Abstract
Background:
Alzheimer’s disease (AD) is the most common form of dementia, particularly in older adults, with clinical manifestations of progressive cognitive decline and functional impairment. The prevalence of AD and related dementia is mounting worldwide, but its etiology remains unresolved, with no available preventative or ameliorative therapy. Emerging evidence suggests that the gut microbiota of patients with AD is different from cognitively normal counterparts.
Summary:
Communication between gut and brain (gut-brain axis) plays a crucial role in AD pathology. Bacteria inhabiting the gut strongly influence this gut-brain axis and thus may participate in AD pathology. Diet, one of the strongest modulators of gut microbiota, also strongly influences brain health and AD pathology. Gut microbiota metabolites including short-chain fatty acids, pro-inflammatory factors, and neurotransmitters may also affect AD pathogenesis and associated cognitive decline. Therefore, investigation of diet-microbiota-brain axis is important to better understand its contribution in AD pathology and its potential use as a target to prevent and treat AD. Herein, we discuss the link between AD and gut microbiota and ponder how microbiota modulation through nutritional approaches may offer avenues for discovering novel preventive and therapeutic strategies against AD.
Key Message:
A strong association exists between lifestyle factors and AD prevalence wherein unhealthy dietary factors have been linked to neurodegeneration. Specific prudent dietary patterns might help in preventing or delaying AD progression by affecting β-amyloid production and tau processing and regulating AD-associated inflammation, metabolism and oxidative stress, plausibly via modulating gut microbiota.
Keywords: Amyloid, Cognition, Dementia, Microbiota, Neurodegenerative disease, Tau
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder affecting over 50 million people worldwide and is the leading cause of dementia and cognitive decline, especially in older adults [1]. Antecedently, AD prevalence was highest in high-income countries (West Europe and North America); however, dementia incidences have started to increase in middle- to low-income countries, with a projected 63% of all cases being in middle- to low-income countries by 2030 and 71% of all cases by 2050 [1]. AD majorly affects the elderly (>65 years), indicating aging as one of the major risk factors, where aging-associated AD progression is called sporadic AD [2]. While the reasons for increasing AD prevalence remain unclear, emerging evidence has linked dietary habits to the AD pathology [3]. For instance, obesity and type-2 diabetes are potential risk factors for AD, and studies have linked high-fat diet and high-carb diet with the risk of AD development. Conversely, the Mediterranean (MD) and ketogenic diets have been linked with healthier brain aging and lower AD risk [4–7]. Further corroborating this evidence, there is an increase in AD prevalence following the epidemiological shift seen in developing countries where there are increasing proportions of obese and diabetic people [1]. In addition to these macroscopic issues, bacteria living in our gut (gut microbiota) may play an important role in AD pathology [2, 8–10]. While mechanisms are still not fully understood, studies suggest that the gut microbiota may influence the brain via inflammation, neurotransmitters, and hormones. Thus, reversing gut microbiota abnormalities through healthy diets could benefit the brain and reduce AD risk. Herein, we discuss current research linking diet and gut microbiota with AD pathology based on the gut-brain axis.
The Pathophysiology of AD
Although the pathology and contributing factors for AD remain unclear, there are genetic mutations such as the extra copy of the amyloid precursor protein (APP) gene and trisomy of chromosome 21, which enhance the neurodegenerative amyloid-beta (Aβ)-protein deposition associated with familial AD [11]. Pathologically, extracellular neurofibrillary plaques and intracellular hyperphosphorylated tau (pTau) tangles are found throughout the cortical parenchyma of the AD brain, especially in the temporal lobes [12]. Profound cerebral atrophy may also support the diagnosis of AD. These visible characteristics have been viewed as main factors of AD pathology for a long time. The buildup of Aβ plaques and neurofibrillary tangles of pTau in the neocortex causes inflammation, oxidative stress, and ultimate neurodegeneration. These symptoms are also mediated by reactive oxygen species, which are normally kept in check via enzymatic antioxidants. However, when antioxidant levels decrease, reac tive oxygen species can cause oxidative stress and lead to neurodegeneration [13]. The inflammation in the brain is also linked with gut leakiness that is common in AD patients, where immune-system agents such as polymorphonuclear neutrophils are able to leak out of the intestinal walls. Continual inflammation from the gut can start to degrade the blood-brain barrier, allowing these inflammatory agents to enter the brain and cause further inflammation. Biomarkers used in AD diagnosis include low Aβ−42 and high tau levels in the brain, decreased fluorodeoxyglucose uptake on PET, and structural cerebral atrophy on MRI. The clinical diagnosis of AD varies from patient to patient and is difficult to characterize with a prescribed set of symptoms. However, symptoms that are more severe than those typical of old age in the ten major diagnostic categories can be a red flag for diagnosticians. These 10 areas of concern in the AD patient are: degradation/difficulty with memory, problem-solving, familiar task-completion, time/place recognition, comprehension of images or spatial relationships, communication, retracing steps, judgment/interpersonal interactions, work/social activities, and mood/personality [14].
Because of incomprehensibility of amyloid cascade hypothesis, studies are investigating Aβ plaques and pTau as markers of a system that has been disrupted from many ends. Studies are hypothesizing that β-amyloids are antimicrobial peptides which may accumulate in the AD brain due to higher carriage of nonbeneficial microbes possibly coming from the degraded blood-brain barrier resulting from inflammation [2]. These amyloids are formed by the cleavage of APP and the activation of myeloid differentiation primary response 88 pathway by toll-like receptor 2 [2]. In addition to brain amyloidosis, there are bacterial amyloids located in the gut, which resemble CNS amyloids in tertiary structure and might play a role in stimulating the immune system in the brain as the immune system learns to recognize the amyloids in the gut and then has an enhanced attack of the amyloids in the brain, leading to inflammation. The leaky gut syndrome that allows for inflammation and neurodegeneration in AD is further mediated by diet, for example, high-fat diet. Thus, the measurable symptoms of AD have complex causes that correlate with gut microbiota.
Gut-Brain Axis in AD
The Aβ plaque and tau tangles hypotheses were the ideal model for AD pathophysiology. However, emerging research has established AD as a component of systemic dysfunction at least in-part mediated by chronic, systemic, and neuronal inflammation, along with gut microbiota. Within this, neuroinflammation hypothesis lies the gut-brain axis, which links the gut microbiota activities to neuronal health and dysfunction (Fig. 1). The effects of gut microbiota in AD are mediated by microbial metabolites that either act on local neurons in the gut and surrounding tissues and send signals to the brain, and/or get absorbed from the gut and reach to brain through circulation. Such examples are monoamines, short-chain fatty acids (SCFAs), gamma-aminobutyric acid (GABA), beta-methylamino-l-alanine, brain-derived neurotrophic factor, serotonin, and dopamine [8, 10].
Fig. 1.
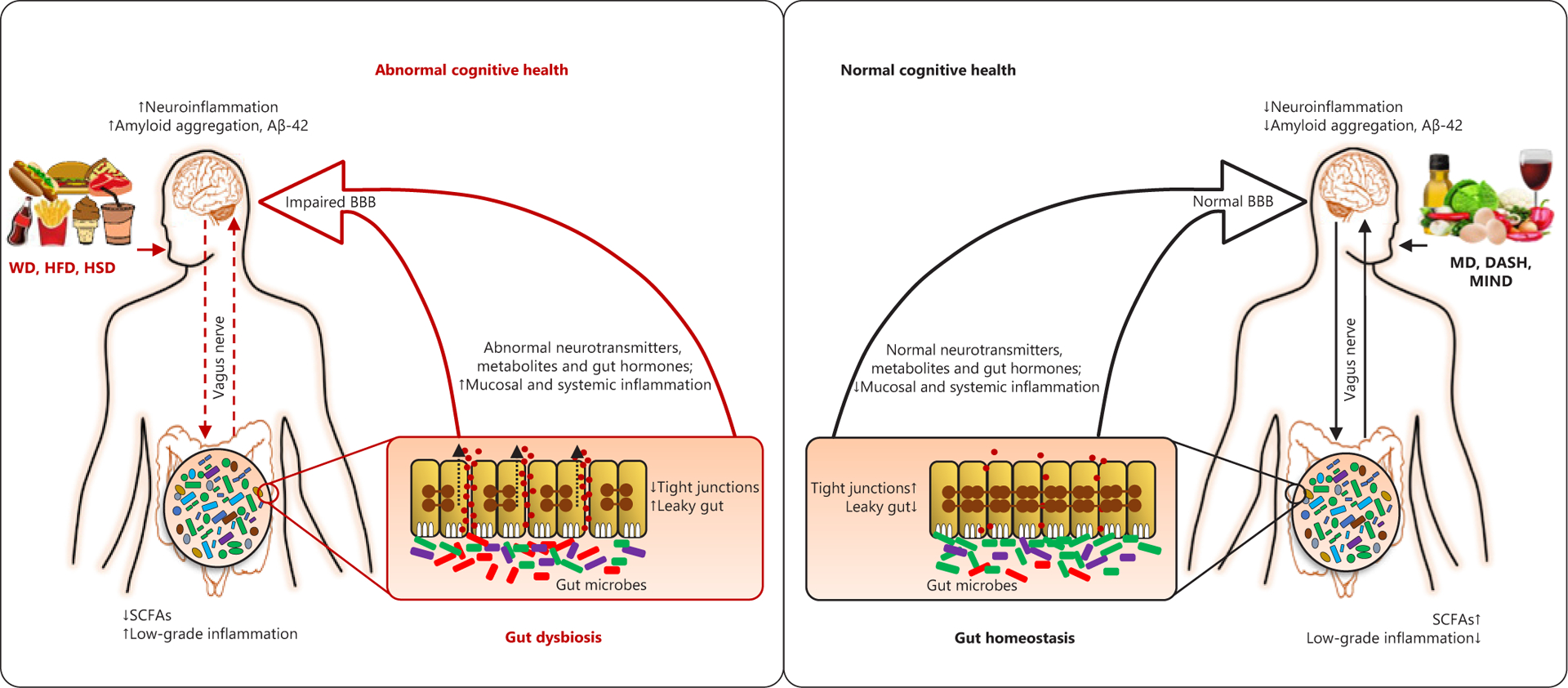
Schematic representation of the putative links connecting diet-microbiome interaction with brain and cognitive health in subjects with MCI and AD. Aβ, amyloid-beta; BBB, blood-brain barrier; BDNF, brain-derived neurotrophic factor; DASH, dietary approaches to stop hypertension diet; HFD, high-fat diet; HSD, high-sugar diet; MD, Mediterranean diet; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay diet; NMDAR, N-methyl-d-aspartate receptor; SCFA, short-chain fatty acids; WD, Western-style diet; AD, Alzheimer’s disease; MCI, mild cognitive impairment.
Emerging human studies including ours have revealed distinct microbiota patterns in AD patients and/or subjects with mild cognitive impairment (MCI) [7, 9]. The already-present microbiota dysbiosis further impacts the aging brain as the natural aging-related gut dysbiosis begins to develop and weakens the intestinal epithelia, causing gut leakiness and inflammation [15]. Increased systemic inflammation instigates immune impairments, allowing β-amyloid buildup in the brain [15]. This indicates that patients typically develop AD during old age even when risk factors were present from early life, for example, genetic mutation. Patients with MCI and AD also show higher levels of gut microbiota-derived trimethylamine N-oxide (TMAO) in the cerebrospinal fluid [16], which correlate with AD biomarkers including pTau, total Tau, and Aβ42. TMAO treatment reduces cognitive function and aging signs in mice, by ameliorating neuronal senescence and mitochondrial dysfunction [17]. TMAO and its precursors have inflammatory biomarkers, possibly contributing to AD-related leaky gut although mechanisms of TMAO in AD remain unclear. The Western diet consists of low-fiber, high-fat, and high-protein foods, where it is common to eat fatty red meats and eggs that are rich in TMA and choline, thus increase TMAO production. This might partly explain higher AD cases in Western countries because Western diet is enriched with these ingredients. Additionally, TMAO-associated brain damage and cognitive deficits may be mediated by increased oxidative stress [17]. Because brain consumes a large amount of oxygen and the neurons have high metabolic rate, the neurons in the brain are at higher risk for oxidative stress in an average person. However, oxidative stress can be both cause and consequence of AD pathology. Inflammation instigates oxidative stress, which can lead to Aβ and pTau accumulation; however, vice-versa is also possible in a destructive cycle. Additionally, oxidative stress can cause mitochon drial dysfunction, and gut microbiota-derived metabolites influence mitochondrial functions. Oxidative stress can be modulated by diet, for example, high-fruit-and-vegetable diets improve cognition, which is linked with decreased oxidative stress in elderly; whereas Western diet is not enriched with fruits and vegetables, which might also explain higher AD prevalence in Western countries.
The Implication of Gut Microbiota in AD
Gut microbiota is vital for our metabolic, immune, and brain health. Under healthy homeostatic conditions, the gut microbiota maintains a symbiotic relationship with the host and exerts important functions for host nutrition and metabolism, colonization resistance to pathogens, intestinal barrier integrity, and immune regulation. Gut microbiota has implications in brain diseases including depression, anxiety, hypertension, and Parkinson’s disease. Recent studies also link gut microbiota with AD; however, it should be noted that majority of these studies are cross-sectional investigations, and hence, more longitudinal intervention studies are needed to identify casual links between microbiota and AD pathology. We recently reported lower abundance of Bacteroidetes and higher abundance of Firmicutes and Proteobacteria (enterobacteriaceae) in MCI patients in addition to several differences at the genus level [7]. Other studies also reported lower diversity and lower Firmicutes-to-Bacteroidetes ratio in AD subjects [9], and lower Bacteroides in demented patients [18], while lower diversity, lower Firmicutes, and higher Proteobacteria were found in AD patients compared to MCI patients and normal subjects [19]. One study demonstrated involvement of butyrate-producing bacteria in cognitive function, where butyrate is classified as a post-biotic as it is the metabolic output (SCFA) of bacteria and has numerous benefits to the gut [20]. Interestingly, we also showed that a MD-ketogenic diet improved the AD biomarkers viz. amyloid and tau proteins in the cerebrospinal fluids of MCI patients, wherein these changes linked with increased gut butyrate [7].
Furthermore, an association of Aβ accumulation with microbiota has been investigated. For instance, APP-PS1 mice, the most-widely used AD model, demonstrate Aβ accumulation in the brain in an age-dependent manner; however, its microbiota differs from wild-type mice. Further, mice transplanted with AD microbiota tend to have higher Aβ accumulation. Further, Aβ aggregation can be inhibited by microbiota-derived valerate and butyrate [10]. Besides, bacterial endotoxins may also be involved in AD-amyloidosis-related inflammation. For example, lipopolysaccharide (LPS), an outer cell wall component of Gram-negative bacteria that can be highly pro-inflammatory, can enhance Aβ accumulation in brain and induce cognitive dysfunction [21]. AD patients show higher LPS levels in blood plasma [22], and neocortex and hippocampus [23]. LPS can also cause chronic neuroinflammation, nerve cell death in entorhinal cortex, and impairment of synaptic plasticity of neurons in hippo-campus [24]. Specific bacteria, for example, Escherichia coli, Bacillus subtilis, Salmonella typhimurium, and Salmonella enterica can also produce amyloids [25]; however, these are not typical and consistent inhabitants of the human gut microbes. In addition, the relationship between AD-associated amyloidosis versus bacterial amyloids remains unclear. Nevertheless, bacterial amyloid might activate specific signaling pathways involved in AD pathogenesis, hinting that the gut microbiota might exacerbate amyloidosis-associated inflammation. These studies link microbiota with the accumulation of amyloids via microbial extracellular components, pro-inflammatory factors, toxins, SCFAs, amyloid, and neurotransmitters; and underscore further investigation of links between gut microbiota and host neurodegenerative health [23]. Considering rapidly emerging studies, the future boom in AD prevention and cure research would foreseeably as well be directed toward microbiota research.
Diet-Microbiota Interaction in the Amelioration of AD
Given the emerging data on microbiota disparities between AD patients versus healthy subjects, researchers have started exploring ways to modulate the microbiome with the ambition to hopefully ameliorate AD pathology. Although gut microbiota can be manipulated through many methods including the use of probiotics, prebiotics, synbiotics, and antibiotics or change of diet, diet is the superlative modulator of gut microbiota. Dietary modulations focused on unsaturated fats, fruits and vegetables, and whole grains can confer benefits on AD-related cognitive health (Fig. 1). For instance, MD correlates with less brain atrophy in key-AD areas (indicating positive effect on AD pathology) [5], besides decreased inflammation, a major symptom of AD, via increasing plasma carotenoids and decreasing C-reaction protein levels [4]. Higher consumption of fish products, which are rich in docosahexaenoic acid (a type of n-3 polyunsaturated fatty acid), has been linked with lower AD risks [3]. Further more, fish, which is rich in vitamin D3, and dairy products, which are rich in vitamin D, promote neural growth factor protein, which can protect against brain inflammation and aging [26]. Prompted by these ameliorative effects of MD against AD, one human study examining the effects of MD-DASH Intervention for Neurodegenerative Delay (MIND) diet, which is a combination of MD with DASH diet (Dietary Approaches to Stop Hypertension), which is rich in fruits, vegetables, whole grains, low-fat dairy, and lean protein, demonstrated that MIND diet was more effective than MD or DASH alone, although all 3 diets demonstrated benefits at reducing AD pathology [6]. In addition to certain dietary lifestyles, individual foods can confer benefits against AD pathology. For instance, consumption of red wine has been shown to prevent the generation of Aβ peptides and reduce AD risk in mouse models [27]. Specifically, it has been reported that moderate alcohol consumption could decrease GABAergic sprouting of axon terminals that is potentially partially responsible for the neurodegeneration found in AD [28]. Flavonoids-rich foods, for example, black currants, grapes, citrus, and green tea, have been shown to inhibit Aβ deposition and prevent hyperphosphorylation of tau proteins, while improving other biomarkers of AD [29].
While the majority of studies examine the impact of healthy diet on the amelioration of AD pathology, several studies have explored the use of supplementation directly targeting the gut bacteria in AD. A randomized double-blind controlled human trial looking at the effects of a 12-weeks consumption of a probiotic combination of Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum, reported significant positive effects on cognitive functioning, although no effects on oxidative stress or inflammation was seen [30]. A mouse study examining the effects of the post-biotic sodium butyrate on Aβ levels and behavioral symptoms in early AD mice found significant reduction in Aβ levels and increase in behavioral responses after 12-weeks of sodium butyrate supplementation [31]. Synbiotics, a combination of probiotics and prebiotics, also show benefits to AD. One human study observed improvement in mental capabilities and reduced inflammation and oxidative stress in AD patients after consuming milk fermented with kefir grains (forming a symbiotic substance) for 90 days [32]. Taken together, the current literature on diet and supplementation influencing the gut microbiota and improve AD pathology is promising, but more studies are warranted. Additionally, research has targeted GABA by testing Tramiprosate, which is an analogue of GABA, and found that it showed promise in increasing the long-term potentiation that had been inhibited by Aβ in AD models as well as clinical trials [33–35]. The drug trazodone, a serotonin agonist and reup-take inhibitor, has also indicated a correlation with delaying cognitive impairment in AD patients 2.6 times more than nontrazadone users [36].
Conclusion
AD is rapidly rising worldwide, but no cure is available hitherto, underscoring the need for effective strategies such as dietary factors to modulate AD-related neuroinflammation and prevent or slow down AD progression. Current evidence shows that both pro-inflammatory and anti-inflammatory capacities of dietary components may play a role in AD management. Diets rich in simple sugars, saturated/trans fats, advanced glycation end-products, and processed meats may incite a pro-inflammatory influence on the brain of AD patients while potentially accelerating obesity, hypertension, dyslipidemia, atherosclerosis, and type-2 diabetes. Contrastingly, complex dietary patterns (e.g., MD, DASH, and MIND) rich in vegetables, fruits, salads, nuts, legumes, berries, polyunsaturated fatty acids, vitamins, flavonoids, polyphenols, probiotics/prebiotics, and whole grains may help in preventing or slowing down the cognitive decline and AD progression. Studies have suggested the involvement of gut microbiota in AD pathology via gut-brain axis. Diet strongly modulates the gut microbiota, which might be one of the mechanisms underlying the benefits of these dietary patterns in ameliorating AD-related perturbations in gut-brain axis. Mechanistic understanding of interaction of lifestyle factors with AD may elucidate links between changing lifestyle patterns and increased AD prevalence while including factors such as microbiota that are crucial for this interaction between host lifestyle and health. Indeed, decoding the connections between diet, microbiota, lifestyle, and dementia would help revealing mechanisms underlying AD pathology while facilitating the discovery of novel strategies for prevention/treatment of AD and related dementia and cognitive decline.
Funding Sources
The authors acknowledge the funding support from National Institutes of Health grant R01 AG018915 and the Pepper Older Americans for Independence Center (P30AG21332), and the Department of Defense funding W81XWH-18-PRARP-NIRA, and funds and services from the Center for Diabetes, Obesity, and Metabolism, Wake Forest Baptist Medical Center, and the National Center for Advancing Translational Sciences (NCATS), the National Institutes of Health-funded Wake Forest Clinical and Translational Science Institute (WF CTSI) through Grant Award Number UL1TR001420.
Footnotes
Conflict of Interest Statement
Dr. Yadav is Chief Scientific Officer and Co-founder of the Post-biotics Inc.; however, he and other authors have no conflict of interest regarding this work and their duties.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013. Jan;9(1):63–e2. [DOI] [PubMed] [Google Scholar]
- 2.Alonso R, Pisa D, Fernández-Fernández AM, Carrasco L. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front Aging Neurosci. 2018;10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczechowiak K, Diniz BS, Leszek J. Diet and Alzheimer’s dementia: nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019. Sep;184:172743. [DOI] [PubMed] [Google Scholar]
- 4.Blum S, Aviram M, Ben-Amotz A, Levy Y. Effect of a mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann Nutr Metab. 2006; 50(1):20–4. [DOI] [PubMed] [Google Scholar]
- 5.Mosconi L, Murray J, Tsui WH, Li Y, Davies M, Williams S, et al. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer’s disease. J Prev Alzheimers Dis. 2014. Jun;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015. Sep;11(9): 1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019. Sep;47:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017. Oct 19;7(1):13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018. Jan;18(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potter H, Granic A, Caneus J. Role of trisomy 21 mosaicism in sporadic and familial Alzheimer’s disease. Curr Alzheimer Res. 2016; 13(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuster-Matanzo A, Llorens-Martín M, Hernández F, Avila J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: therapeutic approaches. Mediators Inflamm. 2013;2013:260925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxid Med Cell Longev. 2016;2016:8590578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atri A The Alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019. Mar;103(2):263–93. [DOI] [PubMed] [Google Scholar]
- 15.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017. Apr 12;21(4):455–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther. 2018. Dec 22;10(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell. 2018. Aug;17(4):e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019. Jan 30;9(1):1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019. Aug;80:633–43. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TTT, Fujimura Y, Mimura I, Fujii Y, Nguyen NL, Arakawa K, et al. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J Micro-biol. 2018. Oct;56(10):760–71. [DOI] [PubMed] [Google Scholar]
- 21.Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016. Nov 29;87(22): 2324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2009. Jan 3;206(1–2):121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Micro-biol. 2017;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauss-Wegrzyniak B, Wenk GL. Beta-amyloid deposition in the brains of rats chronically infused with thiorphan or lipopolysaccharide: the role of ascorbic acid in the vehicle. Neurosci Lett. 2002. Apr 5;322(2): 75–8. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz K, Boles BR. Microbial amyloids: functions and interactions within the host. Curr Opin Microbiol. 2013. Feb;16(1):93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippo-campal neurons. Neurosci Lett. 2003. Jun 5; 343(2):139–43. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, et al. Moderate consumption of cabernet sauvignon attenuates abeta neuropathology in a mouse model of Alzheimer’s disease. Faseb j. 2006. Nov;20(13):2313–20. [DOI] [PubMed] [Google Scholar]
- 28.Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, et al. Nutraceutical properties of mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis. 2010;22(3):715–40. [DOI] [PubMed] [Google Scholar]
- 29.Ayaz M, Sadiq A, Junaid M, Ullah F, Ovais M, Ullah I, et al. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front Aging Neurosci. 2019;11:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernando WMADB, Martins IJ, Morici M, Bharadwaj P, Rainey-Smith SR, Lim WLF, et al. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J Alzheimers Dis. 2020;74(1):91–9. [DOI] [PubMed] [Google Scholar]
- 32.Ton AMM, Campagnaro BP, Alves GA, Aires R, Côco LZ, Arpini CM, et al. Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid Med Cell Longev. 2020;2020:2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, et al. A phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006. Nov 28;67(10):1757–63. [DOI] [PubMed] [Google Scholar]
- 34.Sabbagh MN. Drug development for Alzheimer’s disease: where are we now and where are we headed? Am J Geriatr Pharmacother. 2009. Jun;7(3):167–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res. 2012. Dec;24(6): 580–7. [DOI] [PubMed] [Google Scholar]
- 36.La AL, Walsh CM, Neylan TC, Vossel KA, Yaffe K, Krystal AD, et al. Long-term trazo-done use and cognition: a potential therapeutic role for slow-wave sleep enhancers. J Alzheimers Dis. 2019;67(3):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


