Abstract
Background and Objective
The prevalence of venous thrombus embolism (VTE) in patients with chronic obstructive pulmonary disease (COPD) is higher than in patients without COPD. Owing to the similarity of clinical symptoms between PE and acute exacerbation COPD (AECOPD), PE is likely to be overlooked or underdiagnosed in patients with AECOPD. The aim of the study was to investigate the prevalence, risk factor, clinical characteristics, and prognostic impact of VTE in patients with AECOPD.
Methods
This multicenter, prospective, cohort study was conducted in 11 research centers of China. Data on the baseline characteristics, VTE-related risk factors, clinical symptoms, laboratory examination results, computed tomography pulmonary angiography (CTPA) and lower limb venous ultrasound of AECOPD patients were collected. Patients were followed up for 1 year.
Results
A total of 1580 AECOPD patients were included in the study. The mean (SD) age was 70.4 (9.9) years and 195 (26%) patients were women. The prevalence of VTE was 24.5% (387/1580) and PE was 16.8% (266/1580). VTE patients were older; had higher BMI; and longer course of COPD than non-VTE patients. The history of VTE, cor pulmonale, less purulent sputum, increased respiratory rate, higher D-dimer, and higher NT-proBNP/BNP were independently associated with VTE in hospitalized patients with AECOPD. The mortality at 1-year was higher in patients with VTE than patients without VTE (12.9% vs 4.5%, p<0.01). There was no significant difference in the prognosis of patients with PE in segmental or subsegmental arteries and in main pulmonary arteries or lobar arteries (P>0.05).
Conclusion
VTE is common in COPD patients and is associated with poor prognosis. Patients with PE at different locations had poorer prognosis than patients without PE. It is necessary to perform active screening strategy for VTE in AECOPD patients with risk factors.
Keywords: chronic obstructive pulmonary disease, venous thromboembolism, computed tomography pulmonary angiography, prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide.1 COPD patients may frequently suffer episodes of acute exacerbation of symptoms (AECOPD), defined as acute worsening of respiratory symptoms that requires additional therapy and increases healthcare costs.2 Although most cases of AECOPD are caused by infection and/or air pollution,3 the exact cause of up to 30% of the cases remains unknown.4 Patients with COPD reportedly have approximately twice the risk of developing venous thromboembolic events (VTEs) than those without COPD.5 As pulmonary embolism (PE) manifestations may be similar to AECOPD, it is often difficult to distinguish between the two conditions in COPD patients.6 Thus, the diagnosis of PE based on clinical suspicion alone is not accurate enough. COPD patients tend to have a higher average age and they are more likely to have additional diseases than those without COPD.7 If VTE is missed, it may cause adverse consequences and poor prognosis.5 A recent meta-analysis found that the mortality of patients with VTE was higher than that of those without VTE and the prevalence of VTE in studies where computed tomography pulmonary angiography (CTPA) was performed in all patients was significantly higher than that in studies in which CTPA was not performed in all patients (23% vs 6%, p<0.001).8 However, the meta-analysis only included a few studies with patients in whom CTPA was performed, and the sample size was relatively small; moreover, it only reported on the mortality during hospitalization or within 3 months after discharge. There are few studies on the long-term prognosis of patients with COPD combined with VTE. Therefore, the objectives of our study were to assess the prevalence of VTE in a large cohort of patients with AECOPD who underwent CTPA. In addition, the effect of VTE on prognosis was evaluated in terms of length of hospital stay and 1-year mortality.
Methods
Study Design and Participants
This multicenter, prospective, cohort study was conducted from January 2017 to January 2021 and was supported by the National Key Research and Development Program of China (project number: 2016YFC1304402) and is registered in ClinicalTrials.gov (Clinical Trials ID: NCT 03182309). The study was approved by the central independent ethics committee (Fuwai Hospital, Approval Number: 2017-879, date of approval: October 21, 2016), and complied with the Declaration of Helsinki. The written informed consent was obtained from all patients before inclusion. All the patients admitted for acutely worsening respiratory symptoms of COPD to 1 of the 11 participating centers (Beijing Chaoyang Hospital, Capital Medical University Daxing Teaching Hospital, The Second Hospital of Hebei Medical University, Beijing Fangshan District Liangxiang Hospital, Tongji Hospital, Shanxi Bethune Hospital, Fuwai Hospital, Beijing Sixth Hospital, The First Affiliated Hospital of Nanchang University, Shenzhen People’s Hospital, Peking University International Hospital) in China were included in the study. The inclusion criteria were as follows: 1) Age >40 years; 2) COPD was diagnosed according to the criteria of the 2016 Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guidelines, pulmonary function tests showing the ratio of forced expiratory volume in the first second of expiration (FEV1) to forced vital capacity (FVC) was <70% after postbronchodilator use and that was confirmed by a senior physician; 3) Patients hospitalized for AECOPD, acutely worsening respiratory symptoms of COPD were defined as a sustained worsening of patients’ baseline symptoms (dyspnea, cough, sputum) beyond normal variation and requiring treatment modification. The main exclusion criteria were as follows: 1) Patients with COPD hospitalized for other conditions; 2) Contraindication to undergoing CTPA; 3) Active malignant tumors; 4) Anticoagulant treatment before admission; 5) Pneumothorax at admission; 6) Life expectancy <3 Months; and 7) Inability to give written informed consent.
Procedures
Baseline characteristics of patients including age, sex, height, weight, body mass index (BMI), and smoking status were collected at admission. Clinical evaluations for all participants, including medical history inquiry and physical examination were conducted by the physician in charge and ultimately confirmed by the senior physician. Concomitant diseases; risk factors for VTE including bedridden state/immobility ≥3 days, surgery/traumatic fractures in the past 3 months, oral contraceptive use, deep vein puncture in 1 month, hormone replacement therapy, diuretics treatment, and history of VTE; clinical symptoms; and clinical signs of patients also were recorded, including chest distress, dyspnea, syncope, cough, sputum, fever, hemoptysis, palpitations, lower-limb swelling, lower-limb skin pigmentation, temperature, pulse, respiration rate, blood pressure, rhonchi, moist crackles, varicose veins, and edema in lower extremities. Laboratory results were collected within 24 h after admission, including blood routine examination, prothrombin time (PT), activated partial thrombin time (APTT), fibrinogen (Fbg), D-dimer, alanine transaminase (ALT), aspartate amino transferase (AST), creatinine, N-terminal pro-brain natriuretic peptide (NT-proBNP) or brain natriuretic peptide (BNP), arterial blood gas analysis, cholesterol, triglyceride, and glucose. The PE probability score of Well’s score,9 and revised Geneva score10 were recorded. All patients underwent CTPA, Electrocardiogram (ECG), lower limb venous ultrasound, and cardiac ultrasound within 48 h of admission. The diagnostic criteria for pulmonary embolism by CTPA were as follows: failure of contrast material to fill the entire lumen, the artery may be enlarged, as compared with similar arteries; a partial filling defect surrounded by contrast material on a cross-sectional image; contrast material between the central filling defect and the artery wall on an in-plane, longitudinal image.11 The ultrasonographic criteria for lower limb deep venous thrombosis (DVT) were non-compressibility or incomplete compressibility of the vein in the horizontal axis.12 Although some patients had a previous clinical diagnosis of COPD or were treated for COPD, a spirometry examination was performed when the patient was relatively stable. Specialized investigators at each research center filled in the data in respective case report forms and recorded them into electronic archives; all data were cross-checked and centrally validated. In patients with confirmed VTE, anticoagulation was administered according to current guidelines.10 All patients were followed up at 3, 6, and 12 months. During the follow-up period, patients reported to the study center immediately if any symptoms or signs indicating VTE or bleeding or AECOPD were observed. At the end of the follow-up, all included patients were interviewed by their physicians through structured questionnaires or telephone interviews. All events that occurred after discharge were noted and the reason for readmission was determined.
Sample Size and Statistical Methods
According to previous studies, the prevalence of VTE during COPD exacerbation ranged from 2.1% to 29.1%.13–20 Our study set the expected prevalence of VTE at 20%, and the sample size was calculated to be 1537 cases.
Continuous variables were described as mean (SD) or median (interquartile range [IQR]), according to the Shapiro–Wilk normality tests; categorical variables were described as proportions. Student’s t-test or Mann–Whitney U-test was adopted for group comparisons of continuous quantitative variables, and the chi-square test (or Fisher’s exact test) was used to compare differences in proportion. Variables with P values <0.02 in the univariate analysis and those variables that might be associated with VTE were included in the logistic regression model. The missing outcome data were not imputed. The results of multivariate analysis were reported as adjusted odds ratios with 95% confidence intervals (CI). All statistical analyses were performed with the use of the SPSS software package, version 25 (SPSS). P<0.05 was considered to indicate statistically significant differences.
Results
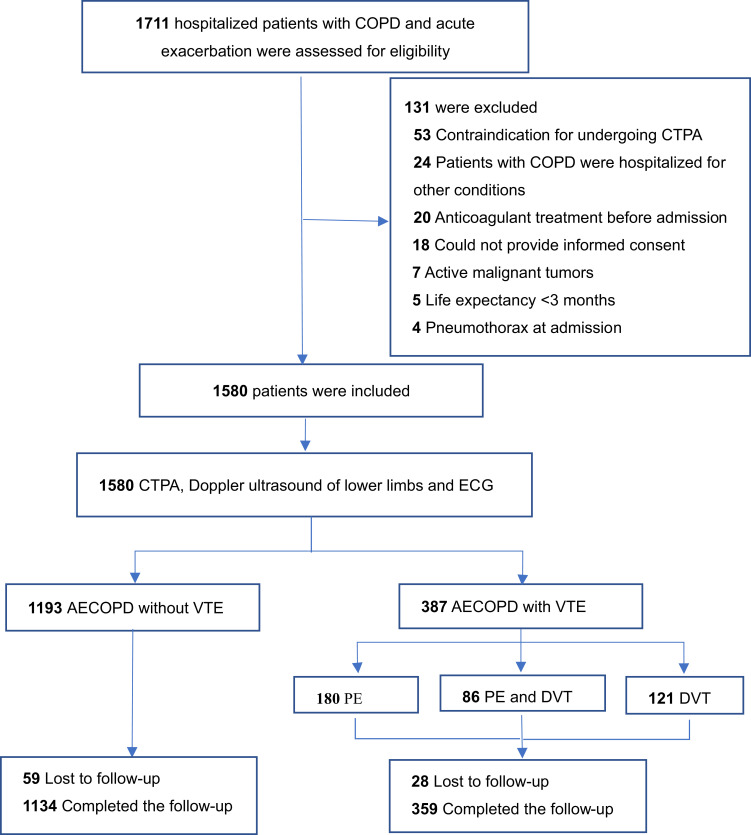
From January 2017 to January 2021, 1711 consecutive patients with COPD admitted to the 11 participating hospitals for acute exacerbation were screened for inclusion in this study. Of these, 131 patients were excluded, mainly related to contraindication of CTPA and hospitalization for conditions other than AECOPD (Figure 1). The mean (SD) age of the remaining 1580 patients was 70.4 (9.9) years, and 195 (26%) patients were women. In total, 1336 (84.6%) patients had comorbidities accompanying COPD. The distribution of COPD patients enrolled according to GOLD stage (GOLD I–IV) was as follows: 9.8%, 32.0%, 36.6%, and 21.6%, respectively (Table 1). In total,1493 patients completed the follow-up, and in all 87 (5.5%) patients were lost to follow-up in the next 1 year.
Figure 1.
Flowchart of patients hospitalized with AECOPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; CTPA, computed tomography pulmonary angiography; AECOPD, acute exacerbation of COPD; VTE, venous thromboembolism.
Table 1.
Baseline Characteristics of Subjects
| Characteristics | All Subjects (n=1580) | Non-VTE (n=1193) | VTE (n=387) | P |
|---|---|---|---|---|
| Age, mean (SD), years | 69.5 (9.0) | 68.8 (9.2) | 71.5 (8.4) | <0.001 |
| Sex, male | 1230 (77.8) | 959 (80.4) | 271 (70.0) | <0.001 |
| BMI, mean (SD) | 23.8 (4.1) | 23.7 (4.1) | 24.3 (4.1) | 0.050 |
| Smokers | 1231 (77.9) | 959 (80.4) | 188 (70.7) | <0.001 |
| Course of disease, median (IQR), years | 10.0 (16.0) | 10.0 (16.0) | 11.3 (17.0) | 0.037 |
| Length of hospital stay, mean (SD), days | 12.0 (6.0) | 11.4 (5.4) | 13.7 (7.3) | <0.001 |
| FEV1 after administration of albuterol, median (IQR) | 1.0 (0.7) | 1.2 (0.7) | 1.5 (0.9) | 0.027 |
| FEV1, %predicted, mean (SD) | 48.9 (25.3) | 47.3 (26.4) | 53.0 (20.5) | <0.001 |
| GOLD stage of obstruction | <0.001 | |||
| I (mild) | 155 (9.8) | 101 (8.5) | 54 (14.0) | |
| II (moderate) | 505 (32.0) | 367 (30.8) | 138 (35.7) | |
| III (severe) | 579 (36.6) | 444 (37.2) | 135 (34.9) | |
| IV (very severe) | 341 (21.6) | 281 (23.6) | 60 (15.5) | |
| Wells score, median (IQR) | 0 (3) | 0.9 (1.5) | 2.9 (4.5) | <0.001 |
| Revised Geneva score, mean (SD) | 4.1 (2.1) | 3.9 (1.9) | 5.0 (2.0) | <0.001 |
| Diuretic | 81 (5.1) | 46 (3.9) | 35 (9.0) | <0.001 |
| Bedridden/immobility ≥3 days | 26 (1.6) | 11 (0.9) | 15 (3.9) | <0.001 |
| Surgery/traumatic fractures <3 month | 20 (1.3) | 9 (0.8) | 11 (2.8) | 0.001 |
| History of VTE | 149 (9.4) | 29 (2.4) | 120 (31.0) | <0.001 |
| Varicosity | 40 (2.5) | 22 (1.8) | 18 (4.7) | 0.021 |
| Lower limbs swelling | 349 (21.9) | 239 (20.0) | 107 (27.6) | 0.002 |
| Deep vein puncture | 8 (4.8) | 6 (4.4) | 2 (6.1) | 0.663 |
| Comorbid conditions | ||||
| Hypertension | 722 (45.7) | 534 (44.8) | 188 (48.6) | 0.192 |
| Coronary artery disease | 463 (29.3) | 336 (28.2) | 127 (32.8) | 0.814 |
| Cor pulmonale | 307 (19.4) | 210 (17.6) | 97 (25.1) | 0.001 |
| Hyperlipemia | 188 (11.9) | 138 (11.6) | 50 (12.9) | 0.483 |
| Atrial fibrillation | 61 (3.9) | 34 (2.8) | 27 (7.0) | <0.001 |
| Pneumonia | 117 (7.4) | 91 (7.6) | 26 (6.7) | 0.551 |
| Diabetes mellitus | 284 (18.0) | 222 (18.76) | 62 (16.0) | 0.254 |
| Cerebrovascular disease | 228 (13.6) | 162 (12.6) | 66 (17.1) | 0.023 |
Abbreviations: VTE, venous thromboembolism; FEV1, forced expiratory volume in 1 second.
The Prevalence of VTE
The prevalence of VTE was 24.5% (387/1580, 95% CI: 24.4–26.6%). PE was confirmed in 266 patients (16.8%; 95% CI: 15.0–18.8%), and DVT was confirmed in 207 patients (13.1%, 95% CI: 11.5–14.9%). There were 180 (67.7%) PE patients without DVT and 86 (32.3%) PE patients with DVT.
The Localization of VTE
Of the 266 patients with PE, 49 (18.4%) had thrombus located in the main pulmonary arteries, 117 (44.0%) had thrombus located in the lobar arteries, and 100 (37.6%) had thrombus located in the segmental or subsegmental arteries. Among the 207 patients with DVT, 37 (17.9%) had thrombus located in the proximal deep vein, and 170 (82.1%) had thrombus located in the distal deep vein. Of the PE patients without DVT (n=180), 52.2% (94/180) thrombi were located in the segmental and subsegmental arteries. In 86 PE patients with DVT, only 6 (6.9%) thrombi were located in the segmental and subsegmental arteries (Table 2).
Table 2.
Localization of the Thrombi on CTPA and Doppler Ultrasonography of the Lower Limbs
| Characteristics | Pulmonary Embolism | Total | ||||
|---|---|---|---|---|---|---|
| No PE | Main Pulmonary Artery, n (%) | Lobar Artery, n (%) | Segmental or Subsegmental, n (%) | |||
| Deep venous thrombosis | No DVT | 0 (0) | 32 (8.3) | 54 (14.0) | 94 (24.3) | 180 (46.5) |
| Proximal deep vein | 12 (3.1) | 8 (2.1) | 14 (3.6) | 3 (0.8) | 37 (9.6) | |
| Distal deep vein | 109 (28.2) | 9 (23.3) | 49 (12.7) | 3 (0.8) | 170 (43.9) | |
| Total | 121 (31.3) | 49 (1.3) | 117 (30.2) | 100 (25.8) | 387 (100) | |
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism.
The Clinical Probability of PE
According to the Wells criteria, 7.4% (n=85) patients with low clinical probability determination, 38.4% (n=153) PE patients with moderate clinical probability, and 73.7% (n=28) patients with high clinical probability determination were confirmed to have PE. Meanwhile, 11.6% (n=57) patients with low clinical probability, 20.0% (n=205) patients with moderate clinical probability, and 50% (n=4) patients with high clinical probability were confirmed to have PE based on the revised Geneva score (Supplemental Table 1).
The Clinical Characteristics and Risk Factors of VTE
In our study, patients with VTE were older than those without (P<0.01), the course of disease in patients without VTE was longer than that in patients with VTE (13.7 years vs 11.4 years. P<0.05). In terms of concomitant diseases, the prevalence of atrial fibrillation, cerebrovascular disease, and cor pulmonale in patients with VTE was higher than in patients without VTE (P<0.05). The FEV1 and FEV1% in patients without VTE were lower than those in patients with VTE (P<0.05). There were statistically significant differences in the distribution of GOLD stages between the groups with and without VTE (P<0.05; Table 1). There were also statistically significant differences between the two groups with respect to history of VTE, varicose veins, lower limbs swelling, bedridden duration ≥3 days, traumatic fracture/surgery within 3 months, and diuretic use (P<0.05; Table 1).
Clinical presentation including cough, purulent sputum, and rhonchi were significantly more prevalent among those without VTE (P<0.05); however, chest distress, chest pain, higher respiratory rate, and moist crackles were significantly more prevalent among those diagnosed with VTE (P<0.05) (Supplemental Table 2). Levels of D-dimers, pH values, NT-proBNP/BNP, prothrombin time (PT), and activated partial thromboplastin time (APTT) were significantly higher among patients with VTE than among those without VTE (P<0.05). However, the level of hemoglobin was lower in patients with VTE than in those without (Supplemental Table 3).
In the multivariate analysis, the values of NT-proBNP/BNP were converted into categorical variables according to each center NT-proBNP/BNP threshold (higher than the threshold is assigned as 1, lower than or equal to the threshold is assigned as 0). Multiple logistic regression analysis revealed that the history of VTE (OR=15.16, 95% CI: 9.29–24.73); cor pulmonale (OR=2.039, 95% CI: 1.40–2.98); higher respiratory rate (OR=1.09, 95% CI: 1.03–1.16); longer PT (OR=1.06, 95% CI: 1.01–1.10); higher D-dimer (OR=1.12, 95% CI: 1.06–1.19); and higher NT-proBNP/BNP (OR=1.44, 95% CI: 1.04–2.00) were independent variables that predicted the presence of VTE in patients with COPD. However, purulent sputum (OR=0.43, 95% CI: 0.23–0.79) and lower PaCO2 (OR=0.97, 95% CI: 0.93–0.99) were independent variables that predicted the absence of VTE in patients with COPD (Table 3).
Table 3.
Multivariate Logistic Regression
| Variables | Odds Ratio | 95% CI | P |
|---|---|---|---|
| History of VTE | 15.158 | 9.291–24.728 | <0.001 |
| Cor pulmonale | 2.039 | 1.393–2.984 | <0.001 |
| Purulent sputum | 0.429 | 0.233–0.787 | 0.006 |
| Respiratory rate | 1.092 | 1.027–1.162 | 0.013 |
| PaCO2 | 0.973 | 0.930–0.985 | 0.032 |
| Prothrombin time | 1.058 | 1.007–1.100 | 0.023 |
| d-dimer | 1.120 | 1.056–1.189 | <0.001 |
| NT-proBNP/BNP | 1.443 | 1.040–2.000 | 0.028 |
Abbreviations: PaCO2, arterial partial pressure of carbon dioxide; NT-proBNP, N-terminal pro brain natriuretic peptide; BNP, brain natriuretic peptide.
The Prognosis of VTE
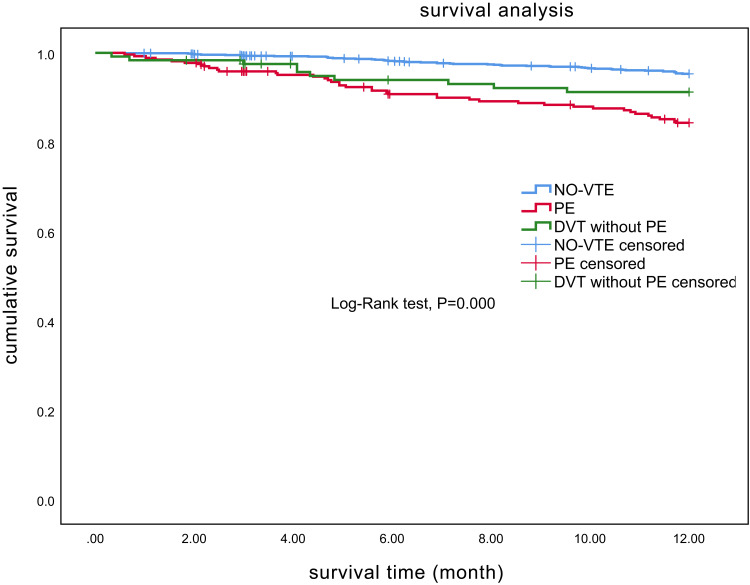
The mean length of hospitalization was longer in patients with VTE than without VTE (13.7 vs 11.4 days, P<0.01). The overall 1-year mortality rate was 6.6% (104/1580; 95% CI, 5.4–7.9%). The mortality of patients with PE was 15.0% (40/266, 95% CI: 11.0–19.9%) and the mortality of patients with DVT but without PE was 8.3% (10/121, 95% CI: 4.0–14.7%). The mortality of patients with VTE (12.9%,50/387, 95% CI: 9.7–16.7%) was higher than that of patients without VTE (4.5%, 54/1193, 95% CI: 3.4–5.9%, P<0.001) (Figure 2).
Figure 2.
Kaplan–Meier curves showing the mortality rates in chronic obstructive pulmonary disease patients with PE, with DVT-only and without VTE.
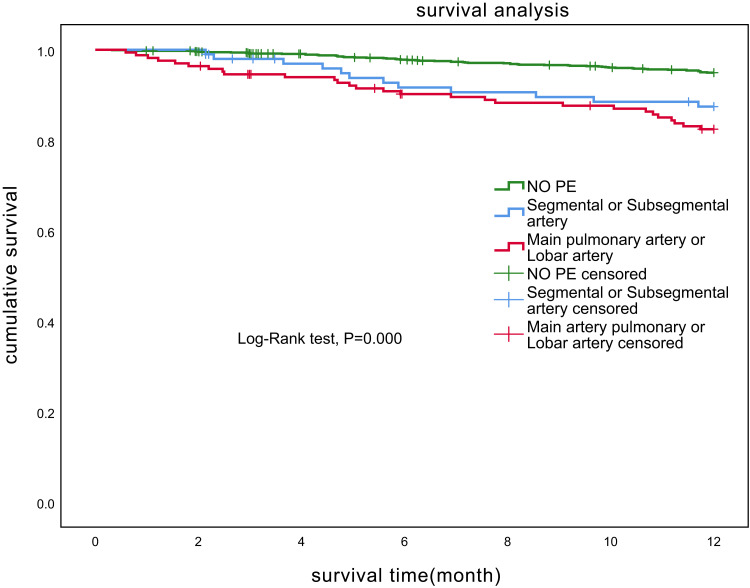
The mortality of patients with PE in the segmental or subsegmental arteries was 12.0% (12/100, 95% CI: 6.4–20.0%). The mortality of patients with PE in the main pulmonary arteries or lobar arteries was 16.9% (28/166, 95% CI: 11.5–23.4%), and there was no statistical difference among patients with PE at different locations (P>0.05) (Figure 3).
Figure 3.
Kaplan–Meier curves showing the mortality rates in chronic obstructive pulmonary disease patients with PE of main pulmonary artery or lobar artery, with PE of segmental or subsegmental artery and without PE.
Discussion
This was a prospective, cohort, multicenter study about the prevalence of VTE based on CTPA and lower limb venous ultrasound in AECOPD patients. The results showed that PE was present in 16.8% (95% CI: 15.0–18.8%) patients who were hospitalized for exacerbation of COPD. DVT was present in 13.1% (95% CI: 11.5–14.9%) patients; therefore, the prevalence of venous thromboembolism was 24.5% (95% CI: 24.4–26.6%) and the prevalence of PE was higher than that of DVT (16.8% vs 13.1%). Previous studies showed that the prevalence of VTE varied greatly, which was mainly because of variations in race, enrollment criteria, study design, sample size, and research settings. Some studies only enrolled patients who were admitted to the hospital for exacerbation of unknown origin and did not require invasive mechanical ventilation,21 while others only analyzed patients with AECOPD requiring mechanical ventilation or those who were admitted to in the Intensive Care Unit (ICU).15,17 In addition, in one study, CTPA or lower-limb Doppler ultrasonography was only performed in patients according to a pre-designed protocol,13 while other studies18,21 included all COPD subjects who underwent CTPA. Thus, it is difficult to draw a definitive conclusion. Gunen et al22 found that COPD patients more frequent had PE than DVT (59% vs 41%), and Schneider et al23 estimated the relative risk of having PE was 2.5-times, but the risk of having DVT was only 1.3 times in COPD patients than non-COPD patients. A recent meta-analysis included 17 studies involving 3170 patients and found that the prevalence of PE and DVT in AECOPD patients was 17.2% (95% CI: 13.4–21.3%) and 7.1% (95% CI: 3.7–11.4%), respectively.24 Our results were consistent with those studies.
Previous studies suggested that >90% of PE originates from DVT in the lower extremities.25 However, our results showed that 67.7% (180/266) patients with AECOPD had PE in the absence of DVT. This may be a particular characteristic of PE in COPD patients, other likely reasons were that the clot can also come from other sites such as the pelvic venous plexus, and the computed tomography venography has not been widely used for detecting VTE in those locations.12 If the presence of VTE is determined only by lower limb venous ultrasonography, 46.5% (180/387) patients with VTE may be missed. Delfina et al26 found that the location of the thrombus in patients with COPD and/or pulmonary tract infection were mostly in the segmental and subsegmental arteries. Our study showed that among 266 patients with PE, 100 (37.6%) had a thrombus located in the segmental or subsegmental artery. Patients with segmental or subsegmental PE are less likely to be distinguished from AECOPD, because the clinical symptoms are less pronounced. A systematic CTPA during COPD exacerbation could not only assist in identifying more unsuspected PE cases but also determine the location of PE. Therefore, the implementation of CTPA for patients with AECOPD is necessary.
Regarding the clinical characteristics of VTE, our study results showed that patients with purulent sputum had a lower incidence of VTE. Several previous studies14,15,19 also showed that increased purulent sputum was a predictor of absence of PE, indicating that AECOPD was caused by bronchial or pulmonary infection but not PE. The increased respiratory rate and lower PaCO2 level were associated with PE. Previous studies also showed that lower PaCO2 level was associated with PE in AECOPD patients,14,18,27 which may be because of increased respiratory rate responsiveness in patients with PE, resulting in excessive exhalation of carbon dioxide that is a predictive factor for the prevalence of VTE in patients with AECOPD. Consistent with previous studies,16,21 our study also demonstrated that the history of VTE was an independent risk factor for VTE. Most patients with cor pulmonale are complicated with congestive heart failure, and our study showed that cor pulmonale was an independent risk factor for VTE, consistent with previous studies.5,28 PT was as an independent factor in the multivariate analysis conducted in our study. The underlying mechanism is likely the large amount of plasminogen that will be consumed during the procedure of VTE, resulting in hyperfibrinolysis and increasing of PT value. However, this study showed that the median value of PT was still in the normal range in both the VTE group and the non-VTE group, so its clinical significance remains to be discussed. It is well known that BNP is mainly synthesized and secreted by ventricular myocytes. When PE occurs, increased right ventricular afterload and ventricular wall dilatation lead to the release of BNP.12 Our results showed that NT-Pro-BNP or BNP was an independent predictive factor for VTE in COPD patients, consistent with other studies.18,19,22 All of the above clinical features can help us accurately identify VTE in patients with AECOPD.
In our study, the 1-year mortality among patients with VTE was higher (12.9% vs 4.5%) and the length of hospitalization was longer than that among patients without VTE (13.7 vs 11.4 days). Previously, some studies only reported the in-hospital mortality15,17,19 or 3-month mortality11 of AECOPD patients with VTE. Undoubtedly, the prolonged hospital stays and higher mortality rate both increased the burden of medical expenses. We further analyzed the survival of patients with PE at different locations, and the results showed that the mortality of patients with PE in the segmental or subsegmental artery was 12.0% and the mortality of patients with PE in the main lung arteries or lobar arteries was 16.9%. There was no statistical difference in prognosis among patients with PE at different locations. Both groups had significantly poorer survival than those without PE. Therefore, timely detection of PE in patients with COPD and performing active treatment is very important. Currently, there is controversy over whether to adopt an active diagnosis to screen for PE in patients with AECOPD. Proposals include the use of CTPA in all patients with AECOPD18,21,22,29 and the fairly judicious use of CTPA.13,15,19,20,30,31 Those who opt for a more restricted approach point out that universal screening for all patients increases radiation exposure, the risk of kidney disease induced by contrast, and increases the economic burden of patients. However, if CTPA is not performed, patients with PE in the segmental or subsegmental artery will be underdiagnosed. Accordingly, anticoagulant therapy is not administered to this subset of patients, which will lead to a worse prognosis. To our knowledge, this is the largest prospective study that has assessed the prevalence and prognosis of VTE in hospitalized COPD patients with acute exacerbations. This study provides a valid and reliable prevalence and prognosis estimate of VTE in the given setting.
As all subjects in our study had COPD, all were assessed as being in the moderate-risk group according to the Simplified Pulmonary Embolism Severity Index (sPESI); the traditional risk stratification of sPESI for PE was not appropriate for COPD patients with PE. Therefore, this study did not conduct risk stratification for those patients with PE.
Our study has several limitations. First, patients with mild acutely worsening respiratory symptoms who were not admitted to hospital were not included in this study, and patients for whom CTPA is contraindicated were also excluded; therefore, these two types of patients are likely underrepresented in our cohort. Second, this study is a multicenter study, and there may have been differences in the interpretation of laboratory examination results and imaging results across different research centers. Third, although the vast majority of clinical parameters were collected in >99% patients, some of the laboratory parameters could not be provided in a percentage of patients, eg, 4.7% patients did not have detailed lung function results. Fourth, owing to the impact of the pandemic, some patients could not maintain a regular and timely follow-up schedule, because of which compliance of anticoagulant therapy decreased that could have affected the prognosis of patients with VTE. However, our study included 11 research centers and was able to provide reliable information on the “real world” management of these patients.
Our study’s strengths include the large sample size and multicenter study design, making it the largest cohort thus far to assess the prevalence of VTE in patients with AECOPD. Prospective data collection ensured the reliability of the study data. Second, all enrolled patients underwent CTPA and Doppler ultrasound of the lower limb, making the results of the research on the of prevalence of VTE more objective. Third, our study provided better patient profiling and 1-year mortality information of COPD patients with VTE than other previous studies.
Conclusion
In conclusion, VTE is common in COPD patients and is associated with poor prognosis, and the prevalence of PE is higher than the prevalence of DVT in patients with AECOPD. Patients with PE in the segmental or subsegmental arteries as well as those with PE in the main pulmonary arteries or lobar arteries have poorer prognosis than patients without PE. Therefore, it is necessary to implement an active screening strategy using CTPA, especially for AECOPD patients with history of VTE, cor pulmonale, less purulent sputum, increased respiratory rate, higher D-dimer, and higher NT-proBNP/BNP, and adopt appropriate anticoagulation treatment for patients with AECOPD.
Acknowledgment
The authors would appreciate the following hospitals and responsible persons for their efforts. Capital Medical University Daxing Teaching Hospital, Capital Medical University: Prof. Ying Zhao; Beijing Sixth Hospital: Prof. Hong Wang and Jing Wang; The First Affiliated Hospital of Nanchang University: Prof. Xiuhua Kang; Shenzhen People’s Hospital: Prof. Yingyun Fu; Peking University International Hospital: Prof. Shuang Liu.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Disclosure
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed January 10, 2021.
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/s0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53(3):128–149. doi: 10.1016/j.arbres.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Borvik T, Braekkan SK, Enga K, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47(2):473–481. doi: 10.1183/13993003.00402-2015 [DOI] [PubMed] [Google Scholar]
- 5.Keramidas G, Gourgoulianis KI, Kotsiou OS. Venous thromboembolic disease in chronic inflammatory lung diseases: knowns and unknowns. J Clin Med. 2021;10(10):10. doi: 10.3390/jcm10102061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong F, Huang K, Ren X, et al. Factors associated with inpatient length of stay among hospitalised patients with chronic obstructive pulmonary disease, China, 2016–2017: a retrospective study. BMJ Open. 2021;11:2. doi: 10.1136/bmjopen-2020-040560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aleva FE, Voets LWLM, Simons SO, de Mast Q, van der Ven AJAM, Heijdra YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD a systematic review and meta-analysis. Chest. 2017;151(3):544–554. doi: 10.1016/j.chest.2016.07.034 [DOI] [PubMed] [Google Scholar]
- 8.Sato R, Hasegawa D, Nishida K, Takahashi K, Schleicher M, Chaisson N. Prevalence of pulmonary embolism in patients with acute exacerbations of COPD: a systematic review and meta-analysis. Am J Emerg Med. 2021;50:606–617. doi: 10.1016/j.ajem.2021.09.041 [DOI] [PubMed] [Google Scholar]
- 9.Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;2:199–207. doi: 10.1001/jama.295.2.199 [DOI] [PubMed] [Google Scholar]
- 10.Klok FA, Mos ICM, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131–2136. doi: 10.1001/archinte.168.19.2131 [DOI] [PubMed] [Google Scholar]
- 11.Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317–2327. doi: 10.1056/NEJMoa052367 [DOI] [PubMed] [Google Scholar]
- 12.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 13.Couturaud F, Bertoletti L, Pastre J, et al. Prevalence of pulmonary embolism among patients with COPD hospitalized with acutely worsening respiratory symptoms. JAMA. 2021;325(1):59–68. doi: 10.1001/jama.2020.23567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dentali F, Pomero F, Di Micco P, et al. Prevalence and risk factors for pulmonary embolism in patients with suspected acute exacerbation of COPD: a multi-center study. Eur J Intern Med. 2020;80:54–59. doi: 10.1016/j.ejim.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Hassen MF, Tilouche N, Jaoued O, Elatrous S. Incidence and impact of pulmonary embolism during severe COPD exacerbation. Respir Care. 2019;64(12):1531–1536. doi: 10.4187/respcare.06661 [DOI] [PubMed] [Google Scholar]
- 16.Pang H, Wang L, Liu J, et al. The prevalence and risk factors of venous thromboembolism in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(11):2573–2580. doi: 10.1111/crj.12959 [DOI] [PubMed] [Google Scholar]
- 17.Bahloul M, Chaari A, Tounsi A, et al. Incidence and impact outcome of pulmonary embolism in critically ill patients with severe exacerbation of chronic obstructive pulmonary diseases. Clin Respir J. 2015;9(3):270–277. doi: 10.1111/crj.12131 [DOI] [PubMed] [Google Scholar]
- 18.Akpinar EE, Hosgun D, Akpinar S, Atac GK, Doganay B, Gulhan M. Incidence of pulmonary embolism during COPD exacerbation. J Bras Pneumol. 2014;40(1):38–45. doi: 10.1590/s1806-37132014000100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi K-J, Cha S-I, Shin K-M, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration. 2013;85(3):203–209. doi: 10.1159/000335904 [DOI] [PubMed] [Google Scholar]
- 20.Ristic L, Rancic M, Radovic M, Ciric Z, Kutlesic Kurtovic D. Pulmonary embolism in chronic hypoxemic patients with and without secondary polycythemia--analysis of risk factors in prospective clinical study. Med Glas. 2013;10(2):258–265. [PubMed] [Google Scholar]
- 21.Tillie-Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med. 2006;144(6):390–396. doi: 10.7326/0003-4819-144-6-200603210-00005 [DOI] [PubMed] [Google Scholar]
- 22.Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35(6):1243–1248. doi: 10.1183/09031936.00120909 [DOI] [PubMed] [Google Scholar]
- 23.Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253–260. doi: 10.1007/s10654-010-9435-7 [DOI] [PubMed] [Google Scholar]
- 24.Fu X, Zhong Y, Xu W, et al. The prevalence and clinical features of pulmonary embolism in patients with AE-COPD: a meta-analysis and systematic review. PLoS One. 2021;16:9. doi: 10.1371/journal.pone.0256480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard P, Sanchez O, Leroyer C, et al. Deep venous thrombosis in patients with acute pulmonary embolism - prevalence, risk factors, and clinical significance. Chest. 2005;128(3):1593–1600. doi: 10.1378/chest.128.3.1593 [DOI] [PubMed] [Google Scholar]
- 26.Fletcher-Sanfeliu D, Redon J, Garcia-Granero A, et al. Pulmonary thrombosis in situ': risk factors, clinic characteristics and long-term evolution. Blood Coagulation Fibrinolysis. 2020;31(7):469–475. doi: 10.1097/mbc.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 27.Davoodi M, Rezvankhah B, Gohari Moghadam K, Hashemi Taheri AP. The prevalence and predicting factors of pulmonary thromboembolism in patients with exacerbated chronic obstructive pulmonary disease. Adv Respir Med. 2018;86(4):168–171. doi: 10.5603/ARM.a2018.0025 [DOI] [PubMed] [Google Scholar]
- 28.Cao Y-Q, Dong L-X, Cao J. Pulmonary embolism in patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J. 2018;131(14):1732–1737. doi: 10.4103/0366-6999.235865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T-S, Su X-L, Mao Y-M, Sun Y-X. Pulmonary embolism in patients with chronic obstructive pulmonary disease and exacerbations of unknown origin. Asian Biomed. 2013;7(4):529–535. doi: 10.5372/1905-7415.0704.208 [DOI] [PubMed] [Google Scholar]
- 30.Rutschmann OT, Cornuz J, Poletti P-A, et al. Should pulmonary embolism be suspected in exacerbation of chronic obstructive pulmonary disease? Thorax. 2007;62(2):121–125. doi: 10.1136/thx.2006.065557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapira-Rootman M, Beckerman M, Soimu U, Nachtigal A, Zeina A-R. The prevalence of pulmonary embolism among patients suffering from acute exacerbations of chronic obstructive pulmonary disease. Emerg Radiol. 2015;22(3):257–260. doi: 10.1007/s10140-014-1280-7 [DOI] [PubMed] [Google Scholar]