Abstract
Background
The short-term effectiveness of a 2-dose regimen of the BioNTech/Pfizer BNT162b2 vaccine for adolescents has been demonstrated. However, little is known about the long-term effectiveness in this age group. It is known, however, that waning of vaccine-induced immunity against infection in adult populations is evident within a few months.
Methods
Leveraging the database of Maccabi Healthcare Services (MHS), we conducted a matched case-control design for evaluating the association between time since vaccination and the incidence of infections, where 2 outcomes were evaluated: documented SARS-CoV-2 infection (regardless of symptoms) and symptomatic infection (COVID-19). Cases were defined as individuals aged 12–16 with a positive polymerase chain reaction (PCR) test occurring between 15 June and 8 December 2021, when the Delta variant was dominant in Israel. Controls were adolescents who had not tested positive previously.
Results
We estimated a peak vaccine effectiveness between 2 weeks and 3 months following receipt of the second dose, with 85% (95% confidence interval [CI]: 84–86%) and 90% (95% CI: 89–91%) effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19), respectively. However, in line with findings for adults, waning effectiveness was evident. Long-term protection was reduced to 73% (95% CI: 68–77%) against infection and 79% (95% CI: 73–83%) against COVID-19 3–5 months after the second dose and waned to 53% (95% CI: 46–60%) against infection and 66% (95% CI: 59–72%) against COVID-19 after 5 months.
Conclusions
Although vaccine-induced protection against both infection and COVID-19 continues over time in adolescents, the protection wanes with time since vaccination, starting 3 months after inoculation and continuing for more than 5 months.
Keywords: SARS-CoV-2, COVID-19 vaccine, waning of vaccine-induced immunity
The vaccine confers excellent short-term protection following 2 doses in adolescents; however, effectiveness significantly wanes after 3 months, in line with data from adults.
The BioNTech/Pfizer mRNA BNT162b2 vaccine was approved for adolescents by the US Food and Drug Administration (FDA) and the European Medicines Agency in May 2021 [1, 2]. Shortly after, on 2 June 2021, Israel, an early adopter of the vaccination campaign in adults, launched a vaccination campaign for adolescents [3] and has since continued to promote it [4].
The short-term effectiveness of a two-dose regimen of the BioNTech/Pfizer mRNA BNT162b2 vaccine for adolescents has been demonstrated both in clinical trials [5] and real-world studies [6]. However, little is known about the long-term effectiveness of the vaccine in this age group. It is known, though, that waning of vaccine-induced immunity of the BNT162b2 vaccine in adult populations is evident within a few months of administration [7–11], although protection against severe disease is more sustained [12]. The question of waning immunity in the adolescent population has important implications for future areas of vaccine research and policy. For example, the US FDA and Israel have already extended the eligibility of the booster (third) dose for ages 12–15 [13, 14], and other countries are considering this decision.
To address this issue, we conducted a retrospective matched case-control study aimed at evaluating the duration of protection conferred by the BNT162b2 vaccine on adolescents aged 12 to 16, leveraging data from Maccabi Healthcare Services (MHS), Israel’s second largest Health Maintenance Organization, which covers 2.5 million members. The 6-month follow-up period of the study, from 15 June to 8 December 2021, represents the longest published on this age group to date, and corresponds to a time when the Delta (B.1.617.2) variant was dominant in Israel, accounting for >90% isolates as of 8 December 2021, prior to the surge of the Omicron variant [15]. By the end of the study period, the Omicron variant was already present though still at low levels, with percentages increasing from 0.30% on 29 November 2021, to 7.2% on 13 December 2021 [16].
METHODS
Data Sources
MHS is a 2.5-million-member, not-for-profit health-fund in Israel. It is the second largest in Israel, covering 26.7% of the population and providing a representative sample of the Israeli population. MHS has maintained a centralized database of Electronic Medical Records (EMRs) for 3 decades, with <1% disengagement rate among its members, allowing for a comprehensive longitudinal medical follow-up. The centralized dataset includes extensive demographic data, clinical measurements and evaluations, outpatient and hospital diagnoses and procedures, medications dispensed, imaging performed and comprehensive laboratory data from a single central laboratory.
Study Population and Data Collection
The study population consisted of MHS members, aged 12–16 years, who received either 1 or 2 doses of the BNT162b2 vaccine. The fraction of adolescents receiving 3 doses was negligible. We excluded adolescents aged 17 years or older to examine a more coherent exposure group, because the former group became eligible for vaccination months earlier (ie, February 2021), and thus exposure and time since- vaccination was considerably different for this age group. Anonymized EMRs were retrieved from MHS’s centralized computerized database. Analyses focused on the period from 15 June 2021, to 8 December 2021, when the Delta variant was dominant in Israel. Participants were those enrolled in MHS as of 15 June 2021, who had not tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by polymerase chain reaction (PCR) test prior to the start of the follow-up period.
Individual-level data for the study population included age, biological sex, and a coded geographical statistical area (GSA; the smallest geostatistical unit of the Israeli census, which correspond to neighborhoods assigned by Israel’s National Bureau of Statistics). Data collected also encompassed the last documented body mass index (BMI); obesity was defined as BMI ≥ 30. Coronavirus disease 2019 (COVID-19)-related information included dates of the first and second dose of the BNT162b2 vaccine and results of any PCR tests for SARS-CoV-2, including tests performed outside of MHS. PCR tests were freely available to MHS members during the study period, for reasons including clinical symptoms, suspected exposure to infected individuals, event attendance requirements, and so forth. Additionally, the data set contained information on symptoms for some of the participants who tested positive. The information about COVID-19-related symptoms was extracted from EMRs, where they were recorded by the primary care physician or a certified nurse who conducted in-person or phone visits with each infected individual. Information on symptoms was only available for individuals who had a positive test for SARS-CoV-2. For individuals who had multiple positive tests, the date of diagnosis was defined as the date of the first positive PCR.
Statistical Analysis
Two main outcomes were evaluated: SARS-CoV-2 infection (regardless of the presence of symptoms) and symptomatic SARS-CoV-2 infection (COVID-19).
Analysis 1: Matched Case-Control Analysis for Breakthrough Infections
We used a matched case-control design [17–19] for evaluating the association between time since vaccination and the incidence of infections. Cases were defined as individuals with a positive PCR test occurring after 15 June 2021, among those 12–16 years of age who did not have a previous positive test recorded. Eligible controls were individuals who had not tested positive prior to the date of the positive PCR of their matched case, that is, individuals who either had a negative PCR test result or who did not obtain a PCR test. Controls were matched by residential socioeconomic status and biological sex. We attempted to match up to 20 controls per case from the entire population, resulting in 90% of the cases matched to 20 controls, 96% of the cases had at least 10 controls, and 99% of the cases had at least 5 controls; 99.8% of cases were matched to at least 1 control.
The analysis sought to estimate the reduction in the odds of a positive test at different time intervals following receipt of the first and second vaccine doses (0–6 days following the first dose; 7 days after dose 1 to 13 days after dose 2; and 14–89 days, 90–149 days and 150–180 days following the second dose). The 7-day and 14-day cutoffs for the first and second doses were chosen based on previous research on vaccine effectiveness (VE) in adults [20, 21]; the 0–6-day period following the first dose was intended to be used as a negative control [22]. The reference group in the analysis was those who were unvaccinated. We included age (in years) and obesity (ie, BMI ≥ 30, which has been linked to severity of COVID-19 symptoms) as covariates [23–25]. We also tested our model by including an additional covariate consisting of the number of tests performed before the study period, that is, between 1 March 2020, and 14 June 2021, to adjust for potential differences in health-seeking behavior [17]. The rationale is that there is a correlation between the number of tests before the study period and the one during it, potentially correcting for a possible detection bias [7, 9]. However, repeated testing was significantly lower compared to adults [7, 17, 26], and results were not materially changed.
A conditional logistic regression model was fit to the data. The VE of the first and second doses (compared to being unvaccinated) was calculated as 100%*[1-(odds ratio)] for each of the time-since-vaccination categories.
Additionally, we carried out a complementary analysis utilizing a test-negative design. Details can be found in the Supplementary Materials.
Analysis 2: Matched Case-Control Analysis for Symptomatic Breakthrough Infections
Similar to the analysis of infections, we performed a matched case-control analysis for symptomatic infection (COVID-19). Cases were individuals who tested positive and exhibited COVID-19-related symptoms after 15 June 2021. Eligible controls were individuals who had not tested positive. Matching was performed as for the infection case-control analysis. Again, at least 1 and up to 20 controls per case were drawn from the entire population; 89% of cases were matched to 20 controls, 96% of cases had at least 10 controls, and 99% of cases had at least 5 controls; 99.8% of cases were matched to at least 1 control. A conditional logistic regression was fit to the data, adjusting for age and obesity, as specified in analysis 1.
Analyses were performed using R version 4.0.5. The analysis conformed to the STROBE checklist for case-control studies.
Ethics Declaration
This study was approved by the MHS (Maccabi Healthcare Services) Institutional Review Board (IRB). Due to the retrospective design of the study, informed consent was waived by the IRB, and all identifying details of the participants were removed before computational analysis.
RESULTS
During the follow-up period, 274 431 PCR tests were performed among 129 909 MHS members 12–16 years of age who did not have a previous documented infection. Baseline characteristics of the participants are given in Table 1. Overall, vaccinated individuals had a notably lower percent of tests that were positive; 6.6% of tests among unvaccinated individuals were positive, compared with <1.4% for those who received their second dose 14–149 days before the test, and 3.6% for those who received their second dose ≥150 days before the test (Supplementary Table 1). There were 14 hospitalizations for COVID-19 and no deaths reported.
Table 1.
Demographic Characteristics of Individuals Who Were Tested Between 15 June and 8 December 2021
| Characteristics | Control (n = 226 201) | Case(n = 11 822) | P Value | Overall(n = 238 023) |
|---|---|---|---|---|
| Age, y, mean (SD) | 14.0 (1.4) | 13.7 (1.4) | <.0001a | 14.0 (1.4) |
| Sex, no. (%) | .9b | |||
| Female | 117 697 (52.0%) | 6143 (52.0%) | 123 840 (52.0%) | |
| Male | 108504 (48.0%) | 5679 (48.0%) | 114 183 (48.0%) | |
| Comorbidities, no. (%) | ||||
| ȃObesity (BMI ≥30) | 7633 (3.4%) | 327 (2.8%) | <.0001b | 7960 (3.3%) |
| Socioeconomic status by GSA | .4c | |||
| ȃHigh (7–10) | 94 810 (41.9%) | 4902 (41.5%) | 99 712 (41.9%) | |
| ȃMedium (5–6) | 82 813 (36.6%) | 4360 (36.9%) | 87 173 (36.6%) | |
| ȃLow (0–4) | 45 034 (19.9%) | 2377 (20.1%) | 47 411 (19.9%) | |
| ȃMissing | 3544 (1.6%) | 183 (1.5%) | 3727 (1.6%) |
Case: Positive polymerase chain reaction (PCR) test.
Abbreviations: BMI, body mass index; GSA, geographical statistical area; SD, standard deviation.
Based on 2 sample t test.
Based on 2 proportion z-test.
Based on χ2 test.
Vaccine Effectiveness Against Breakthrough Infections
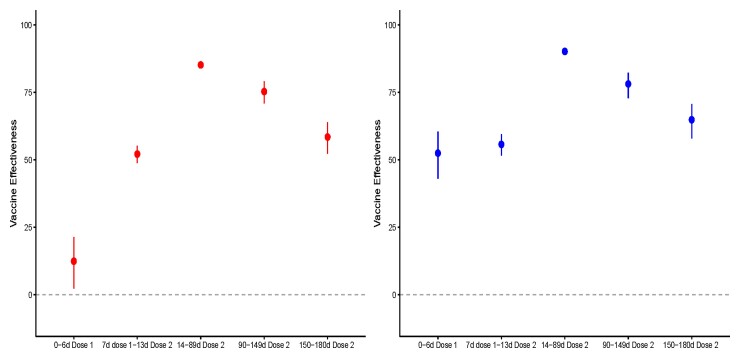
The effectiveness of the first and second doses compared to the unvaccinated population initially increased over time following receipt of the vaccine, with a small reduction in the odds of testing positive in days 0–6 following the first dose (VE = 12%, 95% confidence interval [CI]: 2%, 21%), moderate effectiveness for the period from day 7 after the first dose to day 13 after the second dose (VE = 52%, 95% CI: 48%, 55%), and high effectiveness in the period from days 14 to 89 following dose 2 (VE = 85%, 95% CI: 84%, 86%) (Supplementary Table 2, Figure 1). The effectiveness of the vaccine against infection was subsequently reduced to 73% (95% CI: 68%, 77%) and 53% (95% CI: 46%, 60%) after 90–149 days and 150–180 days following receipt of the second dose, respectively. The results of the test-negative design showed a similar decline in vaccine effectiveness (Supplementary Table 3).
Figure 1.
Reduction in the odds of testing positive for SARS-CoV-2 (left panel) and in having a positive test with symptoms (right panel) among individuals who had received 1 or 2 doses of BNT162b2 vaccine compared to unvaccinated adolescents, by time since vaccination. In red, vaccine effectiveness against SARS-CoV-2 infection, and in blue, against symptomatic infection. Vertical bars represent 95% confidence intervals. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Vaccine Effectiveness Against Symptomatic Breakthrough Infections
We next evaluated vaccine effectiveness against symptomatic infection, comparing vaccinated to unvaccinated individuals. In those partially vaccinated (ie, persons between 7 days after the first dose up to 13 days after the second dose), we observed a 56% (95% CI: 52%, 60%) reduction in the odds of having a symptomatic SARS-CoV-2 infection compared to unvaccinated adolescents. This reduction was more marked 14–89 days following the second dose, where the vaccine effectiveness against COVID-19 was 90% (95% CI: 89%, 91%) (Supplementary Table 4). Similar to the analysis of all infections (regardless of symptomatic presentation), the estimated effectiveness of the vaccine against COVID-19 subsequently decreased, with VE = 79% (95% CI: 73%, 83%) for days 90–149 and VE = 66% (95% CI: 59%, 72%) for days 150–180 after the second dose.
DISCUSSION
In this study, we found that the BioNTech/Pfizer mRNA BNT162b2 vaccine provided strong short-term protection against any SARS-CoV-2 infection and symptomatic infection (COVID-19) with the Delta variant in adolescents, confirming previous findings [5, 6]. Using a matched case-control analysis, we estimated a peak vaccine effectiveness between 2 weeks and 3 months following receipt of the second dose, with 85% (95% CI: 84%, 86%) and 90% (95% CI: 89%, 91%) effectiveness against SARS-CoV-2 infection and COVID-19, respectively. However, in line with previous findings for adults [7–9], waning of vaccine effectiveness was evident in adolescents as well. Long-term protection conferred by the vaccine was reduced to 73% (95% CI: 68%, 77%) against infection and 79% (95% CI: 73%, 83%) against COVID-19 3 to 5 months after the second dose and waned to 53% (95% CI: 46%, 60%) against infection and 66% (95% CI: 59%, 72%) against symptomatic infection after 5 months.
We evaluated vaccine effectiveness against infection and COVID-19 but did not evaluate effectiveness against severe disease. Even in the absence of vaccination, adolescents have a much lower risk of hospitalization and death compared to adults [27, 28], and the number of events in this population was small [29]. Other studies in adults have found that vaccine effectiveness against severe outcomes has been maintained at higher levels than effectiveness against infection [12]. Decisions to vaccinate and to use a booster dose among adolescents, as well as prioritization of vaccines among different age and risk groups, will therefore depend on the policy goals, that is, reducing transmission of SARS-CoV-2 in the population in the short term versus reducing the burden of disease in the population.
This study has several limitations. The estimates of vaccine-induced protection against symptomatic infection should be interpreted with caution, because a protective effect of the vaccine was already evident a few days after the receipt of the first dose (53% effectiveness at 0–6 days with 95% CI: 43%, 60%). We did not expect to find a significant effect of vaccination in the first week after receipt of the first dose, because it presumably takes time for the vaccine to induce an immune response. We observed a small but significant reduction in the odds of infection in the 6 days following the first dose and a larger reduction in the odds of symptomatic infection, which could indicate a potential bias. It is possible that individuals are less likely to be tested immediately after vaccination (e.g., because they attribute symptoms to temporary side effects) and are therefore less likely to be detected (rather than less likely to be infected) compared to unvaccinated individuals, especially symptomatic ones. This effect could also be explained as a healthy user bias, that is, those who are experiencing symptoms may be less likely to get vaccinated. However, the bias would most likely be short-lived, so the estimates of vaccine effectiveness against COVID-19 could be more reliable a couple of weeks after receipt of the first dose [9, 17].
A second limitation is with regards the generalizability of our findings in light of the emergence of novel SARS-CoV-2 variants. Our study only estimates the short- and long-term vaccine effectiveness against the Delta variant, which was the dominant strain in Israel at the time of our study [15]. The protection conferred against other strains, including the Omicron variant, cannot be inferred, although recent studies show an important reduction of vaccine effectiveness against SARS-CoV-2 infection with the Omicron variant, and particularly symptomatic infection, following 2 doses of BNT162b2 in the adult population (ie, 18+ years old) [30].
CONCLUSION
Our analyses suggest that, compared to those who are unvaccinated, adolescents aged 12–16 who received 2 doses of the BNT162b2 vaccine have a lower risk of contracting SARS-CoV-2 infection, as detected by PCR, and a lower risk of symptomatic infection. However, like adults, vaccine-induced protection against both SARS-CoV-2 infection and symptomatic infection wanes with time, starting 3 months after inoculation and continuing for more than 5 months.
Supplementary Material
Contributor Information
Ottavia Prunas, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Daniel M Weinberger, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Virginia E Pitzer, Department of Epidemiology of Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Sivan Gazit, Kahn Sagol Maccabi (KSM) Research & Innovation Center, Maccabi Healthcare Services, Tel Aviv, Israel; Maccabitech Institute for Research and Innovation, Maccabi Healthcare Services, Tel Aviv, Israel.
Tal Patalon, Kahn Sagol Maccabi (KSM) Research & Innovation Center, Maccabi Healthcare Services, Tel Aviv, Israel; Maccabitech Institute for Research and Innovation, Maccabi Healthcare Services, Tel Aviv, Israel.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Data availability statement. According to the Israel Ministry of Health regulations, individual-level data cannot be shared openly. Specific requests for remote access to de-identified community-level data should be referred to KSM, Maccabi Healthcare Services Research and Innovation Center.
Code availability statement. Specific requests for remote access to the code used for data analysis should be referred to KSM, Maccabi Healthcare Services Research and Innovation Center.
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) (USA) under award number R01AI137093. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH (USA).
References
- 1. Food and Drug Administration (FDA) . Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic [Internet]. Food Drug Adm.2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use. Accessed 18 May 2021. [Google Scholar]
- 2. First COVID-19 vaccine approved for children aged 12 to 15 in EU. European Medicines Agency; [Internet]. Available at: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu. Accessed 31 December 2021. [Google Scholar]
- 3. Ministry of Health’s Position Regarding the Expansion of the Vaccination Operation to Ages 12–16 Years. Ministry of Health; [Internet]. Available at: https://www.gov.il/en/departments/news/02062021-01. Accessed 31 December 2021. [Google Scholar]
- 4. Campaign to Motivate Teenagers to Get Vaccinated. Ministry of Health; [Internet]. Available at:https://www.gov.il/en/Departments/news/24082021-04. Accessed 1 January 2022. [Google Scholar]
- 5. Frenck RW, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021; 385:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis 2021; 27:2919–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021; 12:6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021; 385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med 2021;27:2108–2110. [DOI] [PubMed] [Google Scholar]
- 12. Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among aults—United States, March–July 2021. Morb Mortal Wkly Rep 2021; 70:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. F.D.A . Plans to allow 12- to 15-year-olds to receive Pfizer boosters - the New York Times [Internet]. Available at: https://www.nytimes.com/2021/12/30/us/politics/boosters-12-15-year-olds-omicron.html. Accessed 1 January 2022.
- 14. The Vaccination Committee Recommends: 3 Weeks Between the First and Second Dose Also for Children 5-11. Ministry of Health; [Internet]. Available at: https://www.gov.il/en/departments/news/21112021-04. Accessed 1 January 2022. [Google Scholar]
- 15. SARS-CoV-2 variants in analyzed sequences, Israel [Internet]. Available at: https://ourworldindata.org/grapher/covid-variants-area?country=~ISR. Accessed 30 December 2021.
- 16. Share of SARS-CoV-2 sequences that are the omicron variant, Nov 29, 2021, to Mar 7, 2022 [Internet]. Available at: https://ourworldindata.org/grapher/covid-cases-omicron?tab=chart&time=2021-11-29.2022-03-31&country=∼ISR. Accessed 20 March 2022. [Google Scholar]
- 17. Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med 2022; 182:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Essebag V, Platt R W, Abrahamowicz Ml, Pilote L. Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol 2005; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zakeri R, Bendayan R, Ashworth M, et al. A case-control and cohort study to determine the relationship between ethnic background and severe COVID-19. EClinicalMedicine 2020:100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis 2022;74:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010; 21:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020; 43:e72–4. [DOI] [PubMed] [Google Scholar]
- 24. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020; 28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alberca RW, Oliveira LM, Branco ACCC, Pereira NZ, Sato MN. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr 2021; 61:2262–76. [DOI] [PubMed] [Google Scholar]
- 26. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith C, Odd D, Harwood R, et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat Med 2022;28:185–92. [DOI] [PubMed] [Google Scholar]
- 28. Saxena S, Skirrow H, Wighton K. Should the UK vaccinate children and adolescents against covid-19? BMJ 2021;374:n1866. [DOI] [PubMed] [Google Scholar]
- 29. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J 2021; 40:e482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022:NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.