Abstract
Aims
To test the hypothesis that the activation of the growth hormone-releasing hormone (GHRH) receptor signalling pathway within the myocardium both prevents and reverses diastolic dysfunction and pathophysiologic features consistent with heart failure with preserved ejection fraction (HFpEF). Impaired myocardial relaxation, fibrosis, and ventricular stiffness, among other multi-organ morbidities, characterize the phenotype underlying the HFpEF syndrome. Despite the rapidly increasing prevalence of HFpEF, few effective therapies have emerged. Synthetic agonists of the GHRH receptors reduce myocardial fibrosis, cardiomyocyte hypertrophy, and improve performance in animal models of ischaemic cardiomyopathy, independently of the growth hormone axis.
Methods and results
CD1 mice received 4- or 8-week continuous infusion of angiotensin-II (Ang-II) to generate a phenotype with several features consistent with HFpEF. Mice were administered either vehicle or a potent synthetic agonist of GHRH, MR-356 for 4-weeks beginning concurrently or 4-weeks following the initiation of Ang-II infusion. Ang-II-treated animals exhibited diastolic dysfunction, ventricular hypertrophy, interstitial fibrosis, and normal ejection fraction. Cardiomyocytes isolated from these animals exhibited incomplete relaxation, depressed contractile responses, altered myofibrillar protein phosphorylation, and disturbed calcium handling mechanisms (ex vivo). MR-356 both prevented and reversed the development of the pathological phenotype in vivo and ex vivo. Activation of the GHRH receptors increased cAMP and cGMP in cardiomyocytes isolated from control animals but only cAMP in cardiac fibroblasts, suggesting that GHRH-A exert differential effects on cardiomyocytes and fibroblasts.
Conclusion
These findings indicate that the GHRH receptor signalling pathway(s) represents a new molecular target to counteract dysfunctional cardiomyocyte relaxation by targeting myofilament phosphorylation and fibrosis. Accordingly, activation of GHRH receptors with potent, synthetic GHRH agonists may provide a novel therapeutic approach to management of the myocardial alterations associated with the HFpEF syndrome.
Keywords: Heart failure with preserved ejection fraction, Diastolic dysfunction, Excitation–contraction coupling, Growth hormone-releasing hormone analogues, Cardiomyocytes
Graphical Abstract
Graphical Abstract.
Proposed mechanism of action for GHRH-As to modulate the Excitation-Contraction Coupling (ECC). It is well known that activation of GHRHRs (GPCR) induces the adenylyl cyclase (AC)/cyclic AMP/protein kinase A (PKA) pathway. PKA can both phosphorylate sarcomeric and calcium handling proteins and activate soluble guanylyl cyclase (sGC). This pathway also promotes reparative mechanisms at transcriptional level. On the other hand, has been well demonstrated that GHRH-As also promote activation of AKT (PKB) among other kinases. Endothelial Nitric Oxide Synthase eNOS) is also activated by GHRH. The generated nitric oxide (NO) in turn activates sGC to produce the second messenger cGMP, an activator of the protein kinase G (PKG). This kinase is also able to phosphorylate the above-mentioned proteins. This pathway has been proposed to be activated downstream GHRHRs through activation of PI3K by the Gβγ proteins or β-arrestins (among other possible mechanisms).
1. Introduction
Heart failure (HF) remains a leading cause of death worldwide and is associated with two general clinical variants—HF with reduced ejection fraction (HFrEF), characterized by systolic dysfunction, and HF with preserved ejection fraction (HFpEF), predominantly due to diastolic dysfunction.1–3 HFpEF accounts for ∼50% of HF cases, a proportion that is predicted to increase relative to HFrEF.4 While numerous classes of drugs treat HFrEF, only a few are effective in HFpEF.5,6–9 The HFpEF syndrome is characterized by endothelial dysfunction, hypertension, impaired relaxation and contractile reserve, ventricular stiffness and fibrosis.2,10 The underlying molecular mechanisms are incompletely understood due to heterogenous aetiology, generally involving multi-organ pathological conditions.11–13 Importantly, it is only recently that clinical trials of SGLTL2i’s have produced positive outcomes for HfpEF,5 and there are many examples of failed efforts,6,8,14,15 thereby creating a healthcare crisis.16–18
The development of new potent synthetic growth hormone-releasing hormone agonists (GHRH-As) and their application in different models of myocardial injury (ischaemic19–23 or non-ischaemic24) demonstrates that activation of myocardial GHRH receptors (GHRHRs) effectively reduces fibrosis or hypertrophy, respectively, and improves cardiac performance.20–24 In a porcine chronic kidney disease (CKD)-induced HFpEF model, we recently demonstrated that GHRH-As improve the several aspects of cardiac diastolic hemodynamics.25 GHRHR signalling extends beyond the hypothalamic–pituitary axis.26 In this regard, GHRHRs are present on cardiomyocytes,20–22 and GHRH-A signalling in the myocardium acts in a manner largely independent of the growth hormone (GH)/insulin-like growth factor I (IGF-I) axis.21
Few animal models reproduce features of HFpEF and none recapitulate the entire spectrum.12,25,27–31 We used chronic infusion of low dose angiotensin-II (Ang-II) in mice, which exhibit a cardiac pathophysiologic phenotype that resembles HFpEF,32 to study the effects of a synthetic GHRH-A, MR-356, on myocardial performance in vivo and cardiomyocyte performance ex vivo. MR-356 is ∼150× more potent than human GHRH(1-29)NH2 (in terms of GH production) and binds the GHRHR with 7.5-fold greater affinity.19 Therefore, we hypothesized that GHRH-As improve cardiomyocyte performance and that activation of GHRHR signalling prevents and reverses myocardial features of HFpEF by targeting both impaired cardiomyocyte relaxation and remodelling, thereby improving myocardial function.
2. Methods
2.1. Experimental design and animal model
Male CD1 mice (Envigo) (8-week-old) were implanted with a mini-osmotic pump (Alzet) to deliver Ang-II (0.2 mg/kg/day; Sigma-Aldrich Co., Saint Louis, MO) for 4 weeks. One set of mice received daily injections of GHRH-Agonist [GHRH-A (MR-356): 200 μg/kg] or vehicle (DMSO + propylene-glycol) for 4 weeks. The second group of mice initially received only the mini-pump, which was replaced for an additional 4-week Ang-II infusion, at which point mice were randomized to receive 4 weeks of daily injection of MR-356 or vehicle. Unmanipulated age-matched CD1 mice acted as normal controls.
All the procedures were performed in mice anaesthetized with isoflurane (3–5% in an induction chamber and between 0.5 and 2% for maintenance, depending on the procedure and its duration). For echocardiography and mini-pump implantation, the anaesthesia was maintained using a facemask with a scavenging system. For haemodynamic and pressure–volume loop experiments, the anaesthesia was delivered via endotracheal intubation. At the end point, mice were euthanized with isoflurane inhalation (3–5%) followed by cervical dislocation, thoracotomy and exsanguination in hearts harvested for cardiomyocyte isolation or intracardiac injection of saturated KCl (1–2 mEq/Kg) if hearts were collected for histology and molecular assays.
2.2. Study approval
All protocols and experimental procedures were reviewed and approved by the University of Miami Institutional Animal Care and Use Committee and comply with all Federal and State guidelines concerning the use of animals in research and teaching as defined by The Guide for the Care and use of Laboratory Animals (NIH Publication No. 85-234, revised 2011). Animal Welfare Assurance # A-3224-01, approved through 30 November 2023. Protocol # 18-043 (Approval: 03/14/2018; Expiration: 03/13/2021). The University of Miami has received full accreditation with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International), site 001069.
2.3. Materials
MR-356 [(N-Me-Tyr,1 Gln,8 Orn,12 Abu,15 Orn,21 Nle,27 Asp,28 Agm29) hGHRH(1-29)] was synthetized by solid phase methods and purified by HPLC in our laboratories.19
2.4. Echocardiography
Transthoracic echocardiography was performed as previously described with minor modifications.33 All echocardiographic measurements were performed offline over three to five heart beats.
2.5. Speckle-Tracking Echocardiography
Strain analysis was conducted using speckle-tracking software, Vevostrain TM analysis (Visual Sonics). Global longitudinal strain was calculated using B-mode cine images in the LV parasternal long-axis view acquired at a high frame rate.34
2.6. Haemodynamic measurements
Hemodynamic studies were performed using a micro-tipped micromanometer-conductance (pressure–volume) catheter (SPR-839; ADInstruments) as described35 with minor modifications. All analyses were performed using LabChart Pro version 8.1.5 software (ADInstruments).
2.7. Cardiomyocyte isolation
Cardiomyocytes were isolated and prepared from hearts of mice by retrograde perfusion through the aorta in a modified Langendorff system with collagenase Type 2 and protease Type XIV as described previously.36
2.8. Intracellular calcium and sarcomere length measurement
Intracellular Ca2+ was measured using Fura-2 and a dual-excitation (340/380 nm) spectrofluorometer (IonOptix LLC) in cardiomyocytes electric field stimulated at 1, 2, and 4 Hz. Calcium concentration ([Ca2+]i) was calculated as described.36 Simultaneously, sarcomere length (SL) was recorded with an IonOptix iCCD camera.
Hysteresis loops were obtained by plotting SL vs. [Ca2+]i from cardiomyocytes paced at 4 Hz.
SRCa2+ leakage was assessed with 1 mmoL/L tetracaine (Sigma-Aldrich) as described by Shannon et al.37 with some modifications.38 SR Ca2+ content was assessed on the same cell by 20 mmoL/L caffeine pulse as described previously.38 Measurements were carried out at 37°C.
2.9. Western blot immunoanalysis
Protein expression of SERCA2a, PLB, cTnI, cMyBPC, collagen I and III, TGF-β, SMA-α, P4HA1, and protein phosphorylation analyses were assessed by western blot. Briefly, supernatants from isolated cardiomyocyte or heart tissue homogenates were collected and protein concentration determined. Samples were electrophoresed, transferred to PVDF or nitrocellulose membranes (Bio-Rad Laboratories) and detected by immunoblotting as indicated.
2.10. Interstitial fibrosis quantification
Paraffin-fixed heart sections were subjected to Masson’s trichrome staining for assessment of fibrillary collagen deposition. Images were acquired by an Olympus VS120 scanner and analyzed with ImageJ.
2.11. Immunofluorescence
Mice heart tissue sections 4- to 5 μm-thick were immunolabelled with a fluorescent antibody against phosphorylated Histone H3 (pHH3) (Abcam, Cambridge, MA; ab207233) and Dapi (Sigma-Aldrich; D9542). Positive nuclei for pHH3 were quantified and data expressed as fold change vs. the control group.
2.12. Quantitative RT-PCR
Total RNA was isolated from cardiomyocytes using RNAeasy mini kit (Qiagen, Hilden, Germany). RNA (1 μg) was reverse-transcribed (Applied Biosystems, Foster City, CA) using Taqman® Universal Master Mix (Applied Biosystems, Foster City, CA) in a Bio-Rad CFX96™ Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA). Change in mRNA expression was normalized to the change in 18S, and values were expressed as 2ΔCt.
2.13. Cyclic AMP and cyclic GMP immunoassay
Competitive ELISA kits (Thermo Fisher Scientific; Catalog #EMSCAMPL and #EMSCGMPL) for quantification of cyclic AMP (cAMP) and cyclic GMP (cGMP), respectively, in left ventricle, isolated ventricular cardiomyocytes or cardiac fibroblasts were used as directed by the manufacturer.
2.14. Mouse cardiac fibroblast culture
Cardiac fibroblasts from neonatal mice were obtained from iXCell Biotechnologies (catalogue # 10MU-015) and grown as recommended by the manufacturer. For cAMP determination, fibroblasts were treated with 50 nM MR-356 or 300 nM mouse recombinant GHRH (mrGHRH) in culture medium. For mRNA isolation, fibroblasts were pre-treated for 24 h with 1 nM Ang-II. Then, culture media were replaced by fresh media treated with either 1 nM Ang-II or 1 nM Ang-II + 50 nM MR-356 for an additional 48 h. Cells were then washed with PBS and mRNA extracted.
Detailed methodology and procedures are provided in the Supplementary material.
2.15. Statistical analysis
Data are reported as mean ± standard error of the mean (SEM). Statistical significance between three groups was determined by one-way or two-way ANOVA (unless otherwise indicated) followed by Tukey’s or Bonferonni’s post hoc tests, as recommended by the software. For comparisons of two groups, Student’s two-tailed t-test was used. Analyses were performed using Graph Pad Prism, version 9.0.0. The null hypothesis was rejected at P < 0.05.
3. Results
3.1. GHRH-A prevents the appearance of myocardial remodelling and diastolic dysfunction induced by Ang-II infusion in vivo.
Four-weeks of continuous Ang-II infusion induced a cardiovascular phenotype that mimicked HFpEF phenotypically,32 specifically, myocardial hypertrophy with increased heart weight normalized by body weight or tibia length (Figure 1A and Supplementary material online, Table S1), increased relative wall thickness in diastole (RWTd, Figure 1B and Supplementary material online, Table S2) and increased cardiomyocyte width and cross-sectional area (Figures 1C and D, respectively) without changes in length (see Supplementary material online, Figure S1). Myocardial fibrosis increased in Ang-II-treated mice (Figures 1E and F), a result that was supported by the increase in content of collagen III, the transforming growth factor-β (TGF-β, a cytokine which mediates inflammation and fibrosis) and the protein expression of alpha-smooth muscle actin (α-SMA, as a marker of the transition to myofibroblast phenotype) (Figures 1G–I). Hydroxyproline residues was slightly increased while prolyl-4-hydroxylase-α-1 (P4HA1) and collagen I did not change (see Supplementary material online, Figures S2A–C). Co-administration of the GHRH-A, MR-356, prevented the increases in RWTd and cardiomyocyte size (Figures 1B–D and Supplementary material online, Table S2). Moreover, MR-356 prevented fibrosis (Figures 1E–G) and the increase of TGF-β and α-SMA expression seen with Ang-II infusion (Figures 1H and I). In addition, in vitro treatment of murine cardiac fibroblasts with 1 nM Ang-II induced an increase of TNF-α gene transcription, which was attenuated by 50 nM MR-356 (Figure 1J), demonstrating that direct activation of GHRHRs on fibroblasts might promote at least part of the anti-fibrotic effect of GHRH-As. Our experiments in vitro confirmed that activation of GHRHRs by both MR-356 and mrGHRH, directly activate the cyclic AMP/protein kinase A (PKA) signalling pathway in cardiac fibroblasts (see Supplementary material online, Figure S2D).
Figure 1.
GHRH-A MR-356 prevents cardiomyocyte growth and interstitial fibrosis. (A) Heart weight normalized to body weight (N = 15 control, N = 9 Ang-II and N = 9 Ang-II + MR-356 mice). (B) Relative wall thickness in diastole (RWTd) assessed by echocardiography (N = 9 control, N = 22 Ang-II and N = 19 Ang-II + MR-356 mice). (C) Width of isolated cardiomyocytes from control (n = 251 cells, N = 2 mice), Ang-II-treated mice (n = 259 cells, N = 4 mice) and Ang-II + MR-356-treated (n = 380 cells, N = 4 mice). (D) Average and individual values of cross-sectional area (CSA) of cardiomyocytes. (E) Representative images of Masson’s trichrome-stained myocardium sections. (F) Average percentage of interstitial fibrosis (*P < 0.05 vs. control and †P < 0.05 vs. Ang-II, subjected to non-parametric Kruskal–Wallis test). Panels D and F summarize the average count of three different fields analyzed per mouse (individual dots represent each studied mouse [four per group]). (G) Collagen III protein expression in heart homogenates. Top, representative images of Western blots. Bottom, averaged and individual results (N = 6 control, N = 6 Ang-II and N = 4 Ang-II + MR-356 mice). (H) TGF-β protein expression (N = 3 mice per group) and (I) α-smooth muscle actin (α-SMA) (N = 3 mice per group) protein expression in heart homogenates. (J) TNF- α gene expression in cultured cardiac fibroblast untreated (N = 4) or treated with 1 nM Ang-II for 72 h (N = 3, one outlier extremely high expression was removed) or 1 nM Ang-II for 72 h + 50 nM MR-356 for 48 h (N = 4). Ang-II induced the pathological signal which was partially reduced by MR-356. *P < 0.05, **P < 0.01 and ***P < 0.001 in Ang-II vs. control; †P < 0.05, ††P < 0.01 and †††P < 0.001 in Ang-II + MR-356-treated vs. Ang-II; One-way ANOVA. ‡P < 0.05 vs. baseline, Two-way ANOVA.
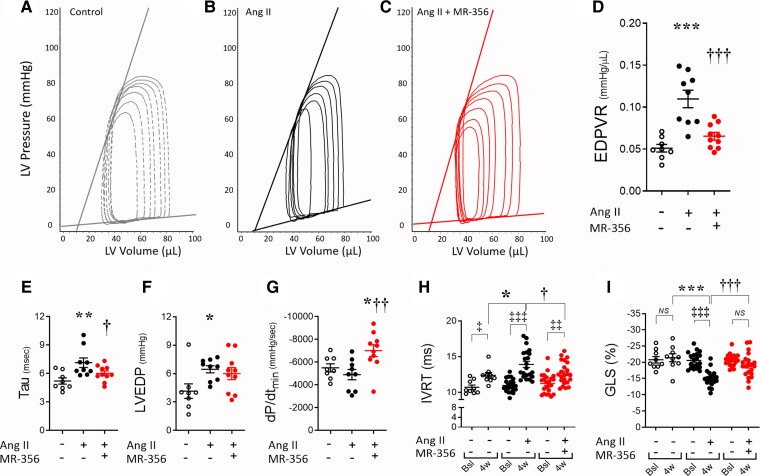
Echocardiographic evaluation demonstrated that Ang-II infusion did not reduce ejection fraction (EF) (see Supplementary material online, Table S2), either alone or during co-treatment with MR-356. Likewise, chamber dimensions were unaffected in systole between groups (see Supplementary material online, Table S2). Invasive hemodynamic assessment (Table 1) demonstrated increased arterial blood pressure and clear evidence of diastolic dysfunction with increased end-diastolic pressure–volume relationship (EDPVR) (Figures 2A–D), relaxation time constant (tau) and left ventricular end-diastolic pressure (LVEDP) in Ang-II-treated mice compared with control (Figures 2E and F). Isovolumic relaxation time (IVRT) increased at 4-weeks (Figure 2H) (within group comparison—4-weeks vs. baseline), and this increase was greater in the Ang-II-treated mice. Global longitudinal strain (GLS) increased (reduced absolute values) with Ang-II treatment, to an extent according to that observed in diastolic dysfunction39 (Figure 2I and Supplementary material online, Table S2). Co-infusion of MR-356 completely prevented the development of diastolic dysfunction (Figures 2C–I) and promoted a robust acceleration in dP/dtmin (Figure 2G). Of note, MR-356 did not have a significant effect on echocardiographic or haemodynamic parameters in control CD1 mice (see Supplementary material online, Figures S3A–H). Together, these results demonstrate that stimulation of GHRH pathway with MR-356 effectively counteracted the development of the Ang-II-induced phenotype.
Table 1.
Haemodynamic assessments on the 4-week Ang-II infusion + MR-356 injection model
| Control (N = 8) | Ang-II (N = 9) | Ang-II + MR-356 (N = 10) | |
|---|---|---|---|
| HR (bpm) | 535.9 ± 11.7 | 543.9 ± 7.2 | 523.5 ± 14.2 |
| Integrated Performance | |||
| EF (%) | 51.0 ± 4.0 | 55.7 ± 5.8 | 58.7 ± 2.7 |
| SW (mmHg.μL) | 2638 ± 182 | 2250 ± 149 | 2576 ± 224 |
| SV (μL) | 37.5 ± 2.0 | 37.7 ± 2.4 | 38.8 ± 2.9 |
| CO (mL/min) | 19.9 ± 1.0 | 20.8 ± 1.5 | 20.2 ± 1.5 |
| Ea/Ees | 0.72 ± 0.05 | 1.14 ± 0.15* | 1.11 ± 0.14 |
| Afterload | |||
| Systolic ABP (mmHg) | 79.7 ± 2.2 | 103.0 ± 4.7* | 88.5 ± 4.6† |
| Diastolic ABP (mmHg) | 54.7 ± 2.7 | 75.5 ± 3.7* | 64.4 ± 4.2 |
| Ea (mmHg/μL) | 1.88 ± 0.25 | 1.86 ± 0.17 | 2.02 ± 0.19 |
| LVESP (mmHg) | 66.4 ± 2.0 | 63.4 ± 2.4 | 72.3 ± 3.4 |
| Preload | |||
| LVEDP (mmHg) | 4.13 ± 0.89 | 6.46 ± 0.38* | 6.01 ± 0.64 |
| LVEDV (μL) | 78.5 ± 7.0 | 66.8 ± 8.2 | 64.7 ± 3.3 |
| Contractility | |||
| ESPVR (mmHg/μL) | 2.71 ± 0.28 | 1.85 ± 0.32* | 1.961 ± 0.19 |
| dP/dtmax (mmHg/sec) | 7328 ± 346 | 6866 ± 519 | 7433 ± 476 |
| dP/dtmax-EDV (mmHg/μL.sec) | 110.7 ± 13.9 | 99.0 ± 6.3 | 91.1 ± 9.6 |
| PRSW (mmHg/μL) | 74.5 ± 3.1 | 63.2 ± 3.9 | 62.4 ± 5.4 |
| Lusitropy | |||
| dP/dtmin (mmHg/sec) | −5494 ± 353 | −4897 ± 451 | −7004 ± 519*† |
| Tau (msec) | 5.21 ± 0.33 | 7.13 ± 0.50* | 6.00 ± 0.26† |
| EDPVR (mmHg/μL) [linear] | 0.051 ± 0.004 | 0.110 ± 0.010* | 0.065 ± 0.004† |
All values represent mean ± SEM. *P < 0.05 vs. control; †P < 0.05 Ang-II + MR-356 vs. Ang-II, one-way ANOVA. N represents number of studied animals.
Figure 2.
Effects of the GHRH-A, MR-356, on diastolic dysfunction in Ang-II-infused mice. (A) Representative pressure–volume (P–V) loops showing the linear regression for ESPVR and EDPVR in a control, (B) Ang-II-treated, or (C) Ang-II + MR-356 mouse. (D) Averaged and individual values of the slopes from the EDPVR linear fitting. (E) Hemodynamic assessment of the relaxation time constant, tau, at 4-week time point, (F) left ventricle end-diastolic pressure (LVEDP) and (G) Maximum rate of pressure decline (dP/dtmin). Animals used for panels D-G: N = 8 control, N = 9 Ang-II and N = 9 Ang-II + MR-356 mice. (H) Isovolumic relaxation time (IVRT) as measured by transthoracic echocardiography at baseline or after 4-week treatment (N = 9 control, N = 22 Ang-II and N = 19 Ang-II + MR-356 mice). (I) Global longitudinal strain (GLS) analysis of the left ventricle (N = 9 control, N = 20 Ang-II and N = 21 Ang-II + MR-356 mice). GLS represents the function of sub-endocardial longitudinal myocardial fibres that are more sensitive to reduced coronary perfusion and increased wall stress. Moreover, GLS reflects changes in the myocardial interstitium including the extent of myocardial fibrosis. ‡P < 0.05, ‡‡P < 0.01 and ‡‡‡P < 0.001 at 4-week vs. baseline, Two-way ANOVA. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control; †P < 0.05, ††P < 0.01 and †††P < 0.001 in Ang-II + MR-356-treated vs. Ang-II; one-way ANOVA.
3.2. GHRH-A prevents sarcomere and contractile dysfunction in cardiomyocytes from Ang-II-treated mice.
To test whether GHRH agonists improve cardiomyocyte function, isolated cardiomyocytes were field stimulated at 1, 2, and 4 Hz. Cardiomyocytes from Ang-II-infused mice exhibited shorter resting (diastolic) SL compared to those from control animals (Figures 3A and B), indicating an inability to relax. In parallel, quiescent cardiomyocytes from the Ang-II-infused mice exposed to 1.8 mmoL/L extracellular CaCl2 were consistently shorter than control cardiomyocytes, while there was no difference between groups in Ca2+-free buffer in the presence of 2,3-butanedione monoxime (see Supplementary material online, Figure S4A). These cardiomyocytes from Ang-II-treated mice also exhibited depressed contractile response to pacing (Figures 3A, C). However, MR-356 administration during Ang-II infusion, both prevented impairment of resting sarcomere tension (Figures 3A and B), and preserved quiescent cardiomyocyte length in the presence of extracellular Ca2+ (see Supplementary material online, Figure S4A, right panel). Treatment with MR-356 also preserved sarcomere shortening and contractile response to pacing similar to control cardiomyocytes (Figures 3A, C). There were no significant differences in Ca2+ transient amplitude (Δ[Ca2+]) between groups (Figures 3D and E), which suggests an altered responsiveness to Ca2+. However, when absolute values of Δ[Ca2+] were normalized by the respective Δ[Ca2+] at 1 Hz (of each group), an impaired response to pacing was observed in Ang-II-treated cardiomyocytes, while the MR-356-treated cardiomyocytes conserved the positive response to pacing similar to controls (Figure 3F). This finding correlates with their contractile response (Figure 3C).
Figure 3.
Sarcomere and contractile dysfunction, and calcium handling alterations in the Ang-II-induced model are prevented by co-treatment with GHRH-A MR-356. (A) Representative traces of sarcomere twitches of cardiomyocytes, electric field stimulated at 1- and 4 Hz, from male CD1 control mice (N = 4, n = 30 cells) and mice that underwent either Ang-II (N = 5, n = 41 cells) or Ang-II + MR-356 treatment (N = 5, n = 38 cells). (B) Resting SL in cardiomyocytes from each group at different pacing rates. (C) Sarcomere shortening in response to pacing. (D) Representative traces of intracellular [Ca2+] in electric field-stimulated cardiomyocytes. (E) Δ[Ca2+] transient amplitude in response to pacing. (F) Δ[Ca2+] normalized (with respect to 1 Hz) in response to pacing. (G) Representative traces of intracellular [Ca2+] in cardiomyocytes illustrating the method for measuring SRCa2+ leak using tetracaine. Upper (Blue) trace corresponds to Ca2+ from a cardiomyocyte not exposed to tetracaine; lower (red) trace is the Ca2+ from the same cardiomyocyte treated with tetracaine for 70 s after pacing was stopped. (H) SRCa2+ leak-load relationship in cardiomyocytes from control (N = 4 mice, n = 31 cells), Ang-II-treated (N = 5 mice, n = 38 cells) or Ang-II + MR-356-treated (N = 5 mice, n = 35 cells) CD1 mice. (I) Average and individual SRCa2+ leak in cardiomyocytes from all groups at matched SRCa2+ load = 99.1 μmoL/L. From each mouse, a total of 5-9 cells were studied. *P < 0.05, ***P < 0.001 in Ang-II vs. control, and †P < 0.05, ††P < 0.01 in Ang-II + MR-356-treated vs. Ang-II; one- or two-way ANOVA as appropriate.
Increased SR Ca2+ leak is a hallmark of HF and contribute to depressed contractile reserve. We assessed sarcoplasmic reticulum (SR) Ca2+ leak with tetracaine (Figure 3G, and see Supplementary material online, Figure S3G). We found that Ang-II-treated myocytes exhibited a steeper rise in SRCa2 + leak with increasing SRCa2+ loads compared to controls (P < 0.05) and this load-leak curve was flattened by treatment with MR-356 (P < 0.0001; Figure 3H). Thus, at matched SRCa2+ load = 99.1 ± 11.5 μmol/L, the Ca2+ leak was elevated in cardiomyocytes from Ang-II-infused mice and partially prevented by treatment with MR-356 (Figure 3I). Treatment of control mice with MR-356 did not affect the cardiomyocyte performance (see Supplementary material online, Figures S3I–L).
3.3. Effect of GHRH-A on the Ca2+ handling in cardiomyocytes from Ang-II-treated mice.
We found slower [Ca2+] decay in cardiomyocytes from Ang-II-treated mice compared with controls while treatment with MR-356 suppressed the delay in Ca2+ decline (Figures 4A and B). The Ca2+ content in the SR of these dysfunctional cardiomyocytes, estimated by a rapid caffeine pulse, was not different from control, but Ang-II + MR-356 co-treatment did increase the SR [Ca2+] in comparison to control (Figure 4C). We investigated the proteins participating in Ca2+ handling. SR Ca2+ ATPase (SERCA2a) protein expression was reduced in cardiomyocytes from Ang-II-treated mice injected with either placebo or MR-356 (Figure 4D). Phospholamban (PLB) phosphorylation at serine 16 (p-Ser16) or threonine 17 (p-Thr17) was increased by Ang-II treatment, an effect further enhanced by co-treatment with MR-356 (Figures 4E and F).
Figure 4.
Ca2+ decay is delayed in cardiomyocytes from the Ang-II-infused model but preserved by treatment with MR-356. (A) Normalized Ca2+ transient re-constructed by using averaged Ca2+ traces from control untreated, Ang-II-treated or Ang-II + MR-356-treated cardiomyocytes paced at 1 Hz. (B) Times to different degrees of the Ca2+ peak decline: time to 10% (tt10%), time to 50% (tt50%), decay time constant (τ) and time to 90% (tt90%) from n = 20–30 control, n = 35–41 Ang-II and n = 30–38 Ang-II + MR-356 cardiomyocytes (from N = 4, N = 5 and N = 5 mice, respectively).  P < 0.05 vs. control, Student’s t-test. (C) SR Ca2+ content in cardiomyocytes paced at 1 Hz (n = 31 control, n = 40 Ang-II and n = 37 Ang-II + MR-356 cardiomyocytes). (D) SERCA2a protein expression; top, representative immunoblotting image with Hsp90 as loading control and bottom, normalized individual and average values. (E) PLB phosphorylation at serine 16 and total PLB. Number of animals used for panels D and E: N = 9 control, N = 9 Ang-II and N = 9 Ang-II + MR-356 mice. *P < 0.05 or **P < 0.01 vs. control, †P < 0.05 vs. Ang-II-treated; one-way ANOVA. (F) Phosphorylation of PLB at threonine 17 and total PLB (data subjected to Kruskal–Wallis non-parametric test). Top, representative images and bottom, individual and average values normalized by total PLB expression (N = 5 control, N = 6 Ang-II and N = 6 Ang-II + MR-356 mice).
P < 0.05 vs. control, Student’s t-test. (C) SR Ca2+ content in cardiomyocytes paced at 1 Hz (n = 31 control, n = 40 Ang-II and n = 37 Ang-II + MR-356 cardiomyocytes). (D) SERCA2a protein expression; top, representative immunoblotting image with Hsp90 as loading control and bottom, normalized individual and average values. (E) PLB phosphorylation at serine 16 and total PLB. Number of animals used for panels D and E: N = 9 control, N = 9 Ang-II and N = 9 Ang-II + MR-356 mice. *P < 0.05 or **P < 0.01 vs. control, †P < 0.05 vs. Ang-II-treated; one-way ANOVA. (F) Phosphorylation of PLB at threonine 17 and total PLB (data subjected to Kruskal–Wallis non-parametric test). Top, representative images and bottom, individual and average values normalized by total PLB expression (N = 5 control, N = 6 Ang-II and N = 6 Ang-II + MR-356 mice).
3.4. Impaired cardiomyocyte relaxation in the Ang-II-infused model is improved by GHRH-A
Myocardial stiffness and deficient relaxation are hallmarks of the HFpEF syndrome and may be due to factors external to the cardiomyocyte or intrinsically associated with sarcomeric proteins. At the cardiomyocyte level, this mouse model exhibited reduced ability to relax as showed by the impaired maximum relaxation rate (dSL/dtmin, Figure 5B), mainly during the late part of the relaxation phase (tt90%, Figures 5A, C). Treatment with MR-356 significantly improved all phases of sarcomere relaxation (Figures 5A–C). Comparing this effect of MR-356 with the modest effect on Ca2+ decline rate, suggests a direct targeting on myofilaments. Accordingly, we analyzed SL-[Ca2+] hysteresis loops of cardiomyocyte contractions induced by electric-field stimulation, to detect differences in responsiveness to Ca2+. Overall, loop morphology was greatly affected in the Ang-II-infused model (Figure 5D, insert panel). In the relaxation phases (Figure 5D), there was Ang-II-induced sensitization of myofilaments (relaxEC50Ang-II = 220.2 ± 1.5 nmol/L vs. relaxEC50Control = 240.0 ± 0.9 nmol/L, P < 0.0001, Figure 5D) consistent with the delayed Ca2+ decline in this model. Co-treatment with MR-356 induced a robust desensitizing effect that correlated with the exacerbated lusitropic effect of MR-356 on isolated cardiomyocytes (relaxEC50Ang-II+MR = 299.7 ± 1.6 nmol/L, Figure 5D). We explored phosphorylation on sites of cardiac troponin I (cTnI) with regulatory activity, serine 23/24. TnI phosphorylation was impaired in Ang-II-treated cardiomyocytes while co-treatment with MR-356 promoted increased phosphorylation (Figure 5E). Similarly, failing cardiomyocytes exhibited reduced phosphorylation at serine 282 of the myosin binding protein C (MyBPC), which was prevented in Ang-II + MR-356 cardiomyocytes (Figure 5F). These findings consistently correlate with the data of responsiveness to Ca2+ (Figure 5D).
Figure 5.
Impaired sarcomere relaxation due to myofilament sensitization in cardiomyocytes from Ang-II-infused mice is prevented by treatment with MR-356. (A) Normalized sarcomere twitch re-constructed by using the averaged SL traces at 1 Hz from control untreated, Ang-II-treated or Ang-II + MR-356-treated cardiomyocytes. (B) Times to different degrees of sarcomere relaxation at 1 Hz: tt10, tt50%, tau (τ) and tt90% from n = 20–30 control, n = 35–41 Ang-II and n = 30–38 Ang-II + MR-356 cardiomyocytes (from N = 4, N = 5 and N = 5 mice, respectively). (C) Maximum sarcomere relaxation rate (dSL/dtmin) at all stimulation frequencies. (D) SL-[Ca2+] hysteresis loops in control, Ang-II- or Ang-II + MR-356-treated cardiomyocytes with overlapped sigmoidal fitting of the relaxation phase (insert panel), and normalized relaxation phase of SL-[Ca2+] loops indicating (arrow heads) the effective [Ca2+] of the relaxation phase (relaxEC50s, right). (E) Phosphorylation of cTnI at serine 23/24 and total cTnI protein expression by western blot. (F) Phosphorylation of cMyBPC at serine 282 and total cMyBPC protein. Top, representative images; bottom, individual and average normalized values. Used animals for panels E and F: N = 8 control, N = 9 Ang-II and N = 9 Ang-II + MR-356 mice. *P < 0.05, **P < 0.01 vs. control, †P < 0.05, ††P < 0.01, †††P < 0.001 vs. Ang-II; One-way or two-way ANOVA.  P < 0.05 vs. control, Student’s t-test.
P < 0.05 vs. control, Student’s t-test.
3.5. GHRH-A improves relaxation and partially reverts the HFpEF-like phenotype induced by 8-week Ang-II infusion in CD1 mice
Given the ability of GHRH-As to prevent the onset of HFpEF-like features induced by Ang-II, we next assessed the ability of GHRH-As to reverse the established phenotype. We infused mice with a low dose of Ang-II for 8 weeks and initiated daily treatment with either MR-356 or vehicle beginning at Week 4, once the HFpEF-like phenotype was already apparent. As with the 4-week protocol, myocardial hypertrophy (increased LV mass) induced by Ang-II was not significantly reduced by MR-356 treatment (Figure 6A). Similarly, these mice exhibited pulmonary oedema as indicated by an increased fluid content in the lungs, which was not completely reversed by MR-356 (Figure 6B). However, the molecular remodelling (as indicated by the altered ratio between the adult (α) to fetal (β) isoform of the myosin heavy chain gene expression, MHY6/MHY7) and the cross-sectional area of cardiomyocytes, were normalized by MR-356 treatment (Figures 6C and D). The increased interstitial myocardial fibrosis in Ang-II-infused mice was consistently reverted by MR-356 (Figures 6E and F). MR-356 also promoted proliferation in the treated group as depicted by the trend toward increased number of cells stained for phosphorylated Histone H3 (pHH3, Figures 6G and H), indicative of enhanced mitotic activity.
Figure 6.
Myocardial remodelling is attenuated by treatment with GHRH-A. (A) LV mass assessed by echocardiography in control, Ang-II-treated and Ang-II-treated + MR-356 mice assessed at baseline, and 4- and 8-weeks (N = 12 animals per group). (B) Lung weight wet/dry ratio (N = 12 animals per group). (C) Ratio between the gene expression of αMHC (adult isoform) and βMHC (foetal isoform), as an indicator of the pathologic reprogramming (N = 2 control, N = 4 Ang-II and N = 2 Ang-II + MR-356 mice). (D) Cardiomyocyte cross-sectional area (CSA). (E) Representative Masson’s trichrome-stained heart sections showing interstitial collagen deposition in control, Ang-II and Ang-II + MR-356 mice. (F) Percentage of fibrotic area. (G) Representative images of immunostaining for phosphorylated Histone H3 (pHH3). (H) pHH3 positive nuclei count. Panels D, F, and H summarize the average count of three different fields analyzed per mouse [individual dots represent each studied mouse (4 per group)]. ‡P < 0.05, ‡‡P < 0.01 vs. baseline, two-way ANOVA *P < 0.05, ***P < 0.001 vs. control, †P < 0.05, †††P < 0.001 vs. Ang-II; One-way ANOVA.
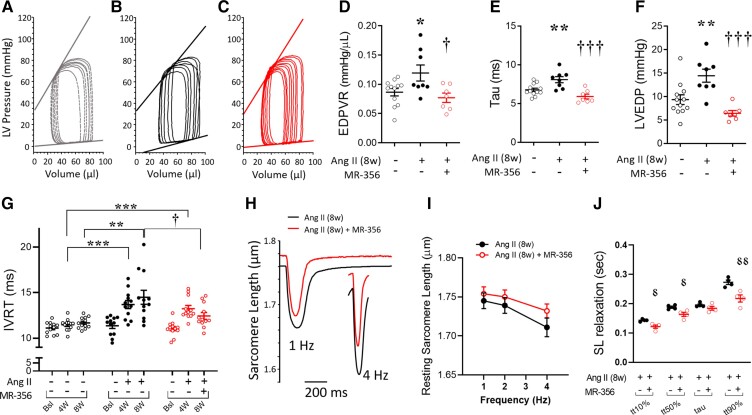
The in vivo haemodynamic assessments yielded similar results as obtained with the 4-week protocol, although in this case no changes in arterial blood pressure or the ventricular-vascular coupling (Ea/Ees) were found (Table 2). We also measured capillary density by isolectin B4 staining and found no differences between groups (see Supplementary material online, Figure S5). These hemodynamic data revealed that MR-356 restored EDPVR, relaxation time constant (τ) and LVEDP (Figures 7A–F and Table 2) to normal in Ang-II-infused animals. The prolongation in IVRT during the first 4-week treatment with Ang-II in both groups, continued to increase in the Ang-II-treated group but MR-356 treatment reduced these values (Figure 7G). The beneficial effects on diastolic function were also seen in isolated cardiomyocytes, since MR-356 restored the resting SL (in diastole, Figures 7H and I). Moreover, sarcomere relaxation rate was accelerated by MR-356 treatment in comparison with the 8-week Ang-II group (Figure 7J). Treatment with MR-356 also improved the delayed Ca2+ decay rate (see Supplementary material online, Figure S6A), consistent with the enhanced transcription (mRNA) of the SERCA2a gene (see Supplementary material online, Figure S6B).
Table 2.
Hemodynamic assessments on the 8-week Ang-II infusion + 4-week MR-356 injection model
| Control (N = 12) | Ang-II (N = 8) | Ang-II + MR-356 (N = 7) | |
|---|---|---|---|
| HR (bpm) | 539.7 ± 8.9 | 542.0 ± 9.8 | 538.8 ± 8.2 |
| Integrated Performance | |||
| EF (%) | 54.2 ± 2.8 | 52.7 ± 4.9 | 56.1 ± 3.4 |
| SW (mmHg.μL) | 3812 ± 327 | 4187 ± 636 | 4037 ± 158 |
| SV (μL) | 47.0 ± 4.2 | 51.4 ± 3.5 | 54.1 ± 2.7 |
| CO (mL/min) | 25.2 ± 2.1 | 27.8 ± 1.8 | 29.1 ± 1.5 |
| Ea/Ees | 1.44 ± 0.16 | 1.78 ± 0.20 | 1.19 ± 0.19 |
| Afterload | |||
| Systolic ABP (mmHg) | 112.8 ± 3.4 | 111.9 ± 7.9 | 110.7 ± 3.3 |
| Diastolic ABP (mmHg) | 82.2 ± 2.6 | 81.9 ± 6.1 | 81.7 ± 2.4 |
| Ea (mmHg/μL) | 1.60 ± 0.10 | 1.71 ± 0.16 | 1.43 ± 0.16 |
| LVESP (mmHg) | 80.3 ± 2.6 | 81.4 ± 4.4 | 75.2 ± 4.7 |
| Preload | |||
| LVEDP (mmHg) | 9.3 ± 1.0 | 14.4 ± 1.4* | 6.4 ± 0.6† |
| LVEDV (μL) | 83.6 ± 5.1 | 99.2 ± 6.3 | 95.7 ± 3.5 |
| Contractility | |||
| ESPVR (mmHg/μL) | 1.33 ± 0.16 | 1.19 ± 0.26 | 1.43 ± 0.31 |
| dP/dtmax (mmHg/sec) | 7456 ± 367 | 7045 ± 445 | 6939 ± 383 |
| PRSW (mmHg/μL) | 63.7 ± 3.4 | 56.9 ± 7.5 | 64.5 ± 3.17 |
| Lusitropy | |||
| dP/dtmin (mmHg/sec) | –7628 ± 407 | –6602 ± 318 | –7288 ± 765 |
| Tau (msec) | 6.77 ± 0.22 | 8.08 ± 0.39* | 5.91 ± 0.31† |
| EDPVR (mmHg/μL) [linear] | 0.087 ± 0.006 | 0.119 ± 0.014* | 0.077 ± 0.008† |
All values represent mean ± SEM. *P < 0.05 vs. control; †P < 0.05 Ang-II + MR-356 vs. Ang-II, one-way ANOVA. N represents number of animals.
Figure 7.
Effect of MR-356 on the 8-week Ang-II-iduced HFpEF-like phenotype. (A) Representative pressure–volume (P–V) loops showing the linear regression for ESPVR and EDPVR in control, (B) 8-week Ang-II-treated, or (C) Ang-II (8-weeks)+MR-356 (4-weeks)-treated mice. (D) Slopes from the EDPVR linear fitting. (E) Hemodynamic assessment of the relaxation time constant (tau) at the 8-week time point. (F) Left ventricular end-diastolic pressure (LVEDP). Animals used for panels D-F: N = 12 control, N = 8 Ang-II and N = 7 Ang-II + MR-356 mice. *P < 0.05, **P < 0.01 vs. control and †P < 0.05, †††P < 0.001 vs. Ang-II (8-week); One-way ANOVA. (G) IVRT as measured by transthoracic echocardiography at baseline, and 4- and 8-weeks (N = 12 animals per group). (H) Representative SL traces comparing Ang-II (8-weeks)-treated and Ang-II (8-week)+MR-356 (4-weeks) cardiomyocytes at 1- and 4-Hz pacing rates. (I) Resting sarcomere length of Ang-II (8-weeks)-treated compared with Ang-II (8-week)+MR-356-treated (4-week) murine cardiomyocytes paced at 1-, 2-, and 4 Hz. Data correspond to averaged values of n = 49 Ang-II and n = 41 Ang-II + MR-356 cardiomyocytes from N = 4 mice per group. (J) Times to different degrees of SL relaxation: tt10, tt50%, tau (τ) and tt90% in 8-week-treated cardiomyocytes paced at 1 Hz (n = 49 Ang-II cells and n = 41 Ang-II + MR-356 cells, from N = 4 mice per group). *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control; Two-way ANOVA.  P < 0.05,
P < 0.05,  P < 0.01 vs. Ang-II (8-weeks); Student’s t-test.
P < 0.01 vs. Ang-II (8-weeks); Student’s t-test.
3.6. Activation of GHRHRs on cardiomyocytes induces different mechanisms shared with GH and IGF-I as well as independent.
To corroborate the direct effect of GHRH-A, we treated freshly isolated cardiomyocytes with mrGHRH (300 nM) for 10 min to activate the GHRHRs and assessed the activation of adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) and soluble guanylyl cyclase (GC)/cGMP/protein kinase G (PKG) pathways by measuring cAMP and cGMP, respectively. Mouse recombinant growth hormone (mrGH, 30 nM) and mouse recombinant insulin-like growth factor I (mrIGF-I, 30 nM) were used as control to determine whether the effects on cardiomyocytes were exclusively mediated by GHRH-A or if the GH/IGF-I axis was also involved. We observed increased cAMP with mrGHRH but no change with mrGH or mrIGF-I (see Supplementary material online, Figure S7A). cGMP was also significantly increased by mrGHRH, while mrGH and mrIGF-I did not induce significant changes (see Supplementary material online, Figure S7B). These findings demonstrate that GHRH directly activates both cAMP/PKA and cGMP/PKG signalling pathways in murine cardiomyocytes ex vivo, suggesting that these mechanisms might underlie the robust lusitropic effect of MR-356 observed in vivo.
4. Discussion
The incidence of the HFpEF syndrome is rising and it is unresponsive to most conventional HF treatments.6–8 Given this increased clinical burden, it is imperative that effective treatments are found. Here we show that a novel class of peptide compounds, GHRH-As,19 previously shown to ameliorate pathological myocardial remodelling,19–24 also effectively restores diastolic dysfunction, a hallmark of HFpEF, to normal in this mouse model. GHRH-As act broadly through mechanisms involving fibrosis reduction22 as well as myocyte calcium cycling and the response of myofilaments to calcium.
4.1. In vivo effect of GHRH-A treatment
Chronic administration of Ang-II to mice reproduces structural and functional myocardial changes compatible with HFpEF. In contrast to Regan et al.,32 after 4-weeks of Ang-II infusion, our mice demonstrated moderated increase in blood pressure (Table 1), while at 8-weeks it was dissipated (Table 2). A majority of patients with HFpEF exhibit hypertension, which might represent a contributory risk factor.40 These animals showed preserved EF and other contractility indices (although specifically ESPVR is reduced) but depressed overall function, as observed with GLS,39 associated with elevated LVEDP, myocardial fibrosis and impaired diastolic function, as measured by IVRT, EDPVR and the relaxation constant tau. Within this pathological context, our Ang-II-infused animals exhibited myocardial hypertrophy that was redirected into a physiological hypertrophy by MR-356. Overall, this treatment improved myocardial performance associated with reduced fibrosis, preserved cardiomyocyte size and diastolic RWT. MR-356 treatment was able to partially prevent the increase in blood pressure induced by Ang-II in the 4-weeks experimental model, where the treatment was initiated simultaneously with the Ang-II infusion (Table 1). MR-356 did not exert a significant effect on arterial blood pressure in control CD1 mice (see Supplementary material online, Figures S3E and F). Thus, although an activation of the sGC/cGMP pathway was detected in isolated cardiomyocytes, further specific studies aimed to determine whether this GHRH-A has a blood pressure lowering effect per se on the peripheral vasculature are needed. Interestingly, animals infused with Ang-II for 8 weeks did not exhibit hypertension and MR-356 did not reduce arterial blood pressure or affect the myocardial capillary density (in Supplementary material online, Figure S5). Based on the unchanged ventricular-vascular coupling (Ea/Ees), it is likely that most of the effects of MR-356 are exerted at the myocardial level.
MR-356 failed to prevent the increase in ventricular weight (in the 4-week mice) or revert the augmented LV mass (in the 8-week mice). Accordingly, a concomitant reduction of interstitial myocardial fibrosis and enhanced proliferation induced by MR-356 treatment would explain the persistent increase in heart size after MR-356 treatment, an effect that might be directly mediated by activation of GHRHRs on the myocardium and/or by the action of GH or IGF-I released after MR-356 injections (see below). Our results show that the above-mentioned phenotype, consistent with the HFpEF syndrome, was both prevented and reversed by the treatment with the GHRH-A, MR-356.
4.2. GHRH-A targets myofilaments and calcium handling proteins
Isolated cardiomyocytes from Ang-II-infused mice exhibited lusitropic impairment as well as alterations in Ca2+ handling, consistent with deficient myocardial relaxation and diastolic dysfunction. Our data strongly suggest that deficient myofilament relaxation in these cardiomyocytes is associated with higher affinity of myofilaments for Ca2+, favouring binding of cytosolic Ca2+ to TnC, thereby delaying cessation of the interaction between thin and thick filaments. This idea is supported by the finding of myofilament sensitization in cardiomyocytes from Ang-II-infused mice (leftward shift in the relaxation phase of SL-Ca2+ loops) associated with reduced phosphorylation of cTnI at serine 23/24 and cMyBPC at serine 282, which play important roles in regulating responsiveness of myofilaments to Ca2+.41,42 Ca2+ bound to myofilaments represents ∼50% of the Ca2+ released from the SR in a typical heartbeat.43 Therefore, increased affinity of myofilaments for Ca2+ has a substantial impact on cytosolic [Ca2+], being able to buffer large amounts of Ca2+, including the Ca2+ leaked from the SR in the failing cardiomyocytes. Moreover, the higher affinity for Ca2+ would make detachment from the troponin complex slower, impacting the cytosolic [Ca2+] decay rate.44,45 Phospholamban (PLB) exerts an inhibitory effect on SERCA2 by protein-protein interaction and PLB phosphorylation releases that repression on SERCA2. Increased PLB phosphorylation in failing cardiomyocytes does not appear sufficient to compensate against both higher affinity for Ca2+ of myofilaments and diminished SERCA2 expression. Enhanced phosphorylation induced by MR-356 on PLB, together with the reduction of SR Ca2+ leak and its desensitizing effect on myofilaments, more efficiently counteracted and prevented slowing of Ca2+ decay rate (Figure 4B for the 4-week and Supplementary material online, Figure S6A in the 8-week Ang-II-induced pathological models).
Myofilament responsiveness to Ca2+ was greatly decreased in cardiomyocytes from Ang-II + MR-356-treated mice, an effect previously reported to be also induced by GH.46,47 This lower affinity of myofilaments for Ca2+ might explain the molecular basis for faster relaxation, preventing increased Ca2+ buffering capacity of myofilaments, since this mechanism facilitates Ca2+ dissociation from cardiac myofilaments in response to increasing pacing.48 Phosphorylation of cTnI and cMyBPC accelerates relaxation either under β-adrenergic stimulation or increasing pacing frequency and may prevent diastolic dysfunction.49–52
The increased SRCa2+ leak is likely to impact on the ATP consumption, increasing the energetic demands necessary to compensate for the extra flux of Ca2+ to the cytosol, thus affecting the energetic balance of the cell (not addressed in this study), which might underlie the depressed inotropic response of failing cardiomyocytes to high demanding conditions in vivo. In addition to the relevance of an elevated SRCa2+ leak, there may also be an inability to properly cycle cross-bridges. cMyBPC directly promotes force generation by enhancing cross-bridge binding and cooperative recruitment53 and acts as a brake on cross-bridge kinetics by slowing down actin-myosin detachment,54 an effect removed by phosphorylation of cMyBPC.53,55,56 MR-356 treatment prevented depressed phosphorylation of cMyBPC at serine 282 in cardiomyocytes from Ang-II-infused mice and might account, in part, for the preserved contractile reserve in cardiomyocytes from Ang-II + GHRH-A-treated mice.
4.3. Synthetic GHRH-As in animal models of cardiac diseases
GHRH-As have beneficial effects in rodent19–22 and porcine23 models of ischaemic cardiomyopathy and more recently, in a novel porcine model of CKD-induced HFpEF,25 where we showed that treatment with the GHRH-A, MR-409, improves myocardial function, including end-diastolic pressure, end-diastolic pressure–volume ratio, and stroke work compared to placebo injections.25 In this murine study, we used MR-356, which combines high GHRH activity with significant reduction of infarct size and prevention of ventricular remodelling compared with GHRH-As that exhibit high GHRHR activation but limited myocardial repair,19 possibly by stimulating reparative pathways, including an AC/PKA signalling as observed with GHRH20 and GC/PKG, as we showed in isolated cardiomyocytes. Other kinases might also play a role in phosphorylating sarcomeric and calcium handling proteins, particularly Akt (PKB) which phosphorylates PLB,57 activates eNOS and affects myofilament sensitivity to Ca2+.58 MR-356 also promotes survival of cardiac stem cells and induces proliferation in vitro, possibly mediated by ERK1/2 and PI3K/Akt pathways59, consistent with our results that Ang-II-infused mice treated with MR-356 exhibited increased mitosis in the myocardium.
A recent study using a mouse model of TAC-induced pressure overload,24 which exhibits characteristics of HFrEF, demonstrates that MR-409 attenuates hypertrophy and improves myocardial function. These beneficial effects are consistent with previous studies.21–23 Our murine Ang-II-infused model exhibited ventricular hypertrophy that was not prevented or reversed by treatment with MR-356 (in contrast to the observations of Gesmundo et al.24). However, MR-356 abolished the cardiomyocyte hypertrophy but also induced proliferation, which might explain the absence of reduction in heart weight and ventricular mass. These effects were accompanied by an evident anti-fibrotic effect compared with the untreated Ang-II-infused mice. Reducing cardiac fibrosis is a characteristic effect of GHRH-As22,23 although the mechanism is unclear. In this regard, although we did not investigate the precise mechanism by which MR-356 prevented and reversed fibrosis in our murine model, our results show that Ang-II induced pro-inflammatory and pro-fibrotic signalling evidenced by an increased TGF-β levels which correlated with the transition (of fibroblasts or other cellular type60) to myofibroblasts, as shown by the elevated expression of α-SMA61 in the myocardium. These effects were prevented by MR-356, which suggests that activation of myocardial GHRHRs by this agonist might inhibits pro-inflammatory signalling pathways,62 preventing the pathologic deposition of collagen in the extracellular matrix. Our results also indicate that a significant part of these effects is mediated by direct action of GHRH-As on cardiac fibroblasts, as the induction of TNF-α expression in isolated murine fibroblasts was partially reverted by MR-356 in vitro.
JI-38, another GHRH-A with features that are comparable with MR-356 in terms of preventing ventricular remodelling, promotes up-regulation of the transcription factor GATA-4, mitosis and proliferation within the myocardium.21 Despite the effectiveness of JI-38 in inducing GH secretion, circulating levels of GH were not different from control untreated animals. MR-356 injection also produces a transient increase of GH19 that would not sustain long-term GH concentrations or maintain pathological levels. We showed here that activation of GHRHRs with mrGHRH induced the AC/cAMP/PKA signalling pathway independently of GH or IGF-I in isolated CD1 murine cardiomyocytes. Interestingly, this experiment also showed that activation of GHRHRs induces a parallel increase in the production of the cGMP. However, we cannot rule out the possible simultaneous action of GHRH-As and GH and/or IGF-1 in vivo as mediators of the beneficial effects of MR-356 since treatment with either mrGH or mrIGF-I showed a non-significant trend to increase this cGMP/PKG pathway in isolated cardiomyocytes ex vivo, but this might have relevance in vivo. The important role of the GH/IGF-1 axis in sustaining the cardiovascular system63,64 has been widely demonstrated; however, there are no studies that address the use of such hormones as a treatment for HFpEF. In contrast, there is sufficient previous evidence, and novel findings in this work, to support that GHRH-As directly activates GHRHRs on the myocardium, in a GH/IGF-1-independent manner.21
As mentioned above, we have synthetized and tested several synthetic GHRH-As, previously demonstrating that MR-356 and MR-409 promote myocardial repair.19 MR-356 is a C-terminal ‘agmatine’ (Agm) substituted peptide of hGHRH(1-29)NH2 (see Supplementary material). It has a 7.5-fold higher binding affinity (EC50: 1.02 nM) and ∼150-fold greater potency compared with hGHRH(1–29)NH2. We previously used MR-409,24 a C-terminal ‘Arg-N-methylamide’ substituted peptide series analogue ([N-Me-Tyr,1 D-Ala2, Orn,12 Abu,15 Orn,21 Nle,27 Asp,28 Arg29-NHCH3]hGHRH(1–29)NH2) which exhibits similar binding affinity (EC50: 1.01 nM) but much higher potency than MR-356 (∼380-fold greater than hGHRH)(1–29)NH2 in male rats19 (see Supplementary material online, Table S3). Cumulative information has been reported about the benefits of MR-409 on various tissues and pathologies, including experimental heart diseases.65 However, relatively less has been described about the therapeutics effects of MR-356. Importantly, MR-356 appears to improve diastolic dysfunction better than MR-409, also exhibiting beneficial effects on fibrosis and cell survival,59 whereas MR-409 may preferentially enhance systolic function through effects at the chamber structural level, improving ejection fraction.19,24 These differences suggest that MR-356 might preferentially activate pathways that target specific pathophysiologic characteristics of HFpEF. Future studies are needed to optimize and pharmacologically test GHRH-As for indication-specific translational development.
In summary, we showed that activation of GHRHR with a synthetic GHRH-A prevents the appearance of pathophysiologic features of HFpEF and reverses the HFpEF-like phenotype once established in mice, including dysfunctional diastolic relaxation in myocytes and intact animals and pathological myocardial remodelling. Increased SR Ca2+ leak together with higher affinity of myofilaments for Ca2+, underlie the dysfunctional contractile response and lusitropy in these failing cardiomyocytes, and may in part account for the pathological phenotype. Myofibrillar and Ca2+ handling proteins appear to be relevant targets for treatment with GHRH agonists.
4.4. Study limitations
An important limitation of our study is that our model is single drug-induced (Ang-II), which does not mimic the broad spectrum of long-term risk factors and co-morbidities associated with the HFpEF syndrome in humans. There is extensive bibliography on animal models of continued Ang-II infusion that lead to HFrEF; however, under conditions of chronic low dose Ang-II,32 many of the key features of HFpEF were observed in CD1 mice. New literature suggest that the pathologic mechanism of Ang-II is mediated by vascular hypertension and its effect on myocardium would counteract that with an hypertrophic response.66 Thus, the increase in blood pressure induced by Ang-II might promote the phenotype observed in this model. However, at 8-week the pathological phenotype persists even in the absence of hypertension. We acknowledge that further evaluation, such as assessing the response of these mice to exercise, would substantially contribute to the characterization of the model. The age of the animals and factors associated with the molecular mechanisms in this species (in contrast with the main features in humans) might also account for potentially different response to GHRH-As in mice vs. human patients.
5. Conclusion
Our work focused on a highly relevant clinical problem with major unmet needs, the HFpEF syndrome and the results presented here show for first time that GHRH-As may, at least in part, prevent and reverse a HFpEF-like phenotype, particularly diastolic dysfunction and fibrosis. Thus, based on the proven properties for cardiac repair of GHRH-As and the robust data obtained in the present study, we consider that GHRH-As are potentially useful agents for the management of HFpEF and indicate that synthetic analogues of GHRH represent novel therapeutic compounds for treating patients with the HFpEF syndrome.
Author contributions
R.A.D. was involved in designing research studies, and conducted experiments, acquiring data, analyzing data, discussion and interpretation of results, and writing the manuscript. R.M.K.-T. was mainly responsible for designing research studies, she also conducted experiments, acquiring and analyzing data, and discussing results. L.M.T. conducted experiments, acquired and analyzed data. A.G.S. conducted experiments and analyzed data. S.K. analyzed and interpreted echocardiographic measurements. A.C.B.A.W. conducted experiments and analyzed data. W.B. participated in discussing results, writing and editing of the manuscript. M.S.S.R.Z. analyzed echocardiographic measurements. R.C. synthesized reagents. A.V.S. provided reagents and contributed to writing of the manuscript. J.M.H. conceived the project, participated in data and results interpretation, provided scientific and financial support for the project, and contributed to writing of the manuscript.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgments
We thank Christopher Zeuthen and Chloe Moulin for their technical assistance.
Contributor Information
Raul A Dulce, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA.
Rosemeire M Kanashiro-Takeuchi, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA; Department of Molecular and Cellular Pharmacology, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Lauro M Takeuchi, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA.
Alessandro G Salerno, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA.
Amarylis C B A Wanschel, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA.
Shathiyah Kulandavelu, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA; Department of Pediatrics, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Wayne Balkan, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA; Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Marilia S S R Zuttion, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA.
Renzhi Cai, Endocrine, Polypeptide and Cancer Institute, Veterans Affairs Medical Center, FL 33125, USA.
Andrew V Schally, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136, USA; Endocrine, Polypeptide and Cancer Institute, Veterans Affairs Medical Center, FL 33125, USA; Division of Hematology/Oncology, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Joshua M Hare, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, 1501 N.W. 10th Avenue, Room 908, Miami, FL 33136, USA; Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Funding
This study was supported by NIH, grants 1R01 HL13735 and 1R01 HL107110 to JMH. J.M.H. is also supported by NIH grants 5UM1 HL113460, 1R01 HL134558, 5R01 CA136387, The Starr, Lipson, and Soffer Family Foundations. A.G.S. is funded by the São Paulo Research Foundation (FAPESP) grant# 2016/01044-0.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Berlot B, Bucciarelli-Ducci C, Palazzuoli A, Marino P. Myocardial phenotypes and dysfunction in HFpEF and HFrEF assessed by echocardiography and cardiac magnetic resonance. Heart Fail Rev 2020; 25:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis GA, Schelbert EB, Williams SG, Cunnington C, Ahmed F, McDonagh TA, Miller CA. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;70:2186–2200. [DOI] [PubMed] [Google Scholar]
- 3. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 4. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 6. Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB, Meyer M. Association of beta-blocker use with Heart Failure Hospitalizations and Cardiovascular disease mortality among patients with Heart Failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open 2019;2:e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 8. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON-HF Investigators and Committee . Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 9. O'Connor CM, deFilippi C. PARAGON-HF—Why we do randomized, controlled clinical trials. N Engl J Med 2019;381:1675–1676. [DOI] [PubMed] [Google Scholar]
- 10. Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:432–441. [DOI] [PubMed] [Google Scholar]
- 11. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obokata M, Kane GC, Reddy YNV, Melenovsky V, Olson TP, Jarolim P, Borlaug BA. The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3707–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kjeldsen SE, von Lueder TG, Smiseth OA, Wachtell K, Mistry N, Westheim AS, Hopper I, Julius S, Pitt B, Reid CM, Devereux RB, Zannad F. Medical therapies for heart failure with preserved ejection fraction. Hypertension 2020;75:23–32. [DOI] [PubMed] [Google Scholar]
- 15. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, NHLBI Heart Failure Clinical Research Network . Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerjee P. Heart failure with preserved ejection fraction: a clinical crisis. Int J Cardiol 2016;204:198–199. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Ruiz I. The search for an effective HFpEF treatment continues. Nat Rev Cardiol 2019;16:647. [DOI] [PubMed] [Google Scholar]
- 18. Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, Granzier H, Hummel SL, Kass DA, Redfield MM, Sam F, Wang TJ, Desvigne-Nickens P, Adhikari BB. Research priorities for heart failure with preserved ejection fraction: national heart, lung, and blood institute working group summary. Circulation 2020;141:1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai R, Schally AV, Cui T, Szalontay L, Halmos G, Sha W, Kovacs M, Jaszberenyi M, He J, Rick FG, Popovics P, Kanashiro-Takeuchi R, Hare JM, Block NL, Zarandi M. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides 2014;52:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Granata R, Trovato L, Gallo MP, Destefanis S, Settanni F, Scarlatti F, Brero A, Ramella R, Volante M, Isgaard J, Levi R, Papotti M, Alloatti G, Ghigo E. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res 2009;83:303–312. [DOI] [PubMed] [Google Scholar]
- 21. Kanashiro-Takeuchi RM, Takeuchi LM, Rick FG, Dulce R, Treuer AV, Florea V, Rodrigues CO, Paulino EC, Hatzistergos KE, Selem SM, Gonzalez DR, Block NL, Schally AV, Hare JM. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI). Proc Natl Acad Sci U S A 2012;109:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanashiro-Takeuchi RM, Tziomalos K, Takeuchi LM, Treuer AV, Lamirault G, Dulce R, Hurtado M, Song Y, Block NL, Rick F, Klukovits A, Hu Q, Varga JL, Schally AV, Hare JM. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci U S A 2010;107:2604–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bagno LL, Kanashiro-Takeuchi RM, Suncion VY, Golpanian S, Karantalis V, Wolf A, Wang B, Premer C, Balkan W, Rodriguez J, Valdes D, Rosado M, Block NL, Goldstein P, Morales A, Cai RZ, Sha W, Schally AV, Hare JM. Growth hormone-releasing hormone agonists reduce myocardial infarct scar in swine with subacute ischemic cardiomyopathy. J Am Heart Assoc 2015;4:e001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gesmundo I, Miragoli M, Carullo P, Trovato L, Larcher V, Di Pasquale E, Brancaccio M, Mazzola M, Villanova T, Sorge M, Taliano M, Gallo MP, Alloatti G, Penna C, Hare JM, Ghigo E, Schally AV, Condorelli G, Granata R. Growth hormone-releasing hormone attenuates cardiac hypertrophy and improves heart function in pressure overload-induced heart failure. Proc Natl Acad Sci U S A 2017;114:12033–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rieger AC, Bagno LL, Salerno A, Florea V, Rodriguez J, Rosado M, Turner D, Dulce RA, Takeuchi LM, Kanashiro-Takeuchi RM, Buchwald P, Wanschel A, Balkan W, Schulman IH, Schally AV, Hare JM. Growth hormone-releasing hormone agonists ameliorate chronic kidney disease-induced heart failure with preserved ejection fraction. Proc Natl Acad Sci U S A 2021;118:e2019835118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology 1995;136:4147–4150. [DOI] [PubMed] [Google Scholar]
- 27. Primessnig U, Schonleitner P, Holl A, Pfeiffer S, Bracic T, Rau T, Kapl M, Stojakovic T, Glasnov T, Leineweber K, Wakula P, Antoons G, Pieske B, Heinzel FR. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 2016;18:987–997. [DOI] [PubMed] [Google Scholar]
- 28. Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, Rau C, Warren CM, Klutho PJ, Alex L, Ferreira-Nichols SC, Ivey JR, Thorne PK, McDonald KS, Krenz M, Baines CP, Solaro RJ, Wang Y, Ford DA, Domeier TL, Padilla J, Rector RS, Emter CA. Western diet-fed, aortic-banded ossabaw swine: a preclinical model of cardio-metabolic heart failure. JACC Basic Transl Sci 2019;4:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valero-Munoz M, Backman W, Sam F. Murine models of heart failure with preserved ejection fraction: a “Fishing Expedition”. JACC Basic Transl Sci 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2021;18:400–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang D, Xu TT, Zhang SJ, Cai Y, Min SD, Zhao Z, Lu CQ, Wang YC, Ju S. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp Biol Med (Maywood) 2021;246:2511–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regan JA, Mauro AG, Carbone S, Marchetti C, Gill R, Mezzaroma E, Valle Raleigh J, Salloum FN, Van Tassell BW, Abbate A, Toldo S. A mouse model of heart failure with preserved ejection fraction due to chronic infusion of a low subpressor dose of angiotensin II. Am J Physiol Heart Circ Physiol 2015;309:H771–H778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC, Kanashiro-Takeuchi RM, Hare JM, Szczesna-Cordary D. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci U S A 2015;112:E4138–E4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winterberg PD, Jiang R, Maxwell JT, Wang B, Wagner MB. Myocardial dysfunction occurs prior to changes in ventricular geometry in mice with chronic kidney disease (CKD). Physiol Rep 2016;4:e12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varghese P, Harrison RW, Lofthouse RA, Georgakopoulos D, Berkowitz DE, Hare JM. beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest 2000;106:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 2012;109:4314–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2 + leak-load relationship. Circ Res 2002;91:594–600. [DOI] [PubMed] [Google Scholar]
- 38. Dulce RA, Yiginer O, Gonzalez DR, Goss G, Feng N, Zheng M, Hare JM. Hydralazine and organic nitrates restore impaired excitation-contraction coupling by reducing calcium leak associated with nitroso-redox imbalance. J Biol Chem 2013;288:6522–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J 2008;29:1283–1289. [DOI] [PubMed] [Google Scholar]
- 40. Tadic M, Cuspidi C, Frydas A, Grassi G. The role of arterial hypertension in development heart failure with preserved ejection fraction: just a risk factor or something more? Heart Fail Rev 2018;23:631–639. [DOI] [PubMed] [Google Scholar]
- 41. Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol 2004;558:927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tong CW, Nair NA, Doersch KM, Liu Y, Rosas PC. Cardiac myosin-binding protein-C is a critical mediator of diastolic function. Pflugers Arch 2014;466:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J 2000;78:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J 2001;80:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kataoka A, Hemmer C, Chase PB. Computational simulation of hypertrophic cardiomyopathy mutations in troponin I: influence of increased myofilament calcium sensitivity on isometric force, ATPase and [Ca2+]i. J Biomech 2007;40:2044–2052. [DOI] [PubMed] [Google Scholar]
- 46. Cittadini A, Ishiguro Y, Stromer H, Spindler M, Moses AC, Clark R, Douglas PS, Ingwall JS, Morgan JP. Insulin-like growth factor-1 but not growth hormone augments mammalian myocardial contractility by sensitizing the myofilament to Ca2 + through a wortmannin-sensitive pathway: studies in rat and ferret isolated muscles. Circ Res 1998;83:50–59. [DOI] [PubMed] [Google Scholar]
- 47. Stromer H, Cittadini A, Douglas PS, Morgan JP. Exogenously administered growth hormone and insulin-like growth factor-I alter intracellular Ca2 + handling and enhance cardiac performance. In vitro evaluation in the isolated isovolumic buffer-perfused rat heart. Circ Res 1996;79:227–236. [DOI] [PubMed] [Google Scholar]
- 48. Baker AJ, Weiner MW. Force decline during muscle relaxation promotes calcium release to the cytosol. Am J Physiol 1997;273:C85–C91. [DOI] [PubMed] [Google Scholar]
- 49. Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, Fitzsimons DP, Irving TC, Moss RL. Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium. J Mol Cell Cardiol 2012;53:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 2007;292:H2212–H2219. [DOI] [PubMed] [Google Scholar]
- 51. Dweck D, Sanchez-Gonzalez MA, Chang AN, Dulce RA, Badger CD, Koutnik AP, Ruiz EL, Griffin B, Liang J, Kabbaj M, Fincham FD, Hare JM, Overton JM, Pinto JR. Long term ablation of protein kinase A (PKA)-mediated cardiac troponin I phosphorylation leads to excitation-contraction uncoupling and diastolic dysfunction in a knock-in mouse model of hypertrophic cardiomyopathy. J Biol Chem 2014;289:23097–23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res 2004;94:496–504. [DOI] [PubMed] [Google Scholar]
- 53. Coulton AT, Stelzer JE. Cardiac myosin binding protein C and its phosphorylation regulate multiple steps in the cross-bridge cycle of muscle contraction. Biochemistry 2012;51:3292–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saber W, Begin KJ, Warshaw DM, VanBuren P. Cardiac myosin binding protein-C modulates actomyosin binding and kinetics in the in vitro motility assay. J Mol Cell Cardiol 2008;44:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem 2009;284:12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sadayappan S, Gulick J, Osinska H, Barefield D, Cuello F, Avkiran M, Lasko VM, Lorenz JN, Maillet M, Martin JL, Brown JH, Bers DM, Molkentin JD, James J, Robbins J. A critical function for Ser-282 in cardiac Myosin binding protein-C phosphorylation and cardiac function. Circ Res 2011;109:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, Santonastasi M, Bellacosa A, Brown JH, Condorelli G. Akt increases sarcoplasmic reticulum Ca2 + cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem 2009;284:28180–28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, Stromer H, Sorriento D, Peschle C, Trimarco B, Sacca L, Condorelli G. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther 2006;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Florea V, Majid SS, Kanashiro-Takeuchi RM, Cai RZ, Block NL, Schally AV, Hare JM, Rodrigues CO. Agonists of growth hormone-releasing hormone stimulate self-renewal of cardiac stem cells and promote their survival. Proc Natl Acad Sci U S A 2014;111:17260–17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 2009;105:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 2004;95:253–260. [DOI] [PubMed] [Google Scholar]
- 62. Recinella L, Chiavaroli A, Orlando G, Ferrante C, Marconi GD, Gesmundo I, Granata R, Cai R, Sha W, Schally AV, Brunetti L, Leone S. Antinflammatory, antioxidant, and behavioral effects induced by administration of growth hormone-releasing hormone analogs in mice. Sci Rep 2020;10:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Isgaard J, Arcopinto M, Karason K, Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine 2015;48:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arcopinto M, Bobbio E, Bossone E, Perrone-Filardi P, Napoli R, Sacca L, Cittadini A. The GH/IGF-1 axis in chronic heart failure. Endocr Metab Immune Disord Drug Targets 2013;13:76–91. [DOI] [PubMed] [Google Scholar]
- 65. Schally AV, Zhang X, Cai R, Hare JM, Granata R, Bartoli M. Actions and potential therapeutic applications of growth hormone-releasing hormone agonists. Endocrinology 2019;160:1600–1612. [DOI] [PubMed] [Google Scholar]
- 66. Zouein FA, Altara R, Massoud GP, Booz GW. The angiotensin II Type 1(AT1) receptor and cardiac hypertrophy: did we have it wrong all along? J Cardiovasc Pharmacol 2021;77:531–535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.