Abstract
Background
Developing a cross-clade, globally effective HIV vaccine remains crucial for eliminating HIV.
Methods
This placebo-controlled, double-blind, phase 1/2a study enrolled healthy HIV-uninfected adults at low risk for HIV infection. They were randomized (1:4:1) to receive 4 doses of an adenovirus 26-based HIV-1 vaccine encoding 2 mosaic Gag and Pol, and 2 mosaic Env proteins plus adjuvanted clade C gp140 (referred to here as clade C regimen), bivalent protein regimen (clade C regimen plus mosaic gp140), or placebo. Primary end points were safety and antibody responses.
Results
In total 152/155 participants (clade C, n = 26; bivalent protein, n = 103; placebo, n = 26) received ≥1 injection. The highest adverse event (AE) severity was grade 3 (local pain/tenderness, 12%, 2%, and 0% of the respective groups; solicited systemic AEs, 19%, 15%, 0%). HIV-1 mosaic gp140-binding antibody titers were 79 595 ELISA units (EU)/mL and 137 520 EU/mL in the clade C and bivalent protein groups (P < .001) after dose 4 and 16 862 EU/mL and 25 162 EU/mL 6 months later. Antibody response breadth against clade C gp140 and clade C/non-clade C gp120 was highest in the bivalent protein group.
Conclusions
Adding mosaic gp140 to the clade C regimen increased and broadened the elicited immune response without compromising safety or clade C responses.
Clinical Trials Registration. NCT02935686.
Keywords: Ad26 HIV vaccine, broad immunogenicity, cross-clade, heterologous regimen, mosaic HIV antigen, tetravalent
With 38 million people living with human immunodeficiency virus (HIV) worldwide, a safe, globally effective vaccine remains essential to curb further HIV transmission [1]. To date, 4 preventive HIV vaccine concepts have been tested in efficacy studies, only 1 of which demonstrated vaccine efficacy (31% in the RV144 trial) [2, 3]. The vaccines’ immunogens’ inability to elicit a sufficiently broad and durable immune response to counter the extensive diversity of HIV-1 is likely one of the main reasons for their poor efficacy [4].
A multivalent HIV vaccine based on the replication-incompetent recombinant adenovirus serotype 26 (Ad26) vector was developed using a mosaic immunogen approach to elicit broader responses against diverse variants of HIV-1 [5–7]. The in silico-designed mosaic antigens include a maximum number of potential epitopes from group M variants of HIV-1 envelope (Env), group-specific antigen (Gag), and polymerase (Pol) proteins [8]. These Ad26-delivered HIV-1 mosaic antigens elicited broad cellular immune responses and partially protected against neutralization-resistant simian-human immunodeficiency virus SHIVSF162P3 in rhesus monkeys [6, 9]. Furthermore, the addition of adjuvanted recombinant glycoprotein (gp)140 to the Ad26-based mosaic vaccine substantially improved protection in rhesus monkeys [10]. Env-specific binding antibodies were repeatedly identified as a correlate of protection for these mosaic vaccines [7, 10, 11]. Specifically, Env-directed antibodies targeting diverse sequences within the first and second hypervariable regions (V1V2) of gp120 correlated inversely with the HIV-1 infection risk in the RV144 efficacy study, with V1V2 antibodies potentially contributing to protection [12–14]. Moreover, in the HVTN 702 phase 2b/3 efficacy trial, high levels of V1V2-binding antibody responses along with CD4+ T-cell responses correlated with decreased HIV-1 acquisition [15]. These findings highlight the potential of Ad26-delivered HIV-1 mosaic antigens combined with recombinant gp140 as vaccine immunogens.
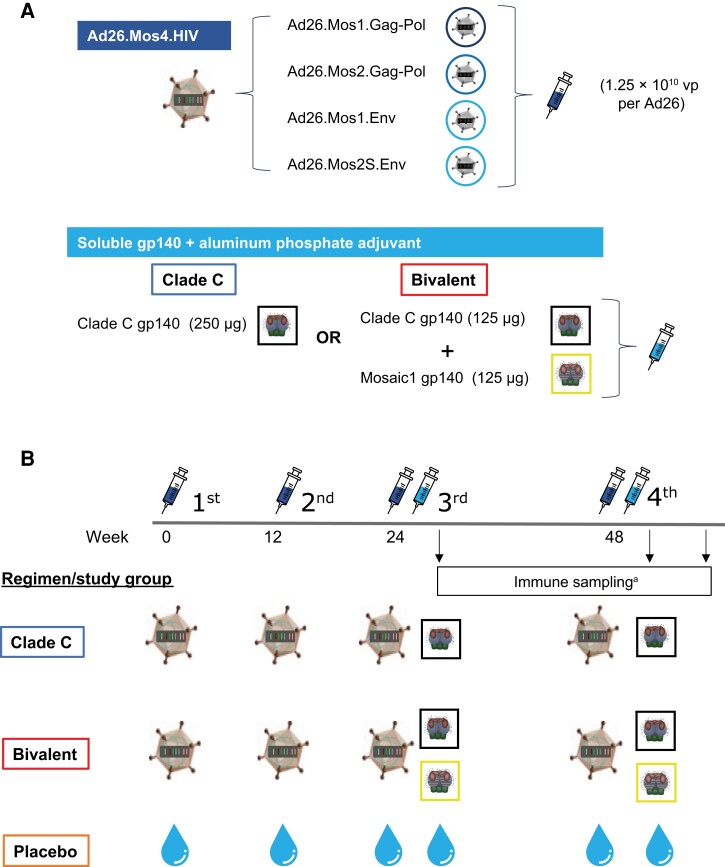
A vaccine regimen consisting of Ad26-delivered HIV-1 mosaic antigens (2 mosaic gag-pol genes and 1 mosaic env gene) and high-dose clade C gp140 induced a robust and comparable immune response in both humans and rhesus monkeys, and protected the monkeys against repetitive, intrarectal, heterologous challenges with SHIVSF162P3 [7]. Adding a fourth Ad26 vector encoding a second, complementary, mosaic env gene induced a significantly greater and broader immune response against diverse HIV-1 strains [16]; this regimen was evaluated in the phase 2b Imbokodo efficacy study (ClinicalTrials.gov identifier, NCT03060629), wherein the primary analysis demonstrated that vaccine efficacy, assessed in women at risk for HIV acquisition, was not significantly different from 0% [17]. The goal of this first-in-human study was to determine whether adding a second gp140, based on mosaic (Mos) env encoded by Ad26, to the previously evaluated clade C gp140, given with the Ad26-based tetravalent HIV vaccine [16] (Figure 1A ), would increase the breadth of humoral immune responses against diverse HIV clades without compromising safety or clade C responses.
Figure 1.
Vaccine composition, regimens, and immunization schedules. A, Composition of the vaccine regimen components used in the study: Ad26.Mos4.HIV, clade C gp140, and Mos1 gp140. B, Participants were administered Ad26.Mos4.HIV or placebo at day 0 and week 12, followed by Ad26.Mos4.HIV in combination with either clade C gp140 (clade C regimen) or clade C gp140 and Mos1 gp140 (bivalent protein regimen) at weeks 24 and 48. aSera and peripheral blood mononuclear cells were collected for analysis of humoral and cellular immune responses at day 0 and at weeks 28 (4 weeks after third vaccination), 52 (4 weeks after fourth vaccination), and 72 (6 months after fourth vaccination). Abbreviations: gp, glycoprotein; HIV, human immunodeficiency virus; Mos, mosaic; vp, viral particle.
METHODS
Vaccines
Three candidate HIV-1 vaccine products manufactured in PER.C6 cells were evaluated: Ad26.Mos4.HIV, clade C gp140, and Mos1 gp140. The Ad26.Mos4.HIV vaccine product is a recombinant, tetravalent, replication-incompetent, Ad26-based vaccine encoding 2 mosaic HIV-1 Gag and Pol and 2 mosaic Env proteins [6, 16, 18] in a 1:1:1:1 virus particle ratio administered at doses of 5 × 1010 virus particles in 0.5 mL. Clade C gp140 contains trimeric, recombinant clade C HIV-1 Env gp140 (C97ZA012.012; GenBank: AF286227.1) administered at doses of 250 µg glycoprotein and 425 µg aluminum phosphate adjuvant in 0.5 mL. The bivalent gp140 vaccine contains clade C gp140 mixed 1:1 with trimeric, recombinant HIV-1 Env gp140 engineered to contain Env motifs of multiple HIV-1 clades [19] administered at doses of 250 µg glycoprotein (ie, 125 µg clade C gp140 plus 125 µg Mos1 gp140) and 425 µg aluminum phosphate in 0.5 mL.
Study Design
This randomized, double-blind, placebo-controlled, phase 1/2a study (ClinicalTrials.gov identifier, NCT02935686) was conducted at 13 sites in the United States, Kenya, and Rwanda. Healthy HIV-uninfected adults aged 18 to 50 years who were at low risk for HIV-1 infection and gave informed consent were enrolled (additional eligibility criteria are listed in the Supplementary Methods). The study regimen consisted of Ad26.Mos4.HIV or placebo administered intramuscularly at day 0 and week 12; at weeks 24 and 48, Ad26.Mos4.HIV was coadministered (1 injection in each arm) with either clade C gp140 (further referred to as the clade C regimen in this article), clade C gp140 and Mos1 gp140 (referred to here as the bivalent protein regimen), or placebo. Participants were randomized (1:4:1) to receive either the clade C, bivalent protein, or placebo regimen, respectively (Figure 1B ). For reference, the clade C regimen uses the same vaccine components as were evaluated in the phase 2b Imbokodo efficacy study [17].
The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory guidelines. The study protocol was approved by institutional review boards at each study site. Participants or their legally acceptable representatives provided written informed consent to participate at the time of screening.
Immunogenicity Assays
Sera and peripheral blood mononuclear cells (PBMCs) to be analyzed for humoral and cellular immune responses, respectively, were obtained from blood samples collected on day 0 (baseline) and at weeks 28 (4 weeks after third vaccination), 52 (4 weeks after fourth vaccination), and 72 (6 months after fourth vaccination; Figure 1B ). Fine-needle aspirates of local draining lymph nodes after the third vaccination were evaluated for germinal center B-cell responses of 8 participants.
Vaccine-induced binding antibody responses were analyzed using 5 HIV-1 Env clade-specific enzyme-linked immunosorbent assays (ELISAs) against gp140 antigens. Vaccine-induced binding immunoglobulin (Ig) G1 and IgG3 subclass responses were investigated using clade C (C97ZA.012)-specific ELISAs. The functionality of vaccine-induced antibodies was evaluated in a virus neutralization assay (VNA) using TZM-bl cells and Env-pseudotyped viruses. The HIV-1 binding antibody multiplex assay was used to determine the magnitude and breadth of antibody isotype (IgG and IgA) and subclass (IgG3) responses to a broad panel of HIV-1 Env using a global antigen panel against gp120, gp140, gp41, and V1V2-gp70 scaffolds (Supplementary Table 1) [20].
Enzyme-linked immunosorbent spot (ELISPOT) assays were used to analyze T-cell responses to stimulation with pools of HIV-1 immunogens. Intracellular cytokine staining was used to detect interferon-γ (IFN-γ) and/or interleukin 2 (IL-2)-producing CD4+ and CD8+ T cells in response to HIV-1 mosaic immunogens. Immune responses against Ad26 were evaluated using an adeno-VNA. These assays are further described in the Supplementary Methods.
End Points
The primary end points were (1) the safety and tolerability of the vaccine regimens (ie, solicited local and systemic adverse events [AEs] over 7 days after each vaccination; AEs over 28 days after each vaccination; discontinuations due to AEs; and serious AEs and AEs of special interest, ie, HIV infection, during the course of the study); and (2) the titers and breadth of HIV-1 Env binding antibody responses to the 2 vaccine regimens. Secondary end points included titers and breadth of Env-specific neutralizing antibodies for tier 1 HIV viruses; Env-specific antibody functionality; titers and breadth of Env-specific binding antibody isotypes; and T-cell responses. T-cell responses were characterized in terms of IFN-γ response, CD4+ and CD8+ T-cell functionality, and T-cell memory differentiation. Exploratory end points included B-cell responses, lymph node immune responses, and immune responses to the Ad26 viral vector. Presented here are results of the final analysis, performed once all participants remaining on study completed their final main study visit.
Statistical Analysis
The sample size was deemed sufficient by the relevant authorities to assess the safety and immunogenicity of the 2 vaccine regimens. The full analysis set (FAS; safety analyses) included all participants who received ≥ 1 study injection. The per protocol immunogenicity set (immunogenicity analyses) included participants who received at least the first 3 injections within the protocol-specified time window (± 2 weeks), did not contract HIV during the study, and had ≥ 1 blood sample analyzed. For pooled analysis, the clade C group immunogenicity data were enriched with data from participants in our previous study [16] to allow statistical analysis. No statistical hypothesis was tested in the immunogenicity analysis, but differences in the magnitudes of immune responses between the vaccinated groups were explored either by a 2-sample t test on the log10 data, if those were normally distributed, or by a Wilcoxon rank sum test, using SAS 9.4 (Supplementary Methods).
RESULTS
Participant Disposition
Between 31 March 2017 and 21 March 2019, 281 volunteers were screened and 155 were randomized to the clade C (n = 26), bivalent protein (n = 103), or placebo (n = 26) groups (Supplementary Figure 1). Three participants in the bivalent protein group were not vaccinated; thus, 152 participants received ≥1 injection and were included in the FAS. Of these, 70% were recruited in the United States, 26% in Rwanda, and 3% in Kenya. The median age was 30 years (range, 18–50 years) and 59% were women (Table 1). Demographics were generally balanced between study groups.
Table 1.
Demographic and Baseline Characteristics in the Full Analysis Set
| Characteristic | Clade C (n = 26) | Bivalent (n = 100) | Placebo (n = 26) |
|---|---|---|---|
| Age, y, mean (range) | 28.7 (19–43) | 31.8 (19–50) | 30.7 (18–50) |
| Body mass index, kg/m², mean (range) | 26.0 (17.3–39.0) | 24.9 (15.9–44.4) | 23.7 (17.2–38.0) |
| Sex | |||
| ȃFemale | 16 (62) | 59 (59) | 15 (58) |
| ȃMale | 10 (39) | 41 (41) | 11 (42) |
| Race | |||
| ȃWhite | 12 (46) | 47 (47) | 12 (46) |
| ȃBlack | 10 (39) | 38 (38) | 9 (35) |
| ȃAsian | 2 (8) | 7 (7) | 2 (8) |
| ȃOther | 2 (8) | 8 (8) | 3 (12) |
| Country | |||
| ȃUnited States | 18 (69) | 70 (70) | 19 (73) |
| ȃRwanda | 7 (27) | 26 (26) | 7 (27) |
| ȃKenya | 1 (4) | 4 (4) | 0 |
Data are No. (%) except where indicated.
Eighty-six percent of participants received all 4 vaccinations (Supplementary Figure 1); 14% discontinued prematurely, due to AEs (1%), pregnancy (1%), loss to follow-up (3%), withdrawal (5%), or other reasons (3%) (Supplementary Table 2). The 2 AEs that led to discontinuation (one grade 2 muscular weakness [clade C group]; one grade 1 celiac disease [bivalent protein group]) were deemed not related to study injection. The 2 pregnant participants did not experience any medical disorders during pregnancy. One pregnancy resulted in a healthy full-term infant, while the other was electively aborted at 8 weeks of gestation.
Safety and Tolerability
Most AEs were grade 1 or 2; grade 3 was the highest severity reported for any AE (Table 2). The rates and severity of solicited local AEs were comparable between the clade C and bivalent protein groups and did not increase with subsequent vaccinations (Supplementary Figure 2). Solicited local AEs were reported by 92% (12% grade 3) and 94% (2% grade 3) in the respective groups, and all were pain/tenderness (Table 2 and Supplementary Figure 2). Solicited systemic AEs were reported by 92% (19% grade 3) and 94% (15% grade 3) in the respective vaccine groups and by 65% in the placebo group; fatigue, headache, and myalgia were the most common (Table 2). There were no reported cases of grade 3 or 4 pyrexia. The frequency of solicited systemic AEs was highest after the first injection, then decreased after subsequent vaccinations (Supplementary Figure 2).
Table 2.
Summary of Solicited and Unsolicited AEs Within 28 Days of Any Injection and Other AEs of Interest in the Full Analysis Set
| Adverse Events | Clade C (n = 26) | Bivalent (n = 100) | Placebo (n = 26) |
|---|---|---|---|
| Any solicited AEs | 25 (96) | 96 (96) | 22 (85) |
| Solicited local AEs | 24 (92) | 94 (94) | 17 (65) |
| ȃGrade 3 pain/tenderness | 3 (12) | 2 (2) | 0 |
| Solicited systemic AEs | 22 (85) | 90 (90) | 19 (73) |
| ȃGrade 3 solicited systemic AEs | 5 (19) | 15 (15) | 0 |
| ȃFatigue | 19 (73) | 78 (78) | 18 (69) |
| ȃHeadache | 15 (58) | 69 (69) | 16 (62) |
| ȃMyalgia | 15 (58) | 68 (68) | 9 (35) |
| Any unsolicited AEs | 13 (50) | 79 (79) | 21 (81) |
| ȃGrade 3 | 0 | 1 (1)a | 0 |
| ȃGrade 4 | 0 | 0 | 0 |
| Unsolicited AEs related to study injection | 0 | 9 (9) | 1 (4) |
| ȃGrade 3 or 4 | 0 | 0 | 0 |
| ȃGastrointestinal disorders, vomiting | 0 | 4 (4) | 0 |
| ȃNervous system disorders, postural dizziness | 0 | 2 (2) | 0 |
| ȃRespiratory, thoracic, and mediastinal disorders | 0 | 2 (2) | 0 |
| ȃEczema | 0 | 0 | 1 (4) |
| AEs leading to discontinuation of study vaccine | 1 (4) | 1 (1) | 0 |
| ȃRelated to vaccine | 0 | 0 | 0 |
| ȃCeliac disease | 0 | 1 (1) | 0 |
| ȃMuscular weakness for 7 days | 1 (4) | 0 | 0 |
| Grade 3 laboratory abnormalities | 0 | 1 (1)b | 0 |
| Grade 3 vital sign abnormalities | 1 (4) | 2 (2) | 1 (4) |
| ȃBradycardia | 1 (4) | 0 | 1 (4) |
| ȃTachycardia | 0 | 2 (2) | 0 |
| ȃConsidered as AEs | 0 | 0 | 0 |
| Serious AEs | 0 | 0 | 0 |
| HIV infection during the study | 0 | 0 | 0 |
| Death | 0 | 0 | 0 |
Data are No. (%).
Abbreviations: AE, adverse event; HIV, human immunodeficiency virus.
Blood creatinine increased.
Absolute neutrophil count decreased.
Unsolicited AEs related to vaccination (all grade 1 or 2) occurred in no participants in the clade C group, 9% in the bivalent protein group (mostly vomiting), and 4% in the placebo group (Table 2). No deaths, serious AEs, or HIV infections occurred.
HIV-1 Env-Specific Antibody Responses
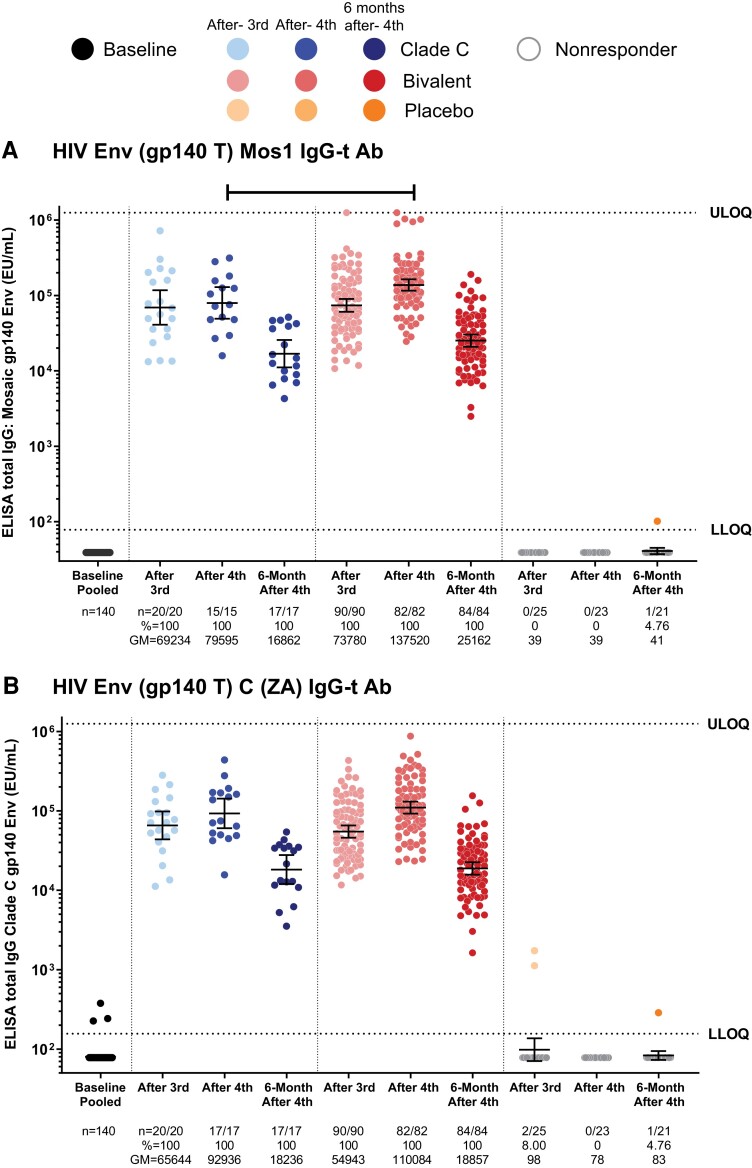
All vaccinees developed IgG responses against HIV-1 Env gp140 after the third and fourth vaccinations (Figure 2A and 2B ). Peak antibody titers to Mos1 gp140 in the bivalent protein group (137 520 ELISA units [EU]/mL) were significantly higher than in the clade C group (79 595 EU/mL; P < .001). These titers waned 6 months after the fourth vaccination but remained higher than at baseline in all vaccinees in both the clade C (16 862 EU/mL) and bivalent protein groups (25 162 EU/mL; Figure 2A ). The binding antibody responses against clade C gp140 were similar between the vaccine groups (Figure 2B ). These data suggest that the Mos1 gp140 component caused no immunologic interference in the antibody response against clade C.
Figure 2.
Humoral binding antibody immune responses: total IgG responses. Total IgG response against vaccine-matched gp140 mosaic (A) and clade C (B) proteins. The analysis was performed using the data from the per-protocol immunogenicity set. Dots represent data from each participant. Geometric means and 95% confidence intervals of the magnitude are presented for each group at each time point. Horizontal bar at the top represents significant difference in 2-sample t test at P < .05. Abbreviations: Ab, antibody; ELISA, enzyme-linked immunosorbent assay; Env, envelope; EU, ELISA units; GM, geometric mean; HIV, human immunodeficiency virus; IgG, immunoglobulin G; LLOQ, lower limit of quantification; ULOQ, upper limit of quantification.
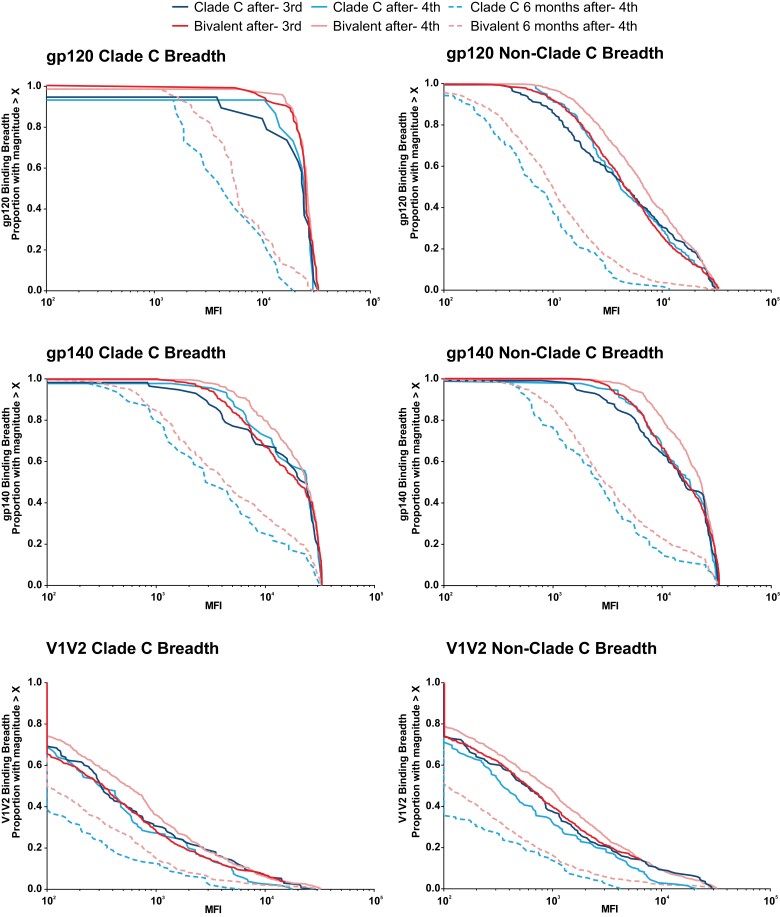
The binding antibody multiplex assay was used to measure the breadth and durability of the binding antibody response against cross-clade panels of gp120, gp140, and V1V2 antigens. The bivalent protein regimen consistently resulted in greater breadth, magnitude, and durability of binding antibody responses than the clade C regimen against gp120 and gp140 clade C, gp120 and gp140 non-clade C, and an extended gp120 panel of clade C antigens after the third and fourth vaccinations, and 6 months after fourth vaccination (Figure 3 and Supplementary Figure 3). Responses against V1V2 had lower magnitude, breadth, and durability compared to other antigens in both vaccine groups. After the third vaccination, cross-clade breadth of humoral immunity was improved in the bivalent protein versus the clade C group (area under magnitude-breadth curve ratios [AUCR] of bivalent protein to clade C regimen for gp140, 1.22, P = .069; gp120, 1.20, P = .035; and V1V2, 1.32, P = .031). The antibody binding breadth increased further after the fourth vaccination (AUCR gp140, 1.17, P = .036; gp120, 1.21, P = .012; and V1V2, 1.55, P = .001) and was consistent 6 months postvaccination (AUCR gp140, 1.24, P = .091; gp120, 1.40, P = .004; and V1V2, 1.47, P < .001), most clearly against the V1V2 antigens. B-cell responses evaluated in 3 participants after the third vaccination showed vaccine-induced Env-specific B cells (Supplementary Figure 4).
Figure 3.
Humoral immune responses: binding antibody breadth. Binding antibody multiplex assay breadth panels of multiple clade C and non-clade C gp120, gp140, and V1V2 antigen panels. The analysis was performed using the data from the per-protocol immunogenicity set. Lines represent the proportion of participants in each group with magnitude > X at each time point. Abbreviations: MFI, mean fluorescence intensity; V1V2, first and second hypervariable regions.
Neutralizing antibody responses against HIV-1 clade C and B viruses were limited to tier 1A virus strains clade C MW965.26 (100% responders in both groups) and clade B SF162.LS (at week 28, 90% in the clade C and 97.8% in the bivalent protein groups responded), whereas no autologous or heterologous tier 2 neutralization was detected (Supplementary Table 3).
HIV-1–Specific Cellular Immune Responses
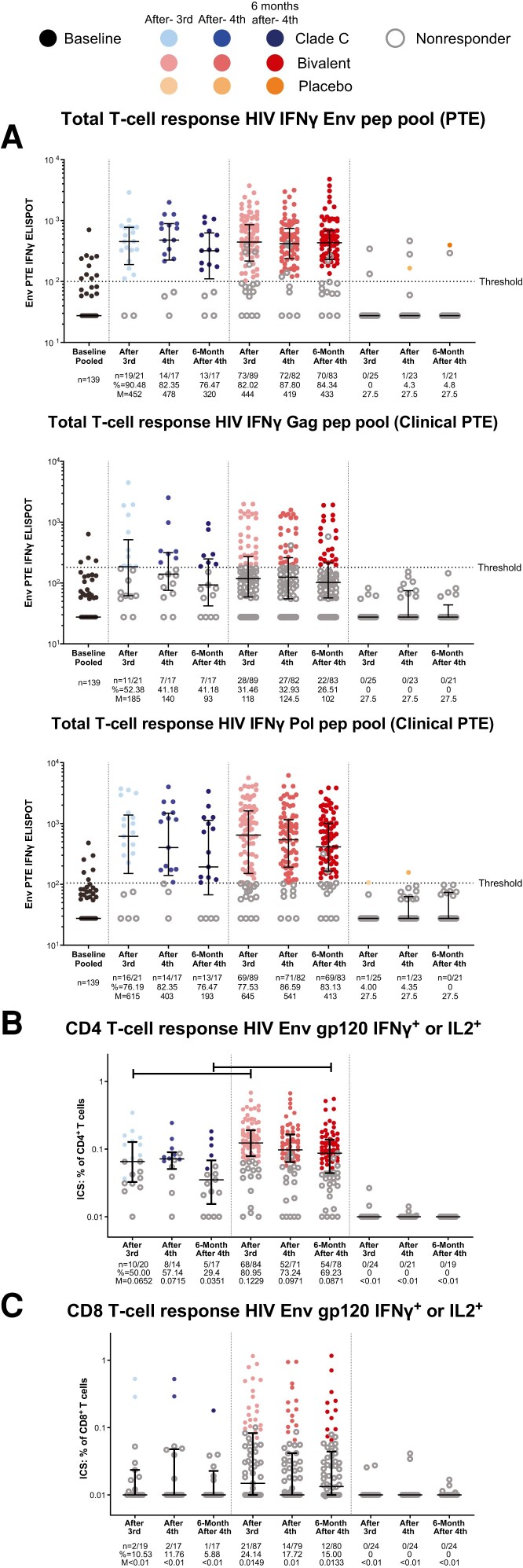
Comparable cellular immune responses against HIV-1 Env were detected in the clade C (median 452 spot-forming cells [SFCs]/106 PBMCs) and bivalent protein (median 444 SFCs/106 PBMCs) groups after the third vaccination and maintained after the fourth vaccination and 6 months later (Figure 4A ). The cellular immune response against Gag and Pol proteins showed no difference in the magnitude and kinetics between vaccinated groups (Figure 4A ). T-cell responses were generally higher against the Env and Pol immunogens than against Gag antigens (Figure 4A ).
Figure 4.
Cellular immune responses. IFN-γ ELISPOT response to stimulation with PTE pools of HIV-1 Env, Gag, and Pol immunogens (A), and ICS to detect IFN-γ– and/or IL-2–producing CD4+ (B) and CD8+ T cells (C) in response to HIV-1 Env gp120 vaccine-matched mosaic immunogens. The analysis was performed using the data from the per-protocol immunogenicity set. ELISPOT responders were defined as a 3-fold increase from baseline for participants with baseline values greater than the threshold, or a postvaccination result greater than threshold for participants with baseline values less than the threshold. ICS responders were defined by Fisher exact test comparison between stimulated and unstimulated cells with resultant P < 1 × 10−5. Dots represent the data from each participant, open circles represent nonresponders, closed circles represent vaccine responders. Median and interquartile range (bars) of the magnitude are presented for each group at each time point. Horizontal bar at the top represents significant difference in Wilcoxon rank sum test at P < .05. Abbreviations: ELISPOT, enzyme-linked immunosorbent spot; Env, envelope; Gag, group-specific antigen; HIV, human immunodeficiency virus; ICS, intracellular cytokine staining; IFN-γ, interferon γ; IL, interleukin; M, median; pep, peptide; Pol, polymerase; PTE, potential T-cell epitope.
Intracellular cytokine staining against vaccine-matched HIV-1 Env gp120 peptide pools showed that both regimens induced both CD4+ and CD8+ T-cell responses (Figure 4B and 4C and Supplementary Figure 5). The magnitude and response rate of CD4+ T-cell responses were consistent throughout the study in both groups; those in the bivalent protein group were significantly higher than those in the clade C group after the third vaccination (0.123% vs 0.065% CD4+ T cells, 81% vs 50% responders, P < .001) and 6 months after the fourth vaccination (0.087% vs 0.035% CD4+ T cells, 69% vs 29% responders, P < .001; Figure 4B ). The participants having the strongest CD4+ T-cell responses to Env were also those with the greatest magnitude of binding antibodies to Mos1 gp140 (Supplementary Figure 6), demonstrating the role of strong T-cell help facilitating greater humoral immune response development. The magnitude and rate of CD8+ T-cell responses against Env protein were generally lower than those of CD4+ T-cell responses in both vaccinated groups (Figure 4C and Supplementary Figure 5B).
The magnitude and rate of CD8+ T-cell responses to Gag (0.014% vs 0.016% CD8+ T cells, 23% vs 35% responders after the fourth vaccination, P > .05) and Pol protein (0.082% vs 0.131% CD8+ T cells, 49% vs 59% responders after the fourth vaccination, P > .05) were higher than those of CD4+ T-cell responses for both regimens (Supplementary Figure 7).
Within these vaccine-specific T-cell populations, large numbers of central and effector memory CD4+ T cells were detected in both groups (Supplementary Figure 8A), indicating the induction of long-lived and durable T-cell responses [21, 22]. The CD8+ T-cell response rate against Env gp120 peptide pools was generally low, but CD8+ effector memory T cells were detected in both groups (Supplementary Figure 8B).
Overall, these data indicate that, in general, the vaccines induce cellular immune responses consistently across the vaccinated populations at rates similar to the peak observed after the third or fourth vaccination, through 6 months after the fourth vaccination.
A forest plot to visualize the binding antibody and T-cell responses induced (Supplementary Figure 9) showed that antibody responses against clade B and clade C (consensus sequence) and the T-cell response against Env after the fourth vaccination were higher in the bivalent protein group than in the clade C group. Other responses were generally comparable between vaccinated groups.
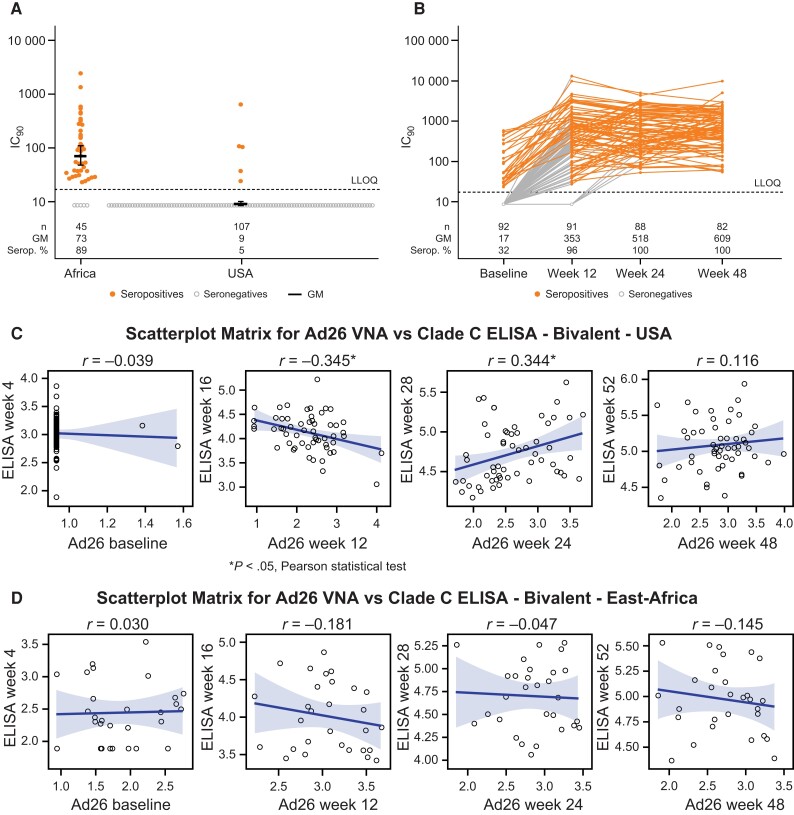
To understand the role of preexisting and vaccine-induced vector immunity in the response to vaccination, Ad26 neutralization titers were evaluated over the course of the vaccination series. Preexisting, naturally induced neutralizing antibodies to Ad26 were detected in most participants from East Africa (89%) compared to 5% of those from the United States (Figure 5A ). Ad26-neutralizing antibody titers increased after the first vaccination but were not substantially boosted upon subsequent vaccinations (Figure 5B ). Among the predominantly Ad26-seronegative participants from the United States, a low negative correlation (r = –0.345, P < .05) between Ad26 VNA and ELISA responses at the second vaccination, and a low positive correlation (r = 0.344, P < .05) after the third vaccination were observed (Figure 5C ). However, no significant correlations between prevaccination Ad26 titers and postvaccination responses were detected among participants from East Africa (Figure 5D ). No difference in the magnitude of ELISA responses was seen between the United States and East Africa after completion of the vaccination schedule (Supplementary Figure 10). Hence, Ad26 vector-specific immunity prior to vaccination had little, if any, influence on the magnitude of induced immune response.
Figure 5.
Vector immunity prevaccination and vaccine-induced immune response. The baseline Ad26 neutralization titers of participants in the United States and East Africa (A), and the Ad26 neutralization titers of participants in the bivalent group prior to each vaccination (B) were measured. Correlation analysis was performed in the population described in (A) to assess the association between Ad26 neutralization titers prevaccination and the corresponding IgG response against clade C postvaccination among participants receiving the regimen with the bivalent protein vaccine in the United States (C) and East Africa (D). A, Individual data, geometric mean, and 95% confidence interval are presented. C and D, line represents the Pearson correlation and the shaded areas indicate the 95% confidence interval. *P < .05, Pearson statistical test. Abbreviations: ELISA, enzyme-linked immunosorbent assay; GM, geometric mean; IC90, 90% inhibitory concentration; IgG, immunoglobulin G; LLOQ, lower limit of quantification; Serop. %, percentage seropositive; VNA, virus neutralization assay.
DISCUSSION
This first-in-human study evaluated the safety and immunogenicity of adding the Mos1 gp140 component to the previously tested Ad26.Mos4.HIV and clade C gp140 vaccine regimen [16]. Adding the in silico designed Mos1 gp140 improved both the magnitude and breadth of humoral immune responses without increasing reactogenicity. Both regimens were generally safe and well tolerated, consistent with the results of earlier studies on Ad26-based mosaic HIV vaccines [7, 11, 16]. Solicited and unsolicited AEs were mostly mild and comparable between the 2 groups, with the rates of solicited systemic AEs decreasing after the first injection. No HIV infections, serious AEs, or deaths occurred.
Both regimens elicited humoral and cellular anti–HIV-1 immune responses in 100% and up to 94% of recipients, respectively. The immunogenicity profiles observed in both groups are generally consistent with earlier studies [7, 11, 16]. Binding antibody titers to Mos1 gp140 increased after each vaccination, peaking after the fourth vaccination in both groups. Neutralizing antibody responses were only raised against tier 1 HIV strains, in line with previous findings [7, 11, 16]. This vaccination schedule demonstrated that the immune response specificities elicited by the first 2 Ad26.Mos4.HIV injections can develop into broad humoral and cellular immunity, and their specificities can be differentially boosted depending on the composition of the subsequent gp140 vaccines. The bivalent gp140 regimen induced a humoral response of greater magnitude and breadth than the clade C gp140 regimen, and was not associated with any diminution in clade C-specific responses, thus ruling out antigenic competition, an important concern in multivalent HIV vaccine development [23]. The improved performance of the bivalent regimen in eliciting a greater magnitude and breadth of V1V2 responses is a potentially important difference, as these responses have been consistently associated with reduced risk of HIV-1 infection and increased vaccine efficacy across trials [14, 15]. These humoral immune response data, taken together, show that adding the Mos1 gp140 component improved vaccine-induced humoral immune responses.
Induction of long-lived immunological memory was indicated by persistence of vaccine-elicited CD4+ and CD8+ T-cell responses 6 months after the final injection at similar magnitude as the peak, combined with balanced effector and central memory CD4+ T-cell phenotype and persistent, and broad antibody responses. The durability of immune responses elicited by both vaccine regimens was consistent with the favorable durability profiles reported for Janssen's Ad26-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [24] and Zika vaccines [25].
All participants generated Ad26-neutralizing antibodies irrespective of their baseline serostatus. The magnitude of vaccine-induced HIV-specific humoral responses was comparable between populations that were predominantly Ad26-seropositive (Kenya and Rwanda) and -seronegative (United States) and was not impacted by preexisting Ad26-specific immunity. These results may be of relevance for situations where Ad26-based vaccines will be more widely used [26]. Very rare cases of vaccine-induced thrombotic thrombocytopenia (VITT) have been reported among recipients of Ad26-based SARS-CoV-2 vaccines [27], but no cases of VITT were reported in either this study or this HIV vaccine development program, nor with any other Ad26-vectored vaccines developed by Janssen, aside from the SARS-CoV-2 vaccine. Given the few participants with evaluable fine-needle aspirate results, no inferences regarding differences in B-cell responses between the vaccine regimens can be drawn.
In conclusion, adding a mosaic gp140 Env component to the regimen containing Ad26.Mos4.HIV and clade C gp140 [16] increases the humoral and CD4+ T-cell immune responses while maintaining the vaccine's safety and tolerability profile. The bivalent protein vaccine consistently induced the greatest breadth of antibody responses against multiclade Env panels, including (although to a lesser extent) against V1V2 clade C antigens. In the HVTN 702 study, high levels of V1V2-directed binding antibody responses were associated with decreased HIV-1 acquisition [15], even though overall, vaccine efficacy was not demonstrated [28]. The phase 2b Imbokodo study (HVTN 705) evaluating efficacy of the Ad26.Mos4.HIV and the clade C gp140 regimen in women in Southern Africa started during execution of the ASCENT trial, but the primary analysis subsequently demonstrated that vaccine efficacy did not differ significantly from 0% [17]. Nevertheless, based on these results from ASCENT, the efficacy of the Ad26.Mos4.HIV and bivalent gp140 vaccine regimen is being evaluated in men who have sex with men and transgender individuals in the Mosaico study (NCT03964415) in Western Europe and the Americas, where clade B is most prevalent. These complementary studies will demonstrate how the different immunologic responses induced by these vaccine regimens will impact vaccine efficacy in populations that are geographically distinct, and in which route of HIV transmission differs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. J. S., D. H. B., and F. T. drafted the primary manuscript. D. J. S., D. H. B., C. C., M. S., K. E. S., S. R. W., S. S., J. H., G. D. T., J. G. K., M. J. M., K. W. C., S. C. D., G. A., G. F., D. M., P. M., S. N., K. C., P. A. G., S. E., E. K., L. C., L. R. B., M. G. P., H. S., and F. T. contributed to the clinical study design, study execution, data analysis, and manuscript editing. D. J. S., D. H. B., S. S., G. D. T., J. G. K., M. J. M., S. C. D., G. A., G. F., D. M., K. C., and M. S. S. assisted with the immunogenicity analyses, data analysis, and manuscript editing. All coauthors reviewed the manuscript, approved the final version, and are fully responsible for all content and editorial decisions.
Acknowledgments. We acknowledge the contributions of Nicole Frahm (Fred Hutchinson Cancer Center, Seattle, WA, USA) for the memory T-cell data. We are grateful to Carol Marty, Todd Haight, Terri Stewart, Lisa Bunts, Shannon Grant, Anisa Gravelle, and Sara Thiebaud for statistical support at the Fred Hutchinson Cancer Center. We thank Mike Archibald, David Beaumont, Caroline Brackett, Yong Lin, Kristy Long, Judith T. Lucas, Brooke Maness, Tara McNair, Kaia Quinn Lyons, MPA, Marcella Sarzotti-Kelsoe, Kelly Seaton, Sherry Stanfield-Oakley, Nicole L. Yates, and Lu Zhang from the HIV Vaccine Trials Network Laboratory at Duke University. We are grateful to the Janssen Compound Development Team (Mo Weijtens, Iedo Beeksma, Ad Knaapen, Richard Verhage, Sabrina Spinosa, Valérie Oriol Mathieu, Carla Truyers), Janssen Clinical Team (Johan De Decker, Imre Laszlo, Cornelia Linthicum, Chris McShane, Raphaele Roten, Avila Theresa, Elke Theuwissen, Anick Vandingenen, Evelien van den Broecke, Olive Yuan), Janssen Subunit Vaccine Design, Biomarkers, and Clinical Immunology Team (Lidia Roman Gonzalez, Annemart Koornneef, Lucy Rutten, Danielle van Manen), and their management (Macaya Douoguih, Jenny Hendriks) for their role in the ASCENT study. We thank Fadi Ghantous from the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, Boston, MA, USA. Medical writing and publication management support was provided by Widagdo Widagdo and Marialuisa Quadri, both employed by Janssen, and by Jacob Watson (Zoetic Science), funded by Janssen. Additional medical writing and editorial assistance were provided by Samantha Santangelo, PhD, and Courtney St Amour, PhD, of Lumanity Communications Inc, and was funded by Janssen. The ASCENT/HVTN118/HPX2003 study team includes Julie A. Ake, Susan Buchbinder, Trevor A. Crowell, Zelda Euler, Ian Frank, Dimitri Goedhart, Jennifer A. Johnson, Michael Keefer, Colleen F. Kelley, Kenneth H. Mayer, Joseph Nkolola, Lauren Peter, Merlin L. Robb, Nadine Rouphael, Lorenz Scheppler, Magda Sobieszczyk, Hong Van Tieu, Matthew H. Collins, and Varun K. Phadke.
Financial support. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant numbers UM1AI069412, UL1TR001102, UM1AI068618, and UM1AI069481); Henry M Jackson Foundation for the Advancement of Military Medicine and the US Department of Defense (grant number W81XWH-17–0072); Ragon Institute of MGH, MIT, and Harvard; Bill and Melinda Gates Foundation (grant number OPP1156831); and Janssen Vaccines and Prevention.
Data sharing. Janssen has an agreement with the Yale Open Data Access (YODA) Project to serve as the independent review panel for evaluation of requests for clinical study reports and participant-level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Data will be made available following publication and approval by YODA of any formal requests with a defined analysis plan. For more information on this process or to make a request, please visit The YODA Project site. The data sharing policy of Janssen (Pharmaceutical Companies of Johnson and Johnson) is available online.
Supplementary Material
Contributor Information
Daniel J Stieh, Janssen Vaccines and Prevention Leiden, the Netherlands.
Dan H Barouch, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA; Ragon Institute of MGH, MIT, and Harvard, Cambridge, Massachusetts, USA.
Christy Comeaux, Janssen Vaccines and Prevention Leiden, the Netherlands.
Michal Sarnecki, Janssen Vaccines, Bern, Switzerland.
Kathryn E Stephenson, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA.
Stephen R Walsh, Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Sheetal Sawant, Department of Surgery, Center for Human Systems Immunology, and Duke Human Vaccine Institute, Duke University, Durham, North Carolina, USA.
Jack Heptinstall, Department of Surgery, Center for Human Systems Immunology, and Duke Human Vaccine Institute, Duke University, Durham, North Carolina, USA.
Georgia D Tomaras, Department of Surgery, Center for Human Systems Immunology, and Duke Human Vaccine Institute, Duke University, Durham, North Carolina, USA.
James G Kublin, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
M Juliana McElrath, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Kristen W Cohen, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Stephen C De Rosa, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Galit Alter, Ragon Institute of MGH, MIT, and Harvard, Cambridge, Massachusetts, USA.
Guido Ferrari, Department of Surgery, Center for Human Systems Immunology, and Duke Human Vaccine Institute, Duke University, Durham, North Carolina, USA.
David Montefiori, Department of Surgery, Center for Human Systems Immunology, and Duke Human Vaccine Institute, Duke University, Durham, North Carolina, USA.
Philipp Mann, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Steven Nijs, Janssen Research and Development, Beerse, Belgium.
Katleen Callewaert, Janssen Research and Development, Beerse, Belgium.
Paul A Goepfert, Division of Infectious Disease, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Srilatha Edupuganti, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Etienne Karita, Rwanda Zambia HIV Research Group, Kigali, Rwanda.
Michael S Seaman, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA.
Lawrence Corey, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lindsey R Baden, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Maria G Pau, Janssen Vaccines and Prevention Leiden, the Netherlands.
Hanneke Schuitemaker, Janssen Vaccines and Prevention Leiden, the Netherlands.
Frank Tomaka, Janssen Research and Development, Titusville, New Jersey, USA.
the ASCENT/HVTN118/HPX2003 Study Team:
Julie A Ake, Susan Buchbinder, Trevor A Crowell, Zelda Euler, Ian Frank, Dimitri Goedhart, Jennifer A Johnson, Michael Keefer, Colleen F Kelley, Kenneth H Mayer, Joseph Nkolola, Lauren Peter, Merlin L Robb, Nadine Rouphael, Lorenz Scheppler, Magda Sobieszczyk, Hong Van Tieu, Matthew H Collins, and Varun K Phadke
References
- 1. World Health Organization . HIV key facts.https://www.who.int/news-room/fact-sheets/detail/hiv-aids. Accessed 15 March 2022.
- 2. Ng'uni T, Chasara C, Ndhlovu ZM. Major scientific hurdles in HIV vaccine development: historical perspective and future directions. Front Immunol 2020; 11:590780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 4. Stephenson KE, Wagh K, Korber B, Barouch DH. Vaccines and broadly neutralizing antibodies for HIV-1 prevention. Annu Rev Immunol 2020; 38:673–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, Walsh SR, Seaman MS, et al. First-in-human randomized, controlled trial of mosaic HIV-1 immunogens delivered via a modified vaccinia Ankara vector. J Infect Dis 2018; 218:633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barouch DH, O'Brien KL, Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 2010; 16:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018; 392:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fischer W, Perkins S, Theiler J, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 2007; 13:100–6. [DOI] [PubMed] [Google Scholar]
- 9. Barouch DH, Stephenson KE, Borducchi EN, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 2013; 155:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barouch DH, Alter G, Broge T, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015; 349:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stephenson KE, Wegmann F, Tomaka F, et al. Comparison of shortened mosaic HIV-1 vaccine schedules: a randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) and a preclinical study in rhesus monkeys (NHP 17-22). Lancet HIV 2020; 7:e410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yates NL, Liao HX, Fong Y, et al. Vaccine-induced Env V1–V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moodie Z, Dintwe O, Sawant S, et al. Analysis of the HVTN 702 phase 2b-3 HIV-1 vaccine trial in South Africa assessing RV144 antibody and T-cell correlates of HIV-1 acquisition risk. J Infect Dis 2022; 226:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baden LR, Stieh DJ, Sarnecki M, et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV 2020; 7:e688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gray G, Mngadi K, Lavreys L, et al. Phase 2b efficacy trial of mosaic HIV-1 vaccine regimen in African women (Imbokodo). Conference on Retroviruses and Opportunistic Infections (CROI), 12–16 February 2022, virtual conference. Abstract 121. [Google Scholar]
- 18. Barouch D, Schuitemaker J, Pau MG, et al. Inventor. US patent application, 2019/0022212 A1, 2019.
- 19. Nkolola JP, Bricault CA, Cheung A, et al. Characterization and immunogenicity of a novel mosaic M HIV-1 gp140 trimer. J Virol 2014; 88:9538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yates NL, deCamp AC, Korber BT, et al. HIV-1 envelope glycoproteins from diverse clades differentiate antibody responses and durability among vaccinees. J Virol 2018; 92:e01843-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–62. [DOI] [PubMed] [Google Scholar]
- 22. Sharpe S, White A, Sarfas C, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016; 101:174–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kallas EG, Grunenberg NA, Yu C, et al. Antigenic competition in CD4+ T cell responses in a randomized, multicenter, double-blind clinical HIV vaccine trial. Sci Transl Med 2019; 11:eaaw1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salisch NC, Stephenson KE, Williams K, et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti-Zika virus vaccine. Ann Intern Med 2021; 174:585–94. [DOI] [PubMed] [Google Scholar]
- 25. Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021; 385:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Food and Drug Administration . FDA issues emergency use authorization for third COVID-19 vaccine.https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine. Accessed 15 March 2022.
- 27. Shay DK, Gee J, Su JR, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep 2021; 70:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gray GE, Bekker LG, Laher F, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N Engl J Med 2021; 384:1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.