grandiflorolic acid natural products.
2. Results and Discussion
An early study from our group disclosed a single example of β,γ-dehydrogenation and subsequent vinyl C─H olefination of 1-propylcyclohexane-1-carboxylic acid in low yield (41%) using five-membered chelating pyridine-pyridone ligand L6 (Scheme 2).3 Despite extensive efforts to improve this reaction, yields remained low and other cyclic carboxylic acids (e.g. cyclopentane, cycloheptane, and cyclooctane) and acyclic substrates were unreactive (Scheme 2). We decided to further explore this curious reactivity using the commercially available substrate 1-methyl-1-cyclohexanecarboxylic acid 1a, since such α-methylated carboxylic acids are widespread among natural products such as terpenes. Under the reported conditions using L6, we were able to observe a 22% 1H NMR (nuclear magnetic resonance) yield for the product 3a resulting from the desired tandem β,γ-dehydrogenation, vinyl C─H olefination, and lactonization sequence. Extensive screening of reaction conditions revealed that the yield could be improved to 66% through two crucial modifications: a 10:1 HFIP:MeCN solvent mixture and use of KF as a base (see Table S3 and S4).
Scheme 2.
Limitations of Previous Reported β,γ-Dehydrogenation and Vinyl C─H Olefination Protocol
We next searched for ligands that could further improve the reactivity of the catalyst (Table 1).9,10 A variety of different ligand classes previously developed in our laboratory to enable the C─H functionalization of free carboxylic acids, such as pyridine L1,10a 2-pyridone L2,10b and mono-N-protected amino acid (MPAA) ligand L3,9b promoted primary C─H activation to deliver the undesired β-olefination product 3a′ in 10–34% yields. The bidentate thioether ligand L4, developed for the β-olefination of free aliphatic acids, afforded a mixture of methylene and methyl C─H activation products in 10% and 24% yields, respectively.6f Modifications to the bite angle and electronic and steric properties of ligand L6 failed to identify a more potent ligand (e.g. 20% yield of 3a with L5, 60% with L7, and 66% with L6). At this point, we considered previous computational results, which had revealed that the 2-pyridone moiety was likely serving as an internal base to promote C─H bond cleavage, in a similar manner to an NHAc group.3,11 Bearing this in mind, we concluded that five-membered chelate analogs of L6, which kept the pyridone intact but replaced the pyridine moiety with other σ-donors, merited further investigation. We first prepared a range of oxime ether-pyridone ligands (L8─L11), inspired by the well-documented directing group ability of oxime-ethers.12 Among these, we were pleased to obtain a significant improvement in reactivity using methyl ketone oxime ether ligand L9 with 84% 1H NMR yield (80% isolated yield) of 3a and moderate diastereoselectivity (4/1). Introducing sterically bulky groups was detrimental to the reactivity of this new catalyst (e.g. 6% with L10 and 0% with L11). Because of the more diffuse lone pair of the sp3 nitrogen relative to the sp2 nitrogen of pyridine, we also designed and tested a series of tertiary amine-pyridone ligands (L12─L15), with morpholine-pyridone ligand L15 affording a comparably high yield (78%).6e Control experiments showed that only the undesired β-C─H olefination product 3a′ (deriving from methyl C─H activation) was obtained in the absence of these ligands, indicating the importance of the bidentate oxime ether-pyridone L9 and morpholine-pyridone L15 ligands for the observed reactivity and exclusive methylene selectivity.
Table 1.
Investigation of Ligands for the β,γ-Dehydrogenation and Vinyl C─H Olefination of Free Carboxylic Acidsa,b

|
Conditions: 1a (0.1 mmol), benzyl acrylate 2a (0.2 mmol), Pd(OAc)2 (10 mol%), ligand (L) (13 mol%), KF (2.0 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1.0 mL/0.1 mL), 100 °C, 24 h.
The yields of 3a/3a′ were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard. The dr values of 3a were determined by 1H NMR analysis of the crude product (~4/1).
Isolated yield of 3a.
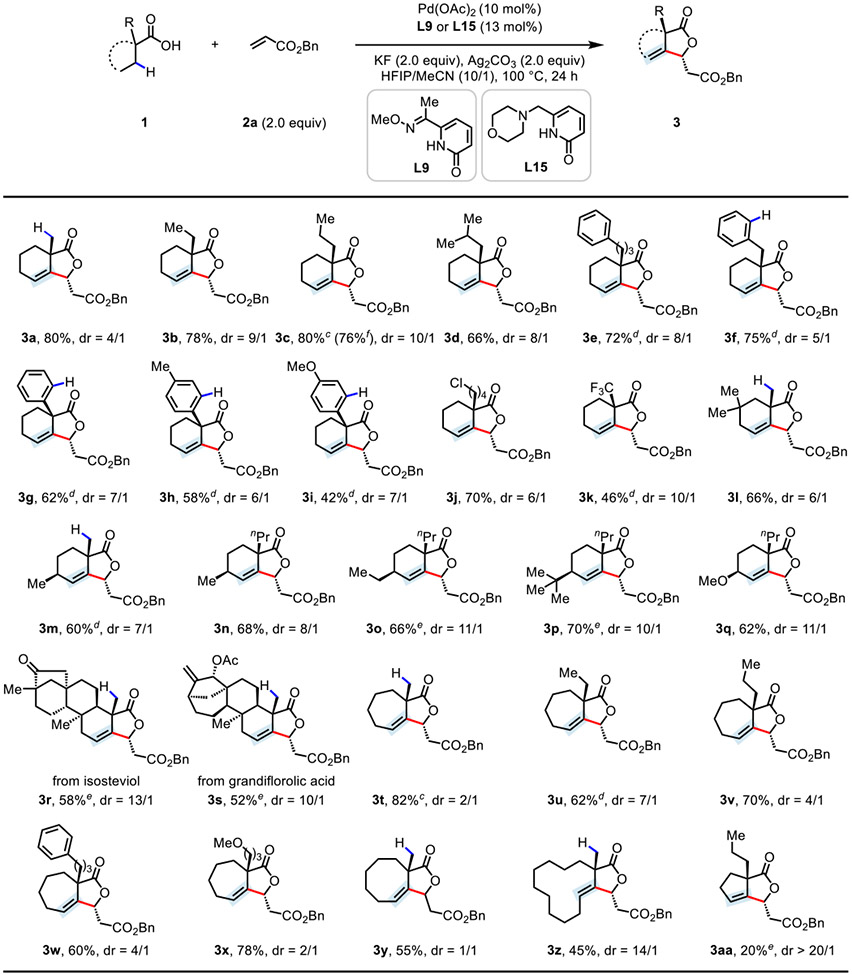
With the optimal ligand and reaction conditions in hand, we next evaluated the substrate scope of the tandem β,γ-C─H dehydrogenation-vinyl olefination reaction (Table 2). A wide range of free cyclic aliphatic acids including six- (1a–s), seven- (1t–x), eight- (1y), and twelve-membered (1z) rings was compatible, affording the fused γ-lactone products (3a–3z) in moderate to good yields (45–82%) with good diastereoselectivities (up to >20/1), albeit cyclopentane substrate 1aa was low yielding (3aa, 20%). It is noteworthy that remaining unreacted carboxylic acid substrates were observed from crude 1H NMR. No byproducts including β,γ-dehydrogenation product was observed during the reaction. The observed selective functionalization of methylene C─H bonds in the presence of primary (3a, 3l, 3m, 3r–t, 3y, and 3z) or aryl (3f–i) C─H bonds is unique compared to the vast majority of C─H activation reactions. A variety of functionalities such as chloro (3j), trifluoromethyl (3k), methoxy (3i, 3q, and 3x), ketone (3r), and allylic acetate (3s) were all well-tolerated, with the chloro moiety (3j) serving as a useful synthetic handle for subsequent derivatization. In addition to the carboxylic acids containing an α-quaternary center, cyclohexanecarboxylic acids bearing substitution at the 3 or 4 position (1l and 1m–q) consistently provided the corresponding products (3l–q) in good yields (60–70%). The utility of this protocol has been further demonstrated by the late-stage functionalization of complex natural products such as isosteviol 1r and grandiflorolic acid 1s,13 thus providing densely functionalized γ-lactones with potential biological activity in synthetically useful yields (58% and 52%, respectively). Piperidine and tetrahedropyran substrates failed to afford desired products, probably due to the instability of the resulting enamine and enol ether from β,γ-dehydrogenation (see Table S6). Additionally, carboxylic acids containing an α-hydrogen afforded α,β-dehydrogenation-vinyl olefination products in low yields under the standard conditions, probably because of the double-bond migration of β,γ-dehydrogenation products to form more stable γ-butenolides (see Table S6). The reaction can be reliably scaled up to a 1.0 mmol scale, delivering 3c in 76% yield.
Table 2.
Cyclic Carboxylic Acid Scope for the β,γ-Dehydrogenation and Vinyl C─H Olefination of Free Carboxylic Acidsa,b
|
Conditions: 1 (0.1 mmol), benzyl acrylate 2a (0.2 mmol), Pd(OAc)2 (10 mol%), L9 (13 mol%), KF (2.0 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1.0 mL/0.1 mL), 100 °C, 24 h.
Isolated yields. The dr values were determined by 1H NMR analysis of the crude product.
LiF (1.0 equiv) and HFIP/MeCN (1.0 mL/0.15 mL) were used.
L15 was used instead of L9.
L6 was used instead of L9.
The reaction was run on a 1.0 mmol scale.
Acyclic carboxylic acids containing less reactive methylene C─H bonds were compatible with the optimal catalyst, delivering the corresponding γ-lactones (5a–l) in moderate to good yields (34–74%) with exclusive methylene selectivity (Table 3). Our previous study reported that aliphatic acids bearing an α-gem-dimethyl group (4a–c) or a single methyl group (4d) preferentially reacted at the methyl C─H bonds using bidentate thioether ligand L4.6f The newly developed ligand scaffolds reversed this inherent methyl selectivity to methylene, highlighting the importance of ligand design for chemoselective (i.e. methylene vs. methyl) C(sp3)─H functionalization. The facile construction of β-methylene-γ-butyrolactones (5a and 5d–l) is extremely valuable, due to their widespread presence among many furanoid terpenes, and the corresponding dearth of existing methods for the synthesis of this scaffold.14 A wide range of functional groups, such as methoxy (5c and 5j), cyclohexane (5i), fluoro (5k), and chloro (5l), was tolerated. This protocol can be conducted on a 1.0 mmol scale to afford 5a in 56% yield.
Table 3.
Acyclic Carboxylic Acid Scope for the β,γ-Dehydrogenation and Vinyl C─H Olefination of Free Carboxylic Acidsa,b

|
Conditions: 1 (0.1 mmol), benzyl acrylate 2a (0.2 mmol), Pd(OAc)2 (10 mol%), L9 (13 mol%), NaOAc (2.0 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (0.95 mL/0.05 mL), 100 °C, 16 h.
Isolated yields. The dr values were determined by 1H NMR analysis of the crude product.
L15 was used instead of L9.
Pd(OAc)2 (15 mol%) and L9 (16 mol%) were used.
L6 was used instead of L9.
The reaction was run on a 1.0 mmol scale.
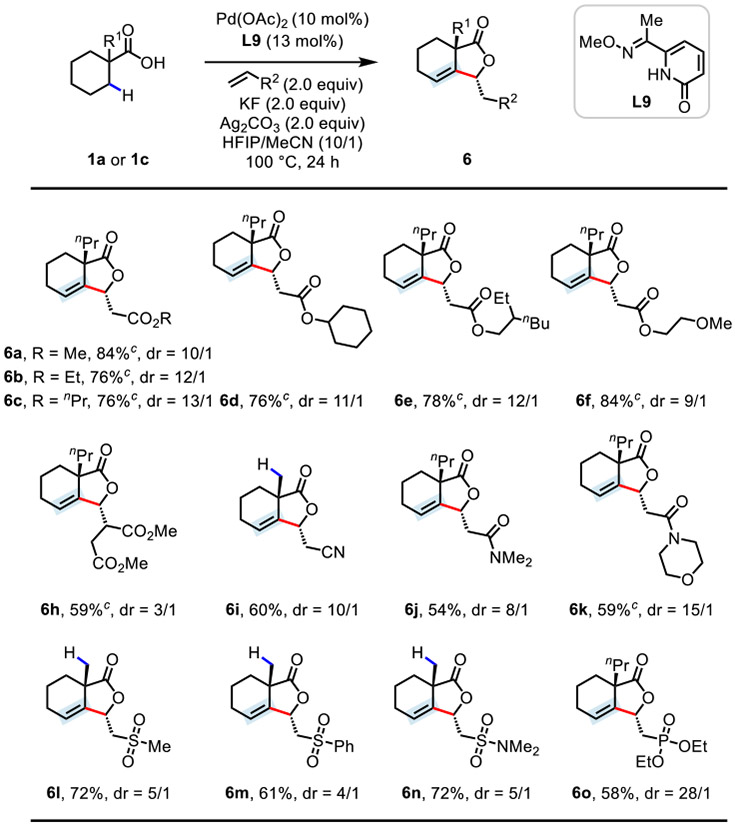
Next, the scope of the olefin coupling partners was evaluated using 1a or 1c as the model acid substrate (Table 4). In addition to various acrylate derivatives (2a–h), a broad range of Michael acceptors, such as acrylonitrile (2i), acrylamide (2j and 2k), vinyl sulfone (2l and 2m), vinyl sulfonamide (2n), and vinyl phosphonate (2o), were compatible with the optimal catalyst, providing the desired β-alkylidene-γ-lactone products (6a–o) in good yields (54–84%). Dimethyl fumarate 2h, a challenging coupling partner in C─H olefination reactions, was well-tolerated to deliver the corresponding product 6h in 59% yield.
Table 4.
|
Conditions: 1a or 1c (0.1 mmol), olefin 2 (0.2 mmol), Pd(OAc)2 (10 mol%), L9 (13 mol%), KF (2.0 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1.0 mL/0.1 mL), 100 °C, 24 h.
Isolated yields. The dr values were determined by 1H NMR analysis of the crude product.
LiF (1.0 equiv) and HFIP/MeCN (1.0 mL/0.15 mL) were used.
To highlight the synthetic applications of these methods, γ-lactone products 3c and 5a were then successfully transformed into several structurally distinct products (Scheme 3). Opening of γ-lactone 3c using NaOH through retro-Michael addition to unmask the olefin with subsequent benzyl protection afforded a synthetically useful diene product 7a in 83% yield. The [4+2] cycloaddition between diene 7a and o-silylaryl triflates provided tricyclic compound 7b in high yield (88%).15 Removal of the Bn protecting group in γ-lactone 5a in the presence of HCl gave the free carboxylic acid 7c in high yield (94%), with the terminal olefin remaining untouched. To investigate the potential biological activity of this β-methylene γ-lactone, an aniline containing an alkynyl group for protein labeling can be subsequently coupled with 7c to deliver 7d in 82% yield.
Scheme 3.
Synthetic Applications
To investigate the role of the ligand in this cascade reaction, control experiments were conducted under either the standard or ligandless conditions (Scheme 4). First, under the standard conditions without an acrylate coupling partner, we observed the formation of β,γ-dehydrogenation product 3a″ in 18% yield; 3a″ was not formed under the ligandless conditions (eq 1). These results highlighted the crucial role of the bidentate ligand for the dehydrogenation process and indicated that product inhibition likely contributes to the low yield of the reaction. Although β,γ-dehydrogenation of free carboxylic acids was a highly enabling transformation, initial ligand investigation failed to further improve the yield (see Table S7). Next, using dehydrogenation product 3a″ as the substrate, the desired vinyl C─H olefination product 3a could be obtained in 38% yield, but only in the presence of optimal ligand L9 (eq 2). Taken together, these results suggest that the bidentate oxime ether-pyridone ligand L9 was responsible for both the β,γ-dehydrogenation and the vinyl C─H olefination pathways. We have also conducted H/D exchange experiments to elucidate the role of new ligand using the standard conditions without an acrylate. In the absence of L9, only 14% deuteration at methyl C─H bonds of 1a was observed (eq 3). However, in the presence of L9, we observed 57% deuteration at methyl C─H bonds, 40% deuteration at β-methylene C─H bonds, and 41% deuteration at γ-methylene C─H bonds from 1a;6o additionally, β,γ-dehydrogenation product 3a″ was formed with 31% deuteration at methyl C─H bonds, 61% deuteration at a vinyl β-C─H bond, and 19% deuteration at a vinyl γ-C─H bond (eq 4). These results suggested that (1) methyl C─H activation was reversible; (2) the observed chemoselectivity (methylene vs. methyl) is probably due to the second vinyl olefination step; and (3) vinyl C─H activation was more facile than methyl C─H activation.
Scheme 4.
Control Experiments and H/D Exchange Experiments
Based on the above control experiments and reported dehydrogenation reactions via β-C─H activation,3,4 we propose that our transformation proceeds via a PdII/Pd0 catalytic cycle outlined in Scheme 5. First, coordination of Pd(OAc)2 to a bidentate oxime ether-pyridone or morpholine-pyridone ligand generates the active LPdII(OAc) species. After coordination of the model substrate 1a to Pd to form int-I, both the countercation K+ and the pyridone ligand accelerate the selective cyclopalladation of the β-methylene C─H bond to form int-II. Next, β-hydride elimination from palladacycle int-II delivers β,γ-dehydrogenation product int-III, which is a possible catalyst inhibitor. After the regeneration of the PdII species by a AgI oxidant, bidentate pyridone ligand-promoted C(sp2)─H activation of int-IV generates vinyl palladacycle int-V. This intermediate can be subsequently coupled with acrylate through olefin insertion to yield int-VI. Finally, the diastereoselective Michael addition of int-VI delivers the fused γ-lactone product 3a, with reoxidation of Pd0 by AgI closing the catalytic cycle.
Scheme 5.
Proposed Mechanism via a PdII/Pd0 Catalytic Cycle
3. Conclusion
In summary, we have realized an effective tandem reaction permitting the sequential β,γ-dehydrogenation and vinyl C─H olefination reactions of ubiquitous free aliphatic acids, including natural products such as isosteviol and grandiflorolic acid. This protocol affords a method for the one-step synthesis of a wide range of β-alkylidene-γ-lactones. Two new classes of pyridone-based ligands, oxime ether-pyridone and morpholine-pyridone, have been developed to enable an initial methylene-selective C─H activation and subsequent vinyl C─H activation.
4. Experimental Section
The general procedure for the β,γ-dehydrogenation and vinyl C─H olefination reaction is as follows: In a sealed tube equipped with a magnetic stir bar was charged with Pd(OAc)2 (2.2 mg, 10 mol%), L9 (2.2 mg, 13 mol%), the appropriate carboxylic acid substrate 1 (0.1 mmol), benzyl acrylate 2a (32.4 mg, 0.2 mmol), Ag2CO3 (55.0 mg, 0.2 mmol), KF (11.6 mg, 0.2 mmol). HFIP (1.0 mL) and MeCN (0.1 mL) were then added. The reaction mixture was then stirred at the rate of 600 rpm at 100 °C for 24 h. After being allowed to cool to room temperature, the mixture was diluted with ethyl acetate and acidified with 0.5 mL of formic acid. The mixture was passed through a pad of Celite with acetone as the eluent to remove any insoluble precipitate. The resulting solutions was concentrated, and the residual mixture was isolated using pTLC (hexane/ethyl acetate = 8/1 to 5/1).
Supplementary Material
Acknowledgements.
We gratefully acknowledge the NIH (NIGMS, R01GM084019), Bristol Myers Squibb, and The Scripps Research Institute for financial support. We also acknowledge China Scholarship Council (fellowship to T.S, China).
Footnotes
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Experimental details, full characterization of new compounds including 1H and 13C NMR spectra, IR, HRMS data (PDF)
The authors declare no competing financial interest.
References
- 1.Gnaim S; Vantourout JC; Serpier F; Echeverria PG; Baran PS Carbonyl desaturation: where does catalysis stand? ACS Catal. 2021, 11, 883–892. [Google Scholar]
- 2.Zhao Y; Chen Y; Newhouse TR Allyl-palladium catalyzed α,β-dehydrogenation of carboxylic acids via enediolates. Angew. Chem., Int. Ed 2017, 56, 13122–13125. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z; Hu L; Chekshin N; Zhuang Z; Qian S; Qiao JX; Yu J-Q Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C─H activation. Science 2021, 374, 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giri R; Maugel N; Foxman BM; Yu J-Q Dehydrogenation of inert alkyl groups via remote C─H activation: converting a propyl group into a π-allylic complex. Organometallics 2008, 27, 1667–1670. [Google Scholar]
- 5.(a) Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chuentragool P; Parasram M; Shi Y; Gevorgyan V General, mild, and selective method for desaturation of aliphatic amines. J. Am. Chem. Soc 2018, 140, 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) For C(sp3)─H functionalization reactions of free carboxylic acids, see: Giri R; Maugel N; Li J-J; Wang D-H; Breazzano SP; Saunder LB; Yu J-Q Palladium-catalyzed methylation and arylation of sp2 and sp3 C─H bonds in simple carboxylic acids. J. Am. Chem. Soc 2007, 129, 3510–3511. [DOI] [PubMed] [Google Scholar]; (b) Chen G; Zhuang Z; Li G-C; Saint-Denis TG; Hsiao Y; Joe CL; Yu J-Q Ligand-enabled β-C─H arylation of α-amino acids without installing exogenous directing groups. Angew. Chem., Int. Ed 2017, 56, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu Y; Chen X; Yuan C; Li G; Zhang J; Zhao Y Pd-catalysed ligand-enabled carboxylate-directed highly regioselective arylation of aliphatic acids. Nat. Commun 2017, 8, 14904. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ghosh KK; van Gemmeren M Pd-catalyzed β-C(sp3)─H arylation of propionic acid and related aliphatic acids. Chem. - Eur. J 2017, 23, 17697–17700. [DOI] [PubMed] [Google Scholar]; (e) Shen P-X; Hu L; Shao Q; Hong K; Yu J-Q Pd(II)-catalyzed enantioselective C(sp3)─H arylation of free carboxylic acids. J. Am. Chem. Soc 2018, 140, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhuang Z; Yu C-B; Chen G; Wu Q-F; Hsiao Y; Joe CL; Qiao JX; Poss MA; Yu J-Q Ligand-enabled β-C(sp3)─H olefination of free carboxylic acids. J. Am. Chem. Soc 2018, 140, 10363–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Hu L; Shen P-X; Shao Q; Hong K; Qiao JX; Yu J-Q PdII-catalyzed enantioselective C(sp3)─H activation/cross-coupling reactions of free carboxylic acids. Angew. Chem., Int. Ed 2019, 58, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ghosh KK; Uttry A; Koldemir A; Ong M; van Gemmeren M Direct β-C(sp3)─H acetoxylation of aliphatic carboxylic acids. Org. Lett 2019, 21, 7154–7157. [DOI] [PubMed] [Google Scholar]; (i) Zhuang Z; Yu J-Q Lactonization as a general route to β-C(sp3)─H functionalization. Nature 2020, 577, 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhuang Z; Herron AN; Fan Z; Yu J-Q Ligand-enabled monoselective β-C(sp3)─H acyloxylation of free carboxylic acids using a practical oxidant. J. Am. Chem. Soc 2020, 142, 6769–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ghiringhelli F; Uttry A; Ghosh KK; van Gemmeren M Direct β- and γ-C(sp3)─H alkynylation of free carboxylic acids. Angew. Chem., Int. Ed 2020, 59, 23127–23131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Zhuang Z; Herron AN; Liu S; Yu J-Q Rapid construction of tetralin, chromane, and indane motifs via cyclative C─H/C─H coupling: four-step total synthesis of (±)-russujaponol F. J. Am. Chem. Soc 2021, 143, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhuang Z; Herron AN; Yu J-Q Syntheses of cyclic anhydrides via ligand-enabled C─H carbonylation of simple aliphatic acids. Angew. Chem., Int. Ed 2021, 60, 16382–16387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Uttry A; Mal S; van Gemmeren M Late-stage β-C(sp3)─H deuteration of carboxylic acids. J. Am. Chem. Soc 2021, 143, 10895–10901. [DOI] [PubMed] [Google Scholar]; (o) Chan HSS; Yang J-M; Yu J-Q Catalyst-controlled site-selective methylene C─H lactonization of dicarboxylic acids. Science 2022, DOI: 10.1126/science.abq3048. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) For a review, see: Lucas EL; Lam NYS; Zhuang Z; Chan HSS; Strassfeld DA; Yu J-Q Palladium-catalyzed enantioselective β-C(sp3)─H activation reactions of aliphatic acids: a retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res 2022, 55, 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) For reviews, see: Chen X; Engle KM; Wang D-H; Yu J-Q Palladium(II)-catalyzed C─H activation/C─C cross-coupling reactions: versatility and practicality. Angew. Chem., Int. Ed 2009, 48, 5094–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Daugulis O; Roane J; Tran LD Bidentate, monoanionic auxiliary-directed functionalization of carbon─hydrogen bonds. Acc. Chem. Res 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lyons TW; Sanford MS Palladium-catalyzed ligand-directed C─H functionalization reactions. Chem. Rev 2010, 110, 1147–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencher PA; Hoskin JF; Wong JJ; Chen X; Yu J-Q; Houk KN; Sorensen EJ Pd(II)-catalyzed synthesis of benzocyclobutenes by β-methylene-selective C(sp3)─H arylation with a transient directing group. J. Am. Chem. Soc 2021, 143, 20035–20041. [DOI] [PubMed] [Google Scholar]
- 9.(a) For reviews, see: He J; Wasa M; Chan KSL; Shao Q; Yu J-Q Palladium-catalyzed transformations of alkyl C─H bonds. Chem. Rev 2017, 117, 8754–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shao Q; Wu K; Zhuang Z; Qian S; Yu J-Q From Pd(OAc)2 to chiral catalysts: the discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C─H activation reactions. Acc. Chem. Res 2020, 53, 833–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) He J; Li S; Deng Y; Fu H; Laforteza BN; Spangler JE; Homs A; Yu J-Q Ligand-controlled C(sp3)─H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science 2014, 343, 1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen Y-Q; Wang Z; Wu Y; Wisniewski SR; Qiao JX; Ewing WR; Eastgate MD; Yu J-Q Overcoming the limitations of γ- and δ-C─H arylation of amines through ligand development. J. Am. Chem. Soc 2018, 140, 17884–17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z; Wang Z; Chekshin N; Qian S; Qiao JX; Cheng PT; Yeung KS; Ewing WR; Yu J-Q A tautomeric ligand enables directed C─H hydroxylation with molecular oxygen. Science 2021, 372, 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Baldwin JE; Jones RH; Najera C; Yus M Functionalisation of unactivated methyl groups through cyclopalladation reactions. Tetrahedron 1985, 41, 699–711. [Google Scholar]; (b) Desai LV; Hull KL; Sanford MS Palladium-catalyzed oxygenation of unactivated sp3 C─H Bonds. J. Am. Chem. Soc 2004, 126, 9542–9543. [DOI] [PubMed] [Google Scholar]
- 13.(a) Ullah A; Munir S; Mabkhot Y; Badshah SL Bioactivity profile of the diterpene isosteviol and its derivatives. Molecules 2019, 24, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Piozzi F; Sprio V; Passannanti S; Mondelli R Structura dell’acido gradiflorolico. Gazz. Chim. Ital 1968, 98, 907–910. [Google Scholar]
- 14.(a) Wenkert E; Alonso ME; Buckwalter BL; Chou KJ A method of synthesis of β-methylfurans and α-methylene and β-methylene γ-lactones. Two menthofuran syntheses. J. Am. Chem. Soc 1977, 99, 4778–4782. [Google Scholar]; (b) Greene AE; Coelho F; Depres J-P A synthesis of β-methylene-γ-butyrolactones. J. Org. Chem 1985, 50, 1973–1975. [Google Scholar]; (c) Devon TK and Scott AI, “Handbook of Naturally Occurring Compounds”, Vol. II, Academic Press, New York, N.Y., 1972. [Google Scholar]
- 15.Wang Y-C; Huang Y-H; Tsai H-C; Basha RS; Chou C-M Palladium-catalyzed proaromatic C(alkenyl)─H olefination: synthesis of densely functionalized 1,3-dienes. Org. Lett 2020, 22, 6765–6770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







