Abstract
Escherichia coli is a facultative anaerobe found in a wide range of environments. Commonly described as the laboratory workhorse, E. coli is one of the best characterized bacterial species to date, however much of our understanding comes from studies involving the laboratory strain E. coli K-12. Resistance-nodulation-division efflux pumps are found in Gram-negative bacteria and can export a diverse range of substrates, including antibiotics. E. coli K-12 has six RND pumps; AcrB, AcrD, AcrF, CusA, MdtBC and MdtF, and it is frequently reported that all E. coli strains possess these six pumps. However, this is not true of E. coli ST11, a lineage of E. coli , which is primarily composed of the highly virulent important human pathogen, E. coli O157:H7. Here we show that acrF is absent from the pangenome of ST11 and that this lineage of E. coli has a highly conserved insertion within the acrF gene, which when translated encodes 13 amino acids and two stop codons. This insertion was found to be present in 97.59 % of 1787 ST11 genome assemblies. Non-function of AcrF in ST11 was confirmed in the laboratory as complementation with acrF from ST11 was unable to restore AcrF function in E. coli K-12 substr. MG1655 ΔacrB ΔacrF. This shows that the complement of RND efflux pumps present in laboratory bacterial strains may not reflect the situation in virulent strains of bacterial pathogens.
Keywords: Escherichia coli, RND, efflux, ST11, acrF
Data Summary
Supplementary tables and figures are found at the end of this manuscript. The pangenome dataset is available at 10.6084 /m9.figshare.c.6147189 and the 1787 ST11 genome assemblies are available at 10.6084 /m9.figshare.21975647.
Impact Statement.
RND pumps are involved in a wide range of physiological functions. However, it is their role in antimicrobial resistance and bacterial virulence that are of significant clinical interest. Still, most of what we know is based upon data from single representative type strains. Here we demonstrate that the study of single type strains results in genetic diversity being missed.
Introduction
Escherichia coli is a Gram-negative bacterium found in a diverse range of environments, including soil, water and both the human and avian digestive tracks. The first published E. coli genome was of the well-studied laboratory strain, E. coli K-12 substrain MG1655 [1]. K-12 is commonly taken as a representative of the E. coli species, however the first genome sequence of a pathogenic E. coli strain, E. coli O157:H7, demonstrated wide genetic diversity and genome size variation [1, 2].
As genome sequencing has become more accessible, several methods for classifying E. coli assemblies have been defined. E. coli assemblies can be split into seven main phylogroups (A, B1, B2, D, E, F, G), which are groups of genomes clustered by phylogenetic similarity [3]. Isolates can also be typed by multilocus sequence typing (MLST), a method based upon the sequence of seven housekeeping genes. For example, using the Warwick MLST scheme, E. coli O157:H7, which is a serological typing, is classified as sequence type (ST) ST11. Interestingly genome size has been shown to vary not only between ST but trends are also seen between the different phylogroups, with niche-specific bacteria having much smaller genome sizes than those that live in the environment [4, 5]. Furthermore pangenome size has also been shown to vary between ST groups [6]; pangenome referring to all genes within the given population.
Resistance-nodulation-division (RND) efflux pumps are tripartite complexes found in Gram-negative bacteria. Many have broad substrate ranges, which include antimicrobials, dyes, detergents and solvents [7], however some, such as CusABC, which exports copper and silver ions, have very narrow substrate ranges. The best characterized RND system is AcrAB-TolC, homologs of which are found in E. coli, Salmonella and Klebsiella . Antimicrobial resistance can occur via this system due to mutations in regulators resulting in increased expression or mutations within the pump itself leading to the modification of substrate specificity [8–10]. Furthermore, RND efflux pumps have been shown to be involved in virulence across Gram-negative bacteria, including but not limited to Klebsiella pneumoniae, Salmonella enterica, Enterobacter cloacae and Erwinia amylovora [11–14]. The RND pump AcrF is a close homolog of the major pump AcrB; in E. coli the AcrF protein shares 77 % identity with AcrB, and 84 % nucleotide identity [15]. Yet unlike acrAB, acrEF is not expressed constitutively under laboratory conditions due to HNS silencing [16–18].
As mentioned, E. coli O157:H7 (ST11), is a frequent foodborne human pathogen though its primary reservoir is the ruminant digestive tract [19]. Disease in humans is caused through the secretion of the Shiga-toxin (Stx), which following host cell uptake is cleaved into two subunits, with subunit A targeting the ribosome resulting in protein synthesis inhibition and cellular apoptosis [20, 21]. Stx-producing E. coli infections can be asymptomatic, however symptoms of disease can vary from watery diarrhoea to haemolytic uraemic syndrome (HUS) and subsequently kidney failure [22]. Despite changes in UK hygiene practices, the number of E. coli O157 infections annually between 1983 and 2012 remained consistent, with an average of 887 cases a year [23].
In this work, the conservation of both the AcrAB and AcrEF efflux systems, plus their associated outer membrane protein (OMP), TolC, was determined across the pangenomes of 18 E. coli ST. The absence of AcrF across the ST11 lineage was confirmed at the individual assembly level and the conserved protein sequence was proven to be non-functional in vivo.
Methods
Pangenome construction
Pangenome construction is described in [24]. Briefly, assemblies were downloaded from the Enterobase E. coli/Shigella database [25]. Duplicate assemblies were removed from the analysis using mash (v1.1.1) [26] prior to annotation using prokka (v1.12) [27]. Pangenomes for each ST were then constructed using Roary (v3.10.2) using the default parameters; core-gene alignments were produced using mafft [28]. Table 1 lists all STs used and the number of assemblies included in the analysis. Assemblies are available at 10.6084 /m9.figshare.c.6147189. The STs selected were chosen to span the main phylogroups of E. coli as well as multiple pathotypes and commensal lineages. Extraintestinal pathogenic E. coli (ExPEC) lineages were of particular interest due to their clinical and AMR significance.
Table 1.
Phylogroups and sequence types included in the analysis
|
Phylogroup |
No. of assemblies |
Sequence type |
|---|---|---|
|
A |
2370 |
ST10 |
|
B1 |
40 |
ST3 |
|
B1 |
1884 |
ST17 |
|
B1 |
2441 |
ST21 |
|
B2 |
283 |
ST12 |
|
B2 |
62 |
ST14 |
|
B2 |
46 |
ST28 |
|
B2 |
873 |
ST73 |
|
B2 |
758 |
ST95 |
|
B2 |
232 |
ST127 |
|
B2 |
3186 |
ST131 |
|
B2 |
91 |
ST141 |
|
B2 |
65 |
ST144 |
|
B2 |
54 |
ST372 |
|
D |
696 |
ST69 |
|
E |
5137 |
ST11 |
|
F |
269 |
ST117 |
|
F |
382 |
ST648 |
Identification of RND gene alleles across the pangenome
The gene sequences of acrA, acrB, acrE, acrF and tolC were downloaded from E. coli K-12 substrain MG1655 (accession: NC_000913.3) and aligned using blast (v2.10.0) [29] to the Roary reference genome of each ST pangenome. Sequences with ≥95 % identity to the known sequence from MG1655 were grouped together and deemed to be a single gene ‘allele’. Matches were included in the analysis providing they had an identity percentage ≥95 %, frequency ≥1 % and the expected length for the gene in question (acrA 1194 bp, acrB 3150 bp, acrE 1158 bp, acrF 3105 bp, tolC 1482 bp). Those that did not exactly meet all three criteria were checked manually to determine whether they were in fact sequences of the gene in question.
Alignment of acrF gene regions
To further investigate the presence and absence of acrF in ST11, the region between flanking genes, acrE and yhdV, was downloaded from 11 ST11 sequences available on NCBI (Table S1, available in the online version of this article). The regions were then aligned using muscle (v3.8.1551) [30] to the acrF nucleotide sequence of E. coli K-12 substr. MG1655 (accession: NC_000913.3 : 3415033–3418137). Nucleotide sequences were translated to determine the ST11 protein sequence within the region.
Confirming the acrF insertion in over 1700 ST11 assemblies
Additional genome assemblies of ST11 (n=1999) were downloaded from the Enterobase E. coli/Shigella database and duplicate sequences were removed again using mash (genome assemblies are available at 10.6084/m9.figshare.21975647). These assemblies were then aligned to the 3150 nucleotide acrF sequence determined in the above section from E. coli O157:H7 str. TW14359 (accession: CP001368.1) using blast.
Cloning of acrF from ST11 into pET21a
The acrF gene from E. coli K-12 substr. MG1655 and E. coli O157:H7 (NCTC 12900) were both amplified from the respective chromosome using Q5 hot start high-fidelity polymerase (NEB) and the following primers; 5′-ttgaccatatgGCAAACTTTTTTATTCGAC and 5′-ggtgctcgagTTATCCTTTAAAGCAACGGC. The primers introduced the NdeI and XhoI sites, allowing the incorporation of amplimers into the high-copy vector, pET21a (Invitrogen). Both vectors were then transformed into E. coli K-12 substr. MG1655, E. coli K-12 substr. MG1655 ΔacrB, E. coli K-12 substr. MG1655 ΔacrB ΔacrF.
Measurement of minimum inhibition concentration (MIC)
The MIC of ethidium bromide, rhodamine 6G and erythromycin were determined by broth microdilution according to the EUCAST guidelines [31]. Strains used in this assay are listed in Table 2. Fold changes greater than twofold were deemed significant.
Table 2.
Strains used in this study
Results
Both acrAB and acrEF are highly conserved across E. coli lineages
Pangenomes of 18 ST were constructed from a total of 18 869 genome assemblies and the variation of acrA, acrB, acrE, acrF and tolC was determined across. In this context, allele refers to a group of sequences with ≥95 % sequence identity to one another. Across the 18 lineages, the mean number of alleles for acrA, acrB, acrE and tolC was between 1.1 and 1.3 (Table 3). However, acrF had a mean of 1.7 alleles per ST (Table 3).
Table 3.
Alleles of acrA, acrB, acrE, acrF and tolC across the pangenomes of 18 E. coli ST
Allele conservation for five resistance-nodulation-division (RND) genes across 18 sequence types (ST) of E. coli . Here, allele refers to a group of sequences with ≥95 % sequence identity to one another. ST11 had no acrF allele.
|
Resistance-nodulation-division gene |
|||||||
|---|---|---|---|---|---|---|---|
|
Phylogroup |
n |
Lineage |
acrA |
acrB |
acrE |
acrF |
tolC |
|
A |
2370 |
ST10 |
1 |
1 |
2 |
5 |
2 |
|
B1 |
40 |
ST3 |
1 |
1 |
1 |
1 |
1 |
|
B1 |
1884 |
ST17 |
1 |
1 |
1 |
2 |
1 |
|
B1 |
2441 |
ST21 |
1 |
1 |
2 |
1 |
1 |
|
B2 |
283 |
ST12 |
1 |
2 |
2 |
4 |
1 |
|
B2 |
62 |
ST14 |
1 |
1 |
1 |
2 |
1 |
|
B2 |
46 |
ST28 |
1 |
1 |
1 |
1 |
1 |
|
B2 |
873 |
ST73 |
2 |
2 |
1 |
1 |
1 |
|
B2 |
758 |
ST95 |
2 |
1 |
1 |
1 |
1 |
|
B2 |
232 |
ST127 |
2 |
1 |
1 |
1 |
1 |
|
B2 |
3186 |
ST131 |
1 |
2 |
2 |
2 |
1 |
|
B2 |
91 |
ST141 |
1 |
1 |
1 |
1 |
1 |
|
B2 |
65 |
ST144 |
1 |
1 |
1 |
2 |
1 |
|
B2 |
54 |
ST372 |
1 |
1 |
1 |
1 |
1 |
|
D |
696 |
ST69 |
1 |
1 |
2 |
2 |
1 |
|
E |
5137 |
ST11 |
2 |
2 |
1 |
0 |
1 |
|
F |
269 |
ST117 |
1 |
1 |
1 |
2 |
2 |
|
F |
382 |
ST648 |
1 |
1 |
1 |
2 |
1 |
|
Total |
18 869 |
Mean number of alleles |
1.2 |
1.2 |
1.3 |
1.7 |
1.1 |
The gene encoding tolC, the OMP for five of the six E. coli RND systems, was the most conserved across the 18 lineages (Table 3). Both acrA and acrB were also found to be highly conserved with most STs included in the analysis possessing only a single allele across the respective pangenome (Table 3). The PAP associated with acrF, acrE, had a mean of 1.3 alleles per lineage. However, the most diverse gene across the dataset was acrF. ST10 was found to have five acrF alleles across the lineage yet no alleles were present in the ST11 pangenome. Moreover, aligning the 5137 individual assemblies used to construct the ST11 pangenome against the acrF gene from MG1655 using blastn did not identify an acrF gene, suggesting acrF in ST11 isolates is not the same as in E. coli K-12.
The acrF gene in ST11 has a conserved 45 bp insertion
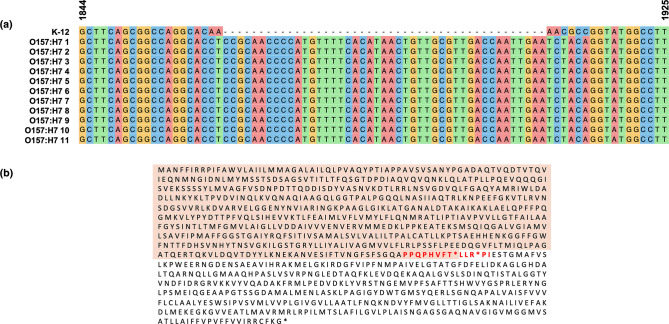
To confirm the absence of acrF across ST11, the region downstream of acrE where acrF is located was downloaded from 11 E. coli O157:H7 genomes available on NCBI as O157:H7 is a very well-characterized ST11 strain. Aligning these sequences using acrF from E. coli K-12 substr. MG1655 as a reference revealed an insertion of 45 nucleotides between nucleotides 1861 and 1914 (Fig. 1a), extending the acrF gene from 3105 to 3150 nucleotides. In silico translation of this 45 bp product revealed an insertion of 13 amino acids plus two internal stop codons suggesting a truncated product (Figs. 1b and S1).
Fig. 1.
acrF in E. coli ST11 contains a 45-nucleotide insertion. (a) Alignment of 11 ST11 acrF sequences downloaded from NCBI database confirms an insertion, not present in E. coli K-12 substr. MG1655. (b) Translation of these genes (example O157:H7 2) confirms the insertion contains two internal stop codons.
To validate this finding 1999 assemblies of E. coli ST11 were downloaded from the Enterobase E. coli/Shigella database. A total of 212 assemblies were removed from the analysis as they were duplicates. The remaining 1787 assemblies were then used to confirm whether the insertion found in the 11 reference ST11 genomes was conserved in a larger sample size. Using the acrF sequence from E. coli O157:H7 strain TW14359, an assembly used in the preliminary alignment, it was confirmed that 1744 out of the 1787 assemblies (97.59 %) had a gene that matched the length expected for ST11 (3150 nucleotides). Sequence identity of those matches ranged from 99.56–100 %, with the average percentage identity at 99.94 %, showing that the insertion is highly conserved within ST11 genomes. Of the 43 genome assemblies that did not have the 3150 bp sequence, 7(0.39 %) had the K-12 sequence. In the remaining 36 genome assemblies, no acrF gene sequences were identified in alignments against either the K-12 and O157 sequences because either there were additional deletions within acrF, the acrF gene was entirely absent, or the gene locations on the assembly contigs prevented identification of acrF.
AcrF in E. coli ST11 is non-functional
While the presence of two stop codons suggested AcrF was non-functional in ST11, the acrF sequences from MG1655 and ST11 NCTC 12900 were each cloned into the high-copy vector pET21a. In order to investigate the function of AcrF, acrB was also inactivated as it encodes the major RND efflux pump AcrB, which is known to mask the subtle phenotype of acrF expression. The function of AcrF was determined by measuring susceptibility to the known AcrF substrates, ethidium bromide, rhodamine 6G and erythromycin.
For the three substrates tested, deletion of acrB significantly reduced the MIC when compared to E. coli K-12 subsp. MG1655, but the additional interruption of acrF had no additive effect. Complementation with the acrF sequence from E. coli K-12 did not alter erythromycin susceptibility, however the MICs for ethidium bromide and rhodamine 6G increased from 8 µg ml−1 to 64 µg ml−1 and 16 µg ml−1 to 64 µg ml−1, respectively. Complementation with the ST11 sequence had no significant effect on susceptibility for any of the compounds tested confirming that AcrF is non-functional in E. coli ST11 (Table 4).
Table 4.
Susceptibility of E. coli K-12 substr. MG1655 ΔacrB ΔacrF following complementation with acrF from E. coli K-12 substr. MG1655 and E. coli O157:H7 (ST11)
|
MIC (µg ml−1) |
|||
|---|---|---|---|
|
Strain |
ERY |
R6G |
EtBr |
|
E. coli ATCC 25922 |
64 |
1024 |
256 |
|
E. coli K-12 substr. MG1655 |
64 |
512 |
512 |
|
E. coli K-12 substr. MG1655 ΔacrB |
4 |
8 |
8 |
|
E. coli K-12 substr. MG1655 ΔacrB ΔacrF |
4 |
8 |
8 |
|
E. coli K-12 substr. MG1655 ΔacrB ΔacrF+pET21 a |
4 |
16 |
8 |
|
E. coli K-12 substr. MG1655 ΔacrB ΔacrF+pET21 a K-12 sequence |
8 |
64 |
64 |
|
E. coli K-12 substr. MG1655 ΔacrB ΔacrF+pET21 a ST11 sequence |
4 |
16 |
8 |
ERY, erythromycin; EtBr, ethidium bromide; R6G, rhodamine 6G.
Discussion
Across the Gram-negative bacteria it is generally assumed that efflux pump sequences are conserved across a species, with genes present in common laboratory strains often taken as representatives of a whole species. This is despite several studies demonstrating that clinical isolates of Acinetobacter baumannii can lack adeB, which encodes the RND component of the AdeABC system associated with reduced antimicrobial susceptibility [32–34]. Here we show that while it is assumed that all E. coli isolates possess AcrB, AcrD, AcrF, CusA, MdtBC and MdtF, this is not true for the ST11 lineage.
E. coli ST11 is a highly virulent E. coli lineage, which includes the Stx producing Escherichia coli O157:H7. While the main reservoir of O157:H7 is the recto-anal junction of ruminants [35], it is also a frequent foodborne pathogen in humans causing bloody diarrhoea and in severe cases, HUS through the secretion of Stx [36].
AcrB is regarded as the major RND efflux pump in E. coli as it is expressed constitutively under laboratory conditions and has been demonstrated to transport a wide range of substrates, including clinically relevant antimicrobials and host molecules such as bile salts [37, 38]. It was therefore unsurprising that acrB was more conserved than acrF across the pangenomes of 18 E. coli STs (1.2 alleles per pangenome for acrB compared with a mean of 1.7 for acrF) because despite the high nucleotide and amino acid similarity [15], AcrF has a much narrower substrate range than AcrB and unlike acrB, the acrF operon is HNS silenced [15, 18].
The absence of AcrF from the core genome of phylogroup E, the phylogenetic group to which ST11 (and therefore O157:H7) belongs, has been previously noted [39]. We believe the absence of this gene in both our work and a previously published pangenome study is due to the highly conserved insertion of 45 nucleotides, which when translated encodes 13 amino acids and two stop codons, thus resulting in two truncated products.
The loss of RND pumps has been linked to virulence attenuation in many Gram-negative bacteria including Neisseria gonorrhoeae, E. amylovora and Vibrio cholerae [13, 40, 41]. For example, Salmonella spp. possess a close homolog of AcrAB-TolC, and deletion of acrB in Salmonella enterica serovar Typhimurium has been shown to reduce pathogen colonization within the host [42]. Moreover, targeted inactivation of the RND pump AcrB via a single D408A point mutation has been demonstrated to result in decreased expression of Salmonella pathogenicity island genes, which facilitate bacterial invasion and replication within host epithelial cells [43]. Crucially, inactivation of acrF in Salmonella was shown to significantly reduce mortality in mice and reduce adhesion to and invasion of INT 407 cells [42, 44]. Given the well-documented virulence of ST11 E. coli within the human host, the lack of AcrF in this lineage is surprising and perhaps suggests that the importance of AcrF in virulence is species, or even lineage, dependent. It is possible this could be due to functional redundancy of RND efflux pumps in Gram-negative bacteria meaning the loss of AcrF function does not have a significant impact on the lifestyle of this E. coli lineage. Nevertheless, the findings presented here add to the growing notion that RND pump diversity and conservation is a key factor in the development of future antimicrobials and efflux pump inhibitors.
Supplementary Data
Funding information
This work was supported by BBSRC MIBTP DTP grant BB/M01116X/1 awarded to HLP and MRC IMPACT DTP grant MR/N013913/1 awarded to PS.
Acknowledgements
We would like to thank Dr Douglas Browning, Dr Helen McNeil and Dr Enea Sancho Vaello for their valuable input throughout the project.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ExPEC, Extraintestinal pathogenic Escherichia coli; HUS, Haemolytic uremic syndrome; MIC, Minimum inhibition concentration; MLST, Multilocus sequence typing; OMP, Outer membrane protein; PAP, Periplasmic adaptor protein; RND, Resistance-nodulation-division; ST, Strain type; Stx, Shiga toxin.
One supplementary figure and one supplementary table are available with the online version of this article.
References
- 1.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- 4.Horesh G, Blackwell GA, Tonkin-Hill G, Corander J, Heinz E, et al. A comprehensive and high-quality collection of Escherichia coli genomes and their genes. Microb Genom. 2021;7:000499. doi: 10.1099/mgen.0.000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta C, Paul S. Microbial lifestyle and genome signatures. CG. 2012;13:153–162. doi: 10.2174/138920212799860698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins EA, Hall RJ, Connor C, McInerney JO, McNally A. Pangenome evolution in Escherichia coli is sequence type, not phylogroup. bioRxiv. 2022 [Google Scholar]
- 7.Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta. 2009;1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsolioutsou A, Martins EA, White DG, Levy SB, Demple B. A soxRS-constitutive mutation contributing to antibiotic resistance in a clinical isolate of Salmonella enterica (Serovar typhimurium) Antimicrob Agents Chemother. 2001;45:38–43. doi: 10.1128/AAC.45.1.38-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koutsolioutsou A, Peña-Llopis S, Demple B. Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates. Antimicrob Agents Chemother. 2005;49:2746–2752. doi: 10.1128/AAC.49.7.2746-2752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair JMA, Bavro VN, Ricci V, Modi N, Cacciotto P, et al. AcrB drug-binding pocket substitution confers clinically relevant resistance and altered substrate specificity. Proc Natl Acad Sci U S A. 2015;112:3511–3516. doi: 10.1073/pnas.1419939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla E, Llobet E, Doménech-Sánchez A, Martínez-Martínez L, Bengoechea JA, et al. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother. 2010;54:177–183. doi: 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil HE, Alav I, Torres RC, Rossiter AE, Laycock E, et al. Identification of binding residues between periplasmic adapter protein (PAP) and RND efflux pumps explains PAP-pump promiscuity and roles in antimicrobial resistance. PLoS Pathog. 2019;15:e1008101. doi: 10.1371/journal.ppat.1008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burse A, Weingart H, Ullrich MS. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora . Mol Plant Microbe Interact. 2004;17:43–54. doi: 10.1094/MPMI.2004.17.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Pérez A, Poza M, Fernández A, Fernández M del C, Mallo S, et al. Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae . Antimicrob Agents Chemother. 2012;56:2084–2090. doi: 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli . J Bacteriol. 2001;183:5803–5812. doi: 10.1128/JB.183.20.5803-5812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg EY, Ma D, Nikaido H. AcrD of Escherichia coli is an aminoglycoside efflux pump. J Bacteriol. 2000;182:1754–1756. doi: 10.1128/JB.182.6.1754-1756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino K, Hayashi-Nishino M, Yamaguchi A. H-NS modulates multidrug resistance of Salmonella enterica serovar Typhimurium by repressing multidrug efflux genes acrEF. Antimicrob Agents Chemother. 2009;53:3541–3543. doi: 10.1128/AAC.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino K, Yamaguchi A. Role of histone-like protein H-NS in multidrug resistance of Escherichia coli . J Bacteriol. 2004;186:1423–1429. doi: 10.1128/JB.186.5.1423-1429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roe AJ, Yull H, Naylor SW, Woodward MJ, Smith DGE, et al. Heterogeneous surface expression of EspA translocon filaments by Escherichia coli O157:H7 is controlled at the posttranscriptional level. Infect Immun. 2003;71:5900–5909. doi: 10.1128/IAI.71.10.5900-5909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pianciola L, Rivas M. Genotypic features of clinical and bovine Escherichia coli O157 strains isolated in countries with different associated-disease incidences. Microorganisms. 2018;6:36. doi: 10.3390/microorganisms6020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruyand M, Mariani-Kurkdjian P, Gouali M, de Valk H, King LA, et al. Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med Mal Infect. 2018;48:167–174. doi: 10.1016/j.medmal.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Butt S, Jenkins C, Godbole G, Byrne L. The epidemiology of Shiga toxin-producing Escherichia coli serogroup O157 in England, 2009-2019. Epidemiol Infect. 2022;150:e52. doi: 10.1017/S0950268822000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams NL, Byrne L, Smith GA, Elson R, Harris JP, et al. Shiga toxin-producing Escherichia coli O157, England and Wales, 1983-2012. Emerg Infect Dis. 2016;22:590–597. doi: 10.3201/eid2204.151485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor CH, Zucoloto AZ, Yu I-L, Corander J, McDonald B, et al. Multi-drug resistant E. coli displace commensal E. coli from the intestinal tract, a trait associated with elevated levels of genetic diversity in carbohydrate metabolism genes. Microbiology. 2022;2022 doi: 10.1101/2022.11.25.517930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Alikhan NF, Mohamed K, Fan Y, Agama Study G, et al. The enterobase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020;30:138–152. doi: 10.1101/gr.251678.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 28.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical M, Infectious D EUCAST definitive document E.DEF 3.1, June 2000: determination of minimum inhibitory concentrations (mics) of antibacterial agents by agar dilution. Clin Microbiol Infect. 2000 doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 32.Leus IV, Adamiak J, Trinh AN, Smith RD, Smith L, et al. Inactivation of AdeABC and AdeIJK efflux pumps elicits specific nonoverlapping transcriptional and phenotypic responses in Acinetobacter baumannii . Mol Microbiol. 2020;114:1049–1065. doi: 10.1111/mmi.14594. [DOI] [PubMed] [Google Scholar]
- 33.Nowak J, Seifert H, Higgins PG. Prevalence of eight resistance-nodulation-division efflux pump genes in epidemiologically characterized Acinetobacter baumannii of worldwide origin. J Med Microbiol. 2015;64:630–635. doi: 10.1099/jmm.0.000069. [DOI] [PubMed] [Google Scholar]
- 34.Darby EM, Bavro VN, Dunn S, McNally A, Blair JMA. RND pumps across the Acinetobacter genus; AdeIJK is the ancestral efflux system. bioRxiv. 2022;2022 doi: 10.1101/2022.10.19.512856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, et al. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect Immun. 2003;71:1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauder AB, Kendall MM. After the fact(or): posttranscriptional gene regulation in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2018;200:19. doi: 10.1128/JB.00228-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, et al. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli . Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 38.Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A. Indole induces the expression of multidrug exporter genes in Escherichia coli . Mol Microbiol. 2005;55:1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- 39.Teelucksingh T, Thompson LK, Cox G. The Evolutionary conservation of Escherichia coli drug efflux pumps supports physiological functions. J Bacteriol. 2020;202:22. doi: 10.1128/JB.00367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jerse AE, Simms AN, Crow ET, Snyder LA, et al. A gonococcal efflux pump system enhances bacterial survival in A female mouse model of genital tract infection. Infect Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bina XR, Howard MF, Taylor-Mulneix DL, Ante VM, Kunkle DE, et al. The Vibrio cholerae RND efflux systems impact virulence factor production and adaptive responses via periplasmic sensor proteins. PLoS Pathog. 2018;14:e1006804. doi: 10.1371/journal.ppat.1006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishino K, Latifi T, Groisman EA. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2006;59:126–141. doi: 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang-Kan X, Blair JMA, Chirullo B, Betts J, La Ragione RM, et al. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica serovar Typhimurium. mBio. 2017;8:e00968-17. doi: 10.1128/mBio.00968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blair JMA, Smith HE, Ricci V, Lawler AJ, Thompson LJ, et al. Expression of homologous RND efflux pump genes is dependent upon AcrB expression: implications for efflux and virulence inhibitor design. J Antimicrob Chemother. 2015;70:424–431. doi: 10.1093/jac/dku380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.