Abstract
VSA-2 is a recently developed semisynthetic saponin immunostimulant. It is prepared by incorporating a terminal-functionalized side chain to the branched trisaccharide domain at the C3 position of Momordica saponin II (MS II) isolated from the seeds of perennial Momordica cochinchinensis Spreng. Direct comparison of VSA-2 and the clinically proven saponin adjuvant QS-21 shows that VSA-2 is comparable to QS-21 in enhancing humoral and cellular immune responses. Structure–activity relationship studies show that structural changes in the side chain have a significant impact on saponins’ adjuvant activity. However, with the VSA-2 molecular framework intact, the new VSA-2 analogues with various substitution(s) at the terminal benzyl group of the side chain retain the ability of potentiating antigen-specific humoral and cellular responses.
Graphical Abstract

INTRODUCTION
Adjuvants are an indispensable element of modern vaccines.1–11 They (a) enhance the ability of a vaccine to elicit strong and durable immune responses including in immunologically compromised individuals,12 (b) reduce antigen dose and the number of necessary immunizations, and (c) modulate the nature of immune response (e.g., to favor humoral or cellular responses). Despite the increasingly important role of adjuvants, there are only a few structurally and/or functionally defined immunostimulating compounds (in different formulations and/or combinations) approved by the FDA for human use.10,13–16 To achieve broad humoral and cellular immune responses, potent adjuvants that are capable of stimulating a balanced Th1/Th2 immunity are highly desirable. QS-21, containing a pair of natural saponin isomers isolated from tree bark of evergreen Quillaja saponaria Molina (QS) tree native to central Chile, is known for inducing strong and balanced humoral and cellular immune responses. It was recently approved as a component of the combination adjuvant AS01b17,18 for GlaxoSmithKline’s shingles vaccine (Shingrix) and malaria vaccine (Mosquirix). The QS-21-containing vaccines demonstrated safety, efficacy, and durable protection. However, their severely limited supply, dose-limiting toxicity, laborious and low-yielding purification, and chemical instability hinder QS-21’s wider use.19,20
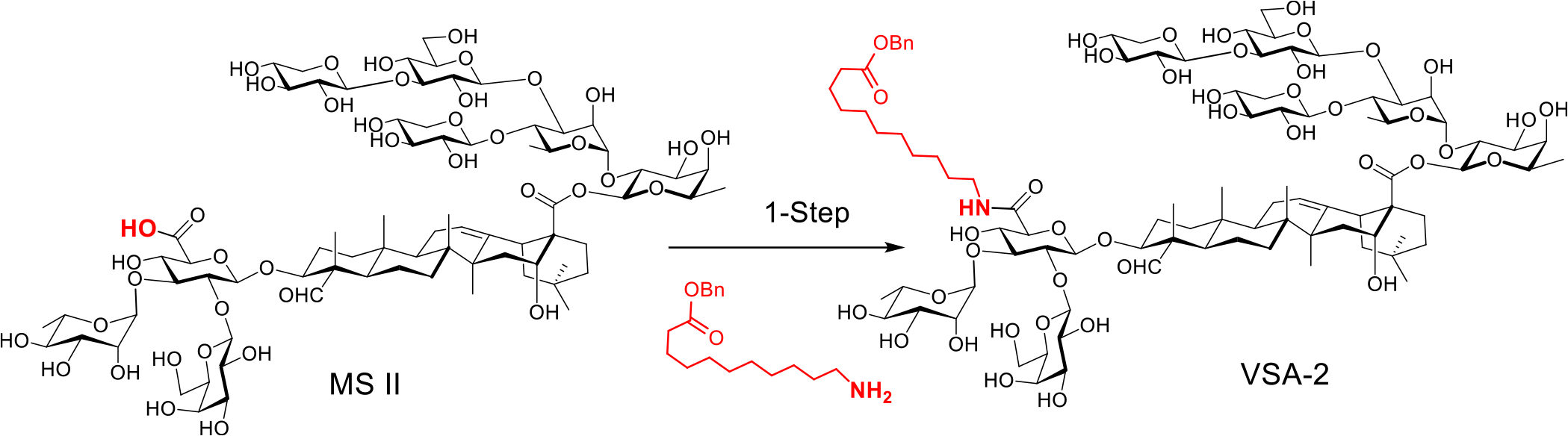
VSA-2 can be a practical alternative to QS-21. It is a semisynthetic derivative prepared in one step from naturally occurring Momordica saponin II (MS II) by incorporating a terminal functionalized side chain to the C3 glucuronic acid unit of its quillaic acid core. The natural saponin precursor is isolated from the seeds of Momordica cochinchinensis SPRENG, a widely available perennial vine that grows in many places around the world (Scheme 1).21 In this work, we will compare adjuvant activity of VSA-2 and QS-21 in enhancing antigen-specific humoral and cellular immune responses. Early studies revealed that the structure of the incorporated artificial side chain significantly affected the adjuvanticity of the MS II derivatives. For example, with a plain aliphatic dodecyl side chain installed at the same site as making VSA-2, the corresponding derivative did not show high adjuvant activity (especially regarding IgG2a responses) comparable to that of VSA-2.21,22 Therefore, it would be important to continue structure–activity relationship (SAR) studies of MS II derivatives to gain more insights into the role of side chains in affecting adjuvanticity. Herein, we report the design, synthesis, and immunological evaluation of VSA-2 analogues 1D–1H and 2D–2H.
Scheme 1. Preparation of VSA-2 Adjuvant from Natural Momordica Saponin II.
RESULTS AND DISCUSSION
We first designed and synthesized MS II derivatives 1D–1H (Scheme 2). 1D has an isomeric side chain similar to that of VSA-2; that is, 1D has a benzoyl group capping the terminal hydroxyl group, while VSA-2 has a benzyl ester group at the terminal. These two isomers have the same hydrophile–lipophile balance (HLB).23,24 1E is similar to 1D, except that the terminal ester in 1D changes to the amide counterpart. 1F is also like 1D, but the benzoyl group of 1D changes to a benzyl ether moiety. Different from 1D–1F, 1G does not have a terminal aromatic moiety; instead, it has a free hydroxyl group. 1H has a terminal amino group in the side chain. In earlier SAR studies of QS-21 analogues, Chea et al. observed that their amino-containing QS-21 analogues did not show adjuvant activities in boosting antibody titers.25 Marciani et al. postulated that incorporation of an amino group in the acyl side chain would lead to its formation of an imine group with the C4 aldehyde moiety of the quillaic acid core in an intramolecular or intermolecular manner.26 The C4 aldehyde moiety was believed to be responsible for reacting with cell surface amino group to deliver a costimulatory signal crucial for T cell activation.27,28 Comparison of MS derivatives 1H and 1G (having a terminal hydroxyl group) will reveal whether a terminal amino group reduces adjuvanticity more than a hydroxyl group.
Scheme 2. Synthesis of MS II derivatives 1D–1Ha.

aReagents and conditions: (a) Boc2O, NaHCO3, DMF, 0 °C—r.t.; (b) BzCl, Et3N, DCM; (c) BnBr, NaH, DMF, 0 °C—r.t.; (d) TFA, DCM; (e) NMM, HOBt, EDC.HCl, R′NH2, H2O/EtOH, r.t.; (f) Pd/C, H2 (55 psi), MeOH/H2O; (g) benzoyl NHS ester, methanol.
Derivatives 1D, 1F, and 1H were easily prepared by coupling MS II with the corresponding side chain by using the published procedure.21 Two new side chains 4a and 4b were obtained from commercially available 11-amino-1-undecanol via standard protecting manipulations (Scheme 2). The side chain of 1H, that is, 1,10-decanediamine, is also commercially available, and 1E was obtained from 1H by reacting it with benzoyl NHS ester. 1G was obtained from either hydrolysis of 1D or debenzylation of 1F.
With 1D–1H in hand, we first compared their ability in potentiating antigen-specific antibody responses. We used chicken egg ovalbumin (OVA) as antigen and QS-21 and VSA-2 as the positive controls. Thus, groups of female BALB/c mice (8–10 weeks of age, six per group) were immunized via the subcutaneous route (s.c.) with OVA (20.0 μg) alone or with QS-21 (20.0 μg), VSA-2 (50.0 μg), or a new MS saponin derivative (50.0 μg) on days 0, 14, and 28. Mice were weighed, and serum samples were collected prior to each immunization and at 2 weeks following the last immunization. The levels of serum antibody activity to OVA were determined by enzyme-linked immunosorbent assay (ELISA).
All groups of mice showed a serum anti-OVA IgG response 2 weeks after the initial immunization and a marked increase in the level of IgG antibodies 2 weeks after the second immunization (week 4) and the third immunization (week 6) (Figure 1A). VSA-2 and QS-21 showed significantly higher IgG antibody activity compared to the OVA-alone group at weeks 2, 4, and 6, but there was no significant difference between VAS-2 and QS-21. Among the new derivatives, only 1D and 1F showed significantly higher antibody IgG activity than the OVA-alone group at both weeks 4 and 6. VSA-2 showed significantly higher IgG antibody activity than 1D–1H at week 2 and higher activity than all the new derivatives except 1F at weeks 4 and 6. Since production of IgG1 and IgG2a in mice is enhanced by the respective Th2 and Th1 cytokines,26,29 we have been using the relative amount of IgG1 and IgG2a as a tentative indication of involvement of Th2 and Th1 immunity potentiated by the adjuvants. The anti-OVA IgG1 responses in the various groups were similar to the total IgG antibody response (Figure 1B). On the contrary, QS-21 and VSA-2 potentiated the anti-OVA IgG2a responses by week 2, and the level of IgG2a antibody increased steadily two weeks after the second and third immunizations. There was no significant difference in IgG2a antibody levels between the QS-21 and VSA-2 groups. Among the new MS derivatives, the IgG2a antibody responses in groups 1D, 1E, and 1F were not significantly different; however, the induced IgG2a titers were significantly higher than that of the OVA-alone group and the 1G and 1H groups by week 6 and lower than that of the QS-21 and VSA-2 groups.
Figure 1.
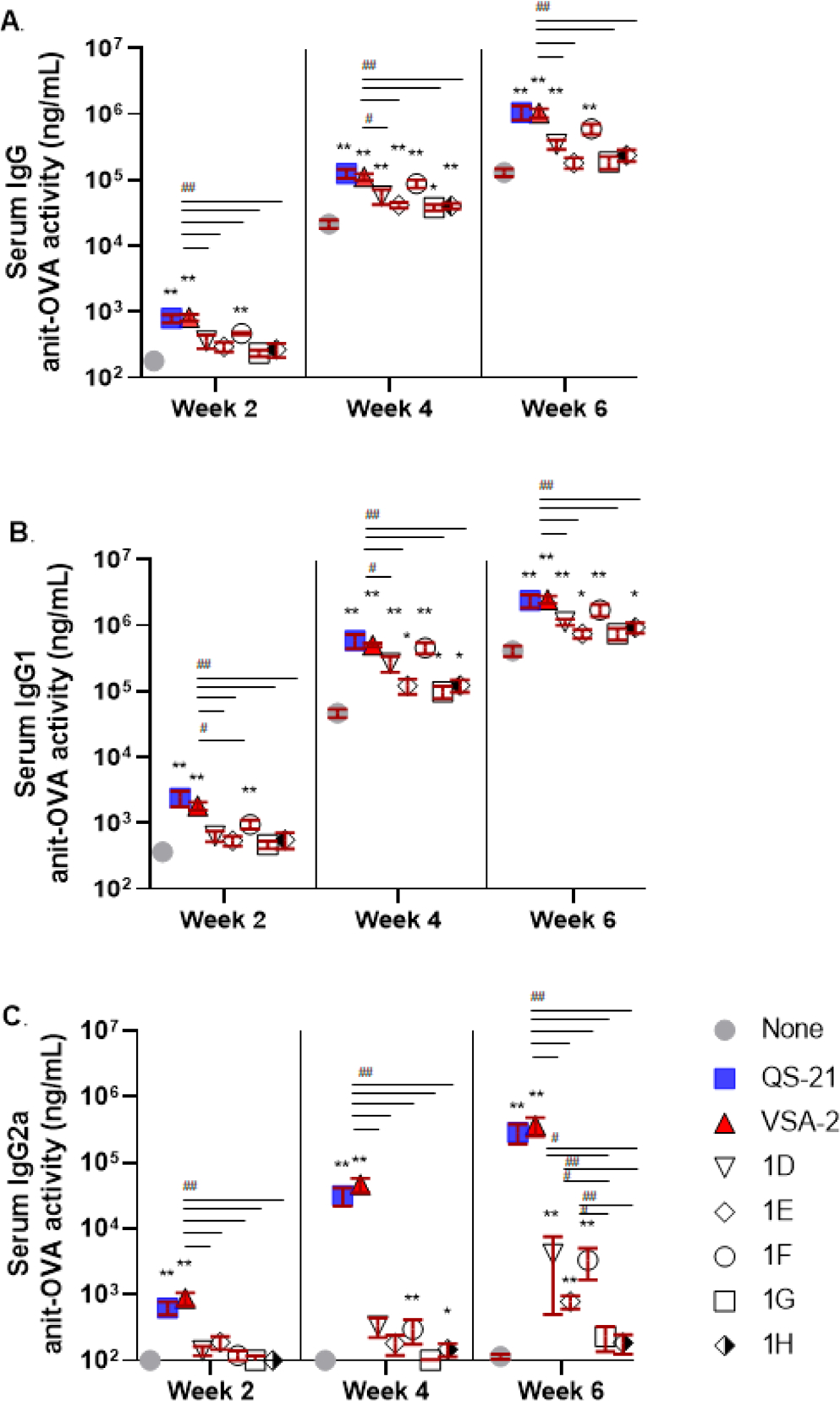
Serum IgG (A), IgG1 (B), and IgG2a (C) anti-OVA responses in mice immunized by the s.c. route with OVA (20.0 μg) alone (none) or with QS-21 (20.0 μg) or an MS derivative (50.0 μg). Mice were immunized on days 0, 14, and 28. Serum samples were collected prior to each immunization and at 6 weeks after the initial immunization. Values are expressed as mean ± SEM. Statistical significance in antibody responses was evaluated by unpaired t tests (nonparametric and Mann–Whiteny test). *P < 0.05 and **P < 0.01 compared with mice immunized with OVA alone. #P < 0.05 and ##P < 0.01.
These results revealed that VSA-2 (50 μg) and QS-21 (20 μg) potentiated similar antigen-specific IgG, IgG1, and IgG2a antibody responses. Structural deviation from VSA-2, as shown with 1D–1H, led to a reduction in antibody responses, especially IgG2a antibody responses. Given the similar low adjuvant activity of 1G and 1H, it remained inconclusive whether a terminal amine reduces saponin’s adjuvanticity by forming imine with the carbonyl group of the quillaic acid core. The significantly lower adjuvant activities of 1G and 1H, however, suggested that it is crucial to keep the terminal benzene moiety to maintain high adjuvant activity, especially for IgG2a antibody activity. Thus, we designed five new VSA-2 derivatives, that is, 2D–2H, by keeping the VSA-2 structural framework (Scheme 3). We introduced different substitution to the terminal benzene ring of the side chain. Saponin 2D with two meta methoxy groups was used to explore how multiple substituents affect adjuvanticity. 2E differs from 2D with only one meta methoxyl group. Saponin 2F, like 2E, has a meta electron-donating group, but the phenoxyl group is of much larger size than the methoxyl group. A comparison of 2E and 2F would reveal how the size of the electronically similar substituent group at the same site affects adjuvanticity. Unlike 2D–2F, saponin 2G is equipped with a nitro group, a strong electron-withdrawing group, at its meta position. Therefore, a comparison of 2D–2F with 2G would reveal how the electronic property of the substituted phenyl group affects adjuvanticity. 2H has a fluoro group at its para position. F’s electronic property is in between that of alkoxyl and nitro groups; its location at the para position should not affect the chemical property of the terminal ester moiety. Derivatives 2D–2H were synthesized by coupling MS II and side chain compounds 5D–5H with the known procedure (Scheme 3).
Scheme 3. Synthesis of MS II derivatives 2D–2Ha.
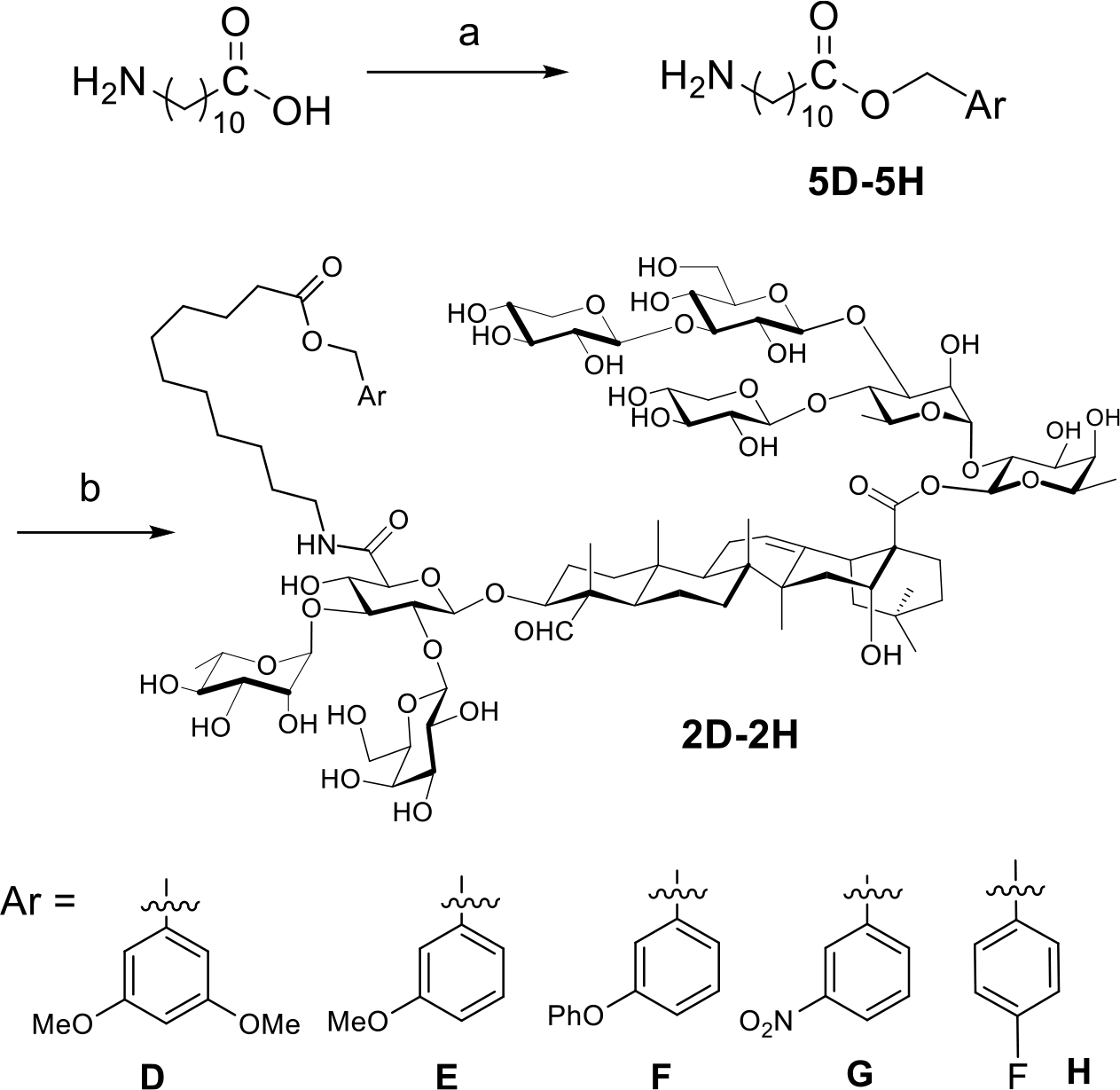
aReagents and conditions: (a) SOCl2, ArCH2OH, r.t.—70 °C; (b) NMM, HOBt, EDC.HCl, RNH2, H2O/EtOH, r.t.
We then evaluated the ability of 2D–2H to potentiate IgG, IgG1, and IgG2a anti-OVA antibody responses. Groups of female BALB/c mice (8–10 weeks of age, six per group) were immunized by the s.c. with OVA (20 μg) alone or with QS-21 (20 μg), VSA-2 (100 μg), or a new MS saponin derivative at 50 μg dose on days 0, 14, and 28. In this study, we increased the dose of VSA-2 to 100 μg in order to see whether an increase in the dose would further enhance its adjuvant activity, compared with QS-21. Like the 1D–1H series, serum anti-OVA IgG (Figure S1) and IgG1 (Figure 2) titers in all groups increased over time. For instance, all groups immunized with OVA + adjuvant (except 2H in week 4 and 2E in week 6) showed significantly higher IgG1 titers than the OVA-alone group (Figure 2A). In week 4, the VSA-2 group had significantly higher serum IgG1 antibody levels than QS-21, 2D, 2E, and 2F, but by week 6, the VSA-2 group was only higher than the 2H group. In terms of IgG2a responses, QS-21 and VSA-2 potentiated significantly higher IgG2a titers than the OVA-alone group at weeks 4 and 6, but no significant difference in IgG2a antibody responses was seen between VSA-2 and QS-21. Unlike at week 4, the new derivatives showed significantly higher IgG2a antibody activities than the OVA control group by week 6; however, their IgG2a antibody activities were lower than that of the VSA-2 group (Figure 2B).
Figure 2.
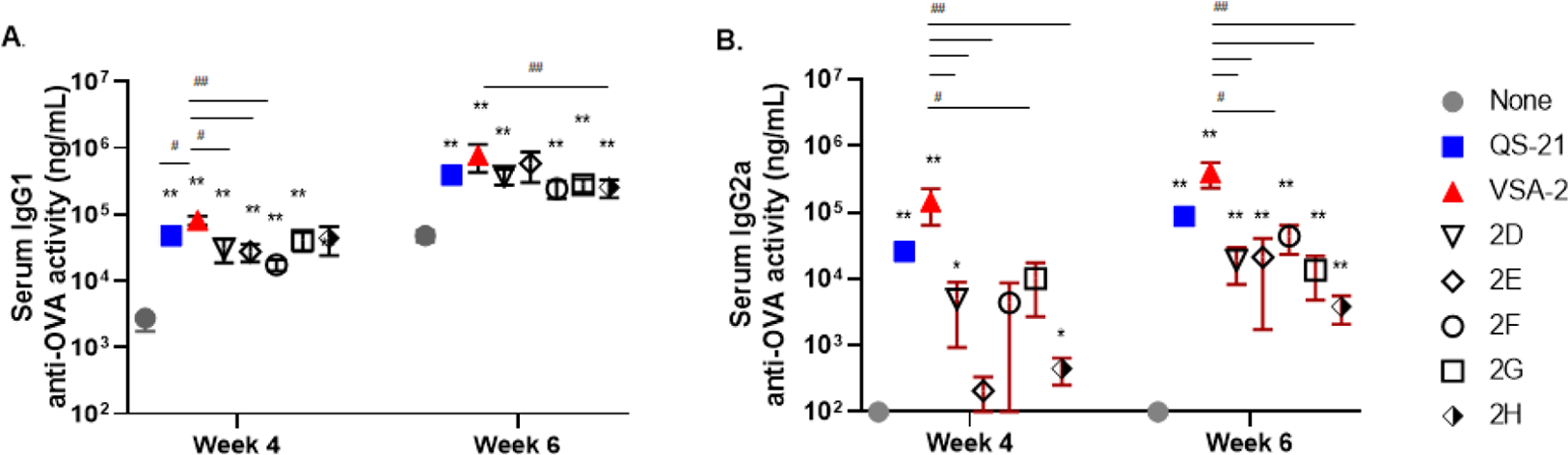
Serum IgG1 (A) and IgG2a (B) anti-OVA responses in mice immunized by the s.c. route with OVA (20 μg) alone (none) or with QS-21 (20 μg), VSA-2 (100 μg), or an MS derivative (50 μg). Mice were immunized on days 0, 14, and 28. Serum samples were collected prior to each immunization and at 6 weeks after the initial immunization. Values are expressed as mean ± SEM. Statistical significance in antibody responses was evaluated by unpaired t tests (nonparametric and Mann–Whiteny test). *P < 0.05 and **P < 0.01 compared with mice immunized with OVA alone. #P < 0.05 and ##P < 0.01.
We also analyzed the T-cell and B-cell responses of mouse spleen cells at week 6 post-immunization with flow cytometry. Compared to the OVA-alone group, both QS-21 (20 μg) and VSA-2 (100 μg) potentiated the activation of CD8+ and CD4+ T cells, as judged by the increased surface expression of PD-1 and intracellular levels of IFNγ in both cell types and increased expression of the cytotoxic molecule, granzyme B, in CD8+ T cells (Figure 3A–E). Spleen cells from mice immunized the new saponins, 2E, 2G, and 2H showed significantly higher intracellular levels of IFNγ in CD8+ T cells than the OVA-alone group (Figure 3B) but significantly lower than that potentiated with QS-21 or VSA-2. Spleen cells from the 2E and 2G groups showed higher IFNγ production in CD4+ effector T cells (Teff) than the OVA-alone group but lower than that of the QS-21 or VSA-2 group (Figure 3E). Splenic CD4+ Teff cells from groups of mice receiving the new saponins (except 2D) showed significantly higher surface expression of PD-1 than the antigen-alone group but lower than the VSA-2 group (Figure 3D), whereas the splenic CD8+ T cells from these mice did not show much difference compared to the OVA control group (Figure 3A). None of the new saponins increased granzyme B production in CD8+ T cells, unlike QS-21 or VSA-2 (Figure 3C). Both QS-21 and VSA-2 also significantly increased IL-4-expressing CD4+ Teff cells, compared with the OVA-alone group (Figure 3F). The preferential increase in IFNγ over IL-4 in QS-21 and VSA-2 groups suggested a shifted Th1 response induced by these adjuvants. Interestingly, both QS-21 and VSA-2 increased IL-21-expressing CD4+ Teff cells compared with the OVA-alone group (Figure 3G). Among the new saponins, only 2D induced more IL-4-expressing CD4+ Teff cells than the OVA-alone group (Figure 3F), and none of them increased IL-21-expressing CD4+ Teff cells (Figure 3G).
Figure 3.
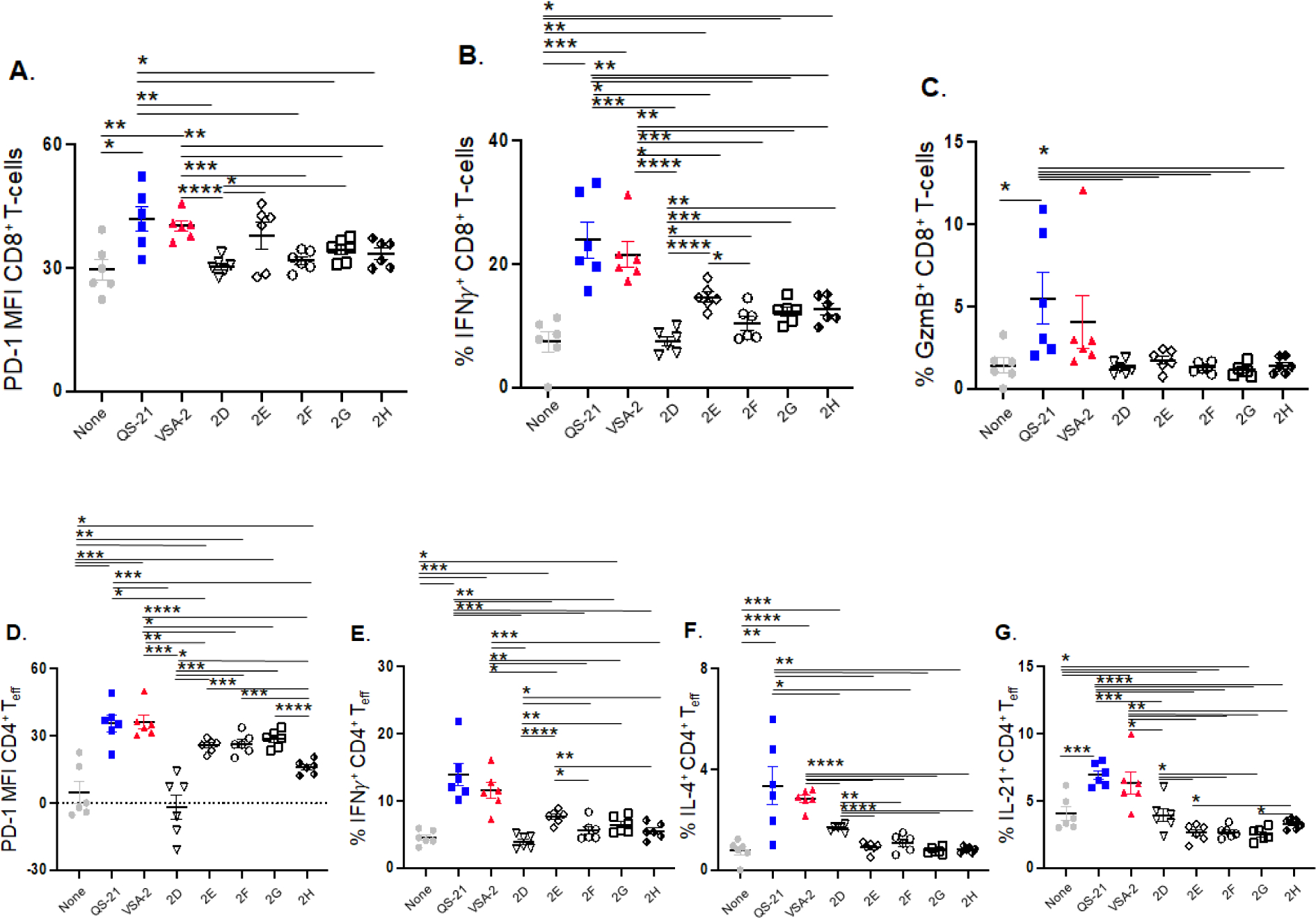
CD8+ T-cell surface expression of PD-1 (A), intracellular levels of IFNγ in CD8+ T-cells (B), expression of granzyme B in CD8+ T-cells (C), CD4+ T-cell surface expression of PD-1 (D), intracellular levels of IFNγ in CD4+ T-cells (E), expression of IL-4 in CD4+ T-cells (F), and expression of IL-21 CD4+ T-cells (G) in mice immunized by the s.c. route with OVA (20 μg) alone (none) or with QS-21 (20 μg), VSA-2 (100 μg), or an MS derivative (50 μg). Mice were immunized on days 0, 14, and 28. Spleens were collected at 6 weeks after the initial immunization. Mean Fluorescence Intensity (MPI) values are expressed as mean ± SEM. Statistical significance in each comparison evaluated by unpaired t tests. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
B cells are responsible for antibody production, which is regulated by follicular helper T cells (TFH) and follicular regulatory T cells (TFR).30–32 TFH cells help B-cells, particularly germinal center (GC) B-cells, to produce high-affinity antibodies, where TFR cells suppress such responses and antibody production. Analysis of these splenic cell types from the various groups of immunized mice revealed that QS-21 and VSA-2 increased TFH (PD-1+Bcl6+Foxp3−CD4+CD3+) (Figure 4A) and CD19+ B-cells (Figure 4D) and reduced TFR cells (PD-1+Bcl6+Foxp3+CD4+CD3+) compared to that seen with OVA alone (Figure 4B). Correspondingly, increased ratios of TFH cells to TFR cells were observed in both QS-21 and VSA-2 groups (Figure 4C). Moreover, the proportions of dark zone GC B-cells (CXCR4hiCD86loFas+GL-7+CD19+), which are responsible for the production of high-affinity antibodies,33 were increased in both QS-21 and VSA-2 groups compared to the OVA-alone group (Figure 4E). In general, the new saponins 2D–2H did not induce these cellular changes, except that 2E, 2G, and 2H increased CD19+ B-cells (Figure 4D), while 2G and 2H increased the ratio of TFH cells to TFR cells (Figure 4C). Taken together, these results suggest that QS-21 and VSA-2 are similar adjuvants in their ability to potentiate strong cellular and humoral antibody responses, while the substitutions to the side-chain terminal benzene moiety affected their adjuvanticity.
Figure 4.
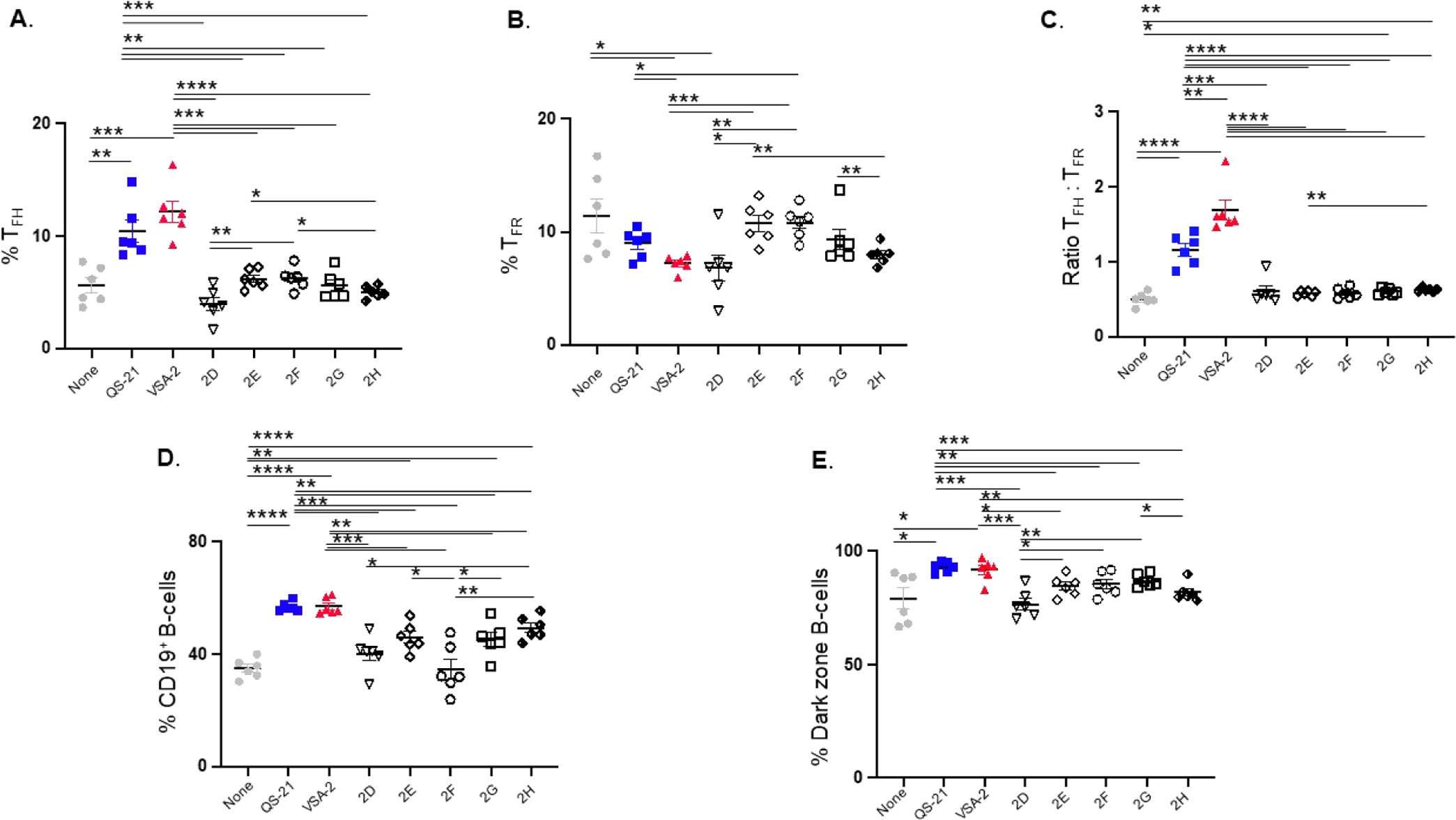
Analysis of splenic cell types affected by saponin adjuvants: follicular helper T cells (A), follicular regulatory T cells (B), ratio of TFH to TRF (C), CD19-expressing B-cells (D), and dark zone B-cells (E) in mice immunized by the s.c. route with OVA (20 μg) alone (none) or with QS-21 (20 μg), VSA-2 (100 μg), or an MS derivative (50 μg). Mice were immunized on days 0, 14, and 28. Spleens were collected at 6 weeks after the initial immunization. Values are expressed as mean ± SEM. Statistical significance in each comparison is evaluated by unpaired t tests. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
CONCLUSIONS
VSA-2 is a potent semisynthetic saponin immunostimulant. Head-to-head comparison of VSA-2 and QS-21, the gold standard of saponin adjuvant, revealed that VSA-2 has an adjuvant activity profile similar to that of QS-21 in terms of enhancing humoral and cellular immune responses induced by an antigen. Many natural saponins are known for their immunostimulatory activity. However, the molecular mechanism for their adjuvanticity remains largely unknown. It is still unclear whether saponins like QS-21 have a molecular receptor in the immune system or their adjuvant activity is mainly due to their amphipathicity within a certain range of HLB. In this work, we designed and synthesized a number of VSA-2 analogues. Some of them have the same or similar HLB to that of VSA-2 but showed different immunological properties from VSA-2. Earlier SAR studies showed that structural changes in the side chain had a significant impact on saponins’ adjuvant activity. In this work, SAR studies of new semisynthetic saponins confirmed the structural effect of the side chain on adjuvanticity. Moreover, as demonstrated with new saponins 2D–2H, when the VSA-2 molecular framework remained intact, substitution(s) at the terminal benzyl group of the side chain can largely retain the saponins’ ability in potentiating antigen-specific humoral responses (e.g., IgG1 and IgG2a) and the cellular responses to a certain extent. The results from this work indicate that SAR studies focussing on the terminal functionalization of the side chain is a viable way to obtain new saponin adjuvants with desired properties and structurally defined molecular probes for mechanistic studies and to provide a new opportunity of using VSA-2-type saponin as a built-in adjuvant by anchoring the saponin through a covalent bond to the terminal benzyl group of the side chain without significantly compromising the adjuvant’s activity profile.
EXPERIMENTAL SECTION
Chemistry.
General.
Organic solutions were concentrated by rotary evaporation at ca. 12 Torr. Flash column chromatography was performed employing 230–400 mesh silica gel. Thin-layer chromatography was performed using glass plates pre-coated to a depth of 0.25 mm with 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Proton and carbon-13 nuclear magnetic resonance (1H NMR or 13C NMR) spectra were recorded on 400, 600, 700, and 850 MHz NMR spectrometers; chemical shifts are expressed in parts per million (δ scale) downfield from tetramethylsilane and are referenced to residual protium in the NMR solvent (CHCl3: δ = 7.26). Data are presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet, and/or multiple resonances), coupling constant in Hertz (Hz), and integration. Anhydrous solvents were used without distillation. Solvents for workup and column chromatography were obtained from commercial vendors and were used without further purification. The purity of the products for immunological studies was determined by a combination of high-performance liquid chromatography (HPLC) and 1H NMR and found to be ≥95%. All in vivo studies were performed in accordance with local IACUC guidelines.
General Procedure of Derivatizing MS II.
The side chain 4 was obtained by treating 3 in dichloromethane with trifluoroacetic acid at room temperature for 3 h. After workup, 4 was used directly for the coupling reaction with MS II. The published general procedure of synthesizing MS derivatives was used under unoptimized conditions.34 The reaction mixture was directly purified with RP HPLC by using a semi-Prep C18, 250 × 10 mm, 5 μm column, and H2O/MeCN gradients (90–10% H2O over 45 min with a 3 mL/min flow rate). The product fraction was concentrated on a rotary evaporator at room temperature to remove MeCN, and the remaining water was then removed on a lyophilizer to provide the derivative as a white solid.
1D (12.4 mg, 35%):
1H NMR (500 MHz, CD3OD) (characteristic protons): δ 9.50 (s, 1H), 8.03 (dd, J = 8.4, 1.3 Hz, 2H), 7.87 (t, J = 5.7 Hz, 1H), 7.63 (tt, J = 7.4, 1.3 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 5.43 (d, J = 1.5 Hz, 1H), 5.33 (b, 1H), 5.23 (d, J = 8.2 Hz, 1H), 5.04 (d, J = 1.4 Hz, 1H), 4.73 (d, J = 7.9 Hz, 1H), 4.56 (d, J = 7.8 Hz, 1H), 4.52 (b, 1H), 4.50–4.40 (m, 3H), 4.35 (t, J = 6.6 Hz, 2H), 4.24 (dd, J = 2.8, 1.9 Hz, 1H), 4.06–4.01 (m, 2H), 2.30 (t, J = 13.6 Hz, 1H), 1.42 (s, 3H), 1.19 (s, 3H), 1.03 (s, 3H), 0.94 (s, 3H), 0.88 (s, 3H), 0.80 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 169.8, 166.7, 143.6, 132.8, 130.2, 129.1, 128.2, 121.5, 104.6, 103.7, 103.5, 102.8, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.5, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.5, 70.8, 70.6, 70.5, 70.0, 68.0, 67.4, 65.7, 64.9, 60.8, 60.6, 54.8, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.2, 32.0, 30.5, 29.9, 29.5, 29.3, 29.2, 29.0, 28.9, 28.4, 26.6, 26.0, 25.8, 24.6, 23.3, 23.0, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C94H148NO42, 1962.9476; found, 1962.9467.
1E (8.6 mg, 56% from 1H with benzoyl N-hydroxysuccinimide ester):
1H NMR (600 MHz, CD3OD) (characteristic protons): δ 9.49 (s, 1H), 7.83 (d, J = 7.4 Hz, 2H), 7.54 (tt, J = 7.4, 1.2 Hz, 1H), 7.48 (d, J = 7.4 Hz, 2H), 5.43 (d, J = 1.6 Hz, 1H), 5.33 (t, J = 3.7 Hz, 1H), 5.23 (d, J = 8.1 Hz, 1H), 5.04 (d, J = 1.7 Hz, 1H), 4.74 (d, J = 7.9 Hz, 1H), 4.56 (d, J = 7.7 Hz, 1H), 4.53 (b, 1H), 4.50–4.46 (m, 2H), 4.45 (d, J = 7.7 Hz, 1H), 4.25 (dd, J = 2.9, 2.2 Hz, 1H), 4.06–4.01 (m, 2H), 3.18 (t, J = 11.3 Hz, 1H), 3.14 (dd, J = 9.4, 8.1 Hz, 1H), 2.90 (dd, J = 14.6, 4.5 Hz, 1H), 2.30 (t, J = 13.7 Hz, 1H), 1.18 (s, 3H), 1.03 (s, 3H), 0.93 (s, 3H), 0.88 (s, 3H), 0.81 (s, 3H); 13C NMR (150.9 MHz, CD3OD): δ 209.6, 175.6, 169.8, 168.8, 143.5, 134.5, 131.1, 128.2, 126.8, 121.6, 104.7, 103.8, 103.5, 102.8, 102.7, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.4, 73.0, 72.4, 72.3, 71.6, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 60.8, 54.8, 48.5, 46.9, 46.6, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.2, 32.8, 31.9, 30.5, 29.9, 29.4, 29.3, 29.1, 28.9, 26.7, 26.5, 25.9, 24.5, 23.3, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C93H147N2O41, 1947.9479; found, 1947.9386.
1F (8.3 mg, 36%) 1H NMR (600 MHz, CD3OD) (characteristic protons):
δ 9.50 (s, 1H), 7.37–7.34 (m, 4H), 7.30 (m, 1H), 5.43 (d, J = 1.5 Hz, 1H), 5.33 (t, J = 3.3 Hz, 1H), 5.30 (d, J = 8.3 Hz, 1H), 5.04 (d, J = 1.6 Hz, 1H), 4.74 (d, J = 8.1 Hz, 1H), 4.56 (d, J = 8.0 Hz, 1H), 4.25 (dd, J = 2.9, 1.7 Hz, 1H), 2.91 (dd, J = 14.2, 4.5 Hz, 1H), 2.30 (t, J = 13.6 Hz, 1H), 1.19 (s, 3H), 1.03 (s, 3H), 0.94 (s, 3H), 0.88 (s, 3H), 0.81 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 169.7, 143.6, 138.5, 121.5, 104.7, 103.8, 103.5, 102.8, 102.7, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.2, 76.8, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.5, 72.4, 72.3, 71.5, 71.4, 70.8, 70.7, 70.5, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 60.8, 60.6, 54.8, 48.4, 41.5, 41.0, 39.7, 38.7, 35.7, 35.2, 32.7, 32.0, 29.9, 29.5, 29.4, 29.3, 29.2, 28.9, 26.6, 25.9, 23.3, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C94H150NO41, 1948.9383; found, 1948.9684.
1 G (7.4 mg, 67% from 1F):
1H NMR (600 MHz, CD3OD) (characteristic protons): δ 9.49 (s, 1H), 5.42 (d, J = 1.6 Hz, 1H), 5.32 (t, J = 3.5 Hz, 1H), 5.22 (d, J = 8.3 Hz, 1H), 5.03 (d, J = 1.4 Hz, 1H), 4.73 (d, J = 7.8 Hz, 1H), 4.55 (d, J = 7.8 Hz, 1H), 4.52 (b, 1H), 4.44 (d, J = 7.6 Hz, 1H), 4.23 (dd, J = 2.8, 1.8 Hz, 1H), 3.17 (t, J = 10.9 Hz, 1H), 3.12 (dd, J = 8.2, 8.1 Hz, 1H), 2.90 (dd, J = 14.0, 4.4 Hz, 1H), 2.30 (t, J = 13.6 Hz, 1H), 1.42 (s, 3H), 1.19 (s, 3H), 1.03 (s, 3H), 0.95 (s, 3H), 0.88 (s, 3H), 0.80 (s, 3H); 13C NMR (213.8 MHz, CD3OD): δ 209.6, 175.6, 169.8, 143.5, 121.5, 104.7, 103.8, 103.6, 102.8, 102.7, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.1, 77.2, 76.9, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.7, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 61.6, 60.8, 60.6, 54.8, 46.6, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.2, 32.7, 32.3, 32.0, 31.7, 30.5, 29.9, 29.5, 29.4, 29.3, 29.2, 29.1, 28.9, 26.5, 25.9, 25.6, 24.5, 23.3, 23.1, 22.3, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C87H144NO41, 1858.9214; found, 1858.9211.
1H (20.4 mg, 27%):
1H NMR (600 MHz, CD3OD) (characteristic protons): δ 9.50 (s, 1H), 5.42 (d, J = 1.1 Hz, 1H), 5.33 (t, J = 3.3 Hz, 1H), 5.24 (d, J = 8.4 Hz, 1H), 5.04 (d, J = 1.4 Hz, 1H), 4.74 (d, J = 8.0 Hz, 1H), 4.56 (d, J = 8.0 Hz, 1H), 4.53 (b, 1H), 4.50–4.46 (m, 2H), 4.45 (d, J = 7.7 Hz, 1H), 4.25 (dd, J = 3.1, 1.9 Hz, 1H), 4.05–4.01 (m, 2H), 3.18 (t, J = 11.1 Hz, 1H), 3.14 (dd, J = 9.4, 8.1 Hz, 1H), 2.31 (t, J = 13.6 Hz, 1H), 1.20 (s, 3H), 1.03 (s, 3H), 0.96 (s, 3H), 0.89 (s, 3H), 0.81 (s, 3H); 13C NMR (150.9 MHz, CD3OD): δ 209.6, 175.6, 169.8, 143.6, 121.5, 104.7, 103.8, 103.5, 102.8, 101.7, 101.9, 99.3, 94.0, 87.4, 84.6, 82.1, 77.3, 76.9, 76.4, 76.0, 75.4, 75.1, 74.3, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.7, 71.5, 70.8, 70.6, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 60.8, 60.7, 54.8, 48.5, 46.6, 41.5, 41.0, 39.7, 39.4, 38.7, 37.9, 35.7, 35.2, 32.7, 32.0, 30.5, 29.9, 29.2, 29.0, 28.9, 28.8, 27.2, 26.4, 26.0, 25.9, 24.5, 23.2, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 15.0, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C86H143N2O40, 1843.9217; found, 1843.9216.
2D (10.0 mg, 56%):
1H NMR (600 MHz, CD3OD) (characteristic protons): δ 9.47 (s, 1H), 6.50 (d, J = 2.3 Hz, 2H), 6.42 (t, J = 2.3 Hz, 1H), 5.41 (d, J = 1.5 Hz, 1H), 5.30 (t, J = 3.6 Hz, 1H), 5.20 (d, J = 8.8 Hz, 1H), 5.05 (s, 2H), 5.02 (d, J = 1.4 Hz, 1H), 4.72 (d, J = 7.8 Hz, 1H), 4.56 (d, J = 8.0 Hz, 1H), 4.53 (d, J = 8.1 Hz, 1H), 4.50 (b, 1H), 4.44 (d, J = 7.6 Hz, 1H), 4.23 (dd, J = 3.0, 1.8 Hz, 1H), 3.15 (t, J = 11.0 Hz, 1H), 3.13 (dd, J = 9.4, 8.1 Hz, 1H), 2.88 (dd, J = 14.1, 4.5 Hz, 1H), 2.38 (t, J = 7.3 Hz, 2H), 2.27 (t, J = 13.4 Hz, 1H), 1.16 (s, 3H), 0.99 (s, 3H), 0.92 (s, 3H), 0.86 (s, 3H), 0.77 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 173.8, 169.8, 161.0, 143.5, 138.6, 121.5, 105.5, 104.7, 103.8, 103.5, 102.8, 101.9, 99.5, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.4, 73.0, 72.4, 72.3, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 65.6, 60.8, 60.6, 54.8, 54.4, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.3, 33.7, 32.8, 32.0, 30.5, 29.9, 29.4, 29.2, 29.1, 29.0, 28.9, 28.7, 26.5, 25.9, 24.7, 23.3, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C96H152NO44, 2022.9687; found 2022.9675.
2E (8.0 mg, 45%):
1H NMR (600 MHz, CD3OD): δ 9.47 (s, 1H), 7.26 (t, J = 7.9 Hz, 1H), 6.93–6.89 (m, 2H), 6.87 (dd, J = 8.2, 2.7 Hz, 1H), 5.40 (d, J = 1.8 Hz, 1H), 5.30 (t, J = 3.7 Hz, 1H), 5.20 (d, J = 8.2 Hz, 1H), 5.09 (s, 2H), 5.01 (d, J = 1.6 Hz, 1H), 4.71 (d, J = 7.9 Hz, 1H), 4.53 (d, J = 7.9 Hz, 1H), 4.49 (b, 1H), 4.42 (d, J = 7.7 Hz, 1H), 4.22 (dd, J = 3.1, 1.9 Hz, 1H), 3.15 (t, J = 10.8 Hz, 1H), 3.11 (dd, J = 9.3, 8.0 Hz, 1H), 2.88 (dd, J = 14.3, 4.1 Hz, 1H), 2.37 (t, J = 7.3 Hz, 2H), 2.27 (t, J = 13.6 Hz, 1H), 1.39 (s, 3H), 1.22 (d, J = 6.3 Hz, 3H), 1.20 (d, J = 6.5 Hz, 3H), 1.16 (s, 3H), 1.00 (s, 3H), 0.91 (s, 3H), 0.85 (s, 3H), 0.78 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 173.8, 169.8, 161.4, 160.0, 143.5, 137.8, 129.2, 121.5, 119.9, 113.2, 104.7, 103.8, 103.5, 102.8, 102.7, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.4, 76.0, 75.5, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.5, 79.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 65.7, 65.6, 60.8, 54.8, 54.3, 41.5, 39.7, 38.8, 35.7, 35.3, 33.7, 32.0, 29.9, 29.4, 29.2, 29.1, 29.0, 28.9, 28.7, 26.5, 25.9, 24.7, 23.3, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C95H149NO43, 1992.9582; found, 1992.9662.
2F (10.0 mg, 54%):
1H NMR (600 MHz, CD3OD): δ 9.51 (s, 1H), 7.42–7.30 (m, 3H), 7.17 (tt, J = 7.4, 2.1 Hz, 1H), 7.14 (d, J = 3.7 Hz, 1H), 7.05–7.01 (m, 3H), 6.97 (dd, J = 8.1, 2.3 Hz, 1H), 5.45 (d, J = 1.6 Hz, 1H), 5.34 (t, J = 3.5 Hz, 1H), 5.25 (d, J = 8.3 Hz, 1H), 5.14 (s, 2H), 5.06 (d, J = 1.5 Hz, 1H), 4.76 (d, J = 7.9 Hz, 1H), 4.58 (d, J = 7.9 Hz, 1H), 4.55 (b, 1H), 4.47 (d, J = 7.7 Hz, 1H), 4.27 (dd, J = 3.0, 1.9 Hz, 1H), 4.07–4.04 (m, 2H), 3.20 (t, J = 11.0 Hz, 1H), 3.16 (dd, J = 9.4, 8.0 Hz, 1H), 2.93 (dd, J = 14.1, 4.4 Hz, 1H), 2.40 (t, J = 7.3 Hz, 2H), 2.32 (t, J = 13.6 Hz, 1H), 1.44 (s, 3H), 1.27 (d, J = 6.2 Hz, 3H), 1.25 (d, J = 6.4 Hz, 3H), 1.21 (s, 3H), 1.04 (s, 3H), 0.96 (s, 3H), 0.90 (s, 3H), 0.82 (s, 3H); 13C NMR (150.9 MHz, CD3OD): δ 209.6, 175.5, 173.7, 169.8, 157.6, 157.1, 138.6, 129.7, 129.6, 123.2, 122.4, 121.5, 118.7, 117.9, 117.7, 104.7, 103.8, 103.5, 102.8, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.4, 76.0, 75.4, 75.1, 74.3, 74.2, 74.0, 73.6, 73.4, 73.0, 72.4, 72.3, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 65.2, 60.8, 60.6, 54.8, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.3, 33.7, 32.0, 30.5, 29.9, 29.4, 29.2, 29.1, 29.0, 28.9, 28.7, 26.5, 26.0, 24.7, 23.3, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C100H152NO43, 2054.9738; found, 2054.9714.
2G (10.0 mg, 56%):
1H NMR (600 MHz, CD3OD): δ 9.51 (s, 1H), 8.29 (s, 1H), 8.24 (dd, J = 8.2, 1.9 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 7.68 (t, J = 7.9 Hz, 1H), 5.44 (d, J = 1.5 Hz, 1H), 5.33 (b, 1H), 5.29 (s, 2H), 5.23 (d, J = 8.2 Hz, 1H), 5.05 (d, J = 1.2 Hz, 1H), 4.75 (d, J = 7.8 Hz, 1H), 4.57 (d, J = 7.8 Hz, 1H), 4.54 (b, 1H), 4.46 (d, J = 7.7 Hz, 1H), 4.26 (b, 1H), 4.07–4.02 (m, 2H), 3.19 (t, J = 11.7 Hz, 1H), 3.15 (dd, J = 9.4, 8.0 Hz, 1H), 2.91 (dd, J = 14.0, 4.4 Hz, 1H), 2.45 (t, J = 7.3 Hz, 2H), 2.30 (t, J = 13.9 Hz, 1H), 1.43 (s, 3H), 1.26 (d, J = 6.3 Hz, 3H), 1.24 (d, J = 6.5 Hz, 3H), 1.20 (s, 3H), 1.03 (s, 3H), 0.94 (s, 3H), 0.89 (s, 3H), 0.81 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 173.5, 169.6, 148.3, 143.6, 138.8, 133.9, 129.6, 124.4, 122.6, 122.3, 121.5, 104.7, 103.7, 103.5, 102.8, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.1, 77.3, 76.8, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 64.4, 60.8, 60.6, 54.8, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.3, 33.6, 32.8, 32.0, 30.5, 29.9, 29.4, 29.2, 29.1, 28.9, 28.8, 26.5, 25.9, 24.7, 23.3, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C94H147N2O44, 2007.9327; found, 2007.9337.
2H (10.0 mg, 56%):
1H NMR (600 MHz, CD3OD): δ 9.51 (s, 1H), 7.45–7.40 (m, 2H), 7.11 (tt, J = 8.7, 2.1 Hz, 1H), 5.44 (d, J = 1.6 Hz, 1H), 5.34 (t, J = 3.4 Hz, 1H), 5.24 (d, J = 8.3 Hz, 1H), 5.13 (s, 2H), 5.05 (d, J = 1.6 Hz, 1H), 4.75 (d, J = 8.0 Hz, 1H), 4.57 (d, J = 7.8 Hz, 1H), 4.54 (b, 1H), 4.46 (d, J = 7.6 Hz, 1H), 4.26 (dd, J = 3.0, 2.1 Hz, 1H), 3.19 (t, J = 11.0 Hz, 1H), 3.15 (dd, J = 9.2, 8.3 Hz, 1H), 2.92 (dd, J = 14.5, 4.4 Hz, 1H), 2.39 (t, J = 7.5 Hz, 2H), 2.31 (t, J = 13.6 Hz, 1H), 1.44 (s, 3H), 1.26 (d, J = 6.3 Hz, 3H), 1.25 (d, J = 6.4 Hz, 3H), 1.21 (s, 3H), 1.04 (s, 3H), 0.95 (s, 3H), 0.89 (s, 3H), 0.82 (s, 3H); 13C NMR (176.0 MHz, CD3OD): δ 209.6, 175.5, 173.7, 169.8, 163.3, 161.9, 143.6, 132.5, 130.2, 130.1, 114.9, 114.8, 104.7, 103.7, 103.5, 102.8, 101.9, 99.3, 94.0, 87.4, 84.6, 84.5, 82.2, 77.3, 76.8, 76.4, 76.0, 75.4, 75.1, 74.2, 74.0, 73.6, 73.3, 73.0, 72.4, 72.3, 71.5, 70.8, 70.7, 70.5, 70.1, 70.0, 69.6, 69.2, 69.1, 68.0, 67.4, 65.7, 65.0, 60.8, 60.6, 54.8, 48.5, 41.5, 41.0, 39.7, 38.7, 38.0, 35.7, 35.3, 33.7, 32.8, 32.0, 30.5, 29.9, 29.4, 29.2, 29.1, 29.0, 28.9, 28.7, 26.5, 25.9, 24.7, 23.3, 23.1, 20.0, 17.0, 16.5, 16.4, 15.1, 9.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C94H147FNO42, 1980.9382; found, 1980.9463.
Synthesis of 3a and 3b.
(BOC)2O (33.0 mg, 0.15 mmol) was added to 11-amino-1-undecanol (20.0 mg, 0.1 mmol) stirring in N,N-dimethylformamide (0.5 mL) and NaHCO3 (5.0 mg, 20%, v/v) at 0 °C. The reaction mixture was stirred overnight at room temperature and then filtered through a silica gel plug [washed with ethyl acetate (EA)] to provide the intermediate with the amino group protected with a Boc group, which was used directly in the synthesis of 3a and 3b.
For the synthesis of 3a, benzoyl chloride (230.0 μl, 2.0 mmol) was added to the intermediate (30.0 mg, 0.1 mmol) in dichloromethane (2.25 mL) and triethylamine (1.39 mL, 10.0 mmol) at room temperature. The mixture was stirred for 48 h and then concentrated. The residue was then dissolved in EtOAc, washed with NaHCO3 (aq.) three times, and dried with anhydrous Na2SO4. The organic layer was then concentrated and purified with column chromatography [silica gel, eluted with petroleum ether (PE) and EA gradient] to afford 3a (15.0 mg, 38%). Rf (PE/EA 9:1) = 0.49; 1H NMR (700 MHz, CDCl3): δ 8.04 (dd, J = 8.0, 1.0 Hz, 2H), 7.56 (tt, J = 7.4, 1.2 Hz, 1H), 7.43 (td, J = 8.0 Hz, 2H), 4.53 (b, 1H), 4.31 (t, J = 6.7 Hz, 2H), 3.10 (b, 2H), 1.76 (quint, J = 7.2 Hz, 2H), 1.44 (b, 11H), 1.35 (quint, J = 7.2 Hz, 2H), 1.27 (b, 12H); 13C NMR (176 MHz, CD3OD): δ 166.6, 155.9, 132.7, 130.5, 129.5, 128.3, 65.1, 40.6, 30.0, 29.7, 29.5, 29.4, 29.2, 28.7, 28.4, 26.8, 26.0; HRMS (ESI-TOF) m/z: [M + H-Boc]+ calcd for C18H30NO2, 292.2277; found, 292.2269.
For the synthesis of 3b, the intermediate (50.0 mg, 0.18 mmol) and sodium hydride (5.0 mg, 0.2 mmol) were stirred in dry N,N-dimethylformamide (1.5 mL) at 0 °C for 15 min, and benzyl bromide (25.0 μL, 0.2 mmol) was added. The mixture was continued to be stirred at room temperature overnight and was then diluted with EtOAc and washed with deionized water three times. The organic layer was dried anhydrous Na2SO4, concentrated and purified with column chromatography (silica gel, eluted with PE and EA gradient) to produce 3b (29.0 mg, 50%). Rf (PE/EA 9:1) = 0.71; 1H NMR (700 MHz, CD3OD) (characteristic protons): δ 7.35–7.32 (m, 4H), 7.28 (m, 1H), 5.30 (s, 1H), 4.50 (s, 2H), 3.46 (t, J = 6.7, Hz, 2H); 3.10 (q, J = 6.1 Hz, 2H); 1.61 (quint, J = 7.1 Hz, 2H); 1.44 (s, 11H), 1.35 (quint, J = 7.0 Hz, 2H), 1.31–1.22 (m, 12H); 13C NMR (176 MHz, CD3OD): δ 155.9, 138.7, 128.3, 127.6, 127.4, 72.8, 70.5, 53.4, 40.6, 30.0, 29.73, 29.67, 29.52, 29.50, 29.47, 29.44, 29.3, 28.4, 26.8, 26.2; HRMS (ESI-TOF) m/z: [M + H-Boc]+ calcd for C18H32NO, 278.2484; found, 278.2480.
General Procedure of Preparing Side Chains 5D–5E (Unoptimized).
A mixture of 11-aminoundecanoic acid (50 mg, 0.25 mmol) and thionyl chloride (36 μL, 0.5 mmol) was stirred at room temperature first and was then heated up to 70 °C in an oil bath and stirred for about 1 h. To the reaction mixture, a substituted benzyl alcohol (0.5 mmol) was added and stirred overnight. The mixture was purified with column chromatography on silica (eluted with DCM/MeOH gradient) to produce the desired side chain.
5D:
3,5-Dimethoxybenzylalcohol (84.0 mg, 0.5 mmol) was used, and purification with column chromatography on silica (eluted with DCM/MeOH gradient) produced 5D (41.0 mg, 47%). Rf (DCM/MeOH 9:1); 1H NMR (700 MHz, CD3OD): δ 6.48 (d, J = 2.2 Hz, 2H), 6.40 (t, J = 2.2 Hz, 1H), 5.03 (s, 2H), 3.77 (s, 6H), 2.97 (t, J = 7.9 Hz, 2H), 2.34 (t, J = 7.6 Hz, 2H), 1.75 (quint, J = 7.8 Hz, 2H), 1.63 (quint, J = 7.8 Hz, 2H), 1.35 (quint, J = 7.3 Hz, 2H), 1.32–1.22 (m, 10H); 13C NMR (176.0 MHz, CD3OD): δ 173.5, 160.9, 138.4, 105.9, 100.1, 65.9, 55.3, 40.0, 34.3, 29.4, 29.3, 29.2, 29.1, 29.0, 27.6, 26.6, 24.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H34NO4, 352.2488; found, 352.2486.
5E:
3-Methoxybenzylalcohol (63.0 mg, 0.5 mmol) and purification with column chromatography on silica (eluted with DCM/MeOH gradient) produced 5E (65.0 mg, 81%). Rf (DCM/MeOH 9:1) 0.56; 1H NMR (700 MHz, CD3OD): δ 7.26 (d, J = 7.9 Hz, 1H), 6.92 (d, J = 7.3 Hz, 1H), 6.88 (s, 1H), 6.85 (dd, J = 7.7, 2.1 Hz, 1H), 5.08 (s, 2H), 3.80 (s, 3H), 2.97 (t, J = 7.7 Hz, 2H), 2.34 (t, J = 7.9 Hz, 2H), 1.76 (quint, J = 7.7 Hz, 2H), 1.63 (quint, J = 7.3 Hz, 2H), 1.37 (quint, J = 7.3 Hz, 2H), 1.33–1.23 (m, 10H); 13C NMR (176.0 MHz, CD3OD): δ 173.6, 159.8, 137.7, 129.6, 120.3, 113.6, 65.9, 55.2, 40.0, 34.3, 29.6, 29.4, 29.3, 29.1, 28.9, 27.7, 26.5, 24.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H32NO3, 322.2382; found, 322.2380.
5F:
3-Phenoxyphenylmethanol (87.0 μL, 0.5 mmol) and purification with column chromatography on silica (eluted with DCM/MeOH gradient) produced 5F (50.0 mg, 52%). Rf (DCM/MeOH 9:1) 0.43; 1H NMR (700 MHz, CD3OD) δ 7.32 (t, J = 17.0 Hz, 2H), 7.29 (t, J = 7.9 Hz, 1H), 7.10 (t, J = 7.4 Hz, 1H), 7.06 (d, J = 7.7 Hz, 1H), 7.00–6.98 (m, 3H), 6.93 (dd, J = 8.2, 2.2 Hz, 1H), 5.07 (s, 3H), 2.97 (t, J = 7.9 Hz, 2H), 2.33 (t, J = 7.5 Hz, 2H), 1.76 (quint, J = 7.7 Hz, 2H), 1.61 (quint, J = 7.4 Hz, 2H), 1.37 (quint, J = 7.4 Hz, 2H), 1.33–1.23 (m, 10H); 13C NMR (176.0 MHz, CD3OD): δ 173.4, 157.5, 157.0, 138.2, 129.8, 129.6, 123.4, 122.6, 119.0, 118.3, 118.2, 65.5, 40.0, 34.3, 29.7, 29.3, 29.2, 29.1, 29.0, 27.7, 26.6, 24.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H34NO3, 384.2539; found, 384.2535.
5G:
3-Nitrobenzylalcohol (60.0 mg, 0.5 mmol) and purification with column chromatography on silica (eluted with DCM/MeOH gradient) produced 5G (64.0 mg, 76%). Rf (DCM/MeOH 9:1) 0.40; 1H NMR (700 MHz, CD3OD): δ 8.21 (s, 1H), 8.13 (d, J = 7.9 Hz, 1H), 7.67 (d, J = 7.6 Hz, 1H), 7.54 (t, J = 7.9 Hz, 1H), 5.20 (s, 2H), 2.96 (t, J = 7.7 Hz, 2H), 2.38 (t, J = 7.6 Hz, 2H), 1.74 (quint, J = 7.6 Hz, 2H), 1.64 (quint, J = 7.4 Hz, 2H), 1.36 (quint, J = 7.3 Hz, 2H), 1.33–1.22 (m, 10H); 13C NMR (176.0 MHz, CD3OD): δ 173.3, 148.4, 138.3, 133.8, 129.5, 123.0, 122.7, 64.5, 40.0, 39.5, 36.8, 34.1, 29.6, 29.4, 29.3, 29.1, 29.0, 27.7, 26.9, 26.5, 26.4, 25.7, 24.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H29N2O4, 337.2127; found, 337.2126.
5H:
4-Fluorobenzylalcohol (55.0 μL, 0.5 mmol) and purification with column chromatography on silica (eluted with DCM/MeOH gradient) produced 5H (49.0 mg, 64%). Rf (DCM/MeOH 9:1) 0.37; 1H NMR (700 MHz, CD3OD): δ 7.31 (dd, J = 8.5, 5.6 Hz, 1H), 7.02 (t, J = 8.5 Hz, 1H), 5.06 (s, 2H), 2.96 (t, J = 7.8 Hz, 2H), 2.31 (t, J = 7.4 Hz, 2H), 1.73 (quint, J = 7.5 Hz, 2H), 1.60 (quint, J = 7.2 Hz, 2H), 1.34 (quint, J = 7.0 Hz, 2H), 1.31–1.21 (m, 10H); 13C NMR (176.0 MHz, CD3OD): δ 173.5, 163.3, 161.9, 132.0, 130.1, 115.5, 115.3, 65.2, 40.1, 39.5, 36.8, 34.2, 29.6, 29.4, 29.3, 29.2, 29.1, 28.8, 27.7, 26.9, 26.6, 26.4, 25.7, 24.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H29FNO2, 310.2182; found, 310.2179.
Antigens.
The chicken egg albumin for in vivo use (Vac-pova) was purchased from InvivoGen.
Mice and Immunization.
BALB/c mice used in this study were purchased from the Frederick Cancer Research (Fredrick, MD) and maintained within an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham (UAB). To assess the adjuvant activity of the MS saponin-based immune adjuvants, groups of female mice (8–10 weeks of age; 6 mice per group) were immunized by the subcutaneous (s.c.) route with OVA (20.0 μg) alone or with antigen plus proper adjuvant such as QS-21 (20.0 μg) or an MS adjuvant (50.0 or 100.0 μg) on days 0, 14, and 28. Prior to each immunization and at 2 weeks post the last immunization, mice were weighed and blood samples were collected from the lateral tail vein by using heparinized capillary pipettes. The serum was obtained after centrifugation and stored at −20 °C until assayed. All studies were performed according to the National Institutes of Health guidelines, and protocols were approved by the UAB Institutional Animal Care and Use Committee.
Evaluation of Antibody Responses.
The levels of specific serum IgG and IgG subclasses against OVA in each group were determined by an ELISA. MaxiSorp microtiter plates (NUNC International, Roskilde, Denmark) were coated with OVA (0.1 μg/mL) or with optimal amounts of goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotechnology Associates, Inc., Birmingham, AL) in borate buffer saline (BBS; 100 mM NaCl, 50 mM boric acid, and 1.2 mM Na2B4O7, pH 8.2) at 4 °C overnight. Plates were blocked with 1% bovine serum albumin and 0.02% sodium azide in BBS for 2 h at room temperature. Serial twofold dilutions of serum samples were added in duplicate to the plates. To generate standard curves, serial dilutions of a mouse immunoglobulin reference serum (MP Biomedicals, Solon, OH) were added to two rows of wells in each plate that had been coated with the appropriate anti-mouse IgG or IgG subclass reagent. After incubation (overnight at 4 °C) and washing of the plates, horseradish peroxidase-conjugated goat anti-mouse IgG or IgG subclass antibody (Southern Biotechnology Associates, Inc.) was added to appropriate wells. After 4 h of incubation at room temperature, plates were washed and developed by o-phenylenediamine substrate with hydrogen peroxide. Color development was recorded at 490 nm. The concentrations of antibodies were determined by interpolation on standard curves generated by using the mouse immunoglobulin reference serum and constructed by a computer program based on four-parameter logistic algorithms (Softmax/Molecular Devices Corp., Menlo Park, CA).
Cell Isolation and Flow Cytometry.
Each mouse spleen was excised and mashed between the frosted ends of two microscope slides to get a single-cell suspension. After removing red blood cells, the cell suspension was passed through a 70 μm filter membrane to eliminate debris. Each single cell suspension was first stained with the fixable viability dye (Biolegend) at 1:1000 in phosphate-buffered saline (PBS) solution for 10 min. After washing with FACS buffer (PBS/2% fetal bovine serum), cells were then incubated with Fc block (anti-mouse CD16/32 antibody) at 1:100 for 10 min, followed by staining with the indicated antibody mixtures for 30 min before washing and flow cytometry analysis. For intracellular staining, cells were fixed and permeabilized using the fixation/permeabilization concentrate and Diluent kit (ThermoFisher Scientific) according to the manufacturer’s protocol, followed by incubation with Fc block and intracellular antibodies for 30 min prior to washing and flow cytometry analysis. For intracellular cytokine detection, cells were stimulated with BD Leukocyte Activation Cocktail, with BD GolgiPlug (BD Biosciences) for 5 h prior to staining. The fluorescence dye-labeled antibodies specific for mouse, CD4 (GK1.5, RM4–5), CD3 (145–2C11), CD8 (53–6.7), CD19 (1D3), Fas (Jo2), T- and B-cell activation antigen (GL7), PD-1 (29F.1A12), CD86 (GL-1), CXCR4 (L276F12), Granzyme B (QA16A02), IFN-γ (XMG1.2), IL-4 (11B11), FoxP3 (FJK-16 s), and Bcl6 (K112–91), were purchased from BD Biosciences, ThermoFisher Scientific and Biolegend. To stain intracellular IL-21, recombinant IL-21R Fc chimera protein (RD systems) was used followed by staining with fluorescence dye-labeled goat anti-human IgG (Biolegend). All steps were performed at 4 °C. Cells were acquired on a BD FACSymphony using FACSDiva software (BD Biosciences) and analyzed with FlowJo software (Treestar).
Statistical Analysis.
Statistical significance in antibody responses was evaluated by t tests (with unpaired, nonparametric, and Mann–Whiteny test) using GraphPad Prism 8. Differences were considered significant at a P value <0.05.
Supplementary Material
ACKNOWLEDGMENTS
P.W. was supported by NIH grant R01 GM120159, and J.W.L. was supported by DoD W81XWH-18-1-0315 and NIH grant R01AI148711.
ABBREVIATIONS
- CTL
cytotoxic T cell
- DCM
dichloromethane
- EDC·HCl
1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide
- ELISA
enzyme-linked immunosorbent assay
- ESI-TOF
electrospray ionization time-of-flight mass spectrometry
- GC
germinal center
- HOBt
hydroxybenzotriazole
- IACUC
International Animal Care and Use Committee
- IgG
immunoglobulin G
- MeCN
acetonitrile
- NHS
N-hydroxysuccinimide
- NMM
N-methylmorpholine
- OVA
ovalbumin
- rha
rhamnose
- s.c.
subcutaneous
- Teff
effector T-cells
- TFH
follicular helper T-cells
- TFR
follicular regulatory T-cells
- Th
T helper cells
- THF
tetrahydrofuran
- xyl
xylose
Footnotes
The authors declare the following competing financial interest(s): P.W. is inventor on patent applications partially based on this work. P.W. and S.M. are co-founders of Adjuvax, LLC.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jmedchem.2c01087
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c01087.
1H and 13C NMR spectra of the new compounds 1D–1H, 2D–2H, 3a, 3b, and 5D–5H (PDF)
Molecular formula strings (CSV)
Contributor Information
Hyunjung Kim, Department of Chemistry, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States.
Di Bai, Department of Chemistry, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States.
Sadashib Ghosh, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States.
Michael L. Franks, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
Xifeng Wang, Department of Chemistry, Central China Normal University, Wuhan, Hubei 430079, P. R. China.
Cheng Yan, Department of Chemistry, Central China Normal University, Wuhan, Hubei 430079, P. R. China.
Zheng Liu, Department of Chemistry, Central China Normal University, Wuhan, Hubei 430079, P. R. China.
Ping Zhang, Department of Pediatric Dentistry, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States.
Suzanne M. Michalek, Department of Microbiology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
Jianmei W. Leavenworth, Department of Neurosurgery, Department of Microbiology, and The O’Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
Pengfei Wang, Department of Chemistry and The O’Neal Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States.
REFERENCES
- (1).Brunner R; Jensen-Jarolim E; Pali-Schöll I The ABC of clinical and experimental adjuvants - a brief overview. Immunol. Lett. 2010, 128, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kensil CR; Mo AX; Truneh A Current vaccine adjuvants: An overview of a diverse class. Front. Biosci. 2004, 9, 2972–2988. [DOI] [PubMed] [Google Scholar]
- (3).Leroux-Roels G Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 2010, 28, C25–C36. [DOI] [PubMed] [Google Scholar]
- (4).Sharp FA; Lavelle EC Discovery of vaccine adjuvants. In Development of Therapeutic Agents Handbook, 1st ed.; Gad SC, Ed.; John Wiley & Sons, Inc: Hoboken, NJ, 2012; pp 533–546. [Google Scholar]
- (5).Wang W; Singh M Selection of adjuvants for enhanced vaccine potency. World J. Vaccine 2011, 01, 33–78. [Google Scholar]
- (6).Weeratna RD; McCluskie MJ Recent advances in vaccine adjuvants. In Emerging Trends in Antibacterial Discovery: Answering the Call to Arms; Miller AA, Miller PF, Eds.; Caister Academic Press: Great Britain, 2011; pp 303–322. [Google Scholar]
- (7).Coffman RL; Sher A; Seder RA Vaccine adjuvants: putting innate immunity to work. Immunity 2010, 33, 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Banday AH; Jeelani S; Hruby VJ Cancer vaccine adjuvants–recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 2015, 37, 1–11. [DOI] [PubMed] [Google Scholar]
- (9).Bastola R; Noh G; Keum T; Bashyal S; Seo JE; Choi J; Oh Y; Cho Y; Lee S Vaccine adjuvants: smart components to boost the immune system. Arch Pharm. Res. 2017, 40, 1238–1248. [DOI] [PubMed] [Google Scholar]
- (10).Pasquale A; Preiss S; Silva F; Garçon N Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccine 2015, 3, 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Temizoz B; Kuroda E; Ishii KJ Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bonam SR; Partidos CD; Halmuthur SKM; Muller S An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [DOI] [PubMed] [Google Scholar]
- (13).O’Hagan DT; Friedland LR; Hanon E; Didierlaurent AM Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [DOI] [PubMed] [Google Scholar]
- (14).Garçon N; Leroux-Roels G; Cheng W-F Vaccine Adjuvants. In Understanding Modern Vaccines Perspectives in Vaccinology Garçon; Stern PL, Cunningham AL, Eds.; Elsevier: Amsterdam, 2011; Vol. 1, pp 89–113. [Google Scholar]
- (15).Del Giudice G; Rappuoli R; Didierlaurent AM Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 2018, 14–21. [DOI] [PubMed] [Google Scholar]
- (16).Shi S; Zhu H; Xia X; Liang Z; Ma X; Sun B Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [DOI] [PubMed] [Google Scholar]
- (17).Didierlaurent AM; Laupèze B; Di Pasquale A; Hergli N; Collignon C; Garçon N Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expet Rev. Vaccine 2017, 16, 55–63. [DOI] [PubMed] [Google Scholar]
- (18).James SF; Chahine EB; Sucher AJ; Hanna C Shingrix: the new adjuvanted recombinant herpes zoster vaccine. Ann. Pharmacother. 2018, 52, 673–680. [DOI] [PubMed] [Google Scholar]
- (19).Martín RS; Briones R Industrial uses and sustainable supply of Quillaja Saponaria (Rosaceae) Saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar]
- (20).Ragupathi G; Gardner JR; Livingston PO; Gin DY Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expet Rev. Vaccine 2011, 10, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wang P; Ding X; Kim H; škalamera Ð; Michalek SM; Zhang P Vaccine adjuvants derivatized from Momordica saponins I and II. J. Med. Chem. 2019, 62, 9976–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang P; Ding X; Kim H; Michalek SM; Zhang P Structural effect on adjuvanticity of saponins. J. Med. Chem. 2020, 63, 3290–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Griffin WC Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- (24).Davies JT, A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent. Gas/Liquid and Liquid/Liquid Interface. Proceedings of the International Congress of Surface Activity; Citeseer, 1957; pp 426–438. [Google Scholar]
- (25).Chea EK; Fernández-Tejada A; Damani P; Adams MM; Gardner JR; Livingston PO; Ragupathi G; Gin DY Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 2012, 134, 13448–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Marciani D Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [DOI] [PubMed] [Google Scholar]
- (27).Rhodes J Covalent chemical events in immune induction: fundamental and therapeutic aspects. Immun. Today 1996, 17, 436–441. [DOI] [PubMed] [Google Scholar]
- (28).Soltysik S; Wu J-Y; Recchia J; Wheeler DA; Newman MJ; Coughlin RT; Kensil CR Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 1995, 13, 1403–1410. [DOI] [PubMed] [Google Scholar]
- (29).Marciani D Effects of N-acylation on the immune adjuvanticity of analogs of the Quillaja saponins derivative GPI-0100. Vaccine 2022, 40, 4169–4173. [DOI] [PubMed] [Google Scholar]
- (30).Leavenworth J; Verbinnen B; Yin J; Huang H; Cantor H A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 2015, 16, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Shen E; Wang Q; Rabe H; Liu W; Cantor H; Leavenworth JW Chromatin remodeling by the NuRD complex regulates development of follicular helper and regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 6780–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Shen E; Rabe H; Luo L; Wang L; Wang Q; Yin J; Yang X; Liu W; Sido JM; Nakagawa H; Ao L; Kim HJ; Cantor H; Leavenworth JW Control of germinal center localization and lineage stability of follicular regulatory T cells by the Blimp1 transcription factor. Cell Rep. 2019, 29, 1848–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Luo L; Hu X; Dixon ML; Pope BJ; Leavenworth JD; Raman C; Meador WR; Leavenworth JW Dysregulated follicular regulatory T cells and antibody responses exacerbate experimental autoimmune encephalomyelitis. J. Neuroinflammation 2021, 18, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Pu YJ; Vaid RK; Boini SK; Towsley RW; Doecke CW; Mitchell D A practical method for functionalized peptide or amide bond formation in aqueous-ethanol media with EDC as Activator. Org. Process Res. Dev. 2009, 13, 310–314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


