Acute decompensated heart failure (ADHF) is the leading cause of hospitalizations in older adults. ADHF hospitalizations are associated with poor quality of life, high financial costs, and high risk for clinical events, with >70% of patients experiencing rehospitalization or death within 1 year of hospital discharge.1 Thus, improving outcomes following hospitalization for ADHF is a national healthcare priority. However, nearly all clinical trials aiming to improve outcomes following heart failure (HF) hospitalization, most of which have only targeted cardiac mechanisms, have been negative. This suggests that adverse outcomes in older patients with ADHF are driven, at least partly, by mechanisms not addressed by current approaches to care.
Traditionally, HF has been viewed solely as a disorder of the heart and cardiovascular system. Recent data strongly support that HF is a systemic disorder initiated, promoted, and progressed by circulating factors, likely systemic inflammation caused by aging, multiple comorbidities, and other contributors.2 Given that the heart never rests and has a continuous, high metabolic rate, circulating factors cause dysfunction of myocardial tissue first. However, these same factors also cause coordinated dysfunction in virtually all organ systems, particularly in skeletal muscle, which shares many features in common with myocardial tissue (Table 1). This broader view of HF as a systemic disorder has multiple profound implications. One of these is that while cardiac muscle is terminally differentiated, skeletal muscle has robust capacity for rapid rejuvenation and regeneration.3 This information would predict that treatments aimed at improving dysfunction of skeletal muscle in HF may be at least as promising as those aimed at cardiac tissue. However, the role of skeletal muscle dys-function and its potential as a therapeutic target in HF has been largely overlooked.
TABLE 1.
Skeletal muscle abnormalities in older patients with heart failure
| Skeletal muscle abnormalities in heart failure |
| Sarcopenia with loss of muscle mass, strength, and function |
| Easy fatigability and reduced tolerance to exercise |
| Increased fatty deposition in skeletal muscle. |
| Anabolic resistance of skeletal muscle proteins to stimuli such as nutrients, insulin, and exercise |
| Reduced proportion of type 1 and type IIa fibers with gain in type IIx fibers |
| Reduced microvascular density (capillarity) |
| Impaired vasodilatory mechanisms |
| Impaired skeletal muscle perfusion |
| Reduced conductive and diffusive oxygen transport |
| Impaired mitochondrial function with higher glycolytic and lower oxidative capacity |
The article by Attaway et al. in this issue of the journal provides considerable impetus for redressing this oversight.4 Sarcopenia is defined as loss of muscle mass with associated weakness and in severe cases, diminished physical function.5 Using International Classification of Diseases-9 (ICD-9) codes from the National Inpatient Sample database, a large all-payer inpatient care database in the United States, they defined a muscle loss phenotype as a surrogate for sarcopenia in a cohort of patients hospitalized for HF and a sample of non-HF general medical patients who were also hospitalized. They then compared characteristics and inpatient outcomes between these two groups, stratified by age (<50, 51–65, >65). Consistent with prior studies, and supporting the validity of this method of identifying sarcopenia, sarcopenia was more common in older age and was associated with a greater burden of comorbidities as well as worse outcomes, including inpatient mortality, length of stay, and inpatient costs.
Among the key findings was that the muscle loss phenotype was approximately eightfold more prevalent in HF (13.5%) versus general medical patients (1.6%), a difference that was even more pronounced among younger patients. Indeed, prevalence of the muscle loss phenotype was relatively stable across age strata in patients with HF (12.3%–14.4% in youngest to oldest) compared to general medical patients, where there was a much sharper age-related rise in the muscle loss phenotype (0.5%–2.7%, youngest to oldest). The relatively low upper age cutoff of 65 years may have obscured the contribution of age to sarcopenia in the oldest patients with HF (e.g., >75 years). However, a similar pattern of reduced age dependence has also been observed in studies of frailty in advanced or hospitalized HF patients,6,7 supporting the central role of the acute and chronic HF disease process in loss of muscle and associated physical function.
The findings by Attaway et al. also implicated comorbidities in the development of the muscle loss phenotype. This is relevant as the burden of comorbidities among hospitalized patients with HF has increased over time and may contribute to the increasing prevalence of coexisting sarcopenia.8 When comparing patients with the muscle loss phenotype, comorbid conditions were also far more frequent among HF patients across all age categories compared to general medical patients. Importantly, when present, the muscle loss phenotype appeared to disproportionally adversely impact older HF patients, who demonstrated worse outcomes compared to older general medical patients who were also identified with muscle loss.
The authors are commended for highlighting, through a novel and creative method, which bears future external validation, the particularly high burden and clinical consequence of sarcopenia in older patients hospitalized with HF, which is frequently unrecognized and unaddressed in HF guidelines and clinical care pathways despite its impact on physical function and associations with adverse clinical outcomes. By relying on clinical recognition and claims data for detecting sarcopenia, the authors may have significantly under-estimated the true rate of sarcopenia in patients hospitalized for HF. Sarcopenia can be easily detected and reproducibly quantified by a number of noninvasive techniques, including dual X-ray absorptiometry, computed tomography, magnetic resonance imaging, and strength testing, among others.9 When formal tests for sarcopenia are applied, rates among patients hospitalized for HF commonly exceed 50%, more than twice the rate in ambulatory HF patients.10 We have observed similar high rates of physical frailty, which shares measures such as gait speed and grip strength with sarcopenia, and is intended to reflect the cumulative global risk of diminished physiologic reserve involving multiple organ systems.11
What explains the surprisingly high rate of sarcopenia among older patients hospitalized for HF, particularly compared to general medicine control patients, and the associated functional impairments? Chronic HF accelerates the aging-associated decline in muscle mass, likely due to the chronic systemic inflammation that is now understood to accompany HF, particularly the HF with preserved ejection fraction—the phenotype that is most common among older persons. The high rates of sarcopenia and associated functional impairments are present across spectrum of EF categories,12 further supporting the systemic impact of clinical HF in general rather than related to any particular cardiomyopathy. Several comorbidities common in older chronic HF patients, such as diabetes mellitus, chronic kidney disease, and chronic lung disease, are also pro-inflammatory and further accelerated muscle loss (Figure 1). These comorbidities also contribute to adverse clinical events in patients hospitalized for HF, >50% of which are due to non-HF causes.13
FIGURE 1.
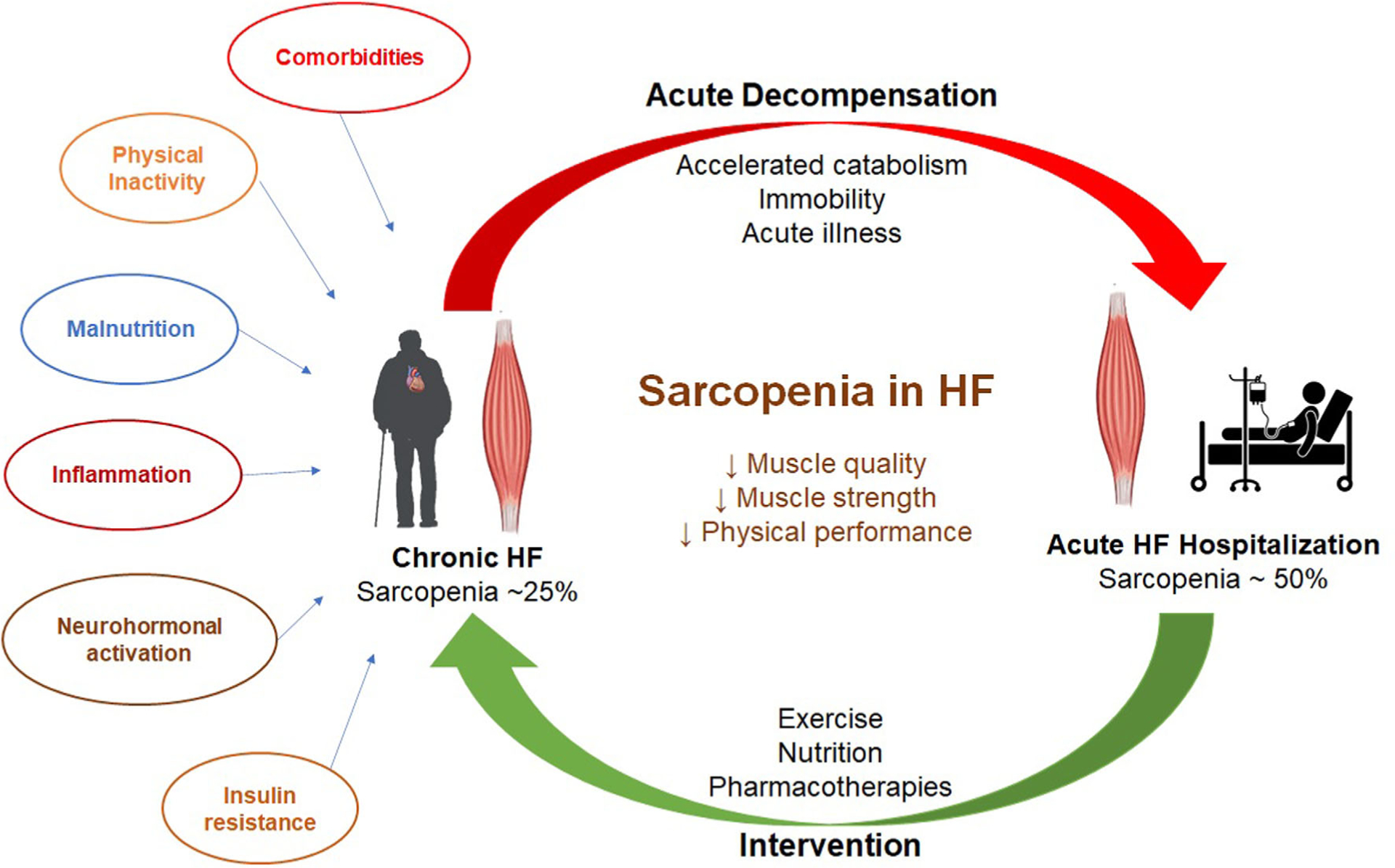
Sarcopenia in heart failure—prevalence, risk factors, and potential therapeutic strategies. Sarcopenia is common in patients with heart failure and worsened by decompensated heart failure hospitalization. Risk factors such as comorbidities, physical inactivity, malnutrition, upregulation of inflammatory pathways, insulin resistance, and neurohormonal activation contribute to the development of sarcopenia in patients with heart failure. Therapeutic approaches such as exercise training, improved nutritional support, and some potential pharmacotherapies (ghrelin analogues and selective androgen receptor modulators) may improve sarcopenia in patients with heart failure
During ADHF, these chronic processes may be exacerbated by an acute rise in inflammatory cytokines and are further compounded by hospital-associated inactivity.14,15 Both the chronic and acute factors preferentially impact skeletal muscle with relative preservation or accumulation of adipose. This leads to sarcopenic obesity, which may be difficult to identify in routine clinical settings without imaging to define body and muscle composition and possibly contributing to the under-recognition of sarcopenia in HF patients. Excess adipose in and around muscle is implicated in local and systemic metabolic pathways conferring generalized risk while diminishing muscle performance and contributing to physical function impairments, which are especially severe across all domains (strength, balance, mobility, and endurance) in patients hospitalized with ADHF.13 These insults to muscle performance may also impair muscle recovery, potentially explaining the persistence of severe functional impairments long after the resolution of ADHF, referred to as the “post hospitalization syndrome.”
Given the well-established risks for adverse clinical events, diminished quality of life, and loss of functional independence associated with sarcopenia in HF, and relative ease of measurement, it is surprising that there have been few efforts to target sarcopenia in HF. We recently found that a novel, early, tailored, multi-domain, progressive, transitional physical rehabilitation intervention targeting individual deficits in functional strength as well as balance, mobility, and endurance can improve functional impairments associated with severe sarcopenia in older patients hospitalized with ADHF.16 Nutritional approaches may be another strategy to mitigate the effects of sarcopenia, and could be complementary with exercise for addressing the adverse effects of sarcopenia in HF.17,18 Pharmacotherapies, such as ghrelin analogues and selective androgen receptor modulators, are also currently under investigation as potential treatments of sarcopenia in HF (Figure 1).19
In conclusion, Attaway and colleagues provide novel and valuable information regarding the importance of muscle loss to outcomes in patients hospitalized with HF. Such data are critical to broaden the therapeutic focus in HF from solely on cardiac tissue to also include coordinated dysfunction throughout the body, and particularly on skeletal muscle. This could help address the large and unmet need in this high-risk population who continue to disproportionally suffer adverse health outcomes following hospitalization. Given that older persons are far more prone to sarcopenia, and that there is a sharp rise in the incidence, prevalence, severity, and poor outcomes of HF with advancing age, there is a mandate for more studies focusing on addressing sarcopenia in older persons with HF, and inserting its detection and treatment into clinical care pathways for older patients with ADHF.
ACKNOWLEDGMENTS
This study was supported in part by the research grants from the National Institutes of Health (R01AG045551, R01AG18915, P30AG021332, U24AG059624), Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, Applied Therapeutics, and the National Institute of Aging GEMSSTAR Grant (1R03AG067960). This study was also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine and by the Oristano Family Fund at Wake Forest School of Medicine.
SPONSOR’S ROLE
Sponsors had no input into this manuscript.
CONFLICT OF INTEREST
Ambarish Pandey has served on the advisory board of Roche Diagnostics. Dalane W. Kitzman reported receiving honoraria outside the present study as a consultant for Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, NovoNordisk, Astra Zeneca, and Novartis, and grant funding outside the present study from Novartis, Bayer, NovoNordisk, and Astra Zeneca, and stock ownership in Gilead Sciences.
REFERENCES
- 1.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168(5):721–730. [DOI] [PubMed] [Google Scholar]
- 2.Upadhya B, Pisani B, Kitzman DW. Evolution of a geriatric syndrome: pathophysiology and treatment of heart failure with preserved ejection fraction. J Am Geriatr Soc. 2017;65(11):2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitzman D, Haykowsky M, Tomczak C. Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:e004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attaway A, Bellar A, Dieye F, Wajda D, Welch N, Dasarathy S. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J American Geriatr Soc. 2021;69(7):1815–1825. 10.1111/jgs.17108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jha SR, Hannu MK, Chang S, et al. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation. 2016;100(2):429–436. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SM, Manghelli JL, Vader JM, et al. Prospective assessment of frailty using the fried criteria in patients undergoing left ventricular assist device therapy. Am J Cardiol. 2017;120(8):1349–1354. [DOI] [PubMed] [Google Scholar]
- 8.Pandey A, Vaduganathan M, Arora S, et al. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation. 2020;142(3):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang J, Ni W, et al. Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021;8: 1007–1017. 10.1002/ehf2.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Kitzman D, Whellan DJ, et al. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail. 2019;7(12):1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi M, Kagiyama N, Kamiya K, et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prev Cardiol. 2020. 10.1093/eurjpc/zwaa117. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019;7(12):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2): 100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of frequency of frailty and severely impaired physical function in patients ≥60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117(12):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves G, Whellan D, O’Connor C, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: The REHAB-HF Pilot Study. JACC Heart Fail. 2016;5: 359–366. [Reference 16 revised on 24th April 2021 after first online publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2019;25(5):380–400. [DOI] [PubMed] [Google Scholar]
- 18.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. [DOI] [PubMed] [Google Scholar]
- 19.Lena A, Anker MS, Springer J. Muscle wasting and sarcopenia in heart failure—the current state of science. Int J Mol Sci. 2020;21(18):6549. [DOI] [PMC free article] [PubMed] [Google Scholar]


