Abstract
Non-alcoholic fatty liver disease (NAFLD), one of the most common causes of liver disease, is an increasingly common cause of hepatocellular carcinoma (HCC). Several demographic, clinical, and genetic factors contribute to HCC risk in NAFLD patients, which may inform risk stratification scores. Proven efficacious approaches to primary prevention approach in patients with non-viral liver disease remain an area of need. Semi-annual surveillance is associated with improved early tumor detection and reduced HCC-related mortality; however, patients with NAFLD have several challenges to effective surveillance, including under-recognition of at-risk patients, low surveillance utilization in clinical practice, and lower sensitivity of current tools for early-stage HCC detection. Treatment decisions are best made in a multidisciplinary fashion and are informed by several factors including tumor burden, liver dysfunction, performance status, and patient preferences. Although patients with NAFLD often have larger tumor burden and increased comorbidities compared to counterparts, they can achieve similar post-treatment survival with careful patient selection. Therefore, surgical therapies continue to provide a curative treatment option for patients diagnosed at an early stage. Although there has been debate about the efficacy of immune checkpoint inhibitors in patients with NAFLD, current data are insufficient to change treatment selection based on liver disease etiology.
Keywords: Non-alcoholic fatty liver disease, Liver cancer, Screening, Treatment
INTRODUCTION
Hepatocellular carcinoma (HCC), the most common type of primary liver cancer, is the third leading cause of cancerrelated death globally,1 and one of the few cancers with an increasing mortality rate.2 HCC mostly arises in a background of advanced chronic liver disease, with the primary etiology differing by geographic location.3 Chronic hepatitis B virus (HBV) infection is the most common underlying etiology of HCC in Asia and many parts of Africa, whereas chronic hepatitis C virus (HCV) infection, alcohol-associated liver disease, and nonalcoholic fatty liver disease (NAFLD) are primary drivers in the Western world.4 With hepatitis B vaccination and hepatitis C treatment programs becoming more widespread, the burden of HCC is increasingly related to non-viral etiologies including alcohol-associated liver disease and NAFLD.5
NAFLD, the hepatic manifestation of the metabolic syndrome, is a chronic disease encompassing a phenotypic spectrum of liver disease. This spectrum ranges from simple fatty infiltration, i.e., NAFL, to inflammatory cellular infiltration, i.e., nonalcoholic steatohepatitis (NASH), which may culminate in fibrosis, cirrhosis, and HCC.6,7 NAFLD is one of the most common causes of liver diseases and is rising further in synchrony with the obesity and metabolic syndrome epidemics across the globe.8 NAFLD currently accounts for approximately 20% of HCC, although this is anticipated to increase in most countries over the next decade.9,10 Herein, we comprehensively review epidemiology, diagnosis, and treatment of NAFLD-related HCC.
EPIDEMIOLOGY
The prevalence of NAFLD is highest in North America and Europe, with nearly one-third of people having NAFLD, including 64 million people in the United States. Of those, approximately 20% have progressive disease and develop cirrhosis. Patients with NASH cirrhosis have an annual HCC incidence of 1-2%, although more than one-fourth of NASH-related HCC occur without underlying cirrhosis.11,12 In fact, NASH is the leading cause of non-cirrhotic HCC. The relatively high prevalence of non-cirrhotic NASH-HCC is due to the large at-risk pool of patients with NASH, as the annual incidence of HCC among patients with non-cirrhotic NASH remains low. The incidence of HCC in NAFLD patients without cirrhosis is approximately 23-46 per 100,000 patient-years.13,14
NAFLD-related HCC is often diagnosed at more advanced stages, typically in context of poor or absent surveillance. Patients are also often older with more co-morbidities, which can preclude curative options such as resection or liver transplantation;15 however, etiology has not been identified as an independent prognostic factor.16
Although cirrhosis is the strongest risk factor for HCC in those with NAFLD, there is a wide variation in HCC risk between patients. Demographic, behavioral, and genetic factors play a role in HCC risk and may inform future risk stratification strategies to identify the highest risk subgroups who would most benefit from surveillance or chemoprevention efforts as well as low risk subgroups who may not warrant surveillance.17 HCC risk factors among those with NAFLD include older age, male sex, Hispanic ethnicity, and metabolic syndrome features like diabetes, obesity, and dyslipidemia.18,19 In those with NAFLD cirrhosis, diabetes is independently associated with increased HCC risk (hazard ratio [HR], 1.3; 95% confidence interval [CI], 1.0-1.7).20 Data from European and USA real-world studies with >18 million patients identified diabetes as the strongest independent metabolic risk factor for HCC.18,21 Among those with diabetes, glycemic control is associated with HCC risk, as patients with adequate glycemic control (HbA1C <7% for more than 80% of time) have a 32% lower risk of HCC than those with suboptimal glycemic control.22 Similarly, obesity and being overweight are associated with a 48-83% increased HCC risk, although some increased risk may be mediated by development of NASH.23
Genetic factors, such as the PNPLA3 148M variant at rs738409, have been found to be associated with disease severity and HCC development in those with obesity and histologically-proven NAFLD.24 The MBOAT7 rs641838 variant is associated with development and severity of NAFLD, via increased hepatic fat content and remodeling of hepatic phosphatidylinositol species.25 This single nucleotide polymorphism is twice as prevalent in NAFLD-HCC compared to NAFLD alone and may predispose to HCC in patients without cirrhosis.26
Clinical risk calculators have been proposed to help stratify NAFLD patients into high- and low-risk groups. For example, a risk calculator to assess HCC risk in patients with either NAFLD or alcoholic liver disease-related HCC incorporated seven predictors: age, gender, diabetes, body mass index, platelet count, serum albumin, and ratio of aspartate aminotransferase to alanine aminotransferase.27 Predicted and observed cumulative incidence curves exhibited overlap, and plotted decision curves showed screening based on risk models had a greater net benefit than a “screen-all” strategy.27 A 133-gene signature, prognostic liver signature (PLS)/-NAFLD, and its four-protein blood-based surrogate secretome signature PLSec-NAFLD may also serve as a NAFLDspecific HCC risk prediction tool.28 These signatures were validated in a global independent cohort of 409 patients with NAFLD, with high-risk etiology-specific PLSec-NAFLD scores significantly associated with HCC development.28 Most risk calculators have been evaluated among patients with NAFLD cirrhosis and similar work is needed to identify high-risk patients with non-cirrhotic NAFLD who may benefit from surveillance. Although clinical risk scores and this blood-based biomarker are promising and fill an area of need, they require further validation prior to routine use in clinical practice.29
PATHOGENESIS AND MOLECULAR ALTERATIONS
Immune system activation is closely related to progression of NAFLD into NASH and NAFLD-related HCC, with many examples of such immunologic cross-talks. Hepatic injury leads to activation of Kupfer cells and resident hepatic macrophages, which have also been implicated in HCC development.30 A decline in cell autophagy has been implicated in the progression of steatosis to NASH and HCC.31 Next-generation sequencing has uncovered cancer driver genes with either oncogenic or tumor suppressive functions that are altered in HCC. Mutations in specific genes such as RPS6KA3-AXIN1 and NFE2L2-CTNNB1 have suggested that Wnt/β-catenin signaling pathway may be implicated in HCC pathogenesis via oxidative stress metabolism and Ras/mitogen-activated kinase pathways.32 Using exome sequencing for better tumor characterization, Guichard et al.32 have identified groups of genes, such as CTNNB1 and TP53 which are related to the risk factors alcohol and HBV, respectively. CTNNB1 and TP53 mutations affect 25-30% of HCC patients, and alongside less frequently mutated genes such as AXIN1, RPS6kA3, TSC1/TSC2, MLL2, ARID2, ARID1A, and KEAP1 help elucidate the main dysregulated pathways in HCC.33 Senescence-associated secretory phenotype (SASP) has been shown to promote obesity-associated HCC in mice, and signs of the same SASP has been observed in hepatic stellate cells of patients with NASH-HCC indicating that a similar pathway may be present in humans.34
PRIMARY PREVENTION
Several risk factors for NASH-related HCC inform potential primary prevention strategies. Targeting components of metabolic syndrome via treating obesity, diabetes, and hypertension can prevent fibrosis and thereby theoretically reduce HCC risk.35 Weight loss strategies that reduce total body weight exceeding 10% have the most substantial effect in addressing NAFLD.36 First-line obesity management includes calorie restricted meals compounded by physical activity.37 Weight loss not only reduces and reverses NAFLD, but also alters the “liver-gut” axis rendering the gut microbiome less implicated in liver fibrosis and HCC development.38 A propensity matched study revealed that bariatric surgery among obese patients was associated with lower incidence of NASH and HCC in a 7-year follow-up period.39 However, there are no data on weight loss leading to HCC risk reduction among NASH patients specifically. Amongst patients with diabetes, treatment with thiazolidinediones and metformin have also been associated with significant reductions in HCC risk.40,41 Statins may serve as another method of chemoprevention, with a meta-analysis of 24 studies (n=59,073 patients) demonstrating decreased HCC risk by 46%.42 Lipophilic statins were associated with a greater HCC risk reduction compared to hydrophilic counterparts,43 which might be explained by increased permeability of lipophilic substances that allows them to exert their cholesterol-dependent effects on HCC development.44 However, professional society guidelines advise against use of these medications solely for HCC chemoprevention outside of other indications.
Outside of specific chemoprevention strategies for NASH-related HCC, coffee has also shown promising results. A meta-analysis of 13 prospective cohort and case-control studies showed that increasing coffee consumption by one cup daily was associated with 15% HCC risk reduction (relative risk [RR], 0.85; 95% CI, 0.82-0.88).45 The potential benefit of coffee might be partly explained by reduced fibrosis, with a meta-analysis of 11 studies demonstrating a lower risk of NAFLD (pooled RR, 0.77; 95% CI, 0.60-0.98) and lower risk of fibrosis among NAFLD patients (RR, 0.68; 95% CI, 0.68-0.79) among coffee users than non-users.46
SURVEILLANCE
The NAFLD patient population presents unique challenges for HCC surveillance and necessitates novel potential solutions (Fig. 1). Professional society guidelines, including those from European Association for the Study of Liver (EASL), American Association for the Study of Liver Diseases (AASLD), and Asian Pacific Association for the Study of the Liver recommend HCC surveillance in at-risk populations.47-49 The best data for HCC surveillance continues to come from a randomized clinical trial from China among >18,000 persons with underlying HBV infection.50 Although Level I data does not exist in patients with cirrhosis, a meta-analysis of cohort studies demonstrated a strong and consistent association between HCC surveillance and improved clinical outcomes including increased early detection and reduced HCC-related mortality.51,52 Although there are few studies exclusively in patients with NASH cirrhosis, results appear consistent in subgroup analyses by proportion of NASH patients in each study.
Figure 1.
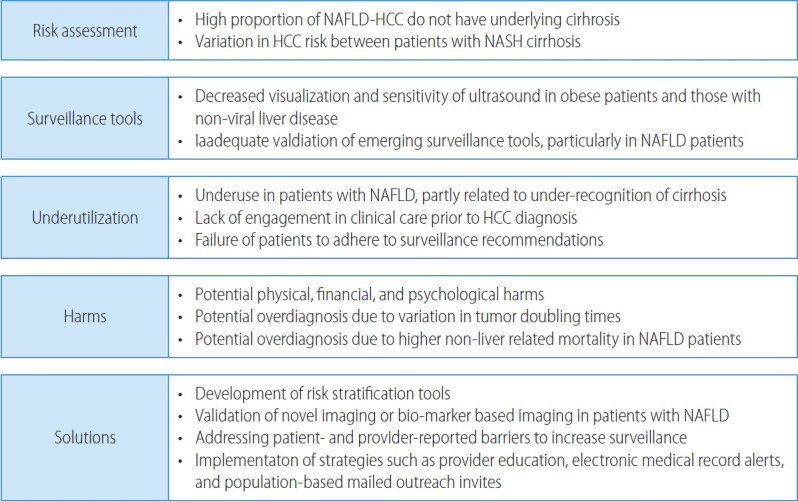
Challenges and potential solutions for HCC surveillance in NAFLD patients. NAFLD, non-alcoholic fatty liver disease; HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis.
Primary populations at risk for HCC include subgroups of patients with high-risk HBV infection and those with cirrhosis from any etiology. Although patients with NASH-related cirrhosis have a lower annual HCC incidence than those with viral-related cirrhosis, their risk exceeds 1% per year and HCC surveillance remains cost-effective.18 In contrast, patients with non-cirrhotic NAFLD have a low annual risk of HCC and decision analyses suggest surveillance is not cost-effective in this population. In a multi-center study from the Veterans Health Administration, HCC incidence was only 0.08 per 1,000 person-years among patients with non-cirrhotic NAFLD. Although there was variation in HCC incidence between subgroups, none demonstrated sufficiently high risk to warrant HCC surveillance.18 A systematic review similarly found HCC incidence at 10 years in NAFLD patients without cirrhosis was only 2.7% compared to 15% for those with cirrhosis.53 Although HCC surveillance is not recommended in all patients with non-cirrhotic NAFLD, the high proportion of HCC arising in the absence of cirrhosis underscores a need for risk stratification tools.
Abdominal ultrasound with or without alpha fetoprotein (AFP) have been the most widely recommended tests for HCC surveillance.47,48,54 However, this strategy misses over one-third of HCC at an early stage, with a meta-analysis demonstrating a pooled sensitivity of only 63%.54 Patients with obesity and those with non-viral liver disease, including NAFLD, have lower ultrasound visualization and sensitivity for early-stage HCC.55 In a cohort of 2,053 patients with cirrhosis, limited visualization was observed in 368 (18.0%) and was independently associated with NAFLD (odds ratio [OR], 2.13; 95% CI, 1.51-3.00) or alcohol-associated (OR, 1.74; 95% CI, 1.25-2.43) cirrhosis and obesity class II (OR, 1.69; 95% CI, 1.06-2.67) or class III (OR, 4.35; 95% CI, 2.82-6.71).56 Limited visualization was observed in less than 15% of patients with normal weight or overweight status, compared to 17.2%, 21.3%, and 40.6% of patients with obesity classes I, II, and III, respectively. Similarly, limited visualization was observed in 23.0% and 29.0% of those with alcohol associated and NAFLD cirrhosis, respectively. The same authors demonstrated the association between ultrasound visualization and test performance with severe visualization limitations significantly associated with missed lesions, i.e., lower sensitivity (OR, 7.94; 95% CI, 1.23-51.2) and moderate limitations associated with increased odds of false positive results, i.e., lower specificity (OR, 1.60; 95% CI, 1.13-2.27).57
Ultrasound-based surveillance is also associated with physical, financial, and psychological harms related to false positive or indeterminate results.51,58 Although HCC has traditionally been assumed to uniformly be an aggressive tumor, recent data suggest variation in tumor doubling times59,60 so overdiagnosis may be another potential harm of HCC surveillance. Overdiagnosis may be particularly prevalent in patients with NAFLD-related HCC given higher risk of non-liver mortality including cardiovascular disease.61 Accounting for surveillance-related harms and benefits, ultrasound and AFP is more cost-effective than ultrasound alone or no surveillance in patients with compensated cirrhosis.62
These data highlight a need for novel imaging or biomarker-based strategies for HCC surveillance in patients with NAFLD. Cohort studies from South Korea suggest that low dose, two-phase computed tomography (CT) and dynamic contrast-enhanced magnetic resonance imaging (MRI) both have higher sensitivity for early-stage HCC than ultrasound;63,64 however, these data were derived in HBV-predominant populations and need validation in broader cohorts, including those with NAFLD. Further, concerns about cost, radiation exposure with CT, and radiologic capacity of MRI may limit their widespread use for HCC surveillance. Abbreviated MRI has been proposed as a means of reducing in-scanner time and costs. Case-control data suggest preserved sensitivity and specificity compared to full diagnostic MRI, with preserved data in patients with NASH cirrhosis;65-67 however, prospective validation is needed.
Therefore, there has been increased interest in biomarkerbased strategies, which may concurrently increase test efficacy and utilization. AFP is the only biomarker to have completed all five phases of biomarker validation, although it has insufficient sensitivity for early-stage HCC detection.68 Tumoral heterogeneity highlights the need for multi-biomarker panels, such as GALAD, to improve sensitivity for early-stage HCC. GALAD was shown to have promising test performance in a multi-center case-control study, with sensitivities of 60-80% for early-stage HCC.69 A subsequent case-control study from Germany similarly demonstrated high accuracy for GALAD in patients with NASH.70 Two large case-control studies have also shown that liquid biopsy techniques, including methylated DNA biomarker panels, have high accuracy for early-stage HCC detection, although there were limited numbers of patients with NASH in both studies.71,72
Another issue that limits the effectiveness of HCC surveillance is underutilization, with a systematic review suggesting less than one in four patients undergo HCC surveillance.73 HCC surveillance underuse was prevalent across subgroups including geographic region and receipt of subspeciality care.73 In a multicenter cohort study, HCC surveillance was underused in a majority of patients.74 Surveillance underuse is related to failures across the screening continuum, including patients not being engaged in clinical care, failure of providers to recognize cirrhosis and order HCC surveillance, and failure of patients to adhere to surveillance recommendations.74-76 One of the consistent correlates for surveillance underuse across studies is the presence of NAFLD cirrhosis, partly related to under-recognition of cirrhosis.73 Studies have also demonstrated the importance of addressing patient- and provider-reported barriers (e.g., transportation and competing interests in clinic, respectively) to increase HCC surveillance.77,78 Several intervention strategies including provider education, electronic medical record alerts, and population-based mailed outreach invitations have been proven efficacious for increasing HCC surveillance use, although studies are needed to evaluate their effectiveness when implemented in clinical practice.73,79,80
RECALL AND DIAGNOSIS
Regardless of liver disease etiology, the recall strategy and diagnostic algorithm for patients with suspected HCC is the same. Patients with subcentimeter liver lesions have a low risk of HCC so should simply undergo repeat ultrasound within 3-6 months.81 Patients with a liver lesion ≥10 mm on ultrasound or AFP ≥20 ng/mL are high risk for HCC and require diagnostic evaluation.47,48,82 Patients with high-risk HBV or cirrhosis from any etiology should undergo diagnostic imaging with 4-phase CT or dynamic contrast-enhanced MRI, as HCC diagnosis can be made based on characteristic radiologic imaging in most patients.
The Liver Imaging and Reporting Data System (LI-RADS) is an imaging algorithm that allows for the characterization of liver lesions in at-risk patients, including radiologic diagnosis of HCC.82 The LI-RADS system classifies lesions from LI-RADS 1 (definitely benign) to LI-RADS 5 (definite HCC), with the latter being defined by arterial phase hyperenhancement, delayed phase washout, and capsule appearance. LI-RADS lesions have an approximately 95% positive predictive value for the presence of HCC, although there has been increasing use of biopsy for diagnostic confirmation and molecular characterization.83 Patients with indeterminate liver nodules (LI-RADS 3 or LI-RADS 4) have an intermediate risk of HCC, between 35-65%, so typically warrant close follow-up imaging, with biopsies used in cases that would change immediate clinical management.84-86 Patients without cirrhosis or high-risk HBV should undergo biopsy for histologic confirmation, as imaging features have insufficient accuracy.49
Although a diagnosis of HCC can be made radiographically in most cases, approximately 10% of HCCs will have atypical imaging features (e.g., arterial phase hyperenhancement alone or delayed washout alone). In these cases, biopsy is needed to make a diagnosis.87 Classical histopathological findings of HCC include the following: well vascularized tumors with wide trabeculae, small cell changes, cytologic atypia, mitotic atypia, reticulin network loss, and a prominent acinar pattern.88 A common histological variant of HCC is steatotic HCC, distinctly associated with underlying metabolic risk factors and NASH cirrhosis.89 Most studies have failed to demonstrate any difference in prognosis, including recurrence after resection, between steatotic HCC and other subtypes.
CLINICAL MANAGEMENT
Although there are several staging systems, the Barcelona Clinic Liver Cancer (BCLC) system includes both prognostic information and a recommended treatment allocation system. BCLC tumor stage is based on multiple factors including tumor burden, degree of liver dysfunction, and patient performance status.90 Tumor stages range from BCLC stage 0 (very early) and stage A (early) to BCLC stage C (advanced) and stage D (terminal) disease. However, there are often multiple treatment options for each tumor stage, and treatment decisions must incorporate other factors including patient preferences and goals of care.
Treatment decisions should be made in a multidisciplinary manner. A multidisciplinary approach ensures that HCC is accurately diagnosed and staged, with treatment decisions being reached in a patient-centered and holistic manner, incorporating the input of all relevant parties. Members of the multidisciplinary team often include hepatologists, liver transplant/hepatobiliary surgeons, medical oncologists, interventional radiologists, radiologists, primary care physicians, and pathologists.91 Multidisciplinary care is associated with increased guideline concordant treatment, increased use of curative therapies, and significantly reduced HCC-related mortality.92,93 Given these benefits, many experts advocate for multidisciplinary care to be standard of care for patients with HCC.
Early-stage HCC
Patients with early-stage HCC are amenable to curative therapies including surgical resection, local ablation, and liver transplantation, which have all been associated with 5-year survival exceeding 70% (Fig. 2). Surgical resection is the treatment of choice in patients without cirrhosis and those with compensated cirrhosis without portal hypertension. In contrast, liver transplantation offers a cure for both the HCC and cirrhosis in those with portal hypertension or hepatic decompensation. The 5-year risk of recurrence exceeds 50% in those who undergo surgical resection or local ablation, compared to approximately 10% after liver transplantation.
Figure 2.
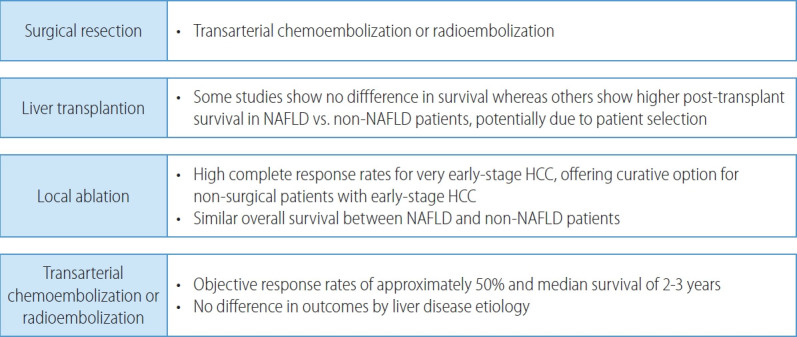
Treatment outcomes for NAFLD patients with early- and intermediate-stage HCC. NAFLD, non-alcoholic fatty liver disease; HCC, hepatocellular carcinoma.
Hepatic resection is a challenging feat in patients with NAFLD-HCC for multiple reasons. Post-operative liver failure in patients undergoing liver resection can be partly explained by steatosis, a hallmark feature of NAFLD, that precludes proper liver regeneration.94 The curative potential of liver resection may be higher in this patient population; however, their comorbidities have serious negative implications.95,96 Metabolic syndrome, a frequent co-morbidity of NAFLD, is associated with a twofold higher risk of complications after liver resection, whether septic, cardiovascular or pulmonary in nature.97 Even in the absence of advanced fibrosis, patients with NAFLD-HCC are not at a lower postoperative risk. The association of steatosis and chronic inflammation also has a bearing on the ability of patients to tolerate resection.98
Liver transplant is the treatment of choice in patients liver dysfunction severe enough to prohibit surgical resection.49 Among patients with NAFLD, assessment and optimization of associated comorbidities including obesity, metabolic syndrome, and cardiovascular disease is particularly important, both while waiting for liver transplantation and afterwards.99 Despite a higher prevalence of these comorbidities, data from the European Liver Transplant Registry showed no difference in survival between NAFLD and non-NAFLD patients.100 An analysis of the UNOS registry in the United States suggests patients with NAFLD may even have higher post-transplant survival (HR, 0.69; 95% CI, 0.63-0.77), potentially related to more rigorous patient selection.101
For patients with early-stage HCC who are not amenable to surgical therapies, local ablation offers another curative approach.102 Local ablation is most commonly performed using thermal-mediated approaches such as radiofrequency ablation or microwave ablation, which can induce complete responses for most lesions ≤3 cm, although response rates decrease once size increases beyond this threshold. NAFLD-HCC, HCV-HCC, and alcoholic liver disease-related HCC appear to have similar overall survival with location ablation.103
Intermediate-stage HCC
Intermediate stage (BCLC-B) HCC is characterized by multifocal HCC (beyond BCLC stage A). Traditionally, TACE has been the recommended treatment for patients with intermediate stage HCC, permitting objective response rates of approximately 50% and a median survival of 2-3 years (Fig. 2).104 There has been increasing use of TARE as an alternative embolic therapy, which is associated with similar overall survival but longer time-to-progression and progression-free survival.105
There has also been increasing recognition of heterogeneity among patients with BCLC stage B disease. One such score that highlights this heterogeneity is the 6-and-12 score, which is based on the sum of number of nodules and largest tumor diameter. Patients with a score <6 have the best survival, score 6-12 have intermediate survival, and those with a score >12 have the worst prognosis (median survival of 35.1, 16, and 7.6 months, respectively).106
Patients with limited tumor burden who have a response to locoregional therapy are considered “downstaged” and can successfully undergo liver transplantation, with near equal outcomes as those who present at an early-stage HCC.107 Although there is no universally accepted threshold for downstaging, data have suggested better outcomes for patients within UNOS-downstaging criteria (one lesion ≤8 cm, 2-3 lesions with largest ≤5 cm, and 3-4 cm ≤3 cm, with total tumor volume ≤8 cm) than those with larger tumor burden.108 The XXL trial, a randomized, controlled, phase IIB/III trial demonstrated liver transplantation after effective and sustained downstaging of eligible HCC beyond Milan criteria was associated with improved 5-year tumor event free survival (HR, 0.20; 95% CI, 0.07-0.57; P=0.003) and overall survival (HR, 0.32; 95% CI, 0.11-0.92; P=0.035) compared to non-transplantation treatments.109 Data from the UNOS database also showed patients who are downstaged can achieve 5-year post- transplant survival of 64%, with an acceptable risk of recurrence (19% at 5 years).110
Conversely, patients with larger intrahepatic tumor burden appear to have lower objective responses and worse survival than those with smaller tumor burden. Any benefit to TACE must be weighed against potential harms, including postembolization syndrome and risk of liver dysfunction. Data from the OPTIMIS study show that objective responses decrease from 40% to 26% from initial to subsequent TACE.111 Considering these data, there has been increasing recognition of “TACE unsuitable” patients, i.e., those in whom the risks of TACE likely outweigh potential benefits. Although there is no consensus definition for TACE unsuitable, some have proposed the up-to-7 criteria, i.e., the sum of the largest tumor diameter and number of lesions exceeding seven.112 A proof-of-concept retrospective propensity-score matched analysis suggested these patients may be better treated with up-front systemic therapy instead of locoregional therapy.113 In this study, patients who were treated with up-front systemic therapy had better survival, primarily due to more prolonged preservation of liver function, than patients who were treated with TACE as initial treatment. Although interesting, these data require validation in prospective clinical trials, which are currently ongoing.
Advanced-stage HCC
Advanced stage (BCLC-C) encompasses patients with vascular invasion or extrahepatic spread, but relatively fit with preserved liver function. These patients should be considered for systemic therapies.90 The first line systemic therapies for HCC include sorafinib, lenvatinib, atezolizumab plus bevacizumab (Atezo-Bev) and durvalumab plus tremelimumab (Durva-Treme), approved in that order (Table 1).
Table 1.
Overall survival, stratified by etiology, in select phase III studies evaluating HCC systemic therapies
| Trial name | Treatment arms | Trial aetiology | OS HR (95% CI) | |
|---|---|---|---|---|
| 1st line | ||||
| SHARP [115] | Sorafenib vs. placebo | HBV (n=111, 18%) | 0.76 (0.38-1.50) | |
| HCV (n=169, 28%) | 0.50 (0.32-0.77) | |||
| Alcohol (n=159, 26%) | 0.76 (0.50-1.16 | |||
| Asia-Pacific [116] | Sorafenib vs. placebo | HBV +ve (n=165, 73%) | 0.74 (0.51-1.06) | |
| HBV -ve (n=61, 27%) | 0.57 (0.29-1.13) | |||
| REFLECT [119] | Lenvatinib vs. sorafenib | HBV (n=479, 50%) | 0.83 (0.68-1.02) | |
| HCV (n=217, 23%) | 0.91 (0.66-1.26) | |||
| Alcohol (n=57, 6%) | 1.03 (0.47-2.28) | |||
| IMbrave150 [122] | Atezolizumab + bevacizumab vs. sorafenib | HBV (n=240, 48%) | 0.51 (0.32-0.81) | |
| HCV (n=108, 22%) | 0.43 (0.22-0.87) | |||
| Non-viral (n=153, 31%) | 0.91 (0.52-1.60) | |||
| HIMALAYA [124] | Tremelimumab + durvalumab vs. sorafenib | HBV (n=241, 31%) | 0.64 (0.48-0.86) | |
| HCV (n=214, 27%) | 1.06 (0.76-1.49) | |||
| Non-viral (n=327, 42%) | 0.74 (0.57-0.95) | |||
| 2nd line | ||||
| RESORCE [125] | Regorafenib vs. placebo | HBV (n=216, 38%) | 0.58 (0.41-0.82) | |
| HCV (n=119, 21%) | 0.79 (0.49-1.26) | |||
| Alcohol (n=145, 25%) | 0.92 (0.61-1.38) | |||
| CELESTIAL [126] | Cabozantinib vs. placebo | HBV (n=267, 38%) | 0.69 (0.51-0.94) | |
| HCV (n=168, 24%) | 1.11 (0.72-1.71) | |||
| Non-viral (n=272, 38%) | 0.72 (0.54-0.96) | |||
| REACH-2 [127] | Ramucirumab vs. placebo | HBV (n=107, 37%) | 0.84 (0.52-1.35) | |
| HCV (n=76, 26%) | 0.76 (0.44-1.33) | |||
| Other (n=109, 37%) | 0.63 (0.38-1.06) | |||
| KEYNOTE-240 [121] | Pembrolizumab vs. placebo | HBV (n=101, 24%) | 0.57 (0.35-0.94) | |
| HCV (n=64, 15%) | 0.96 (0.48-1.92) | |||
| Non-viral (n=248, 60%) | 0.88 (0.64-1.20) | |||
HCC, hepatocellular carcinoma; OS, overall survival; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus.
Both sorafenib and lenvatinib are multitargeted tyrosine kinase inhibitors with antiangiogenic effects through the inhibition of vascular endothelial growth factor (VEGF) receptors.114 Sorafenib was the first systemic therapy to show a survival benefit for patients with advanced stage HCC, demonstrating a significant survival benefit in the SHARP (HR, 0.69; 95% CI, 0.55-0.87) and Asia Pacific (HR, 0.68; 95% CI, 0.50-0.93) trials;115,116 however, the median survival with treatment was still only 10.7 months, leaving room for improvement. The next decade after the SHARP trial was unfortunately met with disappointment given multiple negative trials of agents that failed to further improve survival. In 2018, the REFLECT trial led to the approval of Lenvatinib, which was shown to have non-inferior overall survival (median 13.6 vs. 12.3 months; HR, 0.92; 95% CI, 0.79-1.06) but significant improvements in secondary outcomes including progression-free survival (median 7.4 vs. 3.7 months; HR, 0.66; 95% CI, 0.57-0.77) and objective responses (24.1% vs. 9.2%).117 These improved secondary responses are likely related to greater fibroblast growth factor receptor activity compared to Sorafenib. Although there were few patients with NASH included in the REFLECT trial, subsequent realworld data suggest consistent if not greater activity in NASHrelated HCC than other etiologies. In a European multi-center study of 1,232 patients treated with lenvatinib, of whom 236 had NASH-related HCC, patients with NASH-related HCC had improved overall survival compared to others (median 22.2 vs. 15.1 months; HR, 0.69; 95% CI, 0.56-0.85).118 Similarly, a multi-center study from Japan of 674 patients (103 with NASH) showed improved survival in the NASH-related HCC subgroup (20.5 vs. 16.9 months).119
The recent introduction of immune checkpoint inhibitors has revolutionized the approach to systemic treatment of HCC. Trials involving immune checkpoint inhibitors started with single agent programmed-death 1 (PD-1) inhibitors: nivolumab in the first-line and pembrolizumab in the second-line setting. Both demonstrated durable objective responses in 15-20% of patients in phase II studies;120,121 however, both failed to achieve statistically significant improvements in overall survival in subsequent phase III clinical trials. The disappointment with these failed trials was quickly allayed by positive data from the IMBrave150 trial, leading to the approval of Atezo-Bev in the first-line setting.122 Atezolizumab is a programmed-death ligand 1 (PD-L1) inhibitor, and bevacizumab is a VEGF inhibitor. It is believed that VEGF inhibition can have several immunomodulatory effects that can augment responses, including increased cytotoxic T lymphocytes, increased dendritic cell maturation, decreased tumor associated macrophages, and decreased myeloid derived suppressor cells. Atezolizumab plus bevacizumab was shown to have superior survival compared to sorafenib with median survival of 19.2 vs. 13.4 months.122,123 Atezo-Bev also improved progression-free survival (median 6.9 vs. 4.3 months) and objective responses (30% vs. 11%) compared to sorafenib. Further, the combination was well tolerated with few grade 3-4 adverse events.122 Notably, there were concerns about potential gastrointestinal (GI) bleeding with bevacizumab in patients with underlying cirrhosis; however, the incidence of upper GI bleeding in the trial was acceptable at 7% compared to 4.5% in the sorafenib group.122 The low rate of GI bleeding was likely due to trial design requiring an esophagogastroduodenoscopy within 6 months prior to trial enrollment, and those who were deemed high risk of bleeding were excluded from the trial. Most recently, Durva-Treme was approved based on results from the HIMALAYA trial given significant improvement in overall survival compared to sorafenib (median survival 16.4 vs. 13.8 months; HR, 0.78; 95% CI, 0.65-0.92).124 Of interest, the HIMALAYA trial also provided results from landmark analysis at 3 years, demonstrating 30.7% of patients treated with Durva-Treme were still alive 3 years after randomization, compared to 24.7% of patients treated with durvalumab monotherapy and 20.2% for those treated with sorafenib.
There are several second-line therapies that have been shown to improve survival in phase III trials, including regorafenib (HR, 0.63; 95% CI, 0.50-0.79),125 cabozantinib (HR, 0.76; 95% CI, 0.63-0.92),126 and ramucirumab (HR, 0.71; 95% CI, 0.53-0.95) (Table 1).127 However, there are important differences between the agents based on clinical trial design. For example, the RESORCE trial leading to the approval of regorafenib required tolerance of sorafenib given similar chemical structures between regorafenib and sorafenib;125 REACH-2 required patients to have AFP ≥400 ng/mL given lack of benefit of ramucirumab in patients with lower AFP levels;127 and CELESTIAL had broad inclusion criteria, including 27% of patients who had received two prior lines of therapy (thereby providing data for cabozantinib in both second- and third-lines).126 These trials were all conducted after sorafenib in the first line setting but are extrapolated after newer systemic agents as well.
There has been controversy regarding differential performance of immune checkpoint inhibitors in patients with non-viral etiologies of HCC. Data provided by Pfister et al.128 suggested that HCC due to non-viral etiologies may be less responsive to immunotherapy as opposed to tumors due to viral etiologies. PD-1-targeted immunotherapy in preclinical models of NASH-HCC led to expanded activated CD8+PD-1+T cells but not tumor regression, unveiling impaired tumor immune surveillance.128 A meta-analysis of three randomized phase III clinical trials testing PD-L1 or PD-1 inhibitors in more than 1,600 patients with advanced HCC showed that immunotherapy improved survival in the overall population (HR, 0.77; 95% CI, 0.63-0.94) but failed to do so in those with HCC due to non-viral causes. However, it should be noted that most of these data were post-hoc analyses, limited by residual confounding since trials given randomization was not stratified by liver disease etiology. Further, there was no differences in objective responses in the IMBrave150 trial by liver disease etiology.122 Finally, subgroup analysis of the HIMALAYA study demonstrated a benefit of Durva-Treme in the non-viral subgroup with a HR of 0.74 (95% CI, 0.57-0.95).124 Overall, these data are hypothesis generating although require further validation before changing clinical practice. Therefore, liver disease etiology should likely not defer use of immune checkpoint inhibitor-based therapy in those who are otherwise eligible.
Terminal-stage HCC
End-stage (BCLC-D) patients have major cancer-associated symptoms and/or those with poor liver function. In this case, treatment of HCC, outside of liver transplantation, will not change survival outcomes and the mainstay is symptomatic treatment with palliative care.90
FUTURE DIRECTIONS
Significant strides have been made in recent decades to better understand the pathophysiology of NAFLD-related HCC and how it differs from HCC due to other etiologies. Identifying and developing effective treatments for NAFLD remains an avenue of paramount importance to reduce liverrelated mortality and prevent HCC development. Proper implementation of HCC surveillance remains key, particularly in finding solutions to ultrasound limitations among obese people, who constitute a substantial portion of those with NAFLD. As for therapeutic interventions, it is crucial that additional research be undertaken to refine optimal patient selection and confirm efficacy of immunotherapy-based regimens in this population. Continued research and progress in this growing population is critical to reducing HCC-related mortality in the future.
Footnotes
Conflicts of Interest
Amit Singal has served as a consultant or on advisory boards for FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, GRAIL, Freenome, Universal Dx, Genentech, AstraZeneca, Eisai, Bayer, Exelixis, TARGET RWE. None of the authors have any relevant conflicts of interest.
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IBR approval is not necessary.
Funding Statement
This study was conducted with support from NIH R01 CA256977. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Author Contribution
Conceptualization: AS
Project administration: AS
Writing - original draft: AS, DD
Writing - review and editing: AS, DD, KSED
References
- 1.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci. 2019;64:910–917. doi: 10.1007/s10620-019-05537-2. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 5.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2566. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.Polyzos SA, Mantzoros CS. Nonalcoholic fatty future disease. Metabolism. 2016;65:1007–1016. doi: 10.1016/j.metabol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69:564–568. doi: 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 9.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977. doi: 10.1016/j.cmet.2022.05.003. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696–703. doi: 10.1111/apt.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131. doi: 10.1016/j.cgh.2015.07.019. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reig M, Gambato M, Man NK, Roberts JP, Victor D, Orci LA, et al. Should patients with NAFLD/NASH be surveyed for HCC? Transplantation. 2019;103:39–44. doi: 10.1097/TP.0000000000002361. [DOI] [PubMed] [Google Scholar]
- 14.Pinyopornpanish K, Khoudari G, Saleh MA, Angkurawaranon C, Pinyopornpanish K, Mansoor E, et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21:394. doi: 10.1186/s12876-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geh D, Anstee QM, Reeves HL. NAFLD-associated HCC: progress and opportunities. J Hepatocell Carcinoma. 2021;8:223–239. doi: 10.2147/JHC.S272213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 17.Chrysavgis L, Giannakodimos I, Diamantopoulou P, Cholongitas E. Non-alcoholic fatty liver disease and hepatocellular carcinoma: clinical challenges of an intriguing link. World J Gastroenterol. 2022;28:310–331. doi: 10.3748/wjg.v28.i3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–1837. doi: 10.1053/j.gastro.2018.08.024. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan Y, Kramer JR, Yu X, Li L, Thrift AP, El-Serag HB, et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology. 2020;72:1242–1252. doi: 10.1002/hep.31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71:907–916. doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El-Serag HB, et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420–1428. doi: 10.1002/hep.32244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. 2012;48:2137–2145. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Burza MA, Pirazzi C, Maglio C, Sjöholm K, Mancina RM, Svensson PA, et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037–1041. doi: 10.1016/j.dld.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLDrelated cirrhosis for risk stratification. J Hepatol. 2019;71:523–533. doi: 10.1016/j.jhep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara N, Kubota N, Crouchet E, Koneru B, Marquez CA, Jajoriya AK, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med. 2022;14:e. doi: 10.1126/scitranslmed.abo4474. abo4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160:2572–2584. doi: 10.1053/j.gastro.2021.01.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian Z, Hou X, Liu W, Han Z, Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9:79. doi: 10.1186/s13578-019-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udoh US, Rajan PK, Nakafuku Y, Finley R, Sanabria JR. Cell autophagy in NASH and NASH-related hepatocellular carcinoma. Int J Mol Sci. 2022;23:7734. doi: 10.3390/ijms23147734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copynumber changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 35.Pereira K, Salsamendi J, Casillas J. The global nonalcoholic fatty liver disease epidemic: what a radiologist needs to know. J Clin Imaging Sci. 2015;5:32. doi: 10.4103/2156-7514.157860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zunica ERM, Heintz EC, Axelrod CL, Kirwan JP. Obesity management in the primary prevention of hepatocellular carcinoma. Cancers (Basel) 2022;14:4051. doi: 10.3390/cancers14164051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koumbi L, Eliopoulos AG, Vassilopoulou E. How diet-induced changes in the “gut-liver” axis affect chronic liver disease outcome? Livers. 2021;1:40–48. [Google Scholar]
- 39.Kwak M, Mehaffey JH, Hawkins RB, Hsu A, Schirmer B, Hallowell PT. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: a propensity matched analysis. Am J Surg. 2020;219:504–507. doi: 10.1016/j.amjsurg.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a metaanalysis. Scand J Gastroenterol. 2013;48:78–87. doi: 10.3109/00365521.2012.719926. [DOI] [PubMed] [Google Scholar]
- 42.Islam MM, Poly TN, Walther BA, Yang HC, Jack Li YC. Statin use and the risk of hepatocellular carcinoma: a meta-analysis of observational studies. Cancers (Basel) 2020;12:671. doi: 10.3390/cancers12030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Facciorusso A, Abd El Aziz MA, Singh S, Pusceddu S, Milione M, Giacomelli L, et al. Statin use decreases the incidence of hepatocellular carcinoma: an updated meta-analysis. Cancers (Basel) 2020;12:874. doi: 10.3390/cancers12040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci. 1998;19:26–37. doi: 10.1016/s0165-6147(97)01147-4. [DOI] [PubMed] [Google Scholar]
- 45.Godos J, Micek A, Marranzano M, Salomone F, Rio DD, Ray S. Coffee consumption and risk of biliary tract cancers and liver cancer: a dose-response meta-analysis of prospective cohort studies. Nutrients. 2017;9:950. doi: 10.3390/nu9090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: a meta-analysis of 11 epidemiological studies. Ann Hepatol. 2021;20:100254. doi: 10.1016/j.aohep.2020.08.071. [DOI] [PubMed] [Google Scholar]
- 47.Singal A, Llovet JM, Yarchoan M, Mehta N, Heimbach J, Dawson L, et al. AASLD guidance on prevention, diagnosis and treatment of hepatocellular carcinoma. Hepatology; 2022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. AsiaPacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a metaanalysis. J Hepatol. 2022;77:128–139. doi: 10.1016/j.jhep.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20:283–292. doi: 10.1016/j.cgh.2021.05.002. e10. [DOI] [PubMed] [Google Scholar]
- 54.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a metaanalysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samoylova ML, Mehta N, Roberts JP, Yao FY. Predictors of ultrasound failure to detect hepatocellular carcinoma. Liver Transpl. 2018;24:1171–1177. doi: 10.1002/lt.25202. [DOI] [PubMed] [Google Scholar]
- 56.Schoenberger H, Chong N, Fetzer DT, Rich NE, Yokoo T, Khatri G, et al. Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1561–1569.e4. doi: 10.1016/j.cgh.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong N, Schoenberger H, Yekkaluri S, Fetzer DT, Rich NE, Yokoo T, et al. Association between ultrasound quality and test performance for HCC surveillance in patients with cirrhosis: a retrospective cohort study. Aliment Pharmacol Ther. 2022;55:683–690. doi: 10.1111/apt.16779. [DOI] [PubMed] [Google Scholar]
- 58.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65:1196–1205. doi: 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70:401–407. doi: 10.1136/gutjnl-2020-321040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multicenter cohort of patients with cirrhosis. Hepatology. 2020;72:1654–1665. doi: 10.1002/hep.31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rich NE, Singal AG. Overdiagnosis of hepatocellular carcinoma: prevented by guidelines? Hepatology. 2022;75:740–753. doi: 10.1002/hep.32284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol. 2020;115:1642–1649. doi: 10.14309/ajg.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, et al. MRI with liverspecific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3:456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, et al. A comparison of biannual two-phase low-dose liver CT and US for HCC surveillance in a group at high risk of HCC development. Liver Cancer. 2020;9:503–517. doi: 10.1159/000506834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park HJ, Kim SY, Singal AG, Lee SJ, Won HJ, Byun JH, et al. Abbreviated magnetic resonance imaging vs ultrasound for surveillance of hepatocellular carcinoma in high-risk patients. Liver Int. 2022;42:2080–2092. doi: 10.1111/liv.15110. [DOI] [PubMed] [Google Scholar]
- 66.Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol. 2021;75:108–119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Yokoo T, Masaki N, Parikh ND, Lane BF, Feng Z, Mendiratta-Lala M, et al. Multicenter validation of abbreviated MRI for detecting early-stage hepatocellular carcinoma. Radiology. 2023 Jan 24; doi: 10.1148/radiol.220917. doi: 10.1148/radiol.220917. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parikh ND, Mehta AS, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2020;29:2495–2503. doi: 10.1158/1055-9965.EPI-20-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6. doi: 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 70.Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728–735.e4. doi: 10.1016/j.cgh.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi-analyte cellfree DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022;6:1753–1763. doi: 10.1002/hep4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20:173–182.e7. doi: 10.1016/j.cgh.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology. 2021;73:713–725. doi: 10.1002/hep.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parikh ND, Tayob N, Al-Jarrah T, Kramer J, Melcher J, Smith D, et al. Barriers to surveillance for hepatocellular carcinoma in a multicenter cohort. JAMA Netw Open. 2022;5:e2223504. doi: 10.1001/jamanetworkopen.2022.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marquardt P, Liu PH, Immergluck J, Olivares J, Arroyo A, Rich NE, et al. Hepatocellular carcinoma screening process failures in patients with cirrhosis. Hepatol Commun. 2021;5:1481–1489. doi: 10.1002/hep4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmons OL, Feng Y, Parikh ND, Singal AG. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2019;17:766–773. doi: 10.1016/j.cgh.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19:987–995.e1. doi: 10.1016/j.cgh.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Adamson B, et al. Mailed outreach program increases ultrasound screening of patients with cirrhosis for hepatocellular carcinoma. Gastroenterology. 2017;152:608–615.e4. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13:172–179. doi: 10.1016/j.cgh.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 81.Singal AG, Ghaziani TT, Mehta N, Zhou K, Grinspan L, et al. Recall patterns and risk of primary liver cancer for subcentimeter ultrasound liver observations: a multicenter study. Hepatol Commun. 2023;7:e0073. doi: 10.1097/HC9.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289:816–830. doi: 10.1148/radiol.2018181494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-A systematic review. Gastroenterology. 2019;156:976–986. doi: 10.1053/j.gastro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 84.Onyirioha K, Joshi S, Burkholder D, Yekkaluri S, Parikh ND, Singal AG. Clinical outcomes of patients with suspicious (LI-RADS 4) liver observations. Clin Gastroenterol Hepatol. 2022 Apr 9; doi: 10.1016/j.cgh.2022.03.038. doi: 10.1016/j.cgh.2022.03.038. [DOI] [PubMed] [Google Scholar]
- 85.Arvind A, Joshi S, Zaki T, Burkholder D, Parikh ND, Singal AG. Risk of hepatocellular carcinoma in patients with indeterminate (LIRADS 3) liver observations. Clin Gastroenterol Hepatol. 2021 Dec 10; doi: 10.1016/j.cgh.2021.11.042. doi: 10.1016/j.cgh.2021.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanneganti M, Marrero JA, Parikh ND, Kanwal F, Yokoo T, Mendiratta-Lala M, et al. Clinical outcomes of patients with liver imaging reporting and data system 3 or liver imaging reporting and data system 4 observations in patients with cirrhosis: a systematic review. Liver Transpl. 2022;28:1865–1875. doi: 10.1002/lt.26562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.European Association For The Study Of The Liver EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 88.International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 89.Chan AW, Yu S, Yu YH, Tong JH, Wang L, Tin EK, et al. Steatotic hepatocellular carcinoma: a variant associated with metabolic factors and late tumour relapse. Histopathology. 2016;69:971–984. doi: 10.1111/his.13029. [DOI] [PubMed] [Google Scholar]
- 90.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Byrd K, Alqahtani S, Yopp AC, Singal AG. Role of multidisciplinary care in the management of hepatocellular carcinoma. Semin Liver Dis. 2021;41:1–8. doi: 10.1055/s-0040-1719178. [DOI] [PubMed] [Google Scholar]
- 92.Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. doi: 10.1053/j.gastro.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, Reddick M, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21:1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Meijer VE, Kalish BT, Puder M, Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97:1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 95.Lewis RH, Glazer ES, Bittenbinder DM, O’Brien T, Deneve JL, Shibata D, et al. Outcomes following resection of hepatocellular carcinoma in the absence of cirrhosis. J Gastrointest Cancer. 2019;50:808–815. doi: 10.1007/s12029-018-0152-x. [DOI] [PubMed] [Google Scholar]
- 96.Molinari M, Kaltenmeier C, Samra PB, Liu H, Wessel C, Klem ML, et al. Hepatic resection for hepatocellular carcinoma in nonalcoholic fatty liver disease: a systematic review and meta-analysis of 7226 patients. Annals of Surgery Open. 2021;2:e065. doi: 10.1097/AS9.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhayani NH, Hyder O, Frederick W, Schulick RD, Wolgang CL, Hirose K, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: a national surgical quality improvement program (NSQIP) analysis. Surgery. 2012;152:218–226. doi: 10.1016/j.surg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809–1819. doi: 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 99.Esteban JPG, Asgharpour A. Evaluation of liver transplant candidates with non-alcoholic steatohepatitis. Transl Gastroenterol Hepatol. 2020;7:24. doi: 10.21037/tgh.2020.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong RJ, Chou C, Bonham CA, Concepcion W, Esquivel CO, Ahmed A. Improved survival outcomes in patients with nonalcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002-2012 United Network for Organ Sharing data. Clin Transplant. 2014;28:713–721. doi: 10.1111/ctr.12364. [DOI] [PubMed] [Google Scholar]
- 102.Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 103.Wong CR, Njei B, Nguyen MH, Nguyen A, Lim JK. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;46:1061–1069. doi: 10.1111/apt.14342. [DOI] [PubMed] [Google Scholar]
- 104.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 105.Brown AM, Kassab I, Massani M, Townsend W, Singal AG, Soydal C, et al. TACE versus TARE for patients with hepatocellular carcinoma: overall and individual patient level meta analysis. Cancer Med; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaewdech A, Sripongpun P, Cheewasereechon N, Jandee S, Chamroonkul N, Piratvisuth T. Validation of the “six-and-twelve” prognostic score in transarterial chemoembolization-treated hepatocellular carcinoma patients. Clin Transl Gastroenterol. 2021;12:e00310. doi: 10.14309/ctg.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan DJH, Lim WH, Yong JN, Ng CH, Muthiah MD, Tan EX, et al. UNOS down-staging criteria for liver transplantation of hepatocellular carcinoma: systematic review and meta-analysis of 25 studies. Clin Gastroenterol Hepatol. 2022 Feb 16; doi: 10.1016/j.cgh.2022.02.018. doi: 10.1016/j.cgh.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Frankul L, Frenette C. Hepatocellular carcinoma: downstaging to liver transplantation as curative therapy. J Clin Transl Hepatol. 2021;9:220–226. doi: 10.14218/JCTH.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947–956. doi: 10.1016/S1470-2045(20)30224-2. [DOI] [PubMed] [Google Scholar]
- 110.Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, et al. Liver transplantation outcomes in a U.S. multicenter cohort of 789 patients with hepatocellular carcinoma presenting beyond milan criteria. Hepatology. 2020;72:2014–2028. doi: 10.1002/hep.31210. [DOI] [PubMed] [Google Scholar]
- 111.Peck-Radosavljevic M, Raoul JL, Lee HC, Kudo M, Nakajima K, Cheng AL, et al. OPTIMIS: an international observational study to assess the use of sorafenib after transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC) J Clin Oncol. 2014;32(15_suppl):TPS4155. [Google Scholar]
- 112.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9:245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediatestage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh a liver function: a proof-of-concept study. Cancers (Basel) 2019;11:1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 115.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 116.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 117.Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55:113–122. doi: 10.1007/s00535-019-01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rimini M, Kudo M, Tada T, Shigeo S, Kang W, Suda G, et al. Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open. 2021;6:100330. doi: 10.1016/j.esmoop.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hiraoka A, Kumada T, Tada T, Tani J, Kariyama K, Fukunishi S, et al. Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: multi-center retrospective study. Sci Rep. 2021;11:16663. doi: 10.1038/s41598-021-96089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 121.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 122.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 123.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 124.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022;1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 125.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 126.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 128.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapytreated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


