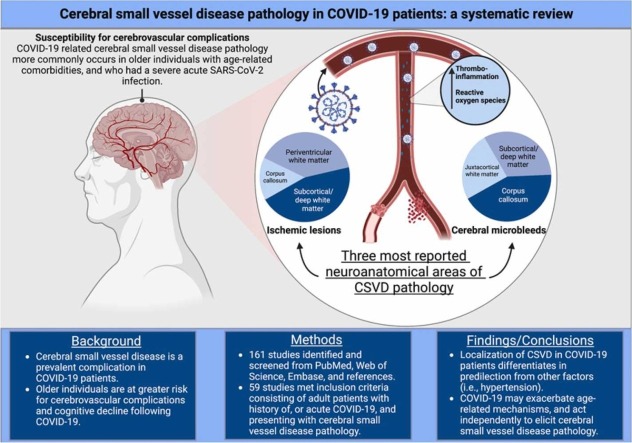
Abstract
Cerebral small vessel disease (CSVD) is the leading cause of vascular cognitive impairment and is associated with COVID-19. However, contributing factors that often accompany CSVD pathology in COVID-19 patients may influence the incidence of cerebrovascular complications. Thus, a mechanism linking COVID-19 and CSVD has yet to be uncovered and differentiated from age-related comorbidities (i.e., hypertension), and medical interventions during acute infection. We aimed to evaluate CSVD in acute and recovered COVID-19 patients and to differentiate COVID-19-related cerebrovascular pathology from the above-mentioned contributing factors by assessing the localization of microbleeds and ischemic lesions/infarctions in the cerebrum, cerebellum, and brainstem. A systematic search was performed in December 2022 on PubMed, Web of Science, and Embase using a pre-established search criterion related to history of, or active COVID-19 with CSVD pathology in adults. From a pool of 161 studies, 59 met eligibility criteria and were included. Microbleeds and ischemic lesions had a strong predilection for the corpus callosum and subcortical/deep white matter in COVID-19 patients, suggesting a distinct CSVD pathology. These findings have important implications for clinical practice and biomedical research as COVID-19 may independently, and through exacerbation of age-related mechanisms, contribute to increased incidence of CSVD.
Keywords: COVID-19, Cerebral small vessel disease, Cognitive impairment, Aging
Graphical Abstract
1. Introduction
Cerebral small vessel disease (CSVD) is the leading cause of vascular dementia (VaD) (Pantoni, 2010) and accounts for up to 25% of ischemic strokes (Li et al., 2018). CSVD is highly heterogenous encompassing pathologic changes such as arteriosclerosis, atherosclerosis, cerebral amyloid angiopathy (CAA), and vasculitides, which affect small perforating arteries, arterioles, venules, and capillaries (Quick et al., 2021). While the pathophysiologic mechanisms remain unclear, endothelial damage resulting in reduced cerebral blood flow and blood-brain barrier disruption is thought to be a direct consequence of this disease (Li et al., 2018, Mustapha et al., 2019). CSVD is characterized by neuroimaging as white matter hyperintensities (WMH), small subcortical infarctions, and microbleeds (MB) (Quick et al., 2021). CSVD is often clinically silent (e.g., silent small subcortical infarct), and difficult to diagnose, making neuroimaging a crucial tool in the early stages to prevent or delay the progression to dementia (Li et al., 2018). Age-related comorbidities such as hypertension, dyslipidemia, and type 2 diabetes are known to contribute to the development of CSVD (Evans et al., 2021). Hence, identification of new risk factors of CSVD is of the utmost importance for preserving cognitive health.
As of February 2023, 102 million cases of Coronavirus 2019 (COVID-19) have been reported, with over 1.1 million deaths in the United States alone. Acute COVID-19 is mild in most individuals, with symptoms consisting of fever, cough, and fatigue (Alimohamadi et al., 2020). However, in the aging population and those with chronic conditions, serious complications can occur with neurologic and vascular injuries being two of the most frequent (Alimohamadi et al., 2020, Mueller et al., 2020, SeyedAlinaghi et al., 2021). Long-COVID, persistence of symptoms greater than 12 weeks after recovery from acute infection (Di Toro et al., 2021, Raveendran et al., 2021), may also have detrimental, long-lasting effects on the nervous and vascular systems (Di Toro et al., 2021, Silva Andrade et al., 2021). Clinical studies have demonstrated that up to 70% of long-COVID patients present with cognitive dysfunction (Guo et al., 2022), including impairments in executive functioning, processing speed, category fluency, memory encoding, and recall (Becker et al., 2021, Hampshire et al., 2021). However, pathophysiological mechanisms of COVID-19-induced cognitive impairment have yet to be fully understood.
Endothelial cells abundantly express angiotensin-converting enzyme-2 (ACE-2) which is the known entry receptor for SARS-CoV-2 (Teuwen et al., 2020). Attachment of spike protein to ACE2 receptor on endothelial cells precipitates a thromboinflammatory environment with excessive reactive oxygen species (ROS) production (Méndez-García et al., 2022, Otifi and Adiga, 2022), causing dysregulation of endothelial function in the macro- and microvasculature in acute and long-COVID patients (Seitz and Ong, 2022). The endothelium represents the inner most layer of the vasculature and is continuous throughout the body, acting as a gate keeper to macro- and microvascular health. Thus, as COVID-19 is an endothelial disease, the acute and recovered phases of SARS-CoV-2 infection cause endothelial dysfunction, affecting pulmonary, hepatic, renal, cardiac, reproductive, and brain organ systems (Davis et al., 2023, Xu et al., 2023). In addition, COVID-19-induced systemic macro- and microvascular endothelial dysfunction have been shown to exacerbate disease severity (Damiani et al., 2020, Lorenzo et al., 2020, Pons et al., 2020, Sabioni et al., 2020, Sardu et al., 2020), persist post-infection (Ambrosino et al., 2021), and have been associated with decreased cognition in recovered COVID-19 patients (Charfeddine et al., 2021, Moretta et al., 2022). Hence, observational, and experimental studies assessing cerebrovascular pathology and function are necessary to understand 1) the cerebral macro- and microvascular pathologies associated with acute and recovered COVID-19 patients and 2) whether persistent cerebromicrovascular dysfunction occurs in COVID-19 patients and if this contributes to long-term cognitive impairment.
The aim of this systematic review was to investigate the contribution of COVID-19 to the development of CSVD in patients with a history of, or active SARS-CoV-2 infection. CSVD will be discussed as a feature of COVID-19, evidenced by neuroimaging, gross pathology or histopathology case reports/series and observational studies from 2020 to 2022.
2. Methods
2.1. Data sources and search strategy
Two investigators (CO and SD) independently conducted a systematic search using PubMed, Web of Science, and Embase databases with the following criteria: ((COVID-19) OR (Long COVID) OR (SARS-CoV-2) OR (post-acute sequelae SARS-CoV-2 infection)) AND ((cerebral small vessel disease) OR (white matter hyperintensity) OR (cerebral amyloid angiopathy) OR (lacunar infarct) OR (subcortical infarct) OR (microbleed)). Searches were conducted from database inception to December 2022. Studies selected for screening were published between 2020 and 2022 and reporting of information included in this review aligns with PRISMA guidelines (Supplementary Table A1).
2.2. Eligibility
Due to the limited time frame in which studies could be published regarding COVID-19-induced cerebromicrovascular complications, case reports and series were eligible for inclusion in this review. Additional studies were included by searching the references of the included articles. Detailed pre-established inclusion and exclusion criteria are outlined below.
2.2.1. Inclusion criteria
(i) Confirmed diagnosis of COVID-19 (e.g., nasopharyngeal swab and RT-PCR) and/or past medical history of confirmed COVID-19 diagnosis.
(ii) Cerebral small vessel disease evidenced by neuroimaging or pathology.
(iii) Adults with COVID-19-induced cerebral small vessel complications.
2.2.2. Exclusion criteria
(i) Studies indicating that cerebral small vessel complications were due to COVID-19 vaccine.
(ii) Publication of articles in a language other than English.
(iii) Pediatric cases (<18 years old) of COVID-19-induced cerebral small vessel complications.
(iii) Published reader responses to articles.
2.3. Data extraction
Two investigators (CO and SD) extracted pertinent data from the included articles. Key information from each article, including authors, year of publication, location, and type of CSVD pathology, COVID-19 status, age and age-related comorbidities, sex, laboratory values, cognitive measurements, and hospital intervention during acute COVID-19 infection, were collected.
2.4. Outcomes
The main outcome measures of our work were to evaluate the type of CSVD-related pathology (i.e., MB and ischemic lesions/infarctions) in COIVD-19 patients, location of the pathology, underlying comorbidities (i.e., aging, hypertension), and levels of thromboinflammatory markers in acute and recovered COVID-19 patients with CSVD pathology.
2.5. Risk of bias of individual studies
Two investigators (CO and CBP) independently performed a risk of bias assessment for each study. To standardize our criteria for risk of bias, a pilot bias assessment was performed on three studies (one case report, one case series, and one observational study). The risk of bias assessment was performed according to the Joanna Briggs Institute (JBI) critical appraisal tools specific to case reports, series, cross-sectional, and case-control studies (JBI, 2020). Disagreements regarding bias assessment were solved through consensus management.
3. Results
3.1. Study selection
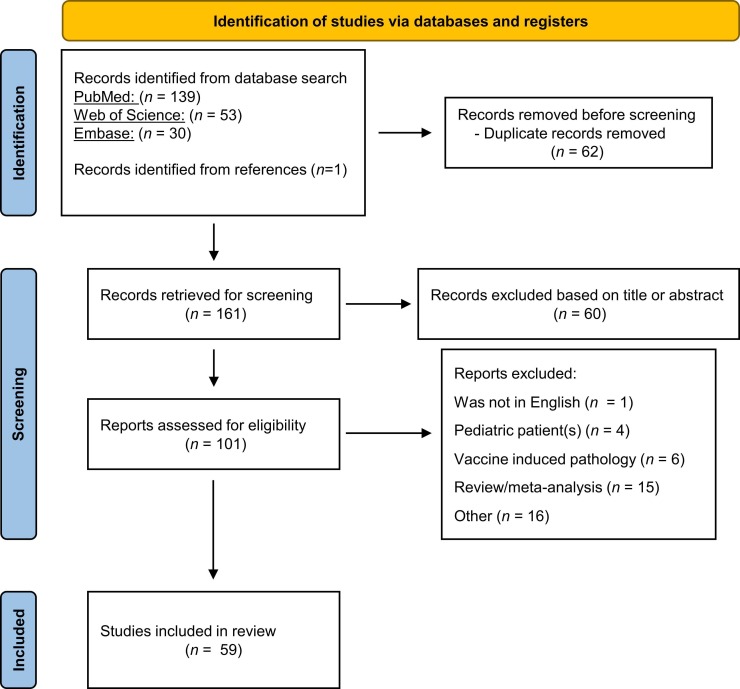
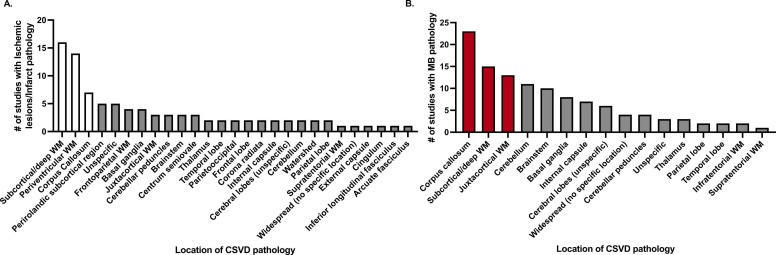
The search criteria yielded 222 publications across three databases (PubMed, Web of Science, and Embase) and one publication through reference. After the removal of duplicate records (n = 62), we screened articles for title and abstract using the above-mentioned pre-determined criteria resulting in exclusion of 60 articles. After full-text evaluation of the remaining 101 articles, 59 publications were included in this review. In-depth paper selection criteria are outlined in the PRISMA diagram (Page et al., 2021) ( Fig. 1). CO and SD independently evaluated each article included in Table 1, Table 2, Table 3, which report a detailed summary of the patient population and neuroradiologic/histopathologic findings in the 59 studies included in this systematic review. Twenty-nine studies were case reports (49.2%), 22 observational studies (37.3%) and eight case series (13.5%). Fig. 2 specifies neuroanatomical areas of CSVD pathology across all included studies, with separate graphs indicating all regions in the cerebrum, cerebellum, and brainstem where microbleeds (Fig. 2A) and ischemic lesions/infarctions (Fig. 2B) occurred.
Fig. 1.
PRISMA flow diagram of study identification and screening. “Other” reasons for exclusion include neuropathology not related to CSVD, reports of patients without COVID-19 diagnosis, reader response publications, abstract with no imaging for MRI or histopathologic results.
Table 1.
Summary of Patient Characteristics and CSVD Findings.
| Citation | Study Type | Patient (s) Characteristics and Timeline | Age | Acute or Recovered COVID-19 | Critical Care | Cardiovascular Comorbidities/ Risk Factors | Cognition | Neuroimaging | CSVD Related Findings |
|---|---|---|---|---|---|---|---|---|---|
| (De Stefano et. al., 2020) | Case-report | COVID-19 positive patient presenting to hospital with flu like symptoms. Admitted to the ICU for respiratory failure 10 days after symptoms onset. No abnormalities on neurologic exam on admission. Weaned off mechanical ventilation after 14 days. | 56 y/o | Acute | Yes | Yes | Persistent executive dysfunction | MRI sequence: T1, T2, DWI, SWI, T2 * GRE, MRA | MRI on day 19 showed MB in juxtacortical WM, CC, and internal capsule. MRA showed no abnormalities (stenosis, vasculitis). |
| (Reichard et. al., 2020) | Case-report | Patient had elective double coronary bypass surgery and 6 days after procedure had pulmonary symptoms and respiratory status became progressively impaired. On day 9 tested positive for COVID-19. Developed AKI, shock, respiratory failure, and died. | 71 y/o | Acute | Yes | Yes | n/a | Postmortem histopathological examination. | Widespread innumerable WM MB. |
| (Hanafi et. al., 2020) | Case-report | COVID-19 positive patient with flu like and pulmonary symptoms and admitted to pulmonary ICU for rapid stabilization. Patient was unarousable after discontinuation of sedation; Chest CT showed ground-glass opacities. | 65 y/o | Acute | Yes | n/a | n/a | CT; MRI sequence: T1, FLAIR, DWI, T2 * GRE, MRA | Globus pallidus MB and hypoxic ischemic lesions in, basal ganglia, cerebellum, CC, and PVWM. MRA shows no abnormalities in large intra or extracranial vessels. |
| (Rudilosso et. al., 2020) | Case-report | COVID-19 positive patient in ICU for COVID-19 bilateral pneumonia. | 50 y/o | Acute | Yes | No | n/a | CT perfusion map; MRI sequence: DWI | Hypoperfusion in paramedian perforation vascular territory suppling L medial thalamus and punctate acute ischemic lesions in bilateral cerebellum. |
| (Planinc et. al., 2020) | Case-report | COVID-19 positive patient with 8-day history of flu like symptoms. CXR showed bilateral pneumonic consolidation. Intubated for respiratory failure on day 7. On day 13, patient was noted to have right sided hemiparesis involving face, arm and leg, clonus, aphasia, and right-sided neglect. Extubated on day 20 and discharged on day 39. | 31 y/o | Acute | Yes | No | n/a | CT; MRI sequence: DWI, SWI | L distal middle cerebral artery acute infarction on CT. Multiple MB in various vascular territories not only within infarcted areas. |
| (Elshereye et. al., 2020) | Case-report | COVID-19 positive patient brought to ED for stroke. On admission L sided stroke symptoms and had a stroke scale of 13/43. CXR revealed bilateral airspace disease. | 75 y/o | Acute | Yes | Yes | n/a | CTA; MRI sequence: DWI | Multiple focal areas in frontal and parietal WM revealing acute lacunar infarctions on MRI; CT revealed PVWM abnormalities. |
| (Williams et. al., 2020) | Case- report | COVID-19 positive patient with genetic confirmation of CADASIL. Patient had sudden onset mild dysarthria with fever, myalgia, anosmia, and ageusia. | 38 y/o | Acute | No | No (other than CADASIL) | n/a | CTA: MRI sequence: FLAIR, DWI, ADC, | CTA of head and neck was unremarkable with no LVO. Chronic small vessel disease evident on MRI day 7 with 11 acute infarcts within internal border zone distribution. WMH in deep WM, PV and subcortical regions. Day 13 MRI showed 1 new infarct in same territory. |
| (Abdi et. al., 2020) | Case-report | COVID-19 positive patient admitted to ED because of decreased level of consciousness and inability to walk with gait symptoms having persisted for 1 month. | 58 y/o | Acute | No | n/a | n/a | MRI sequence: T1, FLAIR | L sided diffuse WMH. |
| (Cannac et. al., 2020) | Case-report | COVID-19 positive patient with MRI performed because of delirium. | 63 y/o | Acute | Yes | n/a | n/a | MRI sequence: T2 * GRE | Innumerable MB in CC, gray/WM interface and scattered in the cerebellum and brain stem. |
| (Vattoth et. al., 2020) | Case-report | COVID-19 positive patient presenting to the hospital for flu-like symptoms. Patient deteriorated with septic shock and AKI. | 66 y/o | Acute | Yes | Yes | n/a | MRI sequence: FLAIR, SWI | Neuroimaging 3 weeks after admitted: MRI showed MB in juxtacortical WM and internal capsule. |
| (Trifan et. al., 2020) | Case-report | COVID-19 positive patient with CADASIL presented to ED with acute onset left leg weakness, dysarthria and ataxia, no flu-like symptoms, CXR negative for consolidation or infiltration. | 37 y/o | Acute | No | Yes | n/a | CT, MRI sequence: FLAIR, DWI, ADC, MRA | CT: negative for acute findings; MRA: no LVO; MRI: lacunar infarct in L cerebellar peduncle and symmetric, bilateral PV and deep WMH. |
| (Shoskes et. al., 2020) | Case-report | COVID-19 positive patient with mental status deterioration. | 69 y/o | Acute | Yes | Yes | n/a | MRI sequence: FLAIR, DWI, ADC, SWI | Brain MRI after mental status deterioration: MB in bilateral juxtacortical WM, CC, basal ganglia, and brainstem. WMH in areas of MB. |
| (Frisullo et. al., 2020) | Case-report | Young and fit COVID-19 positive patient, no flu like symptoms, admitted to ED for sudden onset left hemiparesis, dysarthria and hemianesthesia. | 49 y/o | Acute | No | No | n/a | MRI sequence: FLAIR, DWI, ADC, SWAN | No large vessel occlusion; 2 small cortical acute ischemic lesions in pre and postcentral gyrus. |
| (Haroon et. al., 2020) | Case-report | COVID-19 positive patient admitted to ICU. Developed sepsis, multiorgan failure. Intubation for 3 weeks and needed tracheostomy. MRI after tracheostomy for investigation of delirium. | 72 y/o | Acute | Yes | No | n/a | CT; MRI sequence: FLAIR, DWI, ADC, SWI, 3D TOF | 2 lacunar infarcts in R frontal deep WM and a few small WMH in bilateral periventricular WM. No significant stenosis or occlusion of intracranial arteries. Diffusely distributed MB in bilateral cortical-juxtacortical regions, deep WM, basal ganglia, CC, brain stem and cerebellum. |
| (Gupta et. al., 2020) | Case-report | COVID-19 positive patient admitted to ED with cough, SOB, and fever, and admitted to ICU. Extubated and off ECMO by day 23. Confusion and disorientation warranted brain MRI. | Mid-40’s y/o | Acute | Yes | n/a | n/a | MRI sequence: FLAIR, DWI, T2 * GRE | Diffuse MB on day 23 in bilateral subcortical WM, basal ganglia, CC, brainstem, and cerebellum without signal abnormality T2 FLAIR or DWI |
| (Fraiman et. al., 2020) | Case-report | COVID-19 positive EOAD patient; History of neurologic deficits prior to admission; no PMH of CV RF. On admission, presented stupor (GCS 10) and no signs of localization with normal brainstem reflexes. Patient had dry cough, and abnormal CT scan of lungs. | 38 y/o | Acute | No | No (other than EOAD) | n/a | CT; MRI sequence: T2 * GRE | CT: acute hemorrhage in R frontal lobe; MRI: bilateral hippocampal atrophy and multiple diffuse microbleeds in cerebral lobes and cerebellum. |
| (Neppala et. al., 2021) | Case-reports | Case 1: COVID-19 positive patient with no prior neurologic complications; When taken off sedation at day 30, GCS 3. Case 2 (Son of Case 1): COVID-19 positive patient with same flu like symptoms. GCS 3 after removed from sedation |
Case 1: 68 y/o; Case 2: 49 y/o |
Case 1: Acute Case 2: Acute |
Case 1: Yes Case 2: Yes |
Case 1: Yes Case 2: Yes |
n/a | MRI sequence: FLAIR | Case 1: bilateral PV WMH Case 2: bilateral PV WMH |
| (Toeback et. al., 2021) | Case-reports | COVID-19 positive, critical illness patients. | Case 1: 54 y/o Case 2: 72 y/o |
Acute | Case 1: Yes Case 2: Yes |
Case 1: n/a Case 2: No |
n/a | MRI sequence: FLAIR, SWI | Case 1: multiple WMH and mild brain atrophy and microbleeds in CC. Case 2: WMH in parieto-occipital WM and microbleeds in cortico-juxtacortical junction. 4 weeks later, WMH were increased and surrounding cortical MB. |
| (Rajendran et. al., 2021) | Case-report | COVID-19 positive patient to stroke ward. Previous flu like symptoms for 14 days before admission. Asymptomatic at presentation. NOTCH 3 gene test positive (CADASIL). | 45 y/o | Acute | No | n/a (other than CADASIL) | n/a | CT; MRI sequence: FLAIR, DWI | CT: small white matter hypodensities bilaterally revealing acute infarcts in bilateral centrum semiovale and corona radiata. MRI: subcortical and PV WM hyperintensities prominently in anterior temporal lobe. Chronic lacunar infarct in R centrum semiovale and L basal ganglia. 8 days later repeat MRI w/ new infarcts in bilateral corona radiata. |
| (Zhang et. al., 2021) | Case-report | CADASIL COVID-19 positive patient. Presented with dysphagia, dysarthria, and encephalopathy. No history of cerebrovascular or neurological complications. Neuro exam: sluggish following of commands, aphasia, difficulty handling secretions, dysphagia. Diffuse rhonchi and CXR showed patchy consolidation in R lower lung. Discharged on day 23. | 40’s y/o | Acute | No | Yes | n/a | CT; MRI sequence: FLAIR, DWI, ADC, MRA | MRA of large vessels in head and neck were normal. Acute subcortical ischemic/necrotic changes- abnormal signals in bilateral frontoparietal WM, anterior temporal lobes, basal ganglia, external capsules, and thalami. |
| (Liang et. al., 2021) | Case-report | COVID-19 positive patient in delirious state on admission with 3-week history of fever, rigors, altered sensation, lethargy, headache, and diarrhea. COVID-19 positive on day 10 in hospital. | 74 y/o | Acute | No | Yes | n/a | MRI sequence: T2, T2 * GRE; MRA | CAA with numerous microhemorrhages. |
| (Witvoet et. al., 2021) | Case-report | COVID-19 positive patient presented with flu like symptoms on admission; needed for mechanical ventilation for 22 days. Removal of sedation remained comatose. Neuroimaging day 22. Day 32 patient gradually recovered, GCS 14 and was discharged to rehabilitation center 7 weeks after admission. Able to walk independently 1 month after discharge. | 60 y/o | Acute | Yes | Yes | n/a | CT; MRI sequence: FLAIR, DWI, ADC, SWI, | CT: hypodense areas in supratentorial WM, multiple supratentorial subcortical MB. MRI: confluent WMH in PV and subcortical WM, multiple MB (>50) in subcortical WM, basal ganglia, cerebellum, splenium, and CC. |
| (Pinzon et. al., 2021) | Case-report | Presented 5 weeks after acute COVID-19. Complaints of lack of concentration, sluggishness, and forgetfulness- present 5 days after COVID-19 onset up until 5 weeks at admission. No acute respiratory symptoms. | 40 y/o | Recovered | No | No | Subjective cognitive complaints | CT | Multifocal lacunar infarct in left lateral ventricles. |
| (Piazza et. al., 2021) | Case-report | Admitted for acute onset diplopia that occurred 1 day after onset of fever, myalgia, and headache. Found to be COVID-19 positive and to have cranial nerve six palsy. Brain MRI on admission, treated with high dose steroids which resulted in rapid improvement of diplopia. Repeat brain MRI 1 week later and a third MRI 3 months later showed complete resolution. | 47 y/o | Acute | No | No | n/a | MRI sequence: FLAIR, ADC, SWI, T2 * GRE, CFA-DTI, ASL | Acute infection MRI: WMH in perirolandic subcortical region, centrum semiovale, corona radiata, posterior limb of internal capsule, and pons. MRI repeated 1 week later with complete resolution of neuroradiologic findings. |
| (Backman et. al., 2022) | Case-report | Severe COVID-19 with multi organ failure, bacterial infections, and 35 days of mechanical ventilation. After extubating, presented with delirium. 3 months after infection moderate deficits on MoCA. At 8 months minor deficits in executive function. | 45 y/o | MRI: Acute Cognition: recovered |
Yes | Yes | Deficits on MoCA at 3 months and in executive function at 8 months post infection. | MRI sequence: T1, T2, FLAIR, DWI, SWI | Day 53 after onset of COVID: MB in R temporal lobe, L basal ganglia, and infratentorial MB. |
| (Ahmed et. al., 2022) | Case-reports | Case 1: COVID-19 positive patient with progressively worsening symptoms patient died 7 weeks after hospital admission. Case 2: COVID-19 positive patient eventually transferred to long term facility following severe COVID-19. |
Case 1: 43 y/o; Case 2: 45 y/o |
Acute | Case 1: Yes Case 2: Yes |
Case 1: No Case 2: No |
n/a | MRI sequence: T1, T2, FLAIR, DWI, ADC, T2 * GRE | For both cases: confluent diffuse supratentorial frontoparietal WMH with no MB. |
| (Finsterer, 2022) | Case-report | COVID-19 positive patient with bradykinesia and facial palsy on R side with symptoms of malaise, coughing, and fever. Patient had pre-existing microangiopathy. | 86 y/o | Acute | No | No (other than pre-existing micro-angiopathy) | n/a | MRI: FLAIR, DWI, ADC, SWI | Small subacute ischemic lesion (WMH) in L PVWM and MB in L thalamus. No evidence of carotid stenosis. |
| (Petersson et. al., 2022) | Case- report | Severe COVID-19 patient with Initial MRI performed at 20 days ICU. MRI 7-month follow-up; Cognitive testing 10-month follow-up. | 50’s y/o | Acute and recovered | Yes | Yes | Impaired executive function at 10-month follow-up | MRI sequences: T1, T2, FLAIR, DWI, ADC, SWI | Initial MRI: multiple lacunar infarcts and multiple MB (frontal, parietal, temporal, and supratentorial WM. WMH in splenium of CC and cerebellar peduncles. 7-month MRI: expansion of WMH into large area of deep cortical and periventricular WM cavitation 10 areas of restricted diffusion on initial MRI into lacunes. MB increased from 16 to 17. |
| (Tristán-Samaniego et. al., 2022) | Case- report | Severe COVID-19 patient with MRI on day 19 of ICU; Follow-up MRI 23 days after discharge and cognitive testing at 60 days follow-up. | 46 y/o | Acute and recovered | Yes | No | Impaired executive function at 2-month follow-up | MRI sequences: FLAIR, DWI, SWI | Day 19: bilateral WMH in deep and subcortical WM (splenium and CC) and multiple MB (splenium and subcortical WM); 23 days after discharge: resolution of WM and no change in number or distribution of MB. |
Note. Critical care column includes hospital intervention for respiratory failure/distress (mechanical ventilation, intubation, ECMO). Cardiovascular risk factors and comorbidities include one or more of the following: hypertension, dyslipidemia, hypercholesterolemia, type 2 diabetes, and/or obesity. Abbreviations: Past medical history (PMH), Susceptibility weighted imaging (SWI), Gradient echo (GRE), Magnetic resonance angiography (MRA), Microbleed (MB), White matter (WM), Corpus callosum (CC), Acute kidney injury (AKI), Diffusion weighted imaging (DWI), Fluid attenuated inversion recovery (FLAIR), Periventricular (PV), Left (L), Emergency department (ED), Chest x ray (CXR), Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), Apparent diffusion coefficient (ADC), Computed tomography angiography (CTA), Large vessel occlusion (LVO), White matter hyperintensity (WMH), Glasgow coma scale (GCS), Extracorporeal membrane oxygenation (ECMO), Time of flight (TOF), Right (R), Early onset Alzheimer’s Disease (EOAD), Cardiovascular (CV), Risk factor (RF), Cerebral amyloid angiopathy (CAA), Arterial spin labeling (ASL), colored fractional anisotropy diffusion tensor imaging (CFA-DTI), Susceptibility weighted angiography (SWAN).
Table 2.
Summary of Case Series Patient Characteristics and CSVD Findings.
| Citation | Study Type | Patient (s) Characteristics and Timeline | Age | Severe Acute SARS-CoV-2 infection | Cardiovascular Comorbidities/ Risk Factors | Cognition | Neuroimaging | CSVD Related Findings |
|---|---|---|---|---|---|---|---|---|
| (Fitsiori et. al., 2020) | Case-series | Nine COVID-19 positive patients suffering from severe COVID-19 and had indication for brain MRI. | Mean age: 67.7 | Yes | 8/9 patients | n/a | MRI sequence: DWI, T2, FLAIR, SWI | MB in unusual distribution with specific predilection for CC. Other areas of MB distribution: internal capsule, middle cerebellar peduncles, subcortical regions, basal ganglia. 2 patients with MRI findings of acute ischemic lesions in deep or subcortical WM. |
| (Radmanesh et. al., 2020) | Case- series | Eleven critically ill COVID-19 patients without DIC and on mechanical ventilation at time of imaging. Indication for MRI was deteriorated mental status. | Mean age: 53 y/o | Yes | 7/11 patients | n/a | MRI sequence: DWI, ADC, FLAIR, SWI | Ten out of eleven patients with WMH in bilateral deep and subcortical WM. MB in 7/11 patients varied from 5 to 6 to innumerable in juxtacortical WM and/or corpus callosum (particularly splenium). |
| (Keller et. al., 2020) | Case-series | Eight out of thirty-two COVID-19 positive patients presented with severe neurologic complications. | Mean age: 67.6 y/o | Yes | 7/8 patients | n/a | CT; MRI sequence: SWI, DWI, VWI | Imaging of 8 patients: 2 with single lacunar ischemic lesions on CT, followed up with MRI showing additional lacunar ischemic lesions indicating cerebral small vessel disease. Six out of eight had MB (parietal lobe, R temporal lobe, bi-thalamic, CC, supra and infratentorial) on MRI. CTA or MRA showed no signs of vasculitis. Diagnosis of 8 patients: 6/8 with small vessel disease and 3/6 with small vessel disease as only CNS disorder. |
| (Coolen et. al., 2020) | Case-series | Nineteen Post-mortem brains from COVID-19 death. | Mean age: 77 y/o | Yes | Yes | n/a | Postmortem MRI sequence: T1, T2, FLAIR, DWI, SWI | Subcortical microbleeds (2 decedents), nonspecific WM changes (1 decedent) |
| (Dixon et. al., 2020) | Case-series | Thirty COVID-19 positive critical care. Median time for MRI scan indicated for neurologic signs and symptoms was 37.5 days after admission. All patients had elevated levels of thromboinflammation and majority with AKI. | Median age for 10 MB patients: 56 y/o | Yes | 7/10 MB patients | n/a | MRI sequence: SWI | Ten out of thirty patients with MB distinct from other CNS pathology. MB located in CC, most commonly in the splenium, juxtacortical and subcortical WM, mostly in parietal lobes, and cerebellum and brainstem. Mean number of MB was 35 |
| (Martin et. al., 2022) | Case-series | Six adult deceased patients all attributable to COVID-19. | Mean age: 44 y/o | Yes | Yes | n/a | Histology; Post-mortem MRI sequence: T2, FLAIR, DWI, SWI | One out of six adult brains without pathology. In brains with WMH, they were associated with MB in corpus callosum and splenium. CSVD was defined in 3/6 brains by histology. |
| (Kirschen-baum et. al., 2021) | Case-series | Brains of four deceased COVID-19 patients. Patients were omitted if there was premortem conditions of DIC. All patients had progressive respiratory symptoms. | Ages: 70, 77, 79 and 81 y/o | Yes | Yes | n/a | Postmortem histology; Post-mortem MRI: SWI | Autopsy findings: Endothelitis, intravascular thrombosis, beta-amyloid negative, petechial hemorrhage at gray-white matter junction of neocortex. Postmortem MRI findings: multiple cerebral MB (juxtacortical frontal lobe, CC). |
| (Wierzba-Bobrowicz et. al., 2021) | Case-series | Fifty-two deceased COVID-19 patients who had COVID-19 pneumonia or COVID-19 nucleoprotein in pulmonary tissue. | Median age: 58 y/o | Yes | 48/52 patients | n/a | Histopathology/ immunohistochemistry | Microbleeds in subarachnoid space in all patients most common in gray and white matter of neocortex but also brainstem and cerebellum. Vessel damage in all specimens (endothelial, fibrosis and hyalinization) most prominent in WM. The endothelium of most small vessels showed signs of degeneration, tight junctions significantly widened, and basement membranes of most capillaries showed loss of matrix components. |
Note. Cardiovascular risk factors and comorbidities include one or more of the following: hypertension, dyslipidemia, hypercholesterolemia, type 2 diabetes, and/or obesity. Abbreviations: Diffusion weighted imaging (DWI), Susceptibility weighted imaging (SWI), Fluid attenuated inversion recovery (FLAIR), Microbleed (MB), White matter (WM), Corpus callosum (CC), Disseminated intravascular coagulation (DIC), White matter hyperintensity (WMH), Right (R), Magnetic resonance angiography (MRA), Apparent diffusion coefficient (ADC), Computed tomography angiography (CTA), Vessel wall imaging (VWI), Acute kidney injury (AKI), CSVD (Cerebral small vessel disease).
Table 3.
Summary of Retrospective and Cross-sectional Studies CSVD Findings.
| Citation | Study Type | Groups | Age | Acute or Recovered COVID-19 | Cognition | Neuroimaging | CSVD Related Findings |
|---|---|---|---|---|---|---|---|
| (Agarwal et. al., 2020) | Retrospective | Thirty-five COVID-19 positive patients with MRI findings of leukoencephalopathy and/or cerebral MB; not different in PMH comorbidities with COVID patients with no MB. MB patients had longer hospital stays and were on ventilator longer compared to patients without MB and had worse GCS (6 vs14) at time of MRI. | Median age for MB+ : 61 | Acute | n/a | MRI sequence: SWI, T2 * GRE, ADC, FLAIR, DWI | Eighteen out of twenty-four patients had diffuse and or perirolandic distribution of leukoencephalopathy (30/35 patients), respectively. Distribution of MB in 25/35 patients include: diffuse (10), lobar (15), Pons/cerebellum (5), CC with splenium (15), subcortical WM (19), deep WM (10). |
| (Taylor et. al., 2020) | Retrospective | Twenty-six COVID-19 positive patients with a neurovascular event requiring neuro intensive care/ neurosurgery consultation. | Mean age: 59 y/o | Acute | n/a | CT | Two subcortical small vessel strokes. |
| Kremer et. al (2020) | Retrospective | Thirty-seven severe COVID-19 positive patients. Of those with neurologic symptoms underwent MRI. Majority of patients admitted to ICU because of acute respiratory failure. All patients had elevated thromboinflammation levels. | Mean age: 61 y/o | Acute | n/a | MRI sequence: T1, T2, DWI, T2 * GRE, SWI, FLAIR | 43% of patients had non confluent multifocal WMH in L medial temporal lobe, CC; 24% of patients had WM MB (subcortical WM, CC, internal capsule, cerebellar peduncles). |
| (Lin et. al., 2020) | Retrospective | Neuroimaging for 278 COVID-19 patients, 51 patients with MRI. | Median age: 71.8 y/o | Acute | n/a | MRI sequence: DWI, SWI, FLAIR, T1 | Twenty-six out of fifty-one patients who underwent MRI had MB, 17/26 had 1–3 MB, 7/26 had > 15 MB. Of these 7 patients: 1 with MB distribution in cortex and subcortex, 1 with predominant involvement in basal ganglia and cerebellum, and 2 with MB consistent with previous conditions. The remaining 3 patients (long ICU course with intubation and mechanical ventilation) had greater burden of MB predominantly in splenium of CC along with internal capsule, and juxtacortical WM. |
| (Sawlani et. al., 2021) | Retrospective | One hundred and sixty-seven COVID-19 patients who required hospitalization with neurologic symptoms. | Mean age for abnormal MRI (n = 20): 59.7 y/o | Acute | n/a | MRI sequence: T1, T2, FLAIR, SWI, DWI | Twenty patients had abnormal MRI: 12 patients with MB (splenium of CC); Watershed WMH in 4 patients. 1 patient who had repeat MRI 72 h after initial showed progression of MB. |
| (Lersy et. al., 2021) | Retrospective | Nineteen COVID-19 ARDS ICU patients with MB and 18 COVID-19 ARDS ICU patients without MB. MB vs no-MB- similar ARDS, neurologic complications but normal MRI compared to MB group. | Median age: MB+ (66); MB- (62) | Acute | n/a | MRI sequence: SWI, T1, DWI, FLAIR, MR perfusion. | In MB group, WM MB showed more impaired consciousness and more severe confusion compared to controls. MB in CC (genu and splenium), subtentorial juxtacortical WM, internal capsule, brainstem, middle cerebellar peduncles and cerebellum. |
| (Mendez Elizondo et. al., 2021) | Cross-sectional | Four hundred eighty-one brain MRI. Less than 10% were hospitalized due to COVID-19 pneumonia. | Median/mean age not reported | Acute | n/a | MRI sequence: T2, T1, FLAIR, DWI, ADC, GRE, 3D TOF, SWI | Twenty-six out of four hundred eighty-one patients had MB, of these 26, 6 had positive COVID-19 at time of MRI (23.07 vs 4.15%, COVID-19 vs non-COVID-19). MB due to lipohyalinosis localized to basal ganglia, brainstem, and cerebellum. MB due to CAA localized to cerebral cortex, juxtacortical cerebellum and deep and PV WM. |
| (Hautecloque et. al., 2021) | Retrospective | Nine COVID-19 positive with stroke during infection and 50 non-COVID with stroke. | Median age: COVID+ (69.4); COVID- (70.6) | Acute | n/a | MRI sequence: DWI, ADC, FLAIR, SWI | Significantly more microvascular infarct in COVID-19 patients (4/9) compared to COVID-19 negative (6/50). |
| Conklin et al. (2021) | Retrospective | Sixteen COVID-19 positive patients who were mechanically ventilated at time of MRI. All patients had severe COVID-19 with pulmonary infiltrates in more than 50% of lung field. | Median/mean age not reported | Acute | n/a | MRI sequence: SWI | Punctate foci of abnormal SWI signal in 11/16 cases and 8/16 had more than 10 lesions with 4 patients showing predilection for the CC and the other 4 localizing in the subcortical and deep WM. In total, 69% of patients had microvascular injury in the subcortical and deep WM. |
| Shahjouei et al. (2021) | Cross-sectional | Acute COVID-19 patients with stroke (acute ischemic, intracerebral hemorrhage, SAH, CVST. 27% of patients with ischemic stroke died in the hospital during infection, while the remaining 73% were discharged home or under subacute care. | Mean age of acute ischemic stroke patients: 67.2 y/o | Acute | n/a | n/a | 10.9% of ischemic stroke patients (n = 323) had small vessel ischemic stroke. |
| (Andriuta et. al., 2022) | Cross-sectional | Fourty-six post-acute COVID-19 patients with neurologic complications during acute infection. Exclusion criteria: illiteracy, alcoholism or severe cardiorespiratory or neurological disorders, history of NCD. 1 patient with previous stroke. | Mean age: 50 y/o | Recovered | Cognitive complaints following recovery | MRI sequence: T1, T2, FLAIR, GRE | Thirty-six patients in neuroradiological analysis. Average time from infection to MRI was 202 days. WMH localized to superior frontal and postcentral region, cingulum, cortico-spinal tract, inferior longitudinal fasciculus, internal capsule, and posterior segment of arcuate fasciculus. Increased burden of WMH associated with cognitive performance. |
| (Bungenberg et. al., 2022) | Cross-sectional | Fifty long-COVID patients, with average time since acute COVID-19 was 29 weeks. Grouped by hospitalized (n = 21) [11 ICU and 4 receiving ECMO] and non-hospitalized (n = 29). Over ½ patients had acute respiratory symptoms. | Median age: Hospitalized (57.3); non-hospitalized (45.6) | Recovered | 70% of patients complain of cognitive impairment long term | MRI sequence: T1, T2, DWI, SWI, FLAIR, T2 * GRE | Fourty-two patients in neuroradiologic analysis. PV WMH in 6 hospitalized and 2 non-hospitalized and did not associate with clinical outcome measures. MB only in hospitalized patients and majority seen in patients who received ECMO. MB associated with worse cognitive functioning regardless of MB location. |
| (Uginet et. al., 2022) | Retrospective | Thirty-nine COVID-19 positive patients with acute encephalopathy. | Mean age: 66.5 y/o | Acute | n/a | MRI sequence: T1, T2, DWI, SWI, TOF, MRA, FLAIR | 50% of patients with ischemic lesions had small vessel ischemic lesion and 23/39 patients had microbleeds. |
| Petersen et. al (2022) | Cross-sectional | One hundred eighty-eight non-hospitalized recovered (9.6 months after COVID-19 positive) COVID-19 patients vs. 483 age, sex, and education matched controls. | Median age: COVID+ (55 y/o); Control (57 y/o) | Recovered | n/a | MRI: T1, T2, FLAIR, TOF, MPRAGE | No significant difference in numbers of MB and infarctions between COVID+ and controls. |
| Lersy et. al (2022) | Retrospective | Thirty-one COVID-19 patients with neurologic symptoms during the acute phase underwent MRI. Patients’ average length in ICU was 30 days. 1st follow-up MRI 95 days after baseline; 2nd follow-up MRI 189 days after baseline. All patients had at least one follow-up MRI (30/31 at 3 months), (17/31 at 6 months). No LVO on MRA; 24 patients for ASL assessment. | Mean age: 61 y/o | Acute and recovered | Working memory and executive functions slightly impaired in ½ of patients through follow-ups. | MRI sequence: T1, T2, FLAIR, DWI, SWI, PWI, MRA, FDG PET-CT | Baseline MRI: 19 patients with abnormal brain perfusion (16 with hypoperfusion); 9 cases of diffuse MB (CC, subtentorial juxtacortical WM, internal capsule, brain stem, cerebellar peduncles, cerebellum). 4 cases of small vessel infarct; 4 cases of cerebral vasculitis; Follow-ups: MBs remained stable. Cerebral vasculitis cases normalized on imaging. 1 patient with previous cerebral vasculitis had increase in WMH at 3-month follow-up. Perfusion normalized in 11/19 patients at follow-up. 23 patients underwent FDG PET-CT at 3 months, all but one patient had hypometabolism. At 6 months 5/12 patients who underwent second PET had persisting hypometabolism. |
| (Napolitano et. al., 2022) | Retrospective | Fourteen MB + and 49 MB – patients age and sex matched COVID-19 patients with no difference in acute symptom presentation. MB + patients were hospitalized longer than MB -. | Median age: MB + (62 y/o); MB – (64 y/o) | Acute | n/a | MRI sequence: FLAIR, SWI, DWI | MB + : higher levels of thromboinflammation compared to controls. Subcortical and deep WMH in MB + vs no WMH in MB -. MB distribution was predominantly in CC and juxtacortical area. |
| (Cecchetti et. al., 2022) | Retrospective | Thirty-six recovered COVID-19 patients without major medical illnesses or major systemic, psychiatric, or neurological conditions. MRI imaging was performed at 2 months of discharge. 85% of COVID patients were treated as inpatients with 26.5% requiring mechanical ventilation and 4.1% requiring orotracheal intubation. During acute phase, 73.5% had neurological manifestations. | Mean age: COVID + (60.8 y/o); COVID - (56.9 y/o) | Recovered | COVID-19 positive had worse cognitive function compared to COVID-19 negative | MRI sequence: T2 | Recovered COVID-19 patients presented with increased volume of WMH in right frontal and right parieto-occipital cortices compared to controls, correlating with worse cognitive function and number of cardiovascular RF. |
| (Pelizzari et. al., 2022) | Cross-sectional | Fourty-three age, sex, hypertension, and hyperlipidemia matched individuals who had COVID-19 (n = 22) and did not (n = 21). COVID-19 positive patients all experienced anosmia and neurologic symptoms during acute phase and were never hospitalized due to COVID-19. MRI at 2–12 months after recovery. No patients had history of neurologic disease. | Median age: COVID + (45.7 y/o); COVID – (37.6 y/o) | Recovered | n/a | MRI sequences: FLAIR, DWI, pCASL | No difference between COVID-19 positive and COVID-19 negative groups with respect to WM lesions, GM volume, and CBF. |
| (Poletti et. al., 2022) | Cross-sectional | Forty-nine recovered COVID-19 patients without acute phase ICU treatment, major medical/ brain disorders prior to COVID-19 onset. MRI 61 days after hospital discharge. | Mean age: 55.5 y/o | Recovered | n/a | MRI sequences: T1, T2 FSE, FLAIR,1H-MRS | Increased periventricular, frontal, and parietal WMH negatively associated with GSH level in the ACC. |
| (Fällmar et. al., 2022) | Cross-sectional | Nineteen COVID-19 positive patients with one or more new-onset neurological symptoms. | Median age: 62 y/o | Acute | n/a | MRI sequences: T2 TSE, FLAIR, SWI, DWI | Nine out of nineteen patients with punctate SWI abnormalities; 2/19 with punctate infarcts; 18/19 with WMC (4/18 with changes in CC, MCP, juxtacortical. |
| (Shoskes et. al., 2022) | Retrospective | COVID-19 ARDS vs non-COVID-19 ARDS age matched controls; COVID-19 positive with higher vascular RF. | Median age: COVID + (66 y/o); COVID – (68 y/o) | Acute | n/a | MRI sequences: FLAIR, SWI | Four out of twenty-six COVID-19 positive with diffuse subcortical WMH with underlying MB and multifocal WMH with underlying MB, compared to 0/26 COVID-19 negative controls. |
| Petersen et. al (2022) | Retrospective | Two hundred twenty-three non vaccinated recovered (9 months post-COVID-19) COVID-19 positive patients with acute presentation of mild to moderate COVID-19 and no patients receiving mechanical ventilation or critical care vs 223 age, sex, years of education and cardiovascular risk factors matched controls | Mean age: COVID + (55.54 y/o); COVID- (55.74 y/o) | Recovered | No significant differences 10 months post COVID-19 in executive functioning and memory test scores | MRI sequences: DTI | Recovered COVID-19 patients had higher global free-water and MD in WM, and predicted past SARS-CoV-2 infection, compared to controls. Cortical thickness and markers of CSVD were not different between groups. |
Note. Microbleeds (MB), Past medical history (PMH), Glasgow coma scale (GCS), Corpus callosum, (CC), White matter (WM), Susceptibility weighted imaging (SWI), Gradient echo (GRE), Apparent diffusion coefficient (ADC), Fluid attenuated inversion recovery (FLAIR), Diffusion weighted imaging (DWI), White matter hyperintensity (WMH), Acute respiratory distress syndrome (ARDS), Time of flight (TOF), Cerebral amyloid angiopathy (CAA), Periventricular (PV), Subarachnoid hemorrhage (SAH), Cerebral venous thrombosis (CSVT), Neurocognitive disorder (NCD), Rapid acquisition gradient-echo sequence (MPRAGE), Perfusion weighted imaging (PWI), Fluorodeoxyglucose (FDG)-positron emission tomography (PET)-CT, Magnetic resonance angiography (MRA), Arterial spin labeling (ASL), Large vessel obstruction (LVO), Risk factor (RF), Gray matter (GM), Cerebral blood flow (CBF), Reduced glutathione (GSH), Anterior cingulate cortex (ACC), Proton magnetic resonance spectroscopy(1H-MRS). Turbo spin echo (TSE), Pseudo-continuous arterial spin labeling (pCASL), Fast spin echo (FSE), White matter change (WMC), Middle cerebellar peduncle (MCP), Mean diffusivity (MD), Cerebral small vessel disease (CSVD)
Fig. 2.
Neuroanatomical areas of CSVD pathology in COVID patients. (A) Number of studies that report cerebral, cerebellar, and brainstem locations of small vessel ischemic lesions/infarctions in COVID-19 patients. White bars indicate the three most common areas of insult per study (Subcortical/deep WM, Periventricular WM, Corpus callosum). (B) Number of studies that report cerebral, cerebellar, and brainstem locations of microbleeds in COVID-19 patients. Red bars indicate the three most common areas of insult per study (Corpus callosum, Subcortical/deep WM, Juxtacortical WM). Abbreviations: White matter (WM), Microbleeds (MB).
3.1.1. Case reports
Twenty-nine case reports were included detailing patients with COVID-19-induced cerebromicrovascular complications with neuroimaging indicative of MB and/or small vessel ischemic lesions or infarctions (Table 1). Eighteen out of twenty-nine case reports presented patients who were admitted to the ICU due to respiratory failure/distress and required one or more of the following: intubation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) (Ahmed et al., 2022, Backman et al., 2022, Cannac et al., 2020, De Stefano et al., 2020, Elshereye and Erdinc, 2020, Gupta et al., 2020, Hanafi et al., 2020, Haroon et al., 2020, Neppala et al., 2021, Petersson et al., 2022, Planinc et al., 2020, Reichard et al., 2020, Rudilosso et al., 2020, Shoskes et al., 2020, Toeback et al., 2021, Tristán-Samaniego et al., 2022, Vattoth et al., 2020, Witvoet et al., 2021). MB were seen in 17/29 case reports (Backman et al., 2022, Cannac et al., 2020, De Stefano et al., 2020, Finsterer, 2022, Fraiman et al., 2020, Gupta et al., 2020, Hanafi et al., 2020, Haroon et al., 2020, Liang et al., 2021, Petersson et al., 2022, Planinc et al., 2020, Reichard et al., 2020, Rudilosso et al., 2020, Shoskes et al., 2020, Toeback et al., 2021, Tristán-Samaniego et al., 2022, Vattoth et al., 2020, Witvoet et al., 2021) with 14/17 being in patients with severe pulmonary/respiratory complications. Regarding the three MB cases in which patients did not require intubation, mechanical ventilation or ECMO, patients presented with early onset Alzheimer’s Disease (Fraiman et al., 2020), CAA (Liang et al., 2021), or pre-existing microangiopathy (Finsterer, 2022), all of which are independent risk factors for cerebral MB (Cordonnier and van der Flier, 2011, Woerdeman et al., 2014). All but one report (7/8) presenting with both MB and ischemic lesions/infarctions required pulmonary/respiratory intervention in the ICU (Hanafi et al., 2020, Haroon et al., 2020, Petersson et al., 2022, Shoskes et al., 2020, Toeback et al., 2021, Tristán-Samaniego et al., 2022, Witvoet et al., 2021). Cases of small vessel ischemic lesion/infarct without MB (Abdi et al., 2020, Ahmed et al., 2022, Elshereye and Erdinc, 2020, Frisullo et al., 2020, Neppala et al., 2021, Piazza et al., 2021, Pinzon et al., 2021, Rajendran et al., 2021, Rudilosso et al., 2020, Trifan et al., 2020, Williams et al., 2020, Zhang et al., 2021) were less dependent on ICU status with 8/12 patients not requiring intubation, mechanical ventilation or ECMO. Additionally, in four cases of patients with cerebral autosomal dominant arteriopathy with subcortical infarctions and leukoencephalopathy, COVID-19 exacerbated this genetic condition, with reports of new small vessel ischemic lesions and/or infarctions during acute infection (Rajendran et al., 2021, Trifan et al., 2020, Williams et al., 2020, Zhang et al., 2021).
Laboratory values were recorded for 21/29 case studies of COVID-19 patients presenting with MB or ischemic lesions/infarctions (Abdi et al., 2020, Backman et al., 2022, De Stefano et al., 2020, Elshereye and Erdinc, 2020, Finsterer, 2022, Fraiman et al., 2020, Frisullo et al., 2020, Gupta et al., 2020, Hanafi et al., 2020, Haroon et al., 2020, Liang et al., 2021, Petersson et al., 2022, Piazza et al., 2021, Pinzon et al., 2021, Planinc et al., 2020, Rajendran et al., 2021, Reichard et al., 2020, Shoskes et al., 2020, Tristán-Samaniego et al., 2022, Vattoth et al., 2020, Witvoet et al., 2021). Elevated thromboinflammatory biomarkers (e.g., CRP, IL-6, ferritin, D-dimer) were present in 16/21 case reports. Interestingly, Petersson et al. (2022) published a case of a patient with increased thromboinflammation one month after admission to the hospital, and neuroimaging showed an increased number of MB and expansion of WMH at seven months post-SARS-CoV-2 infection. The patient remained cognitively impaired at 10 months following acute infection , indicating a prolonged thromboinflammatory state, potentially leading to long-term dysregulation of cerebromicrovascular endothelial function, impairing executive function and processing speed. Additionally, multiple studies have shown persistent cognitive dysfunction in COVID-19 patients with CSVD pathology, (Backman et al., 2022, De Stefano et al., 2020, Tristán-Samaniego et al., 2022), and impaired gait patient during acute infection (Abdi et al., 2020), a marker of CSVD-associated cognitive decline (Mukli et al., 2022). Cerebrospinal fluid (CSF) was tested for the presence of SARS-CoV-2 by RT-PCR in nine cases and all were negative for the virus (Abdi et al., 2020, De Stefano et al., 2020, Haroon et al., 2020, Liang et al., 2021, Neppala et al., 2021, Piazza et al., 2021, Rajendran et al., 2021, Tristán-Samaniego et al., 2022, Zhang et al., 2021).
Predisposing factors (comorbidities, sex, age) that may contribute to, or be exacerbated by SARS-CoV-2 infection were reported for most case reports. In total, 32 patients were presented across the 29 studies. Past medical history was available for 26 patients (Ahmed et al., 2022, Backman et al., 2022, De Stefano et al., 2020, Elshereye and Erdinc, 2020, Finsterer, 2022, Fraiman et al., 2020, Frisullo et al., 2020, Haroon et al., 2020, Liang et al., 2021, Neppala et al., 2021, Petersson et al., 2022, Piazza et al., 2021, Pinzon et al., 2021, Planinc et al., 2020, Reichard et al., 2020, Rudilosso et al., 2020, Shoskes et al., 2020, Toeback et al., 2021, Trifan et al., 2020, Tristán-Samaniego et al., 2022, Vattoth et al., 2020, Williams et al., 2020, Witvoet et al., 2021, Zhang et al., 2021) and sex and age data were available from all reports. Thirteen out of twenty-six patients had cardiovascular risk factors (e.g., hypertension, type 2 diabetes, smoking), of whom 10/32 patients were female (De Stefano et al., 2020, Elshereye and Erdinc, 2020, Finsterer, 2022, Fraiman et al., 2020, Frisullo et al., 2020, Rajendran et al., 2021, Trifan et al., 2020, Vattoth et al., 2020, Williams et al., 2020, Zhang et al., 2021) and 12/32 patients were ≥ 60 years old (mean age of 12 patients = 70 years old, range = 60–86 years old) (Cannac et al., 2020, Elshereye and Erdinc, 2020, Finsterer, 2022, Hanafi et al., 2020, Haroon et al., 2020, Liang et al., 2021, Reichard et al., 2020, Shoskes et al., 2020, Toeback et al., 2021, Vattoth et al., 2020, Witvoet et al., 2021).
3.1.2. Case series
Eight case series were included, and detailed neuroimaging/histopathologic findings are included in Table 2. Four studies were observed in the setting of acute COVID-19 (Dixon et al., 2020, Fitsiori et al., 2020, Keller et al., 2020, Radmanesh et al., 2020), while the remaining four were in deceased COVID-19 patients (Coolen et al., 2020, Kirschenbaum et al., 2021, Martin et al., 2022, Wierzba-Bobrowicz et al., 2021). The four studies in acute COVID-19 were similar with respect to the patient population observed in the case reports, all with critical care due to respiratory failure/distress and/or severe neurologic complications, and when reported, elevated thromboinflammatory markers and CSF studies negative for SARS-CoV-2 virus. Thirty-eight patients (29 male, 9 female) were reported across these four studies. Two out of the four studies had a mean age of 67 years old (Fitsiori et al., 2020, Keller et al., 2020) with the remaining two studies having a mean age of 56 years old (Dixon et al., 2020) and 53 years old (Radmanesh et al., 2020). Neuroradiologic findings showed MB in 32/38 patients and ischemic lesions in 14/38 patients; however, not all studies assessed for both MB and ischemic lesions, potentially leading to biased results. In a study showing increased MB in COVID-19 patients, Dixon et al. (2020) reported that several patients post-COVID-19 had ongoing self-reported cognitive dysfunction; however, these symptoms were not further investigated by formal neurocognitive assessment. The majority of the 38 patients had cardiovascular comorbidities (e.g., hypertension, obesity, type 2 diabetes), however, nine patients who presented with CSVD pathology had no predisposing comorbidities or risk factors.
The four post-mortem case series reported gross pathological, histological, and post-mortem MRI findings from patients with COVID-19-related death. Eighty-two patients were reported across these four studies, and when included, thromboinflammatory markers pre-mortem were elevated (22/30 patients) (Coolen et al., 2020, Kirschenbaum et al., 2021, Martin et al., 2022). CSF studies were not included for any of the patients. Sex was included in 3/4 studies with a 2:1 male-female ratio (Kirschenbaum et al., 2021, Martin et al., 2022, Wierzba-Bobrowicz et al., 2021). Of these severe acute COVID-19 case series of deceased patients, post-mortem MRI showed subcortical MB, specifically in the corpus callosum (CC), and WMH (Coolen et al., 2020, Kirschenbaum et al., 2021, Martin et al., 2022). Histology and gross pathological examination revealed CSVD findings, such as endothelitis, intravascular thrombosis, endothelial fibrosis, hyalinization, degeneration, and blood-brain barrier disruption from widened tight junctions and loss of matrix components in the basement membranes of capillaries (Kirschenbaum et al., 2021, Martin et al., 2022, Wierzba-Bobrowicz et al., 2021). All patients had one or more cardiovascular comorbidities, aside from a case series by Wierzba-Bobrowicz et al. (2021) where 92% of patients had comorbidities.
3.1.3. Observational studies
Sixteen observational studies of acute SARS-CoV-2 infection were included, highlighting many of the same conclusions as the case reports and series. Details of patient groups, characteristics, and neuropathological findings for the 16 acute and the seven recovered COVID-19 observational studies are reported in Table 3. The advantage of these studies was to compare different groups and control for comorbidities, past medical history, age, and sex to further solidify COVID-19 as the driver for cerebromicrovascular complications. COVID-19 patients with WMH and/or MB were associated with longer hospital stay, longer duration of mechanical ventilation and worse Glasgow Coma Scale compared to COVID-19 patients without neuropathology (Agarwal et al., 2020, Lersy et al., 2021). COVID-19 patients with MB had an increased burden of WMH compared to age, sex, past medical history, and acute symptom-matched COVID-19 patients with no MB (Napolitano et al., 2022). When comparing COVID-19 positive to COVID-19 negative patients, Mendez et al. examined 481 brain MRIs and found MB in 26/481 scans, 23.07% COVID-19 positive patients compared to 4.15% COVID-19 negative patients (Mendez Elizondo et al., 2021). Hautecloque et al. (2021) compared nine COVID-19 positive patients with stroke vs 50 COVID-19 negative patients with stroke and found significantly more microvascular infarctions in COVID-19 positive (4/9) compared to COVID-19 negative (6/50) patients matched for age, sex, past medical history, and cardiovascular risk factors. To examine the differences between acute respiratory distress syndrome (ARDS) and COVID-19-related cerebral small vessel pathology, Shoskes et al. (2022) measured the burden of WMH and MB in COVID-19 positive ARDS patients vs. COVID-19 negative ARDS age and sex-matched patients. 4/26 COVID-19 positive patients had diffuse and or multifocal WMH with underlying MB and COVID-19 negative ARDS group did not have any patients with this presentation. Multiple observational studies solidified the findings in case reports and series, reporting small vessel infarctions, WMH, and MB in COVID-19 patients (Conklin et al., 2021, Dixon et al., 2020, Fällmar et al., 2022, Kremer et al., 2020, Lin et al., 2020, Sawlani et al., 2021, Shahjouei et al., 2021, Taylor et al., 2020, Uginet et al., 2022).
Seven observational studies of recovered COVID-19 patients were also included, detailing long-term COVID-19-induced cerebromicrovascular complications and their association with cognitive impairment. In a study conducted by Bungenberg et al., 70% of this patient cohort had complaints of long-term cognitive impairment for 29 weeks following acute COVID-19. These patients were grouped into non-hospitalized and hospitalized groups, and MB were only seen in the recovered COVID-19 patients who were hospitalized. Moreover, MB were associated with worse cognitive functioning (Bungenberg et al., 2022). Andriuta et al. (2022) also observed impaired cognitive functioning in recovered COVID-19 patients. This patient cohort underwent neuroradiologic imaging 202 days after infection, and increased WMH burden negatively associated with cognitive performance with areas of executive function most affected. In a longitudinal study conducted by Lersy et al. (2022), COVID-19 patients underwent an MRI during acute infection and follow-ups at 95 and/or 189 days post-infection. Nineteen out of thirty-one patients had abnormal brain perfusion, nine with MB, four with small vessel infarct, and four with cerebral vasculitis at baseline. During follow-ups, MB remained stable, vasculitis resolved, and perfusion was normalized in 11/19 patients. However, at three months, 23 patients who underwent fluorodeoxyglucose (FDG)-positron emission tomography (PET)-CT had hypometabolism and for the 12 patients who had a second scan, five had persisting hypometabolism. In this patient cohort, working memory and executive functions were diminished in half of the patients through follow-ups. Cecchetti et al. (2022) examined recovered COVID-19 patients vs age, sex, and cardiovascular risk factor matched COVID-19 negative controls to assess CSVD pathology and cognitive function. Recovered COVID-19 patients had increased volume of WMH at 2 months after hospital discharge compared to controls. Increased WMH burden correlated with worse cognitive functioning. In a study by Poletti et al. (2022), COVID-19 patients who were recovered for 2 months underwent MRI and proton magnetic resonance spectroscopy to identify the presence of ischemic lesions and reduced glutathione (GSH) levels in the brain and anterior cingulate cortex (ACC), respectively. Increased WMH negatively associated with GSH levels in the ACC. As GSH is the main antioxidant in the brain (Dwivedi et al., 2020), this finding provides mechanistic insight into COVID-19 pathophysiology in the cerebromicrovasculature. Higher mean diffusivity (MD) from diffusion tensor imaging (DTI) revealed disrupted white matter integrity in recovered COVID-19 patients compared to age, sex, and cardiovascular risk factor controls. Although, authors stated that markers for CSVD based on DTI imaging did not differ between controls and recovered COVID-19 patients (Petersen et al., 2022). In addition, studies conducted by Pelizzari et al. (2022) and Petersen et al. (2022) showed that recovered COVID-19 patients had no difference in MB burden, infarctions, or WM lesions compared to COVID-19 negative controls.
3.2. Risk of bias
The risk of bias was assessed using the JBI critical appraisal tools with specific checklists for case reports, case series, cross-sectional, and case-control observational studies. A summary of the assessment results is in Supplementary Table A2–4. For case reports, patient history and presentation of the case as a timeline was unclear for eight studies (Abdi et al., 2020, Cannac et al., 2020, De Stefano et al., 2020, Fraiman et al., 2020, Frisullo et al., 2020, Hanafi et al., 2020, Rudilosso et al., 2020, Shoskes et al., 2020), current clinical condition was unclear for three (Cannac et al., 2020, Hanafi et al., 2020, Zhang et al., 2021), and diagnostic tests/assessment methods were unclear for one (Fraiman et al., 2020). Bias assessement of case-series resulted in three studies that did not have clear inclusion criteria and one that was unclear (Fitsiori et al., 2020, Keller et al., 2020, Kirschenbaum et al., 2021, Martin et al., 2022). Two studies did not have consecutive inclusion of participants (Kirschenbaum et al., 2021, Wierzba-Bobrowicz et al., 2021) and three had either unclear or did not have complete inclusion of participants (Kirschenbaum et al., 2021, Martin et al., 2022, Wierzba-Bobrowicz et al., 2021). One study had unclear reporting of patient demographics and clinical information (Radmanesh et al., 2020). For observational studies, one had groups that were not matched (Agarwal et al., 2020) and three studies had unclear criteria for inclusion and/or the same criteria used for identification of cases and controls (Bungenberg et al., 2022, Cecchetti et al., 2022, Mendez Elizondo et al., 2021; E. L. Petersen et al., 2022). Description of participants was not detailed or unclear in two studies (Mendez Elizondo et al., 2021, Pelizzari et al., 2022) and measuremnt of exposure (i.e., COVID-19) was unclear or not measured in a valid and reliable way in cases and controls in three studies (Cecchetti et al., 2022, Lersy et al., 2022, Shoskes et al., 2022). Five studies did not systematically evaluate confounders (Conklin et al., 2021, Lersy et al., 2022, Lin et al., 2020, Mendez Elizondo et al., 2021, Taylor et al., 2020). Lastly, outcomes were not valid or reliable, or were unclear in the validity or relaibility of measurements in two studies (Conklin et al., 2021, Taylor et al., 2020).
4. Discussion
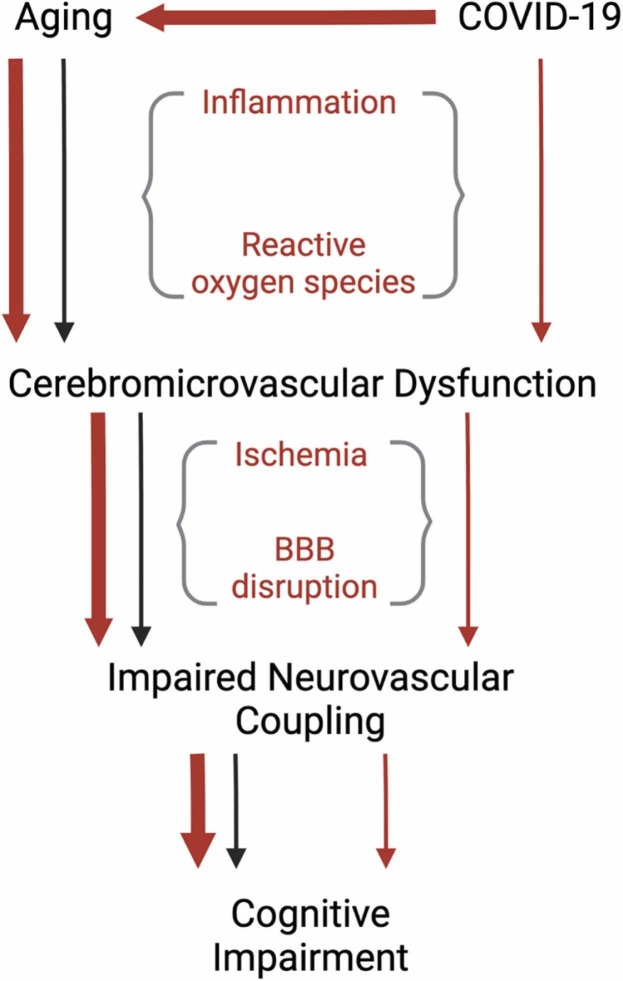
This systematic review investigated the contribution of COVID-19 in the development of CSVD in patients with a history of, or active SARS-CoV-2 infection. We found that CSVD pathology had a specific predilection for the corpus callosum, juxtacortical white matter, subcortical white matter, and periventricular white matter in acute and recovered COVID-19 patients, differing in predilection from other known causes of MB or ischemic lesions (i.e., ARDS, hypertension). Most COVID-19 studies with CSVD pathology were ≥ 55 years old (67%), an important finding as age is the number one non-modifiable risk factor for cerebrovascular accidents and doubles the risk of cerebrovascular complications every decade after 55 years old (Yousufuddin and Young, 2019). Moreover, age-related comorbidities (i.e., hypertension, type 2 diabetes) were highly prevalent among COVID-19 patients with CSVD. Many patients were in severe condition during acute infection, and biomarkers of thromboinflammation (e.g., CRP, IL-6, ferritin, D-dimer) were elevated in most studies, where laboratory data were reported. Furthermore, our review of the literature detailed COVID-19 patients with CSVD complications and patient characteristics consisting of cardiovascular comorbidities, increased severity of acute infection, and advanced age. These factors and concomitant history of, or active SARS-CoV-2 infection may have contributed to endothelial dysfunction and resulting elevations in thromboinflammatory markers, precipitating a CSVD pathology.
There is a strong link between COVID-19 severity and increased age (Neves et al., 2021). The elderly population has a greater amount of acute and post-COVID-19 complications, with two of the most prominent being neurological and vascular chronic complications (Alimohamadi et al., 2020, Mehandru and Merad, 2022, Mueller et al., 2020, Rahmani et al., 2022, SeyedAlinaghi et al., 2021). Aging is also a risk factor for developing long-COVID (Raveendran et al., 2021), in which the majority of patients experience cognitive impairment (Guo et al., 2022). COVID-19 is known to cause elevated levels of inflammation, levels which are chronically elevated in aging individuals (Zuo et al., 2019), in acute (Cao, 2020, Chen et al., 2020, Del Valle et al., 2020, Paliogiannis et al., 2020) and recovered COVID-19 patients (Holms, 2022, Peluso et al., 2021, Schultheiß et al., 2022), and increase the risk, particularly in individuals with age-related comorbidities, of developing endothelial dysfunction (Damiani et al., 2020, Incalza et al., 2018, Lorenzo et al., 2020, Pons et al., 2020, Ruhl et al., 2021, Sabioni et al., 2020, Sardu et al., 2020). Endothelial dysfunction is directly involved in many age-related conditions such as peripheral artery disease (Owens et al., 2022), diabetes, and heart disease (Rajendran et al., 2013). In addition to these pathologies, CSVD, a condition that primarily disrupts the normal functioning of small vessels in the brain (Mustapha et al., 2019) and has an increased risk of development with age (Mu et al., 2022), appears to be exacerbated by COVID-19. However, the exact mechanism underlying SARS-CoV-2 disruption of cerebromicrovascular endothelial function and, in turn, augmented CSVD pathology is unknown.
Two distinct CSVD pathologies seen in COVID-19 patients are small vessel ischemic lesions/infarctions and cerebral MB with each pathology having a predilection for the subcortex and corpus callosum, respectively. Small vessel ischemic lesions/infarctions are more prevalent in the white matter due to increased vulnerability from decreased capillary density (Hase et al., 2019, Pantoni et al., 1996). Ischemic lesions and recent infarctions are commonly seen as hyperintense signals on T2 FLAIR MRI (Chojdak-Łukasiewicz et al., 2021), and when located in the white matter, represented by WMH (Li et al., 2018), are further classified as subcortical/deep and periventricular WMH (Griffanti et al., 2018) and are associated with aging (d’d’d’Arbeloff et al., 2019) and many age-related diseases, including hypertension (Hajjar et al., 2011), type 2 diabetes (Wang et al., 2020), and hypercholesterolemia (Moroni et al., 2020). MB are also associated with aging and age-related diseases (Martinez-Ramirez et al., 2014), with some conditions having specific locations of MB associated with the underlying pathology. In patients with MB due to hypertension, these lesions are located in deep gray matter or the brainstem (Lee et al., 2018), while type 2 diabetes and aging do not have a specific predilection, but are associated with increased burden (Altmann-Schneider et al., 2011, Zhou et al., 2017). However, there is ample evidence that ARDS or mechanical ventilation, a common complication in severe COVID-19, have localization of MB to specific areas of the brain. There is potential for iatrogenic acute lung injury during mechanical ventilation causing hypercapnia and cerebrovascular dilation, resulting in increased cerebral blood flow and intracranial hypertension, a well-documented cause of cerebral MB (Elmståhl et al., 2019, Ziaka and Exadaktylos, 2022). In addition, mechanical ventilation can cause excessive systemic pro-inflammatory cytokine release, further damaging the cerebromicrovascular endothelium and contributing to breakdown of the blood-brain barrier (Yang et al., 2022). In patients with ARDS, cerebrovascular vasoconstriction and concomitant endothelial cell activation may result from prolonged hypoxia, contributing to increased small vessel ischemic lesions/infarctions (Elmståhl et al., 2019). ARDS related cerebral MB have a distinct localization on neuroradiographic imaging, allowing for easier identification of the underlying pathology of CVSD. Similar to microbleeds seen from high altitude exposure, critical illness associated microbleeds (i.e., ARDS) have a predilection for the splenium of the corpus callosum (Riech et al., 2015) with MB sparing the cortex, deep/subcortical and periventricular white matter, basal ganglia and thalami (Fanou et al., 2017). While the corpus callosum was the most reported area for cerebral MB in our systematic review of the COVID-19 literature, subcortical and juxtacortical white matter were the second and third most reported. This indicates the possibility of the SARS-CoV-2 virus exacerbating underlying age-related conditions and medical comorbidities, while also causing direct damage to the cerebromicrovasculature opposed to the above-mentioned diseases and medical interventions.
Elevations in thromboinflammatory markers (e.g., CRP, IL-6, D-dimer) are common in acute (Ali, 2020, Yao et al., 2020) and recovered (Lehmann et al., 2021, Mandal et al., 2021) COVID-19 patients, and were seen throughout this review in most patients where laboratory data was reported. Pathophysiological changes proposed for COVID-19 induced cerebrovascular dysfunction is elevations in pro-inflammatory cytokines and reactive oxygen species (ROS) (Sashindranath and Nandurkar, 2021), acutely and in recovered patients (Vollbracht and Kraft, 2022). SARS-CoV-2, through attachment of spike protein to ACE2 receptor on endothelial cells, causes dysregulation of endothelial functions inducing a pro-thromboinflammatory and pro-oxidative environment, as well as activation of complement, increased endothelial barrier permeability and impaired vasodilatory capacity (Otifi and Adiga, 2022, Xu et al., 2021). Therefore, it is reasonable to assume that the dysregulation of these functions underlies the cerebromicrovascular complications reported in COVID-19 induced CSVD cases. While the ‘cytokine storm’ observed in COVID-19 patients is a well-established systemic manifestation of acute infection (Montazersaheb et al., 2022), multiple research groups, Peluso et. al. and Schulthei et. al., have demonstrated that COVID-19 is associated with long-lasting elevations in inflammation evidenced by increased levels of pro-inflammatory cytokines IL-1, IL-6 and TNF-alpha up to 10 months after infection (Holms, 2022, Peluso et al., 2021, Schultheiß et al., 2022). In addition, Al-Hakeim et. al. reported increased oxidative damage elicited by ROS in recovered COVID-19 patients that correlated with depression and anxiety, neuropsychiatric conditions that often accompany dementia (Lyketsos et al., 2002), 3 months following infection (Al-Hakeim et al., 2022). This, in conjunction with the known deleterious effects of oxidative stress and inflammation on the vasculature and its association with cognitive impairment (Ungvari et al., 2018), provide a strong rationale for the proposed hypothesis of COVID-19 inducing cerebrovascular dysfunction and long-lasting cognitive impairment through promotion of a prolonged thromboinflammatory environment and dysregulation of endothelial function.
Normal brain function critically depends on adequate oxygen and nutrient delivery which is achieved through the fundamental homeostatic mechanism of neurovascular coupling (NVC). NVC is responsible for moment-to-moment adjustment of cerebral blood flow through endothelium-dependent cerebromicrovascular dilation to match the increased metabolic needs of active brain regions. This enables rapid increases in O2 and glucose delivery to active neurons and washout of toxic metabolic by-products (Csipo et al., 2021, Csipo et al., 2019, Tarantini et al., 2017). Preclinical evidence indicates the cerebromicrovascular endothelium and endothelial nitric oxide production as key mediators in the NVC response with pharmacological impairment of brain capillaries attenuating the NVC response, and rescue of cerebromicrovascular endothelial function ameliorating NVC dysfunction and restoring cognitive function (Csiszar et al., 2019, Tarantini et al., 2021, Tarantini et al., 2015, Tarantini et al., 2019, Tarantini et al., 2018, Tarantini et al., 2019, Toth et al., 2014). Impaired NVC is strongly associated with cognitive impairment (Csipo et al., 2021, Owens et al., 2022, Tarantini et al., 2017), Alzheimer’s Disease (Tarantini et al., 2017, Zhu et al., 2022) and VaD (Quick et al., 2021, Shabir et al., 2018). Patients with diseases and conditions known to impair the peripheral macro- and microvascular endothelium (e.g. obesity, peripheral artery disease, aging, diabetes, hypertension) (Balasubramanian et al., 2021, Kazama et al., 2004, Owens et al., 2022, Scioli et al., 2014, Shi and Vanhoutte, 2017, Yannoutsos et al., 2014) have shown decreased cognitive performance (Hay et al., 2020) associated with impaired NVC (Balasubramanian et al., 2021, Csiszar et al., 2017, Duarte et al., 2015, Lasta et al., 2013, Mogi and Horiuchi, 2011, Owens et al., 2022, Tucsek et al., 2014). In addition, atherosclerosis, a systemic thromboinflammatory disease and leading cause of CSVD, is believed to impair NVC (Shabir et al., 2018), indicating the plausibility of generalized endothelial dysfunction extending to the cerebromicrovasculature and disrupting normal cognitive function. Proximal to microcirculatory regulation of NVC, larger cerebral arteries and arterioles regulate cerebral blood flow by the myogenic autoregulatory mechanism. This ensures prevention of high pressure reaching the distal microcirculation, protecting against the detrimental effects of rapid changes in blood pressure, enabling the endothelium to locally regulate blood flow at the microvascular level (Toth et al., 2017). Impairment of cerebral autoregulation, seen in comorbidities commonly associated with vascular cognitive impairment and dementia (i.e., hypertension, diabetes) may also contribute to impairment of NVC due to hypoperfusion or elevated arterial pressure in the distal microcirculation, potentially causing CSVD pathologies of ischemic lesions and microbleeds, respectively (Wang et al., 2022). Moreover, CSVD is associated with impaired NVC (Moretti and Caruso, 2020) and is strongly linked to COVID-19, as is evidenced in this systematic review. This, in conjunction with the deleterious effects COVID-19 has on the vasculature, indicates the plausibility of impaired NVC being the mechanism for which cognitive impairment occurs in recovered COVID-19 patients.
Cerebromicrovascular impairment and resulting impaired cognitive performance has been shown to be reversible in pre-clinical and clinical studies (Csiszar et al., 2019, Sorond et al., 2013, Tarantini et al., 2021, Tarantini et al., 2018, Tarantini et al., 2019, Toth et al., 2014), indicating a promising therapeutic approach to target NVC and to combat long-term cognitive consequences in COVID-19 patients. In brief, these interventions have focused on attenuating oxidative stress through pharmacological treatment or genetic manipulation, which rescue NVC response due to restoration of cerebromicrovascular endothelial health. Due to the immense crosstalk between ROS, inflammation, and coagulation (Wu et al., 2020), targeting one or more of these pathways may prove beneficial for COVID-19 induced CSVD, cognitive impairment, and potentially, impaired NVC. Further, in a recent review of potential therapies for endothelial dysfunction in COVID-19 (Xu et al., 2023), lipid-lowering, anti-inflammatory, anti-coagulatory, and antioxidant drugs have shown protective effects against endothelial dysfunction in COVID-19. Hence, further investigation into the role of NVC dysfunction in COVID-19 induced CSVD, and potential benefits of endothelial protective treatment must be studied to mitigate acute and long-term effects of COVID-19 on cerebrovascular and cognitive health.
5. Limitations and Future Directions
5.1. Limitations of included studies and future directions
COVID-19 induced CSVD is a new phenomenon, with the spread of the SARS-CoV-2 virus and the resulting pandemic beginning in 2020. The limited number of longitudinal observational studies assessing the relationship between COVID-19 and CSVD make it difficult to generalize findings. However, with numerous case reports and series, we observed a trend of CSVD in this patient population in the acute phase and during convalescence. In addition, we noted a different neuroanatomical predilection for COVID-19 patients with CSVD compared to other underlying pathologies. Future studies in more homogeneous groups (e.g., controlled for comorbidities) are needed to assess the direct effect of COVID-19 induced CSVD. More extended cohort studies are needed to fully elucidate the impact of COVID-19 on cerebromicrovascular function. Baseline MRI is necessary to fully understand the long term and acute ischemic and hemorrhagic changes associated with COVID-19. Also, not all studies were assessing for both MB and ischemic lesions/infarctions, leading to a potential involuntary bias among papers only reporting microbleeds or ischemic lesions. While not a specific criterion for this review, neurocognitive testing should be employed in all longitudinal studies as CSVD is the leading cause of VaD and commonly occurs in conjunction with Alzheimer’s Disease pathology (Kim et al., 2020). Future studies should investigate NVC as an area of impairment in this patient subset and as a potential mechanism for cognitive decline given the strong association between impaired NVC and vascular comorbidities associated with, and that contribute to CSVD.
5.2. Limitations of the review
COVID-19 exposure on the effect of CSVD pathology was qualitative rather than quantitative (meta-analysis), limiting the overall generalizability of the review. Bias assessment resulted in a minimal amount of bias regarding the primary outcomes pertinent to this review – COVID-19 status and CSVD pathology. However, key areas need to be addressed for future studies, such as clear information regarding inclusion criteria (e.g., severe COVID-19), and patient demographics/clinical information must be reported as age-related comorbidities are strongly associated with COVID-19 adverse events. Lastly, in case-control and cohort studies, groups need to be matched for comorbidities, age, and sex to assess COVID-19 as an independent factor driving cerebromicrovascular pathologies.
6. Conclusion
CSVD is a prevalent complication in COVID-19 patients with older individuals and those with underlying age-related diseases at increased risk for cerebrovascular complications and cognitive decline ( Fig. 3). While the etiology of CSVD in this patient population has yet to be uncovered, evidence points towards a systemic action of the SARS-CoV-2 virus, inducing elevations in thromboinflammation and ROS, eliciting deleterious effects on the vasculature. Small vessel complications in COVID-19 have a predilection for the subcortical white matter, corpus callosum, juxtacortical white matter, and periventricular white matter, differentiating this CSVD pathology from other known causes (e.g., ARDS, hypertension). While there is a difference in neuroanatomical preference, further studies are needed to differentiate the effect of COVID-19 from past medical history, comorbidities, hospital intervention, and critical care status. With the association of cognitive impairment in COVID-19, and CSVD being a leading cause of vascular cognitive impairment and dementia, NVC should be considered as a mechanism for cognitive dysfunction in this patient population. Identifying a mechanism for COVID-19-induced CSVD may open the door for novel treatments and mitigate the incidence and progression of cognitive impairment in this vulnerable patient population.
Fig. 3.
COVID-19 exacerbation of age-related mechanisms contributing to cognitive impairment. The diagram outlines COVID-19 (red arrows) and aging (black arrows) independent effects on cerebromicrovascular function and COVID-19 exacerbation (large red arrows) on age-related cerebromicrovascular dysfunction and cognitive impairment. We hypothesize that the effect of COVID-19 on cognition is through exacerbation of age-related pathophysiological elevations in inflammation and reactive oxygen species, resulting in cerebromicrovascular dysfunction and contributing to CSVD pathology, concluding with impaired NVC and cognitive impairment.
Funding Statement
Research reported in this publication was supported by the American Heart Association (AHA966924, AHA941290, 23DIVSUP1070991), the National Institute on Aging (R01AG075834, R03AG070479, K01AG073614, RF1AG072295, R01AG055395, R01AG068295, R21AG075639), the National Institute of Neurological Disorders and Stroke (R01NS100782), the Department of Veterans Affairs Clinical Science Research & Development Service (CX000340), the National Cancer Institute (R01CA255840, P30CA225520) of the National Institutes of Health and the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363). This research was also supported by the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS and the Oklahoma Tobacco Settlement Endowment Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs or the American Heart Association.
CRediT authorship contribution statement
Cameron D. Owens: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Camila Bonin Pinto: Conceptualization, Methodology, Validation, Writing – review & editing, Visualization, Funding acquisition. Sam Detwiler: Validation, Investigation, Data curation, Writing – review & editing. Peter Mukli: Writing – review & editing. Anna Peterfi: Writing – review & editing. Zsofia Szarvas: Writing – review & editing. Jordan R. Hoffmeister: Writing – review & editing. Juliette Galindo: Writing – review & editing. Jila Noori: Writing – review & editing. Angelia C. Kirkpatrick: Writing – review & editing. Tarun W. Dasari: Writing – review & editing, Funding acquisition. Judith James: Writing – review & editing, Funding acquisition. Stefano Tarantini: Writing – review & editing, Funding acquisition. Anna Csiszar: Writing – review & editing, Funding acquisition. Zoltan Ungvari: Writing – review & editing, Funding acquisition. Calin I. Prodan: Conceptualization, Writing – review & editing, Funding acquisition. Andriy Yabluchanskiy: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
None.
Acknowledgments
Graphical abstract and figures were created with BioRender.com and GraphPad Prism version 9.5.1 for MacOS, GraphPad Software, San Diego, California USA, www.graphpad.com.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.arr.2023.101962.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
No data was used for the research described in the article.
References
- Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Jain R., Dogra S., Krieger P., Lewis A., Nguyen V., Melmed K., Galetta S. Cerebral microbleeds and leukoencephalopathy in critically Ill patients with COVID-19. Stroke. 2020;51(9):2649–2655. doi: 10.1161/strokeaha.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Gaba W.H., Khan F.M., Mansoori R.A., Adi A.A.K. COVID-related leukoencephalopathy: unusual MRI features and comparability to delayed post hypoxic ischemic encephalopathy. Radio. Case Rep. 2022;17(3):852–855. doi: 10.1016/j.radcr.2021.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hakeim H.K., Al-Rubaye H.T., Al-Hadrawi D.S., Almulla A.F., Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol. Psychiatry. 2022 doi: 10.1038/s41380-022-01836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohamadi Y., Sepandi M., Taghdir M., Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J. Prev. Med. Hyg. 2020;61(3) doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann-Schneider I., Trompet S., de Craen A.J., van Es A.C., Jukema J.W., Stott D.J., Sattar N., Westendorp R.G., van Buchem M.A., van der Grond J. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42(3):638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- Ambrosino P., Calcaterra I., Molino A., Moretta P., Lupoli R., Spedicato G.A., Papa A., Motta A., Maniscalco M., Di Minno M.N.D. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines. 2021;9(8) doi: 10.3390/biomedicines9080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriuta D., Si-Ahmed C., Roussel M., Constans J.M., Makki M., Aarabi A., Basille D., Andrejak C., Godefroy O. Clinical and imaging determinants of neurocognitive disorders in post-acute COVID-19 patients with cognitive complaints. J. Alzheimers Dis. 2022;87(3):1239–1250. doi: 10.3233/jad-215506. [DOI] [PubMed] [Google Scholar]
- Backman L., Möller M.C., Thelin E.P., Dahlgren D., Deboussard C., Östlund G., Lindau M. Monthlong intubated patient with life-threatening COVID-19 and cerebral microbleeds suffers only mild cognitive sequelae at 8-month follow-up: a case report. Arch. Clin. Neuropsychol. 2022;37(2):531–543. doi: 10.1093/arclin/acab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian P., Kiss T., Tarantini S., Nyúl-Tóth Á., Ahire C., Yabluchanskiy A., Csipo T., Lipecz A., Tabak A., Institoris A. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am. J. Physiol. -Heart Circ. Physiol. 2021;320(2):H740–H761. doi: 10.1152/ajpheart.00736.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.H., Lin J.J., Doernberg M., Stone K., Navis A., Festa J.R., Wisnivesky J.P. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungenberg J., Humkamp K., Hohenfeld C., Rust M.I., Ermis U., Dreher M., Hartmann N.K., Marx G., Binkofski F., Finke C., Schulz J.B., Costa A.S., Reetz K. Long COVID-19: Objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022;9(2):141–154. doi: 10.1002/acn3.51496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannac O., Martinez-Almoyna L., Hraiech S. Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology. 2020;95(11):498–499. doi: 10.1212/wnl.0000000000010537. [DOI] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti G., Agosta F., Canu E., Basaia S., Barbieri A., Cardamone R., Bernasconi M.P., Castelnovo V., Cividini C., Cursi M., Vabanesi M., Impellizzeri M., Lazzarin S.M., Fanelli G.F., Minicucci F., Giacalone G., Falini A., Falautano M., Rovere-Querini P., Roveri L., Filippi M. Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J. Neurol. 2022;269(7):3400–3412. doi: 10.1007/s00415-022-11047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charfeddine S., Ibn Hadj Amor H., Jdidi J., Torjmen S., Kraiem S., Hammami R., Bahloul A., Kallel N., Moussa N., Touil I. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front. Cardiovasc. Med. 2021;8:1702. doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zheng K.I., Liu S., Yan Z., Xu C., Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020;19(1):1–7. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojdak-Łukasiewicz J., Dziadkowiak E., Zimny A., Paradowski B. Cerebral small vessel disease: a review. Adv. Clin. Exp. Med. 2021;30(3):349–356. doi: 10.17219/acem/131216. [DOI] [PubMed] [Google Scholar]
- Conklin J., Frosch M.P., Mukerji S.S., Rapalino O., Maher M.D., Schaefer P.W., Lev M.H., Gonzalez R.G., Das S., Champion S.N. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J. Neurol. Sci. 2021;421 doi: 10.1016/j.jns.2021.117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovai A., Trotta N., Taccone F.S., Creteur J., Henrard S., Goffard J.C., Dewitte O., Naeije G., Goldman S., De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95(14):e2016–e2027. doi: 10.1212/wnl.0000000000010116. [DOI] [PubMed] [Google Scholar]
- Cordonnier C., van der Flier W.M. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain. 2011;134(2):335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- Csipo T., Mukli P., Lipecz A., Tarantini S., Bahadli D., Abdulhussein O., Owens C., Kiss T., Balasubramanian P., Nyúl-Tóth Á. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41(5):495–509. doi: 10.1007/s11357-019-00122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T., Lipecz A., Owens C., Mukli P., Perry J.W., Tarantini S., Balasubramanian P., Nyúl-Tóth Á., Yabluchanska V., Sorond F.A. Sleep deprivation impairs cognitive performance, alters task-associated cerebral blood flow and decreases cortical neurovascular coupling-related hemodynamic responses. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-00188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T., Lipecz A., Mukli P., Bahadli D., Abdulhussein O., Owens C.D., Tarantini S., Hand R.A., Yabluchanska V., Kellawan J.M. Increased cognitive workload evokes greater neurovascular coupling responses in healthy young adults. Plos One. 2021;16(5) doi: 10.1371/journal.pone.0250043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A., Tarantini S., Fülöp G.A., Kiss T., Valcarcel-Ares M.N., Galvan V., Ungvari Z., Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39(4):359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A., Yabluchanskiy A., Ungvari A., Ungvari Z., Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41(5):609–617. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Arbeloff T., Elliott M.L., Knodt A.R., Melzer T.R., Keenan R., Ireland D., Ramrakha S., Poulton R., Anderson T., Caspi A., Moffitt T.E., Hariri A.R. White matter hyperintensities are common in midlife and already associated with cognitive decline. Brain Commun. 2019;1(1) doi: 10.1093/braincomms/fcz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E., Carsetti A., Casarotta E., Scorcella C., Domizi R., Adrario E., Donati A. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann. Intensive Care. 2020;10(1):1–3. doi: 10.1186/s13613-020-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Author correction: long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21(6) doi: 10.1038/s41579-023-00896-0. 408-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano P., Nencha U., De Stefano L., Mégevand P., Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin. Neurophysiol. Pr. 2020;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Toro A., Bozzani A., Tavazzi G., Urtis M., Giuliani L., Pizzoccheri R., Aliberti F., Fergnani V., Arbustini E. Long COVID: long-term effects? Eur. Heart J. Suppl. 2021;23(Supplement_E):E1–E5. doi: 10.1093/eurheartj/suab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L., McNamara C., Gaur P., Mallon D., Coughlan C., Tona F., Jan W., Wilson M., Jones B. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon. Stroke Vasc. Neurol. 2020;5(4):315–322. doi: 10.1136/svn-2020-000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J.V., Pereira J.M., Quendera B., Raimundo M., Moreno C., Gomes L., Carrilho F., Castelo-Branco M. Early disrupted neurovascular coupling and changed event level hemodynamic response function in type 2 diabetes: an fMRI study. J. Cereb. Blood Flow. Metab. 2015;35(10):1671–1680. doi: 10.1038/jcbfm.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi D., Megha K., Mishra R., Mandal P.K. Glutathione in brain: overview of its conformations, functions, biochemical characteristics, quantitation and potential therapeutic role in brain disorders. Neurochem. Res. 2020;45(7):1461–1480. doi: 10.1007/s11064-020-03030-1. [DOI] [PubMed] [Google Scholar]
- Elmståhl S., Ellström K., Siennicki-Lantz A., Abul-Kasim K. Association between cerebral microbleeds and hypertension in the Swedish general population "Good Aging in Skåne" study. J. Clin. Hypertens. 2019;21(8):1099–1107. doi: 10.1111/jch.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshereye A., Erdinc B. Multiple lacunar cerebral infarcts as the initial presentation of COVID-19. Cureus. 2020;12(8) doi: 10.7759/cureus.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L.E., Taylor J.L., Smith C.J., Pritchard H.A., Greenstein A.S., Allan S.M. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc. Res. 2021;117(13):2575–2588. doi: 10.1093/cvr/cvab284. [DOI] [PubMed] [Google Scholar]
- Fällmar D., Rostami E., Kumlien E., Ashton N.J., Jackmann S., Pavel R., Blennow K., Hultström M., Lipcsey M., Frithiof R., Westman G., Zetterberg H., Wikström J., Virhammar J. The extent of neuroradiological findings in COVID-19 shows correlation with blood biomarkers, Glasgow coma scale score and days in intensive care. J. Neuroradiol. 2022;49(6):421–427. doi: 10.1016/j.neurad.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanou E.M., Coutinho J.M., Shannon P., Kiehl T.-R., Levi M.M., Wilcox M.E., Aviv R.I., Mandell D.M. Critical illness–associated cerebral microbleeds. Stroke. 2017;48(4):1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Ischemic stroke in a SARS-CoV-2-positive octagenarian without cardiovascular risk factors: a case report. Cureus. 2022;14(3) doi: 10.7759/cureus.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. COVID-19 is associated with an unusual pattern of brain microbleeds in critically Ill patients. J. Neuroimaging. 2020;30(5):593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiman P., Freire M., Moreira-Neto M., Godeiro-Junior C. Hemorrhagic stroke and COVID-19 infection: coincidence or causality? eNeurologicalSci. 2020;21 doi: 10.1016/j.ensci.2020.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisullo G., Bellavia S., Scala I., Piano C., Morosetti R., Brunetti V., Calabresi P., Della Marca G. Stroke and COVID19: Not only a large-vessel disease. J. Stroke Cereb. Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Jenkinson M., Suri S., Zsoldos E., Mahmood A., Filippini N., Sexton C.E., Topiwala A., Allan C., Kivimäki M., Singh-Manoux A., Ebmeier K.P., Mackay C.E., Zamboni G. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: A study in older adults. NeuroImage. 2018;170:174–181. doi: 10.1016/j.neuroimage.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Guo P., Benito Ballesteros A., Yeung S.P., Liu R., Saha A., Curtis L., Kaser M., Haggard M.P., Cheke L.G. COVCOG 2: cognitive and memory deficits in long COVID: a second publication from the COVID and cognition study. Front. Aging Neurosci. 2022:14. doi: 10.3389/fnagi.2022.804937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N.A., Lien C., Iv M. Critical illness-associated cerebral microbleeds in severe COVID-19 infection. Clin. Imaging. 2020;68:239–241. doi: 10.1016/j.clinimag.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I., Quach L., Yang F., Chaves P.H., Newman A.B., Mukamal K., Longstreth W., Jr., Inzitari M., Lipsitz L.A. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation. 2011;123(8):858–865. doi: 10.1161/circulationaha.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafi R., Roger P.A., Perin B., Kuchcinski G., Deleval N., Dallery F., Michel D., Hacein-Bey L., Pruvo J.P., Outteryck O., Constans J.M. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am. J. Neuroradiol. 2020;41(8):1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon K.H., Patro S.N., Hussain S., Zafar A., Muhammad A. Multiple microbleeds: a serious neurological manifestation in a critically Ill COVID-19 patient. Case Rep. Neurol. 2020;12(3):373–377. doi: 10.1159/000512322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase Y., Ding R., Harrison G., Hawthorne E., King A., Gettings S., Platten C., Stevenson W., Craggs L.J., Kalaria R.N. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol. Commun. 2019;7(1):1–12. doi: 10.1186/s40478-019-0666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautecloque G., Kempf C., Stan C., Arentz-Dugay M.H., Vuillemet F., Ahle G., Sellal F., Martinot M. Multifocal and microvascular involvement in ischemic stroke during COVID-19: a cohort study with comparison with Non-COVID-19 stroke. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.732194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M., Barnes C., Huentelman M., Brinton R., Ryan L. Hypertension and age-related cognitive impairment: common risk factors and a role for precision aging. Curr. Hypertens. Rep. 2020;22(10):1–14. doi: 10.1007/s11906-020-01090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms R.D. Long COVID (PASC) is maintained by a self-sustaining pro-inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE signalling, inducing chronic expression of IL-1b, IL-6 and TNFa: anti-inflammatory ezrin peptides as potential therapy. Immuno. 2022;2(3):512–533. 〈https://www.mdpi.com/2673-5601/2/3/33〉 [Google Scholar]
- Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharm. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- JBI. (2020). Critical Appraisal Tools. 〈https://jbi.global/critical-appraisal-tools〉.
- Kazama K., Anrather J., Zhou P., Girouard H., Frys K., Milner T.A., Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase–derived radicals. Circ. Res. 2004;95(10):1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Keller E., Brandi G., Winklhofer S., Imbach L.L., Kirschenbaum D., Frontzek K., Steiger P., Dietler S., Haeberlin M., Willms J., Porta F., Waeckerlin A., Huber M., Abela I.A., Lutterotti A., Stippich C., Globas C., Varga Z., Jelcic I. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020;51(12):3719–3722. doi: 10.1161/strokeaha.120.031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.W., Hong J., Jeon J.C. Cerebral small vessel disease and Alzheimer's disease: a review. Front Neurol. 2020;11:927. doi: 10.3389/fneur.2020.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum D., Imbach L.L., Rushing E.J., Frauenknecht K.B.M., Gascho D., Ineichen B.V., Keller E., Kohler S., Lichtblau M., Reimann R.R., Schreib K., Ulrich S., Steiger P., Aguzzi A., Frontzek K. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021;47(3):454–459. doi: 10.1111/nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., de Sèze J., Ferré J.C., Maamar A., Carsin-Nicol B., Collange O., Bonneville F., Adam G., Martin-Blondel G., Rafiq M., Geeraerts T., Delamarre L., Grand S., Krainik A., Caillard S., Constans J.M., Metanbou S., Heintz A., Helms J., Schenck M., Lefèbvre N., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Lacalm A., Oesterlé H., Bolognini F., Messié J., Hmeydia G., Benzakoun J., Oppenheim C., Bapst B., Megdiche I., Henry Feugeas M.C., Khalil A., Gaudemer A., Jager L., Nesser P., Talla Mba Y., Hemmert C., Feuerstein P., Sebag N., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Comby P.O., Ricolfi F., Thouant P., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Zorn P.E., Matthieu M., Baloglu S., Ardellier F.D., Willaume T., Brisset J.C., Boulay C., Mutschler V., Hansmann Y., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., David J.S., Meyer N., Anheim M., Cotton F. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–e251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasta M., Pemp B., Schmidl D., Boltz A., Kaya S., Palkovits S., Werkmeister R., Howorka K., Popa-Cherecheanu A., Garhöfer G. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 2013;54(1):842–847. doi: 10.1167/iovs.12-10873. [DOI] [PubMed] [Google Scholar]
- Lee J., Sohn E.H., Oh E., Lee A.Y. Characteristics of cerebral microbleeds. Dement Neurocogn Disord. 2018;17(3):73–82. doi: 10.12779/dnd.2018.17.3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A., Prosch H., Zehetmayer S., Gysan M.R., Bernitzky D., Vonbank K., Idzko M., Gompelmann D. Impact of persistent D-dimer elevation following recovery from COVID-19. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0258351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersy F., Willaume T., Brisset J.C., Collange O., Helms J., Schneider F., Chammas A., Willaume A., Meyer N., Anheim M., Cotton F., Kremer S. Critical illness-associated cerebral microbleeds for patients with severe COVID-19: etiologic hypotheses. J. Neurol. 2021;268(8):2676–2684. doi: 10.1007/s00415-020-10313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersy F., Bund C., Anheim M., Mondino M., Noblet V., Lazzara S., Phillipps C., Collange O., Oulehri W., Mertes P.M., Helms J., Merdji H., Schenck M., Schneider F., Pottecher J., Giraudeau C., Chammas A., Ardellier F.D., Baloglu S., Ambarki K., Namer I.J., Kremer S. Evolution of neuroimaging findings in severe COVID-19 patients with initial neurological impairment: an observational study. Viruses. 2022;14(5) doi: 10.3390/v14050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yang Y., Reis C., Tao T., Li W., Li X., Zhang J.H. Cerebral small vessel disease. Cell Transpl. 2018;27(12):1711–1722. doi: 10.1177/0963689718795148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H.W., Mung S.M., Douglass C., Jude E.B. COVID-19-related vasculopathy of the brain. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-242028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Lantos J.E., Strauss S.B., Phillips C.D., Campion T.R., Jr., Navi B.B., Parikh N.S., Merkler A.E., Mir S., Zhang C., Kamel H., Cusick M., Goyal P., Gupta A. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am. J. Neuroradiol. 2020;41(11):2001–2008. doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A.D., Escobar S., Tibiriçá E. Systemic endothelial dysfunction: a common pathway for COVID-19. Cardiovasc. Metab. Dis. 2020;30(8) doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos C.G., Lopez O., Jones B., Fitzpatrick A.L., Breitner J., DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairmentresults from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., Heightman M., Hillman T.E., Jacob J., Jarvis H.C., Lipman M.C.I., Naidu S.B., Nair A., Porter J.C., Tomlinson G.S., Hurst J.R. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Paes V.R., Cardoso E.F., Neto C., Kanamura C.T., Leite C.D.C., Otaduy M.C.G., Monteiro R.A.A., Mauad T., da Silva L.F.F., Castro L.H.M., Saldiva P.H.N., Dolhnikoff M., Duarte-Neto A.N. Postmortem brain 7T MRI with minimally invasive pathological correlation in deceased COVID-19 subjects. Insights Imaging. 2022;13(1):7. doi: 10.1186/s13244-021-01144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ramirez S., Greenberg S.M., Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimer'S. Res. Ther. 2014;6(3):33. doi: 10.1186/alzrt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez Elizondo E.F., Valdez Ramírez J.A., Barraza Aguirre G., Dautt Medina P.M., Berlanga Estens J. Central nervous system injury in patients with severe acute respiratory syndrome coronavirus 2: MRI findings. Cureus. 2021;13(9) doi: 10.7759/cureus.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-García L.A., Escobedo G., Minguer-Uribe A.G., Viurcos-Sanabria R., Aguayo-Guerrero J.A., Carrillo-Ruiz J.D., Solleiro-Villavicencio H. Role of the renin-angiotensin system in the development of COVID-19-associated neurological manifestations. Front. Cell. Neurosci. 2022:16. doi: 10.3389/fncel.2022.977039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ. J. 2011;75(5):1042–1048. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- Montazersaheb S., Hosseiniyan Khatibi S.M., Hejazi M.S., Tarhriz V., Farjami A., Ghasemian Sorbeni F., Farahzadi R., Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol. J. 2022;19(1):92. doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta P., Maniscalco M., Papa A., Lanzillo A., Trojano L., Ambrosino P. Cognitive impairment and endothelial dysfunction in convalescent COVID-19 patients undergoing rehabilitation. Eur. J. Clin. Invest. 2022;52(2) doi: 10.1111/eci.13726. [DOI] [PubMed] [Google Scholar]
- Moretti R., Caruso P. Small vessel disease-related dementia: an invalid neurovascular coupling? Int. J. Mol. Sci. 2020;21(3):1095. doi: 10.3390/ijms21031095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F., Ammirati E., Hainsworth A.H., Camici P.G. Association of white matter hyperintensities and cardiovascular disease: the importance of microcirculatory disease. Circ.: Cardiovasc. Imaging. 2020;13(8) doi: 10.1161/CIRCIMAGING.120.010460. [DOI] [PubMed] [Google Scholar]
- Mu R., Qin X., Guo Z., Meng Z., Liu F., Zhuang Z., Zheng W., Li X., Yang P., Feng Y., Jiang Y., Zhu X. Prevalence and consequences of cerebral small vessel diseases: a cross-sectional study based on community people plotted against 5-year age strata. Neuropsychiatr. Dis. Treat. 2022;18:499–512. doi: 10.2147/ndt.S352651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12(10):9959. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukli P., Detwiler S., Owens C., Csipo T., Lipecz A., Pinto C., Tarantini S., Nyul-Toth A., Balasubramanian P., Hoffmeister J. Gait variability predicts cognitive impairment in older adults with subclinical cerebral small vessel disease. Front. Aging Neurosci. 2022:14. doi: 10.3389/fnagi.2022.1052451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M., Nassir C.M.N.C.M., Aminuddin N., Safri A.A., Ghazali M.M. Cerebral small vessel disease (CSVD)–lessons from the animal models. Front. Physiol. 2019;10:1317. doi: 10.3389/fphys.2019.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano A., Arrigoni A., Caroli A., Cava M., Remuzzi A., Longhi L.G., Barletta A., Zangari R., Lorini F.L., Sessa M., Gerevini S. Cerebral microbleeds assessment and quantification in COVID-19 patients with neurological manifestations. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.884449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neppala S., Sundarakumar D.K., Caravella J.W., Chigurupati H.D., Patibandla P. COVID-19-associated familial acute disseminated encephalomyelitis (ADEM): a case report. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves M.T., de Matos L.V., Vasques A.C., Sousa I.E., Ferreira I., Peres S., Jesus S., Fonseca C., Mansinho K. COVID-19 and aging: identifying measures of severity. SAGE Open Med. 2021;9 doi: 10.1177/20503121211027462. 20503121211027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otifi H.M., Adiga B.K. Endothelial dysfunction in covid-19 infection. Am. J. Med Sci. 2022;363(4):281–287. doi: 10.1016/j.amjms.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens C.D., Mukli P., Csipo T., Lipecz A., Silva-Palacios F., Dasari T.W., Tarantini S., Gardner A.W., Montgomery P.S., Waldstein S.R. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am. J. Physiol. -Heart Circ. Physiol. 2022;322(6):H924–H935. doi: 10.1152/ajpheart.00616.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. bmj. 2021:372. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliogiannis P., Mangoni A.A., Dettori P., Nasrallah G.K., Pintus G., Zinellu A. D-dimer concentrations and COVID-19 severity: a systematic review and meta-analysis. Front. Public Health. 2020;8:432. doi: 10.3389/fpubh.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Pantoni L., Garcia J.H., Gutierrez J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Pelizzari L., Cazzoli M., Lipari S., Laganà M.M., Cabinio M., Isernia S., Pirastru A., Clerici M., Baglio F. Mid-term MRI evaluation reveals microstructural white matter alterations in COVID-19 fully recovered subjects with anosmia presentation. Ther. Adv. Neurol. Disord. 2022;15 doi: 10.1177/17562864221111995. 17562864221111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M.J., Lu S., Tang A.F., Durstenfeld M.S., Ho H.-e, Goldberg S.A., Forman C.A., Munter S.E., Hoh R., Tai V., Chenna A., Yee B.C., Winslow J.W., Petropoulos C.J., Greenhouse B., Hunt P.W., Hsue P.Y., Martin J.N., Daniel Kelly J., Glidden D.V., Deeks S.G., Henrich T.J. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021;224(11):1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E.L., Goßling A., Adam G., Aepfelbacher M., Behrendt C.A., Cavus E., Cheng B., Fischer N., Gallinat J., Kühn S., Gerloff C., Koch-Gromus U., Härter M., Hanning U., Huber T.B., Kluge S., Knobloch J.K., Kuta P., Schmidt-Lauber C., Lütgehetmann M., Magnussen C., Mayer C., Muellerleile K., Münch J., Nägele F.L., Petersen M., Renné T., Riedl K.A., Rimmele D.L., Schäfer I., Schulz H., Tahir E., Waschki B., Wenzel J.P., Zeller T., Ziegler A., Thomalla G., Twerenbold R., Blankenberg S. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City Health Study COVID programme. Eur. Heart J. 2022;43(11):1124–1137. doi: 10.1093/eurheartj/ehab914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M., Nägele F.L., Mayer C., Schell M., Petersen E., Kühn S., Gallinat J., Fiehler J., Pasternak O., Matschke J., Glatzel M., Twerenbold R., Gerloff C., Thomalla G., Cheng B. Brain imaging and neuropsychological assessment of individuals recovered from a mild to moderate SARS-CoV-2 infection. medRxiv. 2022 doi: 10.1101/2022.07.08.22277420. 2022.2007.2008.22277420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson I., Hansen B.M., Svenningsson A., Lundstrom A. Cerebral microvascular injuries in severe COVID-19 infection: progression of white matter hyperintensities post-infection. BMJ Case Rep. 2022;15(9) doi: 10.1136/bcr-2022-249156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza F., Bozzali M., Morana G., Ferrero B., Rizzone M.G., Artusi C.A., Parisi M., Robert A., Imbalzano G., Romagnolo A., Zibetti M., Lopiano L. Early reversible leukoencephalopathy and unilateral sixth cranial nerve palsy in mild COVID-19 infection. Neurol. Sci. 2021;42(12):4899–4902. doi: 10.1007/s10072-021-05545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon R.T., Kumalasari M.D., Kristina H. Ischemic stroke following COVID-19 in a patient without comorbidities. Case Rep. Med. 2021;2021 doi: 10.1155/2021/8178529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planinc D., El-Rekaby A., Sivakumar R., Saksena R., Ngeh J. Acute stroke showing cerebral infarcts and microbleeds in a 31-year-old man with COVID-19 pneumonia. Br. J. Hosp. Med (Lond. ) 2020;81(8):1–3. doi: 10.12968/hmed.2020.0366. [DOI] [PubMed] [Google Scholar]
- Poletti S., Paolini M., Mazza M.G., Palladini M., Furlan R., Querini P.R., Benedetti F. Lower levels of glutathione in the anterior cingulate cortex associate with depressive symptoms and white matter hyperintensities in COVID-19 survivors. Eur. Neuropsychopharmacol. 2022;61:71–77. doi: 10.1016/j.euroneuro.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care. 2020;24(1):1–8. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick S., Moss J., Rajani R.M., Williams A. A vessel for change: endothelial dysfunction in cerebral small vessel disease. Trends Neurosci. 2021;44(4):289–305. doi: 10.1016/j.tins.2020.11.003. [DOI] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M., Borja M.J., Zan E., Fatterpekar G.M. COVID-19-associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology. 2020;297(1):E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani B., Ghashghayi E., Zendehdel M., Baghbanzadeh A., Khodadadi M. Molecular mechanisms highlighting the potential role of COVID-19 in the development of neurodegenerative diseases. Physiol. Int. 2022;109(2):135–162. doi: 10.1556/2060.2022.00019. [DOI] [PubMed] [Google Scholar]
- Rajendran I., Natarajan M.D., Narwani P., Alzouabi O., Kawafi K., Khanna N. A case of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) presenting as post-infectious manifestation of SARS-CoV-2 infection. BJR Case Rep. 2021;7(3) doi: 10.1259/bjrcr.20210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int J. Biol. Sci. 2013;9(10):1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendran A., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab. Syndr.: Clin. Res. Rev. 2021;15(3):869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riech S., Kallenberg K., Moerer O., Hellen P., Bärtsch P., Quintel M., Knauth M. The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit. Care Med. 2015;43(9):e386–e389. doi: 10.1097/CCM.0000000000001150. [DOI] [PubMed] [Google Scholar]
- Rudilosso S., Esteller D., Urra X., Chamorro Á. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J. Stroke Cereb. Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl L., Pink I., Kühne J.F., Beushausen K., Keil J., Christoph S., Sauer A., Boblitz L., Schmidt J., David S. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021;6(1):1–15. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabioni L., De Lorenzo A., Lamas C., Muccillo F., Castro-Faria-Neto H.C., Estato V., Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Micro Res. 2020;134 doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashindranath M., Nandurkar H.H. Endothelial Dysfunction In The Brain: Setting The Stage For Stroke And Other Cerebrovascular Complications of COVID-19. Stroke. 2021;52(5):1895–1904. doi: 10.1161/STROKEAHA.120.032711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawlani V., Scotton S., Nader K., Jen J.P., Patel M., Gokani K., Denno P., Thaller M., Englezou C., Janjua U., Bowen M., Hoskote C., Veenith T., Hassan-Smith G., Jacob S. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin. Radio. 2021;76(2):108–116. doi: 10.1016/j.crad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiß C., Willscher E., Paschold L., Gottschick C., Klee B., Henkes S.-S., Bosurgi L., Dutzmann J., Sedding D., Frese T., Girndt M., Höll J.I., Gekle M., Mikolajczyk R., Binder M. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022;3(6) doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioli M.G., Bielli A., Arcuri G., Ferlosio A., Orlandi A. Ageing and microvasculature. Vasc. Cell. 2014;6(1):1–15. doi: 10.1186/2045-824X-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz A., Ong P. Endothelial dysfunction in COVID-19: A Potential predictor of long-COVID. Int. J. Cardiol. 2022;349:155–156. doi: 10.1016/j.ijcard.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeyedAlinaghi S., Afsahi A.M., MohsseniPour M., Behnezhad F., Salehi M.A., Barzegary A., Mirzapour P., Mehraeen E., Dadras O. Late complications of COVID-19; a systematic review of current evidence. Arch. Acad. Emerg. Med. 2021;9(1) doi: 10.22037/aaem.v9i1.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabir O., Berwick J., Francis S.E. Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci. 2018;19(1):1–16. doi: 10.1186/s12868-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahjouei S., Tsivgoulis G., Farahmand G., Koza E., Mowla A., Vafaei Sadr A., Kia A., Vaghefi Far A., Mondello S., Cernigliaro A., Ranta A., Punter M., Khodadadi F., Naderi S., Sabra M., Ramezani M., Amini Harandi A., Olulana O., Chaudhary D., Lyoubi A., Campbell B.C.V., Arenillas J.F., Bock D., Montaner J., Aghayari Sheikh Neshin S., Aguiar de Sousa D., Tenser M.S., Aires A., Alfonso M.L., Alizada O., Azevedo E., Goyal N., Babaeepour Z., Banihashemi G., Bonati L.H., Cereda C.W., Chang J.J., Crnjakovic M., De Marchis G.M., Del Sette M., Ebrahimzadeh S.A., Farhoudi M., Gandoglia I., Gonçalves B., Griessenauer C.J., Murat Hanci M., Katsanos A.H., Krogias C., Leker R.R., Lotman L., Mai J., Male S., Malhotra K., Malojcic B., Mesquita T., Mir Ghasemi A., Mohamed Aref H., Mohseni Afshar Z., Moon J., Niemelä M., Rezai Jahromi B., Nolan L., Pandhi A., Park J.H., Marto J.P., Purroy F., Ranji-Burachaloo S., Carreira N.R., Requena M., Rubiera M., Sajedi S.A., Sargento-Freitas J., Sharma V.K., Steiner T., Tempro K., Turc G., Ahmadzadeh Y., Almasi-Dooghaee M., Assarzadegan F., Babazadeh A., Baharvahdat H., Cardoso F.B., Dev A., Ghorbani M., Hamidi A., Hasheminejad Z.S., Hojjat-Anasri Komachali S., Khorvash F., Kobeissy F., Mirkarimi H., Mohammadi-Vosough E., Misra D., Noorian A.R., Nowrouzi-Sohrabi P., Paybast S., Poorsaadat L., Roozbeh M., Sabayan B., Salehizadeh S., Saberi A., Sepehrnia M., Vahabizad F., Yasuda T.A., Ghabaee M., Rahimian N., Harirchian M.H., Borhani-Haghighi A., Azarpazhooh M.R., Arora R., Ansari S., Avula V., Li J., Abedi V., Zand R. SARS-CoV-2 and Stroke Characteristics: A Report From the Multinational COVID-19 Stroke Study Group. Stroke. 2021;52(5):e117–e130. doi: 10.1161/strokeaha.120.032927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Vanhoutte P.M. Macro‐and microvascular endothelial dysfunction in diabetes: 糖尿病诱导的内皮细胞功能损伤. J. Diabetes. 2017;9(5):434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- Shoskes A., Migdady I., Fernandez A., Ruggieri P., Rae-Grant A. Cerebral microhemorrhage and purpuric rash in COVID-19: the case for a secondary microangiopathy. J. Stroke Cereb. Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoskes A., Huang M., Gedansky A., Hassett C., Buletko A.B., Duggal A., Uchino K., Cho S.M. MRI of cerebrovascular injury associated with COVID-19 and Non-COVID-19 acute respiratory distress syndrome: a matched case-control study. Crit. Care Med. 2022;50(11):1638–1643. doi: 10.1097/ccm.0000000000005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Andrade B., Siqueira S., de Assis Soares W.R., de Souza Rangel F., Santos N.O., dos Santos Freitas A., Ribeiro da Silveira P., Tiwari S., Alzahrani K.J., Góes-Neto A. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. doi: 10.3390/v13040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond F.A., Hurwitz S., Salat D.H., Greve D.N., Fisher N.D. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Hertelendy P., Tucsek Z., Valcarcel-Ares M.N., Smith N., Menyhart A., Farkas E., Hodges E.L., Towner R., Deak F. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow. Metab. 2015;35(11):1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Tran C.H.T., Gordon G.R., Ungvari Z., Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Valcarcel‐Ares N.M., Yabluchanskiy A., Fulop G.A., Hertelendy P., Gautam T., Farkas E., Perz A., Rabinovitch P.S., Sonntag W.E. Treatment with the mitochondrial‐targeted antioxidant peptide SS‐31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2) doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Valcarcel-Ares M.N., Toth P., Yabluchanskiy A., Tucsek Z., Kiss T., Hertelendy P., Kinter M., Ballabh P., Süle Z., Farkas E., Baur J.A., Sinclair D.A., Csiszar A., Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Yabluchanskiy A., Csipo T., Fulop G., Kiss T., Balasubramanian P., DelFavero J., Ahire C., Ungvari A., Nyúl-Tóth Á. Treatment with the poly (ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41(5):533–542. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S., Balasubramanian P., Delfavero J., Csipo T., Yabluchanskiy A., Kiss T., Nyúl-Tóth Á., Mukli P., Toth P., Ahire C. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. GeroScience. 2021;43(5):2427–2440. doi: 10.1007/s11357-021-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B.E.S., Khandelwal P., Rallo M.S., Patel P., Smith L., Sun H., Nanda A., Singla A., Roychowdhury S., Cheng R.C., Lee K., Gupta G., Johnson S.A. Outcomes and spectrum of major neurovascular events among COVID-19 patients: a 3-center experience. Neurosurg. Open. 2020;1(3):okaa008. doi: 10.1093/neuopn/okaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeback J., Depoortere S.D., Vermassen J., Vereecke E.L., Van Driessche V., Hemelsoet D.M. Microbleed patterns in critical illness and COVID-19. Clin. Neurol. Neurosurg. 2021;203 doi: 10.1016/j.clineuro.2021.106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P., Tarantini S., Tucsek Z., Ashpole N.M., Sosnowska D., Gautam T., Ballabh P., Koller A., Sonntag W.E., Csiszar A. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. -Heart Circ. Physiol. 2014;306(3):H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P., Tarantini S., Csiszar A., Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017;312(1):H1–h20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifan G., Hillmann M., Testai F.D. Acute stroke as the presenting symptom of SARS-CoV-2 infection in a young patient with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Stroke Cereb. Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristán-Samaniego D.P., Chiquete E., Treviño-Frenk I., Rubalcava-Ortega J., Higuera-Calleja J.A., Romero-Sánchez G., Espinoza-Alvarado L., Barrera-Vargas A., Flores-Silva F., González-Duarte A., Vega-Boada F., Cantú-Brito C. COVID-19-related diffuse posthypoxic leukoencephalopathy and microbleeds masquerades as acute necrotizing encephalopathy. Int. J. Neurosci. 2022;132(11):1123–1127. doi: 10.1080/00207454.2020.1865346. [DOI] [PubMed] [Google Scholar]
- Tucsek Z., Toth P., Tarantini S., Sosnowska D., Gautam T., Warrington J.P., Giles C.B., Wren J.D., Koller A., Ballabh P. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2014;69(11):1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uginet M., Breville G., Hofmeister J., Machi P., Lalive P.H., Rosi A., Fitsiori A., Vargas M.I., Assal F., Allali G., Lovblad K.O. Cerebrovascular complications and vessel wall imaging in COVID-19 encephalopathy-a pilot study. Clin. Neuroradiol. 2022;32(1):287–293. doi: 10.1007/s00062-021-01008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ. Res. 2018;123(7):849–867. doi: 10.1161/circresaha.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattoth S., Abdelhady M., Alsoub H., Own A., Elsotouhy A. Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol. J. 2020;33(5):374–376. doi: 10.1177/1971400920939229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbracht C., Kraft K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: implications for the benefit of high-dose intravenous vitamin C. Front Pharm. 2022;13 doi: 10.3389/fphar.2022.899198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.Q., Wang L., Wei M.M., Xia X.S., Tian X.L., Cui X.H., Li X. Relationship between type 2 diabetes and white matter hyperintensity: a systematic review. Front Endocrinol. (Lausanne) 2020;11 doi: 10.3389/fendo.2020.595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tang C., Liu Y., Border J.J., Roman R.J., Fan F. Impact of impaired cerebral blood flow autoregulation on cognitive impairment. Front Aging. 2022;3 doi: 10.3389/fragi.2022.1077302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T., Krajewski P., Tarka S., Acewicz A., Felczak P., Stępień T., M P.G., Grzegorczyk M. Neuropathological analysis of the brains of fifty-two patients with COVID-19. Folia Neuropathol. 2021;59(3):219–231. doi: 10.5114/fn.2021.108829. [DOI] [PubMed] [Google Scholar]
- Williams O.H., Mohideen S., Sen A., Martinovic O., Hart J., Brex P.A., Sztriha L.K. Multiple internal border zone infarcts in a patient with COVID-19 and CADASIL. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvoet E.H., Jiang F.Y., Laumans W., de Bruijn S. COVID-19-related diffuse leukoencephalopathy with microbleeds and persistent coma: a case report with good clinical outcome. BMJ Case Rep. 2021;14(8) doi: 10.1136/bcr-2021-242504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerdeman J., van Duinkerken E., Wattjes M.P., Barkhof F., Snoek F.J., Moll A.C., Klein M., de Boer M.P., IJzerman R.G., Serné E.H. Proliferative retinopathy in type 1 diabetes is associated with cerebral microbleeds, which is part of generalized microangiopathy. Diabetes Care. 2014;37(4):1165–1168. doi: 10.2337/dc13-1586. [DOI] [PubMed] [Google Scholar]
- Wu H., Wang Y., Zhang Y., Xu F., Chen J., Duan L., Zhang T., Wang J., Zhang F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Ilyas I., Little P.J., Li H., Kamato D., Zheng X., Luo S., Li Z., Liu P., Han J. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol. Rev. 2021;73(3):924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- Xu S.-w, Ilyas I., Weng J.-p. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023;44(4):695–709. doi: 10.1038/s41401-022-00998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ran M., Li H., Lin Y., Ma K., Yang Y., Fu X., Yang S. New insight into neurological degeneration: Inflammatory cytokines and blood–brain barrier [Review] Front. Mol. Neurosci. 2022:15. doi: 10.3389/fnmol.2022.1013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoutsos A., Levy B.I., Safar M.E., Slama G., Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J. Hypertens. 2014;32(2):216–224. doi: 10.1097/HJH.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z., Chen X., Chen S., Yu K., Huang Z. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. Intensive Care. 2020;8:1–11. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufuddin M., Young N. Aging and ischemic stroke. Aging. 2019;11(9):2542–2544. doi: 10.18632/aging.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Hirsh E., Zandieh S., Rodricks M.B. COVID-19-associated acute multi-infarct encephalopathy in an asymptomatic CADASIL patient. Neurocrit Care. 2021;34(3):1099–1102. doi: 10.1007/s12028-020-01119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Yang J., Xie P., Dong Y., You Y., Liu J. Cerebral microbleeds, cognitive impairment, and MRI in patients with diabetes mellitus. Clin. Chim. Acta. 2017;470:14–19. doi: 10.1016/j.cca.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Zhu W.M., Neuhaus A., Beard D.J., Sutherland B.A., DeLuca G.C. Neurovascular coupling mechanisms in health and neurovascular uncoupling in Alzheimer’s disease. Brain. 2022 doi: 10.1093/brain/awac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaka M., Exadaktylos A. ARDS associated acute brain injury: from the lung to the brain. Eur. J. Med. Res. 2022;27(1):1–11. doi: 10.1186/s40001-022-00780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L., Prather E.R., Stetskiv M., Garrison D.E., Meade J.R., Peace T.I., Zhou T. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019;20(18):4472. doi: 10.3390/ijms20184472. 〈https://www.mdpi.com/1422-0067/20/18/4472〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
No data was used for the research described in the article.