Abstract
Cardiovascular diseases and specifically heart failure (HF) impact global health and impose a significant economic burden on society. Despite current advances in standard of care, the risks for death and readmission of HF patients remain unacceptably high and new therapeutic strategies to limit HF progression are highly sought. In disease settings, persistent mechanical or neurohormonal stress to the myocardium triggers maladaptive cardiac remodelling, which alters cardiac function and structure at both the molecular and cellular levels. The progression and magnitude of maladaptive cardiac remodelling ultimately leads to the development of HF. Classical therapies for HF are largely protein-based and mostly are targeted to ameliorate the dysregulation of neuroendocrine pathways and halt adverse remodelling. More recently, investigation of novel molecular targets and the application of cellular therapies, epigenetic modifications, and regulatory RNAs has uncovered promising new avenues to address HF. In this review, we summarize the current knowledge on novel cellular and epigenetic therapies and focus on two non-coding RNA-based strategies that reached the phase of early clinical development to counteract cardiac remodelling and HF. The current status of the development of translating those novel therapies to clinical practice, limitations, and future perspectives are additionally discussed.
Keywords: Heart failure, Fibrosis, Epigenetics, Non-coding RNAs, MicroRNAs
This article is part of the Spotlight Issue on Heart Failure.
1. Cardiac fibrosis as one hallmark of the remodelling process
Cardiac fibrosis is defined as the existence of excess extracellular matrix (ECM)-rich tissue in the myocardium. Beneficial cardiac fibrosis is exemplified by reparative scar formation following myocardial infarction (MI), which replaces necrotic cardiomyocytes to maintain myocardial continuity and prevent ventricular rupture. However, this ‘replacement fibrosis’ ultimately reduces contractility and leads to regional or global systolic dysfunction.1 Pathological cardiac fibrosis can also arise from a variety of other stresses, including chronic hypertension, aortic stenosis, rhythm abnormalities, and primary diseases of the cardiac muscle arising from genetic disorders.2–5 Fibrotic remodelling is associated with increased passive myocardial stiffness and the development of diastolic dysfunction (DD), an essential component of heart failure (HF) with preserved ejection fraction (HFpEF),6 and can disrupt cardiac electrical conduction by slowing action potential (AP) propagation, increasing the risk of lethal arrhythmias and other conduction abnormalities.7 Despite clear evidence that fibrosis contributes to the pathogenesis of multiple cardiac diseases, there remain no effective therapeutic approaches to block fibrotic remodelling of the heart. This presumably reflects the failure of existing modalities to target the underlying molecular mechanisms that drive cardiac fibrosis.
Until recently, attempts to correlate fibrosis with cardiac disease were limited to histological detection of fibrotic lesions in explanted or cadaveric hearts, and in some cases, endomyocardial biopsies. However, major advances in imaging now enable quantification of cardiac fibrosis in patients, with cardiac magnetic resonance imaging (CMR) representing the current gold-standard modality for non-invasive evaluation of cardiac fibrosis.8 CMR was recently used to assess the ability of pirfenidone, an FDA-approved medication for the treatment of pulmonary fibrosis, to ameliorate cardiac fibrosis in HFpEF patients. In the PIROUETTE clinical trial, pirfenidone led to a small but significant 1.2% decrease in cardiac fibrosis, as determined by CMR assessment of myocardial extracellular volume, but did not improve diastolic function.9 While these findings were ultimately disappointing, the data validate the use of CMR to assess efficacy of forthcoming anti-fibrotic therapies. New approaches targeting cardiac fibrosis include use of natural-derived compounds, some of which outperformed effects of the established lung anti-fibrotics pirfenidone and nintedanib.10,11 Recently, increased anti-fibrotic efficacy of lead-optimized natural compound modulators also could be translated to living human cardiac tissue and further (pre)clinical development is to be expected.11 In the following sections, we discuss the recent developments in cellular and epigenetic therapies to counteract cardiac fibrosis and remodelling in HF. Additionally, we summarize the most advanced microRNAs (miRNAs) in the early clinical development targeting cardiac remodelling and healing.
2. T-cell immunotherapy targeting cardiac fibroblasts
While multiple cell types contribute to cardiac fibrosis, including cardiomyocytes, endothelial cells (ECs) and leucocytes, it is well-accepted that the primary driver of excessive ECM deposition is the cardiac fibroblast (CF).12 Taking cues from the oncology field, emerging immunotherapy approaches that culminate in suppression of CF activation may prove to be useful for treating cardiac fibrosis.
Adoptive transfer of genetically modified T lymphocytes that express chimeric antigen receptors (CAR-T) targeting cancer cells has revolutionized the treatment of haematological malignancies and is being explored as a possible therapy for a multitude of additional oncologic indications. Recent studies suggest the potential of adapting CAR-T technology to target activated CFs and treat cardiac fibrosis. CAR-T cells were designed to recognize and kill cells expressing fibroblast activation protein (FAP) alpha, which is highly expressed in activated CFs.13 In an initial study, T cells were isolated from mice and infected with a retroviral construct encoding a chimeric T-cell receptor against FAP in culture.14 Subsequent injection of these genetically modified cells was sufficient to attenuate cardiac fibrosis and improve systolic function in mice chronically infused with angiotensin II (Ang II) and phenylephrine (PE). In a follow-up study by the same research group, the mRNA technology used to develop COVID-19 vaccines was harnessed to develop CAR-T cells to target activated CFs.15 Lipid nanoparticles (LNPs) were employed to deliver an mRNA construct and reprogramme T cells endogenously into FAP-targeting CAR-T, referred to as (FAPCAR).15 T-cell selectivity was achieved by coating LNPs with antibodies against the general T-cell surface marker, CD5. Remarkably, intravenous delivery of LNPs harbouring FAPCAR was sufficient to block cardiac fibrosis and improve heart function in the mouse Ang II/PE model. These data provide proof-of-concept evidence that CAR-T cells can be endogenously generated and used to target pathological fibroblasts in the heart. Notably, this approach to creating FAPCAR in vivo using mRNA-loaded LNPs is more cost-effective that exogenous T-cell manufacturing and adoptive transfer (see also Figure 1 for an overview). Furthermore, a major advance of the LNP/mRNA approach is its transient nature, which likely provides a safety advantage over sustained CAR-T therapy with cells infected with retroviral vectors. In this regard, the authors found that FAPCAR-reprogrammed T cells did not persist for longer than a few days following a single bolus injection of encapsulated mRNA.15 Extension of these findings to large animal models that more closely mimic human HF will help define the optimal indications and ultimate utility of FAPCAR as a therapy to treat cardiac fibrosis. Pending the outcome of these studies, future clinical trials, using CMR to quantify cardiac fibrosis as one endpoint, could be rapidly forthcoming.
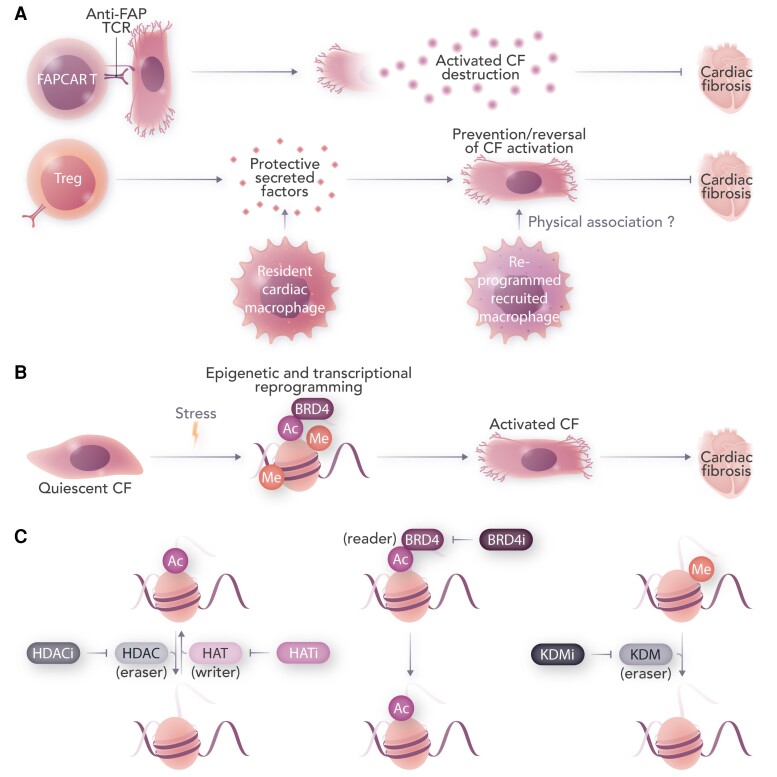
Figure 1.
Immunological and epigenetic strategies to treat cardiac fibrosis. Activated CFs produce excess ECM, directly leading to pathological fibrosis. A: Chimeric antigen receptor T cells can be generated to specifically ablate activated CFs. Regulatory T cells and subsets of cardiac macrophages can also blunt CF activation partly through cytokine-mediated immunomodulation, and potentially through direct cell–cell interaction. B: Stress signalling triggers epigenetic reprogramming in CFs, resulting in activation of pro-fibrotic gene expression. C: Epigenetic writers, erasers, and readers are depicted. Targeting histone modifications through small molecule inhibitors of histone deacetylases (HDACi), histone acetyltransferases (HATi), BRD4 (BRD4i), lysine demethylases (KDMi), or by modulating other pro-fibrotic epigenetic targets yet to be uncovered, can rewire the fibroblast epigenome to selectively attenuate activation and subsequent fibrosis during cardiac stress. FAP, fibroblast activation protein; FAPCAR T, chimeric antigen receptor (CAR) T cells targeted against FAP; TCR, T-cell receptor; Me, methyl group; Ac, acetyl group.
Beyond CAR-T, immunotherapy with regulatory T cells (Tregs) represents another promising cell therapy approach for targeting cardiac fibrosis. While chronic inflammation mediated by T cells clearly promotes pathological cardiac remodelling in pre-clinical models, Tregs represent a subset of lymphocytes that have potent anti-inflammatory and cardioprotective properties.16,17 Treg numbers and function have been shown to be reduced in patients with HFpEF, and the degree of Treg impairment correlated with HF phenotype severity, hospitalization, and mortality.18–20 Consistent with these clinical findings, adoptive transfer experiments demonstrated that Tregs significantly attenuated Ang II-mediated cardiac remodelling in a mouse model, with improved cardiac function correlating with reduced expression of pro-inflammatory cytokines and blunted inflammatory cell infiltration in the heart.21 Subsequent studies revealed that adoptive transfer of Tregs blocked cardiac fibrosis in a mouse model of transverse aortic constriction (TAC), and interleukin-2-mediated expansion of Tregs reversed TAC-induced cardiac remodelling.22,23 In response to MI in mice, there is phenotypic conversion of Tregs into cells that promote inflammation and suppress angiogenesis in the heart, and protective actions of normal Tregs in the setting of MI have been demonstrated.24–27
Adoptive transfer of Tregs is being vigorously pursued as a therapeutic approach for multiple indications, including graft-versus-host disease, inflammatory bowel disease, and multiple sclerosis.28 Based on the aforementioned pre-clinical findings, Treg infusion therapy with cells expanded ex vivo may have salutary actions in patients with cardiac diseases marked by chronic inflammation and fibrosis of the heart. Alternatively, it may be possible to manipulate the function of endogenous Tregs to benefit the heart, such as by using Treg-targeted LNPs that harbour mRNAs encoding factors that promote recruitment, survival, and/or anti-inflammatory function of these lymphocytes, akin to the FAPCAR approach described above. The recent demonstration that CCL17, secreted from activated macrophages, triggers negative signalling in Tregs that culminates in inhibition of recruitment of these protective cells to the heart post-MI also suggests the possibility of therapeutically neutralizing CCL17 signalling as a means of promoting Treg function to combat fibrotic cardiac disease.29 Finally, epigenetic inhibitors could potentially be employed to enhance Treg numbers and function (see the following).
3. Fibro-modulatory actions of cardiac macrophages
It may also be possible to co-opt macrophages as a means of blocking CF activation to treat cardiac fibrosis. Under homeostasis, the adult mammalian heart contains several tissue-resident macrophage subsets characterized by distinct ontogenetic and transcriptional profiles.30 This complexity is magnified in the setting of cardiac disease or under pro-inflammatory conditions, as monocyte recruitment from peripheral tissues contributes additional macrophage subsets to the heart. Within this framework, considerable effort has been made to define how macrophage subpopulations coordinate tissue injury progression vs. resolution in the heart, and cardiac fibrosis in particular. Genetic and pharmacological modulation of tissue-resident vs. recruited macrophages supports distinct effects on the fibrotic response. Following infarction injury, selective genetic ablation of resident CX3CR1+ cardiac macrophages using a pulse-chase protocol to allow recovery of circulating monocytes resulted in greater peri-infarct fibrosis and enhanced mortality in mice.31 More recently, a similar strategy using pulse-chase delivery of anti-CD115 blocking antibody showed that resident macrophage ablation worsened pathological fibrosis in the setting of pressure overload by TAC.32 Genetic ablation of CX3CR1 itself also augmented fibrosis in the mouse heart following Ang II infusion.33 These and other data implicate cardiac-resident macrophage populations such as CX3CR1+ tissue macrophages as cellular attenuators of the fibrotic response. In contrast, the collective evidence suggests macrophages derived from circulating pro-inflammatory monocytes enhance fibrosis and drive pathological outcomes. In particular, recruitment of pro-inflammatory monocytes via the CCR2 signalling axis is associated with adverse fibrotic remodelling and non-resolving inflammation in the heart.34,35 Targeting CCR2 is therefore an attractive anti-fibrotic and immunomodulatory strategy, as genetic or antibody-based blockade of CCR2 reduced fibrosis and adverse remodelling in the setting of TAC,35 as well as in small and large animal models of valvular fibrosis.36 siRNA-based technology to silence CCR2 has also been effective in reducing both post-MI infarct size and atherosclerotic plaque burden in rodent models.34 Although clinically-approved CCR2 antagonists are available and have shown promise in other disease contexts, this has not yet been translated to cardiovascular disease. A degree of CCR2+ monocyte activity is required for acute wound healing, thus targeted approaches will be essential. In this regard, development of imaging platforms for clinical tracking of CCR2+ monocytes in the heart will be particularly valuable in determining the optimal time points and patient populations where CCR2 blockade might be effective.30 Technologies to specifically deliver either small molecules or RNAs to pro-inflammatory macrophages are also under investigation.37,38
Despite strong evidence that macrophages are direct players in the overall cardiac fibrotic response, the specific underlying mechanisms of how macrophages control fibrosis are less well defined. Paracrine signals and cytokine-mediated crosstalk between macrophages and CFs are often invoked and, indeed, a large body of literature has identified specific receptor-ligand interactions between fibroblasts and immune cells including macrophages, the modulation of which can shift fibrotic remodelling.39 Of note, recent encouraging clinical data with monoclonal antibody-based targeting of interleukin-1β (IL-1β) have reinvigorated efforts to target specific cytokine pathways for cardiovascular disease indications. Deletion of the Type I interleukin-1 receptor (IL-1R1) in CFs was shown to improve molecular indices of cardiac fibrosis and pathological remodelling in a mouse MI model.40 CFs with inhibited IL-1R1 signalling also produced lower levels of inflammatory cytokines, suggesting that targeting IL-1 signalling specifically at the level of the CF may be a promising immunomodulatory approach for attenuating fibrosis, with clinically applicable candidate strategies already under investigation. More broadly modulating the macrophage secretome, with a specific focus on candidate crosstalk pathways to CFs, also holds therapeutic promise. For example, deletion of miR-21 specifically in CX3CR1+ macrophages of the pressure overloaded heart was sufficient to mitigate CF activation in vitro and limit adverse remodelling in mice in vivo,41 partly through a reduction in macrophage-derived cytokines such as IL-6 and tumour necrosis factor (TNF). Reciprocal fibroblast-to-macrophage signalling may also elicit subsequent waves of inflammatory activity and contribute to chronic, persistent fibrosis. Over-activation of Yes-associated protein (YAP) in CFs directly stimulated transcriptional upregulation of CCL2, and this was sufficient to elevate CCR2+ monocyte recruitment and trigger a molecular signature of fibrosis in mouse hearts.42 This is consistent with evidence suggesting YAP/transcriptional co-activator with PDZ-binding motif (TAZ) deletion in CFs reduces macrophage accumulation and attenuates remodelling in the heart post-MI.43 In macrophages, YAP/TAZ can also drive expression of pro-inflammatory genes and suppress expression of injury-resolving pathways, in part through a histone deacetylase-3 (HDAC-3)-mediated mechanism.44
There is also evidence to support a direct role for macrophages (and other immune cells) in establishing the ECM itself during cardiac injury and remodelling. Macrophage-derived Type IV collagens were observed as contributing to the scar of cryo-injured zebrafish hearts.45 In particular, macrophage heterogeneity may drive differential ECM regulation in this context, as macrophages expressing the inflammatory cytokine TNF were more highly associated with ECM deposition vs. turnover.46 Circulating immune cells may also directly produce ECM components. In one study, reciprocal bone marrow transplant was used to show that the matricellular ECM protein ‘Secreted protein acidic and rich in cysteine’ (SPARC) contributed to myocardial stiffening post-TAC.47 Although the specific cell types producing SPARC in this setting were not identified, both macrophages and certain populations of Tregs have been implicated as sources of SPARC.48,49 Thus, macrophage-mediated control of fibrosis at the level of the ECM may involve both a direct contribution to ECM content, as well as through crosstalk to CFs which would thereby shape and modulate the ECM.
Therapeutic modulation of macrophages as a means to treat fibrosis remains an unmet clinical goal, although multiple avenues have shown promise in pre-clinical models. This is thanks to a rapidly expanding interest in cardio-immunology over the past decade, combined with crucial technological advances such as large-scaled genomics, single-cell transcriptomics, and genetic systems for interrogating specific macrophage subsets in the heart. Crucially, effective immunomodulatory strategies for cardiac fibrosis must progress beyond broad anti-inflammatory approaches and seek to also harness the advantageous properties of select macrophage subsets. This may include targeted approaches that enhance the ability of macrophages to promote beneficial ECM remodelling though release of MMPs or crosslinking factors.50 Stimulating production or release of pro-resolving molecules by macrophages may also be effective at mitigating pathological CF activation.51 At the same time, attenuating pathways that prolong inflammation and fibrotic remodelling, such as recruitment of CCR2+ monocytes, is theoretically achievable with small molecule or RNA interference approaches and warrants further investigation.
4. Targeting epigenetic histone modifications as anti-fibrotic therapeutic approach
Traditional small molecule drug discovery approaches to directly target activated CFs for the treatment of cardiac fibrosis still hold significant promise.
Epigenetic changes are a recognized hallmark of HF, as such modulating the epigenome represents an attractive new approach for HF therapy.52,53 Here, we focus on inhibitors of epigenetic regulators.
Epigenetic changes which include post-translational modifications (PTMs) of histone tails,54,55 DNA methylation changes56,57 and to a lesser extent, 3D chromatin architecture changes58,59 act through regulating the transcriptome observed in HF phenotypes. At the core of epigenetic changes are chromatin remodellers which regulate the addition or removal of the chromatin modifications, broadly divided into writers, readers and erasers.60 In response to genetic or environmental stresses, these enzymes act together with other factors and co-activators to alter chromatin modifications at regulatory loci, thereby mediating the genome-wide transcriptional changes observed in heart disease.60 While HDAC inhibitors (HDACis) and Bromodomain Extraterminal inhibitors (BETis) are the two most well-developed groups, other classes of remodellers have also shown therapeutic application in pre-clinical models of HF.
However, our understanding of epigenetic regulation of cardiac fibrosis remains in its infancy and represents a deep reservoir for the development of innovative anti-fibrotic therapies. It is our view that epigenetic regulators are among the most auspicious and underappreciated therapeutic targets for the treatment of pathological cardiac fibrosis. In order to advance epigenetic inhibitors into the clinic to treat heart disease, it will be necessary to elucidate functions for specific regulatory proteins to facilitate the development of targeted, as opposed to ‘pan’ inhibitors. For example, in cancer patients, who typically receive maximum tolerated doses (MTDs) of pan-HDAC inhibitors, gastrointestinal disturbance, fatigue, and haematological toxicity have been reported.61 Selective inhibition of one or a small subset of pathogenic HDAC isoforms should help overcome these toxicity issues.
4.1. Reversible histone acetylation
4.1.1. HDAC inhibitors
PTMs of histones come in various flavours including acetylation, methylation, ubiquitination, and phosphorylation.62 These modifications correlate to chromatin accessibility and consequently affect gene expression, with histone acetylation being associated with increased gene expression (H3K27ac and H3K9ac) while methylation could be either associated with repressed genes (H3K27me3 and HeK9me3) or actively transcribed regions (H3K4me1 and H3K4me3).52,62 Histone acetyl transferases (HATs), therefore, generally lead to more accessible chromatin and activation of gene expression while HDACs, to a more compact chromatin and suppression of gene expression.52,62 Besides histone proteins, the same enzymes also acetylate and deacetylate non-histone transcription factors and cellular proteins and thus can modulate other processes in the cell.62
The first suggestion that manipulating epigenetics has the potential to ameliorate cardiac fibrosis came from studies utilizing inhibitors of acetyl-histone ‘erasers’ known as HDACs. One of the earliest indications for the role of HDACs in HF came in 2002 when Class II HDACs were demonstrated as stress-responsive and regulators of the myocyte enhancer factor-2 (MEF2) transcriptional programme in the heart.63 Many more studies have since established the links between HDACs, reactivation of fetal gene programme and cardiac hypertrophy through Ca2+/calmodulin-dependent protein kinase II (CaM Kinase II) signalling and MEF2,64,65 and cardiac fibrosis. There are 18 mammalian HDACs grouped into four classes of HDACs.66 Classes I, II (IIa and IIb), and IV HDAC enzymes depend on zinc for their function while Class III HDACs (sirtuins) require NAD for their action.66
Table 1 is showing different classes of HDACs. A detailed review of HDACis in HF can be found here.66
Table 1.
Summarizing roles of HDACs in cardiac remodelling67–74
| Class | Member | Substrates/Associated proteins | Biological processes | Location |
|---|---|---|---|---|
| I | HDAC 1 | Histones, MEF2, ATM, p53, | Myocardial growth/hypertrophy, morphogenesis, contractility, autophagy | Nucleus |
| HDAC 2 | Histones, GATA, KLF4, | Proliferation, hypertrophy | Nucleus | |
| HDAC 3 | Histones, GATA, NF-kB | Myocyte proliferation, cardiac energy metabolism, hypertrophy | Nucleus | |
| HDAC 8 | HSP70 | Cell proliferation | Nucleus | |
| IIa | HDAC 4 | Histones, GATA4, MEF2, NFAT, MLP, CaM, 14-3-3 | Hypertrophy | Nucleocytoplasm |
| HDAC 5 | Histones, GATA4, MEF2, NFAT, CaM, 14-3-3 | Hypertrophy | Nucleocytoplasm | |
| HDAC 7 | Histones, GATA4, MEF2, NFAT, CaM, 14-3-3, c-Myc | Hypertrophy | Cytoplasm | |
| HDAC 9 | Histones, GATA4, MEF2, NFAT, CaM, 14-3-3 | Hypertrophy | Nucleocytoplasm | |
| IIb | HDAC 6 | Alpha tubulin, Cortactin | Atrial Fibrillation, cytoskeleton | Nucleocytoplasm |
| HDAC 10 | LcoR, PP1 | DNA damage response | Nucleocytoplasm | |
| III | SIRT 1 | Histones, MEF2, Cortactin, p300 | Oxidative stress, apoptosis, senescence | Nucleus/Cytoplasm |
| SIRT 2 | Histones, a-tubulin | Aging, inflammation | Nucleus/Cytoplasm | |
| SIRT 3 | SOD2, SdhA, IDH2 | Oxidative stress, DNA damage response | Mitochondria | |
| SIRT 4 | GDH | Oxidative stress, DNA damage response | Mitochondria | |
| SIRT 5 | CPS1, Cytochrome c | Oxidative stress, DNA damage response | Mitochondria | |
| SIRT 6 | Histones, TNF-α | DNA damage, metabolism | Nucleus | |
| SIRT 7 | P53 | Apoptosis, DNA damage response | Nucleus | |
| IV | HDAC 11 | SHMT2, AKAP12 | DNA replication | Nucleus/Cytoplasm |
Broadly, four main groups of HDACis exist, namely benzamides, hydroxamic acids, cyclic peptides, and short-chain fatty acids. There are either pan-inhibitors of HDACs or those with selective profiles, only affecting a sub-class of HDACs.
The ability of one of these compounds, vorinostat/suberoylanilide hydroxamic acid (SAHA), to block cardiac fibrosis in various pre-clinical models of systolic HF is well-established.75 Vorinostat/SAHA was in 2006 the first FDA-approved HDACi for the treatment of cutaneous T-cell lymphoma. Since then, SAHA along with Trichostatin A (TSA), another HDACi from the same hydroxamic class of HDACi, have been used in pre-clinical models of HF. TSA inhibited the progression of cardiac hypertrophy and fibrosis in mice with overexpression of homeodomain-only protein (HopX)76 and attenuated cardiac hypertrophy in mice subjected to pressure overload.77,78 TSA modulated H3 acetylation, as well as acetylation of other non-histone proteins such as alpha tubulin, and also ameliorated aortic banding-induced autophagy.77–79 Like TSA, SAHA has also been used in pre-clinical models of HF. The cardioprotective effects of SAHA include an increase in autophagic flux, improved myofibril relaxation and decreased cardiomyocyte size. These changes are thought to be mediated through acetylation of histones and other proteins involved in these biological processes.80–82
Other HDACis used in pre-clinical studies include Scriptaid, Valproic acid, and Apicidin-derivative(Api-D), all exhibiting different levels of cardioprotection in mouse models of HF (see Table 2), including attenuation of cardiac hypertrophy and reductions in cardiac fibrosis and cardiac inflammation.66
Table 2.
Roles of HDACis in models of heart failure
| HDACis | Target | Study | Outcomes | References |
|---|---|---|---|---|
| TSA | Class I and II | Mice with aortic banding | Attenuated hypertrophy | 77,83 |
| Transverse aortic constriction (TAC) in Belcin 1 transgenic mice | Attenuated hypertrophy | 79 | ||
| Mice with TAC | Attenuates hypertrophy and increased survival | 78,84 | ||
| Hop transgenic mice | Attenuated hypertrophy, reduced fibrosis and atrial arrhythmia | 76,85 | ||
| SAHA | Rabbits myocardial Ischaemia | Reduced myocardial infarct size | 82 | |
| Dystrophic mice (mdx) | Attenuates arrhythmias | 86 | ||
| Mice Takotsubo-like myocardial injury | Reduced fibrosis | 80 | ||
| Myocardial infarction rats | Improved contractility | 87 | ||
| Cats HFpEF | Improves cardiopulmonary function | 81 | ||
| Scriptaid | Classes I and II | Mice thoracic aortic banding | Attenuated hypertrophy | 77 |
| Valproic acid | Mice with TAC | Attenuates hypertrophy | 84 | |
| Hop transgenic mice | Attenuates hypertrophy | 85 | ||
| Mice Angiotensin II infusion | Attenuates hypertrophy | 83 |
Recent efforts have focused on determining whether HDACis are efficacious in models of DD. ITF2357/givinostat, which is in Phase 3 in patients with Duchenne Muscular Dystrophy,88 improved diastolic function in murine models of hypertension or aging, and SAHA was shown to ameliorate DD in a feline model of HFpEF.81,89,90 In the murine models, cardiac fibrosis was not observed by standard histological readouts, such as picrosirius red staining.89,90 However, evaluation of hearts of mice with DD using more sensitive methods, such as ECM mass spectrometry and atomic force microscopy (AFM), revealed ‘hidden fibrosis’ that was profoundly inhibited by ITF2357/givinostat in a manner that correlated with improved diastolic function.90 Gene expression analysis and studies with cultured cells confirmed a remarkable ability of ITF2357/givinostat to block CF activation, highlighting one mechanism by which the compound blocked fibrosis and improved diastolic function in vivo.
Why are HDACis yet to be tested for anti-fibrotic activity in humans? While HDACis are generally well tolerated, toxicities such as thrombocytopenia have been observed in cancer patients receiving MTDs of the compounds, raising concerns about the use of this epigenetic therapy for chronic conditions such as HF. However, HDACis exhibit salutary effects in animal models of HF at doses far below their MTD and in the absence of detectable haematological toxicity,89 presumably due to transient refinement of epigenetic events resulting in sustained inhibition of pro-fibrotic gene expression. Thus, a low-dose HDACi therapy could be a safe option for the treatment of cardiac fibrosis.
The mechanisms by which HDACis block cardiac fibrosis are likely multifactorial, including direct inhibition of CF activation as well as effects on other cell types such as macrophages,91 and even Tregs. Regarding the latter possibility, HDAC inhibition leads to increased acetylation and activity of FoxP3, a master transcriptional regulator of Treg production and suppressive activity.92 Thus, it is intriguing to speculate that HDACi could be used in combination with Treg immunotherapy as a potent approach to treating cardiac fibrosis.
4.1.2. HAT inhibitors
Counterposing the action of HDACs are HATs, which are acetyl-histone ‘writers’ that catalyze the addition of acetyl-groups to nucleosomal histone tails. The most well-studied HAT in the heart is p300.93 p300 has been implicated in different cellular processes including senescence, apoptosis and cardiac disease through chromatin remodelling to regulate stress response genes94. Indeed, elevated levels of p300 and recruitment of p300 to the regulatory loci of cardiac disease-relevant genes has been observed in cardiac hypertrophy and fibrosis.94 For instance, PE-induced hypertrophy upregulated p300 which in turn acetylated the transcription factor GATA4. Acetylated GATA4 bound to and activated the promoters of endothelin-1 (ET-1) and atrial natriuretic factor (ANF) in cardiomyocytes, driving some of the genomic stress response in cardiac hypertrophy.95 The regulatory effect of p300 on GATA4 expression also includes acetylation of activating marks H3K9 and H3K27 at the promoter of GATA4,96 further demonstrating the important role of HATs in regulating cardiac disease and thus p300 a possible therapeutic target in cardiac disease.
The most common histone acetyltransferase inhibitor (HATi) studied so far in heart disease is the cell-permeable natural compound Curcumin. Curcumin is a polyphenol which gives the yellow colour of turmeric and possesses HAT inhibitory activity with specificity for p300/CBP. In vitro, curcumin repressed cardiomyocyte hypertrophic in neonatal rat cardiac myocytes treated with PE, and also inhibited the induction of ANF and slow cardiac myosin heavy chain (ß-MHC) expressions.62 Similarly, curcumin prevented the progression of HF in vivo, in the salt-sensitive Dahl (DS) hypertensive rat model, as well as improved systolic function in a rat model of MI.97,98 Treatment with curcumin also reduced the levels of apoptosis genes Bax and Caspase 3 and reduced infarct size in another rat model of MI,99 while also reducing cardiac fibrosis.100 Curcumin exerted its beneficial effect mainly through inhibition of p300 mediated histone acetyl transferase activity but also had impact on reduction of oxidative stress as well as activating the cardioprotective JAK2/STAT3 signalling pathway.99
Two other studied HATis are the potent small molecule inhibitors of p300, L002, and C646. Treatment of hypertensive mice with C646 attenuated left ventricular hypertrophy, cardiac fibrosis and DD.101 It also led to a significant improvement of ejection fraction (EF) and fractional shortening in NAD-dependent deacetylase sirtuin-3 (SIRT3) KO mice, while reversing pre-existing cardiac fibrosis in the SIRT3 KO mice.102 Similarly, treatment with L002 attenuated cardiac hypertrophy and cardiac fibrosis in rat models of hypertension-induced HF.101,103 Initial evaluation of p300 inhibition in cardiac fibrosis relied on the use of compounds with pleiotropic actions, lack of selectivity and/or low potency.104 More recent medicinal chemistry efforts have led to the development of superior p300 inhibitors, including A-485, a potent and orally bioavailable small molecule inhibitor that is highly selective for the catalytic domains of p300 and the related HAT, CREB binding protein (CBP). Other compounds, such as CBP112 and CBP30, which target the p300/CBP bromodomain and thereby prevent proper binding of the HATs to acetyl-histone marks on chromatin, provide an alternative approach for inhibiting HATs without suppressing their catalytic activity.105,106 Forthcoming studies with these new p300 inhibitors should shed further light on the roles of HATs in the control of cardiac fibrosis, and the potential of targeting activated CFs with HAT inhibitors as a tactic for blocking fibrotic remodelling of the heart.
4.1.3. Acetyl-histone reading by BRD4
4.1.3.1. Bromodomain Extraterminal Protein inhibitors
Inhibitors of the epigenetic reader Bromodomain Extraterminal protein inhibitors are another well-studied group of epidrugs showing therapeutic promise.107,108 Bromodomain-containing protein 4 (BRD4), a member of the BET family of acetyl-histone binding proteins, has emerged as a critical regulator of CF activation. It is upregulated in cardiac hypertrophy, playing a crucial role in stress response during heart disease. BRD4 is also a central regulator of the activation of CFs in cardiac pathologies through TGF-β pathway and hence inhibition of BRD4 ameliorates cardiac fibrosis.109,110
This chromatin ‘reader’ possesses tandem bromodomains, BD1 and BD2, which mediate binding to acetylated proteins, and a carboxy-terminal domain capable of activating RNA Polymerase II through the positive elongation transcription factor (P-TEFb) complex to initiate gene transcription.111,112 BRD4 inhibition therefore induces BRD4 dissociation from chromatin and abrogates transcriptional pause release during pathological cardiac hypertrophy, and thus suppressing the pathological stress–gene response.113 JQ1, an acetyl-lysine mimetic that competitively displaces BRD4 from chromatin was originally shown to suppress cardiomyocyte hypertrophy, cardiac fibrosis, and systolic dysfunction in the mouse TAC model,113,114 and was subsequently shown to be efficacious in a model of genetic dilated cardiomyopathy caused by a mutant form of phospholamban (PLNR9C).115 Delivery of JQ1 in a therapeutic mode, after cardiac remodelling had occurred, also attenuated systolic dysfunction in both the murine TAC model as well as in post-MI cardiac remodelling in mice.116 It is not known whether JQ1 is efficacious in models of DD (see Table 3).
Table 3.
Role of JQ1 in cardiac remodelling
BRD4 is further known to contribute to the formation of dynamic, cell state-specific enhancers, referred to as super-enhancers (SEs). In CFs, TGF-β signalling targets BRD4 binding to discrete SEs in a p38 kinase-dependent manner, providing a circuit for coupling extracellular cues to the cardiac epigenome to drive pro-fibrotic gene expression.110 Single-cell technologies led to the discovery of a highly regulated enhancer downstream of the gene encoding Meox1, a homeodomain-containing transcription factor whose expression was potently upregulated in CFs after TAC and suppressed by JQ1.118
Regulation of Meox1 expression in CFs involved TAC-inducible association of the Meox1 promoter with a BRD4-targeted enhancer region located >60 kb downstream. Follow-on studies with cultured CFs established a new role for Meox1 as a pro-fibrotic transcription factor in the heart. Thus, these studies uncovered a stress-inducible, BRD4-dependent, long-range chromatin interaction as an important, druggable regulator of cardiac fibrosis. In this regard, safety and efficacy studies with apabetalone, which is a weak BD2-selective BETi, have established the feasibility of targeting BRD4 to treat human cardiovascular disease.119,120 Apabetalone (known as RX-208) is a quinazolone which targets BRD4 (a chromatin reader) to inhibit its interaction with acetylated histones and has shown clinical benefits for atherosclerosis.121 RVX-208 increases the plasma levels of both ApoA-1 and HD cholesterol and inhibits pro-atherosclerotic pathways.122 A clinical trial for RVX-208 called BetonMACE was initiated in 2015 to evaluate safety profile and cardiovascular efficacy of the drug. Although Apabetalone added to standard therapy did not significantly reduce major adverse cardiovascular events after acute coronary syndrome,123 it was associated with fewer hospitalizations in patients with Type 2 diabetes and acute coronary syndrome.120,124 The clinical trial also observed an increase in HDL and a reduction in alkaline phosphatase levels.120,124 We expect that more trials targeting BRD4 are forthcoming.
The fact that inhibitors of acetyl-histone erasers, writers and readers all result in inhibition of CF activation seems paradoxical, but is likely explained by crosstalk between these epigenetic regulatory factors. One example of this is the demonstration that HDAC inhibition with ITF2357 creates new acetyl-histone marks that result in altered localization of BRD4 in the CF genome, resulting in suppression of CF activation.90
4.1.3.2. Histone methylation
Histone methyltransferase inhibitors
Lysine methyltransferases (HMTs) are a class of enzymes that mediate methylation of lysine residues on histones, an epigenetic modification relevant to myocardial fibrosis. In general, methylation at lysine 4 and 36 of histone H3 (H3K4 and H3K36) is usually associated with transcriptional activation, whereas methylation of H3K9 and H3K27 results in gene silencing.125,126 A handful of studies depict the role of HMTs in regulating cardiac pathological remodelling.62 For instance, pressure-overload cardiac stress in mice activates BRG1, a nucleosome remodeller which then recruits G9a/Glp (histone methyltransferase) and DNMT3 to assemble repressive chromatin marks (H3K9 and CpG methylation) on the promoter of Myh6 in mice. Myh6 is thence downregulated, affecting cardiac contraction and contributing to pathological cardiac remodelling.127 Similarly, the H3K9 methyltransferase SUV39H works together with heterochromatin protein 1 gamma (HP1γ) to deposit H3K9 trimethylation on the SIRT1 promoter thus leading to the downregulation of SIRT1, a gene known to confer cardioprotection.128 While studies exploring the effects of HMT inhibition/ablation specifically in the CF population are currently scarce, endothelium-specific depletion of the HMT Suv, Ez, and Trithorax domain 1 (SET1) has been shown to reduce cardiac fibrosis in a model of Ang II-induced hypertension by attenuating transcriptional activation of endothelin-1.129
A mechanistic understanding of the role of HMTs has led to therapeutic development strategies targeting HMTs. Chaetocin, an inhibitor of the H3K9 methyltransferase SUV39H, prolonged survival and restored mitochondrial dysfunction in Dahl salt-sensitive rats with HF.130 Chaetocin also attenuated cardiac damage in a mouse model of MI.128 A recent study in 2022 showed that chaetocin treatment in a mouse ApoE–/– model of atherosclerosis decreased H3K9me3 expression, diminished atherosclerotic plaque formation, and increased plaque stability by decreasing necrotic core area and lipid accumulation.131 This was achieved in part through reduced accumulation of H3K9me3 on contractile gene promoters in vascular smooth muscle cells, leading to upregulation of α-SMA and SM22α and thus promoting a contractile phenotype in these cells.131 Another histone methyltransferase inhibitor (HMTi), UNC0638 in combination with Erythropoietin also restored left ventricular EF and reduced infarct size in a mouse model of acute myocardial infarction (AMI).132 The combination also decreased apoptosis and fibrosis while promoting angiogenesis in this setting.132
Histone demethylase inhibitors
In contrast, lysine demethylases (KDMs) have clearly been directly linked to the control of CF activation. A number of histone demethylases are increased in cardiac disease, these include KDM3A, KDM3C, and KDM6B among others.53,133,134 Histone demethylases regulate cardiac hypertrophy through reduction of repressive marks such as H3K27me3 and H3K9me3, again at the regulatory regions of stress response genes including natriuretic peptides A and B, respectively (Nppa and Nppb).53,133,134 Genetic deletion of selected histone demethylases is cardioprotective in pre-clinical models of cardiac disease.53,135 With Angiotensin II-mediated cardiac hypertrophy as well as pressure-overload-induced cardiac hypertrophy, the pan KDM inhibitor JIB-04 attenuates cardiac hypertrophy and cardiac fibrosis through inhibition of KDM3A/KDM3C mediated chromatin remodelling.53,133,134,136 Similarly, inhibition of JMJD3/KDM6B using GSK-J4, suppressed isoproterenol-induced cardiac hypertrophy in mouse cardiomyocytes in vitro and in vivo.137
Global deletion of the Jumonji C domain-containing K3K9-specific demethylase, KDM3a, blocked TAC-induced cardiac fibrosis in mice, as did treatment with the demethylase inhibitor, JIB-04.138 Transgenic (TG) overexpression of KDM3a in cardiomyocytes was sufficient to trigger fibrotic remodelling of the heart, suggesting that this demethylase mediates pro-fibrotic crosstalk between myocytes and fibroblasts. Thus, it remains unknown whether KDM3a serves a direct function within CFs to promote expression of ECM-encoding genes. H3K4/9-specific lysine specific demethylase 1 (LSD1/KDM1) is an FAD-dependent monoamine oxidase that was shown to be increased in expression in human dilated cardiomyopathy as well as in murine CFs following TAC.135 Knockdown or pharmacological inhibition of LSD1 in cultured CFs attenuated Ang II-mediated activation of the cells, and deletion of the demethylase in activated CFs in mice, using a periostin-MerCreMer driver, reduced cardiac fibrosis and improved function of the heart in mice subjected to TAC in a manner that correlated with suppression of pro-fibrotic TGF-β signalling. The long-standing antidepressant drug, tranylcypromine, which is a monoamine oxidase inhibitor, has been shown to be an effective LSD1 inhibitor, and a variety of additional compounds that target this demethylase are in clinical development.139 Thus, it might be possible to eventually repurpose LSD1 inhibitors for the treatment of cardiac fibrosis.
4.1.4. DNA methylation
4.1.4.1. DNA methyltransferase inhibitors
Mouse and rat models have demonstrated the utility of different DNA methyltransferase inhibitors (DNMTis) to attenuate cardiac hypertrophy and fibrosis.62 For instance, the DNMTi 5-aza-C attenuates cardiac remodelling and cardiac fibrosis in spontaneously hypertensive rats as well as rats receiving a chronic infusion of hypertension-inducing norepinephrine.140,141 Similarly, administration of 5-aza-C reduced cardiac fibrosis and improved arrhythmia in the left ventricle of spontaneously hypertensive rats.142 Treatment with other DNMTis including RG108 and methylene disalicylic acid (MDSA) also attenuated cardiac hypertrophy in a rat model of pressure overload both in vivo,143 and in vitro using engineered heart tissue from rat cardiomyocytes.144 Similar to the DNMTi, genomic deletion of Dnmt1 has also been associated with protection against pathological cardiac remodelling in a rat model of pressure overload, and Adriamycin-induced cardiomyopathy.145 DNMTis are expected to act by modifying the cytosine methylation profiles at loci of stress response genes. 5-aza-C is incorporated into the genomic DNA, thence its clinical utility may be more limited compared with small molecule DNMTi such as RG108. Besides molecular approaches, dietary polyphenols such as tea polyphenols (catechins, epigallocatechin gallate) and bioflavonoids also possess DNMTi properties.62,146 EGCG, a tea polyphenol, has been widely studied in the context of heart disease, with studies showing it attenuates myocardial reperfusion injury and cardiac hypertrophy through different mechanisms including DNA methylation inhibition and modulation of MAP kinase pathway.147–149 While we focus here on DNA methylation, it is important to note that methylation of RNA has emerged as a critical regulator of cardiac homeostasis and remodelling in recent years.150
5. Non-coding RNAs for treatment of cardiac remodelling and HF
In a wider sense non-coding RNAs (ncRNAs) as drug targets can also be viewed as ‘epidrugs’. ncRNAs are generally classified based on their size in small (<200 nucleotides) and long (>200 nucleotides) ncRNAs.151 Whereas miRNAs as small ncRNAs mostly regulate gene expression post-transcriptionally via binding to the 3´ untranslated regions of target mRNA,152,153 long ncRNAs can function as indirect modulators of DNA/histone complexes by recruiting or preventing binding of epigenetic factors, trigger chromatin remodelling and influence post-transcriptional and translational pathways.154–156 Recently circular RNAs as the novel class of long ncRNAs were identified.157,158 This type of RNAs is characterized by covalently linked ends to form a closed circle and can function as miRNA sponges but also modulate transcription, translation and splicing of RNAs.158–160
Mode of action of ncRNAs includes targeting of often rather complete signalling pathways, in contrast to targeting single mRNA/protein targets as conventional (protein-based) drugs do. Thus, ncRNAs have the potential for outperforming conventional drugs because of their wide mode of action especially in rather complex diseases such as HF where multiple genes and pathways are de-regulated.161
Indeed oligonucleotide-based therapies are currently gaining attention as a new treatment option for relatively rare as well as common diseases such as cardiovascular disease. Here, one of the first ncRNAs demonstrating anti-fibrotic properties in the remodelling heart was microRNA-21 (miR-21).162,163 Interestingly, the small non-coding RNA miR-21 is de-regulated in various heart and kidney diseases and has been repeatedly suggested as a therapeutic target. Impressive pre-clinical results have been achieved by an antisense oligonucleotide-based therapy to effectively block the pro-fibrotic traits of miR-21 in small and large animals.164 Since miRNA-mediated pathways are generally very well-conserved, there is considerable commercial interest with regards to clinical translation. Excitingly, the approach to silence miR-21 as an anti-fibrotic therapy was taken up by the pharmaceutical industry and currently there is a clinical Phase 2 study running in patients with Alport syndrome, a chronic kidney disease with a high amount of fibrosis (reviewed in165). There are further multiple ncRNAs including miRNAs, lncRNAs, and circular RNAs, that have been identified as potential targets in HF. NcRNAs involved in cardiac hypertrophy, fibrosis and remodelling processes in HF are extensively reviewed in.155,159,166–170 Some RNA therapies involving miRNAs in cardiovascular diseases coming close to clinical application and summarized in Huang et al.165, but only few have been translated so far to clinical studies. Thus, only those are described here in more detail.
6. Inhibition of miR-92a improves vascularization in cardiac ischaemia and infarction
The conserved miR-17–92 cluster consists of miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a.171 Besides the cluster members’ role in tumor onco- and angiogenesis and regulation of haematopoiesis and immune functions,172 miR-92a gained further attention as an endogenous repressor of the angiogenic programme in ECs involved in adverse remodelling in cardiovascular system.173 Bonauer et al. showed that miR-92a is expressed in human ECs and its forced overexpression in vitro blocks sprout formation in a 3D-model of angiogenesis, inhibits vascular network formation in matrigel assays, and reduces EC migration and impairs EC adhesion to fibronectin.174 Implantation of human umbilical vein endothelial cells (HUVECs) in a matrigel plug into a nude mouse in vivo and subsequential activation of miR-92a reduced the number of invading cells and formation of lectin-positive vessels, indicating that overexpression of miR-92a blocks angiogenesis and vessel formation in vitro and in vivo. Inhibition of miR-92a with specific inhibitors in vitro and in vivo led to the stimulation of vascularization. In the pathological context in a mouse hind-limb ischaemia model, antagomir-92a promoted neovascularization and improved perfusion of the ischaemic limb. Interestingly miR-92a was also upregulated after induction of MI (AMI, via left anterior descending artery occlusion) in mice and intravenous injection of antagomir-92a vs. control antagomir improved systolic and diastolic function of the damaged heart and reduced infarct size. As a directly regulated target for miRNA-92a among others, the integrin subunit α5 (ITGA5) could be confirmed and shown to at least partially improve sprout formation in human ECs.174
In a porcine model of ischaemia and reperfusion, LNA-92a inhibitor (5 mg/kg) was applied via different administration routes, e.g. catheter-based delivery and systemically (intravenous).175 miR-92a expression in heart tissue after 72 h was significantly reduced and regional administration of LNA-92a reduced infarct size and improved EF and left ventricular end-diastolic pressure of the myocardium in LNA-92a- but not in LNA-control injected pigs. Histologically, LNA-92a increased capillary density and reduced inflammation and cell death.175 Bellera et al.176 further showed that a single intracoronary injection of antagomir-92a encapsulated in specific microspheres reduced regional ventricular wall dysfunction and prevented deleterious adverse remodelling of myocardium one month after AMI. More recently, single-cell sequencing of isolated mouse ECs, cardiomyocytes, fibroblasts, and CD45+ haematopoietic cells after AMI and subsequent LNA-92a treatment pointed towards activation of EC autophagy and metabolic-switching processes in cardiomyocytes which could be beneficial in reducing tissue damage and remodelling of the myocardium after AMI.177 Based on the promising therapeutic potential of miRNA-92a inhibition in adverse cardiac remodelling, the first study in humans was conducted.178 This study demonstrated efficiency and target derepression with MRG-110, a LNA-92a-3p inhibitor. Healthy adults received a single intravenous dose of MRG-110 ranging from 0.01 to 1.5 mg/kg body weight or placebo. miR-92a expression in whole blood, CD31+-cells comprising monocytes and circulating ECs, and in circulating extracellular vesicles was efficiently reduced in a dose- and time-dependent manner. miR-92a target gene expressions of ITGA5 and CD93 increased upon LNA-92a treatment, demonstrating efficiency of LNA-92a application in humans and paving the way to further clinical translation (Figure 2).
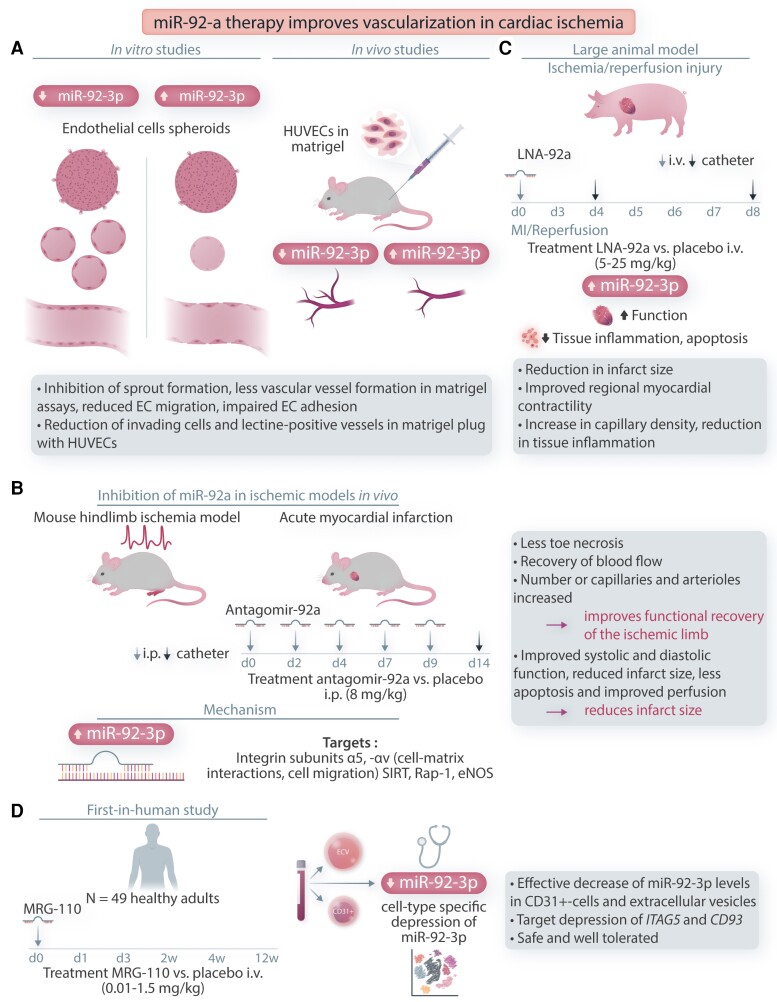
Figure 2.
miR-92a therapy improves vascularization in cardiac ischaemia. A: In-vitro miR-92a overexpression inhibits spout formation in human EC spheroids, vascular network formation, and EC migration. Implantation of HUVECs in matrigel plug in vivo inhibits vessel formation. B: Inhibition of miR-92a with antagomir-92ain ischaemic models in vivo in mouse hind-limb ischaemia model improves perfusion. Inhibition after AMI improves perfusion and reduces infarct size. Treatment regime for antagomir-92a injections in days (d) is indicated. Proposed targets of miR-92a modulation are shown. C: Large animal model of ischaemia/reperfusion for LNA-92a treatment is shown. Treatment regime is indicated in hours (h) or days (d). D. First-in-human study for MRG-110 is illustrated. Treatment regime in hours (h), days (d), and weeks (w) is indicated. ITGA5, integrin subunit α5; SIRT, sirtuin; Rap1, Ras-related protein-1; eNOS, endothelial nitric oxide synthase 3; ECV, extracellular vesicle.
7. Inhibition of miR-132 improves cardiac function and counteracts adverse remodelling
miR-132 arises from the highly conserved miR-132/212 gene cluster and has been shown to play pivotal roles in pathological processes of fibrosis, hypertrophy, apoptosis and angiogenesis in the development of HF.179 To screen for pro-hypertrophic miRNAs, Ucar et al.180 transfected a precursor-miRNA library into neonatal rat cardiomyocytes and determined cardiomyocyte size and brain natriuretic peptide (BNP) secretion. Overexpression of miR-212/132 in vitro and in vivo in mice induced pronounced cardiac hypertrophy. Mechanistically the anti-hypertrophic and pro-autophagic transcription factor Forkhead Box Protein O3 (FoxO3) was shown to be a direct target of miR-212/132. The results indicated that upregulation of miR-212/132 leads to repression of FoxO3 with pro-hypertrophic and anti-autophagic pathway activation resulting in pathological heart growth.180
Foinquinos et al.181 further investigated the mode of action of an optimized synthetic locked nucleic acid mixmer antisense oligonucleotide with fully phosphorylated backbone (antimiR-132) in TG mice overexpressing miR-212/132 cluster. TG animals developed a severe HF phenotype with massive cardiac enlargement at 6 weeks of age, which could be fully prevented by intraperitoneal antimiR-132 injections (20 mg/kg weekly for 4 weeks) leading to reduced miR-132 and increased FoxO3 expression levels in cardiac tissue. At the single-cell level antimiR-132 treatment effectively reduced AP prolongation, calcium transients and contraction of cardiomyocytes isolated from TG mice. Downregulation of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA2a), a predicted direct target of miR-132,182,183 was partially reversed upon treatment. Most importantly, administration of antimiR-132 (at Day 3 and Day 28) in a highly clinically relevant model of HF after AMI in pigs led to improved EF and attenuation of end systolic volume (ESV) as compared with animals treated with anti-miR-132 control. Histological analysis showed a reduction in interstitial fibrosis. No drug-associated adverse effects of treatment occurred.181 Concordant with these findings, suppression of miR-212/132 using engineered circular RNA sponges also showed attenuation of hypertrophy, preservation of cardiac function, and therefore the potential utility of circRNAs as novel therapeutic agents against bono fide miRNA targets.184
Recently, effective reduction of adverse remodelling was confirmed in a pig model of chronic HF after treatment with a specific antisense oligonucleotide against miR-132 (CDR132L).185 Administration of CDR132L efficiently reduced functional levels of miR-132 in the cardiac tissue and in plasma and improved systolic and diastolic cardiac function. At the tissue level CDR132L led to a reduction in fibrosis and cardiomyocyte hypertrophy. Molecular analysis of treated vs. sham untreated cardiac tissue indicated alterations in genes involved in ECM biology (CD44, ECM protein 1, GATA binding protein 3), cardiac hypertrophy (reticulon 4, leukaemia inhibitory factor receptor alpha) and vascular function (bone morphogenic protein receptor Type II, adrenoceptor alpha 1D) upon CDR132L treatment. No therapy-related adverse effects were detected, the monthly CDR132L treatment was efficient, safe and well tolerated.185
Based on these results, a Phase 1b clinical trial with CDR132L in 28 patients with stable HF of ischaemic origin was conducted and recently completed with encouraging results.186 Study treatment was given intravenously twice on days 1 and 28 (0.32, 1, 3, or 10 mg/kg body weight). Circulating plasma levels of miRNA-132 decreased dose-dependently. A combined endpoint of >10% relative NT-proBNP level decrease and/or >2% absolute increase in LVEF from baseline to Day 112 was met in 79% individuals of treated group vs. 46% individuals of non-treated group. Additional fibrosis- and tissue injury-related biomarkers (galectin-3, suppression of tumorigenicity 2 factor, neutrophil gelatinase-associated lipocalin, and matrix metalloproteinase-1) showed a modest decrease, indicating amelioration of disease phenotype. Interestingly QRS complex width was significantly reduced between baseline and study endpoint in patients receiving CDR132L, possibly indicating reduction of AP prolongation and faster conduction of electrical propagation in the heart. CDR132L was well tolerated and no drug-related adverse effects were recorded, encouraging further clinical development of the substance.186 For review on mechanisms and effects of miR-132 manipulations in small and large animals and in humans, refer Figure 3. Recently, antimiR-132 treatment was also shown to improve global cardiac function and to have anti-fibrotic and anti-hypertrophic effects in a pig model of non-ischaemic percutaneous aortic constriction, suggesting that CDR132L could possibly also be used for patients with HF of non-ischaemic origin.187
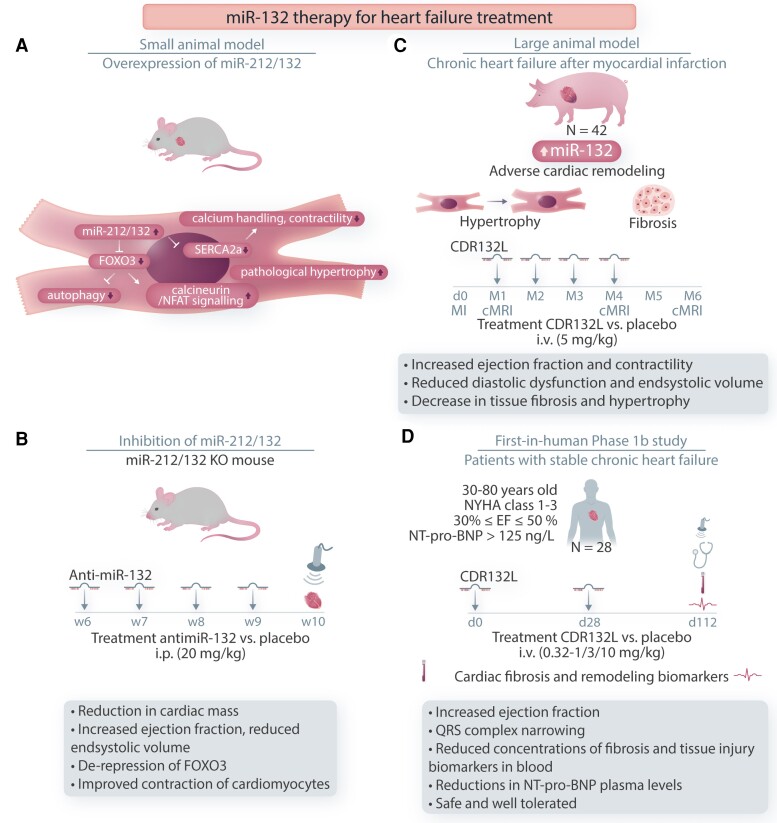
Figure 3.
Role of miR-132 in the cardiac remodelling process. Mechanisms of miR-132 in the cardiac remodelling process and development of anti-miR-132-based therapeutic strategies in mice, pigs, and humans with heart failure. A: Mice model with miR-212/132 overexpression. The proposed mechanism of miR-132 action. B: Inhibition of miR-212/132 in TG mice. Treatment regime in weeks (w) with anti-miR-132 is indicated. C: Large animal model of chronical heart failure after MI. Treatment regime in months (M) with CDR132L is indicated. D: First-in-human Phase 1b study in patients with stable chronic heart failure is illustrated. Inclusion criteria are given. Treatment regime in days (d) with CDR132L is indicated. FoxO3, Forkhead Box Protein O3; SERCA2, sarcoplasmic/endoplasmic reticulum calcium ATPase 2; NFAT, nuclear factor of activated T cells.
Overall the results of the conducted studies so far are very promising in terms of improvement of cardiac function, reduction in adverse remodelling processes and safety and tolerability of CDR132L in patients with subacute and/or chronic HF.
8. Limitations and future directions of ncRNA-therapies
ncRNA-therapies appear attractive to combat cardiovascular diseases in the future due to their capacity to regulate complete signalling pathways rather than only single targets. In the future tissue-specific delivery systems will allow more selective delivery of RNA-based drugs to individual organs and cell types. Cardio-specific nanoparticle formulations for specific delivery of miRNA modulators and novel viral vector variants with high cardio-specificity and less immunogenicity188 could improve targeted delivery of the RNA therapies to the heart cells and prevent adverse effects in other tissue.167 Recent developments of circular RNA sponges for sequestration of specific pathologic miRNAs present further novel therapeutic agents against bono fide miRNA targets and could help to improve dosage regimes and to extend half-lives of the RNA therapies.184 Additionally since many studies are performed in small rodents, where the mode of action compared with human cells could be different, translation of the results to human physiology hampers translational efforts. Therefore cardiovascular studies in large mammals or human tissue directly could further improve clinical translation of RNA therapies. Recently, SarcTrack and CalTrack methods for high-throughput analysis of contractility and calcium transients in human stem cell-derived cardiomyocyte were established.189,190 Such platforms enable preselection of candidates with significant impact on physiological function of the human cardiomyocytes. Further translational models like human living myocardial slices cultivated for weeks to months ex vivo,191–193 enable testing of promising ncRNA-candidates directly in human multicellular model with disease-specific phenotype and to ensure high efficiency and specificity and lack of toxicity of the novel RNA-molecules in patient’s myocardium directly.
9. Concluding remarks
Epidrugs represent a viable therapeutic approach for HF given the widespread epigenetic modifications that we have come to learn to underpin the manifestations and disease sequalae of HF. Although a number of pre-clinical studies have demonstrated therapeutic potential of some molecules, clinical studies are still needed to evaluate safety profiles, dosage, modes of delivery and other pharmacodynamic and pharmacokinetic qualities of the drugs. How do the myriad epigenetic modifications, often at the same genetic loci, present themselves as therapeutic targets at the same time? Is there a hierarchy to achieving the most optimal therapy, or are there different contexts where different targets are more relevant? An additional challenge with many epidrugs is the non-specific, genome-wide effect they have on chromatin remodellers which may lead to side effects in non-target organs, thus limiting their usage. They may indeed serve as co-adjuvant therapies taken together with other HF medications. Future research will likely be geared towards targeted delivery of the drugs to cardiomyocytes or achieving loci-specificity, thus enabling drugs to achieve local rather than global effects. ncRNAs that address key hallmarks of the cardiac remodelling process, such as anti-miR-21 in fibrosis, miR-92a in angiogenesis and miR-132 in pathological hypertrophy, may be used in the future for a more mechanistically orientated drug treatment of HF.
The vast majority of the multitude of epigenetic regulatory proteins and chromatin modifications have yet to be assessed for their roles in the control of cardiac fibrosis and cardiac remodelling. Thus, additional avenues for developing epigenetic therapies to target remodelling of the heart are likely on the horizon.
Contributor Information
Timothy A McKinsey, Department of Medicine, Division of Cardiology, and Consortium for Fibrosis Research & Translation, University of Colorado Anschutz Medical Campus, 12700 E.19th Ave, Aurora, CO, 80045-2507, USA.
Roger Foo, NUHS Cardiovascular Disease Translational Research Programme, NUS Yong Loo Lin School of Medicine, 14 Medical Drive, Level 8, 117599 Singapore, Singapore; Cardiovascular Research Institute, National University Heart Centre, 14 Medical Drive, Level 8, 117599 Singapore, Singapore.
Chukwuemeka George Anene-Nzelu, NUHS Cardiovascular Disease Translational Research Programme, NUS Yong Loo Lin School of Medicine, 14 Medical Drive, Level 8, 117599 Singapore, Singapore; Cardiovascular Research Institute, National University Heart Centre, 14 Medical Drive, Level 8, 117599 Singapore, Singapore; Montreal Heart Institute, 5000 Rue Belanger, H1T 1C8, Montreal, Canada.
Joshua G Travers, Department of Medicine, Division of Cardiology, and Consortium for Fibrosis Research & Translation, University of Colorado Anschutz Medical Campus, 12700 E.19th Ave, Aurora, CO, 80045-2507, USA.
Ronald J Vagnozzi, Department of Medicine, Division of Cardiology, and Consortium for Fibrosis Research & Translation, University of Colorado Anschutz Medical Campus, 12700 E.19th Ave, Aurora, CO, 80045-2507, USA.
Natalie Weber, Institute of Molecular and Translational Therapeutic Strategies (IMTTS), Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany.
Thomas Thum, Institute of Molecular and Translational Therapeutic Strategies (IMTTS), Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany; REBIRTH Center for Translational Regenerative Therapies, Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany; Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Straße 1, 30625 Hannover, Germany.
Funding
This work was supported by the National Institutes of Health (NIH) by grants HL116848, HL147558, DK119594, HL127240, and HL150225 to T.A.M. J.G.T. was supported by the NIH by grant HL147463. R.J.V. was supported by the American Heart Association (AHA) by grant 19CDA34670044. Parts of that work were additionally funded by the ERC Advanced Grant REVERSE (to T.T.) and the DFG TRR267 (to T.T.) and CRC1470 (to T.T.). R.F. and C.G.A.-N. were supported by grants from the Singapore National Medical Research Council and Biomedical Research Council. Figures 2 and 3 were created with BioRender.com.
References
- 1. van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 2010;7:30–37. [DOI] [PubMed] [Google Scholar]
- 2. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Jong S, van Veen TA, van Rijen HV, de Bakker JM. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 2011;57:630–638. [DOI] [PubMed] [Google Scholar]
- 4. Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens 2007; 9:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Junttila MJ, Holmström L, Pylkäs K, Mantere T, Kaikkonen K, Porvari K, Kortelainen ML, Pakanen L, Kerkelä R, Myerburg RJ, Huikuri HV. Primary myocardial fibrosis as an alternative phenotype pathway of inherited cardiac structural disorders. Circulation 2018;137:2716–2726. [DOI] [PubMed] [Google Scholar]
- 6. Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac fibrosis and arrhythmogenesis. Compr Physiol 2017;7:1009–1049. [DOI] [PubMed] [Google Scholar]
- 8. Maron BJ, Maron MS. The remarkable 50 years of imaging in HCM and how it has changed diagnosis and management: from M-mode echocardiography to CMR. JACC Cardiovasc Imaging 2016;9:858–872. [DOI] [PubMed] [Google Scholar]
- 9. Lewis GA, Dodd S, Clayton D, Bedson E, Eccleson H, Schelbert EB, Naish JH, Jimenez BD, Williams SG, Cunnington C, Ahmed FZ, Cooper A, Rajavarma V, Russell S, McDonagh T, Williamson PR, Miller CA. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat Med 2021;27:1477–1482. [DOI] [PubMed] [Google Scholar]
- 10. Schimmel K, Jung M, Foinquinos A, José GS, Beaumont J, Bock K, Grote-Levi L, Xiao K, Bär C, Pfanne A, Just A, Zimmer K, Ngoy S, López B, Ravassa S, Samolovac S, Janssen-Peters H, Remke J, Scherf K, Dangwal S, Piccoli MT, Kleemiss F, Kreutzer FP, Kenneweg F, Leonardy J, Hobuß L, Santer L, Do QT, Geffers R, Braesen JH, Schmitz J, Brandenberger C, Müller DN, Wilck N, Kaever V, Bähre H, Batkai S, Fiedler J, Alexander KM, Wertheim BM, Fisch S, Liao R, Diez J, González A, Thum T. Natural compound library screening identifies new molecules for the treatment of cardiac fibrosis and diastolic dysfunction. Circulation 2020;141:751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kreutzer FP, Meinecke A, Mitzka S, Hunkler HJ, Hobuß L, Abbas N, Geffers R, Weusthoff J, Xiao K, Jonigk DD, Fiedler J, Thum T. Development and characterization of anti-fibrotic natural compound similars with improved effectivity. Basic Res Cardiol 2022;117:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res 2016;118:1021–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Pure E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature 2019;573:430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher C-A, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature 2019;573:430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, Kimura T, Soliman OY, Papp TE, Tam YK, Mui BL, Albelda SM, Puré E, June CH, Aghajanian H, Weissman D, Parhiz H, Epstein JA. CAR T cells produced in vivo to treat cardiac injury. Science 2022;375:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail 2015;8:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theall B, Alcaide P. The heart under pressure: immune cells in fibrotic remodeling. Curr Opin Physiol 2022;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammer A, Sulzgruber P, Koller L, Kazem N, Hofer F, Richter B, Blum S, Hulsmann M, Wojta J, Niessner A. The prognostic impact of circulating regulatory T lymphocytes on mortality in patients with ischemic heart failure with reduced ejection fraction. Mediators Inflamm 2020;2020:6079713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okamoto N, Noma T, Ishihara Y, Miyauchi Y, Takabatake W, Oomizu S, Yamaoka G, Ishizawa M, Namba T, Murakami K, Iwado Y, Ohmori K, Kohno M. Prognostic value of circulating regulatory T cells for worsening heart failure in heart failure patients with reduced ejection fraction. Int Heart J 2014;55:271–277. [DOI] [PubMed] [Google Scholar]
- 20. Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, Cheng Y, Cheng X. Defective circulating CD4CD25 + Foxp3 + CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem 2010;25:451–458. [DOI] [PubMed] [Google Scholar]
- 21. Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009;119:2904–2912. [DOI] [PubMed] [Google Scholar]
- 22. Kanellakis P, Dinh TN, Agrotis A, Bobik A. CD4(+)CD25(+)Foxp3(+) Regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens 2011;29:1820–1828. [DOI] [PubMed] [Google Scholar]
- 23. Wang H, Hou L, Kwak D, Fassett J, Xu X, Chen A, Chen W, Blazar BR, Xu Y, Hall JL, Ge JB, Bache RJ, Chen Y. Increasing regulatory T cells with interleukin-2 and interleukin-2 antibody complexes attenuates lung inflammation and heart failure progression. Hypertension 2016;68:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2019;139:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramjee V, Li D, Manderfield LJ, Liu F, Engleka KA, Aghajanian H, Rodell CB, Lu W, Ho V, Wang T, Li L, Singh A, Cibi DM, Burdick JA, Singh MK, Jain R, Epstein JA. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J Clin Invest 2017;127:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 2012;107:232. [DOI] [PubMed] [Google Scholar]
- 27. Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014;115:55–67. [DOI] [PubMed] [Google Scholar]
- 28. Raffin C, Vo LT, Bluestone JA. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020;20:158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng G, Bajpai G, Ma P, Koenig A, Bredemeyer A, Lokshina I, Lai L, Forster I, Leuschner F, Kreisel D, Lavine KJ. CCL17 Aggravates myocardial injury by suppressing recruitment of regulatory T cells. Circulation 2022;145:765–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhee AJ, Lavine KJ. New approaches to target inflammation in heart failure: harnessing insights from studies of immune cell diversity. Annu Rev Physiol 2020;82:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Revelo XS, Parthiban P, Chen C, Barrow F, Fredrickson G, Wang H, Yucel D, Herman A, van Berlo JH. Cardiac resident macrophages prevent fibrosis and stimulate angiogenesis. Circ Res 2021;129:1086–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falkenham A, de Antueno R, Rosin N, Betsch D, Lee TD, Duncan R, Legare JF. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am J Pathol 2015;185:927–942. [DOI] [PubMed] [Google Scholar]
- 34. Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 2011;29:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim AJ, Xu N, Umeyama K, Hulin A, Ponny SR, Vagnozzi RJ, Green EA, Hanson P, McManus BM, Nagashima H, Yutzey KE. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in marfan syndrome. Circulation 2020;141:132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One 2012;7:e39039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh A, Chakraborty S, Wong SW, Hefner NA, Stuart A, Qadir AS, Mukhopadhyay A, Bachmaier K, Shin JW, Rehman J, Malik AB. Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis. Proc Natl Acad Sci U S A 2022;119:e2121098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buechler MB, Fu W, Turley SJ. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 2021;54:903–915. [DOI] [PubMed] [Google Scholar]
- 40. Bageghni SA, Hemmings KE, Yuldasheva NY, Maqbool A, Gamboa-Esteves FO, Humphreys NE, Jackson MS, Denton CP, Francis S, Porter KE, Ainscough JF, Pinteaux E, Drinkhill MJ, Turner NA. Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight 2019;5:e125074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramanujam D, Schon AP, Beck C, Vaccarello P, Felician G, Dueck A, Esfandyari D, Meister G, Meitinger T, Schulz C, Engelhardt S. MicroRNA-21-Dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circulation 2021;143:1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Francisco J, Zhang Y, Nakada Y, Jeong JI, Huang CY, Ivessa A, Oka S, Babu GJ, Del Re DP. AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis. Sci Rep 2021;11:10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mia MM, Cibi DM, Binte Abdul Ghani SA, Singh A, Tee N, Sivakumar V, Bogireddi H, Cook SA, Mao J, Singh MK. Loss of yap/taz in cardiac fibroblasts attenuates adverse remodeling and improves cardiac function. Cardiovasc Res 2021;118:1785-1804. [DOI] [PubMed] [Google Scholar]
- 44. Mia MM, Cibi DM, Abdul Ghani SAB, Song W, Tee N, Ghosh S, Mao J, Olson EN, Singh MK. YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLoS Biol 2020;18:e3000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simoes FC, Cahill TJ, Kenyon A, Gavriouchkina D, Vieira JM, Sun X, Pezzolla D, Ravaud C, Masmanian E, Weinberger M, Mayes S, Lemieux ME, Barnette DN, Gunadasa-Rohling M, Williams RM, Greaves DR, Trinh LA, Fraser SE, Dallas SL, Choudhury RP, Sauka-Spengler T, Riley PR. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun 2020;11:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bevan L, Lim ZW, Venkatesh B, Riley PR, Martin P, Richardson RJ. Specific macrophage populations promote both cardiac scar deposition and subsequent resolution in adult zebrafish. Cardiovasc Res 2020;116:1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riley HJ, Kelly RR, Van Laer AO, Neff LS, Dasgupta S, Baicu CF, McDonald LT, LaRue AC, Zile MR, Bradshaw AD. SPARC Production by bone marrow-derived cells contributes to myocardial fibrosis in pressure overload. Am J Physiol Heart Circ Physiol 2021;320:H604–H612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McDonald LT, Zile MR, Zhang Y, Van Laer AO, Baicu CF, Stroud RE, Jones JA, LaRue AC, Bradshaw AD. Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis. Am J Physiol Heart Circ Physiol 2018;315:H92–H100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shahneh F, Grill A, Klein M, Frauhammer F, Bopp T, Schafer K, Raker VK, Becker C. Specialized regulatory T cells control venous blood clot resolution through SPARC. Blood 2021;137:1517–1526. [DOI] [PubMed] [Google Scholar]
- 50. Ferrari I, Vagnozzi RJ. Mechanisms and strategies for a therapeutic cardiac immune response. J Mol Cell Cardiol 2021;158:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lantz C, Becker A, Thorp EB. Can polarization of macrophage metabolism enhance cardiac regeneration? J Mol Cell Cardiol 2021;160:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Costantino S, Libby P, Kishore R, Tardif JC, El-Osta A, Paneni F. Epigenetics and precision medicine in cardiovascular patients: from basic concepts to the clinical arena. Eur Heart J 2018;39:4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang W, Song M, Qu J, Liu GH. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018;123:773–786. [DOI] [PubMed] [Google Scholar]
- 54. Gilsbach R, Schwaderer M, Preissl S, Grüning BA, Kranzhöfer D, Schneider P, Nührenberg TG, Mulero-Navarro S, Weichenhan D, Braun C, Dreßen M, Jacobs AR, Lahm H, Doenst T, Backofen R, Krane M, Gelb BD, Hein L. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat Commun 2018;9:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tan WLW, Anene-Nzelu CG, Wong E, Lee CJM, Tan HS, Tang SJ, Perrin A, Wu KX, Zheng W, Ashburn RJ, Pan B, Lee MY, Autio MI, Morley MP, Tam WL, Cheung C, Margulies KB, Chen L, Cappola TP, Loh M, Chambers J, Prabhakar S, Foo RSY. Epigenomes of human hearts reveal new genetic variants relevant for cardiac disease and phenotype. Circ Res 2020;127:761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gilsbach R, Preissl S, Grüning BA, Schnick T, Burger L, Benes V, Würch A, Bönisch U, Günther S, Backofen R, Fleischmann BK, Schübeler D, Hein L. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun 2014;5:5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, Siggens L, Vujic A, Simeoni I, Penkett C, Goddard M, Lio P, Bennett MR, Foo RS. Distinct epigenomic features in end-stage failing human hearts. Circulation 2011;124:2411–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee DP, Tan WLW, Anene-Nzelu CG, Lee CJM, Li PY, Luu TDA, Chan CX, Tiang Z, Ng SL, Huang X, Efthymios M, Autio MI, Jiang J, Fullwood MJ, Prabhakar S, Lieberman Aiden E, Foo RS. Robust CTCF-based chromatin architecture underpins epigenetic changes in the heart failure stress-gene response. Circulation 2019;139:1937–1956. [DOI] [PubMed] [Google Scholar]
- 59. Rosa-Garrido M, Chapski DJ, Schmitt AD, Kimball TH, Karbassi E, Monte E, Balderas E, Pellegrini M, Shih TT, Soehalim E, Liem D, Ping P, Galjart NJ, Ren S, Wang Y, Ren B, Vondriska TM. High-resolution mapping of chromatin conformation in cardiac myocytes reveals structural remodeling of the epigenome in heart failure. Circulation 2017;136:1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anene-Nzelu CG, Lee MCJ, Tan WLW, Dashi A, Foo RSY. Genomic enhancers in cardiac development and disease. Nat Rev Cardiol 2022;19:7–25. [DOI] [PubMed] [Google Scholar]
- 61. Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs 2010;19:1049–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghosh AK, Vaughan DE. Chapter 20 - epigenetic treatment approaches to cardiovascular disease. In Tollefsbol TO (eds). Epigenetics in human disease. 2nd ed. Academic Press; 2018. p607–641. [Google Scholar]
- 63. Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 2002;110:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. Cam kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 2006;116:1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res 2006;98:15–24. [DOI] [PubMed] [Google Scholar]
- 66. McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 2012;52:303–319. [DOI] [PubMed] [Google Scholar]
- 67. Hsu A, Duan Q, McMahon S, Huang Y, Wood SA, Gray NS, Wang B, Bruneau BG, Haldar SM. Salt-inducible kinase 1 maintains HDAC7 stability to promote pathologic cardiac remodeling. J Clin Invest 2020;130:2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ke X, Lin Z, Ye Z, Leng M, Chen B, Jiang C, Jiang X and Li G. Histone deacetylases in the pathogenesis of diabetic cardiomyopathy. Front Endocrinol 2021;12:679655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kee HJ, Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J Biomed Biotechnol 2011;2011:928326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 2007;21:1790–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 2007;13:324–331. [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Wei X, Pang Q, Yi F. Histone deacetylases and their inhibitors: molecular mechanisms and therapeutic implications in dibetes mellitus. Acta Pharmaceutica Sinica B 2012; 2:387–395. [Google Scholar]
- 73. Bagchi RA, Robinson EL, Hu T, Cao J, Hong JY, Tharp CA, Qasim H, Gavin KM, da Silva JP, Major JL, McConnell BK, Seto E, Lin H, McKinsey TA. Reversible lysine fatty acylation of an anchoring protein mediates adipocyte adrenergic signaling. Proc Natl Acad Sci U S A 2022;119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cao J, Sun L, Aramsangtienchai P, Spiegelman NA, Zhang X, Huang W, Seto E, Lin H. HDAC11 Regulates type I interferon signaling through defatty-acylation of SHMT2. Proc Natl Acad Sci U S A 2019;116:5487–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gillette TG, Hill JA. Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ Res 2015;116:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, Stout AL, Epstein JA, Patel VV. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 2008;45:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 2006;113:2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ooi JY, Tuano NK, Rafehi H, Gao XM, Ziemann M, Du XJ, El-Osta A. HDAC Inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics 2015;10:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A 2011;108:4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khurana I, Maxwell S, Royce S, Mathiyalagan P, Karagiannis T, Mazarakis N, Vongsvivut J KNH, Okabe J, Al-Hasani K, Samuel C, El-Osta A. SAHA Attenuates takotsubo-like myocardial injury by targeting an epigenetic ac/dc axis. Signal Transduct Target Ther 2021;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wallner M, Eaton DM, Berretta RM, Liesinger L, Schittmayer M, Gindlhuber J, Wu J, Jeong MY, Lin YH, Borghetti G, Baker ST, Zhao H, Pfleger J, Blass S, Rainer PP, von Lewinski D, Bugger H, Mohsin S, Graier WF, Zirlik A, McKinsey TA, Birner-Gruenberger R, Wolfson MR, Houser SR. HDAC Inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci Transl Med 2020;12:eaay7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 2014;129:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 2006;113:51–59. [DOI] [PubMed] [Google Scholar]
- 84. Ma J, Luo T, Zeng Z, Fu H, Asano Y, Liao Y, Minamino T, Kitakaze M. Histone deacetylase inhibitor phenylbutyrate exaggerates heart failure in pressure overloaded mice independently of HDAC inhibition. Sci Rep 2016;6:34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein hop. J Clin Invest 2003;112:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Colussi C, Berni R, Rosati J, Straino S, Vitale S, Spallotta F, Baruffi S, Bocchi L, Delucchi F, Rossi S, Savi M, Rotili D, Quaini F, Macchi E, Stilli D, Musso E, Mai A, Gaetano C, Capogrossi MC. The histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces cardiac arrhythmias in dystrophic mice. Cardiovasc Res 2010;87:73–82. [DOI] [PubMed] [Google Scholar]
- 87. Nagata S, Marunouchi T, Tanonaka K. Histone deacetylase inhibitor SAHA treatment prevents the development of heart failure after myocardial infarction via an induction of heat-shock proteins in rats. Biol Pharm Bull 2019;42:453–461. [DOI] [PubMed] [Google Scholar]
- 88. Bettica P, Petrini S, D’Oria V, D’Amico A, Catteruccia M, Pane M, Sivo S, Magri F, Brajkovic S, Messina S, Vita GL, Gatti B, Moggio M, Puri PL, Rocchetti M, De Nicolao G, Vita G, Comi GP, Bertini E, Mercuri E. Histological effects of givinostat in boys with duchenne muscular dystrophy. Neuromuscul Disord 2016;26:643–649. [DOI] [PubMed] [Google Scholar]
- 89. Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, Ambardekar AV, Granzier HL, Dinarello CA, McKinsey TA. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med 2018;10:eaao0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Travers JG, Wennersten SA, Pena B, Bagchi RA, Smith HE, Hirsch RA, Vanderlinden LA, Lin YH, Dobrinskikh E, Demos-Davies KM, Cavasin MA, Mestroni L, Steinkuhler C, Lin CY, Houser SR, Woulfe KC, Lam MPY, McKinsey TA. HDAC Inhibition reverses preexisting diastolic dysfunction and blocks covert extracellular matrix remodeling. Circulation 2021;143:1874–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kimbrough D, Wang SH, Wright LH, Mani SK, Kasiganesan H, LaRue AC, Cheng Q, Nadig SN, Atkinson C, Menick DR. HDAC Inhibition helps post-MI healing by modulating macrophage polarization. J Mol Cell Cardiol 2018;119:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang L, Beier UH, Akimova T, Dahiya S, Han R, Samanta A, Levine MH, Hancock WW. Histone/protein deacetylase inhibitor therapy for enhancement of Foxp3+ T-regulatory cell function posttransplantation. Am J Transplant 2018;18:1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wei JQ, Shehadeh LA, Mitrani JM, Pessanha M, Slepak TI, Webster KA, Bishopric NH. Quantitative control of adaptive cardiac hypertrophy by acetyltransferase p300. Circulation 2008;118:934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ghosh AK. P300 in cardiac development and accelerated cardiac aging. Aging Dis 2020;11:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M, Kita T. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol 2003;23:3593–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou W, Jiang D, Tian J, Liu L, Lu T, Huang X, Sun H. Acetylation of H3K4, H3K9, and H3K27 mediated by p300 regulates the expression of GATA4 in cardiocytes. Genes Dis 2019;6:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest 2008;118:868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sunagawa Y, Morimoto T, Wada H, Takaya T, Katanasaka Y, Kawamura T, Yanagi S, Marui A, Sakata R, Shimatsu A, Kimura T, Kakeya H, Fujita M, Hasegawa K. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ J 2011;75:2151–2159. [DOI] [PubMed] [Google Scholar]
- 99. Liu H, Wang C, Qiao Z, Xu Y. Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm Biol 2017;55:1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xiao J, Sheng X, Zhang X, Guo M, Ji X. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther 2016;10:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rai R, Sun T, Ramirez V, Lux E, Eren M, Vaughan DE, Ghosh AK. Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis. J Cell Mol Med 2019;23:3026–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Su H, Zeng H, He X, Zhu SH, Chen JX. Histone acetyltransferase p300 inhibitor improves coronary flow reserve in SIRT3 (sirtuin 3) knockout mice. J Am Heart Assoc 2020;9:e017176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rai R, Verma SK, Kim D, Ramirez V, Lux E, Li C, Sahoo S, Wilsbacher LD, Vaughan DE, Quaggin SE, Ghosh AK. A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis. Epigenetics 2017;12:1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Travers JG, Tharp CA, Rubino M, McKinsey TA. Therapeutic targets for cardiac fibrosis: from old school to next-gen. J Clin Invest 2022;132:e148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hammitzsch A, Tallant C, Fedorov O, O'Mahony A, Brennan PE, Hay DA, Martinez FO, Al-Mossawi MH, de Wit J, Vecellio M, Wells C, Wordsworth P, Muller S, Knapp S, Bowness P. CBP30, A selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proc Natl Acad Sci U S A 2015;112:10768–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Picaud S, Fedorov O, Thanasopoulou A, Leonards K, Jones K, Meier J, Olzscha H, Monteiro O, Martin S, Philpott M, Tumber A, Filippakopoulos P, Yapp C, Wells C, Che KH, Bannister A, Robson S, Kumar U, Parr N, Lee K, Lugo D, Jeffrey P, Taylor S, Vecellio ML, Bountra C, Brennan PE, O’Mahony A, Velichko S, Muller S, Hay D, Daniels DL, Urh M, La Thangue NB, Kouzarides T, Prinjha R, Schwaller J, Knapp S. Generation of a selective small molecule inhibitor of the CBP/p300 bromodomain for leukemia therapy. Cancer Res 2015;75:5106–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Borck PC, Guo LW, Plutzky J. BET Epigenetic reader proteins in cardiovascular transcriptional programs. Circ Res 2020;126:1190–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature 2010;468:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Song S, Liu L, Yu Y, Zhang R, Li Y, Cao W, Xiao Y, Fang G, Li Z, Wang X, Wang Q, Zhao X, Chen L, Wang Y, Wang Q. Inhibition of BRD4 attenuates transverse aortic constriction- and TGF-β-induced endothelial-mesenchymal transition and cardiac fibrosis. J Mol Cell Cardiol 2019;127:83–96. [DOI] [PubMed] [Google Scholar]
- 110. Stratton MS, Bagchi RA, Felisbino MB, Hirsch RA, Smith HE, Riching AS, Enyart BY, Koch KA, Cavasin MA, Alexanian M, Song K, Qi J, Lemieux ME, Srivastava D, Lam MPY, Haldar SM, Lin CY, McKinsey TA. Dynamic chromatin targeting of BRD4 stimulates cardiac fibroblast activation. Circ Res 2019;125:662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 2005;19:523–534. [DOI] [PubMed] [Google Scholar]
- 112. Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 2005;19:535–545. [DOI] [PubMed] [Google Scholar]
- 113. Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, Alaiti MA, Bullard J, Alazem K, Margulies KB, Cappola TP, Lemieux M, Plutzky J, Bradner JE, Haldar SM. BET Bromodomains mediate transcriptional pause release in heart failure. Cell 2013;154:569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Spiltoir JI, Stratton MS, Cavasin MA, Demos-Davies K, Reid BG, Qi J, Bradner JE, McKinsey TA. BET acetyl-lysine binding proteins control pathological cardiac hypertrophy. J Mol Cell Cardiol 2013;63:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Antolic A, Wakimoto H, Jiao Z, Gorham JM, DePalma SR, Lemieux ME, Conner DA, Lee DY, Qi J, Seidman JG, Bradner JE, Brown JD, Haldar SM, Seidman CE, Burke MA. BET Bromodomain proteins regulate transcriptional reprogramming in genetic dilated cardiomyopathy. JCI Insight 2020;5:e138687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Duan Q, McMahon S, Anand P, Shah H, Thomas S, Salunga HT, Huang Y, Zhang R, Sahadevan A, Lemieux ME, Brown JD, Srivastava D, Bradner JE, McKinsey TA, Haldar SM. BET Bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sun Y, Huang J, Song K. BET Protein inhibition mitigates acute myocardial infarction damage in rats via the TLR4/TRAF6/NF-κB pathway. Exp Ther Med 2015;10:2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Alexanian M, Przytycki PF, Micheletti R, Padmanabhan A, Ye L, Travers JG, Gonzalez-Teran B, Silva AC, Duan Q, Ranade SS, Felix F, Linares-Saldana R, Li L, Lee CY, Sadagopan N, Pelonero A, Huang Y, Andreoletti G, Jain R, McKinsey TA, Rosenfeld MG, Gifford CA, Pollard KS, Haldar SM, Srivastava D. A transcriptional switch governs fibroblast activation in heart disease. Nature 2021;595:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kalantar-Zadeh K, Schwartz GG, Nicholls SJ, Buhr KA, Ginsberg HN, Johansson JO, Kulikowski E, Lebioda K, Toth PP, Wong N, Sweeney M, Ray KK, Investigators BE. Effect of apabetalone on cardiovascular events in diabetes, CKD, and recent acute coronary syndrome: results from the BETonMACE randomized controlled trial. Clin J Am Soc Nephrol 2021;16:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nicholls SJ, Schwartz GG, Buhr KA, Ginsberg HN, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Sweeney M, Ray KK, Investigators BE. Apabetalone and hospitalization for heart failure in patients following an acute coronary syndrome: a prespecified analysis of the BETonMACE study. Cardiovasc Diabetol 2021;20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Napoli C, Bontempo P, Palmieri V, Coscioni E, Maiello C, Donatelli F, Benincasa G. Epigenetic therapies for heart failure: current insights and future potential. Vasc Health Risk Manag 2021;17:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gilham D, Wasiak S, Tsujikawa LM, Halliday C, Norek K, Patel RG, Kulikowski E, Johansson J, Sweeney M, Wong NC, Gordon A, McLure K, Young P. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis 2016;247:48–57. [DOI] [PubMed] [Google Scholar]
- 123. Ray KK, Nicholls SJ, Buhr KA, Ginsberg HN, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Sweeney M, Schwartz GG. Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: A randomized clinical trial. JAMA 2020;323:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ray KK, Nicholls SJ, Ginsberg HD, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Cummings JL, Sweeney M, Schwartz GG. Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: rationale, design, and baseline characteristics of the BETonMACE trial. Am Heart J 2019;217:72–83. [DOI] [PubMed] [Google Scholar]
- 125. Zhang X, Wen H, Shi X. Lysine methylation: beyond histones. Acta Biochim Biophys Sin 2012; 44:14–27. [DOI] [PubMed] [Google Scholar]
- 126. Zhang T, Cooper S, Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep 2015;16:1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Han P, Li W, Yang J, Shang C, Lin CH, Cheng W, Hang CT, Cheng HL, Chen CH, Wong J, Xiong Y, Zhao M, Drakos SG, Ghetti A, Li DY, Bernstein D, Chen HS, Quertermous T, Chang CP. Epigenetic response to environmental stress: assembly of BRG1-G9a/GLP-DNMT3 repressive chromatin complex on Myh6 promoter in pathologically stressed hearts. Biochim Biophys Acta 2016;1863:1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang G, Weng X, Zhao Y, Zhang X, Hu Y, Dai X, Liang P, Wang P, Ma L, Sun X, Hou L, Xu H, Fang M, Li Y, Jenuwein T, Xu Y, Sun A. The histone H3K9 methyltransferase SUV39H links SIRT1 repression to myocardial infarction. Nat Commun 2017;8:14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yu L, Yang G, Weng X, Liang P, Li L, Li J, Fan Z, Tian W, Wu X, Xu H, Fang M, Ji Y, Li Y, Chen Q, Xu Y. Histone methyltransferase SET1 mediates angiotensin II-induced endothelin-1 transcription and cardiac hypertrophy in mice. Arterioscler Thromb Vasc Biol 2015;35:1207–1217. [DOI] [PubMed] [Google Scholar]
- 130. Ono T, Kamimura N, Matsuhashi T, Nagai T, Nishiyama T, Endo J, Hishiki T, Nakanishi T, Shimizu N, Tanaka H, Ohta S, Suematsu M, Ieda M, Sano M, Fukuda K, Kaneda R. The histone 3 lysine 9 methyltransferase inhibitor chaetocin improves prognosis in a rat model of high salt diet-induced heart failure. Sci Rep 2017;7:39752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen M-Y, Zhang Z-H, Ke J-F, Li T-T, Li M-F, Lu J-X, Li L-X. Chaetocin attenuates atherosclerosis progression and inhibits vascular smooth muscle cell phenotype switching. J Cardiovasc Transl Res 2022;15:1270–1282. [DOI] [PubMed] [Google Scholar]
- 132. Sung PH, Luo CW, Chiang JY, Yip HK. The combination of G9a histone methyltransferase inhibitors with erythropoietin protects heart against damage from acute myocardial infarction. Am J Transl Res 2020;12:3255–3271. [PMC free article] [PubMed] [Google Scholar]
- 133. Davis K, Azarcon P, Hickenlooper S, Bia R, Horiuchi E, Szulik MW, Franklin S. The role of demethylases in cardiac development and disease. J Mol Cell Cardiol 2021;158:89–100. [DOI] [PubMed] [Google Scholar]
- 134. Zhang S, Lu Y, Jiang C. Inhibition of histone demethylase JMJD1C attenuates cardiac hypertrophy and fibrosis induced by angiotensin II. J Recept Signal Transduct Res 2020;40:339–347. [DOI] [PubMed] [Google Scholar]
- 135. Huo JL, Jiao L, An Q, Chen X, Qi Y, Wei B, Zheng Y, Shi X, Gao E, Liu HM, Chen D, Wang C, Zhao W. Myofibroblast deficiency of LSD1 alleviates TAC-induced heart failure. Circ Res 2021;129:400–413. [DOI] [PubMed] [Google Scholar]
- 136. Tran TA, Zhang QJ, Wang L, Gonzales C, Girard L, May H, Gillette T, Liu ZP, Martinez ED. Inhibition of jumonji demethylases reprograms severe dilated cardiomyopathy and prolongs survival. J Biol Chem 2022;298:101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Guo Z, Lu J, Li J, Wang P, Li Z, Zhong Y, Guo K, Wang J, Ye J, Liu P. JMJD3 Inhibition protects against isoproterenol-induced cardiac hypertrophy by suppressing β-MHC expression. Mol Cell Endocrinol 2018;477:1–14. [DOI] [PubMed] [Google Scholar]
- 138. Zhang QJ, Tran TAT, Wang M, Ranek MJ, Kokkonen-Simon KM, Gao J, Luo X, Tan W, Kyrychenko V, Liao L, Xu J, Hill JA, Olson EN, Kass DA, Martinez ED, Liu ZP. Histone lysine dimethyl-demethylase KDM3A controls pathological cardiac hypertrophy and fibrosis. Nat Commun. 2018;9:5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kanouni T, Severin C, Cho RW, Yuen NY, Xu J, Shi L, Lai C, Del Rosario JR, Stansfield RK, Lawton LN, Hosfield D, O'Connell S, Kreilein MM, Tavares-Greco P, Nie Z, Kaldor SW, Veal JM, Stafford JA, Chen YK. Discovery of CC-90011: A potent and selective reversible inhibitor of lysine specific demethylase 1 (LSD1). J Med Chem 2020;63:14522–14529. [DOI] [PubMed] [Google Scholar]
- 140. Watson CJ, Horgan S, Neary R, Glezeva N, Tea I, Corrigan N, McDonald K, Ledwidge M, Baugh J. Epigenetic therapy for the treatment of hypertension-induced cardiac hypertrophy and fibrosis. J Cardiovasc Pharmacol Ther 2016;21:127–137. [DOI] [PubMed] [Google Scholar]
- 141. Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res 2014;101:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Puertas R D, Meugnier E, Romestaing C, Rey C, Morel E, Lachuer J, Gadot N, Scridon A, Julien C, Tronc F, Chapuis B, Valla C, Janin A, Pirola L, Méjat A, Rome S, Chevalier P. Atrial fibrillation is associated with hypermethylation in human left atrium, and treatment with decitabine reduces atrial tachyarrhythmias in spontaneously hypertensive rats. Transl Res 2017;184:57–67.e5. [DOI] [PubMed] [Google Scholar]
- 143. Stenzig J, Schneeberger Y, Löser A, Peters BS, Schaefer A, Zhao RR, Ng SL, Höppner G, Geertz B, Hirt MN, Tan W, Wong E, Reichenspurner H, Foo RS, Eschenhagen T. Pharmacological inhibition of DNA methylation attenuates pressure overload-induced cardiac hypertrophy in rats. J Mol Cell Cardiol 2018;120:53–63. [DOI] [PubMed] [Google Scholar]
- 144. Stenzig J, Hirt MN, Löser A, Bartholdt LM, Hensel JT, Werner TR, Riemenschneider M, Indenbirken D, Guenther T, Müller C, Hübner N, Stoll M, Eschenhagen T. DNA Methylation in an engineered heart tissue model of cardiac hypertrophy: common signatures and effects of DNA methylation inhibitors. Basic Res Cardiol 2016;111:9. [DOI] [PubMed] [Google Scholar]
- 145. Wu TT, Ma YW, Zhang X, Dong W, Gao S, Wang JZ, Zhang LF, Lu D. Myocardial tissue-specific Dnmt1 knockout in rats protects against pathological injury induced by Adriamycin. Lab Invest 2020;100:974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Napoli C, Grimaldi V, De Pascale MR, Sommese L, Infante T, Soricelli A. Novel epigenetic-based therapies useful in cardiovascular medicine. World J Cardiol 2016;8:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen DD, Dong YG, Liu D, He JG. Epigallocatechin-3-gallate attenuates cardiac hypertrophy in hypertensive rats in part by modulation of mitogen-activated protein kinase signals. Clin Exp Pharmacol Physiol 2009;36:925–932. [DOI] [PubMed] [Google Scholar]
- 148. Crescenti A, Solà R, Valls RM, Caimari A, Del Bas JM, Anguera A, Anglés N, Arola L. Cocoa consumption alters the global DNA methylation of peripheral leukocytes in humans with cardiovascular disease risk factors: A randomized controlled trial. PLoS One 2013;8:e65744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 2007;292:E1378–E1387. [DOI] [PubMed] [Google Scholar]
- 150. Dorn LE, Tual-Chalot S, Stellos K, Accornero F. RNA Epigenetics and cardiovascular diseases. J Mol Cell Cardiol 2019;129:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 2016;96:1297–1325. [DOI] [PubMed] [Google Scholar]
- 152. Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011;469:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ambros V. microRNAs: tiny regulators with great potential. Cell 2001;107:823–826. [DOI] [PubMed] [Google Scholar]
- 154. Devaux Y, Zangrando J, Schroen B, Creemers EE, Pedrazzini T, Chang CP, Dorn GW II, Thum T, Heymans S. Long noncoding RNAs in cardiac development and ageing. Nat Rev Cardiol 2015;12:415–425. [DOI] [PubMed] [Google Scholar]
- 155. Bär C, Chatterjee S, Thum T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 2016;134:1484–1499. [DOI] [PubMed] [Google Scholar]
- 156. Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol 2019;21:542–551. [DOI] [PubMed] [Google Scholar]
- 157. Aufiero S, Reckman YJ, Pinto YM, Creemers EE. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol 2019;16:503–514. [DOI] [PubMed] [Google Scholar]
- 158. Santer L, Bär C, Thum T. Circular RNAs: A novel class of functional RNA molecules with a therapeutic perspective. Mol Ther 2019;27:1350–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I. Circular RNAs in heart failure. Eur J Heart Fail 2017;19:701–709. [DOI] [PubMed] [Google Scholar]
- 160. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019;20:675–691. [DOI] [PubMed] [Google Scholar]
- 161. Bär C, Chatterjee S, Falcão Pires I, Rodrigues P, Sluijter JPG, Boon RA, Nevado RM, Andrés V, Sansonetti M, de Windt L, Ciccarelli M, Hamdani N, Heymans S, Figuinha Videira R, Tocchetti CG, Giacca M, Zacchigna S, Engelhardt S, Dimmeler S, Madonna R, Thum T. Non-coding RNAs: update on mechanisms and therapeutic targets from the ESC working groups of myocardial function and cellular biology of the heart. Cardiovasc Res 2020;116:1805–1819. [DOI] [PubMed] [Google Scholar]
- 162. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 163. Thum T, Chau N, Bhat B, Gupta SK, Linsley PS, Bauersachs J, Engelhardt S. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest 2011;121:461–462. author reply 462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, Dueck A, Thum T, Laugwitz KL, Maegdefessel L, Weber C, Kupatt C, Engelhardt S. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol 2020;75:1788–1800. [DOI] [PubMed] [Google Scholar]
- 165. Huang CK, Kafert-Kasting S, Thum T. Preclinical and clinical development of noncoding RNA therapeutics for cardiovascular disease. Circ Res 2020;126:663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang Y, Liu B. Circular RNA in diseased heart. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 2019;16:661–674. [DOI] [PubMed] [Google Scholar]
- 168. Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, Heymans S, Martelli F, Emanueli C, Devaux Y. Regulatory RNAs in heart failure. Circulation 2020;141:313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhou H, Tang W, Yang J, Peng J, Guo J, Fan C. MicroRNA-related strategies to improve cardiac function in heart failure. Front Cardiovasc Med 2021;8:773083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shen NN, Wang JL, Fu YP. The microRNA expression profiling in heart failure: A systematic review and meta-analysis. Front Cardiovasc Med 2022;9:856358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008;132:875–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bonauer A, Dimmeler S. The microRNA-17-92 cluster: still a miRacle? Cell Cycle 2009;8:3866–3873. [DOI] [PubMed] [Google Scholar]
- 173. Lucas T, Bonauer A, Dimmeler S. RNA Therapeutics in cardiovascular disease. Circ Res 2018;123:205–220. [DOI] [PubMed] [Google Scholar]
- 174. Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009;324:1710–1713. [DOI] [PubMed] [Google Scholar]
- 175. Hinkel R, Penzkofer D, Zühlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation 2013;128:1066–1075. [DOI] [PubMed] [Google Scholar]
- 176. Bellera N, Barba I, Rodriguez-Sinovas A, Ferret E, Asín MA, Gonzalez-Alujas MT, Pérez-Rodon J, Esteves M, Fonseca C, Toran N, Garcia Del Blanco B, Pérez A, Garcia-Dorado D. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J Am Heart Assoc 2014;3:e000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rogg EM, Abplanalp WT, Bischof C, John D, Schulz MH, Krishnan J, Fischer A, Poluzzi C, Schaefer L, Bonauer A, Zeiher AM, Dimmeler S. Analysis of cell type-specific effects of MicroRNA-92a provides novel insights into target regulation and mechanism of action. Circulation 2018;138:2545–2558. [DOI] [PubMed] [Google Scholar]
- 178. Abplanalp WT, Fischer A, John D, Zeiher AM, Gosgnach W, Darville H, Montgomery R, Pestano L, Allée G, Paty I, Fougerousse F, Dimmeler S. Efficiency and target derepression of anti-miR-92a: results of a first in human study. Nucleic Acid Ther 2020;30:335–345. [DOI] [PubMed] [Google Scholar]
- 179. Xu K, Chen C, Wu Y, Wu M, Lin L. Advances in miR-132-based biomarker and therapeutic potential in the cardiovascular system. Front Pharmacol 2021;12:751487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyöngyösi M, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Weber N, Zlabinger K, Hašimbegović E, Winkler J, Fiedler J, Dangwal S, Fischer M, de la Roche J, Wojciechowski D, Kraft T, Garamvölgyi R, Neitzel S, Chatterjee S, Yin X, Bär C, Mayr M, Xiao K, Thum T. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat Commun 2020;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wahlquist C, Jeong D, Rojas-Muñoz A, Kho C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans PA, Hajjar RJ, Mercola M. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 2014;508:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Lei Z, Wahlquist C, El Azzouzi H, Deddens JC, Kuster D, van Mil A, Rojas-Munoz A, Huibers MM, Mercola M, de Weger R, Van der Velden J, Xiao J, Doevendans PA, Sluijter JPG. miR-132/212 impairs cardiomyocytes contractility in the failing heart by suppressing SERCA2a. Front Cardiovasc Med 2021;8:592362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lavenniah A, Luu TDA, Li YP, Lim TB, Jiang J, Ackers-Johnson M, Foo RS. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Mol Ther 2020;28:1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Zlabinger K, Hašimbegović E, Winkler J, Garamvölgyi R, Neitzel S, Gyöngyösi M, Thum T. CDR132L Improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J 2021;42:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, Rode L, Weigt H, Genschel C, Lorch U, Theek C, Levin AA, Bauersachs J, Solomon SD, Thum T. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J 2021;42:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hinkel R, Batkai S, Bähr A, Bozoglu T, Straub S, Borchert T, Viereck J, Howe A, Hornaschewitz N, Oberberger L, Jurisch V, Kozlik-Feldmann R, Freudenthal F, Ziegler T, Weber C, Sperandio M, Engelhardt S, Laugwitz KL, Moretti A, Klymiuk N, Thum T, Kupatt C. AntimiR-132 attenuates myocardial hypertrophy in an animal model of percutaneous aortic constriction. J Am Coll Cardiol 2021;77:2923–2935. [DOI] [PubMed] [Google Scholar]
- 188. Rode L, Bär C, Groß S, Rossi A, Meumann N, Viereck J, Abbas N, Xiao K, Riedel I, Gietz A, Zimmer K, Odenthal M, Büning H, Thum T. AAV Capsid engineering identified two novel variants with improved in vivo tropism for cardiomyocytes. Mol Ther 2022;9:S1525-0016(22)00424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Toepfer CN, Sharma A, Cicconet M, Garfinkel AC, Mücke M, Neyazi M, Willcox JAL, Agarwal R, Schmid M, Rao J, Ewoldt J, Pourquié O, Chopra A, Chen CS, Seidman JG, Seidman CE. Sarctrack. Circ Res 2019;124:1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Psaras Y, Margara F, Cicconet M, Sparrow AJ, Repetti GG, Schmid M, Steeples V, Wilcox JAL, Bueno-Orovio A, Redwood CS, Watkins HC, Robinson P, Rodriguez B, Seidman JG, Seidman CE, Toepfer CN. Caltrack: high-throughput automated calcium transient analysis in cardiomyocytes. Circ Res 2021;129:326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Watson SA, Duff J, Bardi I, Zabielska M, Atanur SS, Jabbour RJ, Simon A, Tomas A, Smolenski RT, Harding SE, Perbellini F, Terracciano CM. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat Commun 2019;10:2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Fischer C, Milting H, Fein E, Reiser E, Lu K, Seidel T, Schinner C, Schwarzmayr T, Schramm R, Tomasi R, Husse B, Cao-Ehlker X, Pohl U, Dendorfer A. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat Commun 2019;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Perbellini F, Thum T. Living myocardial slices: a novel multicellular model for cardiac translational research. Eur Heart J 2020;41:2405–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]