Abstract
Between 10% and 20% of patients with cancer-related pain cannot achieve adequate control following the three-step ladder guidelines by the World Health Organization. Therefore, a “fourth step”, including interventional approaches, has been suggested for those cases. Systematic reviews support the early use of interventional procedures to treat refractory cancer pain, control symptoms and prevent opioid dose escalation. There is strong evidence of the efficacy of celiac plexus or splanchnic neurolysis, vertebroplasty, kyphoplasty and intrathecal drug delivery. Those procedures have been found to be associated with a decrease in the symptom burden and opioid consumption, improved quality of life, and suggested as having a potentially positive impact on survival. Several studies have recommended using specific interventional techniques at earlier stages, possibly even when opioid treatment is first being considered. Conversely, leaving these options as a last analgesic resource might not be advisable since the burden these procedures might impose on too ill patients is significant. The objective of this review was to collect the available evidence published on the use of interventional treatments for refractory cancer pain with a particular interest in comparing early versus late indications. The results of the search demonstrated a very low number and quality of articles particularly addressing this question. This scarce number of evidence precluded performing a systematic analysis. A detailed and narrative description of the potential benefits of integrating interventional techniques into clinical guidelines at the early stages of the disease is provided.
Keywords: cancer pain, interventional pain, neurolysis, cement augmentation, intrathecal drug delivery, early, timing
Introduction
Adequate pain control in any of the stages of cancer is critical to the diagnosis, patients quality of life and survival.1–3 The failure to obtain good pain or symptom relief with strong opioids and coadjuvants prompted the inclusion of a fourth step to the World Health Organization (WHO) analgesic ladder, which includes interventional approaches.4 According to previous research, the procedure bearing the best evidence is the Celiac Plexus Neurolysis (CPN) with a recommendation of 2A+.5 Confirming the above, the latest systematic review and meta-analysis on the efficacy of CPN for pancreatic cancer-related abdominal pain demonstrated significant, albeit short termed, benefits of performing the neurolysis and using standard conventional medical management (CMM).6 Other procedures, such as splanchnic neurolysis, vertebroplasty or kyphoplasty, and intrathecal drug delivery, also received positive recommendations, bearing lower, albeit substantial, scientific evidence.5
The findings of both evidence-based and experience-based medicine seem to point towards suggesting interventional procedures in patients with refractory cancer pain syndromes. In centers where interventional procedures are integrated into routine cancer pain care, the oncology team naturally identify candidates at earlier stages based on previous experiences with patients obtaining good results (authors’ personal experience). Performing interventional procedures at earlier stages, rather than as a last resort is systematically, albeit unjustifiably, suggested in review articles about interventional cancer pain.5 Lack of solid evidence prevents this suggestion from being adopted as routine care, being sufficiently funded, and integrated into clinical guidelines.
The objective of this topical review was to summarize the data and findings from studies on interventional treatments for refractory cancer pain with a particular interest in comparing early versus late indications.
Methods
A narrative review based on a non-systematic literature search of articles listed in PubMed published between January 2000 until June 2023. Articles were identified by the following key words: “Cancer Pain”; “Intractable Pain”; “Nerve Block”; “Radiofrequency Ablation”; “Spinal Injections”; “Implantable Infusion Pumps”; “Vertebroplasty” or “Kyphoplasty”.
To focus the search on the timing of these interventional cancer pain procedures, each article identified was accessed and with a search tool, words such as “timing”, “early” or “late” were searched within the manuscript. Those articles fulfilling the above were the ones included in this review.
Results
Results are presented into three categories: a) Peripheral procedures, b) Vertebral augmentation procedures, and c) Intraspinal drug delivery.
Peripheral Procedures
Studies reviewed addressed early versus late indications using two different perspectives: (1) The diagnosis timing (ie, early vs late cancer stages),7 and (2) The intervention timing concerning the severity of the symptoms and the need for analgesics.8 As can be seen in Table 1, sympathectomies (eg celiac plexus, see Figure 1 and superior hypogastric plexus, see Figure 2) at early cancer stages have been compared with “no intervention” or “placebo” treatments. For example, Okuyama et al compared intraoperative celiac plexus block versus pharmacological therapy alone at the early stages of cancer. The data from this small case series suggest that intraoperative celiac plexus block provided long-lasting pain control, reducing opioid consumption and the adverse effects of high opioid doses.9 Staats et al10 studied patients with pancreatic cancer receiving CPN with alcohol during laparotomy. This randomized placebo-controlled trial showed that intraoperative CPN was associated with a meaningful reduction in pain intensity, reduced pain interference, and increased mood scores and life survival.
Table 1.
Summary of Articles Comparing Early vs Late
| 1st Author and Year | Early Procedure Group –Early Diagnosis | Late Comparison Group | Outcomes Favoring Procedure Group |
|---|---|---|---|
| Okuyama 20029 | Intraoperative celiac plexus alcoholization during exploratory laparoscopy + CMM | Only exploratory laparoscopy + CMM | Lower opioid consumption |
| Staats 200110 | Intraoperative celiac plexus alcoholization during exploratory laparotomy + CMM | Intraoperative celiac plexus placebo injection during exploratory laparotomy + CMM | Lower pain severity Lower pain-related interference Improved mood scores Prolonged survival |
| Wyse 201111 | Celiac plexus alcoholization during diagnostic endoscopic ultrasound exam + CMM | Only diagnostic endoscopic ultrasound exam + CMM | Lower pain scores |
| 1st Author and Year | Early Procedure Group –Before Strong Opioids | Late Comparison Group | Outcomes Favoring Procedure Group |
| Amr 20138 | Celiac plexus neurolysis for patients with severe pain treated with NSAIDs, Acetaminophen and <400mg Tramadol | Celiac plexus neurolysis for patients with mild pain treated with strong opioids | Lower pain scores Slower escalation of narcotics Better QoL |
| Amr 201412 | Visceral sympathetic alcoholization for abdominopelvic cancer pains treated with non-opioids | Visceral sympathetic alcoholization for abdominopelvic cancer pains treated with opioids | More responders Lower subsequent opioid consumption Superior QoL |
| 1st Author and Year | Early Procedure Group –Low Dose Morphine | Late Comparison Group | Outcomes Favoring Procedure Group |
| de Oliveira 200413 | Visceral sympathetic alcoholization for abdominopelvic cancer pains treated with <90mg MEDD | Visceral sympathetic alcoholization for abdominopelvic cancer pain treated with >90mg MEDD | No significant differences between groups |
Abbreviations: CMM, Conventional Medical Management; NSAIDs, Non-steroidal Anti-inflammatory Drugs; QoL, Quality of Life; MEDD, Morphine Equivalence Daily Dose in milligrams.
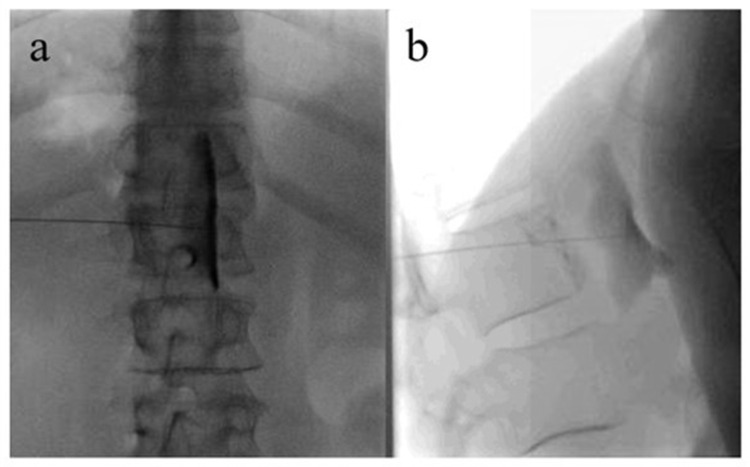
Figure 1.
Celiac plexus neurolysis in a patient with pancreatic cancer-related abdominal pain.
Notes: Anterio-posterior (a) and lateral (b) view of a needle placed anterior to the abdominal aorta. Contrast delineates the anterior margin of the Aorta and the exit of the celiac trunk.
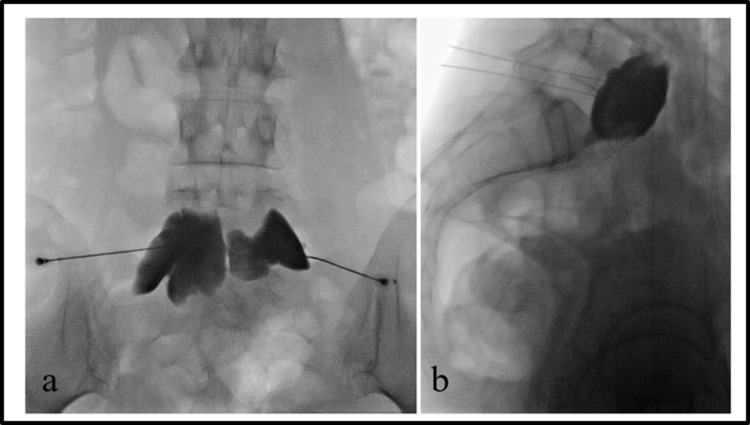
Figure 2.
Superior hypogastric plexus neurolysis in a patient with ovarian cancer-related pelvic pain.
Notes: Anterio-posterior (a) and lateral (b) view of two needles placed anterior to the inferior aspect of the L5 vertebral body. Contrast covers the entire surface of the ventral surface of L5 vertebral body.
Wyse et al11 compared early endoscopic ultrasound-guided celiac plexus neurolysis with conventional treatment. This randomized, double-blinded, controlled trial evaluated pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. Three months after a combined diagnostic-therapeutic endoscopic ultrasound guided CPN, patients reported a significant reduction in pain intensity. There were no significant differences in opioid consumption, quality of life and survival times.
Studies comparing early vs late interventional treatments have evaluated the effects of sympathectomies used before strong opioids or escalation to high doses. For example, Amr et al12 evaluated the effects of performing a CPN before or after controlling severe pain with medications. Patients with severe pancreatic pain (>7/10 on a numerical rating scale, NRS), poorly responding to tramadol, were randomly allocated to receiving a CPN with alcohol immediately upon initial assessment or waiting until CMM with morphine reduced their pain to an NRS<4. Patients who received the celiac bloc after controlling their severe pain with medication had better pain control, required fewer opioids, and reported improved quality of life compared with those receiving the celiac block at the beginning, followed by pharmacotherapy for pain relief.
de Oliveira et al13 compared the effects of early versus late neurolytic sympathetic plexus block on managing abdominal or pelvic cancer pain with an eight-week follow-up. The first group of patients underwent neurolytic blocks of the celiac plexus, the superior hypogastric plexus, or a lumbar sympathetic chain with alcohol if using NSAID and a weak oral opioid or morphine </=90 mg/day and reporting NRS>/=4 (group I). The second group of patients received sympathectomies when using NSAID and morphine at more than 90 mg/day and reporting NRS>/=4 (group II). The third group of patients received only pharmacological therapy (group III). The patients of groups I and II showed a significant reduction in pain intensity, opioid consumption, and better quality of life than those of group III. There were no significant differences between groups I and II. Opioid-related adverse effects were significantly higher in group III. The authors concluded that sympathetic neurolysis should be considered earlier in the treatment of the disease.
Bhatnagar et al14 described early bedside ultrasound-guided sympathetic neurolysis in a case series of patients with abdominopelvic cancer pain receiving less than 30 mg morphine equivalent. The NRS score decreased from 6 ± 0.7 before the block to 2 ± 1.3 after the block and participants significantly reduced their opioid consumption at two months post neurolysis.
Early vs late sympathectomy was also studied in opioid naïve patients compared with those requiring opioids for pain control. Amr et al12 evaluated 109 patients with inoperable abdominopelvic cancer, with moderate-to-severe pain (NRS 4–7), that received nonopioid analgesics. Patients were randomly assigned to two groups. In one group (Group early), patients underwent a sympathectomy before receiving medications from Step 2 of the WHO ladder. Patients of the Group Late initially received analgesics according to the WHO ladder, and when strong opioids failed to control pain, a sympathectomy was performed. The proportion of patients with adequate pain control at 12 months was higher in patients receiving an early sympathectomy. Moreover, patients receiving an early sympathectomy consumed fewer opioids and reported fewer adverse effects. In addition, the proportion of patients having adequate pain control with Tramadol increased during the first five months after an early sympathectomy. As well, the quality of life of patients receiving an early sympathectomy improved until the fifth month after the intervention.
Vertebral Augmentation Procedures (VAP)
Multiple myelomas and metastatic cancers can produce spine instability and vertebral compression fractures. Conventional analgesia, bed rest or bracing are often associated with pain, limiting mobility and self-care. Surgery is not usually an easy option for advanced cancer.15
Evidence from systematic reviews and one metanalysis supports the use and efficacy of vertebroplasty (see visual example in Figure 3) and kyphoplasty in cancer patients with vertebral compression fractures. Vertebral augmentation reduced pain intensity during the initial days after the procedures, opioid consumption, and functional disability related to pain. The benefits of symptom control and the low rates of complication are consistent independently of the cancer populations and the level of vertebral fractures.16,17
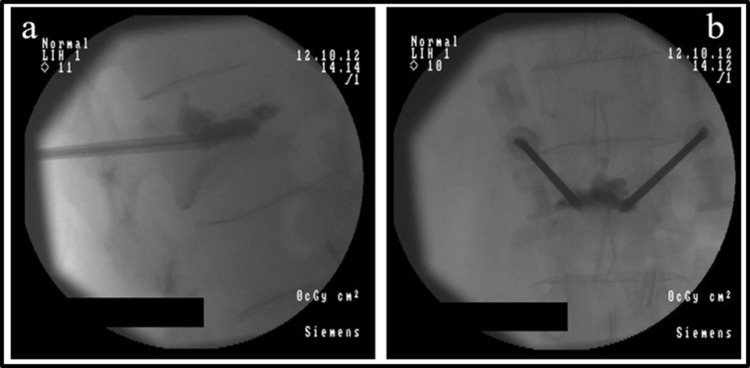
Figure 3.
Percutaneous vertebroplasty in a patient with low back pain related to vertebral fracture.
Notes: Anterio-posterior (a) and lateral (b) view of two needles placed inside L2 vertebral body and injection of radio-opaque acrylic cement in the center of the vertebral collapse.
The International Multiple Myeloma (MM) Working group concluded in their consensus17 that the timing of the treatment with VAP in patients impacts patient-reported outcomes. Compared to non-interventional alternatives, patients undergoing early VAP reported significantly lower back pain, functional recovery, and lower need for analgesic medication, which is essential in MM patients with renal impairment.18
Despite some conflicting controversy on the indication of cement augmentation treatment for osteoporotic-related vertebral fractures,19 the available evidence in non-cancer cases similarly favours early management in pain management.20 Benefits involve patient-reported outcomes and a significant reduction in morbidity and mortality.21 Additional benefits found in cancer pain patients suggest that VPA is cost-effective.22
Aside from being performed early to manage pain better, VPA could have a potential role when performed preventively in cancer patients diagnosed with vertebral metastases at risk of developing pathological fractures. The role of long-term consolidation of vertebral metastases after VPA was assessed in a retrospective cohort of patients. After a mean follow-up of 3.1±2.1 years, group comparison revealed a significantly higher rate of pathological fracture of vertebral metastases in the non-VAP group.23
Intraspinal Drug Delivery (IDD)
Targeted spinal drug administration (see Figure 4) is part of the 4th step of the WHO ladder and is traditionally considered the last analgesic option to manage cancer-related pain when other alternatives fail. However, no studies ever compared IDD use at early or late cancer stages.
Figure 4.
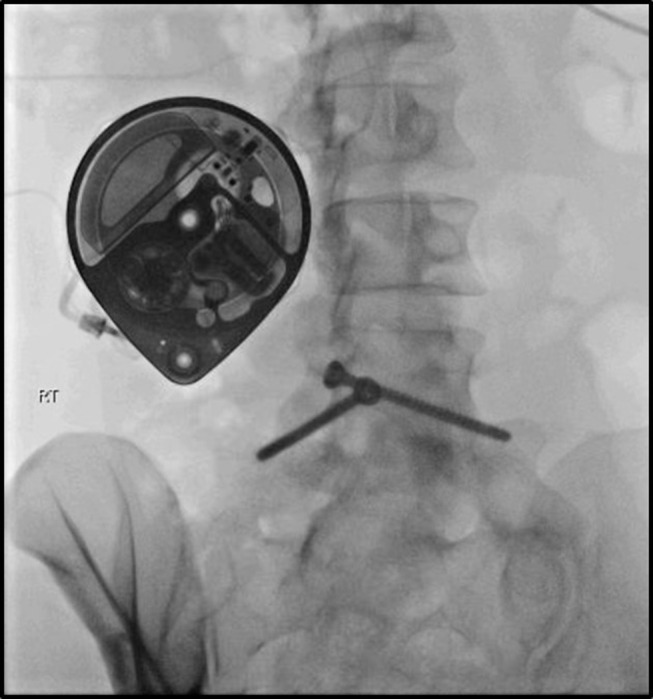
Intrathecal catheter connected to an implanted pump.
The benefits of IDD for cancer pain management have been compared with CMM. Smith et al24 randomized 202 patients with moderate-to-severe cancer pain (NRS >/= 50/100) to receive CMM or IDDS plus CMM. A significant clinical benefit was defined as >/= 20% reduction in NRS scores or equal scores with >/= 20% reduction in toxicity four weeks after randomization. The primary outcome measure was a combined measure of pain intensity and reduction in toxicity. Patients receiving IDD were more likely to have meaningful benefits (84% vs 71%) and a reduction in the toxicity scores (50% IDD vs 17% on CMM) in contrast with those patients who received only CMM. Additionally, patients receiving IDD reported less fatigue and attention interference. Finally, the survival at six months of patients receiving IDD was 54% compared with 37% of those who received only CMM.24 A subsequent analysis of participants “as per treated” (all patients receiving IDD including those crossing over from CMM vs only receiving CMM) revealed that 32% of the patients in the group randomized to CMM that did not cross over to IDD were alive at 6 months. This was a lower survival than the 59% of patients randomized to CMM who received IDD and were alive at six months.25
Sindt et al,26 retrospectively, evaluated the opioid consumption of 173 patients with advanced cancer receiving IDD. Most patients were receiving >200 mg/day of oral morphine equivalents before implant. One month after the IDD implant, 83% of patients discontinued systemic opioids, and 11% reduced the doses to <100 mg daily morphine equivalents.
Patel et al27 described the perspective of family carers of adult patients receiving IDD analgesia for cancer pain. The findings showed that carers considered IDD beneficial when it enabled the patients to be themselves through their final illness. Carers valued quality of life improvements at the end of life by alleviating pain and reducing side effects of CMM.
Patients with IDD incur lower medical utilization and payments during the first year after implant, independently of the initial cost of IDD,28 including hospital admissions, consultation to emergency services and drug expenses.29 Brogan et al,30 retrospectively, evaluated the charts of 36 patients with cancer pain who survived more than four weeks after receiving IDD. The authors modeled the factors associated with the rapid achievement of cost-benefit with IDD. The cost of CMM before IDD and at 4–6 weeks were collected and projected over time. IDD costs included all intrathecal pump implantation and maintenance costs. The cost-benefit compared with conventional therapy was predicted at 344 months. However, the cost-benefit in patients receiving high-dose, non-generic medications or intravenous patient-controlled analgesia before the IDD was achieved at 7.4 months. The use of parenteral therapy, high-dose opioids, and non-generic opioids was associated with earlier attainment of IDD cost-benefit.30
Health Quality Ontario in Canada evaluated the cost burden for IDD’s publicly funded health care system for cancer pain.31 This study included one randomized trial examining the effectiveness and harms of IDD and one case series that reported economic evaluation. Authors concluded that IDD systems are more cost-effective than high-cost oral therapy if administered for at least seven months. However, a conclusion regarding the cost-effectiveness of IDD systems compared with current standards of care for managing refractory cancer pain in adults could not be established for the Canadian health system. Authors also suggested that using IDD for cancer pain would result in a significant increase on the public health system budget.
Patient-reported outcomes, survival and cost-effectiveness are complex to analyze in cancer patients. Nevertheless, IDD is recommended for patients with cancer pain where conservative interventions have failed or are contraindicated. Additionally, IDD is indicated when pain is causing a significant physical and mental health impact independently of life expectancy. However, a minimal proportion of patients with refractory cancer pain received IDD despite the recommendation for its use. Duarte et al32 evaluated the impact of the 2015 National Health System England Clinical Commissioning Policy on the use of IDD pumps for managing cancer pain in England. The authors evaluated the Hospital Episode Statistics of all patients receiving IDD to manage cancer pain between 2014 and January 2020. Additionally, they estimated the number of patients with cancer potentially eligible to receive an IDD pump during the same period. The increase in the number of cancer patients potentially suitable for an IDD did not match the number of IDD implants. Only 30 of 800 patients with cancer eligible for an IDD received the implant between April 2018 and March 2019.
The International Neuromodulation Society consensus33 suggest indication of IDD in cases of intolerable cancer-related pain, refractory to CMM with an appropriate life expectancy.
A recent review34 suggested that IDD called for a change in the paradigm of cancer pain treatment. Given the underuse rate, the potential benefits, and the risks associated with oral opioid medication, IDD should be indicated early in the treatment program. Several factors have been identified as predictors of successful outcomes of IDD initiation, including pre-implant lower opioid consumption,35 better performance status,36 preserved cognitive capabilities, optimized medical comorbidities, good nutritional status, and balanced emotional well-being. Those characteristics are more frequently identified in early rather than advanced cancer or end-of-life patients.37
Conclusions
The main goal in pain management for patients with cancer should be to improve function and comfort while reducing or avoiding the negative adverse effects of strong analgesic medications.38 For those 10–20% of patients with cancer pain refractory to strong opioids,39 the inclusion of a further interventional step has been suggested. Changing the WHO cancer pain ladder paradigm responds to the necessity to provide rapid and effective symptom relief in those cases where the standard approach fails.4,40
For some cancer-related pain syndromes such as intra-abdominal malignancies, the indication of interventional options has been sufficiently demonstrated, yet these procedures remain absent from routine clinical recommendations and guidelines. Insufficient scientific evidence; adequately trained interventional pain physicians; scarce healthcare resources, and poor interdisciplinary communication explain this absence from standard clinical pathways. In those centers where interventional cancer pain is integrated within an interdisciplinary care model, early interventional approaches to prevent symptom aggravation and opioid dose escalation become thus, routine.
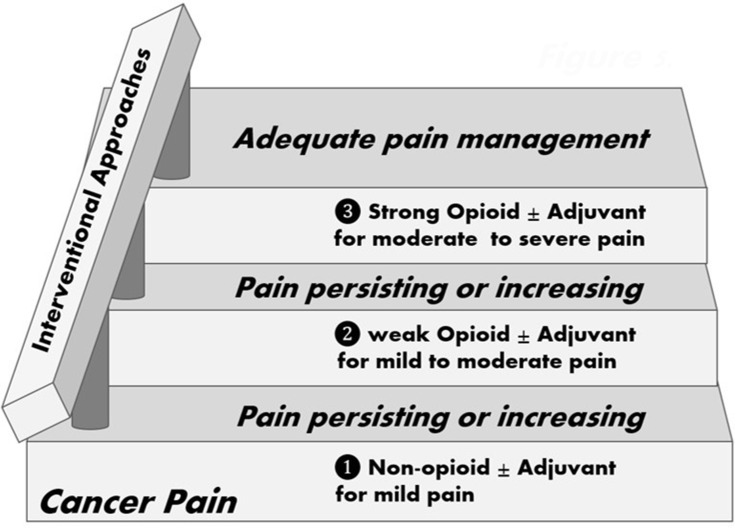
Based on evidence extracted from celiac plexus neurolysis for intra-abdominal cancer-related pain, interventional approaches, rather than a last 4th step resource, should be regarded as a handrail41 accompanying all three steps of the WHO ladder (see Figure 5). For all other interventional options mentioned in this review, evidence is only solid in suggesting the indication when compared to CMM, but no data is available regarding early versus late indication. Interestingly, those same authors providing the above mentioned evidences agree in stating in their conclusions that the sooner the better to perform these procedures based on the assumption that early cancer pain management reduces suffering and improves quality of life.
Figure 5.
Interventional approaches as the handrail to the WHO ladder.
Acknowledgments
Authors want to thank the Louise and Alan Edwards Foundation for their generous support to the Alan Edwards Pain Management Unit of the Montreal General Hospital, the Cancer Pain Clinic at the Cedars Cancer Center, and the Edwards Family Interdisciplinary Centre for Complex Pain of the Montreal Children Hospital, all three programs part of the McGill University Health Center. JM’s work is supported by ICREA Acadèmia, the Government of Catalonia (2017SGR-1321) and the Spanish Ministry of Science.
Ethics Statement
Permission for publication of clinical images was duly obtained from patients or their carers.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7(10):797–809. doi: 10.1038/nrn1914 [DOI] [PubMed] [Google Scholar]
- 2.Gagliese L, Gauthier LR, Rodin G. Cancer pain and depression: a systematic review of age-related patterns. Pain Res Manag. 2007;12(3):205–211. doi: 10.1155/2007/150126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miladinia M, Baraz S, Ramezani M, Malehi AS. The relationship between pain, fatigue, sleep disorders and quality of life in adult patients with acute leukaemia: during the first year after diagnosis. Eur J Cancer Care. 2018;27(1):e12762. doi: 10.1111/ecc.12762 [DOI] [PubMed] [Google Scholar]
- 4.Miguel R. Interventional treatment of cancer pain: the fourth step in the World Health Organization analgesic ladder? Cancer Control. 2000;7(2):149–156. doi: 10.1177/107327480000700205 [DOI] [PubMed] [Google Scholar]
- 5.Vissers KCP, Besse K, Wagemans M, et al. Pain in patients with cancer. Pain Pract. 2011;11(5):453–475. doi: 10.1111/j.1533-2500.2011.00473.x [DOI] [PubMed] [Google Scholar]
- 6.Okita M, Otani K, Gibo N, Matsui S. Systematic review and meta-analysis of celiac plexus neurolysis for abdominal pain associated with unresectable pancreatic cancer. Pain Pract. 2022;22(7):652–661. doi: 10.1111/papr.13143 [DOI] [PubMed] [Google Scholar]
- 7.Wyse JM, Chen YI, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20(9):2186–2192. doi: 10.3748/wjg.v20.i9.2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cancer pain - time effect: a prospective, randomized multicenter study. J Pain Symptom Manage. 2014;48(5):944–956.e2. doi: 10.1016/j.jpainsymman.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 9.Okuyama M, Shibata T, Morita T, et al. A comparison of intraoperative celiac plexus block with pharmacological therapy as a treatment for pain of unresectable pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002;9(3):372–375. doi: 10.1007/s005340200042 [DOI] [PubMed] [Google Scholar]
- 10.Staats PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain Med. 2001;2(1):28–34. doi: 10.1046/j.1526-4637.2001.002001028.x [DOI] [PubMed] [Google Scholar]
- 11.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound–guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29(26):3541–3546. doi: 10.1200/JCO.2010.32.2750 [DOI] [PubMed] [Google Scholar]
- 12.Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer. Clin J Pain. 2013;29(9):807–813. doi: 10.1097/AJP.0b013e3182757673 [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira R, Dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004;110(1–2):400–408. doi: 10.1016/j.pain.2004.04.023 [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar S, Khanna S, Roshni S, et al. Early ultrasound-guided neurolysis for pain management in gastrointestinal and pelvic malignancies: an observational study in a tertiary care center of urban India. Pain Pract. 2012;12(1):23–32. doi: 10.1111/j.1533-2500.2011.00467.x [DOI] [PubMed] [Google Scholar]
- 15.Health Quality Ontario. Vertebral augmentation involving vertebroplasty or kyphoplasty for cancer-related vertebral compression fractures: a systematic review. Ont Health Technol Assess Ser. 2016;16(11):1–202. [PMC free article] [PubMed] [Google Scholar]
- 16.Mattie R, Brar N, Tram JT, et al. Vertebral augmentation of cancer-related spinal compression fractures: a systematic review and meta-analysis. Spine. 2021;46(24):1729–1737. doi: 10.1097/BRS.0000000000004093 [DOI] [PubMed] [Google Scholar]
- 17.Kyriakou C, Molloy S, Vrionis F, et al. The role of cement augmentation with percutaneous vertebroplasty and balloon kyphoplasty for the treatment of vertebral compression fractures in multiple myeloma: a consensus statement from the International Myeloma Working Group (IMWG). Blood Cancer J. 2019;9(3):3. doi: 10.1038/s41408-019-0187-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papanastassiou ID, Vrionis FD. Is early vertebroplasty/kyphoplasty justified in multiple myeloma given the rapid vertebral fracture progression? Spine J. 2016;16(7):833–834. doi: 10.1016/j.spinee.2015.12.083 [DOI] [PubMed] [Google Scholar]
- 19.Buchbinder R, Busija L. Why we should stop performing vertebroplasties for osteoporotic spinal fractures. Intern Med J. 2019;49(11):1367–1371. doi: 10.1111/imj.14628 [DOI] [PubMed] [Google Scholar]
- 20.Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10052):1408–1416. doi: 10.1016/S0140-6736(16)31341-1 [DOI] [PubMed] [Google Scholar]
- 21.Lin JH, Chien LN, Tsai WL, Chen LY, Chiang YH, Hsieh YC. Early vertebroplasty associated with a lower risk of mortality and respiratory failure in aged patients with painful vertebral compression fractures: a population-based cohort study in Taiwan. Spine J. 2017;17(9):1310–1318. doi: 10.1016/j.spinee.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 22.Health Quality Ontario. Vertebral augmentation involving vertebroplasty or kyphoplasty for cancer-related vertebral compression fractures: an economic analysis. Ont Health Technol Assess Ser. 2016;16(12):1–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Delpla A, Tselikas L, de Baere T, et al. Preventive vertebroplasty for long-term consolidation of vertebral metastases. Cardiovasc Intervent Radiol. 2019;42(12):1726–1737. doi: 10.1007/s00270-019-02314-6 [DOI] [PubMed] [Google Scholar]
- 24.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040–4049. doi: 10.1200/JCO.2002.02.118 [DOI] [PubMed] [Google Scholar]
- 25.Smith TJ, Coyne PJ, Staats PS, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol. 2005;16(5):825–833. doi: 10.1093/annonc/mdi156 [DOI] [PubMed] [Google Scholar]
- 26.Sindt JE, Odell DW, Dalley AP, Brogan SE. Initiation of intrathecal drug delivery dramatically reduces systemic opioid use in patients with advanced cancer. Neuromodulation. 2020;23(7):978–983. doi: 10.1111/ner.13175 [DOI] [PubMed] [Google Scholar]
- 27.Patel N, Huddart M, Makins H, et al. ‘Was it worth it?’ Intrathecal analgesia for cancer pain: a qualitative study exploring the views of family carers. Palliat Med. 2018;32(1):287–293. doi: 10.1177/0269216317723777 [DOI] [PubMed] [Google Scholar]
- 28.Stearns LJ, Hinnenthal JA, Hammond K, Berryman E, Janjan NA. Health services utilization and payments in patients with cancer pain: a comparison of intrathecal drug delivery vs. conventional medical management. Neuromodulation. 2016;19(2):196–204. doi: 10.1111/ner.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stearns LJ, Narang S, Albright RE, et al. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open. 2019;2(4):e191549. doi: 10.1001/jamanetworkopen.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brogan SE, Winter NB, Abiodun A, Safarpour R. A cost utilization analysis of intrathecal therapy for refractory cancer pain: identifying factors associated with cost benefit. Pain Med. 2013;14(4):478–486. doi: 10.1111/pme.12060 [DOI] [PubMed] [Google Scholar]
- 31.Health Quality Ontario. Intrathecal drug delivery systems for cancer pain: a health technology assessment. Ont Health Technol Assess Ser. 2016;16(1):1–51. [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte RV, Sale A, Desai P, Marshall T, Eldabe S. The unmet need for intrathecal drug delivery pumps for the treatment of cancer pain in England: an assessment of the hospital episode statistics database. Neuromodulation. 2020;23(7):1029–1033. doi: 10.1111/ner.13264 [DOI] [PubMed] [Google Scholar]
- 33.Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96–132. doi: 10.1111/ner.12538 [DOI] [PubMed] [Google Scholar]
- 34.Dupoiron D, Duarte R, Carvajal G, Aubrun F, Eldabe S. Rationale and recent advances in targeted drug delivery for cancer pain: is it time to change the paradigm? Pain Physician. 2022;25(3):E414–E425. [PubMed] [Google Scholar]
- 35.Brogan SE, Sindt JE, Jackman CM, White J, Wilding V, Okifuji A. Prospective association of serum opioid levels and clinical outcomes in patients with cancer pain treated with intrathecal opioid therapy. Anesth Analg. 2020;130(4):1035–1044. doi: 10.1213/ANE.0000000000004276 [DOI] [PubMed] [Google Scholar]
- 36.Dupoiron D, Leblanc D, Demelliez-Merceron S, et al. Optimizing initial intrathecal drug ratio for refractory cancer-related pain for early pain relief. A retrospective monocentric study. Pain Med. 2019;20(10):2033–2042. doi: 10.1093/pm/pnz096 [DOI] [PubMed] [Google Scholar]
- 37.Gulati A, Puttanniah V, Hung J, Malhotra V. Considerations for evaluating the use of intrathecal drug delivery in the oncologic patient. Curr Pain Headache Rep. 2014;18(2). doi: 10.1007/s11916-013-0391-2 [DOI] [PubMed] [Google Scholar]
- 38.Syrjala KL, Jensen MP, Elena Mendoza M, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol. 2014;32(16):1703–1711. doi: 10.1200/JCO.2013.54.4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afsharimani B, Kindl K, Good P, Hardy J. Pharmacological options for the management of refractory cancer pain-what is the evidence? Support Care Cancer. 2015;23(5):1473–1481. doi: 10.1007/s00520-015-2678-9 [DOI] [PubMed] [Google Scholar]
- 40.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician. 2010;56(6):514–515. [PMC free article] [PubMed] [Google Scholar]
- 41.Hochberg U, Elgueta MF, Perez J. Interventional analgesic management of lung cancer pain. Front Oncol. 2017;7(FEB). doi: 10.3389/fonc.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]