Abstract
Background
Benzodiazepines are considered the gold standard for treatment of alcohol withdrawal syndrome (AWS), a group of symptoms that occur after abrupt cessation of alcohol use, but may be associated with serious adverse effects. Given the safety concerns, alternative treatment options for AWS management have been investigated, including gabapentin and baclofen. Because no available studies have investigated the inpatient use of the gabapentin and baclofen combination for alcohol detoxification, this study aims to evaluate their efficacy and safety in the inpatient hospital setting.
Methods
This retrospective cohort study at the Captain James A. Lovell Federal Health Care Center in North Chicago, Illinois, included patients who were aged ≥ 18 years and who were admitted to the general acute medicine floor for the primary indication of AWS from January 1, 2014, to July 31, 2021. The primary outcome was the length of stay, defined as hours from admission to either discharge or 36 hours with a Clinical Institute Withdrawal Assessment of Alcohol (CIWA) score ≤ 8. Electronic health records were reviewed to collect CIWA scores, alcohol withdrawal seizure and delirium tremens incidence, rates of conversions from gabapentin/baclofen to lorazepam, rates of transitions to a higher level of care, and readmission for AWS within 30 days.
Results
Mean length of stay in the gabapentin/baclofen group was statistically significantly shorter compared with the benzodiazepine group (42.6 vs 82.5 hours, P < .001). The study found no significant difference between the gabapentin/baclofen and benzodiazepine groups in AWS readmission, adjuvant medications for AWS management, and number of patients who transitioned to a higher level of care. Overall, the safety of gabapentin/baclofen vs benzodiazepine were comparable; however, 1 patient experienced a seizure, and 1 patient experienced delirium tremens during admission in the benzodiazepine group.
Conclusions
Gabapentin/baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Alcohol use disorder (AUD) is a chronic disease characterized by an impaired ability to control alcohol use that negatively impacts the social, occupational, and health aspects of patients’ lives.1 It is the third leading modifiable cause of death in the United States.2 About 50% of patients with AUD experience alcohol withdrawal syndrome (AWS) following abrupt cessation of alcohol use. AWS often presents with mild symptoms, such as headaches, nausea, vomiting, and anxiety. However, as many as 20% of patients experience severe and potentially life-threatening symptoms, such as tremors, delirium, hallucinations, and seizures within 48 hours of AWS onset.3
Benzodiazepines, such as lorazepam or chlordiazepoxide, are considered the gold standard for AWS.4 Benzodiazepines act by potentiation of γ-aminobutyric acid (GABA) receptors that produce inhibitory responses in the central nervous system (CNS). This mechanism is similar to the activity of ethanol, which acts primarily at the GABA-A receptors, resulting in facilitation of GABAergic transmission. The Clinical Institute Withdrawal Assessment (CIWA) of Alcohol scale is a commonly used tool to assess the severity of AWS and the appropriate dosing schedule of benzodiazepines.3 Multiple studies have demonstrated the superiority of using benzodiazepines, as they are beneficial for reducing withdrawal severity and incidence of delirium and seizures.5,6
Although benzodiazepines are effective, they are associated with serious adverse effects (AEs), such as respiratory depression, excessive sedation, and abuse potential.4 Older patients are at higher risk of these AEs, particularly oversedation. In addition, sudden discontinuation of a benzodiazepine treatment can result in anxiety, irritability, and insomnia, which might worsen AWS.
Given the safety concerns of benzodiazepines, alternative treatments for AWS management have been investigated, including gabapentin. Previous studies have demonstrated gabapentin might be effective for mild-to-moderate AWS management.7–9 Gabapentin exhibits its action by binding to the α2δ subunit of voltage-activated calcium channels with high affinity. Although the exact mechanism of action of gabapentin in AWS is unknown, it has been proposed that gabapentin normalizes GABA activation in the amygdala, which is associated with alcohol dependence.10 A systemic review conducted by Leung and colleagues found that gabapentin might be an option for the management of mild AWS.11 However, current evidence does not support the use of gabapentin monotherapy in patients with severe AWS, a history of seizures, or those at risk of delirium tremens (DTs) since there is a higher chance of complications.
Baclofen is another medication investigated by researchers for use in patients with AWS. Baclofen works by activating the GABA-B receptor, which results in the downregulation of GABA-A activity. This results in a negative feedback loop leading to a decrease in excitatory neurotransmitters that is similar to the effect produced by alcohol.12 However, there is limited evidence that baclofen is effective as monotherapy for the treatment of AWS. A Cochrane review previously evaluated baclofen use in AWS but found insufficient evidence of its efficacy and safety for this indication.13
The Captain James A. Lovell Federal Health Care Center (CJALFHCC) in North Chicago, Illinois, currently uses a protocol in which the combination of gabapentin and baclofen is an option for AWS management in the inpatient setting. According to the current protocol, the combination of gabapentin and baclofen (g/b) is indicated for patients whose CIWA score is ≤ 8. If the CIWA score is 9 to 15, lorazepam or chlordiazepoxide should be used; if the CIWA score is 16 to 20, lorazepam should be used; and if the CIWA score is greater than 20, then lorazepam and dexmedetomidine are recommended. The protocol also lists certain patient characteristics, such as history of seizures, traumatic brain injury, or long duration of alcohol consumption, in which clinical judgment should be used to determine whether a described detoxification regimen is appropriate or whether the patient should be managed off-protocol.
Because to our knowledge, no current studies have investigated the use of g/b for inpatient AWS, the goal of this study was to evaluate its efficacy and safety. We hypothesized that AWS duration would be significantly different in patients who received g/b for AWS management compared with those treated with benzodiazepines.
METHODS
We performed a retrospective cohort chart review at CJALFHCC. Data were collected from the facility’s electronic health record Computerized Patient Record System (CPRS). This study was approved by the Edward Hines Jr. Veterans Affairs Hospital Institutional Review Board.
Patient records were screened and included if they met the following criteria: (1) Patients aged ≥ 18 years who were hospitalized from January 1, 2014, to July 31, 2021, for the primary indication of AWS; (2) Patients who received a g/b or benzodiazepine protocol during AWS hospitalization. If a patient was admitted multiple times for AWS management, only the first admission was included for primary outcome analysis. Exclusion criteria were patients who were active-duty service members, discharged within 24 hours; patients with a primary seizure disorder; patients with known gabapentin, baclofen, or benzodiazepine allergy or intolerance. Patients who used gabapentin, baclofen, or benzodiazepines in an outpatient setting prior to AWS admission; had concurrent intoxication or overdose involving substances other than alcohol; had a concurrent regimen of gabapentin, baclofen, or benzodiazepines; or had initiation on adjuvant medications for AWS management (eg, divalproex, haloperidol, carbamazepine, or clonidine) also were excluded. Patients were categorized as those who received g/b as the initial therapy after admission or patients who received benzodiazepine therapy.
The primary outcome of this study was the length of stay (LOS), which was defined as the hours from admission to either discharge or 36 hours with a CIWA score ≤ 8. Secondary outcomes included the occurrence of alcohol withdrawal seizure, the occurrence of DTs, rates of conversions from g/b protocol to lorazepam use, rates of transitions to a higher level of care (eg, an intensive care unit), and readmission for AWS within 30 days.
CPRS was used to collect information including baseline demographics, blood alcohol content, CIWA scores throughout hospitalization, number of admissions for alcohol detoxification in the previous year, AWS readmission within 30 days after discharge, prior treatment with g/b, history of alcohol withdrawal seizures and DTs, hospital LOS, outpatient medications for AUD treatment, rates of conversions from g/b protocol to lorazepam, and rates of transition to a higher level of care.
Statistical Analysis
Study data were stored and analyzed using an Excel spreadsheet and IBM SPSS Statistics software. LOS was compared between the g/b and benzodiazepine groups using inferential statistics. An independent 2-sample t test was used to assess the primary outcome if data were normally distributed. If the collected data were not distributed normally, the Mann-Whitney U test was used. All other continuous variables were assessed by using independent t tests and categorical variables by using χ2 tests. A P value < .05 was considered statistically significant. Effect size of d = 0.42 was calculated based on a previous study with a similar research design as our study.9 We determined that if using an independent 2-sample t test for the primary outcome analysis, an estimated sample size of 178 subjects would provide the study with an 80% power to detect a difference at a 2-sided significance level with α = 0.05. If using the Mann-Whitney U test, 186 subjects would be required to provide identical power.
RESULTS
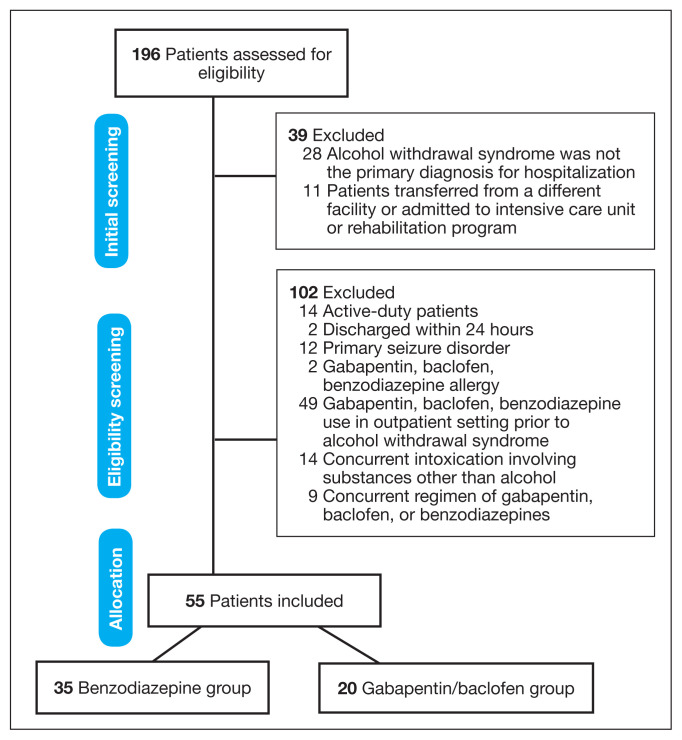
We reviewed 196 patient health records, and 39 were initially excluded. The most common reason was that AWS was not the primary diagnosis for hospitalization (n = 28). After eligibility screening, 102 subjects were excluded with the most common reason for exclusion being the use of gabapentin, baclofen, or benzodiazepines in the outpatient setting before admission (n = 49). Fifty-five patients met the inclusion criteria; 35 patients were in the benzodiazepine group and 20 in the g/b group (Figure 1).
FIGURE 1.
Patient Allocation Flow Diagram
Most patients in both groups were White and male (Table 1). The average admission CIWA score in the benzodiazepine group was higher than the g/b group (6.8 vs 3.9; P = .001). The maximum CIWA score was also higher in the benzodiazepine group compared with the g/b group (12.7 vs 5.5; P < .001).
TABLE 1.
Patient Characteristics
| Characteristics | Benzodiazepine (n = 35) | Gabapentin/baclofen (n = 20) | P value |
|---|---|---|---|
| Age, mean, y | 51 | 52 | .65 |
|
| |||
| Sex, No. (%) | .99 | ||
| Male | 32 (91) | 18 (90) | |
| Female | 3 (9) | 2 (10) | |
|
| |||
| Race, No. (%) | .99 | ||
| African American | 6 (17) | 3 (15) | |
| White | 29 (83) | 16 (80) | |
|
| |||
| Blood alcohol content, mean, mg/dL | 221 | 180 | .20 |
|
| |||
| Charlson Comorbidity Index, mean | 1.5 | 1.4 | .80 |
|
| |||
| Clinical Institute Withdrawal Assessment score, mean | |||
| At admission | 6.8 | 3.9 | .001a |
| Maximum | 12.7 | 5.5 | < .001a |
|
| |||
| Alcohol detoxification admissions in previous year, mean | 0.5 | 0.85 | .39 |
|
| |||
| History, No. (%) | |||
| Treatment with gabapentin/baclofen combination | 0 (0) | 7 (35) | .0004a |
| Alcohol withdrawal seizures and delirium tremens | 17 (49) | 5 (25) | .15 |
| Traumatic brain injury | 0 (0) | 2 (10) | .13 |
| Past or current illicit substance use | 4 (11) | 3 (15) | .70 |
|
| |||
| Outpatient alcohol use disorder medications, No. (%) | 2 (6) | 2 (10) | .62 |
Baseline characteristics that are significantly different between groups.
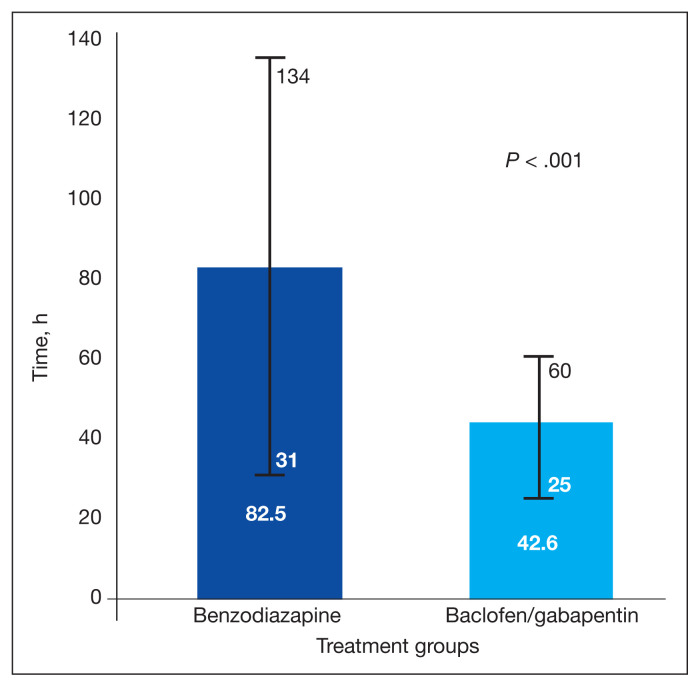
The Shapiro-Wilk tests showed a significant departure from normality in the benzodiazepine group W(35) = 0.805 (P < .001) and g/b group W(20) = 0.348 (P < .001) for the primary outcome. The g/b group average LOS was shorter compared with the benzodiazepine group (42.6 vs 82.5 hours, respectively). By using the Mann-Whitney U Test, a statistically significant difference was found in the primary outcome U = 98; z score = 4.41 (P < .001; Figure 2).
FIGURE 2.
Mean Hours From Admission to Discharge or 36 Hours With Clinical Institute Withdrawal Assessment Score ≤ 8
Additionally, this study examined multiple secondary outcomes (Table 2). Length of hospitalization, defined as hours from admission to discharge, was shorter in the g/b group compared with in the benzodiazepine group (76.8 hours vs 115.4 hours; P = .03). There was no significant difference between the benzodiazepine and g/b groups in AWS readmission within 30 days after discharge, adjuvant medications added for AWS management, and the number of patients transitioned to a higher level of care. However, 3 patients had to be transitioned to the intensive care unit in the benzodiazepine group compared with none in the g/b group. Of note, 2 patients (10%) in the g/b group were switched to benzodiazepines. Also, 1 patient experienced a seizure and 1 patient experienced DTs in the benzodiazepine group during admission, with no incidences of seizures or DTs in the g/b group.
TABLE 2.
Secondary Outcome Analysis
| Secondary outcomes | Benzodiazepine (n = 35) | Gabapentin/baclofen (n = 20) | P value |
|---|---|---|---|
| Length of hospitalization, h | 115.4 | 76.8 | .03 |
| Patients on adjuvant medications for alcohol withdrawal syndrome management, No. | 2 | 0 | .53 |
| Patients transitioned to a higher level of care, No. | 3 | 0 | .29 |
| Alcohol withdrawal syndrome readmissions within 30 d after discharge, No. | 8 | 2 | .30 |
DISCUSSION
This retrospective chart review study found that LOS was shorter in patients with AWS treated with g/b compared with those treated with benzodiazepines, with no significant difference in safety outcomes such as seizures, DTs, or intensive care unit transfers. Although there was a statistically significant difference in the primary outcome between the 2 groups, it appears that patients on benzodiazepine therapy originally had more severe AWS presentation as their admission and maximum CIWA scores were statistically significantly higher compared with the g/b group. Thus, patients who were initially started on g/b had less serious AWS presentations. Based on this information we can conclude that the g/b combination may be an effective option for mild AWS management.
To our knowledge, this is the first study that has investigated the combination of g/b compared with benzodiazepines for AWS management in hospitalized patients. The research design of this project was adapted from the Bates and colleagues study that examined gabapentin monotherapy use for the treatment of patients hospitalized with AWS.9 We specifically used the primary outcome that they defined in their study since their LOS definition aimed to reflect clinically active withdrawal rather than simply hours of hospitalization, which would decrease the risk of confounding the primary outcome. The results of our research were similar to Bates and colleagues as they found that the gabapentin protocol appeared to be an effective and safe option compared with benzodiazepines for patients hospitalized with AWS.9
Limitations
This study has multiple limitations. As it was a retrospective chart review study, the data collection accuracy depends on accurate record keeping. Additionally, certain information was missing, such as CIWA scores for some patients. This study has limited external validity as most of the patients were older, White, and male, and the data collection was limited only to a single center. Therefore, it is uncertain whether the results of this study can be generalized to other populations. Also, this study had a small sample size, and we were not able to obtain the intended number of patients to achieve a power of 80%. Lastly, some background characteristics, such as admission and maximum CIWA scores, were not distributed equally between groups. Therefore, future studies are needed with a larger sample size that examine the LOS in the g/b group compared with the benzodiazepine group and in which CIWA scores are matched to reduce the effect of extraneous variables.
CONCLUSIONS
Gabapentin and baclofen combination seems to be an effective and safe alternative to benzodiazepines and may be considered for managing mild AWS in hospitalized patients, but additional research is needed to examine this regimen.
Acknowledgments
Research committee: Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; Jennifer Kwon, PharmD, BCOP. Co-investigators: Zachary Rosenfeldt, PharmD, BCPS; Kaylee Caniff, PharmD, BCIDP.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest or outside sources of funding with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Ethics and consent
Since this study is retrospective in nature, it presents no more than minimal risk of harm to patients and involves no procedures that would require written consent. This project was approved by the Edward Hines, Jr. Veterans Affairs Hospital Institutional Review Board.
References
- 1.National Institute on Alcohol Abuse and Alcoholism. Understanding alcohol use disorder. 2020. [Accessed February 2, 2023]. Updated April 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder .
- 2.Moss HB. The impact of alcohol on society: a brief overview. Soc Work Public Health. 2013;28(3–4):175–177. doi: 10.1080/19371918.2013.758987. [DOI] [PubMed] [Google Scholar]
- 3.Pace C. Alcohol withdrawal: epidemiology, clinical manifestations, course, assessment, and diagnosis. [Accessed January 26, 2023]. https://www.uptodate.com/contents/alcohol-withdrawal-epidemiology-clinical-manifestations-course-assessment-and-diagnosis .
- 4.Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE 01–VE 07. doi: 10.7860/JCDR/2015/13407.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo-Smith MF. Pharmacological management of alcohol withdrawal A meta-analysis and evidence-based practice guideline American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 6.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of acute alcohol withdrawal. CMAJ. 1999;160(5):649–655. [PMC free article] [PubMed] [Google Scholar]
- 7.Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582–1588. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultationliaison psychiatry service. Psychosomatics. 2018;59(5):496–505. doi: 10.1016/j.psym.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Bates RE, Leung JG, Morgan RJ, 3rd, Fischer KM, Philbrick KL, Kung S. Retrospective analysis of gabapentin for alcohol withdrawal in the hospital setting: the Mayo Clinic experience. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):542–549. doi: 10.1016/j.mayocpiqo.2020.06.002. Published 2020 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897–906. doi: 10.1177/1060028015585849. [DOI] [PubMed] [Google Scholar]
- 12.Cooney G, Heydtmann M, Smith ID. Baclofen and the alcohol withdrawal syndrome-a short review. Front Psychiatry. 2019;9:773. doi: 10.3389/fpsyt.2018.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Wang LN. Baclofen for alcohol withdrawal. Cochrane Database Syst Rev. 2019;2019(11):CD008502. doi: 10.1002/14651858.CD008502.pub6. Published 2019 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]